ABSTRACT
The semicircular canals of the mammalian inner ear are derived from epithelial pouches in which epithelial cells in the central region of each pouch undergo resorption, leaving behind the region at the rim to form a tube-shaped canal. Lack of proliferation at the rim and/or over-clearing of epithelial cells in the center of the pouch can obliterate canal formation. Otic-specific knockout of bone morphogenetic protein 2 (Bmp2) results in absence of all three semicircular canals; however, the common crus and ampullae housing the sensory tissue (crista) are intact. The lack of Bmp2 causes Ntn1 (which encodes netrin 1), which is required for canal resorption, to be ectopically expressed at the canal rim. Ectopic Ntn1 results in reduction of Dlx5 and Lmo4, which are required for rim formation. These phenotypes can be partially rescued by removing one allele of Ntn1 in the Bmp2 mutants, indicating that Bmp2 normally negatively regulates Ntn1 for canal formation. Additionally, non-resorption of the canal pouch in Ntn1−/− mutants is partially rescued by removing one allele of Bmp2. Thus, reciprocal inhibition between Bmp2 and netrin 1 is involved in canal formation of the vestibule.
KEY WORDS: Patterning, Inner ear, Morphogenesis, Mouse
Summary: Bmp2-conditional mutant analyses support the hypothesis that presumptive crista induces canal genesis zone in the canal pouch to express Bmp2, which promotes canal formation by restricting Ntn1 expression to the resorption domain.
INTRODUCTION
The three semicircular canals of the inner ear and their associated sensory tissues, the cristae, are responsible for detecting angular head movements. Morphogenesis of the semicircular canals begins when the epithelia from the dorsal and lateral regions of the developing otocyst extend outward. These two epithelial extensions form pouches containing cavities that are continuous with the lumen of the otocyst (Fig. 1E-H, Fig. S1; Fekete et al., 1997; Martin and Swanson, 1993). Vertical (dorsal) and horizontal canal pouches are converted into separate canals when the two epithelial layers that create the walls of each pouch merge towards each other in the central region of each prospective canal and then fuse, forming fusion plates in response to mesenchymal proliferation and epithelial signaling via molecules such as netrin 1 and fibroblast growth factor 9 (Fgf9) (Pirvola et al., 2004; Salminen et al., 2000). Epithelial cells within the fusion plate resorb via apoptosis and/or retract towards the edge of the fusion plate (Fekete et al., 1997; Martin and Swanson, 1993). In contrast, the epithelia layers at the rim and at the center of the vertical canal pouch do not fuse, thus creating a continuous network of hollow tubes that connect to the remainder of lumen of the ear, forming the anterior and posterior canals that are joined by the common crus in the center. Similar resorption events occur in the horizontal canal pouch, except only the rim region of this pouch is preserved to form the lateral canal.
Fig. 1.
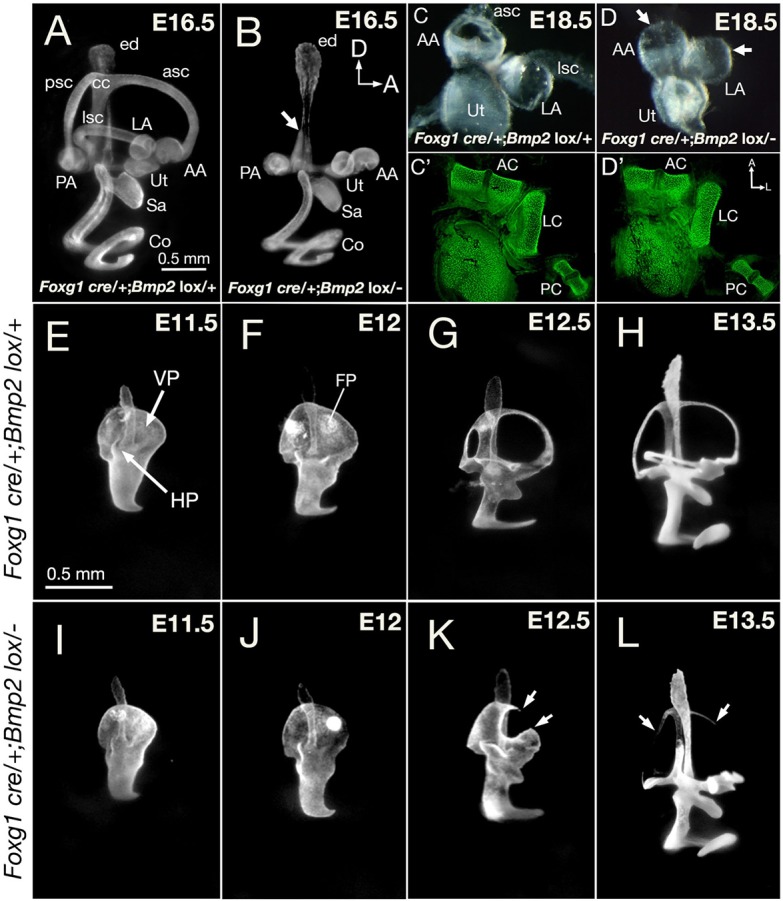
Phenotypes of Foxg1cre/+; Bmp2lox/− inner ears. (A,B) Paint-filled Foxg1cre/+; Bmp2lox/+ (A) and Foxg1cre/+; Bmp2lox/− (B) inner ears at E16.5. (B) The conditional mutants are missing all three semicircular canals, and the common crus is thinner (arrow); however, the endolymphatic duct, utricle, saccule and cochlear duct appear normal. (C,D) Dissected membranous labyrinths of the utricle and the anterior and lateral ampullae in heterozygous controls (C) and in conditional mutants (D) at E18.5. Mutant ampullae have no canal opening (D, arrows) but the cristae within appear intact based on phalloidin staining of sensory hair cells (C′,D′). (E-L) Semicircular canal development in Foxg1cre/+; Bmp2lox/+ (E-H) and Foxg1cre/+; Bmp2lox/− (I-L) ears between E11.5 and E13.5. (E-H) The vertical and lateral canal pouches in heterozygous controls are apparent at E11.5, with fusion plates emerging by E12 and followed by resorption. Canals reach their adult pattern by E13.5, but the size of the canals continues to increase after this age. (I-L) The Foxg1cre/+; Bmp2lox/− canal pouch (I) is slightly smaller than controls (E) at E11.5, but reduction in size is clear by E12 (J). (K) At E12.5, an opening is observed in the anterior region of the vertical canal pouch (arrows). (L) By E13.5, only remnants of the three canals are evident (arrows). AA, anterior ampulla; AC, anterior crista; asc, anterior semicircular canal; CC, common crus; Co, cochlea; ed, endolymphatic duct; FP, fusion plate; HP, horizontal canal pouch; LA, lateral ampulla; LC, lateral crista; lsc, lateral semicircular canal; PA, posterior ampulla; PC, posterior crista; psc, posterior semicircular canal; VP, vertical canal pouch; Sa, saccule; Ut, utricle. Orientations: A, anterior; D, dorsal. Orientation in B also applies to A,E-L. Orientation in D′ applies to C-D. Scale bars: 0.5 mm in A for A,B; 0.5 mm in E, for E-L.
Based on fate mapping and gene expression analyses in the chicken inner ear, it was hypothesized that signaling molecules in the prospective sensory crista induce the adjacent tissue at the rim of the canal pouch to become the canal genesis zone that gives rise to the canals, as well as to some of the cells in the common crus (Fig. S1; Chang et al., 2004; Wu and Kelley, 2012). On the other hand, cells in the rest of the canal pouch largely give rise to the common crus or are resorbed. This unusual growth pattern of the canal pouch is thought to be mediated by Fgfs such as Fgf10, which is secreted from the prospective crista and induces Bmp2 expression in the canal genesis zone (Chang et al., 2004). It is not clear, however, whether this mechanism proposed in the chicken is direct and/or conserved. Other evidence in support of the role for Fgf signaling in Bmp2-mediated canal formation comes from studies showing that Fgf10 has a similar expression pattern in the presumptive cristae in mice (Pauley et al., 2003; Pirvola et al., 2000), and all three canals are missing in Fgf10 knockout mice (Ohuchi et al., 2005; Pauley et al., 2003). While this canal phenotype is consistent with the model of Fgfs secreted in the cristae mediating canal pouch formation, it is still not clear whether this effect of Fgf10 in the mouse inner ear is direct because knockouts of other genes expressed in the presumptive cristae, such as Bmp4 and Jag1 (which encodes a ligand of the Notch signaling pathway), resulted in similar canal phenotypes (Chang et al., 2008; Kiernan et al., 2006; Morrison et al., 1999). Nevertheless, if the canal genesis zone and Bmp2 are involved in canal formation in mammals in a similar manner to that described in chicken (Chang et al., 2004), then Bmp2 should be required for the formation of the canals but not the ampullae or the common crus in mammals. We tested this hypothesis by generating conditional knockout mice in which Bmp2 expression is absent in the developing mouse inner ear. The Bmp2 conditional mutant phenotypes support a model in which crista mediates canal formation via the induction of a canal genesis zone and Bmp2 is an important effector of this zone. Furthermore, our results show that one of the mechanisms whereby Bmp2 promotes canal formation is by the restriction of Ntn1 expression to the resorption domain.
