Abstract
Introduction
In this meta-analysis, we analyzed retrospective cohort studies that assessed the prognostic potential of the pretreatment lymphocyte-to-monocyte ratio (LMR) among patients with ovarian cancer (OC).
Materials and methods
We comprehensively searched electronic databases, including PubMed and Embase, from inception through October 2018. A random-effects model was used to calculate pooled HRs and their 95% CIs for overall survival (OS) and progression-free survival (PFS). The low LMR group was treated as the reference group.
Results
Twelve studies, including 3,346 OC cases at baseline, were included. Overall, our results indicated that LMR was positively associated with both OS (HR: 1.85, 95% CI: 1.50–2.28, P<0.001; I2=76.5%) and PFS (HR: 1.70, 95% CI: 1.49–1.94, P<0.001; I2=24.4%) among OC patients. Stratified analyses indicated that, for OS, the LMR’s protective effect was more evident in studies conducted among younger patients (<55 years) than in those conducted among older patients (≥55 years; P for interaction =0.017), which was confirmed by meta-regression analysis (P=0.004).
Conclusion
This study suggested that a higher pretreatment LMR level was associated with a favorable prognosis among OC patients. Future large-scale prospective clinical trials are needed to confirm the prognostic value of LMR among OC patients.
Keywords: ovarian cancer, lymphocyte-to-monocyte ratio, prognosis, meta-analysis
Introduction
Ovarian cancer (OC) is the fifth most common cause of cancer-related deaths among women, with ~90% of these cases being epithelial ovarian cancer (EOC).1 At the start of 2018, there were an estimated 22,240 newly diagnosed cases and 14,070 deaths due to OC in USA.1 Although OC is less common than other cancers such as breast cancer, OC is attracting increased attention because of its poor prognosis. The 5-year survival rate for OC is only 47.2%. Although great progress has been made in cancer research, the overall prognosis for OC remains poor, because it is often diagnosed late in the disease process and has high recurrence rates after curative resection.2 Therefore, more effective and convenient markers must be identified to estimate the prognosis and select appropriate treatment strategies.
Over the past decades, many theories have been postulated to explain OC’s etiology, and most of them converge on the role of inflammation.3 The systemic inflammatory response is associated with survival in advanced and localized cancers.4 Cancer-related inflammation includes modulating inflammatory cells and mediators such as cytokines and chemokines; however, these markers are not routinely measured despite their direct changes provide a direct surrogate marker of expression (eg, lymphocyte-to-monocyte ratio [LMR]).4 Several recent studies assessed the prognostic effect of pretreatment LMR among patients with OC, but the results were inconsistent. Elevated LMR was shown to increase survival in some,5–7 but not all,8–11 studies. As the statistical power of an individual study may be too weak to identify associations between pretreatment LMR and OC patient survival (sample size of most included studies was <300 OC patients), a meta-analysis combining data from all published studies may be more convincing.
Thus, we conducted a meta-analysis to evaluate the prognostic effect of pretreatment LMR on OC patient survival, which included all eligible publications to date.
Materials and methods
This meta-analysis was conducted in accordance with the PRISMA (Table S1).12
Search strategies
A comprehensive literature search of PubMed, Embase, Web of Science, Chinese National Knowledge Infrastructure (CNKI, http://www.cnki.net), and the Wanfang databases (http://www.wanfangdata.com.cn) was conducted from inception through October 2018. The following search terms were used: (lymphocyte-to-monocyte or lymphocyte monocyte or lymphocyte-monocyte or lymphocyte to monocyte or lymphocyte/monocyte or LMR) AND (cancer* or carcinoma* or neoplasm* or malignan* or tumour* or tumor*) AND (ovary or ovarian) without language restriction (Supplementary materials). Related articles generated by Google Scholar (http://scholar.google.com) and PubMed were retrieved. We also scanned the reference lists of related articles to identify all potential useful studies on OC that might have been missed in our database searches.
Study selection
Inclusion criteria were as follows: 1) studies on patients with OC diagnosed histopathologically; 2) studies that assessed the prognostic value of pretreatment LMR among OC patients; 3) studies that reported the LMR cutoff value; 4) studies that reported sufficient information for calculating the HR and its 95% CI; and 5) studies that used overall survival (OS) and/or progression-free survival (PFS) as outcomes. For studies with overlapping data, only the most relevant articles with the largest datasets were included in the final analysis.
Data extraction
Two independent reviewers (X-PG and Y-HL) evaluated all potential articles for inclusion. Disagreements were resolved by discussion among all coauthors. The following information was collected: the first author’s name, publication year, country (region) and ethnicity of the population, publication type, number of OC patients at baseline, age, year of recruitment, time of follow-up, treatment method, tumor stage, histological type, LMR cutoff value, method of obtaining cutoff value, OC diagnostic criteria, survival analysis methods, and prognostic end points (OS or PFS). HRs were extracted from multivariate or univariate analyses or Kaplan–Meier survival curves. If only Kaplan–Meier curves were provided, we extracted data from the survival curves using Engauge Digitizer v.4.1 software.13
Quality assessment
Each study’s methodological quality was assessed as per the Newcastle–Ottawa Scale (NOS),14 which was used to allocate a maximum of nine stars for selection quality of the study population, comparability, and outcome. The studies’ quality scores ranged from 0 to 9, with 7–9 points indicating a high-quality study and 0–6 points indicating a low-quality study.
Statistical analyses
The DerSimonian and Laird random-effects model of inverse variance methods was used to estimate the pooled HRs and 95% CIs. Unless otherwise stated, we used the most fully adjusted RRs from each study, and the low LMR group was treated as the reference group. If the studies used different reference groups to estimate the LMR HR for OS/PFS, we used an Excel macro file to transform the reference group.15
The random-effects model was chosen a priori, because it is considered to be more conservative than the fixed-effects model and it accounts for both within- and between-study heterogeneity.16 Between-study heterogeneity was tested using Cochran’s Q test and Higgins I2 statistic (higher I2 values denote greater heterogeneity).17 We performed subgroup analyses for both OS and PFS to examine the robustness of the results by age (<55 vs ≥55 years), LMR cutoff value (≤3.0 vs >3.0), sample size (≤200 vs >200), and NOS score (<7 and ≥7 points). Influence analysis was also conducted to assess the effect of a single study on the pooled estimates.18 These variables were also analyzed as covariates in the meta-regression analysis. Publication bias was assessed by visually inspecting funnel plots and quantitatively evaluated using Egger’s and Begg’s linear regression asymmetry tests.17 All data were analyzed using Stata software, version 11.0 (StataCorp LP, College Station, TX, USA), and a two-sided P<0.05 was considered statistically significant.
