Abstract
Purpose
Antimicrobial resistance (AMR) is one of the major threats to human health, and the high frequency of resistant pathogens in the hospital environment can contribute to the transmission of difficult-to-treat health care-associated infections (HAIs). We recently reported that, compared with conventional chemical cleaning, the use of a microbial-based sanitation strategy (Probiotic Cleaning Hygiene System [PCHS]) was associated with remodulation of hospital microbiota and reduction of HAI incidence. Here, we aimed to analyze the impact of PCHS on AMR and related effects, such as HAI-associated antimicrobial drug consumption and costs.
Patients and methods
Five Italian hospitals, enrolled in a multicenter study where conventional sanitation methods were replaced with PCHS, were included in the analysis. The study period included a 6-month observation for each sanitation type. Surface microbiota AMR was analyzed using microarray, nested PCR, antibiogram, and microdilution tests. Drug consumption data and related costs were obtained from the medical records of all hospitalized patients affected by HAIs.
Results
PCHS use was associated with up to 99% decrease of the AMR genes harbored by surface hospital microbiota, independently of the resistance types originally present in each individual setting (Pc<0.01). Functional assays confirmed the molecular data, demonstrating a 33%–100% decrease of resistant strains depending on the antibiotic type. Antimicrobial drug consumption associated with HAI onset showed a global 60.3% decrease, with a 75.4% decrease of the associated costs.
Conclusion
The spread of AMR in the hospital environment can be limited by the use of sanitation methods to remodulate the hospital microbiota, leading to lower antimicrobial consumption and costs. This approach might be considered as part of broader infection prevention and control strategies.
Keywords: AMR, HAI, antimicrobials, drug consumption, costs
Plain language summary
Antimicrobial resistance (AMR) is one of the major threats to human health and is frequently found in infections acquired during hospitalization (health care-associated infections [HAIs]). AMR can make the pharmacological treatment of infected patients difficult or even impossible. Resistant microbes contaminate the hospital environment, contributing to HAI onset, and the chemical sanitation methods typically used for cleaning cannot control AMR, sometimes even contributing to the selection of resistant strains. We previously found that a probiotic-based sanitation method (Probiotic Cleaning Hygiene System [PCHS]) stably reduced microbial contamination and was associated with a reduction in HAIs in five Italian hospitals. In this study, we aimed to analyze the impact of PCHS on AMR, as well as the consumption and costs associated with HAI-related antimicrobial therapy. The results revealed that PCHS use was associated with a decrease of AMR in the surface microbiota by up to 99% compared with conventional chemical sanitation methods. In addition, a 60.3% decrease in HAI-associated antimicrobial consumption was observed, accompanied by a 75.4% decrease in the related costs. In conclusion, these findings indicate that an environmental intervention effective in altering the hospital microbiota can consistently limit the spread of AMR in the hospital environment, contributing to containing the incidence of HAIs and reducing HAI-related antimicrobial drug consumption and costs, and might therefore be considered as a useful component of infection prevention strategies.
Introduction
The prevention and control of health care-associated infections (HAIs) depend on multiple factors, including the adoption of standard and transmission-based precautions, appropriate environmental hygiene, pertinent diagnostics, and prudent antibiotic use. HAIs are a global concern, as they negatively affect patient outcomes, often increasing both the lengths of hospital stay and the associated health care costs. In Europe, over 3 million patients acquire HAIs every year, with 37,000 deaths occurring as a direct consequence of HAIs.1 This is also associated with the increasing antimicrobial resistance (AMR) of HAI-associated pathogens,2,3 such as vancomycin-resistant enterococci, methicillin-resistant staphylococci, and carbapenem-resistant Enterobacteriaceae. HAIs tend to exhibit higher resistance rates to antibiotics compared with community-acquired infections,4 further threatening the outcome of infections in hospitalized patients. Consequently, AMR in HAIs is a recognized threat to global public health.5
The persistent contamination of hospital surfaces is an important contributor to HAI transmission,6–10 as these surfaces serve as reservoirs of common pathogens, including drug-resistant ones.6,7,9–13 In particular, methicillin-resistant Staphylococcus aureus and carbapenem- or even multidrug-resistant (MDR) Enterobacteriaceae and Pseudomonas spp. are frequently detected.6,12,13 Furthermore, we recently observed that even less-common types of AMR can be detected with substantial frequency in the hospital environment, such as mcr-1 plasmid-mediated resistance against colistin (colR), a last resort drug used to treat infections caused by MDR gram-negative bacteria. In fact, up to 8% of gram-negative bacteria isolated from the surfaces of Italian hospitals were found to harbor mcr-1 plasmid-mediated colR.14 This value was higher than that found in isolates obtained from patients, where plasmid-mediated colR was detected in only 1%–2% of isolates, suggesting that the hospital environment may be a reservoir for the further transmission of colR.14
Conventional sanitation techniques based on the use of chemical detergents/disinfectants do not prevent recon-tamination15,16 and can even potentially contribute to the selection of HAI-associated MDR pathogens,17,18 thus further worsening the implications of AMR. In contrast, we recently reported that a sanitation approach based on ecologically sustainable detergents containing spores of Bacillus probiotics (PCHS) could decrease the levels of surface pathogens by up to 90% more than conventional sanitizers,19,20 without inducing the selection of drug-resistant strains, as demonstrated by molecular analyses of the entire microbiota resistome.19,21,22 Furthermore, in a recent multicenter study performed in five Italian hospitals where PCHS was compared with chemical sanitation, we confirmed that PCHS use consistently and stably decreased the levels of surface pathogens including resistant ones, and this was accompanied by a 52% reduction of HAI incidence in hospitalized patients.23
The present study aimed to analyze the AMR characteristics of the hospital microbiota before and after the introduction of PCHS and evaluate the potential impact of PCHS use on HAI-associated antimicrobial drug consumption and associated costs.