RESULTS
Absence of semicircular canals in Foxg1cre/+; Bmp2lox/− embryos
Bmp2 knockout mice die during early embryogenesis prior to sufficient inner ear development (Zhang and Bradley, 1996). Therefore, we generated conditional knockout of Bmp2 in the inner ear using Foxg1cre mice that start to express cre in the invaginating otic placode (Hébert and McConnell, 2000). qRT-PCR results of vestibular tissue at E11.75 confirmed the absence of Bmp2 transcripts in the conditional mutants compared with heterozygous controls (Fig. S2). Analyses of paint-filled Foxg1cre/+; Bmp2lox/− inner ears indicate the absence of all three semicircular canals, although a thin common crus is evident at embryonic day (E) 15.5 or older (Fig. 1B, arrow; n=12; compare with heterozygotes in Fig. 1A). Compared with controls (Fig. 1C,C′), the three ampullae in mutants are intact with no canal opening (Fig. 1D, arrows), and the cristae within the ampullae appear normal based on phalloidin staining of sensory hair cells (Fig. 1D′). This combination of absent canals with retained common crus and ampullae is consistent with the postulated role of Bmp2 in chicken and zebrafish studies (Chang et al., 2004; Hammond et al., 2009). Other inner ear structures, such as the endolymphatic duct, utricle, saccule and cochlear duct, are indistinguishable from controls (Fig. 1A,B).
Semicircular canals develop between E11.5 and E13.5 in control mice (Fig. 1E-H). The two epithelial outpockets, the vertical and horizontal canal pouches, are evident by E11.5 (Fig. 1E, VP and HP). Over time, the opposing epithelia in the center region of the canal pouch merge towards each other to form a fusion plate (Fig. 1F, FP) that undergoes epithelial resorption, leaving the remaining epithelial cells at the rim to form the tube-shaped canal (Fig. 1G,H). Resorption takes place in the prospective anterior canal first, followed sequentially by the posterior and lateral canals (Fig. 1F,G; Morsli et al., 1998). All three canals are formed by E13.5, and they only increase in size after this age (Morsli et al., 1998).
Compared with controls (Fig. 1E,F), Foxg1cre/+; Bmp2lox/− canal pouches are present, although slightly reduced in size, at E11.5, with the size reduction becoming more apparent by E12 (Fig. 1I,J, E11.5-12, n=16). By E12.5, part of the anterior rim is missing in the vertical canal pouch (Fig. 1K, arrows, n=8), and there is no anterior canal. Only remnants of the three canals are evident by E13.5 (Fig. 1L, arrows, n=18). These results indicate that the canal defects observed in the Bmp2 conditional mutants are initiated early during canal development and are not a result of canal degeneration subsequent to formation.
Expression pattern of Bmp2 in developing semicircular canals
To address the role of Bmp2 in canal formation, we investigated its normal expression pattern in the developing inner ear and compared its expression pattern with that of two other genes that are required for canal formation, Lmo4 (which encodes a LIM-only domain transcription regulator) and Dlx5 (which encodes distaless-related 5 homeodomain transcription factor) (Deng et al., 2010; Merlo et al., 2002). Both Lmo4 and Dlx5 are activated prior to canal pouch formation in the lateral region of the otocyst (Deng et al., 2010; Merlo et al., 2002). By the time the canal pouches are evident at E11.5, the expression domains of Lmo4 (Fig. 2B) and Dlx5 (not shown) are restricted to the rim of the canal pouch. In contrast, Bmp2 transcripts are only detectable after canal pouches are morphologically evident and not prior. At E11.75, Bmp2 is expressed strongly in the fusion plate (Fig. 2A, arrowheads) and more weakly at the rim of the canal pouch (Fig. 2A, bracket) that is positive for Lmo4 (Fig. 2B). After canal formation, Bmp2 is initially expressed in the entire canal epithelium at E15.5 (Fig. 2C,D), but its expression domain becomes restricted to the outer rim of the canals over time (Fig. 2E), where it overlaps with the expression domain of Dlx5 (Fig. 2F) but is complementary to that of Ntn1, which is expressed in the inner rim of canals (Fig. 2G).
Fig. 2.
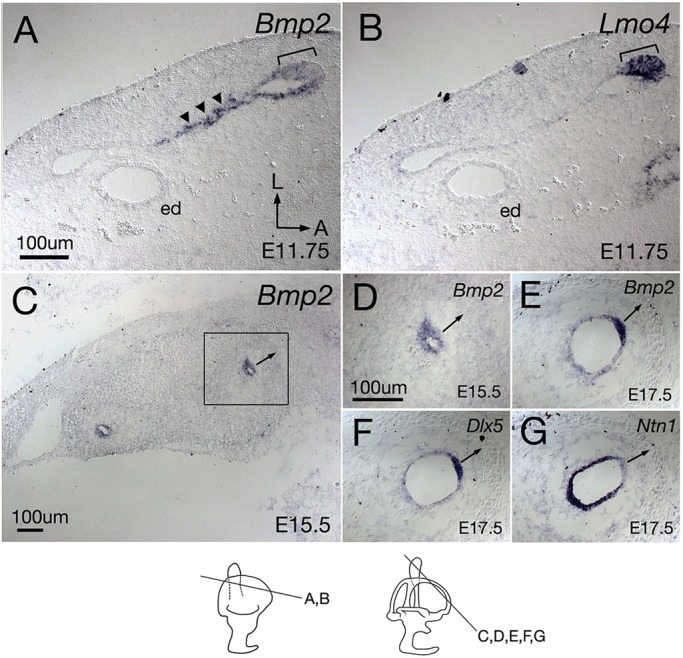
Bmp2 expression during semicircular canal formation. (A) A mid-section through the vertical canal pouch at E11.75. Bmp2 is broadly expressed in the fusion plate (arrowheads) and the rim region of the canal pouch (bracket). (B) Adjacent section to A showing Lmo4 is exclusively expressed in the rim domain (bracket). (C) At E15.5, Bmp2 is expressed in the entire canal epithelium. (D) A high-magnification view of the rectangle in C. (E-G) Adjacent sections of the anterior semicircular canal at E17.5 probed for Bmp2 (E), Dlx5 (F) and Ntn1 (G). Bmp2 is restricted to the outer rim of the canal (E), overlapping with the Dlx5 domain (F). In contrast, Ntn1 shows a complementary expression pattern with Bmp2 (E) and Dlx5 (G). Arrows are pointing away from the outer rim of the canal. ed, endolymphatic duct. Orientations: A, anterior; L, lateral. Orientation in A also applies to B-G. Scale bars: 100 μm in A for A,B; 100 μm in C; 100 μm in D for D-G.
Decreased cell proliferation at the rim of Foxg1cre/+; Bmp2lox/− inner ears
The early reduction in the size of the mutant canal pouch suggests that Bmp2 may be required for epithelial proliferation. Therefore, we examined proliferation in Foxg1cre/+; Bmp2lox/− inner ears using BrdU. The majority of cell proliferation of the canal pouch occurs at the rim (Fig. 3A,C, arrow), which is similar to observations reported in the chicken (Chang et al., 1999, 2002). To quantify BrdU-positive cells, three representative sections in the vertical canal pouch at E11.75 (n=3 embryos) or the anterior and posterior canals at E12.5 (n=3 embryos), were selected from each ear and the average number of BrdU-positive cells per canal epithelial area was calculated as described in the Materials and Methods. No statistical significant difference was observed in the percentages of BrdU-positive epithelial cells between Foxg1cre/+; Bmp2lox/− and heterozygous control pouches at E11.75 (Fig. 3A,B,E; P=0.0736). Despite the absence of a distinct anterior canal, based on Foxg1cre/+; Bmp2lox/− paint-filled ears at E12.5 (Fig. 1K), cryo-sections reveal some residual canal epithelia without a lumen (Fig. 3D, inset). These canal tissues show a 52% reduction in cell proliferation per unit area (Fig. 3D,E; P=0.000003) compared with heterozygous controls (Fig. 3C,E). In contrast, we did not observe a significant difference in BrdU-positive cells between endolymphatic ducts of controls and mutants (P=0.2182).
Fig. 3.
