ABSTRACT
In the adult rodent brain, neural stem cells (NSCs) persist in the ventricular-subventricular zone (V-SVZ) and the subgranular zone (SGZ), which are specialized niches in which young neurons for the olfactory bulb (OB) and hippocampus, respectively, are generated. Recent studies have significantly modified earlier views on the mechanisms of NSC self-renewal and neurogenesis in the adult brain. Here, we discuss the molecular control, heterogeneity, regional specification and cell division modes of V-SVZ NSCs, and draw comparisons with NSCs in the SGZ. We highlight how V-SVZ NSCs are regulated by local signals from their immediate neighbors, as well as by neurotransmitters and factors that are secreted by distant neurons, the choroid plexus and vasculature. We also review recent advances in single cell RNA analyses that reveal the complexity of adult neurogenesis. These findings set the stage for a better understanding of adult neurogenesis, a process that one day may inspire new approaches to brain repair.
KEY WORDS: Differentiation, Neural stem cells, Neurogenesis, Self-renewal, Stem cell heterogeneity, Transcriptomics
Summary: This Review discusses the identification, regulation and heterogeneity of neural stem cells and examines recent insights into their transcriptomic properties and mechanism of persistence into adulthood.
Introduction
Most adult organs retain a population of somatic stem cells that can respond to physiological conditions or injury by producing new cells for tissue homeostasis or repair. The brain was long considered an exception; it was widely assumed that the adult brain contained progenitors to generate glial cells, but that new neurons could only form during embryonic development. This dogma was challenged in the 1960s when Joseph Altman suggested that the addition of newborn neurons could occur in multiple regions of the adult mammalian brain including the hippocampus, olfactory bulb (OB) and cortex (Altman, 1962; Altman and Das, 1965). Direct demonstration for neurogenesis came from studies in songbirds: the laboratory of Fernando Nottebohm identified newly formed cells as neurons based not only on their structure, but also on their electrophysiological properties and functional integration into the song-control nuclei (Paton and Nottebohm, 1984; Burd and Nottebohm, 1985). This suggested that primary progenitors/neural stem cells (NSCs) with the ability for long-term self-renewal and the generation of neurons and glia persisted in the adult brain. A decade or so later, the presence of NSCs in mammals was suggested by the isolation and in vitro propagation of cells with stem cell properties (Reynolds and Weiss, 1992; Richards et al., 1992; Gage et al., 1995). Since then, the presence of adult mammalian NSCs and the addition of new neurons into the adult OB and hippocampus has been widely confirmed (for a review, see e.g. Song et al., 2016; Gonçalves et al., 2016; Lim and Alvarez-Buylla, 2016).
In the adult mammalian brain, the majority of NSCs are found within the ventricular-subventricular zone (V-SVZ) on the walls of the lateral ventricles (LVs). These primary progenitors give rise to young neurons that migrate a long-distance (3-8 mm in mice) to the OB. New OB neurons are thought to contribute to fine odor discrimination and odor-reward association (Li et al., 2018; Grelat et al., 2018; Lledo and Saghatelyan, 2005). NSCs are also found in the subgranular zone (SGZ) of the hippocampus; these generate new excitatory neurons for the dentate gyrus (DG), which plays roles in learning, memory and pattern separation (Ming and Song, 2011). These cells are known by several names: radial astrocytes, radial glia-like cells, radial cells, neural progenitors or type 1 progenitors. We refer to them here as radial astrocytes (RAs), given their original identification as a type of astrocyte (Eckenhoff and Rakic, 1984) before they were identified as NSCs (Seri et al., 2001, 2004).
Although much progress has been made in characterizing adult NSCs, the lineages they generate and the signaling pathways that influence their behavior, we are still lacking a detailed understanding of the mechanisms that sustain the NSC pool while ensuring life-long neurogenesis. For example, the extrinsic and/or intrinsic factors that promote quiescence and activation of NSCs remain largely unknown. Moreover, heterogeneity appears to be a key feature of primary progenitors/NSCs in the mammalian brain, but how this heterogeneity arises and how it affects NSC function is not fully understood.
Here, we review recent findings on adult neurogenesis, focusing on NSCs in the V-SVZ. The responses of NSCs to injury have been reviewed elsewhere (e.g. Sun, 2016; Patel and Sun, 2016; Chang et al., 2016) and are not covered here. We first discuss the identification, regulation and heterogeneity of NSCs. We then review recent insights into the transcriptomic signatures of adult NSCs, and summarize our understanding of NSC modes of division and their mechanisms of persistence in adult mice. Where relevant, we compare NSCs in the two neurogenic regions of the adult mammalian brain and discuss recent controversies on the extent to which neurogenesis continues in the adult human brain.
NSC identities and dynamics in the V-SVZ
Initial clues into the glial nature of NSCs came from work in songbirds. In adult canaries, radial glia persist in the walls of the forebrain ventricles and their division was linked to the production of new neurons (Alvarez-Buylla et al., 1990). In the late 1990s, it became evident that mammalian NSCs also have glial characteristics (for a review, see Kriegstein and Alvarez-Buylla, 2009). Indeed, it was shown that radial glia (RG) and a subset of V-SVZ astrocytes (B1 cells) are the NSCs of the ventricular zone (VZ) of the developing brain (Anthony et al., 2004; Miyata et al., 2001; Noctor et al., 2001; Götz et al., 1998) and of the V-SVZ of the adult forebrain (Doetsch et al., 1999), respectively. Shortly thereafter, NSCs in the SGZ were identified and were also shown to have astroglial properties (Seri et al., 2001, 2004; Garcia et al., 2004; Filippov et al., 2003).
The V-SVZ is the largest germinal zone in the adult brain. In young adult mice, there are roughly 7000 B1 cells per lateral wall of the lateral ventricles (Mirzadeh et al., 2008). B1 cells retain key epithelial properties of radial glia: they contact the cerebrospinal fluid (CSF) with a small apical ending and contact blood vessels with a longer basal process (Fig. 1). However, the ventricular surface in the adult acquires a unique pattern very different to that in the embryo, because of the development of ependymal (E) cells that exhibit large apical surfaces. As such, the small apical domains of B1 cells are surrounded by the large apical surfaces of E cells, forming pinwheel-like structures (Fig. 1). Multiciliated E cells have been suggested to function as NSCs (Johansson et al., 1999), but other studies have shown that they do not divide in the adult (Spassky et al., 2005; Chiasson et al., 1999). Recent work using single cell transcriptomic and lineage analysis has further confirmed that E cells do not possess progenitor properties or function as NSCs (Shah et al., 2018).
Fig. 1.
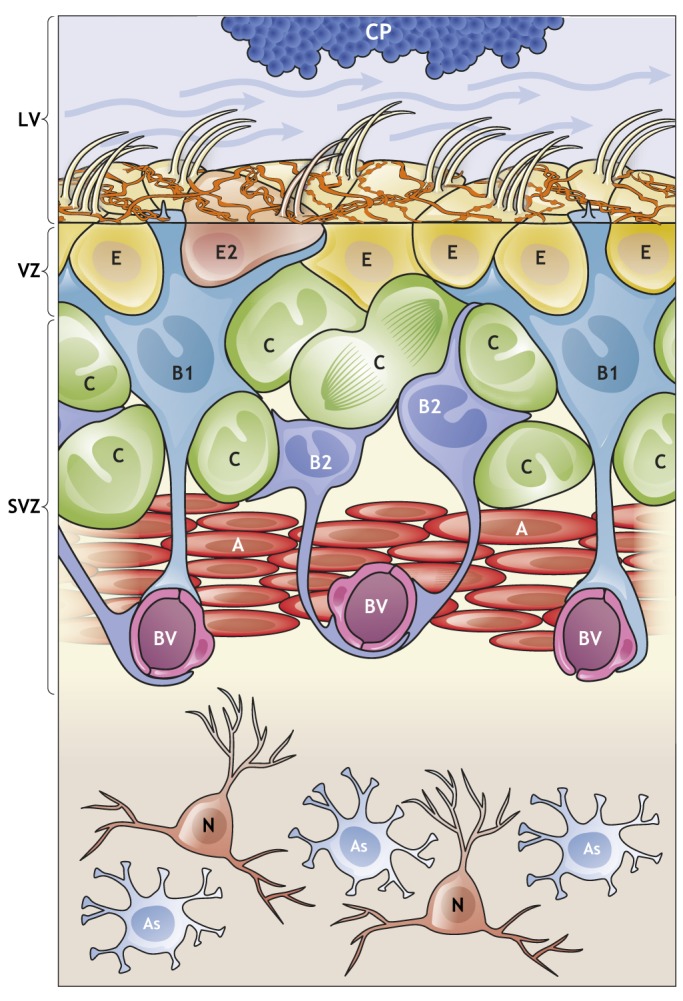
The V-SVZ: a niche for adult neurogenesis. Schematic of the organization and composition of the adult V-SVZ in the walls of the lateral ventricle (LV). B1 cells (light blue) have astroglial characteristics and function as the NSCs in the V-SVZ. They give rise to B2 cells (dark blue), which share many astroglial characteristics with B1 cells, including contacts with blood vessels (BV), but lack an apical contact. B1 cells also generate transient-amplifying cells (C cells; green) that give rise to young neurons (A cells; red). B1 cells are in contact with the cerebrospinal fluid (CSF; blue arrows) through a small apical contact that harbors a primary cilium. The choroid plexus (CP; blue) secretes factors into the CSF that are important for the regulation of B1 cells (see Fig. 2); the CP and CSF are therefore included as part of the niche. B1 cell apical endings are surrounded by the large surfaces of multiciliated and biciliated ependymal cells (E/E2 cells) which form pinwheel-like structures. In addition, supraependymal axons (orange) coursing on the surface of the ventricular wall contact both E cells and B1 cells. Below the apical surface, in the SVZ, B1 cells contact C cells, A cells, BV and B2 cells. Mature neurons (N; orange) and astrocytes (As; pale blue) can be found below the V-SVZ in the striatum.
In young adult mice, B1 cells produce ∼10,000 young migrating interneurons (neuroblasts, also known as A cells; Lois et al., 1996) every day through the generation of transient amplifying intermediate progenitors (IPCs; also known as C cells) (Doetsch et al., 1999). V-SVZ C cells divide three to four times before generating A cells, which also divide to further amplify the number of young neurons generated (Ponti et al., 2013). A cells collect from throughout the V-SVZ and, using chain migration, enter the rostral migratory stream (RMS) in which they continue to migrate to the OB (Lois and Alvarez-Buylla, 1994; Lois et al., 1996; Doetsch and Alvarez-Buylla, 1996; Luskin, 1993). Within the OB, young neurons differentiate into local interneurons that integrate into the existing circuitry (Petreanu and Alvarez-Buylla, 2002; Imayoshi et al., 2008; Luskin, 1993). Interestingly, B1 cells are heterogeneous and generate – depending on their location in the V-SVZ – different subtypes of OB interneurons (discussed below) (Merkle et al., 2007, 2014). B1 cells also generate B2 cells (Obernier et al., 2018), a population of proliferative V-SVZ astrocytes, the function of which is unknown (Doetsch et al., 1997). Interestingly, B1 and B2 cells are coupled through gap-junctions, which allows for calcium waves to travel among groups of B cells (Lacar et al., 2011). B2 cells do not contact the lateral ventricle but, as with B1 cells, they contact the vasculature (Fig. 1). Interestingly, during fetal human development, RG that lose apical contact with the ventricle (known as outer RG or basal RG) also function as primary progenitors of neurons (Hansen et al., 2010). Although the number of B1 cells drastically reduces in the first year of life in mice, the levels of newborn neurons in the OB are less affected by age, suggesting that a second population of NSCs that lack apical contact might exist in the adult rodent brain (Obernier et al., 2018). B1 cells also produce oligodendrocytes (Fogarty et al., 2005; Casper and McCarthy, 2006; Nait-Oumesmar et al., 1999; Menn et al., 2006). However, it remains unknown whether individual NSCs are multipotent in vivo and can generate both neurons and glia.
The B1 cell niche: regulators from near and far
B1 cells receive multiple signals that regulate their renewal and differentiation (Fig. 2). Compared with NSCs in the embryonic brain, adult NSCs receive signals from the fully formed brain with all its complexity and connectivity. Below we discuss the signaling molecules that regulate the behavior of V-SVZ B1 cells. These include factors from immediate NSC neighbors, from the choroid plexus (CP), CSF and vasculature, and from local and distant neuronal sources (Fig. 2). B1 cell-intrinsic factors and their role in V-SVZ neurogenesis have been reviewed elsewhere (e.g. Lim and Alvarez-Buylla, 2016; Jones and Connor, 2012; Bond et al., 2015; Murao et al., 2016) and are not discussed here.