Results
Search results
The electronic database searches identified 1,018 articles (Figure 1), of which 180 duplications were excluded by Endnote. After assessing titles and abstracts and screening full texts, 824 unrelated articles were excluded. For the remaining 14 potentially eligible articles, 2 duplicate studies, 1 duplicate study,19 and 1 study with incomplete data20 were further excluded. Finally, 12 studies were included.5–11,21–25
Figure 1.
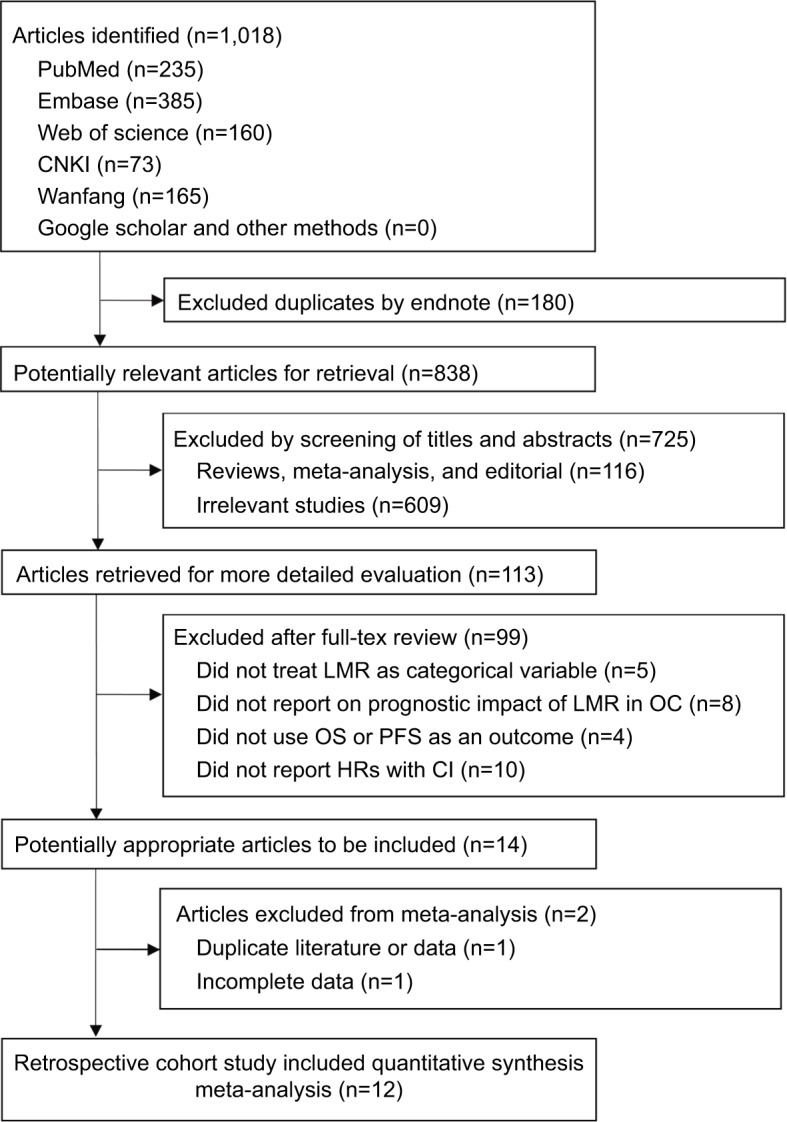
Flowchart of study selection in the current meta-analysis.
Abbreviations: CNKI, Chinese National Knowledge Infrastructure; LMR, lymphocyte-to-monocyte ratio; OC, ovarian cancer; OS, overall survival; PFS, progression-free survival.
Characteristics of the included studies
Table 1 summarizes the characteristics of the included studies. In total, 3,346 OC patients (weighted age: 55.8 years) were included, with a follow-up period ranging from 23.6 to 58 months. All studies were published in 2016 or later. The number of patients per study ranged from 42 to 672. Eight studies were conducted among Chinese patients, three among Korean patients, and one among American Caucasian patients. The LMR cutoff values ranged from 1.85 to 4.35. The overall NOS scores ranged from 5 to 8 points (Table S2). Most cases were EOC, and 76.5% were stage III/IV. Among these studies, three investigated only OS, while nine investigated both OS and PFS.
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Year | Country | Ethnicity | Duration of study conducted | Follow-up duration (months) | Age (years)a | Pathological type | Pathological subtypes, n (%) | No. of patientsb | No. of stage I/II | No. of stage III/IV | Outcome | Cutoff value | Method of obtaining cutoff | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Eo et al7 | 2016 | Korea | Asian | 2006.1– 2013.12 | NA | 54 (14–84) | EOC | Serous: 132 (56.4) Mucinous: 35 (15.0) Clear cell: 35 (15.0) Endometrioid: 21 (10.0) Mixed epithelial: 5 (2.1) Others: 6 (2.6) |
234 | 97 | 137 | OS and PFS | 2.07 | ROC curve | 6 |
| Sun and Song21 | 2016 | China | Asian | 2006.2– 2014.4 | NA | 55 (17–84) | EOC | Serous: 103 (54.5) Others: 86 (45.5) |
189 | 78 | 111 | OS and PFS | 1.85 | ROC curve | 6 |
| Wang et al24 | 2016 | China | Asian | 2000.1– 2013.12 | NA | 56.1±10.2 | OC | Serous: 214 (89.2) Mucinous: 22 (9.2) Endometrioid: 2 (0.8) Clear cell: 2 (0.8) |
240 | 59 | 181 | OS | 3.949 | ROC curve | 6 |
| Kwon et al9 | 2017 | Korea | Asian | 2009.4– 2012.6 2000–2010 |
NA | 70 (65–85) | EOC | Serous: 33 (78.6) Non-serous: 9 (21.4) |
42 | 0 | 42 | OS and PFS | 3.63 | ROC curve | 6 |
| Li et al10 | 2017 | USA | Caucasian | 2000–2010 | 49.5 (0.1–175.3) | 63 (28–93) | EOC | High-grade serous: 525 (80.3) Low-grade serous: 4 (0.6) Endometrioid: 71 (10.9) Clear cell: 37 (5.7) Mucinous: 17 (2.6) |