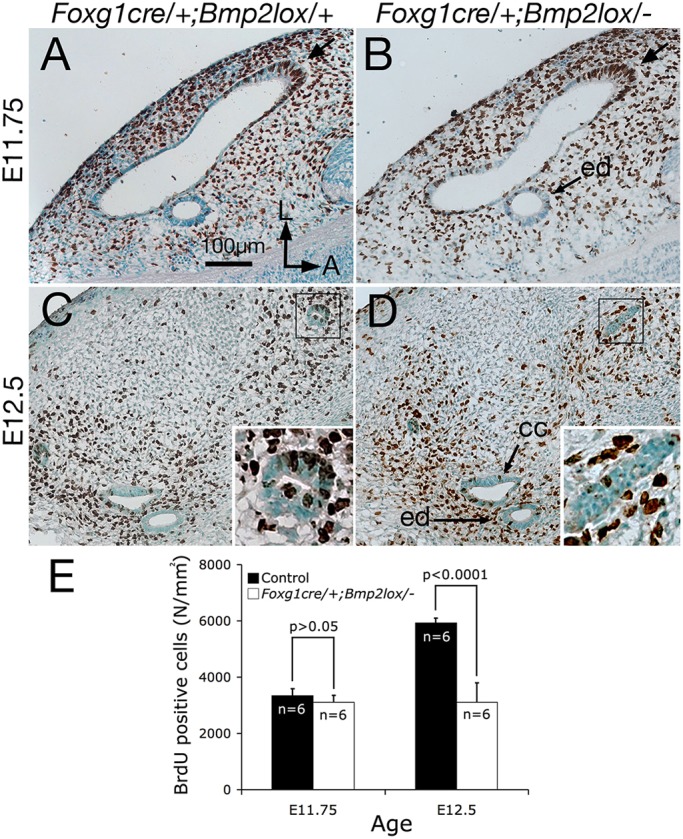
Decreased cell proliferation in the Foxg1cre/+; Bmp2lox/− canal pouch. (A-D) BrdU labeling of Foxg1cre/+; Bmp2lox/+ (A,C) and Foxg1cre/+; Bmp2lox/− (B,D) canal pouches visualized using alkaline phosphatase-conjugated secondary antibodies at E11.75 (A,B) and E12.5 (C,D) to label proliferating cells. At E11.75, the number of BrdU-positive cells per epithelial area is not reduced in Foxg1cre/+; Bmp2lox/− inner ears (B), compared with heterozygous controls (A,E; P>0.05). By E12.5, Foxg1cre/+; Bmp2lox/− inner ears (D) show reduced cell proliferation, compared with controls (C,E; P<0.0001). (E) Quantification of BrdU-labeled cells between Bmp2 heterozygous and conditional knockout canal pouches at E11.75, and anterior and posterior canals at E12.5. Orientation in A applies to B-D. CC, common crus; ed, endolymphatic duct. Scale bar: 100 μm.
As aberrant cell death could also affect normal canal pouch outgrowth, we investigated the role of apoptosis in contributing to the mutant canal phenotype using TUNEL. No obvious difference in the number or distribution of apoptotic cells between control and conditional mutant vestibules at E11.75 and E12.5 was observed (Fig. S3, n=6). Together, these results suggest that Bmp2 is required for maintaining cell proliferation in the canal pouch during canal formation.
Downregulation of pSmad1/5/8, Dlx5 and Lmo4 at the rim of Foxg1cre/+; Bmp2lox/− canals
Reduction of epithelial growth and/or increased resorption can lead to the failure of canal formation. As Bmp2 is broadly expressed in the canal pouch, including both the rim (the prospective canal region) and the resorption domain, it is possible that Bmp2 may have dual roles in canal formation: promoting growth and inhibiting resorption. To investigate the mechanism of Bmp2 function, we focused our analyses on the anterior canal. We asked which cells in the anterior part of the vertical canal pouch are responsive to Bmp2 by investigating the presence of phosphorylated Smad 1/5/8 (pSmad), a downstream transducer of Bmp signaling (ten Dijke et al., 2000). At E11.75, pSmad immunoreactivity is detected strongly at the rim of the canal pouch, as well as in the endolymphatic duct epithelium and in the mesenchymal cells surrounding the endolymphatic duct (Fig. 4A). In Foxg1cre/+; Bmp2lox/− inner ears, there is a marked reduction of pSmad immunoreactivity in the anterior rim of the canal pouch (Fig. 4B; arrow), suggesting that the primary site of Bmp2 action is at the rim of the canal pouch domain. Consistently, both Dlx5 and Lmo4, which are expressed in the canal rim, are also downregulated at the rim of the mutant canal pouch (Fig. 4D,F, arrow), compared with heterozygous controls (Fig. 4C,E). The downregulated Dlx5 and Lmo4 expression patterns and the decreased proliferation observed (Fig. 3) are consistent with the demonstrated function of Dlx5 and Lmo4 in canal formation (Deng et al., 2010; Merlo et al., 2002).
Fig. 4.
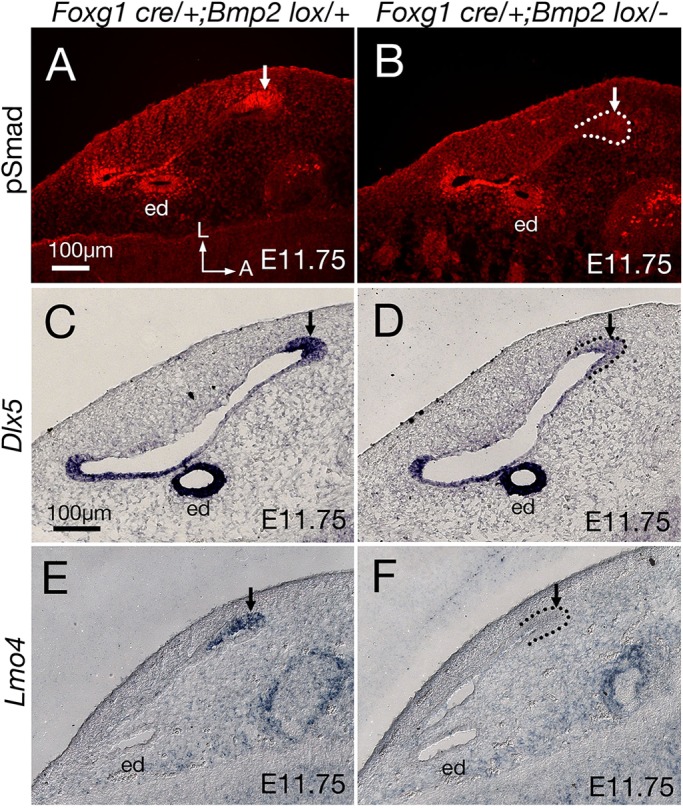
Downregulation of genes expressed in the rim of the Foxg1cre/+; Bmp2lox/− canal pouch. (A,C,E) pSmad (A), Dlx5 (C) and Lmo4 (E) are expressed at the rim of the anterior canal pouch in heterozygous controls (arrow) at E11.75. (B,D,F) Foxg1cre/+; Bmp2lox/− inner ears show reduced expression of pSmad 1/5/8 (B), Dlx5 (D) and Lmo4 (F) in the rim domain (arrow). For easier visualization, parts of the outer rim of the canal epithelia are outlined with dotted lines. Orientation in A also applies to B-F. ed, endolymphatic duct. Scale bars: 100 μm in A for A,B; 100 μm in C for C-F.
Expansion of the Ntn1 expression domain in Foxg1cre/+; Bmp2lox/− canal pouches
As Bmp2 is also expressed in the resorption domain of the canal pouch (Fig. 2A), we examined expression of some of the genes that are specifically expressed in the resorption domain in the Bmp2 conditional mutants. Both Ntn1 and Nor-1 (Nr4a3) are expressed in the resorption domain of the canal pouch, while the rim is devoid of hybridization signals (Fig. 5A,C, arrow; Ponnio et al., 2002; Salminen et al., 2000). After canal formation, these genes are expressed in the inner rim of canals (Fig. 5G; Ponnio et al., 2002; Rakowiecki and Epstein, 2013). Netrin 1 is required for the resorption process in mice as the fusion plate fails to develop and no resorption occurs in Ntn1 knockout mice (Salminen et al., 2000). The function of Nor-1 in canal formation is less clear. Inner ears of Nor-1 knockouts display canals with thinner calibers, which could be caused by reduced proliferation as well as mis-regulated resorption (Ponnio et al., 2002). In Foxg1cre/+; Bmp2lox/− inner ears, the size of the canal rim appears smaller and the Ntn1 expression domain is expanded around the edge of the anterior rim (Fig. 5B, arrow). In contrast to Ntn1 expression (Fig. 5B), the anterior rim of the mutant canal pouch remains devoid of Nor-1 expression (Fig. 5D, arrow) similar to heterozygous controls (Fig. 5C).
Fig. 5.
