Abstract
Growing insight into prognosis of pediatric acute myeloid leukemia (AML) survival has led to improved outcome over time and could be further enhanced through investigation using a large number of patients. To characterize the extent of the association of pediatric AML survival with its identified prognostic factors, we analyzed the United States population-based Surveillance Epidemiology and End Results (SEER) large dataset of 3442 pediatric AML patients diagnosed and followed between 1973 and 2011 using a Cox proportional hazards model stratified by year of diagnosis. Patients diagnosed between 10 and 19 years of age were at a higher risk of death compared to those diagnosed before age 10 (adjusted hazard ratio (aHR): 1.30, 95% confidence interval (CI): 1.17–1.44). African Americans (1.27, 1.09–1.48) and Hispanics (1.15, 1.00–1.32) had an elevated risk of mortality than Caucasians. Compared to the subtype acute promyelocytic leukemia, AML with minimal differentiation (2.44, 1.78–3.35); acute erythroid leukemia (2.34, 1.60–3.40); AML without maturation (1.87, 1.35–2.59); and most other AML subtypes had a higher risk of mortality, whereas AML with inv(16) had a substantially lower risk. Age at diagnosis, race-ethnicity, AML subtype, county level poverty and geographic region appeared as significant prognostic factors of pediatric AML survival in the US. Contrary to previous findings, the subtypes of AML with t (9;11)(p22;q23)MLLT3-MLL, AML without maturation and acute myelomonocytic leukemia emerged to be indicative of poor outcome.
Keywords: SEER, Acute myeloid leukemia, Heterogeneous, Prognostic factors, Stratified Cox-proportional hazard model
1. Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of leukemias that arise from clonal disorder caused by malignant transformation of a bone marrow-derived, self-renewing stem cell or myeloid progenitor, which demonstrates a decreased rate of apoptosis as well as aberrant, and usually limited, differentiation capacity [1]. AML contributes to 18% of childhood leukemia cases [2] and about 50% of all deaths of pediatric leukemia in the United States [3]. The age-adjusted incidence rate of childhood AML in the US has been increasing at 1% per year between 1975 and 2011 and is currently estimated to be 8.5 cases per million [3,4]. The outcomes of pediatric AML patients vary by morphology, phenotype, cytogenetics, white blood cell counts, response to therapy and host factors such as age at diagnosis, race-ethnicity, sex, body mass index, genetic polymorphism and socio-economic status [5–9]. Survival of childhood AML has dramatically improved in recent decades. The five-year survival rate has risen to 64% in 2009 from 21% in 1975 [10]. Contributing factors to the improved survival include refinement in the diagnosis of AML and advancement in therapeutic approaches. The French–British–American (FAB) classification was proposed in 1976 to classify AML subtypes by morphology [11]. The World Health Organization (WHO) developed a more refined classification system upon the FAB classification in 2001 by incorporating cytogenetics information and then expanded the subtyping to include more recent findings in2008 [12,13]. It has been reported that the presence of t(15;17), t (8;21), inv(16), t(16;16), t(9;11), Down syndrome, FAB type M3 and molecular evidence of these abnormalities are favorable; and monosomies of chromosomes 5 or 7, deletion of the long arm of chromosome 5 (del5q), abnormalities of the long arm of chromosome 3, complex karyotypes and resistant disease defined as more than 15% bone marrow blasts on recovery from the first course of chemotherapy are unfavorable prognostic factors of survival. Other cytogenetic abnormalities or a normal karyotype are stated as indicators of a standard risk of mortality [14–19]. After adjustment for the effects of cytogenetic and other prognostic factors, children age 10 years or older at the time of diagnosis are at a greater risk of death than those diagnosed before age 10 [15]. African American patients have a significantly worse outcome than their white counterparts [17,18]. Low socio-economic status is reported to be associated with poor prognosis [6].
Published reports on the associations of pediatric AML with its prognostic factors are based mainly on studies involving relatively small number of patients, which may not be adequate for comprehensive exploration of these heterogeneous associations. Also, the risk of mortality has greatly reduced, and many factors may have lost their prognostic values over time [5]. In this study, we used the Surveillance Epidemiology and End Results’ (SEER) large dataset from patients diagnosed and followed between 1973 and 2011 to characterize the extent of associations of pediatric AML survival with previously identified prognostic factors. The SEER program of the National Cancer Institute is the most comprehensive and reliable source of population-based information in the US on cancer incidence and survival [20]. Being large and well representative of the study population in the US, the SEER dataset is ideal for characterizing the complex associations between pediatric AML survival and its prognostic factors with greater precision. Patients in the SEER dataset were diagnosed over a span of 39 years and were at varying risk of mortality at the time of diagnosis as survival improved over time. We stratified the analysis by the year of diagnosis grouped into 5-year intervals to account for the time-varying survival pattern and performed a sensitivity analysis to assess the potential effect of using unequal interval of follow-up time on the estimates.