654 | 121 | 533 | OS and PFS | 2.22 | ROC curve | 8 |
| Xiang et al11 | 2017 | China | Asian | 2011.1– 2016.3 2007.1– 2015.12 |
23.6 (0.77–69.4) | 53.3±13.6 (20–82) | OC | Serous: 87 (65.4) Mucinous: 14 (10.5) Endometrioid: 10 (7.5) Clear cell: 5 (3.8) Others: 17 (12.8) |
124 | 64 | 69 | OS | 4.35 | ROC curve | 6 |
| Zhang et al5 | 2017 | China | Asian | 2007.1– 2015.12 | NA | 50 (24–76) | OC | Serous: 123 (51.9) Non-serous: 114 (36.3) |
237 | 67 | 170 | OS and PFS | 3.82 | NA | 5 |
| Zhu et al6 | 2017 | China | Asian | 2008.6– 2015.12 | 38 (5–103) | 55 (30–70) | EOC | Serous: 484 (72.0) Non-serous: 188 (28.0) |
672 | 0 | 672 | OS and PFS | 3.45 | ROC curve | 7 |
| Tang et al22 | 2017 | China | Asian | 2005.1– 2015.1 | 46 (2–120) | 52.2±12.0 | EOC | Serous: 146 (68.2) Non-serous: 68 (31.8) |
214 | 99 | 115 | OS | 3.85 | ROC curve | 7 |
| Tian23 | 2017 | China | Asian | 2009.1– 2011.7 | 58 (2–60) | 54 (14–76) | EOC | Serous: 166 (62.2) Mucinous: 21 (7.9) Endometrioid: 65 (24.3) Clear cell: 14 (5.2) Others: 1 (0.4) |
267 | 86 | 181 | OS and PFS | 3.09 (OS) 2.07 (PFS) | ROC curve | 7 |
| Yang and Lo25 | 2017 | China | Asian | 2005.1–2011.5 | 37 (1–112) | 54 (22–78) | EOC | Serous: 206 (56.6) Mucinous: 111 (30.5) Endometrioid: 23 (6.3) Others: 24 (6.6) |
364 | 52 | 312 | OS and PFS | 3.84 | ROC curve | 7 |
| Kwon et al8 | 2018 | Korea | Asian | 2005.1–2011.5 | NA | 50 (24–77) | OCCC | OCCC: 109 (100) | 109 | 64 | 45 | OS and PFS | 4.2 | ROC curve | 6 |
Notes:
Mean, median (range) of age at baseline.
Number of patients at baseline.
Abbreviations: EOC, epithelial ovarian cancer; NA, not available; NOS, Newcastle–Ottawa Scale; OC, ovarian cancer; OCCC, ovarian clear cell carcinoma; OS, overall survival; PFS, progression-free survival; ROC, receiver-operator characteristic.
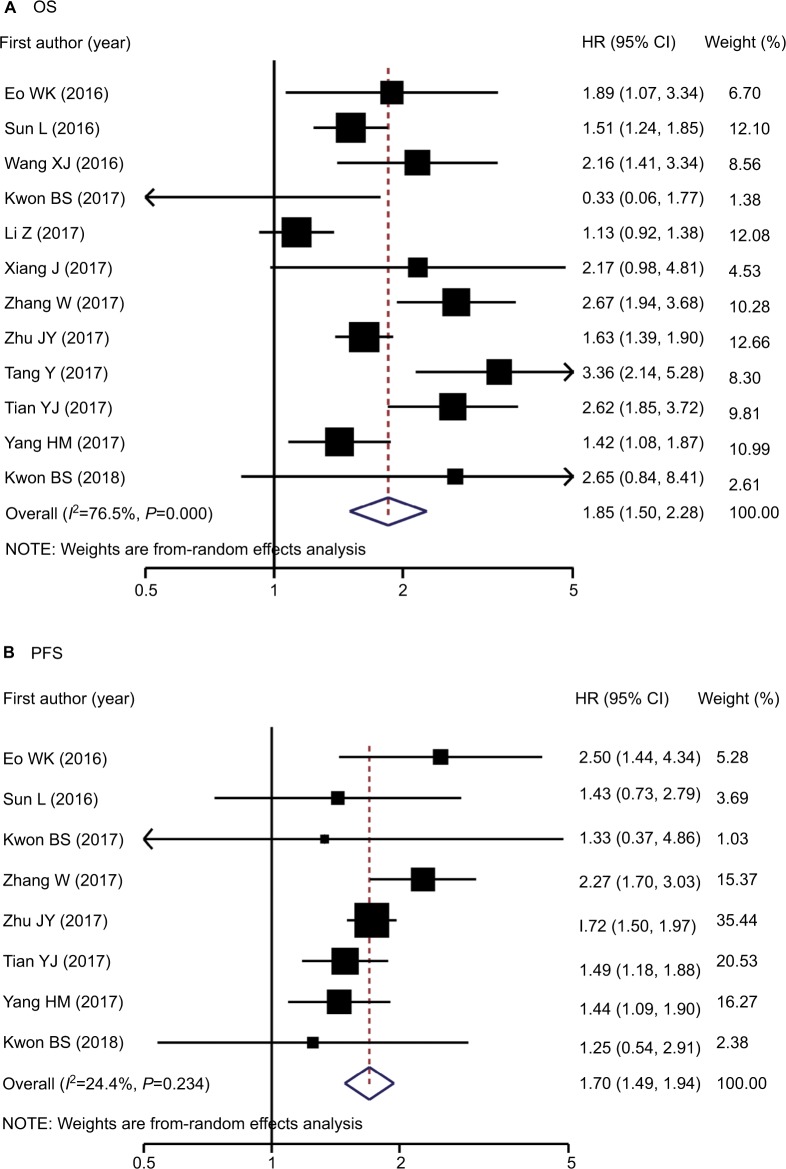
Association between LMR and OS among OC patients
Twelve studies involving 3,346 patients reported LMR and OS data among OC cases.5–11,21–25 Increased LMR was associated with improved OS (pooled HR: 1.85, 95% CI: 1.50–2.28, P<0.001) with significant between-study heterogeneity (P<0.001, I2=76.5%; Table 2 and Figure 2A). The association persisted after reanalyzing studies among Asian patients or those with only EOC. Stratified analyses for age, LMR cutoff values, sample size, and NOS score revealed significant interactions for age (P for interaction =0.017) and LMR cutoff values (P for interaction =0.025). The protective effect of elevated LMR was more evident among younger patients than older patients (HR: 2.28 vs 1.47) and among studies using an LMR cutoff of >3.0 than in those using ≤3.0 (HR: 2.09 vs 1.38). Meta-regression analysis further confirmed that age, but not LMR cutoff values, significantly contributed to inter-study heterogeneity (P for regression =0.004 and 0.153; Table S3).
Table 2.