Patients and methods
Study design
The design and type of the multicenter study, which was performed over 18 months in five Italian hospitals (ISRCTN Registry: ISRCTN58986947), were described previously.23 Briefly, the Internal Medicine wards of the enrolled hospitals were surveyed for 6 months while using the conventional chemical-based sanitation method and then for a further 6 months while using the microbial-based PCHS system. PCHS sanitation was performed daily using ecologically sustainable detergents containing spores of three Bacillus species (Bacillus subtilis, Bacillus pumilus, and Bacillus megaterium). The cleaning staff did not change during the study period and were trained in the appropriate application procedure for PCHS. The training was limited to the correct methods for preparing and using the PCHS cleansers, and no other differences were introduced. Hospital personnel and inpatients were not aware of the change in the type of sanitation method used. All of the participating hospitals provided approval through their local ethics committee and agreed not to introduce any other interventions that could potentially affect HAI incidence during the study period.
The survey was performed between January 1, 2016, and June 30, 2017, and included a 6-month pre-intervention period of observation (pre-PCHS), when hospitals maintained their conventional chemical-based sanitation procedures, and a 6-month post-intervention period of observation (PCHS), when PCHS was routinely applied. Both the environmental bioburden and HAIs were surveyed throughout the entire study period.
Analysis of environmental AMR
Specimens of the hospital surface microbiota were collected monthly by sampling in duplicate three representative points per room (floor, bed footboard, and sink) of three to six randomized rooms per hospital, as previously described.19,21,23 The total microbial population was collected using sterile rayon swabs rubbed on a 10×10 cm area, as previously described.21 Total DNA was extracted from the collected microbiota samples using the QIAamp UCP Pathogen Mini Kit (catalog no. 50214; Qiagen, Hilden, Germany) and analyzed using a quantitative PCR microarray detecting 84 resistance (R) genes (Antibiotic Resistance Genes, catalog no. BAID-1901ZRA; Qiagen).21 In total, 756 samples (378 in the pre-intervention phase and 378 in the intervention phase) were analyzed.
All of the surface samples from both the pre-intervention and intervention phases of the study were also grown in MacConkey broth (catalog no. 402490; Liofilchem, Teramo, Italy), which is selective for the Enterobacteriaceae family, to amplify the Enterobacteriaceae population and analyze the prevalence of the mcr-1 plasmid gene coding for colR. The presence of this gene was analyzed using nested PCR as previously described.14 The molecular findings were also functionally confirmed for individual colonies following culture isolation using a broth microdilution assay (SensiTest Colistin, catalog no. 75001; Liofilchem), as previously described.14 In total, 452 Enterobacteriaceae isolates (223 in the pre-intervention phase and 229 in the intervention phase) were analyzed.
