Abstract
Adenosine is an endogenous ubiquitous purine nucleoside, which is increased by hypoxia, ischaemia and tissue damage and mediates a number of physiopathological effects by interacting with four GPCRs, identified as A1, A2A, A2B and A3. Physiological and acutely increased adenosine is mostly associated with beneficial effects that include vasodilatation and a decrease in inflammation. In contrast, chronic overproduction of adenosine occurs in important pathological states, where long‐lasting increases in the nucleoside levels are responsible for the bad side of adenosine associated with chronic inflammation, fibrosis and organ damage. In this review, we describe and critically discuss the pathological overproduction of adenosine and analyse when, where and how adenosine exerts its detrimental effects throughout the body.
Abbreviations
- AD
Alzheimer's disease
- ADK
adenosine kinase
- Aβ
amyloid‐β
- BBB
blood–brain barrier
- CD39
apyrase
- CD73
5′‐nucleotidase
- COPD
chronic obstructive pulmonary disease
- EAE
experimental autoimmune encephalomyelitis
- HD
Huntington's disease
- HIF
hypoxia‐inducible factor
- IBD
inflammatory bowel diseases
- KO
knockout
- L‐DOPA
levodopa
- MDSC
myeloid‐derived suppressor cells
- MS
multiple sclerosis
- NTs
nucleoside transporters
- PD
Parkinson's disease
- RLS
restless legs syndrome
- αSyn
α‐synuclein
Tables of Links
| TARGETS | ||
|---|---|---|
| GPCRs a | Enzymes b | Transporters c |
| A1 receptor | 5’‐nucleotidase | Glutamate transporter 1 |
| A2A receptor | Adenosine deaminase | SLC 28 and 29 nucleoside transporters |
| A2B receptor | Adenosine kinase | |
| A3 receptor | Apyrase (CD39) | |
| D1 receptor | Caspase 1 | |
| D2 receptor | ERK1 | |
| ERK2 | ||
| JNK | ||
| P38 | ||
| PLC | ||
| PKA |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,cAlexander et al., 2015a,b,c).
Introduction
Adenosine is an endogenous ubiquitous autacoid regulating the function of every tissue and organ in the body (Borea et al., 2016). It is produced both intra‐ and extracellularly following the activity of specific enzymes, and its extracellular concentration is tightly regulated within a range of 30–200 nM (Fredholm et al., 2011). In particular, the main extracellular source of adenosine is the degradation of ATP and AMP by the ectoenzymes apyrase (CD39) and 5′‐nucleotidase (CD73), respectively, whilst for the intracellular level, its generation depends on AMP hydrolysis by an intracellular CD73 or S‐adenosylhomocysteine hydrolase (Zimmermann, 2000; Eltzschig, 2009). Adenosine concentration is maintained in equilibrium through equilibrative solute carrier family 29 (SLC 29) or concentrative solute carrier family 28 (SLC 28) nucleoside transporters (NTs) present in the cell membrane or through its phosphorylation operated by an intracellular adenosine kinase (ADK) (Alexander et al., 2015c). In addition, adenosine signalling can be arrested following adenosine deaminase activity, which is present both inside and outside the cell, transforming adenosine to inosine. Extracellular adenosine increases up to μM levels in hypoxia/ischaemia due to the up‐ and down‐regulation of CD73 and ADK, respectively; this increase in adenosine is characteristic of several important pathological conditions as it is involved in the regulation of energy supply/demand (Fredholm, 2014). Due to its rapid metabolism, adenosine behaves like an autocrine/paracrine rather than a systemic mediator (Figure 1).
Figure 1.
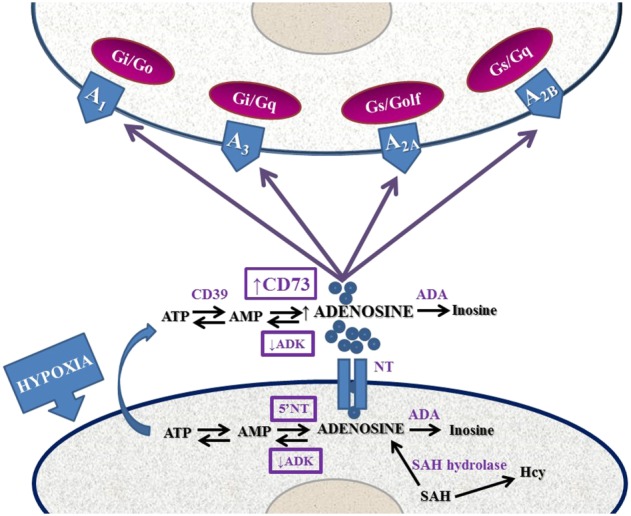
Schematic representation of the adenosinergic system from adenosine formation and release to signalling and removal from the extracellular space. Alterations occurring during hypoxia are shown. 5′NT, 5′‐nucleotidase; ADA, adenosine deaminase; SAH, S‐adenosylhomocysteine.