Total, stratified, and sensitivity analyses of the associations between pretreatment LMR and survival among OC patients
| Groups | OS | PFS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Noa | RR (95% CIs)b | Pc | I2 (%) | Pd | Noa | RR (95% CIs)b | Pc | I2 (%) | Pd | |
|
| ||||||||||
| Overall | 125–11,21–25 | 1.85 (1.50–2.28) | <0.001 | 76.5 | <0.001 | 85–9,21,23,25 | 1.70 (1.49–1.94) | <0.001 | 24.4 | 0.234 |
| Asian only | 115–9,11,21–25 | 1.97 (1.62–2.40) | <0.001 | 67.0 | 0.001 | 85–9,21,23,25 | 1.70 (1.49–1.94) | <0.001 | 24.4 | 0.234 |
| EOC only | 86,7,9,10,21–23,25 | 1.69 (1.34–2.13) | <0.001 | 79.4 | <0.001 | 66,7,9,21,23,25 | 1.64 (1.48–1.82) | <0.001 | 0.0 | 0.483 |
| Subgroup analyses | ||||||||||
| Age (years) | ||||||||||
| <55 | 75,7,8,11,22,23,25 | 2.28 (1.72–3.01) | <0.001 | 61.9 | 0.015 | 55,7,8,23,25 | 1.74 (1.37–2.20) | <0.001 | 54.7 | 0.065 |
| ≥55 | 56,9,10,21,24 | 1.47 (1.17–1.85) | 0.001 | 72.2 | 0.006 | 36,9,21 | 1.70 (1.50–1.94) | <0.001 | 0.0 | 0.811 |
| LMR cutoff values | ||||||||||
| ≤3.0 | 37,10,21 | 1.38 (1.06–1.80) | 0.015 | 64.0 | 0.062 | 27,21 | 1.81 (1.11–2.97) | 0.018 | 65.3 | 0.090 |
| >3.0 | 95,6,8,9,11,22–25 | 2.09 (1.63–2.67) | <0.001 | 70.1 | 0.001 | 65,6,8,9,23,25 | 1.72 (1.48–1.99) | <0.001 | 18.0 | 0.297 |
| Sample size | ||||||||||
| ≤200 | 48,9,11,21 | 1.59 (0.99–2.56) | 0.057 | 37.8 | 0.186 | 38,9,21 | 1.36 (0.83–2.20) | 0.219 | 0.0 | 0.970 |
| >200 | 85–7,10,22–25 | 1.94 (1.50–2.50) | <0.001 | 83.1 | <0.001 | 55–7,23,25 | 1.74 (1.48–2.06) | <0.001 | 52.0 | 0.080 |
| NOS score | ||||||||||
| <7 | 75,7–9,11,21,24 | 1.94 (1.45–2.60) | <0.001 | 57.1 | 0.030 | 55,7–9,21 | 2.06 (1.65–2.59) | <0.001 | 0.0 | 0.429 |
| ≥7 | 56,10,22,23,25 | 1.79 (1.31–2.46) | <0.001 | 86.7 | <0.001 | 36,23,25 | 1.63 (1.46–1.81) | <0.001 | 0.0 | 0.379 |
| Influence analysese | ||||||||||
| Minimal | – | 1.75 (1.43–2.13) | <0.001 | 72.9 | <0.001 | – | 1.64 (1.48–1.81) | <0.001 | 0.0 | 0.560 |
| Maximal | – | 1.97 (1.62–2.40) | <0.001 | 67.0 | 0.001 | – | 1.76 (1.51–2.05) | <0.001 | 23.0 | 0.254 |
Notes:
Number of studies.
RRs and 95% CIs were pooled by using the random-effects model (the DerSimonian and Laird method).
P-value of Z-test for the significance of pooled RRs and 95% CIs.
P-value of Q-test for between-study heterogeneity test.
Influence analysis was conducted by eliminating one study at a time; for OS, the excluded study was the study by Tang et al22 for minimal pooled RRs, and Li et al10 for the maximal pooled RRs; for PFS, the excluded study was the study by Zhang et al5 for minimal pooled RRs, and Tian23 for the maximal pooled RR.
Abbreviations: EOC, epithelial ovarian cancer; LMR, lymphocyte-to-monocyte ratio; NOS, Newcastle–Ottawa Scale; OC, ovarian cancer; OS, overall survival; PFS, progression-free survival.
Figure 2.
Forest plots of studies evaluating HRs of high pretreatment LMR among patients with OC for (A) OS and (B) PFS. Error bars indicate 95% CI.
Abbreviations: LMR, lymphocyte-to-monocyte ratio; OC, ovarian cancer; OS, overall survival; PFS, progression-free survival.
Association between LMR and PFS in OC patients
Eight studies5–9,21,23,25 involving 2,114 patients reported data for the association between LMR and PFS among OC patients, and all studies were conducted among Asian patients. Similar to OS, the random-effects combined analysis demonstrated that LMR was positively and significantly associated with PFS (pooled HR: 1.70, 95% CI: 1.49–1.94, P<0.001) but with low between-study heterogeneity (I2=24.4%; P=0.234; Table 2 and Figure 2B). The result was similar among studies with only EOC cases. Stratified analyses suggested that the association did not differ among NOS scores, LMR cutoff values, and age strata (P interaction range =0.066–0.987). Meta-regression analysis also revealed that publication year, age, NOS score, sample size, and LMR cutoff value did not significantly contribute to heterogeneity (P for regression range =0.086–0.982).
Sensitivity analysis and bias
The sensitivity analyses indicated that the pooled HRs were not obviously influenced by any single study for either OS or PFS (Table 2).
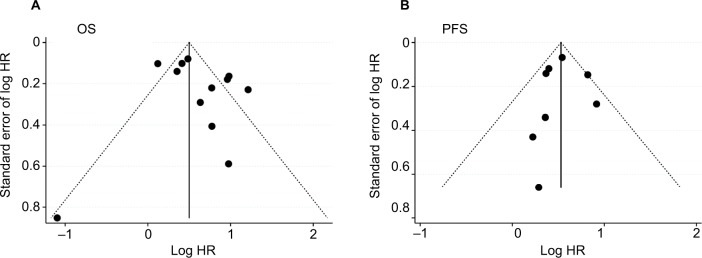
Both Egger’s and Begg’s tests revealed no significant publication bias, and the P-values were 0.732 and 0.272 for OS and 1.000 and 0.887 for PFS. The funnel plots also showed no evidence of publication bias for either OS or PFS (Figure 3).
Figure 3.
Funnel plots of studies evaluating HRs of high pretreatment LMR among patients with OC for (A) OS and (B) PFS.
Abbreviations: LMR, lymphocyte-to-monocyte ratio; OC, ovarian cancer; OS, overall survival; PFS, progression-free survival.
