Abstract
Brugada syndrome is a genetic condition that predisposes to an increased risk of ventricular fibrillation and sudden cardiac death in a structurally normal heart. The Brugada type 1 electrocardiogram (ECG) pattern may occur independently of the actual syndrome, and this clinical phenomenon is often referred to as Brugada phenocopy. There are several other factors which have been known to induce this electrocardiographic pattern, and currently, there is a paucity of literature with respect to the pattern that is observed in patients with electrolyte disturbances, specifically hyponatremia. This case report highlights a suspected hyponatremia-induced Brugada type 1 ECG pattern, which subsequently normalized following resolution of the electrolyte derangement.
Keywords: Brugada syndrome, Brugada phenocopy, Brugada type pattern, hyponatremia
Introduction
Brugada syndrome (BrS), first described as a clinical entity in 1992, is a genetic condition having a diagnostic electrocardiogram (ECG) pattern which is associated with a heightened risk of ventricular arrhythmias (VAs) and sudden cardiac death (SCD).1 It is considered a primary electrical condition without structural heart disease.2 The ECG pattern consists of ST-segment elevation >2 mm in one or more leads from V1 to V3 with “coved type” descending to inverted T-wave or “saddle back” morphology consistent with Brugada type 1 and type 2 patterns, respectively.3,4 The prevalence varies geographically and ethnically with a male preponderance. The risk stratification and management of patients remains challenging.2
The Brugada type 1 ECG pattern may occur independently of the actual syndrome, and this clinical phenomenon is often referred to as Brugada phenocopy (BrP).2 It is typically recommended that these patients are further risk stratified to determine those having true BrS with potentially fatal arrhythmias vs those with Brugada’s phenocopies as seen in a myriad of clinical situations.5,6
Currently, there is a paucity of literature with respect to the Brugada-type ECG pattern that is observed in patients with electrolyte disturbances, specifically hyponatremia.7–9 This case highlights a highly suspicious severe hyponatremia-induced Brugada type 1 ECG phenocopic pattern, which subsequently normalized following resolution of the electrolyte derangement.
Case report
A 49-year-old Caribbean black gentleman with medical history of hypertension on hydrochlorothiazide and angiotensin-converting enzyme inhibitors presented to the emergency department with acute delirium. There was no pertinent history of syncope, presyncope palpitations, or family history of SCD according to relatives upon admission. The physical examination revealed an obtunded patient with tenuous vital signs with systolic blood pressures of 106 mmHg, tachycardia 112 beats per minute, and pulse oximetry of 96% on room air. Initial laboratory results indicated a serum sodium level of 108 mg/dL (normal 135–145 mg/dL), with other electrolytes being within their respective normal ranges. His serum osmolality was 266 mOsm/kg (normal 275–295 mOsm/kg) and urinary osmolality 586 mOsm/kg (normal 300–900 mOsm/kg). A random urinary sodium was <20 mEq/L.
The tentative diagnosis was antihypertensive-induced hypovolemic hyponatremia secondary to the thiazide-like diuretic and angiotensin-converting enzyme inhibitor. The nephrology team was consulted for the management of the severe hyponatremia for which they initiated a 1,000 mL 0.9% normal saline bolus, followed thereafter by a maintenance infusion of 75 mL per hour for 3 days, ensuring an appropriate correction rate of the serum sodium level with surveillance electrolyte testing. On admission, the patient’s ECG displayed a coved ST-segment elevation in V1 and V2, with elevated J points suggestive of a Brugada type 1 pattern (Figure 1). During his 5-day hospitalization, he underwent extensive investigations which included endocrinologic, infectious, and rheumatologic, all of which were all normal. A chest radiograph was unremarkable and transthoracic echocardiogram displayed mild diastolic dysfunction with left atrial enlargement. Gradually, his electrolyte derangements (hyponatremia and hyposmolality) resolved over the ensuing hospitalization and serial daily ECGs revealed subsequent normalization of the ECG abnormalities (Figure 2). His neurological status also steadily improved, returning to baseline.
Figure 1.