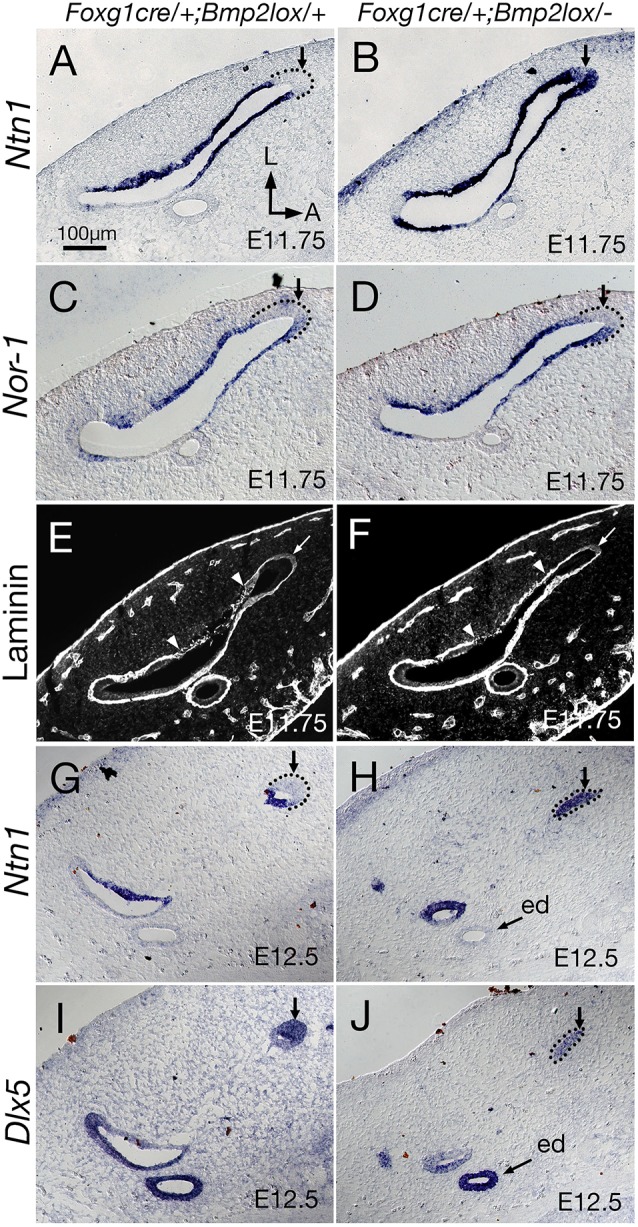
Expansion of Ntn1 expression to the rim of Foxg1cre/+; Bmp2lox/− canal pouch. (A-D) Adjacent sections probed for Ntn1 (A,B) and Nor-1 (C,D) transcripts at E11.75. (A) Ntn1 is expressed in the canal pouch but not the rim of Foxg1cre/+; Bmp2lox/+ controls, whereas Ntn1 expression is expanded around the rim of Foxg1cre/+; Bmp2lox/− canal pouches (B, arrow). (C) Nor-1 expression in heterozygous controls is similar to Ntn1, and is not expressed at the rim (arrow). (D) Unlike Ntn1, the rim of the mutant canal pouch is devoid of Nor-1 expression (arrow). (E,F) Anti-laminin antibody staining of the basement membrane at E11.75, which starts to show intermittent staining pattern in the lateral side of the fusion plate in both controls (E) and mutants (F) (arrowheads), shown in grayscale. No obvious difference in staining pattern between controls and mutants (arrows) is observed at the rim of the canal pouch. (G-J) Expression patterns of Ntn1 (G,H) and Dlx5 (I,J) of Bmp2 heterozygous controls (G,I) and conditional mutants (H,J) at E12.5. (G,I) The complementary expression domain of Ntn1 and Dlx5 in the canal is apparent at E12.5. (H,J) In remnants of Foxg1cre/+; Bmp2lox/− canals, Ntn1 expression is strong (H, arrow) but Dlx5 is weaker (J, arrow). ed, endolymphatic duct. Orientation in A also applies to B-J. Scale bar: 100 μm.
By E12.5, remnant anterior canal tissues of Foxg1cre/+; Bmp2lox/− inner ears lose the normal complementary expression patterns of Ntn1 and Dlx5 observed in controls (Fig. 5G,I; Rakowiecki and Epstein, 2013), showing, instead, prominent Ntn1 expression and diffuse Dlx5 expression throughout the epithelium (Fig. 5H,J). Although this strong expression of Ntn1 at E12.5 in Foxg1cre/+; Bmp2lox/− inner ears is consistent with the earlier upregulation of Ntn1 observed at the rim of the canal pouch at E11.75 (Fig. 5B), it is not clear whether these changes in the Ntn1 expression pattern in the mutant canal pouch represent an actual upregulation of Ntn1 or simply the appearance of upregulation due to a loss of canal rim tissue. Notably, qRT-PCR analyses of the dorsal region of the inner ear, which included the canal pouch, the endolymphatic duct and some surrounding mesenchyme, did not show a significant change in the levels of Ntn1, Dlx5, Nor-1 and Lmo4 transcripts, although the predicted downregulation of Bmp2 between conditional knockouts and heterozygous controls was detected (Fig. S2).
Upregulated Ntn1 expression without expedited basement membrane breakdown in Foxg1cre/+; Bmp2lox/− canal pouches
Within the normal resorption domain, the basement membrane breaks down and epithelial cells disappear or retract towards the edge of the domain (Martin and Swanson, 1993). The failure of basement membrane breakdown is one of the phenotypes associated with loss of Ntn1 (Salminen et al., 2000). Therefore, we investigated whether the change in Ntn1 expression at the rim of the mutant canal pouch (Fig. 5B) is associated with abnormal breakdown of the basement membrane. Anti-laminin staining shows no obvious difference between controls (Fig. 5E) and mutants (Fig. 5F) in the staining pattern at the rim (arrow) or resorption domain (arrowheads) of the E11.75 canal pouches. We did not observe any significant differences in the extent of basement membrane breakdown between the mutants and their littermate controls (ratio of disrupted basement membrane length between controls and mutants=1.09, s.d.=0.16, n=3). However, fusion plate formation followed by basement membrane breakdown is an ongoing dynamic process, so changes in basement membrane breakdown may not be apparent at an early age (E11.75). Hence, we analyzed late stage canal resorption, reasoning that increased basement membrane breakdown should be accumulative and resorption should be more advanced in the mutants than in controls at later stages. By contrast, we found that resorption is more advanced in controls at E12. When some of the canals are already apparent in littermate controls (Fig. 6A-A″), Bmp2 mutants show upregulated Ntn1 expression and presence of a lumen at the canal rim but resorption is not complete (Fig. 6B-B″, n=3), suggesting that there is no premature or enhanced membrane breakdown caused by the upregulated Ntn1 expression in the mutants. By E12.5, this Ntn1-basement membrane relationship is maintained in the canal even though the lumen is collapsed (Figs 5H and 6D), indicating that upregulated Ntn1 expression is not correlated with basement membrane breakdown in the Bmp2 mutants. Together, our results suggest that if the change in Ntn1 expression causes the demise of canal formation in the Bmp2 mutants, this effect is not mediated by premature or expedited basement membrane breakdown. The persistence of Ntn1 expression in the inner rim of normal canals (Figs 5G and 6A) also lends support to the idea that the presence of Ntn1 is not necessarily correlated with epithelial resorption.
Fig. 6.
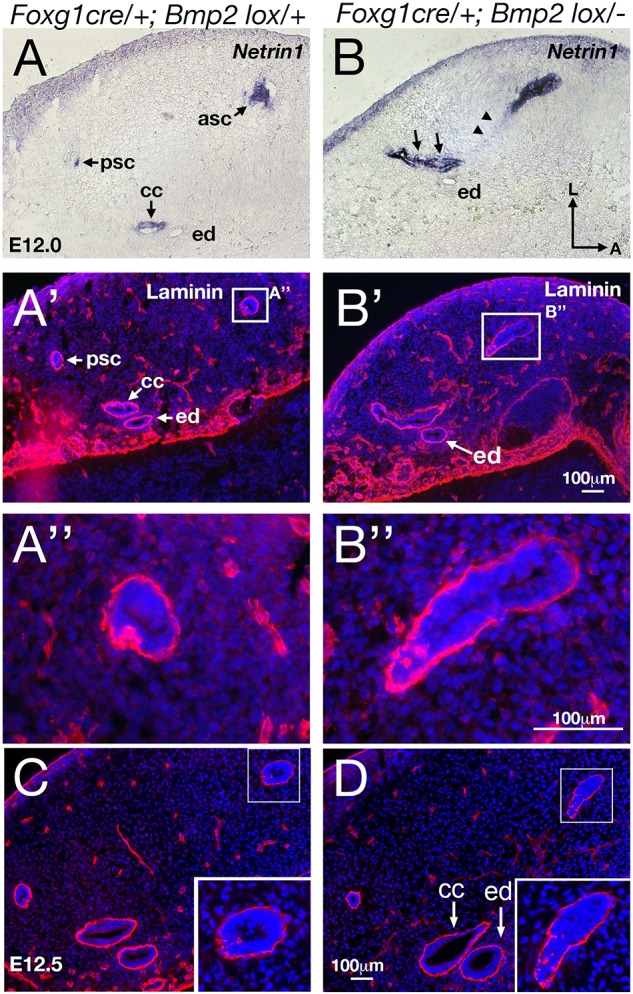
No premature basement membrane breakdown in Bmp2 conditional knockout mutants. Adjacent sections of Foxg1cre/+; Bmp2lox/+ (A-A″,C) and Foxg1cre/+; Bmp2lox/− (B-B″,D) canals/canal pouches at E12 (A-B″) and E12.5 (C,D) that were processed for Ntn1 (A,B) and anti-laminin antibody staining (A′,A″,B′,B″,C,D). At E12, the anterior and posterior canals (asc and psc) in the control show intact basement membrane (A′,A″) with Ntn1 expressed in the inner rim of each canal (A). In contrast, the Bmp2 mutant (B-B″) shows upregulated Ntn1 expression at the canal rim (B) but its basement membrane appears intact (B″). In comparison with the control (A-A″), resorption is not complete in the mutant (B-B″, arrows and arrowheads). There is no distinguishable posterior canal (psc) or common crus (arrows in B), and remnant Ntn1 expression is observed in the resorption domain of the anterior canal pouch region (arrowheads in B). By E12.5, canal resorption seems complete and comparable between the control (C) and mutant (D), but the remnant anterior canal in the mutant (inset in D) looks similar to the one at E12.0 (B″) with an intact basement membrane. asc, anterior semicircular canal; cc, common crus; ed, endolymphatic duct; psc, posterior semicircular canal. Scale bar: 100 μm.