Adenosine interacts with four adenosine receptors named A1, A2A, A2B and A3 belonging to the family of GPCRs (Borea et al., 2015). All of them affect cAMP levels, A1 and A3 receptors being coupled to inhibitory Gi and A2A and A2B receptors to stimulatory Gs proteins respectively. Furthermore, A3 and A2B receptors are also coupled to Gq proteins, leading to the activation of PLC and an increase in calcium levels. Upon activation of A1 and A3 receptors, KATP channels are opened. Furthermore, all adenosine receptors stimulate MAPKs, triggering a variety of intracellular signalling pathways relevant for a number of cell functions including growth and proliferation, apoptosis, necrosis and inflammation (Schulte and Fredholm, 2003).
The wide distribution of adenosine receptors in almost all cells of the organism makes them an attracting target for drug development in several diseases, even though this ubiquity makes the development of selective adenosine receptor agonists and antagonists difficult to realize without the occurrence of side effects (Chen et al., 2013). Recently, we have published a review about the role of adenosine as a guardian angel against cellular damage, highlighting where, when and how adenosine exerts its protective mission under both physiological and pathophysiological conditions, in response to stress, both in the brain and in the periphery (Borea et al., 2016). However, with regard to the ‘guardian angel’ metaphor, we should not forget that there are instances in which a chronic overproduction of adenosine is pathological, particularly in Parkinson's disease (PD), fibrosis, hepatic steatosis, colitis, asthma, diabetes and cancer.
In this review, we focus on the pathological overproduction of adenosine describing when, where and how it exerts its detrimental effects throughout the body.
Pathological role of adenosine in cerebral ischaemia
In the CNS, both neurons and glial cells produce adenosine and express adenosine receptors that affect several homeostatic functions ranging from sleep to arousal, learning, memory and cerebral blood circulation. The principal adenosine receptor subtypes located in the CNS are A1 and A2A receptors, which are crucial in the regulation of neurotransmitter release, neuronal excitability, synaptic plasticity, vasodilatation and neuroinflammation (Sebastiao and Ribeiro, 2009). In general, the role of A1 receptors emerges as neuroprotective being an inhibitor of excitatory transmission, while that of the A2A receptor is detrimental, even though evidence has been obtained showing that its activation has beneficial effects (Melani et al., 2014; Pedata et al., 2016). The molecular bases of the adverse effects of A2A receptor stimulation are attributed to its modulation of neuronal glutamate release, potentiation of NMDA‐mediated effects, central inflammatory processes, glial reactivity, blood–brain barrier (BBB) permeability and infiltration of peripheral immune cells, which are associated with excitotoxicity and the pathogenesis of several brain diseases (Yu et al., 2004, 2008; Chen et al., 2008a,b; Azdad et al., 2009; Carta et al., 2009; Gui et al., 2009; Melani et al., 2009; Dai et al., 2010). Serendipitous pioneering studies about the involvement of A2A receptors in ischaemia found that its antagonism protected the brain against ischaemic insults (Gao and Phillis, 1994; Lubitz et al., 1995; Phillis, 1995; Monopoli et al., 1998). Later, this finding was confirmed in A2A receptor knockout (KO) mice and/or following pharmacological inhibition of the receptor, where a reduction in infarcted area was observed following ischaemic damage (Chen et al., 1999; 2007; Chen and Pedata, 2008). It has been demonstrated that after ischaemia, excitotoxicity is the first phenomenon occurring in the brain in the first 4 h, when the A2A receptor is responsible for the increase in glutamate levels, through both the release of glutamate from glutamatergic terminals and the inhibition of the glutamate‐1 transporter (GLT‐1) in astrocytes. Indeed, it has been shown that A2A receptor blockade protects against excitotoxicity (Matos et al., 2012b, 2013). Furthermore, A2A receptor activation reduces the affinity of agonists for A1 receptors in A1–A2A receptor heteromers, which exert a presynaptic control of striatal glutamate release, resulting in the fine‐tuning of the modulatory effect on striatal glutamatergic neurotransmission (Ciruela et al., 2006).