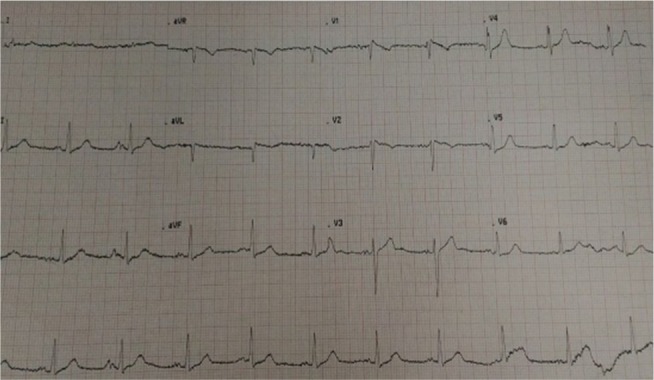
There is the characteristic ST-segment elevation ≥2 mm in ≥1 right precordial lead (V1 to V3), followed by an r'-wave and a straight ST-segment.
Notes: Additionally, the descending ST-segment crosses the isoelectric line and is followed by a negative and symmetric T-wave. At 40 ms of high takeoff, the decrease in amplitude of ST is ≤4 mm, the duration of QRS is longer than in a right bundle branch block and there is a mismatch between V1 and V6 (Figure 2)3. No high-pass filters were applied to attenuate low-frequency noise.14,15
Figure 2.
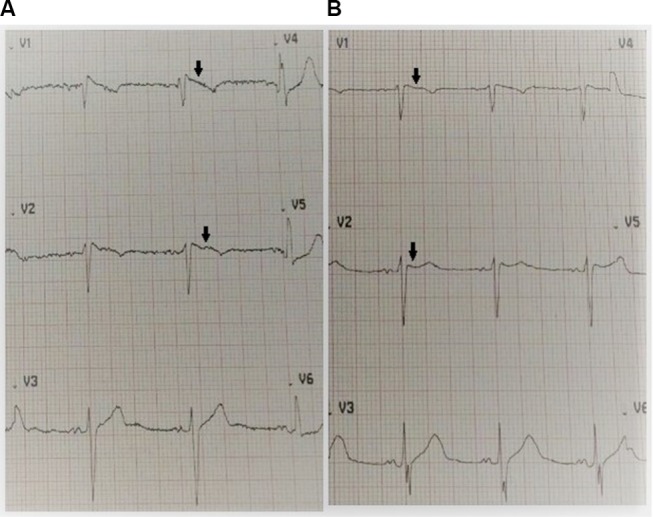
(A) Admission ECG upon presentation indicating a coved ST-segment elevation (black arrows) in V1 and V2, with elevated J points suggestive of a Brugada type 1 pattern. The patient’s serum sodium was 108 mg/dL (normal 135–145 mg/dL). (B) Day 5 ECG indicating resolution of the Brugada type 1 pattern (black arrows) with normalization of serum sodium. The patient’s serum sodium was 135 mg/dL (normal 135–145 mg/dL).
Abbreviation: ECG, electrocardiogram.