Discussion
In this meta-analysis, we first report the prognostic value of pretreatment LMR among OC patients. Our results indicate that higher pretreatment LMR levels are associated with increased OS and PFS among OC patients. Substantial heterogeneity was observed for OS; further subgroup and meta-regression analyses indicated that age contributed to this heterogeneity, and these associations were more evident among younger patients than older populations.
In recent years, several prognostic indicators derived from peripheral blood, such as LMR, have been widely investigated as useful prognostic markers in cancers. LMR has been identified as an independent prognostic factor in patients with various cancers, such as head and neck,26 pancreatic,27 colorectal,28 hepatocellular,29 and breast cancers.30 Our results were consistent with findings from these studies, showing that higher LMR ratios may improve cancer prognoses.
The exact mechanisms by which LMR has some prognostic relevance in OC patients were still unknown. According to the current evidence, lymphopenia might weaken the efficacy of the immune system and be associated with worse prognosis in cancers; cell-mediated cytotoxicity may be attenuated if the level of effector T cells is insufficient.31 Circulating monocytes may contribute to both tumor growth and reduced immunosurveillance through differentiating into macrophages after infiltrating a tumor and then respond to the wide spectrum of chemokines and growth or differentiation factors.31 Thus, the prognostic effect of LMR among OC patients can be assumed to be related to tumor-infiltrating immune cells, such as tumor-infiltrating lymphocytes (TILs), or tumor-associated macrophages. Circulating TILs, as direct measures of intratumoral immunity, may contribute to cancer growth and spread.32 In OC tumor tissue sections, intraepithelial CD8+ TILs correlated with good outcome, and a high ratio of CD8+/FoxP3+ T regulatory cells was beneficial to survival.33 Recent epidemiological studies have also confirmed that the presence of TILs was associated with improved clinical outcomes in OC patients.34–36 Peripheral blood-based parameters (eg, LMR) have been studied as a surrogate measures of intratumoral immunity that reflect a host’s immune response.4 LMR has been shown a statistically significant correlation with CD8+ TILs among patients with breast cancer.37 Tumor-associated macrophages (TAMs) have been suggested to be involved in accelerating angiogenesis, invasion, migration, and metastasis and suppress the body’s autoimmune response against tumor cells.38,39 In addition, LMR had been supposed to reflect the TIL/TAM ratio, as the circulating levels of lymphocytes and monocytes may indicate the formation or the presence of TILs and TAMs, and significant correlation was observed between the LMR and the TIL/TAM ratio.31 Immunotherapy has emerged as one of the most promising approaches for OC treatment,40 and change in the LMR has been supposed to be an early surrogate marker of the efficacy of nivolumab monotherapy.41 Thus, LMR represents the balance between the host’s immune status and the degree of tumor progression, and it may therefore be a prognostic biomarker among OC patients.
Subgroup analyses indicated that the favorable prognostic effect of pretreatment LMR for OS was more evident in studies conducted among younger (<55 years) than older patients (≥55 years; P for interaction =0.017), which was further confirmed by meta-regression analysis (P=0.004). One explanation for our finding is that human aging is characterized by a gradual increase in subclinical chronic inflammation, and older people are more likely to get chronic inflammatory diseases.42 The greater severity of the inflammatory state among older OC patients may weaken the LMR’s protective prognostic effect. In addition, older patients responded more efficiently to immunotherapy, such as programmed death-ligand 1 (nivolumab and pembrolizumab), and PD-L1 (atezolizumab) inhibitors also confirmed this finding.43,44
Some limitations of this meta-analysis should also be considered. First, between-study heterogeneity was significant for OS (I2: 76.5%). Based on subgroup and meta-regression analyses, age was the main source of heterogeneity, and the pooled HR results showed consistent positive relationships. Second, most studies included herein were performed among Asian patients, while only one study examined OS among Caucasian patients,10 and no relevant studies were found for African patients. Thus, the findings of the present study might be limited to Asian patients, and the prognostic effects of LMR for other populations (eg, Caucasian or African) still need further confirmation. Third, the studies included herein differed in how the covariates were adjusted. However, the pooled estimates were similar between the maximal and minimal numbers of covariate adjustment analyses for both OS and PFS, indicating that these confounders were unlikely to significantly bias our findings (data not shown). Fourth, categorical analysis did not allow detecting the best cutoff point, which invites further studies to solve this problem. Fifth, all included studies were retrospective single-center studies, and the bias was unavoidable.
Conclusion
This meta-analysis demonstrated that higher pretreatment LMR values were associated with more favorable outcomes among OC patients, and the associations were stronger for younger patients than older patients. Future large-scale prospective clinical trials are needed to confirm the LMR’s prognostic effect and its cutoff value among OC patients. Therefore, LMR is a readily available, routinely measured, and inexpensive inflammatory biomarker, and if causation and cutoff value of LMR was established, LMR could be easily applied in daily clinical practice.
Supplementary materials
Detailed search strategies for each database
PubMed (N=215)
#1: lymphocyte-to-monocyte OR lymphocyte monocyte OR lymphocyte-monocyte OR lymphocyte to monocyte OR lymphocyte/monocyte OR LMR
#2: ((cancer* OR carcinoma* OR neoplasm* OR malignan* OR tumour* OR tumor*) AND (ovary OR ovarian)) OR “Ovarian Neoplasms”[mesh]
#3: #1 AND #2
Embase (N=385)
#1: ((cancer* OR carcinoma* OR neoplasm* OR malignan* OR tumour* OR tumor*) AND (ovary OR ovarian)) OR (‘ovarian neoplasms’/exp)
#2: (‘lymphocyte to monocyte’) OR (lymphocyte AND monocyte) OR (‘lymphocyte monocyte’) OR (lymphocyte AND to AND monocyte) OR (LMR) OR (lymphocyte?monocyte)
#3: #1 AND #2
Web of science (N=160)
#1 (Ovarian Neoplasms) OR ((cancer* OR carcinoma* OR neoplasm* OR malignan* OR tumour* OR tumor*) AND (ovary OR ovarian))
#2 (lymphocyte-to-monocyte ratio OR “lymphocyte monocyte ratio” OR “lymphocyte to monocyte ratio” OR LMR)
#3: #1 AND #2
Wanfang (N=165)
#1 摘要:(卵巢癌+卵巢肿瘤)*摘要:(淋巴细胞)*摘 要:(单核细胞))
#2 摘要:(卵巢癌+卵巢肿瘤)*摘要:(LMR) #3 题名或关键词:(卵巢癌+卵巢肿瘤)*题名或关键 词:(淋巴细胞)*题名或关键词:(单核细胞)
#4 题名或关键词:(卵巢癌+卵巢肿瘤)*题名或关键 词:(LMR)
#5 主题:(卵巢癌+卵巢肿瘤)*主题:(淋巴细胞) * 主题:(单核细胞)
#6 主题:(卵巢癌+卵巢肿瘤)*主题:(LMR) #7: #1 OR #2 OR #3 OR #4 OR #5 OR #6
Chinese National Knowledge Infrastructure (CNKI; N=73)
#1 AB=(‘卵巢癌’+’卵巢肿瘤’) and AB=’淋巴 细胞’ and AB=’单核细胞’
#2 AB=(‘卵巢癌’+’卵巢肿瘤’) and AB=’LMR’ #3 TI=(‘卵巢癌’+’卵巢肿瘤’) and TI=’淋巴 细胞’ and TI=’单核细胞’
#4 TI=(‘卵巢癌’+’卵巢肿瘤’) and TI=’LMR’ #5 KY=(‘卵巢癌’+’卵巢肿瘤’) and KY=’淋巴 细胞’ and KY=’单核细胞’
#6 KY=(‘卵巢癌’+’卵巢肿瘤’) and KY=’LMR’ #7 SU=(‘卵巢癌’+’卵巢肿瘤’) and SU=’淋巴 细胞’ and SU=’单核细胞’
#8 SU=(‘卵巢癌’+’卵巢肿瘤’) and SU=’LMR’ #9: #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
Table S1.