2. Methods
2.1. SEER data
This study utilized case listings from 17 SEER registries between 1973 and 2011. SEER currently collects data on patient demographics, incidence, mortality, primary tumor site, tumor structure and follow-up information from population-based cancer registries covering approximately 28% of the US population. The SEER covered population is comparable to the general US population with regards to sex, race-ethnicity, measures of poverty and education [20]. Detailed descriptions of the SEER data are available in the SEER Program Coding and Staging Manual [21]. We identified 3450 AML patients aged 0–19 years at the time of diagnosis. Excluding eight children with missing follow-up time, a total of 3442 subjects were used for the analysis. The following variables were included in this study:
2.2. Age at diagnosis
The SEER data included age at diagnosis as <1, 1–4, 5–9, 10–14, 15–19 years. We dichotomized this variable to be <10 and 10–19 years based on previously established clinical cut-offs [15,22,23].
2.3. Race/ethnicity
We used SEER variables “Race recode (W, B, AI, API)” and “Origin recode NHIA (Hispanic, Non-Hisp)” to form the following mutually exclusive race–ethnicity groups: non-Hispanic Caucasian (Caucasian), non-Hispanic African American (AA), Hispanics and others.
2.4. Number of primaries
This variable was defined as the total number of independent reportable tumors of an individual in the SEER data [24]. The minimum number of primaries was 1 and the maximum was 3. In our preliminary exploration, we found 3264 (94.8%), 170 (5%) and 8 (0.2%) subjects with 1, 2 or 3 primaries, respectively. Hence, we collapsed this variable into two categories: 1 and ≥2 primary tumors.
2.5. Receipt of radiation
Information about radiation therapy was recorded in the SEER data as (a) beam radiation, (b) combination of beam radiation with implant or isotopes, (c) none, (d) radiation, not otherwise specified, recommended, (f) refused, and (g) unknown. To recode, (c) and were set to be “no”; (a), (b), (d) were “yes”; (e) and (g) were “unknown”.
2.6. Year of diagnosis
This variable was recorded in single-year interval. We recoded this variable as 7 five-year intervals (1973–1977, 1978–1982, 1983–1987, 1988–1992, 1993–1997, 1998–2002, 2003–2007) and a four-year interval (2008–2011).
2.7. AML subtypes
AML subtypes were classified in SEER data by the International Classification of Diseases for Oncology, third edition (ICD-O-3)/WHO 2008 definitions and were accessed using the SEER variable “ICD-O-3Hist/behav” [21,24]. The AML subtypes as they appeared in the SEER data, along with italicized abbreviations used in this study, are as follows—9840/3: acute erythroid leukemia (M6 type), AEL; 9861/3: acute myeloid leukemia, AML–NOS; 9866/3: acute promyelocytic leukemia (AML with t(15;17)(q22;q12)) PML/RARA, APL; 9867/3: acute myelomonocytic leukemia, AMML; 9897/3: acute myeloid leukemia with (9;11)(p22;q23);MLLT3-MLL, AML t (9); 9910/3: acute megakaryoblastic leukemia, AMKL; 9871/3: AML with inv(16)(p13.1q22) or t(16;16)(p13.1;q22), CBFB-MYH11, AML inv(16); 9872/3: acute myeloid leukemia with minimal differentiation, AML min diff; 9873/3: acute myeloid leukemia without maturation, AML w/o mat; 9874/3: acute myeloid leukemia with maturation, AML w/mat; 9895/3: AML with myelodysplasia-related changes (multilineage dysplasia), AML–MRC; 9896/3: acute myeloid leukemia, t(8;21)(q22;q22) RUNX1-RUNX1T1, AML t(8); 9920/3: therapy-related (acute) myeloid neoplasm, t-AML. The ICD codes 9840/3, 9861/3, 9866/3, 9867/3, 9897/3 and 9910/3 were reportable since 1978, while the rest of the above were reportable since 2001. In addition, 9898/3: myeloid leukemia associated w/Down Syndrome and 9865/3: acute myeloid leukemia w/t(6;9)(p23;q34) DEK-NUP214 became reportable since 2010; only 13 and 1 patients of these two subtypes were in the data, respectively. None of these 14 patients experienced mortality.
2.8. Percentage of families in county with income below poverty level
The variable was a county-level attribute and indicated the percentage of families with income below the poverty based on the Census American Community Survey 2007–2011. The variable was categorized into quartiles. Patients in Quartile 1 were from counties with the minimum percentage of family-income below the poverty level (approximately the lowest 25%), while patients in Quartile 4 were from counties with maximum percentage of family-income below the poverty level (approximately the highest 25%).
2.9. Geographic region
SEER registries are located in 5 regions of contract health service delivery areas (CHSDA) in the US: East, Pacific Coast, Northern Plains, Southwest and Alaska. Thirteen patients were from Alaska, and the hazard rate in this region was comparable to that in the Pacific Coast. We collapsed data from these two regions.
2.10. Follow-up time and survival status
The follow-up time was documented as the duration from the time of diagnosis to death or the last day of the available survival information in the SEER registry. The SEER variables “vital status recode” and “SEER cause-specific death classification” were used to determine overall and AML-specific mortality, respectively. For the sensitivity analysis, those who did not experience death within the first five years after diagnosis were censored.