Furthermore, all of the S. aureus colonies isolated from the sampled surfaces were analyzed for drug susceptibility. Specifically, the same surface points were sampled using contact (replicate organism direct agar contact) plates containing Baird Parker medium (catalog no. 163512; Liofilchem), which is selective for Staphylococcus spp. After 48 hours of incubation at 37°C, presumptive S. aureus colonies were identified by biochemical typing (Staph System 18R, catalog no. 71630; Liofilchem), isolated, and then characterized for antibiotic susceptibility using conventional disk-diffusion Kirby–Bauer antibiograms on Mueller–Hinton agar plates (catalog no. 105437; EMD Millipore, Billerica, MA, USA). The following antibiotics were tested: ampicillin (catalog no. CT0003B; Oxoid), vancomycin (catalog no. CT0058B; Oxoid), oxacillin (catalog no. CT0040B; Oxoid), cefotaxime (catalog no. CT066B; Oxoid), imipenem (catalog no. CT0455B; Oxoid), and penicillin G (catalog no. CT0043B; Oxoid). Inhibition zone diameters were interpreted according to the European Committee on Antimicrobial Susceptibility Testing breakpoint tables for the interpretation of minimum inhibitory concentration (MIC) and inhibition zone diameters24 and the Clinical and Laboratory Standards Institute manual (26th edition).25 In total, 112 S. aureus isolates (80 in the pre-intervention phase and 32 in the intervention phase) were analyzed.
For both S. aureus and Enterobacteriaceae isolates, MDR strains were defined as those showing resistance to three or more antibiotics, and their incidence was compared for the pre-intervention and intervention phases.
Analysis of HAI-related antimicrobial drug consumption
As previously reported,23 all new patients admitted to the enrolled wards during the pre-intervention and intervention phases were evaluated for the development of HAIs. In total, 11,461 patients were surveyed, including all HAI types in the analysis, which were identified according to the criteria defined by the European Center for Disease Prevention and Control.26
Antimicrobial consumption data were obtained from patients’ medical records. For each patient, two electronic clinical records containing general data and information concerning eventual HAI onset, location, etiological agent, drug therapy, and infection resolution/outcome were collected. All data were anonymized and submitted centrally via a secure, password-protected website. Data analyzers were blinded to the intervention time and hospital’s group.
Analysis of HAI-related antimicrobial drug costs
Direct costs related to the drug treatment of HAI were evaluated by analyzing the daily cost per patient, including in the analysis only the costs of consumed antimicrobial drugs (thus excluding estimates of the costs of health care professionals’ time to administer the drugs and the apparatus used, and general bed day costs). Each hospital was asked to provide, for the different types of antimicrobials used, the cost per patient per day of treatment with the standard dose, distinguishing also the costs for different routes of administration. In case of missing data from a hospital for a particular drug, an “average” cost was calculated based on the data provided by the other hospitals. The cost of each individual drug treatment per patient was calculated by multiplying the daily cost by the treatment duration. The total cost per patient was calculated by summing the costs of the different treatments administered. The total costs of antimicrobial usage in the pre-PCHS and PCHS phases were calculated by summing the drug costs for HAI patients in the two groups.
Statistical methods
Statistical analyses were performed using the chi-squared test and the Mann–Whitney test to evaluate the significance of AMR in bacterial isolates and gender differences (male/female) between the pre-PCHS and PCHS patient groups, the Kolmogorov–Smirnov test to evaluate normality, and the parametric Student’s t-test to evaluate differences in micro-array data. P-values of <0.05 were considered to indicate statistical significance. Bonferroni correction for multiple comparisons was applied for the analysis of microarray data (Pc values of <0.05 were considered significant). Analyses were performed using the IBM SPSS 23 software.
Results
Impact of sanitation method on AMR of hospital surface microbiota
Surface bioburden analyses indicated that contamination was mostly attributable to the presence of Staphylococcus spp., which represented up to 90% of the total surface microbiota, although other genera were also present (including Enterobacteriaceae spp., Pseudomonas spp., Acinetobacter, Clos-tridium difficile, and Candida spp.). As reported previously, PCHS use was associated with a mean 83% decrease of all of the detected pathogens, compared with that detected during the pre-intervention phase.23 Detailed analysis of the studied pathogen types revealed that PCHS use led to decreases in the surface loads of 57.2%–93.3% compared with those detected during the pre-PCHS phase, as summarized in Table 1. All differences were statistically significant (P<0.05).
Table 1.
Variations in individual pathogens’ load on hospital surfaces during pre-PCHS and PCHS (CFU/m2)
| Pathogen type | Pre-PCHSa | PCHSa | Decrease (%) |
|---|---|---|---|
| Aspergillus spp. | 181±307 | 12±6 | 93.3 |
| Candida spp. | 2,597±1,798 | 1,108±559 | 57.3 |
| Clostridium difficile | 334±290 | 132±219 | 60.5 |
| Pseudomonas aeruginosa | 970±982 | 415±350 | 57.2 |
| Acinetobacter baumannii | 2,844±841 | 520±726 | 81.7 |
| Enterobacteriaceae spp. | 1,774±901 | 189±135 | 89.3 |
| Staphylococcus spp. | 26,947±17,293 | 4,674±3,799 | 82.7 |
Note:
Results are expressed as mean value of CFU/m2 ± SD detected in the five enrolled hospitals.