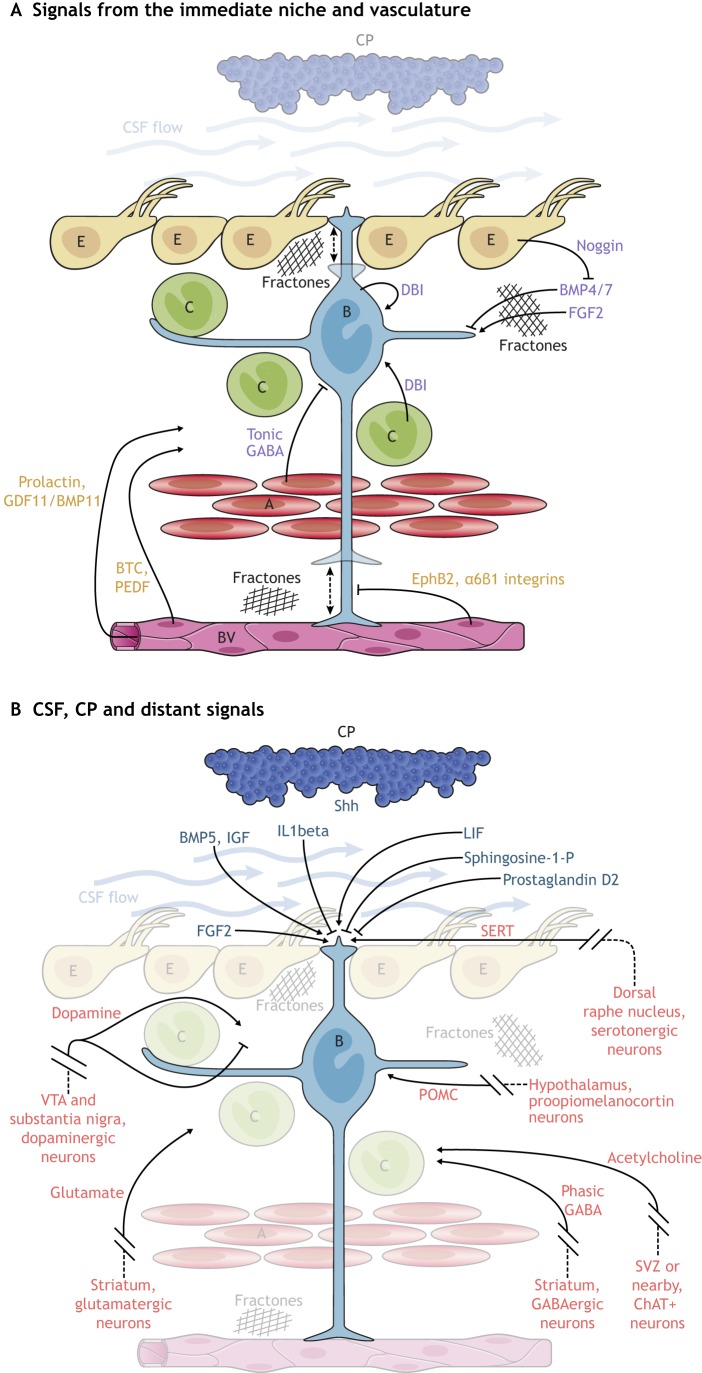
Fig. 2.
Signaling molecules that regulate V-SVZ neurogenesis. (A,B) A number of signaling molecules promote or inhibit the proliferation of B1 cells and hence regulate neurogenesis. These regulators can come from cells in direct contact with B1 cells (the ‘immediate’ niche) or from more distant sources. (A) Nearby cells, such as C cells, A cells, other B1 cells and E cells release factors (purple) that can influence B1 cells. B1 cells also contact blood vessels (BV) with the end foot of their basal process, which allows cell-cell interaction with endothelial cells and access to soluble factors (orange) in the blood. Dashed arrows indicate as yet unknown actions. (B) Factors (dark blue) from the CSF and CP act on B1 cells through their apical contacts. A number of distant signals (red), such as neurotransmitters released from neurons that reside outside the niche, also act on NSCs and their progeny.
Signals from neighboring cells
B1 cells exist in a niche in which they are in intimate contact with other B1, E, A and C cells (the ‘immediate niche’) as well as the vasculature (Fig. 2A; also see Table 1). A cells release gamma-aminobutyric acid (GABA), which activates GABAA-receptors on B1 cells, thereby reducing proliferation (Liu et al., 2005; Fernando et al., 2011). In contrast, the release of the diazepam-binding inhibitor protein (DBI) from B1 cells and C cells increases V-SVZ proliferation and A cell production (Alfonso et al., 2012). Through their lateral processes, B1 cells contact each other and likely exchange signals that are associated with their state of activation and possibly their mode of division. Such cell-cell communication between B1 cells may instruct the activation of one NSC upon the consumption or death of a neighboring NSC.
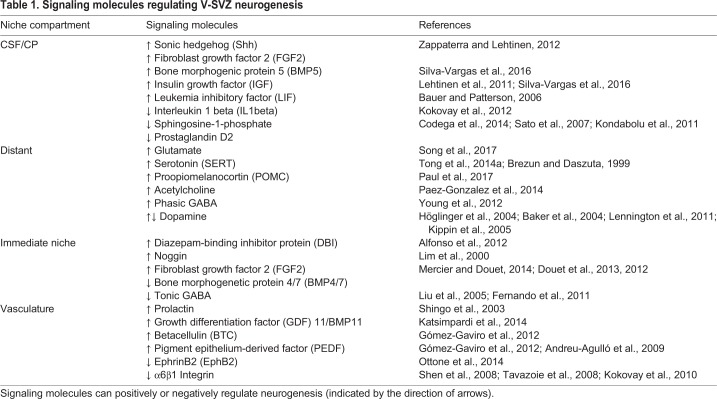
Table 1.
Signaling molecules regulating V-SVZ neurogenesis
Apically, B1 cells are surrounded by E cells, which propel the CSF with bundles of long motile cilia. E cells release noggin, an antagonist of bone morphogenetic protein (BMP) 4 (Lim et al., 2000). BMP4 inhibits cell proliferation (Mercier and Douet, 2014) and favors glial differentiation (Lim et al., 2000). It is thought that noggin releases this inhibition, which promotes V-SVZ cell proliferation and neurogenesis. BMP4/7 together with fibroblast growth factor (FGF) 2b (FGF2), which promotes proliferation in the V-SVZ (Mercier and Douet, 2014; Douet et al., 2013, 2012), are sequestered by extracellular matrix structures called fractones (Kerever et al., 2007). Each fractone is contacted by multiple processes from cells in the V-SVZ, including B1 cells (Obernier et al., 2018; Mercier et al., 2002). A recent report shows that a second atypical bulbar form of fractones next to B1 cells could play important roles in the regulation of neurogenesis (Nascimento et al., 2018). Interestingly, these bulbs, which are rich in laminin α5, appear to be deposited not by blood vessels, but by E cells. Genetic deletion of laminin α5 in E cells increases cell proliferation in the V-SVZ.
In addition to E cells and B1 cells and their progeny, the V-SVZ contains microglia (Doetsch et al., 1997). These blood-derived brain macrophages appear to be particularly abundant in the V-SVZ early in postnatal life (Shigemoto-Mogami et al., 2014), which corresponds to a period of high oligodendrogenesis and neurogenesis activity (Tong et al., 2015; Batista-Brito et al., 2008; Hinds, 1968; Bayer, 1983; Kessaris et al., 2006). Depletion of microglia results in decreased neurogenesis and oligodendrogenesis, and reduced levels of cytokines (Shigemoto-Mogami et al., 2014). Although the number of microglia decreases, some persist in the adult V-SVZ and have a more activated phenotype than those in the brain parenchyma (Mosher et al., 2012; Goings et al., 2006). The function of microglia in the regulation of B1 cells remains unknown, but it has been suggested that progenitor cells may regulate the activation of microglia (Mosher et al., 2012). Microglia have also been shown to be present next to chains of A cells, where they promote the migration of young neurons (Ribeiro Xavier et al., 2015).
These findings highlight that B1 cells derive information from cells that surround them, including lineage-related progeny and other cells within the niche. B1 cells also appear to actively change contacts with neighboring cells through the constant extension and retraction of processes (Obernier et al., 2018). In addition, the extracellular matrix appears to act together with cell-cell contacts, possibly sequestering cytokines and/or growth factors. How these signals work in concert to determine whether B1 cells divide to generate progeny or self-renew remains unknown. Making this regulation even more complex, factors beyond the immediate vicinity of the niche play key roles in regulating B1 cells, as discussed below.
The blood and endothelial cells
The basal end-feet of B1 and B2 cells contact blood vessels (Shen et al., 2008; Obernier et al., 2018; Figs 1 and 2A; also see Table 1). Interestingly, calcium waves that spread through gap junctions between B cells can also propagate into blood vessels (Lacar et al., 2011). This close association of B1 cells with the vasculature appears to be mediated by stromal-derived factor (SDF) 1 (Cxcl12) that is expressed by endothelial cells, as well as through α6β1 integrins, which appear to inhibit proliferation in the V-SVZ (Shen et al., 2008; Tavazoie et al., 2008; Kokovay et al., 2010). Endothelial cells also release betacellulin (BTC), which increases B1 and C cell proliferation and stimulates neurogenesis via EGFR/ErbB4/ERK signaling (Gómez-Gaviro et al., 2012). Pigment epithelium-derived factor (PEDF; Serpinf1) is also released by endothelial cells and enhances Notch signaling in activated NSCs (aNSCs), which promotes symmetric self-renewal (Gómez-Gaviro et al., 2012; Andreu-Agulló et al., 2009). Through endothelial ephrin B2 and jagged 1-mediated inhibition of differentiation, direct cell-cell contact between B1 cells and endothelial cells arrests B1 cells in the G0-G1 phase of the cell cycle and maintains NSCs in a quiescent state (Ottone et al., 2014). Interestingly, the basal processes and end-feet of B1 cells are highly dynamic and can shift along and between blood vessels (Obernier et al., 2018), suggesting that NSCs may select sites and timing of contacts with the vasculature. C cells, and in particular proliferating C cells, are also closely associated with blood vessels in an SDF1 and α6β1 integrin-dependent manner (Shen et al., 2008; Tavazoie et al., 2008). The vasculature appears to also contribute to chain migration of A cells along a vascular scaffold (Snapyan et al., 2009; Bovetti et al., 2007). Moreover, circulating factors in the blood exert their effects on V-SVZ neurogenesis in an age-dependent manner. Young blood displays higher levels of growth differentiation factor (GDF) 11/BMP11 and has ‘rejuvenating’ effects on the V-SVZ of older mice; in heterochronic parabiosis experiments, old mice undergo vascular remodeling and increased neurogenesis in response to young blood (Katsimpardi et al., 2014).
The cerebrospinal fluid
B1 cells directly contact the CSF through a small apical contact that harbors a primary cilium that is likely involved in signal transduction (Rohatgi et al., 2007; Corbit et al., 2005; Tong et al., 2014b). The CSF contains many signaling molecules that are considered important for the regulation of the V-SVZ (Fig. 2B; also see Table 1). These include sonic hedgehog (Shh), FGFs and leukemia inhibitory factor (LIF) (for a review, see Zappaterra and Lehtinen, 2012). LIF promotes NSC self-renewal at the expense of neurogenesis (Bauer and Patterson, 2006). B1 cells also respond to interleukin 1β in the CSF, increasing their expression of vascular cell adhesion molecule (VCAM) 1 and reducing their proliferation (Kokovay et al., 2012). Interestingly, the CP secretome changes during aging, decreasing its proliferation-promoting effects with age (Silva-Vargas et al., 2016; Lehtinen et al., 2011). For example, insulin-like growth factor (IGF) 2 in the CSF, which is known to promote RG proliferation through the IGF1 receptor (Lehtinen et al., 2011), decreases with age. aNSCs from old mice show increased clone formation when cultured with CP-conditioned medium from young mice, which is enriched in BMP5 and IGF1 (Silva-Vargas et al., 2016). Gradients of chemorepulsive signals created by CSF flow help guide A cell migration (Sawamoto et al., 2006). Recent work also suggests that B1 cells sense the flow of CSF through alpha epithelial sodium channel (αENaC), providing a possible mechanism for B1 cells to detect the presence of signals in the apical domain of the V-SVZ (Petrik et al., 2018). Clearly, the CSF contains many factors that modulate B1 cells and their progeny, but whether CSF signals are supportive or instructive in the regulation of NSC self-renewal or differentiation remains unknown.
Neural inputs
B1 cells are also modulated by a number of local neurotransmitters (Fig. 2B; for a review, see Berg et al., 2013) such as GABA, which is released as discussed above. However, in contrast to this tonic activation of B1 cells that promotes quiescence, the phasic activation of GABAA-receptors has been suggested to promote progenitor cell proliferation (Young et al., 2012). GABA from medium spiny and aspiny striatal neurons that project into the V-SVZ appears to provide phasic activation of GABAA-receptors, inducing calcium activity in progenitors (Young et al., 2014). In addition, cholinergic terminals from local choline acetyltransferase (ChAT)+ neurons have been proposed to modulate progenitor proliferation and increase the production of young neurons (Paez-Gonzalez et al., 2014). It has also been shown that glutamate that is released from striatal neurons can increase the proliferation of A cells through AMPA receptor activation (Song et al., 2017).
Neural inputs to the V-SVZ also come from more distant locations (Fig. 2B; also see Table 1). The apical surface of the V-SVZ is covered by a dense network of supraependymal axons that originate from serotonergic neurons in the dorsal raphe nucleus and contact B1 and E cells (Tong et al., 2014a; Brezun and Daszuta, 1999). Serotonin could contribute to the positive regulation of B1 cell proliferation; B1 cells express the serotonin receptors 5HT2C (HTR2C) and 5A (HTR5A) and respond to 5HT receptor agonists (Tong et al., 2014a). Dopaminergic neurons from the substantia nigra and the ventral tegmental area (VTA) also project into the V-SVZ, and ablation of these neurons results in reduced V-SVZ proliferation and neurogenesis (Höglinger et al., 2004; Baker et al., 2004; Lennington et al., 2011). However, dopamine may also inhibit NSC proliferation; dopamine D2-receptor antagonists increase the number of label-retaining NSCs as well as neurogenesis and gliogenesis (Kippin et al., 2005).
Integrating signals in time and space
How V-SVZ NSCs/progenitor cells integrate the many factors from their immediate surroundings and distant sources remains unknown. It is possible that subpopulations of NSCs may differ in their response to extrinsic signals. It is also largely unknown how the combination of both local and distant factors that regulate adult NSCs is linked to the function of new neurons in the OB. After their birth in the V-SVZ, it takes ∼15 days for new neurons to migrate and differentiate (Petreanu and Alvarez-Buylla, 2002). Therefore, the birth of neurons is disconnected in time from the ultimate function these cells have in the OB (Li et al., 2018; Grelat et al., 2018; Lledo and Saghatelyan, 2005; Cecchi et al., 2001). Any regulation at the level of the V-SVZ is therefore likely anticipatory, predisposing the germinal center to increase or decrease neuronal output in advance of events that may change OB discriminatory function. Prolactin, which is produced during early pregnancy and lactation, appears to increase V-SVZ output in female mice, possibly preparing the OB for changes in the olfactory environment as the young are born and weaned (Shingo et al., 2003). Proopiomelanocortin neurons in the hypothalamus also promote proliferation of NSCs in the anterior-ventral V-SVZ, linking neurogenesis to neuroendocrine function (Paul et al., 2017). Alternatively, generation of neurons from the V-SVZ may ensure that a sufficient supply of young neurons is produced and the excess eliminated once the cells become integrated into the OB (Petreanu and Alvarez-Buylla, 2002). Clearly, B1 cells are at the center of many signaling pathways (Fig. 2), yet an integrative view of how these factors regulate self-renewal and differentiation of NSCs, and how this affects physiological function, remains to be investigated.