At the intracellular level, the inhibition of p38 MAPK in microglia and JNK in oligodendrocytes may be responsible for the protective effect mediated by A2A receptor antagonists in ischaemia (Melani et al., 2006, 2009). However, it is also known that later, hours and days after insult, there is a massive infiltration of blood cells and neuroinflammation that may be inhibited by activation of A2A receptors located on blood cells. Accordingly, the chronic administration of an A2A receptor antagonist for 7 days after transient focal ischaemia failed to protect the brain (Melani et al., 2015), suggesting that A2A receptor antagonists should be administered for a limited time window of about 4 h after stroke (Pedata et al., 2016). However, it is important to mention that chronic enhanced levels of adenosine may lead to seizures, through A2A receptor activation and neurotrophins, suggesting that in this context A2A receptor antagonists could be useful clinically (Sandau et al., 2016). Interestingly, recent evidence adds a new pathological aspect to adenosine overproduction related to the development of pain behaviour. This effect seems to be due to the activation A2B receptors on myeloid cells, which are able to transactivate nociceptors of sensory neurons, and induce hypersensitive neurons and chronic pain through a pathway involving IL‐6. Hence, the A2B receptor may play a major role in chronic pain by promoting immune–neuronal interactions (Hu et al., 2016) (Figure 2).
Figure 2.
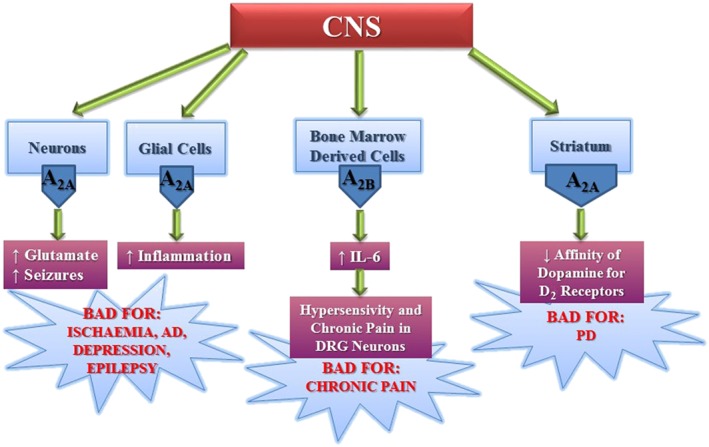
Deleterious effects of adenosine mediated through A2A and A2B receptors expressed in different areas of the CNS. DRG, dorsal root ganglion.
Pathological role of adenosine in neurodegenerative diseases
There is a well‐established allosteric relationship between striatal A2A receptors and dopamine D2 receptors, supported by evidence that A2 receptor activation is responsible for the decreased affinity of agonist binding to D2 receptors (Ferrè et al., 1991). This represented the first proof of concept for the therapeutic utility of A2A receptor antagonists in PD (Armentero et al., 2011; Preti et al., 2015; Ferré et al., 2016) (Figure 2). Interestingly, the well‐known epidemiological consideration that caffeine protects against PD has been confirmed in an animal model of PD and attributed to A2A receptor antagonism (Ross et al., 2000; Ascherio et al., 2001, 2004; Chen et al., 2001; Yu et al., 2008; Xu et al., 2016). A2A receptors and D2 receptor heteroceptor complexes have been detected in cellular models and in the striatum (Hillion et al., 2002; Canals et al., 2003; Azdad et al., 2009; Borroto‐Escuela et al., 2010, 2011, 2013; Varani et al., 2010; Trifilieff et al., 2011; Bonaventura et al., 2015). As the receptors show antagonistic interactions at both membrane and intracellular signalling levels, dopamine depletion may be responsible for an overactivation of A2A receptors and the consequent symptoms of PD (Peterson et al., 2012). Therefore, antagonists of A2A receptors are beneficial for the improvement in motor function in different animal models of PD, as they attenuate the inhibition exerted via A2A receptors on the effects of D2 receptors in the gabaergic striato‐pallidal neurons (Fuxe et al., 2015). Recently, pharmacological data have suggested a new model that indicates A2A receptors and D2 receptor heteromers form heterotetramers, which consist of A2A receptors and D2 receptor homodimers. This model supports the evidence that, at high concentrations, A2A receptor antagonists exert the same effects as A2A receptor agonists, thus reducing D2 receptor‐mediated activity in neurons; these findings could be important from a clinical point of view, when the use of A2A receptor antagonists is being considered for therapy (Bonaventura et al., 2015). Even though the development of A2A receptor antagonists as new drugs for PD treatment has encountered several obstacles in the clinic, istradefylline has recently been registered in Japan, as it has been found to decrease the “off time” when used in combination with levodopa (L‐DOPA). However, it has not receive approval by the American Food and Drug Administration, due to the lack of significant improvement in the phenomenon of “wearing off” in comparison with L‐DOPA. Other potential new drugs like preladenant and vipadenant have failed in phase III clinical studies for PD (Navarro et al., 2016; Oertel and Schulz 2016). The reasons for which these drugs failed were the lack of significant benefits of preladenant combined with L‐DOPA when compared to L‐DOPA