Abbreviations: PCHS, Probiotic Cleaning Hygiene System; CFU, colony forming units.
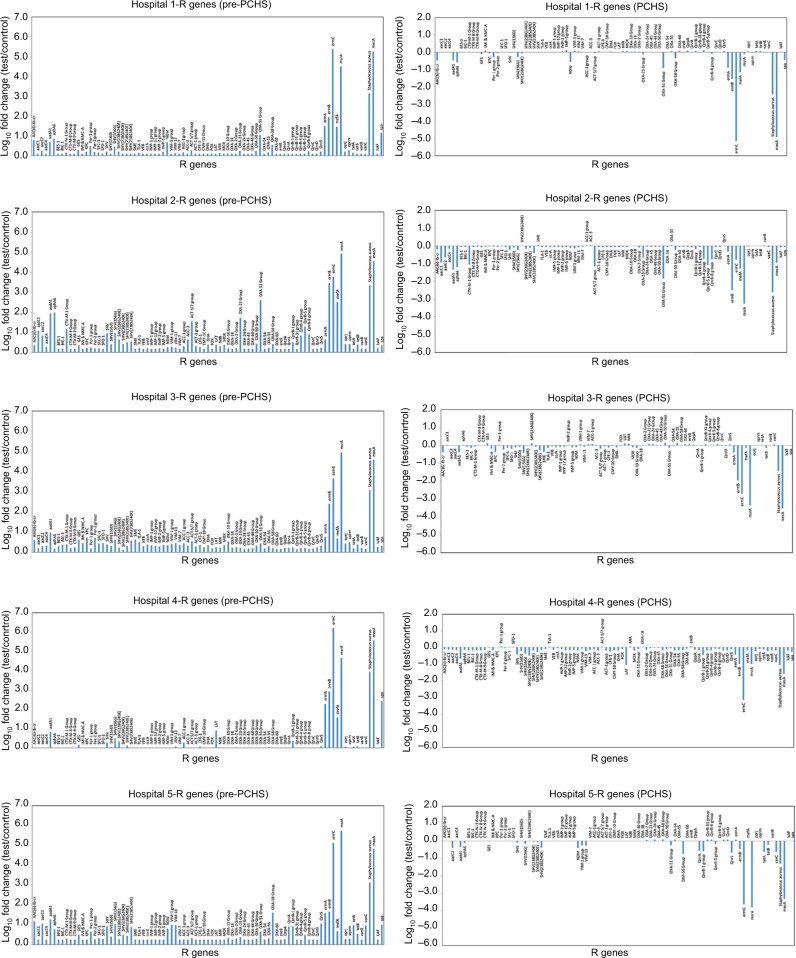
Based on these findings, we aimed to examine in detail the AMR characteristics of the contaminating population in each setting before and after PCHS use. As expected, this analysis revealed that the resistance (R) genes harbored by surface microbiota in the pre-intervention period exhibited different degrees of prevalence in the five enrolled hospitals, potentially reflecting the selective pressure exerted in each setting by the preferential use of certain antibiotics (Figure 1, left panels). Overall, R genes coding for resistance against aminoglycosides, fluoroquinolones, macrolides, methicillin, and vancomycin and for class-A, class-C, and class-D β-lactamases were detected. However, in accordance with the generally high levels of contamination by Staphylococcus spp., the most prevalent R gene was mecA, which codes for methicillin resistance and accounted for 42.7% of all of the detected R genes in the surface micro-biota. The genes ermC (26.9%) and msrA (26.6%) were also found to be prevalent R genes, whereas all the other identified genes represented only 3.8% of the resistome.
Figure 1.
Resistome analysis of the hospital surface microbiota.
Notes: The resistome of the surface contaminant population was analyzed as described in the “Patients and methods” section. The results of the pre-intervention (pre-PCHS, left panels) and intervention (PCHS, right panels) phases are shown for each setting (hospitals 1, 2, 3, 4, and 5). The results of the pre-PCHS phase are expressed as the log10 fold change of each detected R gene compared with the negative controls (NTC), and the results of the PCHS phase are expressed as the log10 fold change of each detected R gene compared with the pre-PCHS phase. The plotted data are the mean values obtained in monthly environmental sampling campaigns (12 sampling campaigns) for all of the sampled points (18 sampled points per hospital per sampling campaign).
Abbreviation: PCHS, Probiotic Cleaning Hygiene System.