Reciprocal negative regulation between Bmp2 and Ntn1 in canal formation
To further determine whether the upregulated Ntn1 expression in Bmp2 conditional mutants has any significant contribution to the loss of canal phenotype, we conducted a genetic experiment by generating Foxg1cre/+; Bmp2lox/− embryos that lack one allele of Ntn1. We reasoned that if Bmp2 mediates its function by negatively regulating Ntn1 expression in the canal pouch, the canal phenotype in the Bmp2 conditional mutants might be partially rescued by removing one allele of Ntn1. Indeed, in Foxg1cre/+; Bmp2lox/−; Ntn1+/− inner ears, longer partial anterior canals (>50% of control length, P<0.0001) and increased frequency of intact posterior canals (P<0.0001) are evident, compared with Foxg1cre/+; Bmp2lox/− inner ears (Fig. 7A-C, Table 1). Ntn1+/− canals are normal (Fig. 7D; Abraira et al., 2008). Gene expression patterns of the anterior canal rim in Foxg1cre/+; Bmp2lox/−; Ntn1+/− inner ears (Fig. 8C-C″) are similar to controls (Fig. 8A-A″) but different from those in Foxg1cre/+; Bmp2lox/− ears (Fig. 8B-B″). The rescued canals show no ectopic Ntn1 expression but pSmad and Dlx5 are present at the rim of the canal pouch (Fig. 8C-C″, n=7). These results provide genetic and cellular evidence that Bmp2 normally negatively regulates Ntn1 expression at the canal rim, which directly or indirectly affects the rim identity.
Fig. 7.

Reciprocal inhibition of Bmp2 and Ntn1 function in canal formation. (A-C) Paint-filled Foxg1cre/+; Bmp2lox/+ (A, control), Foxg1cre/+; Bmp2lox/− (B) and Foxg1cre/+; Bmp2lox/−; Ntn1+/− (C) inner ears at E13.5. (B) Foxg1cre/+; Bmp2lox/− inner ears lack the three semicircular canals. This canal phenotype of Foxg1cre/+; Bmp2lox/− ears is partially rescued by eliminating one allele of Ntn1 (C). In Foxg1cre/+; Bmp2lox/−; Ntn1+/− inner ears (C; P<0.0001), the anterior canal is longer (single arrow) and the posterior canal is intact (double arrows). (D-F) Paint-filled Ntn1+/− (D), Ntn1−/− (E) and Ntn1−/−; Bmp2+/− (F) inner ear at E13.5. (D) Ntn1+/− inner ears are normal. (E) Ntn1−/− inner ears show non-resorption of the canal pouches; this phenotype is partially rescued for the anterior canal in the absence of one allele of Bmp2 (F, asterisk; P<0.05). Scale bar: 100 μm.
Table 1.
Rescue of the Foxg1cre/+; Bmp2lox/− canal phenotype by eliminating one allele of Ntn1
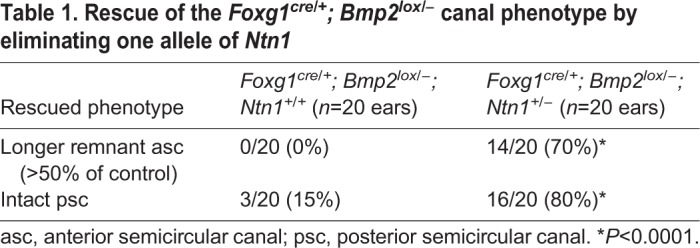
Fig. 8.

Canal identity is recovered in Foxg1cre/+;Bmp2lox/−;Ntn1+/− inner ears. Adjacent sections of the canal pouch in Foxg1cre/+; Bmp2lox/+ (A-A″), Foxg1cre/+; Bmp2lox/− (B-B″) and Foxg1cre/+; Bmp2lox/−; Ntn1+/− (C-C″) inner ears at E12 that were processed for Dlx5 (A,B,C), Ntn1 in situ hybridization (A′,B′,C′) and anti-pSmad antibody staining (A″,B″,C″). In Foxg1cre/+; Bmp2lox/−; Ntn1+/− ears (C-C″), the upregulation of Ntn1 (B′) and the reduction of Dlx5 (B) and pSmad (B″) observed in Foxg1cre/+; Bmp2lox/− canal rims are restored to the expression patterns seen in controls (A-A″). Dotted lines outline the rim of the canal pouch. Scale bar: 100 μm.
Next, to investigate whether there is a reciprocal inhibition of Ntn1 by Bmp2, we generated Ntn1−/− mice in a Bmp2+/− background. The extent of canal non-resorption is variable in the Ntn1 mutants (Table 2); however, in the presence of only one allele of Bmp2, the frequency of a distinct anterior canal increases from 57% to 89% (Fig. 7F, Table 2; P=0.0198). These results indicate that netrin 1 also negatively regulates Bmp2 functions. To address whether Ntn1 normally inhibits Bmp2 function by suppressing Bmp2 expression, we investigated whether Bmp2 expression and pSmad are upregulated in Ntn1−/− ears during canal formation. Although the Ntn1 mutants always show a delay in canal development relative to the littermate controls, we did not observe an obvious upregulation of Bmp2 expression domain or pSmad immunostaining in Ntn1 knockouts, as might be predicted by the genetic results (Fig. 9A,A′,B,B′, arrows, n=12 ranging from E11.75-12.5). In fact, there may be a reduction in Bmp2 expression in the center of the canal pouch (Fig. 9B′, arrowheads, n=3/11) but this reduction was not consistently observed among all the specimens. In addition, there is no obvious expansion of the canal rim identity based on the Dlx5 expression pattern (Fig. 9A″,B″, arrows, n=10). These results suggest that Ntn1 is unlikely to mediate canal resorption by inhibiting Bmp2 expression or restricting Dlx5 expression to the rim, and the role of Ntn1 in negatively regulating Bmp2 functions remains to be determined.
Table 2.
Rescue of Ntn1−/− non-resorption phenotype by eliminating one allele of Bmp2
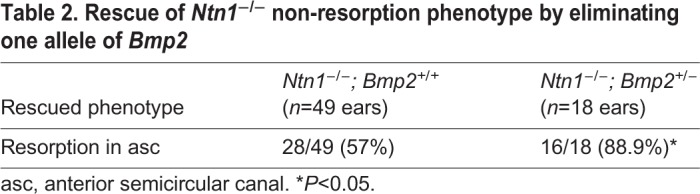
Fig. 9.
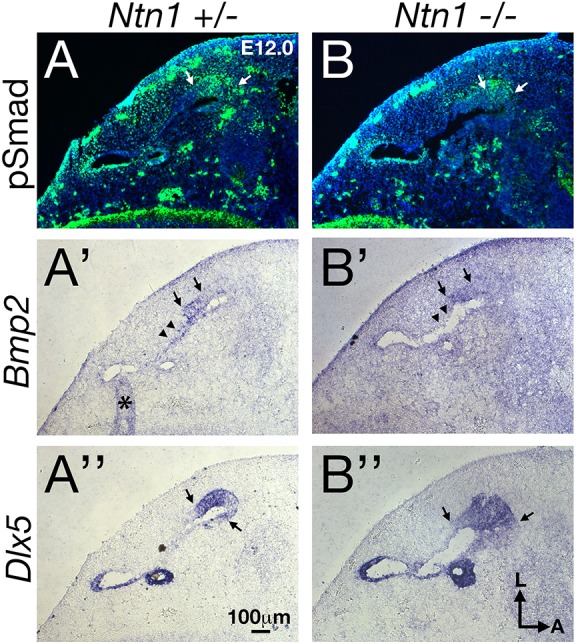
Gene expression analyses of Ntn1−/− canal pouch. Adjacent sections of the canal pouch of Ntn1+/− (A-A″) and Ntn1−/− (B-B″) embryos at E12 that were processed for anti-pSmad antibody staining (A,B), and Bmp2 (A′,B′) and Dlx5 (A″,B″) in situ hybridization. Arrows delineate the approximate borders of strong expression domains and an asterisk represents an artifact created by a fold in the tissue. There is no obvious change in the expression domain of pSmad, Bmp2 and Dlx5 in the Ntn1−/− compared with controls. Arrowheads indicate the fusion plate region, in which Bmp2 expression in the mutant may be reduced (B′). Scale bar: 100 μm.