He was subsequently discharged with a modified antihypertensive regimen and early outpatient clinic appointments to both nephrology and cardiology for which he returned to normal, pre-morbid functioning with complete resolution of his hyponatremia. Of note, an outpatient sodium channel blocker test was not performed due to patient declining the procedure during the informed consent discussion amid his concerns of precipitating lethal arrhythmias,10 nor could the patient afford the genetic testing for SCN5A.11,12 The patient and his family had been extensively counseled with respect to accessing online resources and his case submission to the Brugada Phenocopy international registry and online educational portal for further insight into his condition.13
Discussion
It is challenging to ascertain the true burden of BrS due to the unknown prevalence and dynamic variability of ECGs; however, the incidence of the BrS electrocardiographic pattern has ranged from 0.12% to 0.8% in several studies and has accounted for ~4%–12% of all sudden deaths.16 The mainstay of therapy is an implantable cardioverter-defibrillator, but radiofrequency catheter ablation is also considered a novel management strategy.2 Since its first description in 1992, there has been steady progress with respect to the electrophysiological and genetic mechanisms underlying the disease.2
An ECG displaying a BrS type 1 pattern that is triggered by other factors has been called Brugada ECG phenocopy.17 It is imperative to ascertain the distinction between BrS and BrP as it may completely alter a patient’s prognosis and management.13 Several studies provide robust evidence that BrP and BrS ECG patterns are visually identical and indistinguishable, and consequently, an erroneous diagnosis may have a negative impact on patient morbidity and mortality.18–20
There are a multitude of conditions that display BrP with both type 1 and type 2 patterns such as right ventricular ischemia, pulmonary embolism, left anterior descending artery occlusion, right bundle branch block, pectus excavatum, and arrhythmogenic cardiomyopathy.21 Additionally, there are dynamic factors which disrupt transmembrane ionic currents, such as bradycardia and drugs, and can either unmask or exacerbate a BrP ECG pattern.5,22–25 This induction is described as an “acquired form of BrS”. It remains unknown whether acquired BrS is due to individual susceptibility resulting from an increase in latent ion channel dysfunction.6
The literature is not replete with describing the manifestation of BrP ECG pattern in the presence of severe hyponatremia, and currently there are only a few case reports and series that discuss this possible association5,7,8,26 Mechanistically, it is suspected that severe hyponatremia has a similar potential to diminish the electrochemical ionic gradient, by decreasing the inward sodium current and leaving the transient outward current unopposed. This may result in the loss of the action potential dome in the right ventricular epicardium producing a Brugada phenocopy.27 However, whether induction of the Brugada pattern by severe hyponatremia is associated with increased susceptibility to VAs is currently uncertain.21 This is likely a transient and reversible phenomenon as displayed by the resolution of the electrocardiographic abnormalities when both the serum sodium and transmembrane gradient are normalized over the ensuing hospitalization.
In a study by Xu et al, two cases (7%) displayed BrP with hyponatremia also with a myriad of electrolyte derangements such as acidosis, hyperkalemia, hypocalcemia, hyperphosphatemia, and hyperglycemia. In seven cases (26%), provocative testing using sodium channel blockers was performed, and all failed to reproduce a BrS ECG pattern (BrP class A).7 Additionally, no SCD or malignant VAs were detected.7,28
Our patient’s ECG was mostly consistent with a Brugada “coved type 1” phenocopy for several reasons, although the presence of a negative p-wave component in V2 could possibly suggest electrode malposition.2,4 There is the characteristic ST-segment elevation ≥2 mm in ≥1 right precordial lead (V1 to V3), followed by an rʹ-wave and a straight ST-segment. Additionally, the descending ST-segment crosses the isoelectric line and is followed by a negative and symmetric T-wave. At 40 ms of high takeoff, the decrease in amplitude of ST is ≤4 mm, the duration of QRS is longer than in a right bundle branch block, and there is a mismatch between V1 and V6.3 No high-pass filters were applied to attenuate low-frequency noise.14,15
Our patient was considered to have a low pretest probability of BrS as there was no pertinent medical or familial history of syncope, palpitations, or witnessed nocturnal agonal respiration. He did not undergo provocative testing due to concerns of inducing lethal arrhythmias and SCD.29
As aforementioned, Xu alluded to the fact that all provocative testing failed to replicate a BrP class A patient in similar clinical scenarios. The patient could not afford genetic testing for mutations of the SCN5A gene; however, this is not deemed mandatory since the SCN5A mutation is identifiable in only 20%–30% of probands affected by true BrS.30
As per Anselm et al’s BrP systematic diagnostic criteria, it is likely that our patient did display a type 1, class B, hyponatremia-induced BrP (as drug challenge was not performed) which subsequently resolved after normalization of the electrolyte derangement.31,32 It is imperative to note that while BrS and BrP have identical patterns in precordial leads V1 to V3, patients with BrP have an identifiable underlying condition that elicits these patterns which normalize upon resolution. Furthermore, BrS possesses a high clinical pretest probability of true congenital BrS, whereas conversely, BrP has a low pretest probability based on symptomatology and history. Significantly, patients with BrP have a negative provocative challenge with procainamide, flecainide, or ajmaline, while patients with true congenital BrS have a positive provocative challenge.31
Differentiating between BrS and BrP is important because patients with BrS are at risk of SCD and may require an implantable cardioverter-defibrillator and should refrain from drugs with sodium channel-blocking properties.