Following the introduction of PCHS, significant decreases in the R genes present in the microbiota were observed in each hospital, compared with those detected in the corresponding pre-intervention period (Pc<0.01), independently of the relative abundance of specific R genes in the pre-intervention phase (Figure 1, right panels). Limiting the analysis exclusively to the most prevalent R genes detected in the surveyed settings (ie, those whose amounts were at least one log higher than in the negative controls), the introduction of PCHS appeared to be associated with a significant 70%–99.99% decrease, depending on the type of R gene (Figure 2).
Figure 2.
Resistome analysis of the hospital surface microbiota during the pre-PCHS phase.
Notes: The resistome of the surface contaminant population was analyzed as described in the “Patients and methods” section. The results of the pre-intervention (pre-PCHS) phase are plotted according to the prevalence of R genes. The results are the mean values for all five enrolled hospitals obtained in monthly environmental sampling campaigns (six sampling campaigns) for all of the sampled points (18 sampled points per hospital per sampling campaign).
Abbreviations: PCHS, Probiotic Cleaning Hygiene System; CTR, control.
In addition, as staphylococcal contamination was prevalent in all of the enrolled hospitals, and due to the important role of S. aureus in HAIs, we also examined the drug susceptibilities of all the S. aureus strains isolated from the hospital surfaces during the study. As such, all the S. aureus isolates were analyzed using standard Kirby–Bauer antibiograms, as described in the “Patients and methods” section. The results, which are summarized in Table 2, revealed that, compared with those detected in the pre-intervention phase, the S. aureus isolates from the post-intervention phase were 63.9%–93.5% less resistant to antibiotics, depending on the antibiotic type. In addition, a global 72.4% decrease of MDR S. aureus isolates (defined as those resistant to three or more antibiotics), from 58/81 (71.6%) in the pre-PCHS phase to 16/30 (53.3%) in the PCHS phase, was observed.
Table 2.
Antibiotic resistance in Staphylococcus aureus isolates of pre-PCHS and PCHS phases of the study
| Study period | Isolates (n) | Resistant isolates | ||||||
|---|---|---|---|---|---|---|---|---|
| Penicillin G | Ampicillin | Vancomycin | Oxacillin | Cefotaxime | Imipenem | MDRa | ||
| Pre-PCHS | 81 | 53 (65.4%) | 58 (71.6%) | 31 (38.2%) | 50 (61.7%) | 61 (75.3%) | 42 (51.8%) | 58 (71.6%) |
| <g>PCHS | <g>30 | <g>18 (60.0%) | <g>20 (66.6%) | <g>2 (6.6%) | <g>18 (60.0%) | <g>22 (73.3%) | <g>13 (43.3%) | <g>16 (53.3%) |
| % Decrease (strain number) | –66.0 | –65.5 | –93.5 | –64.0 | –63.9 | –69.0 | –72.4 | |
Notes:
MDR was defined as those strains resistant to three or more antibiotics. Penicillin G (10 IU), ampicillin (10 µg), vancomycin (30 µg), oxacillin (1 µg), cefotaxime (30 µg), imipenem (10 µg).
Abbreviation: PCHS, Probiotic Cleaning Hygiene System.
We also analyzed the prevalence of mcr-1 plasmid-mediated colR, which was not included in the microarray assay. The results revealed that, in contrast to the pre-intervention phase, where 21/223 (9.2%) of gram-negative bacteria harbored the mcr-1 plasmid, only 6/229 (2.6%) of these isolates tested positive for the mcr-1 plasmid R gene in the intervention phase. All of the PCR-positive strains displayed MIC values varying from 4 to 16 mg/L in the broth microdilution assays and exhibited the MDR phenotype, being resistant to three or more antibiotics.
Impact of probiotic-based sanitation method on antimicrobial drug consumption
Data regarding drug therapy were available for 274/284 patients who developed an HAI in the pre-PCHS phase (out of 5,930 total patients surveyed) and for 124/128 patients who developed an HAI in the PCHS phase (out of 5,531 total patients surveyed). Table 3 compares the antimicrobials administered to these patients in terms of the number of prescriptions and therapy duration (expressed in days). Among the antimicrobials used, β-lactams (administered with or without β-lactamase inhibitors) and fluoroquinolones were the most prevalent, accounting for 58.8% of the total number of drugs administered and 55.8% of the total days of therapy. Notably, PCHS introduction was associated with a global 60.3% reduction in drug consumption compared with the pre-intervention phase (range 40.5%–90.9%, depending on the drug type). In accordance with this, the number of days of therapy showed a global 58.6% decrease compared with the pre-intervention phase (range 30.8%–97.1%, depending on the drug type). The 274 patients from the pre-PCHS phase developed 289 episodes of HAI, whereas the 124 patients from the PCHS phase developed 128 episodes of HAI; the mean number of therapy days for each patient was 12.2 and 11.1 days (−9%), respectively, in the two phases, whereas it was 11.8 and 10.8 (−8.5%) when referring to each HAI episode. The detected differences were, however, not statistically significant.