DISCUSSION
Conserved role of Bmp2 in canal formation
Although not necessarily recognized at the time, the earliest results that implicated Bmp2 in canal formation were conducted with chicken embryos, in which treatments with noggin (Nog, an antagonist of Bmp2 and Bmp4) or virus encoding Nog resulted in truncation or loss of inner ear canals (Chang et al., 1999; Gerlach et al., 2000). Subsequent studies (also conducted in ovo) proposed a model in which Fgfs secreted from the presumptive crista mediate canal formation by upregulating Bmp2 in the canal pouch (Chang et al., 2004). More recently, bmp2b was shown to be required for maintaining canal structures in zebrafish (Hammond et al., 2009). Mutant bmp2b zebrafish have no canals but intact ampullae and common crus, similar to the mouse mutants reported here. Taken together, results in both zebrafish and mice strongly suggest that Bmp2 has a conserved role in canal formation. Furthermore, our studies provide insights into the mechanisms of this Bmp2 function.
Timing of the requirements for Bmp2 in canal formation
Both gene expression and phenotypic analyses of Bmp2 conditional mutants indicate that Bmp2 has a slightly different role from other genes implicated in canal formation, such as Dlx5, Hmx2, Hmx3 and Lmo4 (Deng et al., 2010; Hadrys et al., 1998; Merlo et al., 2002; Wang et al., 2004, 1998). Most of these genes are broadly expressed in the otocyst initially and become restricted to the canal pouch by the time the pouch is morphologically evident. In the cases of Dlx5 and Lmo4, their transcripts are restricted to the rim of the canal pouch (Fig. 10A). In contrast, Bmp2 transcripts are detected robustly only after the canal pouches are established. Consistent with these expression patterns, knockout of Dlx5, Hmx2, Hmx3 and Lmo4 generated vestibular phenotypes that are more severe and variable than that of the Bmp2 conditional mutants (Deng et al., 2010; Hadrys et al., 1998; Merlo et al., 2002; Wang et al., 2004, 1998). Severe phenotypes, such as a much-reduced size of the canal pouch or a complete lack of resorption reported in Dlx5 and Hmx mutants, are not observed in the Bmp2 conditional mutants and this is indicative of Dlx5 and Hmx having an earlier and broader role than Bmp2 in canal formation. Nevertheless, we show that the maintenance of some of these early genes, such as Dlx5 and Lmo4, are dependent on Bmp2 function.
Fig. 10.

Role of Bmp2 in semicircular canal formation. (A) Normal canal development from E11.75 to E17.5. At E11.75, pSmad, Dlx5 and Lmo4 (black dots) are expressed in the rim of the canal pouch, whereas Ntn1 (red) is expressed in the resorption domain. Bmp2 is expressed in both of these domains of the canal pouch and it functions to restrict Ntn1 expression to the resorption domain (1) and Ntn1 indirectly restricts rim identity (2). In addition, the rim domain may be another target region of Bmp2 based on pSmad 1/5/8 staining (3). (B) In the absence of Bmp2, there is an expansion of the Ntn1 expression to the rim, which results in a reduction in epithelial cell proliferation and downregulation of Dlx5 and Lmo4, collectively producing the failure of canal formation. The dotted arrows represent postulated effects based on our genetic and gene expression analyses.
Is Bmp2 the downstream mediator of crista signaling in canal formation?
Several lines of evidence in chicken studies suggest that Bmp2 is a downstream mediator of crista signaling. First, its expression domain coincides with the canal genesis domain identified in fate-mapping studies using a lipophilic dye (Chang et al., 2004). Second, manipulating Fgf functions in the prospective crista by implanting beads soaked with Fgf2 or an inhibitor of Fgf receptor, SU5402, to the canal pouch resulted in gain and loss of canal tissues that were preceded by up- and downregulation of Bmp2 expression, respectively (Chang et al., 2004). Consistently, the loss of common crus with Fgf2 treatments can be rescued by the simultaneous presence of noggin (Chang et al., 2004). However, despite the evidence supporting a role for Bmp2 in mediating canal formation, other ligands expressed in the prospective crista, such as jagged 1 (Jag1) and Bmp4 also qualify as candidates for canal induction based on studies in both chicken and mice (Chang et al., 2008; Kiernan et al., 2006). Furthermore, Fgfs, Jag1, Bmp4 and Wnts are dependent on each other for expression in the crista (Chang et al., 2008; Daudet et al., 2007; Rakowiecki and Epstein, 2013). Thus, further studies are required to determine whether Fgfs directly activate Bmp2 in the canal pouch.
Fate mapping studies in chicken suggest that the Bmp2-positive canal genesis zone contributes to most of the cells in the canals but contributes much less to the common crus (Chang et al., 2008). Although there is no direct evidence that a similar canal genesis zone exists in the mouse, the Bmp2 expression domain in the mouse canal pouch, albeit broader, is similar to that of the chicken (Chang et al., 2004, 2008). Thus, it is interesting that the phenotypes obtained from the Bmp2 conditional mutants (a fully penetrant phenotype of loss of canals with intact ampullae and a milder reduction in the size of the common crus) are entirely consistent with the canal genesis zone hypothesis formulated in chicken, suggesting that the establishment of a canal genesis zone is a conserved mechanism of canal formation.
Mechanisms of Bmp2 in canal formation
Reciprocal inhibition between the rim and resorption domain of the canal pouch during canal formation have been well documented (Abraira et al., 2008; Huang et al., 2018). Our results indicate that broad expression of Bmp2 in the canal pouch (Fig. 2) appears to be required for both of these domains during canal formation. First, our results suggest Bmp2 restricts the resorption process via negative regulation of Ntn1 expression (Fig. 10A, #1 and #2). By reducing the gene dose of Ntn1, canals are partially recovered in the Bmp2 conditional mutants (Fig. 7, Table 1). In the recovered canals, Ntn1 is no longer upregulated at the canal rim and Dlx5 and pSmad are expressed normally. These results suggest that Bmp2 negatively regulates Ntn1 expression in the canal rim, which could indirectly affect rim identity (Fig. 10A, #1 and #2). However, if negative regulation of Ntn1 is important for canal rim specification, one might expect the canal rim domain to be expanded in the Ntn1−/− canal pouch. This does not appear to be the case (Fig. 9). No obvious change in rim identity is observed based on pSmad and Dlx5 expression. These results suggest that Bmp2 function is not mediated by regulation of Ntn1 alone.
A possible additional function of Bmp2 is promoting canal rim formation where its transducer, pSmad, is expressed from canal pouch stage to canals (Figs 2 and 10A, #3). It is arguable that the presence of pSmad in the rescued canals of Foxg1cre/+; Bmp2lox/−; Ntn1+/− inner ears indicates that pSmad is not a direct readout of Bmp2 and the site of Bmp2 action remains unclear (Fig. 8), and there is no direct experimental evidence that supports the notion that Bmp2 promotes canal rim formation. However, existing results from both chicken and zebrafish studies support the notion that Bmp2 is also required for the continual growth of the canal after its initial formation and resorption is complete (Chang et al., 1999; Hammond et al., 2009). In summary, both of the above proposed mechanisms for Bmp2 function – in negatively regulating resorption via Ntn1 (Fig. 10, #1 and #2) and in promoting rim identity via pSmad (Fig. 10, #3) – remain plausible.
Negative regulation of Ntn1 by Bmp2 in canal formation
Ntn1 has many functions during embryogenesis. In addition to its well-established role in axonal guidance, it also mediates other cellular processes, such as cell migration, branching morphogenesis of lung and mammary gland, and canal resorption of the inner ear (Cirulli and Yebra, 2007; Lai Wing Sun et al., 2011). Its mechanism in canal resorption is not clear and does not seem to involve its known receptors in other systems (Abraira et al., 2008). Ntn1 is thought to mediate canal resorption by mediating basement membrane breakdown of the resorption domain. The resorption role of Ntn1 in lateral canal formation is negatively regulated by Lrig3, an immunoglobulin superfamily protein (Abraira et al., 2008). In the absence of Lrig3, the Ntn1 expression domain is expanded into the rim of the lateral canal pouch, which is accompanied by an expanded breakdown of the basement membrane. More recent gain-of-function studies of Ntn1 in both chicken and mice suggested additional complexity regarding the role of Ntn1 in basement membrane breakdown (Nishitani et al., 2017). Although gain of Ntn1 function in mice causes moderate effects of canal truncation, which presumably is due to ectopic canal resorption, gain of Ntn1 function in chicken shows an increase in fusion plate formation without resorption. These results suggest that the effect of Ntn1 in canal epithelium may be context dependent.