alone, while vipadenant was found to have toxic effects. It has been hypothesized that administration of this class of compounds should start as early as possible to avoid a structural change in the A2A receptor–D2 receptor heteroceptor complexes that may induce dyskinesias and the onset of “off time” caused by L‐DOPA (Fuxe et al., 2015). Another pharmacological approach for PD treatment is suggested from the results obtained with bivalent drugs acting at A2A receptor–D2 receptor complex (Soriano et al., 2009), with integrated dual acting ligands having an improved efficacy in preliminary BBB permeability tests (Jörg et al., 2015). Recently, a novel protective effect of A2A receptor antagonists has been attributed to the modulation of α‐synuclein (αSyn) effects, as α‐synuclein‐induced damage to striatal neurons was clearly reduced in A2A receptor KO mice and A2A receptor antagonists prevented the neurotoxicity related to α‐synuclein aggregation. These results provide additional evidence in support of antagonists as effective drugs for the treatment of PD and related synucleinopathies (Kachroo and Schwarzschild, 2012; Ferreira et al., 2015). The use of A2A receptor antagonists has been found useful in other pathologies involving neuronal dysfunction including Huntington's (HD) and Alzheimer's (AD) diseases as well as major depression and schizophrenia, epilepsy, acute and chronic stress, restless legs syndrome (RLS) and memory fear (Cunha, 2005, 2016; Canas et al., 2009; Batalha et al., 2013; Krügel, 2016; Laurent et al., 2016; Quiroz et al., 2016; Simões et al., 2016) (Figure 2). Different studies have reported an up‐regulation of A2A receptors in animal models of HD (Varani et al., 2001; Tarditi et al., 2006), and recently, it has been shown that A2A receptor antagonists block working memory deficits at early stages of HD models (Li et al., 2015). Hyperactivation of both D1 and A2A receptors has been found in HD striatum where D1 plus A2A receptor antagonists reduced PKA activity, which is involved in hippocampal‐dependent cognitive dysfunction in HD, further supporting a therapy based on A2A receptor blockade. Nevertheless, evidence in favour of A2A receptor activation as a strategy for HD treatment has also been reported (Gomes et al., 2011; Martire et al., 2013; Tyebji et al., 2015). In addition, a positive action of A2A receptors in the early phases of neurodegeneration has been attributed to the presynaptic facilitation of GABA transmission exerted by A2A receptor agonists, involving brain‐derived neurotrophic factor–tropomyosin receptor kinase B in the hippocampus (Colino‐Oliveira et al., 2016).
A change in the pattern of adenosine receptors with a decrease in A1 and an increase in A2A receptors has been also detected in AD. As a consequence A2A receptor antagonists have been found to prevent neuronal death and the decrease in astrocytic glutamate uptake, caused by an amyloid‐β (Aβ) fragment, that may be responsible for excitotoxicity in AD (Dall'Igna et al., 2003; Canas et al., 2009; Matos et al., 2012a). Importantly, it has been shown that humans with AD have increased levels of A2A receptors in astrocytes. Also, in young and aging mice, their genetic removal increased long‐term memory and increased the levels of an immediate‐early gene necessary for memory (Orr et al., 2015). Furthermore, deletion of A2A receptors has been shown to have a protective effect from spatial memory and hippocampal long‐term depression caused by Tau pathology (Laurent et al., 2016; Müller et al., 2016). In addition, A2A receptors are up‐regulated in CA3 synapses at early stages of AD and their antagonism reversed the block of long‐term synaptic potentiation in a mouse model of AD amyloidosis (Viana da Silva et al., 2016). Caffeine has been found to have a protective effect against cognitive impairment in both human and animal studies (Maia and de Mendonça, 2002; Ritchie et al., 2007; Smith, 2009; Santos et al., 2010). Furthermore, caffeine reduced plasma and brain Aβ levels in an animal model of AD and prevented memory deficits caused by Aβ administration (Dall'Igna et al., 2007; Cao et al., 2009). Interestingly, the initial findings of a case–control study were the first to demonstrate that caffeine/coffee consumption is associated with a reduced risk, or delayed onset, of dementia (Cao et al., 2012). Therefore, it seems that caffeine, the most popular and widely used drug in the world, by antagonizing the effects of adenosine, retains a big potential to counteract different neurodegenerative diseases (Woods et al., 2016).