Table 3.
Drug consumption and therapy days during pre-PCHS and PCHS phases of the survey
| Drug types | Molecules (n) | Therapy days (n) | ||
|---|---|---|---|---|
| Pre-PCHS | PCHS | Pre-PCHS | PCHS | |
| β-Lactamsa | 126 | 75 (−40.5%) | 1,140 | 711 (−37.6%) |
| Fluoroquinolones | 111 | 20 (−82%) | 723 | 102 (−85.9%) |
| Glycopeptides | 43 | 18 (−58.1%) | 442 | 178 (−59.7%) |
| Cephalosporins | 43 | 22 (−48.8%) | 354 | 136 (−61.6%) |
| Antifungals | 31 | 6 (−80.6%) | 287 | 41 (−85.7%) |
| Acid antibiotics | 11 | 1 (−90.9%) | 68 | 2 (−97.1%) |
| Polymixins | 7 | 3 (−57.1%) | 85 | 56 (−34.1%) |
| Sulfamides | 6 | 1 (−83.3%) | 43 | 9 (−79.1%) |
| Aminoglycosides | 5 | 2 (−60.0%) | 39 | 27 (−30.8%) |
| Others | 16 | 9 (−43.7%) | 112 | 98 (−12.5%) |
| Total | 403 | 160 (−60.3%) | 3,339 | 1,382 (−58.6%) |
Note:
With or without β-lactamase inhibitors.
Abbreviation: PCHS, Probiotic Cleaning Hygiene System.
Impact of probiotic-based sanitation method on HAI-related antimicrobial usage costs
Based on the data obtained for the drug consumption associated with HAI onset from each enrolled hospital, we evaluated the direct costs related to the antimicrobial treatment of HAIs, expressed as the daily cost.
The economic analysis focused on those 398 patients who developed at least one HAI during hospitalization and whose medical records reported complete data on drug therapy, that, 289 HAI episodes in 274 patients during the pre-intervention phase and 128 HAI episodes in 124 patients during the intervention phase. Males accounted for 43.1% and 44.4% of the patients in the pre-PCHS and PCHS phases, respectively, and the mean age was 76±12.3 years in the pre-PCHS phase and 78.7±11.3 years in the PCHS phase (Table S1).
Daily costs were available for 281/289 of the HAI episodes in the pre-PCHS phase and 127/128 of the HAI episodes in the PCHS phase. Data analysis revealed that the mean costs for the management of a single HAI episode decreased from € 213.7±915.3 (Q1, 4.5; median, 17.7; Q3, 63.3) in the pre-PCHS period (when conventional chlorine-based sanitizing systems were used) to € 116.3±249.9 (Q1, 6.0; median, 25.0; Q3, 64.4) in the PCHS phase, representing a 45.6% reduction in the cost per HAI episode. The detected differences were, however, not statistically significant. Considering the total number of patients who experienced HAIs, the overall economic impact of HAI-associated drug therapy was € 60,062.17 in the pre-PCHS phase and € 14,767.16 in the PCHS phase, representing a 75.4% reduction in the total antimicrobial costs upon the introduction of PCHS, compared with the pre-PCHS phase.
Discussion
AMR is one of the major threats to human health, and the persistent contamination of the hospital environment acts as a reservoir for the pathogens responsible for HAI onset. The control of environmental contamination is a difficult task, and the conventional sanitation methods used to date, although well intentioned, allow recontamination and can favor the selection of resistant strains.
Our results revealed that PCHS use was associated with significant decreases in all the R genes harbored by the surface microbiota in the surveyed hospitals, independently of the resistance types originally present in each individual setting (Pc<0.01). This reduction was particularly evident for those genes that were highly represented in the pre-PCHS phase in all the five enrolled hospitals, namely, the genes mecA (coding for methicillin resistance), aad1 (aminoglycoside resistance, often present in S. aureus), OXA-51 group (β-lactamases, oxacillinases), ermA-B-C genes (coding for resistance against macrolides, lincosamide, and streptogramin B), and mefA and msrA (both coding for macrolide resistance).
These data were confirmed by the results obtained for S. aureus isolates through standard antibiogram analysis, as PCHS introduction was accompanied by a 61.9%–93.5% reduction in resistant isolated strains, depending on the antibiotic type, and a total 73.7% decrease in the number of strains resistant to three or more antibiotics.