Conclusion
In conclusion, it appears likely that severe hyponatremia may induce a suspected Brugada-type ECG phenocopy that reverts to the pre-existing baseline state upon normalization of this electrolyte derangement as evidenced in this case report. Further in-depth research is required to elucidate the mechanistic effects and to determine any prognostic clinical implications.
Copyright permission
The patient has provided written informed consent to have the details of his case published.
Institutional approval
Institutional approval was not required for publication of this case report.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol. 1992;20(6):1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Brugada J, Campuzano O, Arbelo E, Sarquella-Brugada G, Brugada R. Present status of Brugada syndrome: JACC state-of-the-art review. J Am Coll Cardiol. 2018;72(9):1046–1059. doi: 10.1016/j.jacc.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Bayés de Luna A, Brugada J, Baranchuk A, et al. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J Electrocardiol. 2012;45(5):433–442. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 4.de Luna AB, Garcia-Niebla J, Baranchuk A. New electrocardiographic features in Brugada syndrome. Curr Cardiol Rev. 2014;10(3):175–180. doi: 10.2174/1573403X10666140514101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yap YG, Behr ER, Camm AJ. Drug-induced Brugada syndrome. Europace. 2009;11(8):989–994. doi: 10.1093/europace/eup114. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu W. Acquired forms of the Brugada syndrome. J Electrocardiol. 2005;38(4 Suppl):22–25. doi: 10.1016/j.jelectrocard.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Xu G, Gottschalk BH, Anselm DD, et al. Relation of the Brugada phenocopy to hyperkalemia (from the International registry on Brugada phenocopy) Am J Cardiol. 2018;121(6):715–717. doi: 10.1016/j.amjcard.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez PA, Vázquez Blanco M, Lerman J. Brugada type 1 electrocardiographic pattern induced by severe hyponatremia. Cardiology. 2011;118(2):97–100. doi: 10.1159/000327089. [DOI] [PubMed] [Google Scholar]
- 9.Tamene A, Sattiraju S, Wang K, Benditt DG. Brugada-like electrocardiography pattern induced by severe hyponatraemia. Europace. 2010;12(6):905–907. doi: 10.1093/europace/euq034. [DOI] [PubMed] [Google Scholar]
- 10.Ueoka A, Morita H, Watanabe A, et al. Prognostic significance of the sodium channel blocker test in patients with Brugada syndrome. J Am Heart Assoc. 2018;7(10):e00861710. doi: 10.1161/JAHA.118.008617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarquella-Brugada G, Campuzano O, Arbelo E, Brugada J, Brugada R. Brugada syndrome: clinical and genetic findings. Genet Med. 2016;18(1):3–12. doi: 10.1038/gim.2015.35. [DOI] [PubMed] [Google Scholar]
- 12.Antzelevitch C, Patocskai B. Brugada syndrome: clinical, genetic, molecular, cellular, and ionic aspects. Curr Probl Cardiol. 2016;41(1):7–57. doi: 10.1016/j.cpcardiol.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottschalk BH, Anselm DD, Baranchuk A. Brugada phenocopy international registry and online educational portal. 2014. [Accessed November 30, 2018]. Available from: http://www.brugadaphenocopy.com.