2.11. Statistical analysis
Distribution of the overall and AML-specific mortality were summarized by study variables. Kaplan–Meier survival curve was used to characterize the crude probability of survival over time since diagnosis across groups of prognostic factors. A univariable Cox proportional hazard model was used to determine the association of each study variable with the risk of mortality of pediatric AML patients. A multivariable Cox proportional hazard model was used to determine the significant prognostic factors of pediatric AML in the US. Models were stratified by the year of diagnosis to account for the time-varying survival pattern of pediatric AML. Age at diagnosis, sex, race-ethnicity, number of primaries, radiation, AML subtype, percent families with income below poverty in county and geographic region were used as predictors. Hazard ratios (HR) were reported with associated 95% confidence intervals (CI). The rationale and a brief description of the stratified Cox proportional hazards model are provided in the supplementary materials. In addition, we have repeated the analyses using only the first five-year follow-up data to evaluate the sensitivity of the estimates using unequal follow-up time. All analyses were two-tailed with the level of significance of 0.05. The statistical software SAS 9.3 (SAS Institute, Cary, NC) was used for data analyses.
3. Results
Among 3442 AML patients, there were 48% females, 52% Caucasians, 12% AA, 25% Hispanics, 54% AML–NOS, 12% APL, 9% AMML and 7% AMKL. Approximately 47% patients were diagnosed before 10 years of age. There were 1673 (49%) overall and 1383 (40%) AML-specific deaths.
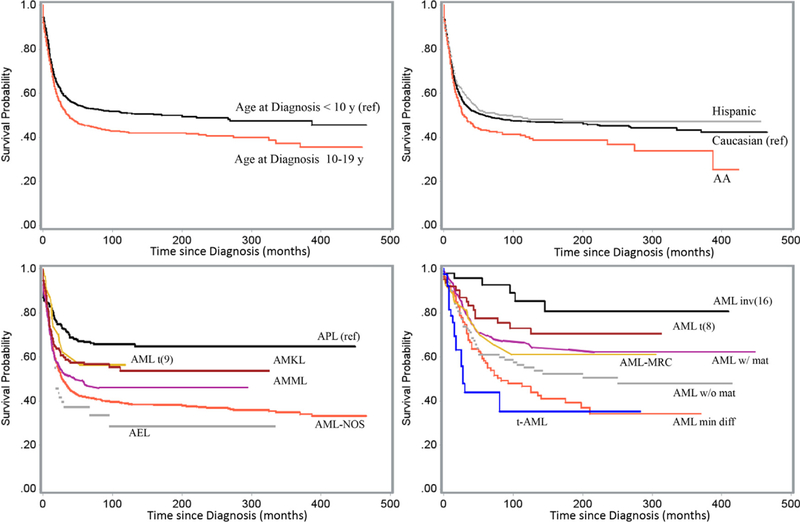
Fig. 1 displays the estimated Kaplan–Meier probability of pediatric AML patients surviving at any given time since diagnosis by age-group at diagnosis, race–ethnicity and subtype. The estimates represented the crude proportions of survivals as the analyses were not accounted for the time varying survival patterns over diagnosis years.
Fig. 1.
Survival of pediatric AML patients by prognostic factors, SEER data, 1973–2011. Survival (unadjusted) by AML subtype was displayed in two plots due to difference in SEER reporting years (Table 1). Subtypes including APL and others (lower left) were available since 1978; data for AML inv(16) and other subtypes (lower right) were available since 2001.
Table 1 presents the distribution of overall and AML-specific mortality and their risks associated with the study variables. The estimation of HR in this table accounted for the time varying survival pattern as the model was stratified by year of diagnosis. No substantial differences were found between the results obtained using overall and AML-specific mortality except the results related to t-AML subtype and number of primaries. Therefore, results using overall mortality were illustrated unless otherwise mentioned. Compared to those who were diagnosed before 10, patients between 10 and 19 years of age at the time of diagnosis showed a higher risk of mortality, HR (CI): 1.23 (1.12,1.36). More on the effect of the age at diagnosis using alternative cut-offs could be found in Supplementary Table S1. No substantial difference in the risks of mortality between males and females was observed (p = 0.20). Although the overall proportion of death was the lowest in Hispanic among the three race–ethnicity groups, AA and Hispanic AML patients had a higher risk of mortality compared to Caucasian patients; HR: 1.29 and 1.16 in AAs and Hispanics, respectively. The presence of multiple primaries was associated with an elevated risk of overall death, HR: 1.25, while risk of AML-specific death in this group was 6 percent of that in the single tumor group, HR (CI): 0.06 (0.02, 0.17). No substantial influence of the receipt of radiation therapy was observed on the risk of mortality (p = 0.80). Differential risks of death were associated with various AML subtypes. Subtypes t-AML, AML min diff, AEL, AMML, AML w/o mat, AML–NOS and AMKL exhibited worse prognosis than that of APL. Although not significant (p>0.05), AML t(9), AML–MRC and AML w/mat also showed a substantially worse outcome. The estimated risk of mortality in AML inv(16) patients was half that in the APL patients. The hazard risk of mortality was comparable between AML t(8) and APL. No AML-specific death occurred in the t-AML subtype. In terms of geographic regions, registries located in the East showed the best prognosis while registries in the Southwest exhibited worst. In addition to the Southwest, registries in the Pacific Coast also exhibited a substantially worse prognosis compared to the registries in the East. In terms of the county level percent families with income below poverty, the risk of mortality in the Quartile 4 was 1.25 times of that in the Quartile 1. The risk was comparable among Quartiles 1–3. Unequal follow-up time of patients was unlikely to influence the estimates. No substantial differences were observed between results using the entire follow-up time in Table 1 and only the first five years of follow-up time in the Supplementary Table S2.