We observed a similar expansion of the Ntn1 expression domain in Bmp2 conditional mutants to that seen in Lrig3 nulls, but without the increase in basement membrane breakdown or programmed cell death of the Lrig3 nulls (Fig. 10B). Although we cannot rule out an increased fusion plate formation in the Bmp2 mutants as a result of Ntn1 expansion, a late stage canal resorption analysis indicates that the basement membrane remains intact at the canal rim despite the upregulated Ntn1 expression (Fig. 6). Thus, the negative regulation of Ntn1 function by Lrig3 and Bmp2 is mechanistically different. For example, Dlx5 is downregulated in the rim domain of the Bmp2 mutants and this is not the case for Lrig3 nulls (Abraira et al., 2008). Furthermore, Nor-1, another gene expressed in the resorption domain, is not expanded in the Bmp2 mutants, suggesting that the rim is not co-opted entirely into the resorption domain. Based on these results, we propose that the absence of canals in Bmp2 conditional knockouts is partially due to upregulation of Ntn1 function, which affects the canal rim identity (expression of Dlx5 and Lmo4 as well as cell proliferation) but does not involve expedited basement membrane breakdown at the rim.
Although there is no evidence of Ntn1 inhibiting Lrig3 (Abraira et al., 2008), Ntn1 negatively regulates Bmp2 functions, at least in the formation of the anterior canal (Fig. 7). The cellular mechanism for this regulation is not clear. Even though we did not observe an obvious expansion of rim domain identity or upregulated Bmp2 expression in the Ntn1 knockout, as postulated based on the genetic results (Figs 7 and 9), it is quite possible that Bmp2 is mediating its effect indirectly via reciprocal inhibition between canal rim and resorption domains (Fig. 10A). Under this scenario, Bmp2 functions in the canal rim. By removing one allele of Bmp2 in the Ntn1 knockouts, it reduces the inhibitory effect of the canal rim on the resorption domain and alleviates the non-resorption phenotype in Ntn1 knockouts (Figs 7 and 10A, Table 2). Such reciprocal inhibition has been described for Lmx1a and Lmo4 recently (Huang et al., 2018). Although Lmx1a and Lmo4 regulate each other in forming transcriptional complexes, the mechanisms underlying the reciprocal inhibition between Bmp2 and Ntn1 will require further investigation. Nevertheless, the combined results in mouse, chicken and zebrafish suggest that Bmp2 has a conserved role in the formation of all three semicircular canals.
MATERIALS AND METHODS
Mice and genotyping
The Bmp2tm1.1Mis/tm1.1Mis (Bmp2lox/lox) and Bmp2+/tm1Brd (Bmp2+/−) mice were maintained on a 129SvEv; C57BL6/J mixed background as previously described (Singh et al., 2008; Zhang and Bradley, 1996). Bmp2 conditional mutants were generated by mating Foxg1cre/+; Bmp2+/− with Bmp2lox/lox mice. The Ntn1+/Gt(ST629)Byg (Ntn1+/−) mice (Serafini et al., 1996) on C57BL/6J background were obtained from Dr Lisa Goodrich (Harvard Medical School, Boston, MA, USA) and were used to generate Foxg1cre/+; Bmp2lox/−; Ntn1+/− or Ntn1−/−; Bmp2+/− mice.
In situ hybridization
For in situ hybridization, RNA probes for Bmp4 (Morsli et al., 1998), Bmp2 (Hwang et al., 2010), Ntn1 (Abraira et al., 2008), Dlx5 (Depew et al., 1999) and Lmo4 (Deng et al., 2006) were prepared as previously described. All in situ hybridization results were repeated at least three times with littermate pairs.
Immunohistochemistry, TUNEL and BrdU labeling
For immunohistochemistry, specimens were fixed in 4% paraformaldehyde for 1 h for anti-pSmad 1/5/8 or overnight at 4°C for anti-laminin staining. The primary antibodies used were anti-pSmad 1/5/8 antibody (a gift from Edward Laufer, Columbia University, NY, USA; 1:1000) and anti-laminin antibody (Millipore, AB2034, 1:500). Secondary antibodies were either Alexa 488 or 568 (Invitrogen, A21206, A10042, 1:200). Apoptotic cells were detected using the TUNEL (Terminal dUTP Nuclear End Labeling) technique (Millipore). For the detection of cell proliferation, the Amersham cell proliferation kit (GE Healthcare) was used. The 5′-bromo-2′deoxyuridine (BrdU) labeling solution (1 ml of a 10 mM BrdU solution per 100 g body weight) was injected intraperitoneally, 2 hours before harvesting. After cryo-sectioning, slides were post-fixed with 4% paraformaldehyde for 10 min at room temperature and treated with an antigen retrieval solution [12.5 ml formamide, 7.5 ml 20×SSC (pH 4.5), 2.5 ml 10% SDS and 2.5 ml dH2O] for 30 min at 65°C. The slides were incubated with anti-BrdU antibody (mouse monoclonal, dilution 1:100) in DNAse-1 solution for 2 h at room temperature and then followed with peroxidase conjugated anti-mouse IgG (dilution 1:200) for 1 h. The stable DAB (diaminobenzidine, Invitrogen) solution was applied for detection and methyl green (Vector) was used for counter-staining. BrdU labeled cells were also detected by using a fluorescent-tagged secondary antibody (Invitrogen).
Data quantification and statistics
To quantify BrdU-positive cells, three representative sections were selected from the vertical canal pouch of E11.75 or anterior and posterior canals of E12.5 ears from wild type (n=3 for each age) and mutants (n=3 for each age). The average number of BrdU-positive cells per entire epithelial area were calculated. The epithelial areas were measured using ImageJ software (available at https://imagej.nih.gov/ij/index.html). As controls, the average number of BrdU-positive cells per endolymphatic duct epithelial area were also measured and calculated. For statistical analysis, BrdU cell counts were analyzed using Student's t-test, and categorized paint fill data were analyzed using Chi-square tests with Yates’ correction.
qRT-PCR analyses
The dorsal halves of E11.5 to E11.75 Bmp2 conditional mutant and littermate inner ears (including the vertical canal pouch, endolymphatic duct and some surrounding mesenchyme) were dissected in ice-cold 1× PBS and flash frozen in a slurry of dry ice and methyl butane. RNA was extracted from the frozen tissue using the RNAqueous Micro RNA Isolation Kit (Ambion). The quality and concentration of the RNA samples were evaluated using an Agilent 2100 bioanalyzer and RNA 6000 Nano Kit. RNA of high quality (RIN>8.0) was converted to cDNA with the Superscript III First-Strand cDNA Synthesis system (Life Technologies) using oligo-dT primers. Relative gene expression levels in Bmp2 conditional mutants and heterozygous control otocysts were evaluated via qRT-PCR performed using Taqman Fast Advanced Master Mix and Taqman Gene Expression Assays on an Applied Biosystems ViiA 7 Real Time PCR system. Each reaction was run in triplicate to assess technical repeatability. Expression levels of target genes (Bmp2, Dlx5, Nor1, Lmo4 and Ntn1) using specific primers (Life Technologies) were normalized to expression levels of reference genes Gapdh and Rpl30.
Supplementary Material
Acknowledgements
We thank Drs Dan Vasiliauskas, Susan Morton, Tom Jessel and Edward Laufer for the gift of anti-phosphosmad antibodies; and Drs Stephen Harris at the University of Texas Health Science Center, San Antonio, Texas, and Yuji Mishina at the University of Michigan for the Bmp2-lox and Bmp2+/− mouse strains, respectively. We also thank Drs Lisa Cunningham, Weise Chang and Rob Morell at the NIDCD for critical reading of the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was funded by a National Institute on Deafness and other Communication Disorders intramural grant (1ZIADC000021) to D.K.W. Deposited in PMC for immediate release.