Not all NSCs are equal: the origins and heterogeneity of B1 cells
B1 cells share many features with embryonic NSCs. They possess a primary cilium that contacts the CSF, they exhibit apico-basal polarity and they are derived from RG, which earlier in embryonic development also generate the majority of forebrain neurons (Merkle et al., 2004). As in embryonic NSCs, B1 cells also exhibit heterogeneity with regard to their origins and the cell types they give rise to (Fig. 3).
Fig. 3.
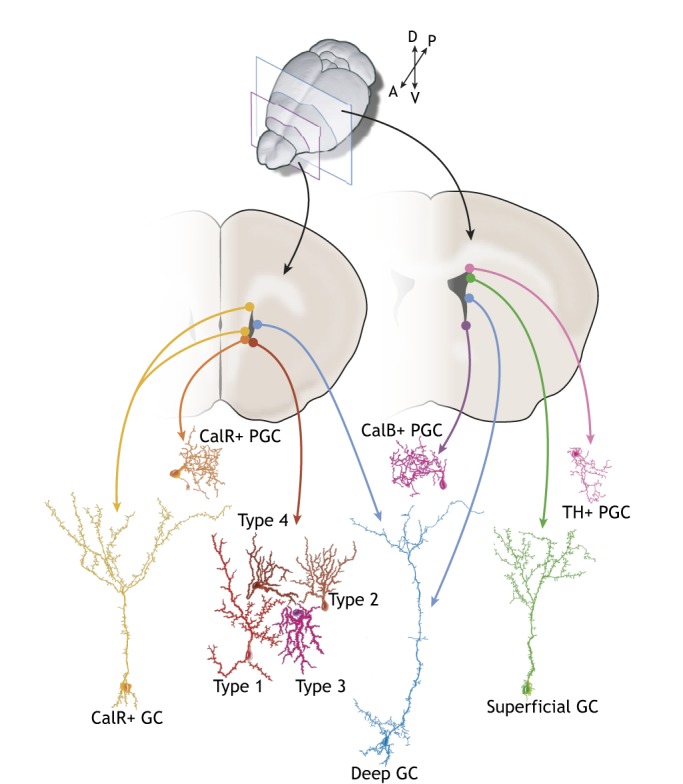
NSC heterogeneity. B1 cells are regionally specified: depending on their location in the V-SVZ along the anterior-posterior and medial-lateral axes, B1 cells generate different subtypes of interneurons that are destined for the OB. For example, B1 cells in the posterior dorsal V-SVZ (indicated in the posterior coronal section on the right) generate tyrosine-hydroxylase (TH)+ periglomerular cells (PGCs; pink) and superficial granule cells (GCs; green), whereas B1 cells in the posterior ventral V-SVZ give rise to calbindin (CalB)+ PGCs (purple). Cells in the anterior medial V-SVZ (shown in the anterior section on the left) generate calretinin (CalR)+ PGCs (orange). Ventral NSCs produce deep GCs (blue), whereas dorsal NSCs generate superficial GCs. A small subset of NSCs in the lateral anterior ventral V-SVZ generates four additional types of interneurons (type 1-4).
During mid-embryonic development, a subpopulation of RG divides and generates pre-B1 cells that express VCAM1 (Fuentealba et al., 2015; Furutachi et al., 2015; Hu et al., 2017), which is also expressed on the apical surface of mature B1 cells and appears to be crucial for stem cell maintenance. Blocking antibodies or shRNA against VCAM1 leads to differentiation and depletion of the NSC population (Kokovay et al., 2012). Then during late embryogenesis, a second subset of RG upregulates glial fibrillary acidic protein (GFAP) expression and acquires the transcriptomic signature of quiescent B1 cells (Yuzwa et al., 2017). These pre-B1 cells upregulate the negative cell cycle regulator p57kip2 (Cdkn1c) and remain largely quiescent until activated postnatally to produce OB interneurons (Fuentealba et al., 2015; Furutachi et al., 2015; Yuzwa et al., 2017). The subsequent and complete transformation of the embryonic VZ into an adult V-SVZ has not been well studied. It is known that transformation occurs progressively during the first ten postnatal days, coinciding with the differentiation of E cells and the expansion of their apical surface (Hu et al., 2017; Tramontin et al., 2003). During this time, B1 cell bodies are displaced into the underlying SVZ, but they maintain the thin apical domain that results in the formation of pinwheels.
As in RG, which generate diverse subsets of neurons depending on their location, B1 cells are heterogeneous in terms of the cell types they generate (Fig. 3) (for a review, see Chaker et al., 2016). Indeed, depending on their location along the anterior-posterior and dorsal-ventral axes of the lateral wall, as well as along the most anterior-ventral tip of the medial wall of the lateral ventricles, B1 cells generate at least ten different subtypes of OB interneurons (Merkle et al., 2007, 2014; Kelsch et al., 2007; Delgado and Lim, 2015; Young et al., 2007; Kohwi et al., 2005, 2007). B1 cells also appear to be highly resilient to changes in fate: transplanting them from ventral to dorsal regions of the V-SVZ or vice versa is not sufficient to change the types of neurons they generate, which suggests that the regional specification of B1 cells is intrinsic (Merkle et al., 2007). Recent single cell RNA sequencing data indicates that transcription factors that are known to be expressed in a spatially restricted manner anti-correlate with each other (Llorens-Bobadilla et al., 2015). Pax6 expression, for example, determines the generation of dopaminergic OB interneurons from NSCs and, whereas Pax6 mRNA is widely expressed in the V-SVZ, Pax6 protein expression is limited to the dorsal V-SVZ, with the microRNA-7a inhibiting Pax6 protein expression in other domains. Accordingly, the inhibition of microRNA-7a (microRNA 7-2) leads to translation of Pax6 mRNA in these regions and an increase in dopaminergic neurons in the OB (de Chevigny et al., 2012). Shh signaling in the ventral V-SVZ is higher than that in the dorsal V-SVZ and is required for the production of ventrally derived deep granule neurons and calbindin+ periglomerular neurons. Experimentally increasing Shh signaling dorsally leads to the generation of neurons in the OB that are similar to those generated from the ventral V-SVZ (Ihrie et al., 2011). Thus, Shh signaling is instructive for regional specification and the generation of specific OB interneuron subtypes.
Retroviral barcode lineage tracing has shown that the regional specification of postnatal progenitor cells can be traced back to early stages of embryonic development. Adult B1 cells inherit their regional signature from RG (Fuentealba et al., 2015). However, primary progenitors at different developmental times (embryonic versus adult) within the same location differ in the types of neurons they generate. This is perhaps most dramatic in the pallium: dorsal RG in the cortical VZ generate excitatory neurons for the different layers of the developing cortex in the embryo, whereas B1 cells in the dorsal V-SVZ generate inhibitory interneurons for the superficial granule cell layer of the OB (Fig. 3) (Merkle et al., 2007; Ventura and Goldman, 2007). Similarly, a postnatal remnant of the medial ganglionic eminence, which in the embryonic brain is a major source of cortical interneurons, gives rise to subsets of OB calretinin+ granule neurons and periglomerular neurons (Merkle et al., 2007). This change in neuronal cell types that are produced from development to postnatal life appears to occur in all the walls of the lateral ventricle (Fuentealba et al., 2015). Subtypes of periglomerular interneurons are further generated in a temporal manner: whereas NSCs during early postnatal life mostly generate calbindin+ periglomerular interneurons, tyrosine hydroxylase+ and calretinin+ periglomerular interneurons are generated during adult life (De Marchis et al., 2007). The extrinsic and intrinsic factors that control the switch in neuronal cell fate as development progresses remain unknown.
It has also been shown that dopaminergic OB interneurons born at different times give rise to functionally different cell types: whereas interneurons born postnatally are anaxonic and have no axon initial segment, those born during embryonic development have an axon and an axon initial segment, and are more excitable than postnatally generated cells (Galliano et al., 2018). It is unclear whether this is because of the integration of young neurons into a circuit that differs in maturity, whether the age of the niche affects the differentiation of primary progenitors or whether primary progenitors generate different cell types based on their own age. It is interesting to speculate that those B1 cells born early during development from RG (pre-B1 cells) have different functions than B1 cells that are generated by self-renewing divisions later in life. Similar to pre-B1 cells, some B1 cells may undergo extended periods of quiescence and function as a reserve for the generation of neurons as animals age. Such dynamics may require NSCs to keep track of their own history, e.g. the number of times the cell (or its predecessors) has already divided.
Overall, these studies reveal that the population of NSCs in the V-SVZ is heterogeneous: they have different origins and, depending on their location and time of division, can generate distinct types of neurons (Fig. 3). The mechanisms underlying this heterogeneity are largely unknown, but lineage-tracing experiments indicate that regional specification is established very early in embryonic development and is inherited by the adult NSCs (Fuentealba et al., 2015).
Gene expression of V-SVZ neural stem/progenitor cells
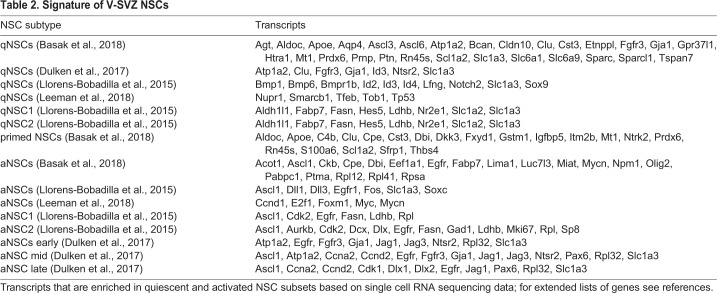
Adult B1 cells are largely quiescent in vivo (Morshead et al., 1994). This explains why anti-mitotic treatments, such as Ara-C, spare B1 cells but kill actively mitotic C cells. Following the anti-mitotic treatment, neurogenesis is re-initiated through the activation of NSCs (Doetsch et al., 1999; Basak et al., 2018). Two populations of NSCs have been proposed in the adult V-SVZ – quiescent (qNSCs) and aNSCs – and are suggested to interconvert in vitro (Codega et al., 2014). qNSCs and aNSCs have been prospectively isolated using FACS to study their transcriptome and in vitro stem cell properties (Codega et al., 2014; Beckervordersandforth et al., 2010; Capela and Temple, 2002; Khatri et al., 2014). These studies have revealed that qNSCs and aNSCs both express GFAP and prominin, but that EGFR expression is limited to aNSCs (Codega et al., 2014). Nestin is not expressed in qNSCs but, together with EGFR, is upregulated upon activation. In vitro, qNSCs generate significantly fewer neurospheres than aNSCs. aNSCs actively proliferate, but this population contains fewer cells positive for Ki67 (Mki67) compared with EGFR+ C cells (Dulken et al., 2017). It has been suggested that both qNSCs and aNSCs contact the LV, but only qNSCs express the G protein-coupled receptors for sphingosine-1-phosphate and prostaglandin D2 (Codega et al., 2014), which are present in the CSF (Sato et al., 2007; Kondabolu et al., 2011). qNSCs are also enriched in genes that are associated with signaling receptors, cell-cell adhesion and ion channels, suggesting that quiescence is actively maintained. By contrast, genes that are involved in cell cycle control, protein synthesis and DNA repair are enriched in aNSCs. Interestingly, qNSCs, but not aNSCs, display invaginations of the nuclear envelope that resemble envelope-limited chromatin sheets (ELCSs), which are also found in subpopulations of RG (Cebrián-Silla et al., 2017). These ELCSs are enriched in telomeres and associated with epigenetic markers that are involved in gene repression, and have been suggested to act as a nuclear compartment for heterochromatin domains that are related to quiescence.
Recent advances in single cell RNA sequencing have confirmed the existence of distinct aNSC and qNSC populations. The molecular profiles of single V-SVZ cells have been examined from FACS-sorted cells that express GFAP, prominin, and EGFR (Dulken et al., 2017; Leeman et al., 2018), or GLAST (Slc1a3) and prominin (NSCs) and PSA-NCAM (A cells) (Llorens-Bobadilla et al., 2015). Despite differences in isolation protocols, these studies uncovered a continuum of cell types that transition from quiescence, to activation, to differentiation (summarized in Table 2). These studies further subdivided qNSCs and aNSCs along this trajectory [into two qNSCs and two aNSCs (Llorens-Bobadilla et al., 2015) or one qNSC and three aNSCs (Dulken et al., 2017)]. qNSCs, which express high levels of Hes5, Blbp (Fabp7), Aldh1L1 and GLAST, enter a primed state before becoming activated and upregulate genes for protein synthesis and ribosomal biogenesis such as Rpl32, but not genes related to cell cycle. The upregulation in protein synthesis genes within the qNSC continuum suggests that the exit from quiescence is accompanied by translational activation (Llorens-Bobadilla et al., 2015).
Table 2.