Even less common types of AMR were affected by PCHS usage, including the colR provided by the mcr-1 plasmid, which decreased by 77.1% in the PCHS phase compared to what was detected in the pre-PCHS phase. Although quite rare, this type of AMR represents a severe threat to inpatients, as colistin is considered a last resort drug for infections caused by multi- or pan-drug-resistant gram-negative bacteria, thus rendering ineffective any therapeutic option. Functional assays, performed by microdilution, confirmed the molecular results, demonstrating a concomitant reduction of gram-negative MDR strains, as all of the colR isolates also exhibited the MDR phenotype.
Overall, these data are consistent with the general decrease in surface pathogens previously reported21,23 and suggest that PCHS is able to antagonize in a nonspecific manner all the pathogens commonly found in the hospital environment, thus rendering the detection of drug-resistant strains extremely infrequent and unlikely. In accordance with this, we previously reported that the number of HAIs was significantly diminished (−52.0%).23
Our data demonstrate reductions in both the overall impact of HAIs (number of patients with at least one HAI, −52%; number of administered drugs, −58.6%; cost, −75.4%; and days of therapy, −58.6%) and the severity of individual HAI episodes (days of therapy, −8.5% and cost, −45.6%). These data show that the overall decreases observed in therapy duration and costs both depended on the significant drop of HAI incidence during the PCHS phase and suggest that they may also have depended on a difference in the treatments necessary for individual HAI episodes.
Limitations
The present study does, however, have some limitations that should be considered. In fact, although the analyses were performed on a very large number of patients, which likely renders the results representative of clinical reality, data analysis was performed by comparing the pre-PCHS and PCHS patient groups without any matching of the patients themselves. Thus, the clinical characteristics of the patients in the two groups may not have been completely identical, leading to possible bias in the results. Furthermore, only patients for whom complete data regarding drug therapy were available were included, and this may have caused underestimation of the total drug consumption and costs.
Furthermore, our analysis focused only on drug costs, but other parameters should also be included to obtain a comprehensive estimate of the management and treatment of HAIs, such as the types of apparatus used, the labor costs for health care professionals, and the bed day costs. In addition, the reduction of HAIs may also have an impact on the length of hospitalization and related hospitalization costs. Taking into account that HAIs and AMR increase the health care costs, length of stay in hospitals, morbidity, and mortality, the results of the present study should be considered conservative estimates of the possible savings, from a health care perspective, that can be obtained using the PCHS system in clinical practice.
Conclusion
The findings described in this study indicate that environmental intervention based on microbiota modulation can significantly limit the spread of AMR in the hospital environment, contributing to containing the risk of infection and reducing antimicrobial consumption and the associated costs. The results of this study suggest that use of the PCHS approach can be considered as part of infection prevention and control strategies, and this approach should be further explored in future studies both in different settings and for its impact on AMR diffusion.
Data sharing statement
The datasets supporting the conclusions of this article are included within the article and its additional files.
Supplementary material
Table S1.
Demographic characteristics of patients
| Patients | Pre-PCHS | PCHS | Differences |
|---|---|---|---|
| No. (%) | No. (%) | P-value | |
| Total | 274 | 124 | |
| Gender (male) | 118 (43.1%) | 55 (44.4%) | n.s. |
| Age (years ± SD) | 76.0±12.3 | 78.7±11.3 | 0.025 |
Abbreviations: n.s., nonsignificant; PCHS, Probiotic Cleaning Hygiene System.