Table 1.
Pediatric AML mortality distribution and hazard risk by study variables, SEER 1973–2011.
| Variables | Death, n (%) | Hazard ratio (95% CI)a | ||||
|---|---|---|---|---|---|---|
| Overall | AML-specific | Overall | p-value | AML-specific | p-value | |
| Age at diagnosis (years) | ||||||
| <10 | 723 (45) | 602 (37) | - | - | - | - |
| 10–19 | 950 (52) | 781 (43) | 1.23 (1.12, 1.36) | <0.0001 | 1.21 (1.09, 1.35) | 0.0004 |
| Sex | ||||||
| Female | 780 (47) | 654 (40) | - | - | - | - |
| Male | 893 (50) | 729 (41) | 1.07 (0.97, 1.17) | 0.20 | 1.03 (0.93, 1.15) | 0.53 |
| Race-ethnicity | ||||||
| Caucasian | 875 (49) | 734 (41) | - | - | - | - |
| Hispanics | 375 (44) | 299 (35) | 1.16 (1.02, 1.32) | 0.02 | 1.13 (0.98, 1.30) | 0.09 |
| AA | 232 (57) | 191 (47) | 1.29 (1.12, 1.49) | <0.0001 | 1.28 (1.09, 1.50) | 0.003 |
| Number of primaries | ||||||
| 1 | 1580 (48) | 1379 (42) | - | - | - | - |
| ≥2 | 93 (52) | 4 (2) | 1.25 (1.01, 1.54) | 0.04 | 0.06 (0.02, 0.17) | <0.0001 |
| Radiation | ||||||
| No | 1463 (48) | 1206 (40) | - | - | - | - |
| Yes | 203 (53) | 171 (45) | 1.00 (0.86, 1.16) | 1 | 1.02 (0.87, 1.20) | 0.80 |
| Subtype | ||||||
| APL | 122 (31) | 107 (27) | - | - | - | - |
| AML-NOS | 1029 (57) | 869 (48) | 1.67 (1.37, 2.02) | <0.0001 | 1.59 (1.30, 1.96) | <0.0001 |
| AEL | 36 (62) | 25 (43) | 2.22 (1.52, 3.22) | <0.0001 | 2.01 (1.34, 2.26) | <0.0001 |
| AMML | 150 (49) | 96 (31) | 1.87 (1.47, 2.38) | <0.0001 | 1.74 (1.34, 2.26) | <0.0001 |
| AML inv(16) | 6 (13) | 4 (8) | 0.47 (0.21, 1.07) | 0.07 | 0.45 (0.18, 1.10) | 0.08 |
| AML min diff | 59 (56) | 39 (37) | 2.30 (1.68, 3.15) | <0.0001 | 2.24 (1.59, 3.14) | <0.0001 |
| AML w/o mat | 54 (45) | 39 (33) | 1.81 (1.31, 2.50) | <0.0001 | 1.71 (1.21, 2.44) | 0.003 |
| AML w/mat | 65 (34) | 52 (27) | 1.23 (0.91, 1.66) | 0.19 | 1.14 (0.82, 1.59) | 0.43 |
| AML-MRC | 10 (37) | 5 (19) | 1.40 (0.73, 2.67) | 0.31 | 1.12 (0.52, 2.41) | 0.77 |
| AML t(8) | 16 (25) | 9 (14) | 1.02 (0.60, 1.72) | 0.95 | 0.74 (0.38, 1.41) | 0.35 |
| AML t(9) | 12 (36) | 8 (24) | 1.57 (0.87, 2.85) | 0.14 | 1.51 (0.79, 2.90) | 0.22 |
| AMKL | 95 (41) | 61 (27) | 1.54 (1.18, 2.02) | 0.002 | 1.44 (1.08, 1.94) | 0.01 |
| t-AML | 19 (49) | 0 | 3.98 (2.43, 6.51) | <0.0001 | − | − |
| Geographic region | ||||||
| East | 396 (43) | 303 (33) | - | - | - | - |
| Pacific Coast | 800 (47) | 664 (39) | 1.10 (0.97, 1.24) | 0.13 | 1.20 (1.04, 1.37) | 0.01 |
| Northern Plains | 303 (61) | 256 (52) | 1.08 (0.92, 1.26) | 0.34 | 1.16 (0.98, 1.38) | 0.09 |
| Southwest | 174 (58) | 160 (53) | 1.24 (1.03, 1.48) | 0.02 | 1.47 (1.21, 1.78) | 0.0001 |
| Percent families below poverty | ||||||
| Quartile 1 | 471 (50) | 387 (41) | - | - | - | - |
| Quartile 2 | 414 (51) | 339 (42) | 0.97 (0.85, 1.11) | 0.67 | 0.97 (0.83, 1.12) | 0.64 |
| Quartile 3 | 469 (44) | 389 (36) | 1.03 (0.90, 1.18) | 0.66 | 1.06 (0.92, 1.23) | 0.42 |
| Quartile 4 | 319 (52) | 268 (44) | 1.25 (1.08, 1.44) | 0.003 | 1.28 (1.10, 1.50) | 0.002 |
| Year of diagnosis | ||||||
| 1973–1977 | 173 (85) | 113 (56) | - | - | - | - |
| 1978–1982 | 155 (81) | 95 (50) | 0.84 (0.67, 1.04) | 0.10 | 0.81 (0.65, 1.03) | 0.08 |
| 1983–1987 | 131 (72) | 73 (40) | 0.71 (0.56, 0.89) | 0.003 | 0.66 (0.52, 0.85) | 0.001 |
| 1988–1992 | 143 (62) | 83 (36) | 0.57 (0.45, 0.71) | <0.0001 | 0.54 (0.42, 0.68) | <0.0001 |