Signature of V-SVZ NSCs
qNSCs were found to express the Notch receptor Notch2 and transcription factors such as Sox9, Id2 and Id3 (Llorens-Bobadilla et al., 2015). In contrast, aNSCs, which express EGFR, upregulate other cell cycle genes, likely related to the transition from non-mitotic to mitotic aNSCs, and express high levels of the Notch ligands Fos and basic helix-loop-helix transcriptional activator achaete-scute family bHLH transcription factor 1 (Ascl1). Ascl1 appears to be a key player in the aNSC transcriptome, as these cells are enriched in genes that are controlled by this transcription factor (Andersen et al., 2014). Ascl1 induces neuronal differentiation and the expression of the Notch ligands delta like canonical Notch ligand 1 (Dll1) and jagged 1 (for a review, see Kopan and Ilagan, 2009). For example, C cells, which express high levels of Ascl1, express Dll1 and through Notch-dependent feedback regulate the quiescence of NSCs that express Notch receptors (Aguirre et al., 2010; Kawaguchi et al., 2013). The activation of Notch receptors in neighboring NSCs leads to translocation of the Notch intracellular domain into the nucleus, in which it forms a transcriptional complex with Rbpj that activates the expression of Hes1 and Hes5. The Hes factors, in turn, repress the expression of Dll1 and pro-neural genes, inhibiting neuronal differentiation in NSCs. Accordingly, the deletion of Rbpj in NSCs results in a transient increase in proliferation in V-SVZ cells and increased neurogenesis (Imayoshi et al., 2010). Interestingly, Rbpj deletion also results in the depletion of NSCs and the failure to regenerate after treatment with Ara-C, which suggests that active Notch signaling maintains the undifferentiated state of qNSCs in the V-SVZ. However, Notch isoforms are differentially expressed in the V-SVZ: NSCs in the ventral and lateral wall also appear to express Notch3, the loss of which leads to exit of quiescence, reduced numbers of qNSCs and increased NSC cell division in a region-specific manner (Kawai et al., 2017). In contrast, Notch1 is preferentially expressed in aNSCs and C cells (Kawai et al., 2017), both of which also express Ascl1 (Dulken et al., 2017; Codega et al., 2014), and its loss leads to reduced NSC proliferation (Basak et al., 2012).
A subset of mitotic aNSCs express genes that are associated with GABAergic neuronal differentiation, such as Dlx1 and Dlx2, and repress glial-associated genes. This suggests that aNSCs initiate a neuronal program that potentially primes them for differentiation. However, whereas cell cycle genes distinguish qNSCs from aNSCs, other gene modules behave more dynamically, which suggests that a single molecular switch that induces differentiation might not exist (Basak et al., 2018).
The transition from qNSC to aNSC is further accompanied by changes in cell metabolism. Genes that are involved in glycolysis are highly expressed in qNSCs, whereas aNSCs are enriched in genes that are associated with oxidative phosphorylation (Llorens-Bobadilla et al., 2015; Leeman et al., 2018). qNSCs also express high levels of Spot14 (Thrsp), a negative regulator of fatty acid synthase (FASN). The fatty acid binding protein Blbp, which is involved in transporting lipids that have been taken up from the circulation, is also expressed at high levels in RG and adult NSCs. Inhibition of de novo lipogenesis or loss of Blbp decreases proliferation and neurogenesis of SGZ and V-SVZ NSCs, whereas the knockdown of Spot14 leads to activation of NSCs in the SGZ (Knobloch et al., 2013).
qNSCs and aNSCs also use different mechanisms for protein homeostasis: aNSCs show high proteasome activity in culture and are enriched with proteasome and chaperone-related genes. By contrast, qNSCS express genes that are associated with lysosomes (Leeman et al., 2018). Although qNSCs have lower protein synthesis rates, they have larger lysosomes and contain more lysosome-associated protein aggregates than do aNSCs. The clearance of protein aggregates by lysosomal activity leads to NSC activation in response to EGF and FGF. Interestingly, aging is paralleled by robust changes in the transcriptome of qNSCs but not of aNSCs (Leeman et al., 2018). During aging, neurogenesis and the number of NSCs decline (Obernier et al., 2018; Enwere et al., 2004), and lysosome activity becomes impaired in qNSCs, leading to higher levels of protein aggregates. This may explain the reduced capability of NSCs to become activated in the aged V-SVZ (Enwere et al., 2004; Capilla-Gonzalez et al., 2014).
Similarities and differences between V-SVZ and SGZ NSCs
NSCs in the two major germinal centers of the adult brain – the V-SVZ and the SGZ – share astroglial features and expression of some molecular markers. There are, however, important differences in their niches, anatomy and molecular properties. Below, we highlight some of the similarities and differences between NSCs in the V-SVZ and SGZ. SGZ NSCs (RAs) have been reviewed in detail elsewhere (e.g. Gonçalves et al., 2016).
Niches and lineages
One key difference between B1 cells and RAs is their anatomical location. The SGZ is a displaced germinal niche, located away from the ventricular walls. The RAs are located deep in the brain, at the interface of the hilus and the granule cell layer in the DG. Therefore, unlike V-SVZ B1 cells, RAs do not contact the ventricle. However, as with B1 cells, RAs contact neighboring NSCs, IPCs and a vascular plexus under the SGZ (Seri et al., 2004; Palmer et al., 2000). Furthermore, through their elaborate radial process, RAs contact mature granule neurons and, in the molecular layer, other glial cells and axonal terminals (Moss et al., 2016). RAs give rise to IPCs (in the SGZ known as D cells or Type 2a/2b progenitors), although how many times these IPCs then divide in the SGZ remains controversial (Encinas et al., 2011; Seri et al., 2004; Lugert et al., 2012). Ultimately, IPCs generate young neurons (Kempermann et al., 2004) that do not move far from the mother RA (Sun et al., 2015) and integrate into the granule cell layer (GCL), in which they differentiate into glutamatergic excitatory granule neurons (van Praag et al., 2002). Therefore, RAs are in close proximity to the very same neurons they generate and other mature neurons of the DG. This is unlike B1 cells, which do not contact the OB or the neurons within it. Another key difference is that neurons born from RAs in the adult SGZ are excitatory, whereas B1 cells generate inhibitory neurons. RAs are also able to produce differentiated astrocytes, but apparently do not generate oligodendrocytes under normal conditions (Steiner et al., 2004). However, they can be induced to generate oligodendrocytes by overexpression of Ascl1 or knockout of the ubiquitin-specific protease 9 X-linked (USP9X) (Oishi et al., 2016; Jessberger et al., 2008).
Similar to the V-SVZ lineage, a continuum from quiescence, to activation, to differentiation has been shown, based on transcriptomics, for SGZ NSCs in the adult hippocampus (Shin et al., 2015; Artegiani et al., 2017). Pseudotime analysis suggests that, during the transition from qNSC to aNSC, genes that are related to the Notch, BMP and MAPK pathways are downregulated, whereas low levels of IPC-related genes and proliferation markers become expressed in late NSCs. As with V-SVZ NSCs, SGZ NSCs switch from glycolysis to oxidative phosphorylation during activation: qNSCs are enriched in genes that are associated with glucose/glycolysis and lipid metabolism, including fatty-acid degradation and sphingolipid metabolism, whereas oxidative-phosphorylation, protein synthesis and cell cycle genes are upregulated in aNSCs. HopX, which has been recently identified as a marker for outer RG in the human embryonic cortical SVZ (Pollen et al., 2015), is also expressed in SGZ qNSCs and is downregulated during the transition to aNSCs (Shin et al., 2015). Interestingly, HopX is not detected in V-SVZ NSCs (Li et al., 2015), which suggests important differences in the expression profiles in NSCs from the two main neurogenic regions of the adult brain.
Extrinsic regulation
There are also some similarities in the regulation of RAs and B1 cells. RAs make contact with blood vessels in a vascular plexus next to the SGZ (Palmer et al., 2000; Seri et al., 2004) and, as in B1 cells, circulating factors in the blood affect RAs in an age-dependent manner, possibly owing to an elevation of the levels of the chemokine CCL11, which reduces neurogenesis (Villeda et al., 2011). Similar to B1 cells, RAs respond to neurotransmitters that are released, in the case of RA, from neurons within the DG or from distant locations. Locally, GABA from DG parvalbumin+ interneurons appears to regulate RA quiescence through tonic activation of GABAA-receptors (Song et al., 2013, 2012; Ge et al., 2007). Interestingly, deletion of the γ2 subunit of GABAA-receptors in RAs promotes symmetric self-renewal (Song et al., 2012). Treatment with DBI, a negative allosteric modulator of GABAA-receptors in NSCs and progenitor cells, also promotes RA symmetric self-renewal and delays the generation of young neurons (Dumitru et al., 2017). As in the V-SVZ, neurogenesis in the SGZ/DG is regulated by input from neurons in distant locations. Serotonin from the raphe nucleus positively regulates SGZ stem/progenitor proliferation, and lesions of the raphe nucleus reduce neurogenesis in the SGZ/DG (Brezun and Daszuta, 1999). Proliferation is also inhibited by the ablation of medial septal cholinergic neurons or midbrain dopaminergic neurons (Fréchette et al., 2009; Van Kampen and Eckman, 2010; Höglinger et al., 2004), or by NMDA receptor agonists and glutamatergic input to the DG, whereas NMDA receptor antagonists increase neurogenesis (Cameron et al., 1995; Berg et al., 2013). The locus coeruleus neurons in the brain stem innervate the hippocampus and release norepinephrine, which increases proliferation in the SGZ by activating quiescent RAs through β-adrenergic receptors, or decreases proliferation and neurogenesis through α2-adrenergic receptors (Jhaveri et al., 2014, 2010; Kulkarni et al., 2002). SGZ neurogenesis also responds to the external environment: hippocampal neurogenesis is reduced under stress (e.g. through the release of glucocorticoids; for a review, see Mirescu and Gould, 2006), whereas environmental enrichment increases proliferation, differentiation and/or cell survival in the hippocampus, possibly through increased levels of vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) (for a review, see Nithianantharajah and Hannan, 2006).
Heterogeneity of NSCs in the SGZ
As seen in the B1 cells of the V-SVZ (discussed above), RAs exhibit functional and morphological heterogeneity (for review, see Berg et al., 2018). For example, whereas RAs are more abundant in the dorsal (temporal) SGZ, RAs in the ventral blade of the DG generate neurons that appear to mature at a faster rate than those born dorsally (Piatti et al., 2011). Furthermore, RAs have been divided into two subtypes: type α and type β. Type α cells, which represent the majority of RAs, have radial processes that branch into the molecular layer. The radial processes of type β RAs, by contrast, are shorter and branch within the GCL, extending to the border of the outer GCL and inner molecular layer. Interestingly, similar to the lineage relationship between B1 and B2 cells in the V-SVZ, type α RAs give rise to type β RAs (Gebara et al., 2016). Besides these RAs, which possess a radial glia-like morphology, horizontal non-radial NSCs have been described in the DG (Suh et al., 2007; Steiner et al., 2006). It remains unclear how the heterogeneity in morphology of B1 cells and RAs is associated with their progenitor functions. Whether these differences are a reflection of stages in quiescence or activation, or whether different morphological types correspond to unique populations of primary progenitors, remains an important question for future research.
Maintenance of V-SVZ neural stem cells
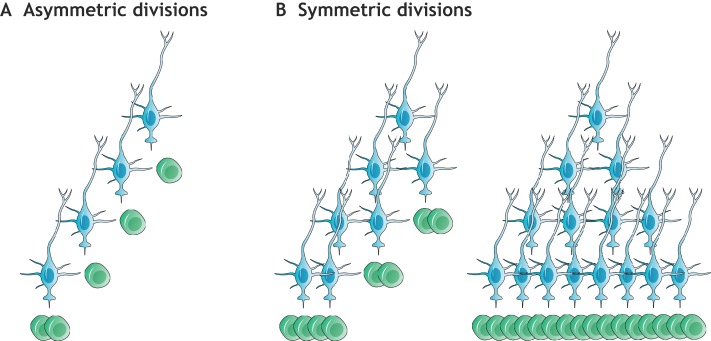
Discovering how primary progenitors are retained throughout postnatal and adult life is essential in understanding adult neurogenesis. Sustained neurogenesis for extended periods of time requires the coordinated regulation of quiescence, self-renewal and differentiation. These processes require the control of cell division mode, i.e. symmetric versus asymmetric cell division (Fig. 4). Symmetric divisions of NSCs can result in either two stem cells (self-renewal) or two IPCs (i.e. stem cell consumption), and therefore affect stem cell numbers. In contrast, NSCs undergoing asymmetric divisions generate one stem cell and one IPC. Asymmetric divisions in theory could continually replenish the stem cell pool, but this mode of division does not allow independent regulation of neurogenesis versus self-renewal. In contrast, symmetric divisions allow self-renewal to be uncoupled and independently regulated from differentiation, which allows for the generation of progeny with minimal replicative burden on individual stem cells. Symmetric divisions have therefore been proposed as a mechanism to protect stem cells from cancer and replicative aging owing to the accumulation of DNA damage (Shahriyari and Komarova, 2013; Hormoz, 2013). Indeed, it is becoming increasingly evident that symmetric divisions fuel the self-renewal of somatic stem cells in various tissues and organisms, e.g. in the brain of Drosophila melanogaster (Mora et al., 2018), during Caenorhabditis elegans germ cell development (Kimble and White, 1981), and in the adult mammalian hematopoietic system (Bernitz et al., 2016), epidermis (Rompolas et al., 2016) and intestinal epithelium (Snippert et al., 2010).
Fig. 4.
Division modes of NSCs. (A) Asymmetric stem cell divisions (proposed e.g. by Calzolari et al., 2015) generate a fixed lineage with one B1 cell (blue) giving rise to another B1 cell and one C cell (green). This mode of division requires repeated divisions of B1 cells for the production of an increased population of progeny before a final (consuming) division generates only C cells. (B) Symmetric divisions can generate either two B1 cells (symmetric self-renewal) or two C cells (symmetric consumption/differentiation), allowing self-renewal and differentiation to be regulated independently, and allowing the generation of progeny with minimal replicative burden on individual stem cells. Note that sister B1 cells can become consumed asynchronously (left), and that multiple self-renewing divisions can precede consuming divisions (right).