Acknowledgments
We thank Dr Filippo Berloco, Prof Gabriele Pelissero, Dr Paola Antonioli, Dr Lorenzo Tognon, Dr Giovanni Villone, and Dr Nelso Trua for providing drug data from the enrolled hospitals. We also thank Maddalena Coccagna for technical support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.ECDC . Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Stockholm, Sweden: ECDC; 2013. [Google Scholar]
- 2.Suetens C, Hopkins S, Kolman J, Diaz Högberg L. Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial use in European Acute Care Hospitals. Stockholm, Sweden: European Centre for Disease Prevention and Control; 2013. [Google Scholar]
- 3.Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 4.Ferjani S, Saidani M, Amine FS, Boutiba Ben Boubaker I. A comparative study of antimicrobial resistance rates and phylogenetic groups of community-acquired versus hospital-acquired invasive Escherichia coli. Med Mal Infect. 2015;45(4):133–138. doi: 10.1016/j.medmal.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 5.OECD, Union E . Health at a Glance: Europe 2016: State of the Health in the EU Cycle. Paris: OECD Publishing; 2016. [Google Scholar]
- 6.Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65(Suppl 2):50–54. doi: 10.1016/S0195-6701(07)60015-2. [DOI] [PubMed] [Google Scholar]
- 7.Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39(8):1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dancer SJ. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27(4):665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otter JA, Yezli S, French GL. The role played by contaminated surfaces in the transmission of nosocomial pathogens. Infect Control Hosp Epidemiol. 2011;32(7):687–699. doi: 10.1086/660363. [DOI] [PubMed] [Google Scholar]
- 10.Dancer SJ. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009;73(4):378–385. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 11.Weber DJ, Anderson D, Rutala WA. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26(4):338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 12.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber DJ, Rutala WA, Miller MB, Huslage K, Sickbert-Bennett E. Role of hospital surfaces in the transmission of emerging health care-associated pathogens: norovirus, Clostridium difficile, and Acinetobacter species. Am J Infect Control. 2010;38(5 Suppl 1):S25–S33. doi: 10.1016/j.ajic.2010.04.196. [DOI] [PubMed] [Google Scholar]
- 14.Caselli E, D’Accolti M, Soffritti I, Piffanelli M, Mazzacane S. Spread of mcr-1-driven colistin resistance on hospital surfaces, Italy. Emerg Infect Dis. 2018;24(9):1752–1753. doi: 10.3201/eid2409.171386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutala WA, Weber DJ. Selection of the ideal disinfectant. Infect Control Hosp Epidemiol. 2014;35(7):855–865. doi: 10.1086/676877. [DOI] [PubMed] [Google Scholar]
- 16.Almatroudi A, Gosbell IB, Hu H, et al. Staphylococcus aureus dry-surface biofilms are not killed by sodium hypochlorite: implications for infection control. J Hosp Infect. 2016;93(3):263–270. doi: 10.1016/j.jhin.2016.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Cornejo-Juárez P, Vilar-Compte D, Pérez-Jiménez C, Ñamendys-Silva SA, Sandoval-Hernández S, Volkow-Fernández P. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Int J Infect Dis. 2015;31:31–34. doi: 10.1016/j.ijid.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Caini S, Hajdu A, Kurcz A, Borocz K. Hospital-acquired infections due to multidrug-resistant organisms in Hungary, 2005–2010. Euro Surveill. 2013;18(2):20352. [PubMed] [Google Scholar]
- 19.Vandini A, Temmerman R, Frabetti A, et al. Hard surface biocontrol in hospitals using microbial-based cleaning products. PLoS One. 2014;9(9):e108598. doi: 10.1371/journal.pone.0108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fauci VL, Costa GB, Anastasi F, Facciol AC. An innovative approach to hospital sanitization using probiotics: in vitro and field trials. J Microb Biochem Technol. 2015;7(3):5. [Google Scholar]
- 21.Caselli E, D’Accolti M, Vandini A, et al. Impact of a probiotic-based cleaning intervention on the microbiota ecosystem of the hospital surfaces: focus on the resistome remodulation. PLoS One. 2016;11(2):e0148857. doi: 10.1371/journal.pone.0148857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caselli E. Hygiene: microbial strategies to reduce pathogens and drug resistance in clinical settings. Microb Biotechnol. 2017;10(5):1079–1083. doi: 10.1111/1751-7915.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caselli E, Brusaferro S, Coccagna M, et al. Reducing healthcare-associated infections incidence by a probiotic-based sanitation system: a multicentre, prospective, intervention study. PLoS One. 2018;13(7):e0199616. doi: 10.1371/journal.pone.0199616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EUCAST The European Committee on Antimicrobial Susceptibility Testing – EUCAST. 2017. Oct 20, [Accessed July 24, 2018]. 2017. Available from: http://www.eucast.org/
- 25.CLSI Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. 2018. [Accessed July 24, 2018]. Available from: http://clsi.org/standards/products/microbiology/documents/m100/
- 26.ECDC European surveillance of healthcare associated infections in intensive care units. 2015. [Accessed July 24, 2018]. Available from: http://ecdc.europa.eu/en/publications/publications/healthcare-associated-infections-hai-icu-protocol.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Demographic characteristics of patients
| Patients | Pre-PCHS | PCHS | Differences |
|---|---|---|---|
| No. (%) | No. (%) | P-value | |
| Total | 274 | 124 | |
| Gender (male) | 118 (43.1%) | 55 (44.4%) | n.s. |
| Age (years ± SD) | 76.0±12.3 | 78.7±11.3 | 0.025 |
Abbreviations: n.s., nonsignificant; PCHS, Probiotic Cleaning Hygiene System.