| 1993–1997 | 224 (60) | 119 (32) | 0.53 (0.44, 0.65) | <0.0001 | 0.46 (0.37, 0.57) | <0.0001 |
| 1998–2002 | 336 (50) | 237 (35) | 0.42 (0.35, 0.50) | <0.0001 | 0.39 (0.32, 0.47) | <0.0001 |
| 2003–2007 | 334 (40) | 266 (32) | 0.33 (0.27, 0.39) | <0.0001 | 0.28 (0.23, 0.34) | <0.0001 |
| 2008–2011 | 177 (24) | 137 (18) | 0.28 (0.23, 0.34) | <0.0001 | 0.24 (0.19, 0.30) | <0.0001 |
Hazard ratios were estimated with stratification by year of diagnosis intervals.
Table 2 shows the adjusted hazard ratios (aHR) of pediatric AML patients associated with the influential prognostic factors estimated using a multivariable Cox proportional hazards regression model stratified by the years of diagnosis. In the multivariable model, age at diagnosis, race–ethnicity, AML subtypes, geographic region and percentage of families below poverty in the county remained as significant prognostic factors for the survival of pediatric AML patients. Number of primaries failed to retain the significance that was detected in the univariable model. Patients diagnosed with AML at or after the age of 10 years on average had a 30% increase in the risk of mortality compared to those diagnosed before 10. Similarly, AA (aHR: 1.27) and Hispanic (aHR: 1.15) pediatric AML patients had worse outcome than their Caucasian counterparts. The discrepancy in the risk of mortality between AML subtypes remained as it was in the univariable model—t-AML, AML with min diff, AEL, AMML, AML w/o mat, AMKL and AML–NOS were associated with elevated risk of mortality compared to that of APL, while, AML inv(16) exhibited an average of two-fold survival advantage. The outcome of AML t(8) was comparable to that of APL.
Table 2.
Association between prognostic factors and overall survival of pediatric AML patients in the multivariable Cox proportional hazards model stratified by year of diagnosis, SEER data, 1973–2011.
| Variables | aHRa (95% CI*) | p-value |
|---|---|---|
| Age at diagnosis (years) | ||
| <10 | - | - |
| 10–19 | 1.30 (1.17,1.44) | <0.0001 |
| Sex | ||
| Female | - | - |
| Male | 1.02 (0.93, 1.13) | 0.66 |
| Race-ethnicity | ||
| Caucasian | - | - |
| Hispanics | 1.15 (1.00, 1.32) | 0.05 |
| AA | 1.27 (1.09, 1.48) | 0.002 |
| Number of primaries | ||
| 1 | - | - |
| ≥2 | 1.11 (0.88, 1.40) | 0.39 |
| Radiation | ||
| No | - | - |
| Yes | 0.94 (0.81, 1.10) | 0.44 |
| Subtype | ||
| APL | - | - |
| AML-NOS | 1.77 (1.45, 2.15) | <0.0001 |
| AEL | 2.34 (1.60, 3.40) | <0.0001 |
| AMML | 1.96 (1.54, 2.49) | <0.0001 |
| AML inv(16) | 0.48 (0.21, 1.10) | 0.08 |
| AML min diff | 2.44 (1.78, 3.35) | <0.0001 |
| AML w/o mat | 1.87 (1.35, 2.59) | 0.0002 |
| AML w/mat | 1.27 (0.94, 1.72) | 0.13 |
| AML-MRC | 1.58 (0.82, 3.02) | 0.17 |
| AML t(8) | 1.08 (0.64, 1.82) | 0.79 |
| AML t(9) | 1.80 (0.99, 3.27) | 0.06 |
| AMKL | 1.83 (1.39, 2.42) | <0.0001 |
| t-AML | 3.77 (2.20, 6.45) | <0.0001 |
| Geographic region | ||
| East | - | - |
| Pacific Coast | 1.15 (1.01, 1.32) | 0.03 |
| Northern Plains | 1.08 (0.92, 1.26) | 0.38 |
| Southwest | 1.28 (1.06, 1.54) | 0.01 |
| Percent families below poverty | ||
| Quartile 1 | - | - |
| Quartile 2 | 0.97 (0.85, 1.11) | 0.65 |
| Quartile 3 | 0.98 (0.86, 1.13) | 0.82 |
| Quartile 4 | 1.19 (1.02, 1.39) | 0.03 |
CI: confidence interval.
aHR: adjusted hazard ratio.