In the rodent brain, the mode of NSC division changes depending on developmental stage. Early embryonic neuroepithelial cells in the neural tube expand through symmetric self-renewal (Kriegstein and Alvarez-Buylla, 2009). In contrast, RG in the cortical VZ have been shown to mostly undergo asymmetric cell divisions to both self-renew and generate cortical neurons, either directly or through IPCs (Haubensak et al., 2004; Noctor et al., 2004). In the adult V-SVZ, GLAST+ NSCs have been suggested to undergo a rapid sequence of asymmetric cell divisions before becoming consumed by a terminal symmetric division, thereby lacking long-term self-renewal capabilities (Calzolari et al., 2015) (Fig. 4A). However, clonal analysis using barcoding techniques has revealed that the majority of OB interneurons born after P28 do not have clonal relationships to OB neurons that were born earlier (Fuentealba et al., 2015), which suggests that repeated asymmetric divisions of NSCs do not occur. Consistently, recent data shows that the majority of GFAP+ V-SVZ NSCs divide symmetrically to either self-renew or differentiate (Fig. 4B), but exhibit a greater probability to undergo consuming divisions (Fig. 5), thereby leading to a decline in B1 cells over time (Obernier et al., 2018). Similar results were obtained upon lineage tracing of Troy+ or Ki67+ NSCs (Basak et al., 2018). It will be interesting to determine whether self-renewing NSCs and those primed to differentiate differ in their transcriptomic signatures.
Fig. 5.
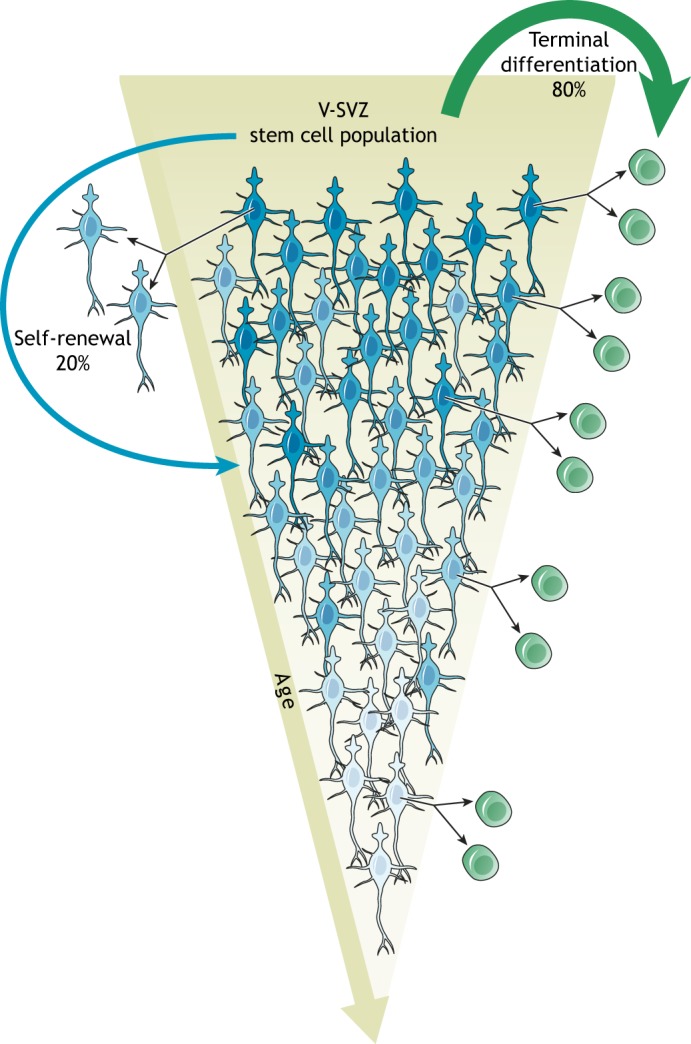
B1 cells decline over time. B1 cells (blue) divide symmetrically. The majority (∼80%) of B1 cells becomes consumed by the generation of C cells (green), leading to a declining B1 cell population as the animal ages (terminal differentiation; green arrow). A smaller fraction (∼20%) of B1 cells symmetrically self-renews, which generates two B1 cells. Given the high level of terminal differentiation, this process allows neurogenesis to be maintained throughout the lifespan of the animal (albeit at lower levels). Note that B1 cells can undergo more than one round of symmetric self-renewal, enter extended periods of quiescence, and generate progeny at different times. In addition, each self-renewing division may lead to intrinsic changes in B1 cells (aging of the stem cell lineage), here illustrated by different shades of blue.
B1 cells can symmetrically self-renew more than once before they undergo a final differentiative division (Fig. 5), thereby transiently amplifying their lineage. Interestingly, self-renewing B1 cells can reside in the V-SVZ for several months before their consumption (Obernier et al., 2018), which suggests long-term self-renewal capacities of at least a subset of NSCs. Compared with asymmetric cell divisions, this sequence of symmetric self-renewal followed by symmetric differentiation could allow for either the generation of larger numbers of differentiating progeny or the distribution of replicative burden between lineage-related B1 cells. Stem cells in the hematopoietic system have similarly been shown to asynchronously undergo four rounds of symmetric amplification, with age-related changes linked to ‘cellular memory’ and the division history of the cell (Bernitz et al., 2016). It is known that B1 cells can enter extended periods of quiescence of up to several months (Obernier et al., 2018; Basak et al., 2018) but it remains unclear whether the sporadic re-activation of NSCs is linked to the cell history, i.e. the number of previous self-renewing divisions. Moving forward, it will be interesting to determine whether repeated self-renewal changes the transcriptomic signature of NSCs.
Thus, in mice, the processes of NSC activation, maintenance and differentiation seem to be highly regulated throughout the life span of the animal to maintain neurogenesis, albeit at progressively reduced levels. The extent of neurogenesis, and the number of NSCs that continue to generate neurons, in the human adult brain, however, remains controversial. The protracted development and longer life-span in humans and other species may limit the processes of neurogenesis described here in the adult mouse brain to fetal and infant stages. Indeed, in children younger than one year of age, many migrating young neurons are present not only in the RMS, but also throughout the frontal lobe (Sanai et al., 2011; Paredes et al., 2016b), although the time of birth and origin of these young neurons remains unknown. In the adult human brain, rare young neurons are observed in the walls of the lateral ventricles (Sanai et al., 2011; Wang et al., 2011) but a clear RMS or evidence of migration into the OB is missing. Surprisingly, in the same adult human brains in which young neurons are present in the walls of the ventricles, cells with clear marker expression and morphology of young neurons are not present in the DG or are extremely rare (Sorrells et al., 2018; Cipriani et al., 2018; Knoth et al., 2010; Dennis et al., 2016), which suggests minimal levels of adult neurogenesis. However, other studies suggest that neurogenesis, even in the aged human DG, continues robustly (Spalding et al., 2013; Boldrini et al., 2018), whereas still other studies report a sharp decline and a small and negligible contribution in adults (Knoth et al., 2010; Dennis et al., 2016). The reasons that underlie these discrepancies are unclear. It is possible that, as brain size increases (as occurs in humans) NSCs which, as discussed above, become regionally specified early during embryonic development, could become spatially dissociated from sites of integration (Paredes et al., 2016a). In addition, the large size of the human brain with its very extensive regions of white matter may impose limits on postnatal NSC deposition and long-range neuronal migration. Overall, further studies are clearly needed to fully understand whether or not neurogenesis occurs in the adult human brain.
Conclusions
We are now beginning to build a detailed molecular description of the cell types in the V-SVZ, their behaviors and the cell-cell interactions that take place in this niche. However, a number of key questions remain open. For example, how do the different subpopulations that have been defined by transcriptomic analyses relate to the cell types that exist in vivo? High-resolution microscopic approaches combined with multiplex mRNA detection should allow a better identification of cell diversity in vivo. Advances in live imaging will also soon make it possible to observe the behavior of B1 cells in vivo, as has been recently achieved for RAs in the hippocampus (Pilz et al., 2018). B2 cells are particularly intriguing as they are derived from B1 cells (Obernier et al., 2018), and it will be interesting to understand their role in the generation of the neurogenic lineage. Here too, single cell transcriptomic analyses may provide new tools to lineage trace B2 cells. How NSCs integrate different signals to regulate adult neurogenesis also remains elusive. Another fascinating issue is whether NSCs have a ‘molecular memory’ of their own history (Fig. 4); self-renewing NSCs and their daughter NSCs may differ from each other depending on their age, number of previous divisions and duration of quiescence between divisions.
It is also becoming evident that intrinsic factors, apparently inherited from the developmental history of each stem cell, determine the regional heterogeneity of B1 cells. How this regional heterogeneity is generated and maintained remains a fascinating problem to resolve. Single cell RNA-sequencing and lineage tracing approaches could also help to understand regional differences among B1 cells. It will be equally important to determine what controls the switch from the generation of forebrain neurons from embryonic RG, to the generation of OB interneurons from postnatal B1 cells. The next few years will no doubt shed light on some of these areas, and will provide a more detailed understanding of the regulation of adult neurogenesis. This fascinating process, which was long thought to be impossible, is already revealing basic mechanisms of sustained neuronal birth and differentiation, and may one day provide a strategy to replace neurons for brain repair.
Acknowledgements
We thank Kenneth X. Probst for preparation of the illustrations and Stephanie Redmond, Arantxa Cebrian-Silla and Cristina Guinto from the Alvarez-Buylla laboratory for critical reading of the manuscript. Owing to limited space, we could not cover all of the exciting contributions to the field.
Footnotes
Competing interests
A.A.-B. is co-founder and on the Scientific Advisory Board of Neurona Therapeutics.
Funding
This work is funded by the National Institutes of Health (NS28478 and HD32116) and the John G. Bowes Research Fund. A.A.-B. is the Heather and Melanie Muss Endowed Chair of Neurological Surgery at the University of California, San Francisco. Deposited in PMC for release after 12 months.
References
- Aguirre A., Rubio M. E. and Gallo V. (2010). Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 467, 323-327. 10.1038/nature09347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso J., Le Magueresse C., Zuccotti A., Khodosevich K. and Monyer H. (2012). Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 10, 76-87. 10.1016/j.stem.2011.11.011 [DOI] [PubMed] [Google Scholar]
- Altman J. (1962). Are new neurons formed in the brains of adult mammals? Science 135, 1127-1128. 10.1126/science.135.3509.1127 [DOI] [PubMed] [Google Scholar]
- Altman J. and Das G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319-335. 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Theelen M. and Nottebohm F. (1990). Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron 5, 101-109. 10.1016/0896-6273(90)90038-H [DOI] [PubMed] [Google Scholar]
- Andersen J., Urbán N., Achimastou A., Ito A., Simic M., Ullom K., Martynoga B., Lebel M., Göritz C., Frisén J. et al. (2014). A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 83, 1085-1097. 10.1016/j.neuron.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu-Agulló C., Morante-Redolat J. M., Delgado A. C. and Fariñas I. (2009). Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat. Neurosci. 12, 1514-1523. 10.1038/nn.2437 [DOI] [PubMed] [Google Scholar]
- Anthony T. E., Klein C., Fishell G. and Heintz N. (2004). Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41, 881-890. 10.1016/S0896-6273(04)00140-0 [DOI] [PubMed] [Google Scholar]
- Artegiani B., Lyubimova A., Muraro M., Van Es J. H., Van Oudenaarden A. and Clevers H. (2017). A single-cell RNA sequencing study reveals cellular and molecular dynamics of the hippocampal neurogenic niche. Cell Rep. 21, 3271-3284. 10.1016/j.celrep.2017.11.050 [DOI] [PubMed] [Google Scholar]
- Baker S. A., Baker K. A. and Hagg T. (2004). Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur. J. Neurosci. 20, 575-579. 10.1111/j.1460-9568.2004.03486.x [DOI] [PubMed] [Google Scholar]
- Basak O., Giachino C., Fiorini E., Macdonald H. R. and Taylor V. (2012). Neurogenic subventricular zone stem/progenitor cells are Notch1-dependent in their active but not quiescent state. J. Neurosci. 32, 5654-5666. 10.1523/JNEUROSCI.0455-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak O., Krieger T. G., Muraro M. J., Wiebrands K., Stange D. E., Frias-Aldeguer J., Rivron N. C., Van De Wetering M., Van Es J. H., Van Oudenaarden A. et al. (2018). Troy+ brain stem cells cycle through quiescence and regulate their number by sensing niche occupancy. Proc. Natl. Acad. Sci. USA 115, E610-E619. 10.1073/pnas.1715911114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista-Brito R., Close J., Machold R. and Fishell G. (2008). The distinct temporal origins of olfactory bulb interneuron subtypes. J. Neurosci. 28, 3966-3975. 10.1523/JNEUROSCI.5625-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S. and Patterson P. H. (2006). Leukemia inhibitory factor promotes neural stem cell self-renewal in the adult brain. J. Neurosci. 26, 12089-12099. 10.1523/JNEUROSCI.3047-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer S. A. (1983). 3H-thymidine-radiographic studies of neurogenesis in the rat olfactory bulb. Exp. Brain Res. 50, 329-340. 10.1007/BF00239197 [DOI] [PubMed] [Google Scholar]
- Beckervordersandforth R., Tripathi P., Ninkovic J., Bayam E., Lepier A., Stempfhuber B., Kirchhoff F., Hirrlinger J., Haslinger A., Lie D. C. et al. (2010). In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell 7, 744-758. 10.1016/j.stem.2010.11.017 [DOI] [PubMed] [Google Scholar]
- Berg D. A., Belnoue L., Song H. and Simon A. (2013). Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain. Development 140, 2548-2561. 10.1242/dev.088005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. A., Bond A. M., Ming G.- and Song H. (2018). Radial glial cells in the adult dentate gyrus: what are they and where do they come from? F1000Research 7, 277 10.12688/f1000research.12684.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernitz J. M. Kim H. S., Macarthur B., Sieburg H. and Moore K. (2016). Hematopoietic stem cells count and remember self-renewal divisions. Cell 167, 1296-1309.e10. 10.1016/j.cell.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M., Fulmore C. A., Tartt A. N., Simeon L. R., Pavlova I., Poposka V., Rosoklija G. B., Stankov A., Arango V., Dwork A. J. et al. (2018). Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589-599.e5. 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond A. M., Ming G.-L. and Song H. (2015). Adult mammalian neural stem cells and neurogenesis: five decades later. Cell Stem Cell 17, 385-395. 10.1016/j.stem.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovetti S., Hsieh Y.-C., Bovolin P., Perroteau I., Kazunori T. and Puche A. C. (2007). Blood vessels form a scaffold for neuroblast migration in the adult olfactory bulb. J. Neurosci. 27, 5976-5980. 10.1523/JNEUROSCI.0678-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezun J. M. and Daszuta A. (1999). Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience 89, 999-1002. 10.1016/S0306-4522(98)00693-9 [DOI] [PubMed] [Google Scholar]