PRISMA 2009 checklist
| Section/topic | No. | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 3 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 4, 5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (eg, Web address), and, if available, provide registration information including registration number | NA |
| Eligibility criteria | 6 | Specify study characteristics (eg, PICOS and length of follow-up) and report characteristics (eg, years considered, language, and publication status) used as criteria for eligibility, giving rationale | 6 |
| Information sources | 7 | Describe all information sources (eg, databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 5, Supplementary materials, pages 1–2 |
| Study selection | 9 | State the process for selecting studies (ie, screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 6 |
| Data collection process | 10 | Describe method of data extraction from reports (eg, piloted forms, independently, and in duplicate) and any processes for obtaining and confirming data from investigators | 6 |
| Data items | 11 | List and define all variables for which data were sought (eg, PICOS and funding sources) and any assumptions and simplifications made | 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 7, Table S2 |
| Summary measures | 13 | State the principal summary measures (eg, risk ratio and difference in means) | 7 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (eg, I2) for each meta-analysis | 7 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (eg, publication bias and selective reporting within studies) | 7, 8 |
| Additional analyses | 16 | Describe methods of additional analyses (eg, sensitivity or subgroup analyses, meta- regression), if done, indicating which were prespecified | 7 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 8, Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (eg, study size, PICOS, and follow-up period) and provide the citations | 8, Table 1 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 10, Table S3 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and CIs, ideally with a forest plot | Figure 2 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measures of consistency | 9, 10, Table 2, Figure 2 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | 10, Table S3 |
| Additional analysis | 23 | Give results of additional analyses, if done (eg, sensitivity or subgroup analyses, meta- regression [see item 16]) | 9, 10 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (eg, health care providers, users, and policy makers) | 10 |
| Limitations | 25 | Discuss limitations at study and outcome level (eg, risk of bias), and at review level (eg, incomplete retrieval of identified research and reporting bias) | 12, 13 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence and implications for future research | 13 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (eg, supply of data); role of funders for the systematic review | 13 |
Note: Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009: 6(7): e1000100. https://doi.org/10.1371/journal.pmed.1000100. For more information, visit: www.prisma-statement.org.
Abbreviation: NA, not available.
Table S2.
Methodological quality of all studies based on NOS for assessing the quality of each included study
| Study | Representa- tiveness of exposed cohort | Selection of non- exposed cohort | Assessment of exposure | Outcome not present at the start of the study | Compara- bility based on the design or analysis | Assessment of outcome | Follow- up long enough for outcomes | Adequacy of follow-up | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Eo et al1 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Sun and Song2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Wang et al3 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Kwon et al4 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Li et al5 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Xiang et al6 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 6 |
| Zhang et al7 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 5 |
| Zhu et al8 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Tang et al9 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Tian10 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Yang and Lo11 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Kwon et al12 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
Abbreviation: NOS, Newcastle–Ottawa Scale.
Table S3.
Meta-regression analyses of the associations between pretreatment LMR and survival among OC patients
| Coefficient | Standard error | T-value | P-value | 95% CI of intercept | |
|---|---|---|---|---|---|
|
| |||||
| OS | |||||
| Year of publication | 0.0820097 | 0.2491714 | 0.33 | 0.749 | (–0.4731787, 0.6371981) |
| Age | −0.0738882 | 0.0200856 | −3.68 | 0.004 | (−0.1186417, −0.0291346) |
| Sample size | −0.0008594 | 0.0005413 | −1.59 | 0.143 | (−0.0020655, 0.0003466) |
| LMR cutoff value | 0.2008357 | 0.129754 | 1.55 | 0.153 | (−0.0882742, 0.4899455) |
| NOS score | −0.1728062 | 0.1330581 | −1.30 | 0.223 | (−0.4692782, 0.1236658) |
| PFS | |||||
| Year of publication | −0.1952826 | 0.2098772 | −0.93 | 0.388 | (−0.7088336, 0.3182684) |
| Age | −0.0333006 | 0.0275148 | −1.21 | 0.272 | (−0.1006267, 0.0340256) |
| Sample size | −0.0000113 | 0.0004759 | −0.02 | 0.982 | (−0.0011757, 0.0011531) |
| LMR cutoff value | 0.0189409 | 0.1054612 | 0.18 | 0.863 | (−0.2391134, 0.2769952) |
| NOS score | −0.1618594 | 0.0787455 | −2.06 | 0.086 | (−0.3545426, 0.0308238) |
Abbreviations: LMR, lymphocyte-to-monocyte ratio; NOS, Newcastle–Ottawa Scale; OC, ovarian cancer; OS, overall survival; PFS, progression-free survival.