4. Discussion
Studies recognized a growing list of prognostic factors of AML survival over the past few decades. The better understanding of this disease allowed more precise treatment and eventually contributed to improved survival patterns over time. Age at diagnosis, race–ethnicity, socio-economic status, and cytogenetic and morphologic features emerged as the crucial factors in the prognosis of pediatric AML survival. The present study confirmed previous findings and provided estimates with greater precision utilizing a large population-based dataset with long-term follow-up to quantify the heterogeneous association between prognostic factors and AML survival.
Likewise in previous studies [14–16], age at diagnosis remained as a significant independent factor in our study. The risk of mortality in patients who were diagnosed at an older age (10–19 years) was 1.30 times that of those who were diagnosed at a younger age. This estimate was 1.63 in the study by Razzouk et al. [15]. Age-associated biological features and their response to therapy might be the contributors of this difference in the survival of AML patients [7,15,18]. Disparity in the distribution of AML subtypes over age groups in the SEER dataset could partially support the age dependence of biological features. The prevalenc of APL, AMML, AML inv(16), AML w/o mat, AML w/mat, AML t(8) and t-AML were higher among patients diagnosed at ages ≥10 years; while most patients with AMKL were diagnosed at ages <10 years. AML inv(16), the most survival–favorable subtype in our study, exhibited an age dependence response. Six out of 32 patients of ages 10–19 years at diagnosis died in this subtype, while none of 16 patients diagnosed at a age <10 years died.
AA and Hispanics had been identified to have a worse outcome than their Caucasian counterparts [3,17,18]. Our study demonstrated that AAs had the poorest outcome (aHR: 1.27), followed by Hispanic patients (aHR: 1.15). The racial differences in AML survival may be attributed to the genetics, differential access to the medical facilities and differential response to therapies [17,18,25]. In SEER data, proportions of AA patients were relatively lower in AML inv (16) and AML t(8), two subtypes with survival advantages. The worst survival in AA could be partly explained by the worse county level poverty. About 23% of patients in Quartile 4 of the percentage of families in the county with income below poverty level were AA, while the percentages of AA were 5–12%, monotonically increasing in Quartiles 1–3.
Advances in identifying morphological and cytogenetic risk factors helped in classifying AML patients into high-, low-, and intermediate-risk groups of mortality. Differential survival outcomes of AML subtypes were demonstrated in numerous studies [7,14,15,18,26]. In our study, AML with inv(16) and t-AML showed the lowest and highest risk of mortality, respectively. The order of the other subtypes from higher to lower risk compared to that of APL are as follows: AML min diff, AEL, AMML, AML w/o mat, AMKL, AML t(9), AML–NOS, AML–MRC, AML w/o mat. While others reported AML t(9), AML w/o mat and AMML as survival favorable subtypes [6,7,27,28], findings of our study revealed these as highrisk subtypes, which could partly be attributed to the worse response to therapy [29,30]. AEL is a heterogeneous subtype, and survival outcome was reported to vary by cytogenetic abnormalities [28]. Our study demonstrated this as a high-risk subtype. Findings of our study also demonstrated AMKL and AML–NOS as pediatric survival unfavorable subtypes and AML–MRC with intermediate mortality risk. Like other studies, our study indicated that AML w/mat, AML t(8), AML inv(16) and APL were survival favorable subtypes. All 19 patients having t-AML died from non-AML causes. Since t-AML is a clinical syndrome recognized as a complication after cytotoxic and/or radiation therapy [30], this result came as no surprise.
Our study confirmed the role of socio-economic status on the prognosis of AML survival reported by Petridou et al. [6]. Patients from counties with higher percentages of families below poverty level showed a worse prognosis. Our study identified patients from the Southwest region of the US with the worst prognosis. No previous studies reported this fact for AML.
Findings of our study did not suggest association of sex, radiation therapy, and number of primaries with pediatric AML survival after adjustment for other prognostic factors.
Like most epidemiological studies, the current study was not without limitations. First, our results might be driven in part by the effect of unmeasured confounders. There was limited information of treatment in the SEER data. Secondly, event-free survival, an important oncologic outcome, is missing in the SEER data. Thirdly, the follow-up periods tended to be shorter for patients diagnosed more recently. However, our results were not limited by this variability of the follow-up time, as verified by the sensitivity analysis. Lastly, AML survival improved dramatically over time, causing the baseline hazard to differ during the study period; also, proportion of Hispanics patients increased over time in SEER data. These improved survival and increased proportion of Hispanic patients over time induced an artifact (Fig. 1) in the non-stratified analysis that Hispanics had better outcomes than Caucasians. The stratified analysis handled this constraint of the population-based data successfully (Tables 1 and 2). This is the result of the well-known Simpson’s paradox.