- Burd G. D. and Nottebohm F. (1985). Ultrastructural characterization of synaptic terminals formed on newly generated neurons in a song control nucleus of the adult canary forebrain. J. Comp. Neurol. 240, 143-152. 10.1002/cne.902400204 [DOI] [PubMed] [Google Scholar]
- Calzolari F., Michel J., Baumgart E. V., Theis F., Götz M. and Ninkovic J. (2015). Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 18, 490-492. 10.1038/nn.3963 [DOI] [PubMed] [Google Scholar]
- Cameron H. A., Mcewen B. S. and Gould E. (1995). Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 15, 4687-4692. 10.1523/JNEUROSCI.15-06-04687.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela A. and Temple S. (2002). LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35, 865-875. 10.1016/S0896-6273(02)00835-8 [DOI] [PubMed] [Google Scholar]
- Capilla-Gonzalez V., Cebrian-Silla A., Guerrero-Cazares H., Garcia-Verdugo J. M. and Quiñones-Hinojosa A. (2014). Age-related changes in astrocytic and ependymal cells of the subventricular zone. Glia 62, 790-803. 10.1002/glia.22642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper K. B. and Mccarthy K. D. (2006). GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Mol. Cell. Neurosci. 31, 676-684. 10.1016/j.mcn.2005.12.006 [DOI] [PubMed] [Google Scholar]
- Cebrián-Silla A., Alfaro-Cervelló C., Herranz-Pérez V., Kaneko N., Park D. H., Sawamoto K., Alvarez-Buylla A., Lim D. A. and García-Verdugo J. M. (2017). Unique organization of the nuclear envelope in the post-natal quiescent neural stem cells. Stem Cell Rep. 9, 203-216. 10.1016/j.stemcr.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G. A., Petreanu L. T., Alvarez-Buylla A. and Magnasco M. O. (2001). Unsupervised learning and adaptation in a model of adult neurogenesis. J. Comput. Neurosci. 11, 175-182. 10.1023/A:1012849801892 [DOI] [PubMed] [Google Scholar]
- Chaker Z., Codega P. and Doetsch F. (2016). A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip. Rev. Dev. Biol. 5, 640-658. 10.1002/wdev.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Adorjan I., Mundim M. V., Sun B., Dizon M. L. and Szele F. G. (2016). Traumatic brain injury activation of the adult subventricular zone neurogenic niche. Frontier. Neurosci. 10, 332 10.3389/fnins.2016.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson B. J., Tropepe V., Morshead C. M. and Van Der Kooy D. (1999). Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J. Neurosci. 19, 4462-4471. 10.1523/JNEUROSCI.19-11-04462.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani S., Ferrer I., Aronica E., Kovacs G. G., Verney C., Nardelli J., Khung S., Delezoide A.-L., Milenkovic I., Rasika S. et al. (2018). Hippocampal radial Glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer's disease adults. Cereb. Cortex 28, 2458-2478. 10.1093/cercor/bhy096 [DOI] [PubMed] [Google Scholar]
- Codega P., Silva-Vargas V., Paul A., Maldonado-Soto A. R., Deleo A. M., Pastrana E. and Doetsch F. (2014). Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 82, 545-559. 10.1016/j.neuron.2014.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit K. C., Aanstad P., Singla V., Norman A. R., Stainier D. Y. R. and Reiter J. F. (2005). Vertebrate smoothened functions at the primary cilium. Nature 437, 1018-1021. 10.1038/nature04117 [DOI] [PubMed] [Google Scholar]
- De Chevigny A., Coré N., Follert P., Gaudin M., Barbry P., Béclin C. and Cremer H. (2012). miR-7a regulation of Pax6 controls spatial origin of forebrain dopaminergic neurons. Nat. Neurosci. 15, 1120-1126. 10.1038/nn.3142 [DOI] [PubMed] [Google Scholar]
- De Marchis S., Bovetti S., Carletti B., Hsieh Y.-C., Garzotto D., Peretto P., Fasolo A., Puche A. C. and Rossi F. (2007). Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J. Neurosci. 27, 657-664. 10.1523/JNEUROSCI.2870-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R. N. and Lim D. A. (2015). Embryonic Nkx2.1-expressing neural precursor cells contribute to the regional heterogeneity of adult V-SVZ neural stem cells. Dev. Biol. 407, 265-274. 10.1016/j.ydbio.2015.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C. V., Suh L. S., Rodriguez M. L., Kril J. J. and Sutherland G. T. (2016). Human adult neurogenesis across the ages: an immunohistochemical study. Neuropathol. Appl. Neurobiol. 42, 621-638. 10.1111/nan.12337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F. and Alvarez-Buylla A. (1996). Network of tangential pathways for neuronal migration in adult mammalian brain. Proc. Natl. Acad. Sci. USA 93, 14895-14900. 10.1073/pnas.93.25.14895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., García-Verdugo J. M. and Alvarez-Buylla A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046-5061. 10.1523/JNEUROSCI.17-13-05046.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F., Caillé I., Lim D. A., García-Verdugo J. M. and Alvarez-Buylla A. (1999). Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97, 703-716. 10.1016/S0092-8674(00)80783-7 [DOI] [PubMed] [Google Scholar]
- Douet V., Arikawa-Hirasawa E. and Mercier F. (2012). Fractone-heparan sulfates mediate BMP-7 inhibition of cell proliferation in the adult subventricular zone. Neurosci. Lett. 528, 120-125. 10.1016/j.neulet.2012.08.077 [DOI] [PubMed] [Google Scholar]
- Douet V., Kerever A., Arikawa-Hirasawa E. and Mercier F. (2013). Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone. Cell Prolif. 46, 137-145. 10.1111/cpr.12023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulken B. W., Leeman D. S., Boutet S. C., Hebestreit K. and Brunet A. (2017). Single-cell transcriptomic analysis defines heterogeneity and transcriptional dynamics in the adult neural stem cell lineage. Cell Rep. 18, 777-790. 10.1016/j.celrep.2016.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru I., Neitz A., Alfonso J. and Monyer H. (2017). Diazepam binding inhibitor promotes stem cell expansion controlling environment-dependent neurogenesis. Neuron 94, 125-137.e5. 10.1016/j.neuron.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Eckenhoff M. F. and Rakic P. (1984). Radial organization of the hippocampal dentate gyrus: a Golgi, ultrastructural, and immunocytochemical analysis in the developing rhesus monkey. J. Comp. Neurol. 223, 1-21. 10.1002/cne.902230102 [DOI] [PubMed] [Google Scholar]
- Encinas J. M., Michurina T. V., Peunova N., Park J.-H., Tordo J., Peterson D. A., Fishell G., Koulakov A. and Enikolopov G. (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566-579. 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enwere E., Shingo T., Gregg C., Fujikawa H., Ohta S. and Weiss S. (2004). Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354-8365. 10.1523/JNEUROSCI.2751-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando R. N., Eleuteri B., Abdelhady S., Nussenzweig A., Andang M. and Ernfors P. (2011). Cell cycle restriction by histone H2AX limits proliferation of adult neural stem cells. Proc. Natl. Acad. Sci. USA 108, 5837-5842. 10.1073/pnas.1014993108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov V., Kronenberg G., Pivneva T., Reuter K., Steiner B., Wang L.-P., Yamaguchi M., Kettenmann H. and Kempermann G. (2003). Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol. Cell. Neurosci. 23, 373-382. 10.1016/S1044-7431(03)00060-5 [DOI] [PubMed] [Google Scholar]
- Fogarty M., Richardson W. D. and Kessaris N. (2005). A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development 132, 1951-1959. 10.1242/dev.01777 [DOI] [PubMed] [Google Scholar]
- Fréchette M., Rennie K. and Pappas B. A. (2009). Developmental forebrain cholinergic lesion and environmental enrichment: behaviour, CA1 cytoarchitecture and neurogenesis. Brain Res. 1252, 172-182. 10.1016/j.brainres.2008.11.082 [DOI] [PubMed] [Google Scholar]
- Fuentealba L. C., Rompani S. B., Parraguez J. I., Obernier K., Romero R., Cepko C. L. and Alvarez-Buylla A. (2015). Embryonic origin of postnatal neural stem cells. Cell 161, 1644-1655. 10.1016/j.cell.2015.05.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutachi S., Miya H., Watanabe T., Kawai H., Yamasaki N., Harada Y., Imayoshi I., Nelson M., Nakayama K. I., Hirabayashi Y. et al. (2015). Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 18, 657-665. 10.1038/nn.3989 [DOI] [PubMed] [Google Scholar]
- Gage F. H., Coates P. W., Palmer T. D., Kuhn H. G., Fisher L. J., Suhonen J. O., Peterson D. A., Suhr S. T. and Ray J. (1995). Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. USA 92, 11879-11883. 10.1073/pnas.92.25.11879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliano E., Franzoni E., Breton M., Chand A. N., Byrne D. J., Murthy V. N. and Grubb M. S. (2018). Embryonic and postnatal neurogenesis produce functionally distinct subclasses of dopaminergic neuron. eLife 7, 491 10.7554/eLife.32373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia A. D. R., Doan N. B., Imura T., Bush T. G. and Sofroniew M. V. (2004). GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 7, 1233-1241. 10.1038/nn1340 [DOI] [PubMed] [Google Scholar]
- Ge S., Pradhan D. A., Ming G.- and Song H. (2007). GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 30, 1-8. 10.1016/j.tins.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Gebara E., Bonaguidi M. A., Beckervordersandforth R., Sultan S., Udry F., Gijs P.-J., Lie D. C., Ming G.-L., Song H. and Toni N. (2016). Heterogeneity of radial glia-like cells in the adult hippocampus. Stem Cells 34, 997-1010. 10.1002/stem.2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goings G. E., Kozlowski D. A. and Szele F. G. (2006). Differential activation of microglia in neurogenic versus non-neurogenic regions of the forebrain. Glia 54, 329-342. 10.1002/glia.20381 [DOI] [PubMed] [Google Scholar]
- Gómez-Gaviro M. V., Scott C. E., Sesay A. K., Matheu A., Booth S., Galichet C. and Lovell-Badge R. (2012). Betacellulin promotes cell proliferation in the neural stem cell niche and stimulates neurogenesis. Proc. Natl. Acad. Sci. USA 109, 1317-1322. 10.1073/pnas.1016199109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves J. T., Schafer S. T. and Gage F. H. (2016). Adult neurogenesis in the hippocampus: from stem cells to behavior. Cell 167, 897-914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- Götz M., Stoykova A. and Gruss P. (1998). Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 21, 1031-1044. 10.1016/S0896-6273(00)80621-2 [DOI] [PubMed] [Google Scholar]
- Grelat A., Benoit L., Wagner S., Moigneu C., Lledo P.-M. and Alonso M. (2018). Adult-born neurons boost odor-reward association. Proc. Natl. Acad. Sci. USA 115, 2514-2519. 10.1073/pnas.1716400115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D. V., Lui J. H., Parker P. R. L. and Kriegstein A. R. (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554-561. 10.1038/nature08845 [DOI] [PubMed] [Google Scholar]
- Haubensak W., Attardo A., Denk W. and Huttner W. B. (2004). Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc. Natl. Acad. Sci. USA 101, 3196-3201. 10.1073/pnas.0308600100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds J. W. (1968). Autoradiographic study of histogenesis in the mouse olfactory bulb. II. Cell proliferation and migration. J. Comp. Neurol. 134, 305-321. 10.1002/cne.901340305 [DOI] [PubMed] [Google Scholar]
- Höglinger G. U., Rizk P., Muriel M. P., Duyckaerts C., Oertel W. H., Caille I. and Hirsch E. C. (2004). Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 7, 726 10.1038/nn1265 [DOI] [PubMed] [Google Scholar]
- Hormoz S. (2013). Stem cell population asymmetry can reduce rate of replicative aging. J. Theor. Biol. 331, 19-27. 10.1016/j.jtbi.2013.04.018 [DOI] [PubMed] [Google Scholar]
- Hu X.-L., Chen G., Zhang S., Zheng J., Wu J., Bai Q.-R., Wang Y., Li J., Wang H., Feng H. et al. (2017). Persistent expression of VCAM1 in radial glial cells is required for the embryonic origin of postnatal neural stem cells. Neuron 95, 309-325.e6. 10.1016/j.neuron.2017.06.047 [DOI] [PubMed] [Google Scholar]
- Ihrie R. A., Shah J. K., Harwell C. C., Levine J. H., Guinto C. D., Lezameta M., Kriegstein A. R. and Alvarez-Buylla A. (2011). Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron 71, 250-262. 10.1016/j.neuron.2011.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Ohtsuka T., Takao K., Miyakawa T., Yamaguchi M., Mori K., Ikeda T., Itohara S. and Kageyama R. (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153-1161. 10.1038/nn.2185 [DOI] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Yamaguchi M., Mori K. and Kageyama R. (2010). Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 30, 3489-3498. 10.1523/JNEUROSCI.4987-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S., Toni N., Clemenson G. D. Jr, Ray J. and Gage F. H. (2008). Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat. Neurosci. 11, 888-893. 10.1038/nn.2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri D. J., Mackay E. W., Hamlin A. S., Marathe S. V., Nandam L. S., Vaidya V. A. and Bartlett P. F. (2010). Norepinephrine directly activates adult hippocampal precursors via beta3-adrenergic receptors. J. Neurosci. 30, 2795-2806. 10.1523/JNEUROSCI.3780-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhaveri D. J., Nanavaty I., Prosper B. W., Marathe S., Husain B. F., Kernie S. G., Bartlett P. F. and Vaidya V. A. (2014). Opposing effects of α2- and β-adrenergic receptor stimulation on quiescent neural precursor cell activity and adult hippocampal neurogenesis. PLoS ONE 9, e98736 10.1371/journal.pone.0098736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C. B., Momma S., Clarke D. L., Risling M., Lendahl U. and Frisén J. (1999). Identification of a neural stem cell in the adult mammalian central nervous system. Cell 96, 25-34. 10.1016/S0092-8674(00)80956-3 [DOI] [PubMed] [Google Scholar]