References
- 1.Eo WK, Chang HJ, Kwon SH, et al. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian cancer. J Cancer. 2016;7(3):289–296. doi: 10.7150/jca.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun L, Song Y. Effects of lymphocyte and monocyte ratio on prognosis of epithelial ovarian cancer. Chinese Clinical Oncology. 2016;10:909–912. [Google Scholar]
- 3.Wang X, Yuan Z, Qiu H. The relationship between preoperative blood lymphocyte-to-monocyte ratio and the prognostic of epithelial ovarian cancer. Progi Obstet Gynecol. 2016;9:654–657. [Google Scholar]
- 4.Kwon BS, Lee HJ, Yang J, Song YJ, Suh DS, Lee DH, Kim KH. Prognostic value of preoperative lymphocyte-monocyte ratio in elderly patients with advanced epithelial ovarian cancer. Obstet Gynecol Sci. 2017;60(6):558–564. doi: 10.5468/ogs.2017.60.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Hong N, Robertson M, Wang C, Jiang G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep. 2017;7:43001. doi: 10.1038/srep43001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiang J, Zhou L, Li X, et al. Preoperative Monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. 2017;10(1):33–39. doi: 10.1016/j.tranon.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Ye B, Liang W, Ren Y. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep. 2017;7(1):9548. doi: 10.1038/s41598-017-10328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu JY, Liu CC, Wang L, Zhong M, Tang HL, Wang H. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer. 2017;8(5):737–743. doi: 10.7150/jca.17668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang Y, Li J, Xu F, Hu H. Association between monocyte-to-lymphocyte ratio and prognosis of patients with epithelial ovarian cancer. Am J Obstet Gynecol Pediat. 2017;5:532–538. [Google Scholar]
- 10.Tian Y. Analysis of Prognostic Factors of Epithelial Ovarian Cancer. He Bei, China: Hebei Medical University; 2017. [Google Scholar]
- 11.Yang HM, Lou G. The relationship of preoperative lymphocyte-monocyte ratio and the clinicopathological characteristics and prognosis of patients with epithelial ovarian cancer. Zhonghua Zhong Liu Za Zhi. 2017;39(9):676–680. doi: 10.3760/cma.j.issn.0253-3766.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Kwon BS, Jeong DH, Byun JM, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer. 2018;9(7):1127–1134. doi: 10.7150/jca.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Acknowledgments
The study was supported by research grants from National Natural Science Foundation of China (81602853).
Footnotes
Author contributions
Study concept and design: F-FZ and SZ. Data extraction and analysis: X-PG and Y-HL. Manuscript drafting: X-PG and Y-HL. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vergote I, Tropé CG, Amant F, et al. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 3.Macciò A, Madeddu C. Inflammation and ovarian cancer. Cytokine. 2012;58(2):133–147. doi: 10.1016/j.cyto.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Dupré A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. 2018;44(5):566–570. doi: 10.1016/j.ejso.2018.02.209. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W, Ye B, Liang W, Ren Y. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep. 2017;7(1):9548. doi: 10.1038/s41598-017-10328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu JY, Liu CC, Wang L, Zhong M, Tang HL, Wang H. Peripheral blood lymphocyte-to-monocyte ratio as a prognostic factor in advanced epithelial ovarian cancer: a multicenter retrospective study. J Cancer. 2017;8(5):737–743. doi: 10.7150/jca.17668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eo WK, Chang HJ, Kwon SH, et al. The lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian cancer. J Cancer. 2016;7(3):289–296. doi: 10.7150/jca.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon BS, Jeong DH, Byun JM, et al. Prognostic value of preoperative lymphocyte-monocyte ratio in patients with ovarian clear cell carcinoma. J Cancer. 2018;9(7):1127–1134. doi: 10.7150/jca.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon BS, Lee HJ, Yang J, Song YJ, Suh DS, Lee DH, Kim KH. Prognostic value of preoperative lymphocyte-monocyte ratio in elderly patients with advanced epithelial ovarian cancer. Obstet Gynecol Sci. 2017;60(6):558–564. doi: 10.5468/ogs.2017.60.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Hong N, Robertson M, Wang C, Jiang G. Preoperative red cell distribution width and neutrophil-to-lymphocyte ratio predict survival in patients with epithelial ovarian cancer. Sci Rep. 2017;7:43001. doi: 10.1038/srep43001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiang J, Zhou L, Li X, et al. Preoperative monocyte-to-lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. 2017;10(1):33–39. doi: 10.1016/j.tranon.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17(24):2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Hamling J, Lee P, Weitkunat R, Ambühl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27(7):954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 16.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Mander A, Clayton D. Assessing the influence of a single study in meta-analysis. Stata Tech Bull. 1999;8:108–110. [Google Scholar]
- 19.Wang X. The Relationship Between Preoperative Blood Lymphocyte-To-Monocyte Ratio and the Prognostic of Epithelial Ovarian Cancer. Zheng Zhou, China: The First Affiliated Hospital of Zheng Zhou University; 2017. [Google Scholar]
- 20.Romito A, Marchetti C, di Santo G. Monocyte-to-lymphocyte ratio as predictor of survival and response to treatment in ovarian cancer. Int J Gynecol Cancer. 1913;2017:27. [Google Scholar]
- 21.Sun L, Song Y. Effects of lymphocyte and monocyte ratio on prognosis of epithelial ovarian cancer. Chinese Clin Oncol. 2016;10:909–912. [Google Scholar]
- 22.Tang Y, Li J, Xu F, Hu H. Association between monocyte-to-lymphocyte ratio and prognosis of patients with epithelial ovarian cancer. Am J Obstet Gynecol Pediat. 2017;5:532–538. [Google Scholar]
- 23.Tian Y. Analysis of Prognostic Factors of Epithelial Ovarian Cancer. He Bei, China: Hebei Medical University; 2017. [Google Scholar]
- 24.Wang X, Yuan Z, Qiu H. The relationship between preoperative blood lymphocyte-to-monocyte ratio and the prognostic of epithelial ovarian cancer. Prog Obstet Gynecol. 2016;9:654–657. [Google Scholar]
- 25.Yang HM, Lou G. The relationship of preoperative lymphocyte-monocyte ratio and the clinicopathological characteristics and prognosis of patients with epithelial ovarian cancer. Zhonghua Zhong Liu Za Zhi. 2017;39(9):676–680. doi: 10.3760/cma.j.issn.0253-3766.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Tham T, Olson C, Khaymovich J, Herman SW, Costantino PD. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol. 2018;275(7):1663–1670. doi: 10.1007/s00405-018-4972-x. [DOI] [PubMed] [Google Scholar]
- 27.Hu RJ, Ma JY, Hu G. Lymphocyte-to-monocyte ratio in pancreatic cancer: prognostic significance and meta-analysis. Clin Chim Acta. 2018;481:142–146. doi: 10.1016/j.cca.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Song W, Wang K, Zhang RJ, Zou SB. Prognostic value of the lymphocyte monocyte ratio in patients with colorectal cancer: a meta-analysis. Medicine (Baltimore) 2016;95(49):e5540. doi: 10.1097/MD.0000000000005540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song W, Tian C, Wang K, Zhang RJ, Zou SB. The pretreatment lymphocyte to monocyte ratio predicts clinical outcome for patients with hepatocellular carcinoma: a meta-analysis. Sci Rep. 2017;7:46601. doi: 10.1038/srep46601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu RJ, Liu Q, Ma JY, Zhou J, Liu G. Preoperative lymphocyte-to-monocyte ratio predicts breast cancer outcome: a meta-analysis. Clin Chim Acta. 2018;484:1–6. doi: 10.1016/j.cca.2018.05.031. [DOI] [PubMed] [Google Scholar]