Author contributions
Conceptualization: D.K.W.; Methodology: C.H.H., J.K., C.R., S.O., D.K.W.; Validation: S.O., D.K.W.; Formal analysis: C.H.H., J.K., C.R., S.O., D.K.W.; Investigation: D.K.W.; Data curation: C.H.H., J.K., C.R., S.O., D.K.W.; Writing - original draft: C.H.H., D.K.W.; Writing - review & editing: J.K., S.O., D.K.W.; Visualization: C.H.H., C.R., S.O., D.K.W.; Supervision: D.K.W.; Project administration: D.K.W.; Funding acquisition: D.K.W.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.174748.supplemental
References
- Abraira V. E., Del Rio T., Tucker A. F., Slonimsky J., Keirnes H. L. and Goodrich L. V. (2008). Cross-repressive interactions between Lrig3 and netrin 1 shape the architecture of the inner ear. Development 135, 4091-4099. 10.1242/dev.029330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W., Nunes F. D., De Jesus-Escobar J. M., Harland R. and Wu D. K. (1999). Ectopic noggin blocks sensory and nonsensory organ morphogenesis in the chicken inner ear. Dev. Biol. 216, 369-381. 10.1006/dbio.1999.9457 [DOI] [PubMed] [Google Scholar]
- Chang W., ten Dijke P. and Wu D. K. (2002). BMP pathways are involved in otic capsule formation and epithelial-mesenchymal signaling in the developing chicken inner ear. Dev. Biol. 251, 380-394. 10.1006/dbio.2002.0822 [DOI] [PubMed] [Google Scholar]
- Chang W., Brigande J. V., Fekete D. M. and Wu D. K. (2004). The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development 131, 4201-4211. 10.1242/dev.01292 [DOI] [PubMed] [Google Scholar]
- Chang W., Lin Z., Kulessa H., Hebert J., Hogan B. L. M. and Wu D. K. (2008). Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 4, e1000050 10.1371/journal.pgen.1000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V. and Yebra M. (2007). Netrins: beyond the brain. Nat. Rev. Mol. Cell Biol. 8, 296-306. 10.1038/nrm2142 [DOI] [PubMed] [Google Scholar]
- Daudet N., Ariza-McNaughton L. and Lewis J. (2007). Notch signalling is needed to maintain, but not to initiate, the formation of prosensory patches in the chick inner ear. Development 134, 2369-2378. 10.1242/dev.001842 [DOI] [PubMed] [Google Scholar]
- Deng M., Pan L., Xie X. and Gan L. (2006). Differential expression of LIM domain-only (LMO) genes in the developing mouse inner ear. Gene Expr. Patterns 6, 857-863. 10.1016/j.modgep.2006.02.005 [DOI] [PubMed] [Google Scholar]
- Deng M., Pan L., Xie X. and Gan L. (2010). Requirement for Lmo4 in the vestibular morphogenesis of mouse inner ear. Dev. Biol. 338, 38-49. 10.1016/j.ydbio.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depew M. J., Liu J. K., Long J. E., Presley R., Meneses J. J., Pedersen R. A. and Rubenstein J. L. (1999). Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126, 3831-3846. [DOI] [PubMed] [Google Scholar]
- Fekete D. M., Homburger S. A., Waring M. T., Riedl A. E. and Garcia L. F. (1997). Involvement of programmed cell death in morphogenesis of the vertebrate inner ear. Development 124, 2451-2461. [DOI] [PubMed] [Google Scholar]
- Gerlach L. M., Hutson M. R., Germiller J. A., Nguyen-Luu D., Victor J. C. and Barald K. F. (2000). Addition of the BMP4 antagonist, noggin, disrupts avian inner ear development. Development 127, 45-54. [DOI] [PubMed] [Google Scholar]
- Hadrys T., Braun T., Rinkwitz-Brandt S., Arnold H. H. and Bober E. (1998). Nkx5-1 controls semicircular canal formation in the mouse inner ear. Development 125, 33-39. [DOI] [PubMed] [Google Scholar]
- Hammond K. L., Loynes H. E., Mowbray C., Runke G., Hammerschmidt M., Mullins M. C., Hildreth V., Chaudhry B. and Whitfield T. T. (2009). A late role for bmp2b in the morphogenesis of semicircular canal ducts in the zebrafish inner ear. PLoS ONE 4, e4368 10.1371/journal.pone.0004368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert J. M. and McConnell S. K. (2000). Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev. Biol. 222, 296-306. 10.1006/dbio.2000.9732 [DOI] [PubMed] [Google Scholar]
- Huang Y., Hill J., Yatteau A., Wong L., Jiang T., Petrovic J., Gan L., Dong L. and Wu D. K. (2018). Reciprocal negative regulation between Lmx1a and Lmo4 is required for inner ear formation. J. Neurosci. 38, 5429-5440. 10.1523/JNEUROSCI.2484-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang C. H., Guo D., Harris M. A., Howard O., Mishina Y., Gan L., Harris S. E. and Wu D. K. (2010). Role of bone morphogenetic proteins on cochlear hair cell formation: analyses of Noggin and Bmp2 mutant mice. Dev. Dyn. 239, 505-513. 10.1002/dvdy.22200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A. E., Xu J. and Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2, e4 10.1371/journal.pgen.0020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Wing Sun K., Correia J. P. and Kennedy T. E. (2011). Netrins: versatile extracellular cues with diverse functions. Development 138, 2153-2169. 10.1242/dev.044529 [DOI] [PubMed] [Google Scholar]
- Martin P. and Swanson G. J. (1993). Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev. Biol. 159, 549-558. 10.1006/dbio.1993.1263 [DOI] [PubMed] [Google Scholar]
- Merlo G. R., Paleari L., Mantero S., Zerega B., Adamska M., Rinkwitz S., Bober E. and Levi G. (2002). The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev. Biol. 248, 157-169. 10.1006/dbio.2002.0713 [DOI] [PubMed] [Google Scholar]
- Morrison A., Hodgetts C., Gossler A., Hrabé de Angelis M. and Lewis J. (1999). Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech. Dev. 84, 169-172. 10.1016/S0925-4773(99)00066-0 [DOI] [PubMed] [Google Scholar]
- Morsli H., Choo D., Ryan A., Johnson R. and Wu D. K. (1998). Development of the mouse inner ear and origin of its sensory organs. J. Neurosci. 18, 3327-3335. 10.1523/JNEUROSCI.18-09-03327.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani A. M., Ohta S., Yung A. R., Del Rio T., Gordon M. I., Abraira V. E., Avilés E. C., Schoenwolf G. C., Fekete D. M. and Goodrich L. V. (2017). Distinct functions for netrin 1 in chicken and murine semicircular canal morphogenesis. Development 144, 3340-3360. 10.1242/dev.144519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi H., Yasue A., Ono K., Sasaoka S., Tomonari S., Takagi A., Itakura M., Moriyama K., Noji S. and Nohno T. (2005). Identification of cis-element regulating expression of the mouse Fgf10 gene during inner ear development. Dev. Dyn. 233, 177-187. 10.1002/dvdy.20319 [DOI] [PubMed] [Google Scholar]
- Pauley S., Wright T. J., Pirvola U., Ornitz D., Beisel K. and Fritzsch B. (2003). Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn. 227, 203-215. 10.1002/dvdy.10297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U., Spencer-Dene B., Xing-Qun L., Kettunen P., Thesleff I., Fritzsch B., Dickson C. and Ylikoski J. (2000). Fgf/Fgfr-2(IIIb) signaling is essential for inner ear morphogenesis. J. Neurosci. 20, 6125-6134. 10.1523/JNEUROSCI.20-16-06125.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirvola U., Zhang X., Mantela J., Ornitz D. M. and Ylikoski J. (2004). Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev. Biol. 273, 350-360. 10.1016/j.ydbio.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Ponnio T., Burton Q., Pereira F. A., Wu D. K. and Conneely O. M. (2002). The nuclear receptor Nor-1 is essential for proliferation of the semicircular canals of the mouse inner ear. Mol. Cell. Biol. 22, 935-945. 10.1128/MCB.22.3.935-945.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowiecki S. and Epstein D. J. (2013). Divergent roles for Wnt/beta-catenin signaling in epithelial maintenance and breakdown during semicircular canal formation. Development 140, 1730-1739. 10.1242/dev.092882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen M., Meyer B. I., Bober E. and Gruss P. (2000). Netrin 1 is required for semicircular canal formation in the mouse inner ear. Development 127, 13-22. [DOI] [PubMed] [Google Scholar]
- Serafini T., Colamarino S. A., Leonardo E. D., Wang H., Beddington R., Skarnes W. C. and Tessier-Lavigne M. (1996). Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 87, 1001-1014. 10.1016/S0092-8674(00)81795-X [DOI] [PubMed] [Google Scholar]
- Singh A. P., Castranio T., Scott G., Guo D., Harris M. A., Ray M., Harris S. E. and Mishina Y. (2008). Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex. Dev. 2, 134-141. 10.1159/000143431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Dijke P., Miyazono K. and Heldin C.-H. (2000). Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem. Sci. 25, 64-70. 10.1016/S0968-0004(99)01519-4 [DOI] [PubMed] [Google Scholar]
- Wang W., Van De Water T. and Lufkin T. (1998). Inner ear and maternal reproductive defects in mice lacking the Hmx3 homeobox gene. Development 125, 621-634. [DOI] [PubMed] [Google Scholar]
- Wang W., Grimmer J. F., Van De Water T. R. and Lufkin T. (2004). Hmx2 and Hmx3 homeobox genes direct development of the murine inner ear and hypothalamus and can be functionally replaced by Drosophila Hmx. Dev. Cell 7, 439-453. 10.1016/j.devcel.2004.06.016 [DOI] [PubMed] [Google Scholar]
- Wu D. K. and Kelley M. W. (2012). Molecular mechanisms of inner ear development. Cold Spring Harb. Perspect. Biol. 4, a008409 10.1101/cshperspect.a008409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H. and Bradley A. (1996). Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122, 2977-2986. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