- Jones K. S. and Connor B. (2012). Intrinsic regulation of adult subventricular zone neural progenitor cells and the effect of brain injury. Am. J. Stem Cells 1, 48-58. [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L., Litterman N. K., Schein P. A., Miller C. M., Loffredo F. S., Wojtkiewicz G. R., Chen J. W., Lee R. T., Wagers A. J. and Rubin L. L. (2014). Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 344, 630-634. 10.1126/science.1251141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi D., Furutachi S., Kawai H., Hozumi K. and Gotoh Y. (2013). Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat. Commun. 4, 1880 10.1038/ncomms2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H., Kawaguchi D., Kuebrich B. D., Kitamoto T., Yamaguchi M., Gotoh Y. and Furutachi S. (2017). Area-specific regulation of quiescent neural stem cells by Notch3 in the adult mouse subependymal zone. J. Neurosci. 37, 11867-11880. 10.1523/JNEUROSCI.0001-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsch W., Mosley C. P., Lin C. W. and Lois C. (2007). Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 5, e300 10.1371/journal.pbio.0050300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Jessberger S., Steiner B. and Kronenberg G. (2004). Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447-452. 10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- Kerever A., Schnack J., Vellinga D., Ichikawa N., Moon C., Arikawa-Hirasawa E., Efird J. T. and Mercier F. (2007). Novel extracellular matrix structures in the neural stem cell niche capture the neurogenic factor fibroblast growth factor 2 from the extracellular milieu. Stem Cells 25, 2146-2157. 10.1634/stemcells.2007-0082 [DOI] [PubMed] [Google Scholar]
- Kessaris N., Fogarty M., Iannarelli P., Grist M., Wegner M. and Richardson W. D. (2006). Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat. Neurosci. 9, 173-179. 10.1038/nn1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri P., Obernier K., Simeonova I. K., Hellwig A., Hölzl-Wenig G., Mandl C., Scholl C., Wölfl S., Winkler J., Gaspar J. A. et al. (2014). Proliferation and cilia dynamics in neural stem cells prospectively isolated from the SEZ. Sci. Rep. 4, 3803 10.1038/srep03803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J. E. and White J. G. (1981). On the control of germ cell development in Caenorhabditis elegans. Dev. Biol. 81, 208-219. 10.1016/0012-1606(81)90284-0 [DOI] [PubMed] [Google Scholar]
- Kippin T. E., Kapur S. and Van Der Kooy D. (2005). Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J. Neurosci. 25, 5815-5823. 10.1523/JNEUROSCI.1120-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch M., Braun S. M. G., Zurkirchen L., Von Schoultz C., Zamboni N., Araúzo-Bravo M. J., Kovacs W. J., Karalay Ö., Suter U., Machado R. A. C. et al. (2013). Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 493, 226-230. 10.1038/nature11689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth R., Singec I., Ditter M., Pantazis G., Capetian P., Meyer R. P., Horvat V., Volk B. and Kempermann G. (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5, e8809 10.1371/journal.pone.0008809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M., Osumi N., Rubenstein J. L. and Alvarez-Buylla A. (2005). Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J. Neurosci. 25, 6997-7003. 10.1523/JNEUROSCI.1435-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohwi M., Petryniak M. A., Long J. E., Ekker M., Obata K., Yanagawa Y., Rubenstein J. L. R. and Alvarez-Buylla A. (2007). A subpopulation of olfactory bulb GABAergic interneurons is derived from Emx1- and Dlx5/6-expressing progenitors. J. Neurosci. 27, 6878-6891. 10.1523/JNEUROSCI.0254-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E., Goderie S., Wang Y., Lotz S., Lin G., Sun Y., Roysam B., Shen Q. and Temple S. (2010). Adult SVZ lineage cells home to and leave the vascular niche via differential responses to SDF1/CXCR4 signaling. Cell Stem Cell 7, 163-173. 10.1016/j.stem.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokovay E., Wang Y., Kusek G., Wurster R., Lederman P., Lowry N., Shen Q. and Temple S. (2012). VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell 11, 220-230. 10.1016/j.stem.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Kondabolu S., Adsumelli R., Schabel J., Glass P. and Pentyala S. (2011). Evaluation of prostaglandin D2 as a CSF leak marker: implications in safe epidural anesthesia. Local Reg. Anesth. 4, 21-24. 10.2147/LRA.S18053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R. and Ilagan M. X. G. (2009). The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216-233. 10.1016/j.cell.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A. and Alvarez-Buylla A. (2009). The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149-184. 10.1146/annurev.neuro.051508.135600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni V. A., Jha S. and Vaidya V. A. (2002). Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur. J. Neurosci. 16, 2008-2012. 10.1046/j.1460-9568.2002.02268.x [DOI] [PubMed] [Google Scholar]
- Lacar B., Young S. Z., Platel J.-C. and Bordey A. (2011). Gap junction-mediated calcium waves define communication networks among murine postnatal neural progenitor cells. Eur. J. Neurosci. 34, 1895-1905. 10.1111/j.1460-9568.2011.07901.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman D. S., Hebestreit K., Ruetz T., Webb A. E., Mckay A., Pollina E. A., Dulken B. W., Zhao X., Yeo R. W., Ho T. T. et al. (2018). Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science 359, 1277-1283. 10.1126/science.aag3048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen M. K., Zappaterra M. W., Chen X., Yang Y. J., Hill A. D., Lun M., Maynard T., Gonzalez D., Kim S., Ye P. et al. (2011). The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69, 893-905. 10.1016/j.neuron.2011.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennington J. B., Pope S., Goodheart A. E., Drozdowicz L., Daniels S. B., Salamone J. D. and Conover J. C. (2011). Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J. Neurosci. 31, 13078-13087. 10.1523/JNEUROSCI.1197-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Takeda N., Jain R., Manderfield L. J., Liu F., Li L., Anderson S. A. and Epstein J. A. (2015). Hopx distinguishes hippocampal from lateral ventricle neural stem cells. Stem Cell Res. 15, 522-529. 10.1016/j.scr.2015.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W. L., Chu M. W., Wu A., Suzuki Y., Imayoshi I. and Komiyama T. (2018). Adult-born neurons facilitate olfactory bulb pattern separation during task engagement. eLife 7, e33006 10.7554/eLife.33006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D. A. and Alvarez-Buylla A. (2016). The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harbor Perspect. Biol. 8, a018820 10.1101/cshperspect.a018820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D. A., Tramontin A. D., Trevejo J. M., Herrera D. G., García-Verdugo J. M. and Alvarez-Buylla A. (2000). Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28, 713-726. 10.1016/S0896-6273(00)00148-3 [DOI] [PubMed] [Google Scholar]
- Liu X., Wang Q., Haydar T. F. and, Bordey A. (2005). Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 8, 1179-1187. 10.1038/nn1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledo P.-M. and Saghatelyan A. (2005). Integrating new neurons into the adult olfactory bulb: joining the network, life–death decisions, and the effects of sensory experience. Trends Neurosci. 28, 248-254. 10.1016/j.tins.2005.03.005 [DOI] [PubMed] [Google Scholar]
- Llorens-Bobadilla E., Zhao S., Baser A., Saiz-Castro G., Zwadlo K. and Martin-Villalba A. (2015). Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell 17, 329-340. 10.1016/j.stem.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Lois C. and Alvarez-Buylla A. (1994). Long-distance neuronal migration in the adult mammalian brain. Science 264, 1145-1148. 10.1126/science.8178174 [DOI] [PubMed] [Google Scholar]
- Lois C., García-Verdugo J.-M. and Alvarez-Buylla A. (1996). Chain migration of neuronal precursors. Science 271, 978-981. 10.1126/science.271.5251.978 [DOI] [PubMed] [Google Scholar]
- Lugert S., Vogt M., Tchorz J. S., Müller M., Giachino C. and Taylor V. (2012). Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1(high) intermediate progenitors. Nat. Commun. 3, 670 10.1038/ncomms1670 [DOI] [PubMed] [Google Scholar]
- Luskin M. B. (1993). Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11, 173-189. 10.1016/0896-6273(93)90281-U [DOI] [PubMed] [Google Scholar]
- Menn B., Garcia-Verdugo J. M., Yaschine C., Gonzalez-Perez O., Rowitch D. and Alvarez-Buylla A. (2006). Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26, 7907-7918. 10.1523/JNEUROSCI.1299-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F. and Douet V. (2014). Bone morphogenetic protein-4 inhibits adult neurogenesis and is regulated by fractone-associated heparan sulfates in the subventricular zone. J. Chem. Neuroanat. 57-58, 54-61. 10.1016/j.jchemneu.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Mercier F., Kitasako J. T. and Hatton G. I. (2002). Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J. Comp. Neurol. 451, 170-188. 10.1002/cne.10342 [DOI] [PubMed] [Google Scholar]
- Merkle F. T., Tramontin A. D., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2004). Radial glia give rise to adult neural stem cells in the subventricular zone. Proc. Natl. Acad. Sci. USA 101, 17528-17532. 10.1073/pnas.0407893101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F. T., Mirzadeh Z. and Alvarez-Buylla A. (2007). Mosaic organization of neural stem cells in the adult brain. Science 317, 381-384. 10.1126/science.1144914 [DOI] [PubMed] [Google Scholar]
- Merkle F. T., Fuentealba L. C., Sanders T. A., Magno L., Kessaris N. and Alvarez-Buylla A. (2014). Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 17, 207-214. 10.1038/nn.3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G.-L. and Song H. (2011). Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687-702. 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C. and Gould E. (2006). Stress and adult neurogenesis. Hippocampus 16, 233-238. 10.1002/hipo.20155 [DOI] [PubMed] [Google Scholar]
- Mirzadeh Z., Merkle F. T., Soriano-Navarro M., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2008). Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265-278. 10.1016/j.stem.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Kawaguchi A., Okano H. and Ogawa M. (2001). Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron 31, 727-741. 10.1016/S0896-6273(01)00420-2 [DOI] [PubMed] [Google Scholar]
- Mora N., Oliva C., Fiers M., Ejsmont R., Soldano A., Zhang T.-T., Yan J., Claeys A., De Geest N. and Hassan B. A. (2018). A temporal transcriptional switch governs stem cell division, neuronal numbers, and maintenance of differentiation. Dev. Cell 45, 53-66.e5. 10.1016/j.devcel.2018.02.023 [DOI] [PubMed] [Google Scholar]
- Morshead C. M., Reynolds B. A., Craig C. G., Mcburney M. W., Staines W. A., Morassutti D., Weiss S. and Van Der Kooy D. (1994). Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron 13, 1071-1082. 10.1016/0896-6273(94)90046-9 [DOI] [PubMed] [Google Scholar]
- Mosher K. I., Andres R. H., Fukuhara T., Bieri G., Hasegawa-Moriyama M., He Y., Guzman R. and Wyss-Coray T. (2012). Neural progenitor cells regulate microglia functions and activity. Nat. Neurosci. 15, 1485-1487. 10.1038/nn.3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Gebara E., Bushong E. A., Sánchez-Pascual I., O'Laoi R., El M'Ghari I., Kocher-Braissant J., Ellisman M. H. and Toni N. (2016). Fine processes of Nestin-GFP-positive radial glia-like stem cells in the adult dentate gyrus ensheathe local synapses and vasculature. Proc. Natl. Acad. Sci. USA 113, E2536-E2545. 10.1073/pnas.1514652113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murao N., Noguchi H. and Nakashima K. (2016). Epigenetic regulation of neural stem cell property from embryo to adult. Neuroepigenetics 5, 1-10. 10.1016/j.nepig.2016.01.001 [DOI] [Google Scholar]
- Nait-Oumesmar B., Decker L., Lachapelle F., Avellana-Adalid V., Bachelin C. and Van Evercooren A. B. (1999). Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 11, 4357-4366. 10.1046/j.1460-9568.1999.00873.x [DOI] [PubMed] [Google Scholar]
- Nascimento M. A., Sorokin L. and Coelho-Sampaio T. (2018). Fractone bulbs derive from ependymal cells and their laminin composition influence the stem cell niche in the subventricular zone. J. Neurosci. 38, 3880-3889. 10.1523/JNEUROSCI.3064-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J. and Hannan A. J. (2006). Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat. Rev. Neurosci. 7, 697-709. 10.1038/nrn1970 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Flint A. C., Weissman T. A., Dammerman R. S. and Kriegstein A. R. (2001). Neurons derived from radial glial cells establish radial units in neocortex. Nature 409, 714-720. 10.1038/35055553 [DOI] [PubMed] [Google Scholar]
- Noctor S. C., Martínez-Cerdeño V., Ivic L. and Kriegstein A. R. (2004). Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 7, 136-144. 10.1038/nn1172 [DOI] [PubMed] [Google Scholar]
- Obernier K., Cebrian-Silla A., Thomson M., Parraguez J. I., Anderson R., Guinto C., Rodas Rodriguez J., Garcia-Verdugo J.-M. and Alvarez-Buylla A. (2018). Adult neurogenesis is sustained by symmetric self-renewal and differentiation. Cell Stem Cell 22, 221-234.e8. 10.1016/j.stem.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi S., Zalucki O., Premarathne S., Wood S. A. and Piper M. (2016). USP9X deletion elevates the density of oligodendrocytes within the postnatal dentate gyrus. Neurogenesis 3, e1235524 10.1080/23262133.2016.1235524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottone C., Krusche B., Whitby A., Clements M., Quadrato G., Pitulescu M. E., Adams R. H. and Parrinello S. (2014). Direct cell-cell contact with the vascular niche maintains quiescent neural stem cells. Nat. Cell Biol. 16, 1045-1056. 10.1038/ncb3045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paez-Gonzalez P., Asrican B., Rodriguez E. and Kuo C. T. (2014). Identification of distinct ChAT+ neurons and activity-dependent control of postnatal SVZ neurogenesis. Nat. Neurosci. 17, 934-942. 10.1038/nn.3734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer T. D., Willhoite A. R. and Gage F. H. (2000). Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 425, 479-494. [DOI] [PubMed] [Google Scholar]