In conclusion, the study identified age at diagnosis, race-ethnicity, AML subtypes, county poverty level and geographic regions as key prognostic factors of AML survival in the United States SEER large dataset. It also demonstrated the importance of employing a proper strategy to address complicacies in the analysis of population-based dataset. Although survival improved over time, prognostic values of host, disease, socio-economic and environmental factors remained significant. Complex interactions of these factors, unknown biological processes, and response to therapy might give rise to these associations. Future research could focus on better understanding of interactions of these processes, and elucidating their mechanisms.
Supplementary Material
Acknowledgments
We thank to the editor and reviewers for careful review and insightful comments which have led to a significant improvement of the article. This study was partially supported by the National Institutes of Health (NIH) COBRE Grant (PI: Shaffer) and an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the NIH under Grant no. U54-GM104941 (PI: Binder-Macleod).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.canep.2015.06.009.
References
- [1].National Cancer Institute: PDQ® Childhood Acute Myeloid Leukemia/Other Myeloid Malignancies Treatment. Bethesda, MD: National Cancer Institute. Available from: http://cancer.gov/cancertopics/pdq/treatment/childAML/HealthProfessional (accessed 11.26.14.). [Google Scholar]
- [2].Linabery AM, Ross JA, Trends in childhood cancer incidence in the U.S. (1992–2004), Cancer 112 (2008) 416–432, doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- [3].Howlader N, Noone AM, Krapcho M, et al. , Seer Cancer Statistics Review 1975–2009 (Vintage 2009 Populations), MD: National Cancer Institute, Bethesda, 2012. [Google Scholar]
- [4].Fast Stats An interactive tool for access to SEER cancer statistics. Surveillance Research Program, National Cancer Institute. http://seer.cancer.gov/faststats (accessed 4.01.15.).
- [5].Radhi M, Meshinchi S, Gamis A, Prognostic factors in pediatric acute myeloid leukemia, Curr. Hematol. Malig. Rep 5 (October (4)) (2010) 200–206, doi: 10.1007/s11899-010-0060-z. [DOI] [PubMed] [Google Scholar]
- [6].Petridou ET, Sergentanis TN, Perlepe C, Papathoma P, Tsilimidos G, Kontogeorgi E, Kourti M, Baka M, Moschovi M, Polychronopoulou S, Sidi V, Hatzipantelis E, Stiakaki E, Iliadou AN, La Vecchia C, Skalkidou A, Adami H, Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis, Ann. Oncol 26 (March (3)) (2015) 589–597, doi: 10.1093/annonc/mdu572 Epub 2014 December 19. [DOI] [PubMed] [Google Scholar]
- [7].Meshinchi S, Arceci RJ, Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia, Oncologist 12 (March (3)) (2007) 341–355. [DOI] [PubMed] [Google Scholar]
- [8].Davies SM, Robison LL, Buckley JD, Tjoa T, Woods WG, Radloff GA, Ross JA, Perentesis JP, Glutathione S-transferase polymorphisms and outcome of chemotherapy in childhood acute myeloid leukemia, J. Clin. Oncol 19 (March (5)) (2001) 1279–1287. [DOI] [PubMed] [Google Scholar]
- [9].Damm F, Heuser M, Morgan M, Yun H, Grosshennig A, Göhring G, Schlegelberger B, Döhner K, Ottmann O, Lübbert M, Heit W, Kanz L, Schlimok G, Raghavachar A, Fiedler W, Kirchner H, Döhner H, Heil G, Ganser A, Krauter J, Single nucleotide polymorphism in the mutational hotspot of WT1 predicts a favorable outcome in patients with cytogenetically normal acute myeloid leukemia, J. Clin. Oncol 28 (Feburary (4)) (2010) 578–585, doi: 10.1200/JCO.2009.23.0342 Epub 2009 December 28. [DOI] [PubMed] [Google Scholar]
- [10].Cancer Facts & Figures, American Cancer Society, American Cancer Society, Atlanta, 2014. [Google Scholar]
- [11].Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C, Proposals for the classification of the acute leukaemias. French–American–British (FAB) co-operative group, Br. J. Haematol 33 (August (4)) (1976) 451–458. [DOI] [PubMed] [Google Scholar]
- [12].Vardiman JW, Harris NL, Brunning RD, The World Health Organization (WHO) classification of the myeloid neoplasms, Blood 10 0 (October (7)) (2002) 2292–2302. [DOI] [PubMed] [Google Scholar]
- [13].Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD, The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes, Blood 114 (July (5)) (2009) 937–951, doi: 10.1182/blood-2009-03-209262 Epub 2009 April 8. [DOI] [PubMed] [Google Scholar]