- 31.Zhu Y, Li M, Bo C, et al. Prognostic significance of the lymphocyte-to-monocyte ratio and the tumor-infiltrating lymphocyte to tumor-associated macrophage ratio in patients with stage T3N0M0 esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2017;66(3):343–354. doi: 10.1007/s00262-016-1931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 33.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 36.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KH, Kim EY, Yun JS, Park YL, Do SI, Chae SW, Park CH. The prognostic and predictive value of tumor-infiltrating lymphocytes and hematologic parameters in patients with breast cancer. BMC Cancer. 2018;18(1):938. doi: 10.1186/s12885-018-4832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCloskey CW, Rodriguez GM, Galpin KJC, Vanderhyden BC. Ovarian cancer immunotherapy: preclinical models and emerging therapeutics. Cancers (Basel) 2018;10(8):E244. doi: 10.3390/cancers10080244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekine K, Kanda S, Goto Y, et al. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer. 2018;124:179–188. doi: 10.1016/j.lungcan.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Elisia I, Lam V, Hofs E, et al. Effect of age on chronic inflammation and responsiveness to bacterial and viral challenges. PLoS One. 2017;12(11):e0188881. doi: 10.1371/journal.pone.0188881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kugel CH, Douglass SM, Webster MR, et al. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 2018;24(21):5347–5356. doi: 10.1158/1078-0432.CCR-18-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elias R, Giobbie-Hurder A, McCleary NJ, Ott P, Hodi FS, Rahma O. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer. 2018;6(1):26. doi: 10.1186/s40425-018-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
PRISMA 2009 checklist
| Section/topic | No. | Checklist item | Reported on page number |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | 1 |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | 3 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 4, 5 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (eg, Web address), and, if available, provide registration information including registration number | NA |
| Eligibility criteria | 6 | Specify study characteristics (eg, PICOS and length of follow-up) and report characteristics (eg, years considered, language, and publication status) used as criteria for eligibility, giving rationale | 6 |
| Information sources | 7 | Describe all information sources (eg, databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 5, Supplementary materials, pages 1–2 |
| Study selection | 9 | State the process for selecting studies (ie, screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 6 |
| Data collection process | 10 | Describe method of data extraction from reports (eg, piloted forms, independently, and in duplicate) and any processes for obtaining and confirming data from investigators | 6 |
| Data items | 11 | List and define all variables for which data were sought (eg, PICOS and funding sources) and any assumptions and simplifications made | 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 7, Table S2 |
| Summary measures | 13 | State the principal summary measures (eg, risk ratio and difference in means) | 7 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (eg, I2) for each meta-analysis | 7 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (eg, publication bias and selective reporting within studies) | 7, 8 |
| Additional analyses | 16 | Describe methods of additional analyses (eg, sensitivity or subgroup analyses, meta- regression), if done, indicating which were prespecified | 7 |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 8, Figure 1 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (eg, study size, PICOS, and follow-up period) and provide the citations | 8, Table 1 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 10, Table S3 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and CIs, ideally with a forest plot | Figure 2 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measures of consistency | 9, 10, Table 2, Figure 2 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | 10, Table S3 |
| Additional analysis | 23 | Give results of additional analyses, if done (eg, sensitivity or subgroup analyses, meta- regression [see item 16]) | 9, 10 |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (eg, health care providers, users, and policy makers) | 10 |
| Limitations | 25 | Discuss limitations at study and outcome level (eg, risk of bias), and at review level (eg, incomplete retrieval of identified research and reporting bias) | 12, 13 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence and implications for future research | 13 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (eg, supply of data); role of funders for the systematic review | 13 |
Note: Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009: 6(7): e1000100. https://doi.org/10.1371/journal.pmed.1000100. For more information, visit: www.prisma-statement.org.
Abbreviation: NA, not available.
Table S2.
Methodological quality of all studies based on NOS for assessing the quality of each included study
| Study | Representa- tiveness of exposed cohort | Selection of non- exposed cohort | Assessment of exposure | Outcome not present at the start of the study | Compara- bility based on the design or analysis | Assessment of outcome | Follow- up long enough for outcomes | Adequacy of follow-up | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Eo et al1 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Sun and Song2 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Wang et al3 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Kwon et al4 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
| Li et al5 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 8 |
| Xiang et al6 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 6 |
| Zhang et al7 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 5 |
| Zhu et al8 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Tang et al9 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Tian10 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 7 |
| Yang and Lo11 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 0 | 7 |
| Kwon et al12 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 6 |
Abbreviation: NOS, Newcastle–Ottawa Scale.
Table S3.
Meta-regression analyses of the associations between pretreatment LMR and survival among OC patients
| Coefficient | Standard error | T-value | P-value | 95% CI of intercept | |
|---|---|---|---|---|---|
|
| |||||
| OS | |||||
| Year of publication | 0.0820097 | 0.2491714 | 0.33 | 0.749 | (–0.4731787, 0.6371981) |
| Age | −0.0738882 | 0.0200856 | −3.68 | 0.004 | (−0.1186417, −0.0291346) |
| Sample size | −0.0008594 | 0.0005413 | −1.59 | 0.143 | (−0.0020655, 0.0003466) |
| LMR cutoff value | 0.2008357 | 0.129754 | 1.55 | 0.153 | (−0.0882742, 0.4899455) |
| NOS score | −0.1728062 | 0.1330581 | −1.30 | 0.223 | (−0.4692782, 0.1236658) |
| PFS | |||||
| Year of publication | −0.1952826 | 0.2098772 | −0.93 | 0.388 | (−0.7088336, 0.3182684) |
| Age | −0.0333006 | 0.0275148 | −1.21 | 0.272 | (−0.1006267, 0.0340256) |
| Sample size | −0.0000113 | 0.0004759 | −0.02 | 0.982 | (−0.0011757, 0.0011531) |
| LMR cutoff value | 0.0189409 | 0.1054612 | 0.18 | 0.863 | (−0.2391134, 0.2769952) |
| NOS score | −0.1618594 | 0.0787455 | −2.06 | 0.086 | (−0.3545426, 0.0308238) |
Abbreviations: LMR, lymphocyte-to-monocyte ratio; NOS, Newcastle–Ottawa Scale; OC, ovarian cancer; OS, overall survival; PFS, progression-free survival.