- Paredes M. F., Sorrells S. F., Garcia-Verdugo J. M. and Alvarez-Buylla A. (2016a). Brain size and limits to adult neurogenesis. J. Comp. Neurol. 524, 646-664. 10.1002/cne.23896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes M. F., James D., Gil-Perotin S., Kim H., Cotter J. A., Ng C., Sandoval K., Rowitch D. H., Xu D., Mcquillen P. S. et al. (2016b). Extensive migration of young neurons into the infant human frontal lobe. Science 354, aaf7073 10.1126/science.aaf7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K. and Sun D. (2016). Strategies targeting endogenous neurogenic cell response to improve recovery following traumatic brain injury. Brain Res. 1640, 104-113. 10.1016/j.brainres.2016.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton J. A. and Nottebohm F. N. (1984). Neurons generated in the adult brain are recruited into functional circuits. Science 225, 1046-1048. 10.1126/science.6474166 [DOI] [PubMed] [Google Scholar]
- Paul A., Chaker Z. and Doetsch F. (2017). Hypothalamic regulation of regionally distinct adult neural stem cells and neurogenesis. Science 356, 1383-1386. 10.1126/science.aal3839 [DOI] [PubMed] [Google Scholar]
- Petreanu L. and Alvarez-Buylla A. (2002). Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J. Neurosci. 22, 6106-6113. 10.1523/JNEUROSCI.22-14-06106.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik D., Myoga M. H., Grade S., Gerkau N. J., Pusch M., Rose C. R., Grothe B. and Götz M. (2018). Epithelial sodium channel regulates adult neural stem cell proliferation in a flow-dependent manner. Cell Stem Cell 22, 865-878.e8. 10.1016/j.stem.2018.04.016 [DOI] [PubMed] [Google Scholar]
- Piatti V. C., Davies-Sala M. G., Esposito M. S., Mongiat L. A., Trinchero M. F. and Schinder A. F. (2011). The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. J. Neurosci. 31, 7715-7728. 10.1523/JNEUROSCI.1380-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz G.-A., Bottes S., Betizeau M., Jörg D. J., Carta S., Simons B. D., Helmchen F. and Jessberger S. (2018). Live imaging of neurogenesis in the adult mouse hippocampus. Science 359, 658-662. 10.1126/science.aao5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollen A. A., Nowakowski T. J., Chen J., Retallack H., Sandoval-Espinosa C., Nicholas C. R., Shuga J., Liu S. J., Oldham M. C., Diaz A. et al. (2015). Molecular identity of human outer radial glia during cortical development. Cell 163, 55-67. 10.1016/j.cell.2015.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G., Obernier K., Guinto C., Jose L., Bonfanti L. and Alvarez-Buylla A. (2013). Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc. Natl. Acad. Sci. USA 110, E1045-E1054. 10.1073/pnas.1219563110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A. and Weiss S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255, 1707-1710. 10.1126/science.1553558 [DOI] [PubMed] [Google Scholar]
- Ribeiro Xavier A. L., Kress B. T., Goldman S. A., Lacerda De Menezes J. R. and Nedergaard M. (2015). A Distinct population of microglia supports adult neurogenesis in the subventricular zone. J. Neurosci. 35, 11848-11861. 10.1523/JNEUROSCI.1217-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards L. J., Kilpatrick T. J. and Bartlett P. F. (1992). De novo generation of neuronal cells from the adult mouse brain. Proc. Natl Acad. Sci. USA 89, 8591-8595. 10.1073/pnas.89.18.8591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R., Milenkovic L. and Scott M. P. (2007). Patched1 regulates hedgehog signaling at the primary cilium. Science 317, 372-376. 10.1126/science.1139740 [DOI] [PubMed] [Google Scholar]
- Rompolas P., Mesa K. R., Kawaguchi K., Park S., Gonzalez D., Brown S., Boucher J., Klein A. M. and Greco V. (2016). Spatiotemporal coordination of stem cell commitment during epidermal homeostasis. Science 352, 1471-1474. 10.1126/science.aaf7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanai N., Nguyen T., Ihrie R. A., Mirzadeh Z., Tsai H.-H., Wong M., Gupta N., Berger M. S., Huang E., Garcia-Verdugo J.-M. et al. (2011). Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382-386. 10.1038/nature10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Malchinkhuu E., Horiuchi Y., Mogi C., Tomura H., Tosaka M., Yoshimoto Y., Kuwabara A. and Okajima F. (2007). HDL-like lipoproteins in cerebrospinal fluid affect neural cell activity through lipoprotein-associated sphingosine 1-phosphate. Biochem. Biophys. Res. Commun. 359, 649-654. 10.1016/j.bbrc.2007.05.131 [DOI] [PubMed] [Google Scholar]
- Sawamoto K., Wichterle H., Gonzalez-Perez O., Cholfin J. A., Yamada M., Spassky N., Murcia N. S., Garcia-Verdugo J. M., Marin O. and Rubenstein J. L. (2006). New neurons follow the flow of cerebrospinal fluid in the adult brain. Science 311, 629-632. 10.1126/science.1119133 [DOI] [PubMed] [Google Scholar]
- Seri B., García-Verdugo J. M., McEwen B. S. and Alvarez-Buylla A. (2001). Astrocytes give rise to new neurons in the adult mammalian hippocampus. J. Neurosci. 21, 7153-7160. 10.1523/JNEUROSCI.21-18-07153.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B., García-Verdugo J. M., Collado-Morente L., McEwen B. S. and Alvarez-Buylla A. (2004). Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 478, 359-378. 10.1002/cne.20288 [DOI] [PubMed] [Google Scholar]
- Shah P. T., Stratton J. A., Stykel M. G., Abbasi S., Sharma S., Mayr K. A., Koblinger K., Whelan P. J. and Biernaskie J. (2018). Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell 173, 1045-1057.e9. 10.1016/j.cell.2018.03.063 [DOI] [PubMed] [Google Scholar]
- Shahriyari L. and Komarova N. L. (2013). Symmetric vs. asymmetric stem cell divisions: an adaptation against cancer? PLoS ONE 8, e76195 10.1371/journal.pone.0076195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q., Wang Y., Kokovay E., Lin G., Chuang S.-M., Goderie S. K., Roysam B. and Temple S. (2008). Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3, 289-300. 10.1016/j.stem.2008.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y., Hoshikawa K., Goldman J. E., Sekino Y. and Sato K. (2014). Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 34, 2231-2243. 10.1523/JNEUROSCI.1619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J., Berg D. A., Zhu Y., Shin J. Y., Song J., Bonaguidi M. A., Enikolopov G., Nauen D. W., Christian K. M., Ming G.-L. et al. (2015). Single-cell RNA-seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell 17, 360-372. 10.1016/j.stem.2015.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T., Gregg C., Enwere E., Fujikawa H., Hassam R., Geary C., Cross J. C. and Weiss S. (2003). Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117-120. 10.1126/science.1076647 [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V., Maldonado-Soto A. R., Mizrak D., Codega P. and Doetsch F. (2016). Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell 19, 643-652. 10.1016/j.stem.2016.06.013 [DOI] [PubMed] [Google Scholar]
- Snapyan M., Lemasson M., Brill M. S., Blais M., Massouh M., Ninkovic J., Gravel C., Berthod F., Gotz M., Barker P. A. et al. (2009). Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 29, 4172-4188. 10.1523/JNEUROSCI.4956-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippert H. J., van Der Flier L. G., Sato T., Van Es J. H., Van Den Born M., Kroon-Veenboer C., Barker N., Klein A. M., Van Rheenen J., Simons B. D. et al. (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134-144. 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Song J., Zhong C., Bonaguidi M. A., Sun G. J., Hsu D., Gu Y., Meletis K., Huang Z. J., Ge S., Enikolopov G. et al. (2012). Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489, 150-154. 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Sun J., Moss J., Wen Z., Sun G. J., Hsu D., Zhong C., Davoudi H., Christian K. M., Toni N. et al. (2013). Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci. 16, 1728-1730. 10.1038/nn.3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Olsen R. H. J., Sun J., Ming G.-L. and Song H. (2016). Neuronal circuitry mechanisms regulating adult mammalian neurogenesis. Cold Spring Harbor Perspect. Biol. 8, a018937 10.1101/cshperspect.a018937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M., Yu S. P., Mohamad O., Cao W., Wei Z. Z., Gu X., Jiang M. Q. and Wei L. (2017). Optogenetic stimulation of glutamatergic neuronal activity in the striatum enhances neurogenesis in the subventricular zone of normal and stroke mice. Neurobiol. Dis. 98, 9-24. 10.1016/j.nbd.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells S. F., Paredes M. F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K. W., James D., Mayer S., Chang J., Auguste K. I. et al. (2018). Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377-381. 10.1038/nature25975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding K. L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H. B., Boström E., Westerlund I., Vial C., Buchholz B. A. et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219-1227. 10.1016/j.cell.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N., Merkle F. T., Flames N., Tramontin A. D., García-Verdugo J. M. and Alvarez-Buylla A. (2005). Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25, 10-18. 10.1523/JNEUROSCI.1108-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner B., Kronenberg G., Jessberger S., Brandt M. D., Reuter K. and Kempermann G. (2004). Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 46, 41-52. 10.1002/glia.10337 [DOI] [PubMed] [Google Scholar]
- Steiner B., Klempin F., Wang L., Kott M., Kettenmann H. and Kempermann G. (2006). Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia 54, 805-814. 10.1002/glia.20407 [DOI] [PubMed] [Google Scholar]
- Suh H., Consiglio A., Ray J., Sawai T., D'amour K. A. and Gage F. H. (2007). In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1, 515-528. 10.1016/j.stem.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. (2016). Endogenous neurogenic cell response in the mature mammalian brain following traumatic injury. Exp. Neurol. 275, 405-410. 10.1016/j.expneurol.2015.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G. J., Zhou Y., Stadel R. P., Moss J., Yong J. H. A., Ito S., Kawasaki N. K., Phan A. T., Oh J. H., Modak N. et al. (2015). Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc. Natl Acad. Sci. USA 112, 9484-9489. 10.1073/pnas.1508545112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie M., Van Der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J. M. and Doetsch F. (2008). A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279-288. 10.1016/j.stem.2008.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C. K., Chen J., Cebrián-Silla A., Mirzadeh Z., Obernier K., Guinto C. D., Tecott L. H., García-Verdugo J. M., Kriegstein A. and Alvarez-Buylla A. (2014a). Axonal control of the adult neural stem cell niche. Cell Stem Cell 14, 500-511. 10.1016/j.stem.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C. K., Han Y.-G., Shah J. K., Obernier K., Guinto C. D. and Alvarez-Buylla A. (2014b). Primary cilia are required in a unique subpopulation of neural progenitors. Proc. Natl. Acad. Sci. USA 111, 12438-12443. 10.1073/pnas.1321425111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C. K., Fuentealba L. C., Shah J. K., Lindquist R. A., Ihrie R. A., Guinto C. D., Rodas-Rodriguez J. L. and Alvarez-Buylla A. (2015). A dorsal SHH-dependent domain in the V-SVZ produces large numbers of oligodendroglial lineage cells in the postnatal brain. Stem Cell Rep. 5, 461-470. 10.1016/j.stemcr.2015.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin A. D., García-Verdugo J. M., Lim D. A. and Alvarez-Buylla A. (2003). Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb. Cortex 13, 580-587. 10.1093/cercor/13.6.580 [DOI] [PubMed] [Google Scholar]
- Van Kampen J. M. and Eckman C. B. (2010). Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharmacology 58, 921-929. 10.1016/j.neuropharm.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H., Schinder A. F., Christie B. R., Toni N., Palmer T. D. and Gage F. H. (2002). Functional neurogenesis in the adult hippocampus. Nature 415, 1030-1034. 10.1038/4151030a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R. E. and Goldman J. E. (2007). Dorsal radial glia generate olfactory bulb interneurons in the postnatal murine brain. J. Neurosci. 27, 4297-4302. 10.1523/JNEUROSCI.0399-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S. A., Luo J., Mosher K. I., Zou B., Britschgi M., Bieri G., Stan T. M., Fainberg N., Ding Z., Eggel A. et al. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 477, 90-94. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Liu F., Liu Y.-Y., Zhao C.-H., You Y., Wang L., Zhang J., Wei B., Ma T., Zhang Q. et al. (2011). Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 21, 1534-1550. 10.1038/cr.2011.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. M., Fogarty M., Kessaris N. and Richardson W. D. (2007). Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J. Neurosci. 27, 8286-8296. 10.1523/JNEUROSCI.0476-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. Z., Taylor M. M., Wu S., Ikeda-Matsuo Y., Kubera C. and Bordey A. (2012). NKCC1 knockdown decreases neuron production through GABA(A)-regulated neural progenitor proliferation and delays dendrite development. J. Neurosci. 32, 13630-13638. 10.1523/JNEUROSCI.2864-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. Z., Lafourcade C. A., Platel J.-C., Lin T. V. and Bordey A. (2014). GABAergic striatal neurons project dendrites and axons into the postnatal subventricular zone leading to calcium activity. Frontier. Cell. Neurosci. 8, 10 10.3389/fncel.2014.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa S. A., Borrett M. J., Innes B. T., Voronova A., Ketela T., Kaplan D. R., Bader G. D. and Miller F. D. (2017). Developmental emergence of adult neural stem cells as revealed by single-cell transcriptional profiling. Cell reports 21, 3970-3986. 10.1016/j.celrep.2017.12.017 [DOI] [PubMed] [Google Scholar]
- Zappaterra M. W. and Lehtinen M. K. (2012). The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell. Mol. Life Sci. 69, 2863-2878. 10.1007/s00018-012-0957-x [DOI] [PMC free article] [PubMed] [Google Scholar]