- [14].Webb DK, Harrison G, Stevens RF, Gibson BG, Hann IM, Wheatley K, MRC childhood leukemia working party. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia, Blood 98 (September (6)) (2001) 1714–1720. [DOI] [PubMed] [Google Scholar]
- [15].Razzouk BI, Estey E, Pounds S, Lensing S, Pierce S, Brandt M, Rubnitz JE, Ribeiro RC, Rytting M, Pui CH, Kantarjian H, Jeha S, Impact of age on outcome of pediatric acute myeloid leukemia: a report from 2 instutions, Cancer 106 (June (11)) (2006) 2495–2502, doi: 10.1002/cncr.21892. [DOI] [PubMed] [Google Scholar]
- [16].Mistry AR, Pedersen EW, Solomon E, Grimwade D, The molecular pathogenesis of acute promyelocytic leukaemia: implications for the clinical management of the disease, Blood Rev. 17 (June (2)) (2003) 71–97. [DOI] [PubMed] [Google Scholar]
- [17].Children’s Oncology Group, Aplenc R, Alonzo TA, Gerbing RB, Smith FO, Meshinchi S, Ross JA, Perentesis J, Woods WG, Lange BJ, Davies SM, Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group, Blood 108 (July (1)) (2006) 74–80 Epub 2006 March 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rubnitz JE, Razzouk BI, Lensing S, Pounds S, Pui CH, Ribeiro RC, Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia, Cancer 109 (January (1)) (2007) 157–163. [DOI] [PubMed] [Google Scholar]
- [19].Trueworthy R, Shuster J, Look T, Crist W, Borowitz M, Carroll A, Frankel L, Harris M, Wagner H, Haggard M, Ploidy of lymphoblasts is the strongest predictor of treatment outcome in B-progenitor cell acute lymphoblastic leukemia of childhood: a Pediatric Oncology Group study, J. Clin. Oncol 10 (April (4)) (1992) 606–613. [DOI] [PubMed] [Google Scholar]
- [20].SEER as a Research Resource, 2010, February. Retrieved November 26, 2014, from http://seer.cancer.gov/about/factsheets/SEER_Research_Brochure.pdf.
- [21].Adamo M, Dickie L, Ruhl J, SEER Program Coding and Staging Manual, National Cancer Institute, Bethesda, MD, 2015 20850–9765, January 2015. [Google Scholar]
- [22].Creutzig U, Zimmermann M, Reinhardt D, Dworzak M, Stary J, Lehrnbecher T, Early deaths and treatment-related mortality in children undergoing therapy for acute myeloid leukemia: analysis of the multicenter clinical trials AML-BFM 93 and AML-BFM 98, J. Clin. Oncol 22 (November (21)) (2004) 4384–4393. [DOI] [PubMed] [Google Scholar]
- [23].Rubnitz JE, Lensing S, Zhou Y, Sandlund JT, Razzouk BI, Ribeiro RC, Pui CH, Death during induction therapy and first remission of acute leukemia in childhood: the St. Jude experience, Cancer 101 (October (7)) (2004) 1677–1684. [DOI] [PubMed] [Google Scholar]
- [24].Ruhl J, Adamo M, Dickie L, Hematopoietic And Lymphoid Neoplasm Coding Manual, National Cancer Institute, Bethesda, MD, 2015. 20850–9765, January. [Google Scholar]
- [25].Lange BJ, Gerbing RB, Feusner J, Skolnik J, Sacks N, Smith FO, Alonzo TA, Mortality in overweight and underweight children with acute myeloid leukemia, JAMA 293 (January (2)) (2005) 203–211. [DOI] [PubMed] [Google Scholar]
- [26].Manola KN, Cytogenetics of pediatric acute myeloid leukemia, Eur. J. Haematol 83 (November (5)) (2009) 391–405, doi: 10.1111/j.1600-0609.2009.01308.x Epub 2009 June 25. [DOI] [PubMed] [Google Scholar]
- [27].Creutzig U, van den Heuvel-Eibrink MM, Gibson B, Dworzak MN, Adachi S, de Bont E, Harbott J, Hasle H, Johnston D, Kinoshita A, Lehrnbecher T, Leverger G, Mejstrikova E, Meshinchi S, Pession A, Raimondi SC, Sung L, Stary J, Zwaan CM, Kaspers GJ, Reinhardt D, AML Committee of the International BFM Study Group. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel, Blood 120 (October (16)) (2012) 3187–3205, doi: 10.1182/blood-2012-03-362608 Epub 2012 August 9. [DOI] [PubMed] [Google Scholar]
- [28].Mihova D, Zhang L, Acute erythroid leukemia: a review, N. Am. J. Med. Sci 5 (2) (2012) 110–118. [Google Scholar]
- [29].Tarlock K, Meshinchi S, Pediatric acute myeloid leukemia: biology and therapeutic implications of genomic variants, Pediatr. Clin. North Am 62 (Feburary (1)) (2015) 75–93, doi: 10.1016/j.pcl.2014.09.007 Epub 2014 October 29. [DOI] [PubMed] [Google Scholar]
- [30].Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, von Lilienfeld-Toal M, Wilhelm S, Kündgen A, Götze K, Rummel M, Nachbaur D, Schlegelberger B, Göhring G, Späth D, Morlok C, Zucknick M, Ganser A, Döhner H, Schlenk RF, German–Austrian AMLSG. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML, Blood 117 (Feburary (7)) (2011) 2137–2145, doi: 10.1182/blood-2010-08-301713 Epub 2010 December 2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.