- 14.García-Niebla J, Llontop-García P, Valle-Racero JI, Serra-Autonell G, Batchvarov VN, de Luna AB. Technical mistakes during the acquisition of the electrocardiogram. Ann Noninvasive Electrocardiol. 2009;14(4):389–403. doi: 10.1111/j.1542-474X.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight BP, Pelosi F, Michaud GF, Strickberger SA, Morady F. Clinical consequences of electrocardiographic artifact mimicking ventricular tachycardia. N Engl J Med. 1999;341(17):1270–1274. doi: 10.1056/NEJM199910213411704. [DOI] [PubMed] [Google Scholar]
- 16.Quan XQ, Li S, Liu R, Zheng K, Wu XF, Tang Q. A meta-analytic review of prevalence for Brugada ECG patterns and the risk for death. Medicine (Baltimore) 2016;95(50):e5643. doi: 10.1097/MD.0000000000005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranchuk A, Nguyen T, Ryu MH, et al. Brugada phenocopy: new terminology and proposed classification. Ann Noninvasive Electrocardiol. 2012;17(4):299–314. doi: 10.1111/j.1542-474X.2012.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottschalk BH, Anselm DD, Brugada J, et al. Expert cardiologists cannot distinguish between Brugada phenocopy and Brugada syndrome electrocardiogram patterns. Europace. 2016;18(7):1095–1100. doi: 10.1093/europace/euv278. [DOI] [PubMed] [Google Scholar]
- 19.Gottschalk BH, Baranchuk A. Differentiation between Brugada syndrome and Brugada phenocopy ECG patterns: is it possible? Brugada Phenocopy. 2018;1:119–124. [Google Scholar]
- 20.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/ HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm Society. Heart Rhythm. 2018;15(10):e190–e252. doi: 10.1016/j.hrthm.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 21.Moncayo-Arlandi J, Brugada R. Unmasking the molecular link between arrhythmogenic cardiomyopathy and Brugada syndrome. Nat Rev Cardiol. 2017;14(12):744–756. doi: 10.1038/nrcardio.2017.103. [DOI] [PubMed] [Google Scholar]
- 22.Postema PG, Neville J, de Jong JS, Romero K, Wilde AA, Woosley RL. Safe drug use in long QT syndrome and Brugada syndrome: comparison of website statistics. Europace. 2013;15(7):1042–1049. doi: 10.1093/europace/eut018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res. 1999;85(9):803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 24.Yan GX, Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100(15):1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 25.Junttila MJ, Gonzalez M, Lizotte E, et al. Induced Brugada-type electrocardiogram, a sign for imminent malignant arrhythmias. Circulation. 2008;117(14):1890–1893. doi: 10.1161/CIRCULATIONAHA.107.746495. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal Y, Aggarwal S, Kalavakunta JK, Gupta V. All that looks like “Brugada” is not “Brugada”: Case series of Brugada phenocopy caused by hyponatremia. J Saudi Heart Assoc. 2016;28(4):274–277. doi: 10.1016/j.jsha.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maheshwari A, Sreenivasan S, Benditt D. Brugada Phenocopy: The Art of Recognizing the Brugada ECG Pattern. 1st ed. Elsevier; 2018. [Accessed November 30, 2018]. pp. 87–92. Available from: https://market.android.com/details?id=book-kyk0D-wAAQBAJ. [Google Scholar]
- 28.Abu Shama R, Bayes de Luna A, Baranchuk A. Tachycardia-dependent Brugada phenocopy due to hyperkalemia. J Cardiovasc Electrophysiol. 2017;28(9):1084–1085. doi: 10.1111/jce.13269. [DOI] [PubMed] [Google Scholar]
- 29.Antzelevitch C, Brugada P, Borggrefe M, et al. Brugada syndrome: report of the second consensus conference: endorsed by the heart rhythm society and the European heart rhythm association. Circulation. 2005;111(5):659–670. doi: 10.1161/01.CIR.0000152479.54298.51. [DOI] [PubMed] [Google Scholar]
- 30.Probst V, Wilde AA, Barc J, et al. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet. 2009;2(6):552–557. doi: 10.1161/CIRCGENETICS.109.853374. [DOI] [PubMed] [Google Scholar]
- 31.Anselm DD, Baranchuk A. Terminological clarification of Brugada phenocopy, Brugada syndrome, and the Brugada ECG pattern: Re. early repolarization pattern in patients with provocable Brugada phenocopy: a marker of additional arrhythmogenic cardiomyopathy. Int J Cardiol. 2014;171(2):288. doi: 10.1016/j.ijcard.2013.11.080. [DOI] [PubMed] [Google Scholar]
- 32.Anselm DD, Gottschalk BH, Baranchuk A. Brugada phenocopies: consideration of morphologic criteria and early findings from an international registry. Can J Cardiol. 2014;30(12):1511–1515. doi: 10.1016/j.cjca.2014.09.023. [DOI] [PubMed] [Google Scholar]