Due to their modulation of glutamatergic and monoaminergic transmission in the striatum, A2A receptors may also be involved in depression (Krügel, 2016). In behavioural animal models, A2A receptor antagonists produced antidepressant effects (El Yacoubi et al., 2003; Hodgson et al., 2009). However, istradefylline was found to exert them independently from monoaminergic transmission (Yamada et al., 2014a,b). Furthermore, caffeine or selective A2A receptor antagonists reversed the performance deficits in reserpine‐treated rats and prevented mood and memory dysfunctions induced by chronic stress (Batalha et al., 2013; Kaster et al., 2015; Minor and Hanff, 2015). In addition, in a broad‐based model of depression mediated by an increase in hippocampal A2A receptors, which control synaptic glutamatergic function, caffeine was able to prevent memory, but not mood, deficits (Machado et al., 2016).
Overall, based on the progress obtained in clarifying the molecular mechanisms underlying CNS diseases, adenosine reveals its bad side essentially through the activation of A2A receptors, which may be considered as targets of therapeutic strategies and may represent a promising future in the battle against a wide spectrum of unmet central diseases.
Pathological role of adenosine in autoimmune diseases
Immune cells express all adenosine receptors, which means adenosine can affect inflammatory and immune events (Cekic and Linden, 2016). In particular, it was found that adenosine modulates immune function by exerting inhibitory effects on neutrophils, lymphocytes, monocytes/macrophages and dendritic cells (Mills et al., 2012). Therefore, a possible area of investigation for novel therapeutic strategies through adenosine receptor modulation is the study of adenosine's role in the immunopathogenesis of multiple sclerosis (MS).
Interestingly, it has been observed that mice with CD73 knocked‐down, the critical enzyme for generating extracellular adenosine in various cells including T cells, are protected against experimental autoimmune encephalomyelitis (EAE, an animal model for MS) and lack the CNS lymphocyte infiltrates associated with disease progression (Mills et al., 2008).
Moreover, caffeine decreases the incidence of EAE and attenuates it at the behavioural, histological and neurochemical levels in mice and rats (Tsutsui et al., 2004; Chen et al., 2010). Furthermore, it shifted the status of the cytokine levels from Th1, pro‐inflammatory to Th2, anti‐inflammatory in EAE, then ameliorated inflammatory injury in the brain and spinal cord, suggesting it has a neuroprotective effect via its role as an immunomodulator (Chen et al., 2010).
Surprisingly, even though the A2A receptor is recognized as a major mediator of anti‐inflammatory responses, it has been reported that the pharmacological blockade of this subtype attenuates EAE pathology in CD73 KO mice (Mills et al., 2008). Furthermore, A2A receptor blockade prevented oligodendrocyte damage through a reduction of JNK/MAPK signalling in these cells (Melani et al., 2009). However, to explain the beneficial effects of A2A receptor antagonists in EAE, it has been suggested that although the A2A receptor present on lymphocytes is anti‐inflammatory, that expressed in the CNS plays a pro‐inflammatory role and appears to be essential for EAE development (Mills et al., 2012). However, an A2A receptor agonist worsened experimental autoimmune neuritis through an inhibitory effect of T‐cell proliferation and IL‐2 secretion (Zhang et al., 2016). The role of A2B receptors in the pathogenesis of MS has also been investigated by knocking out this subtype or blocking it with selective antagonists. A2B receptors have been found to be up‐regulated in peripheral blood leukocytes of MS patients and the peripheral lymphoid tissues of EAE mice. Furthermore, pharmacological blockade of the A2B receptor ameliorated the clinical symptoms of EAE and reduced immune damage in the CNS. Importantly in A2B receptor KO mice, EAE was less severe. The mechanism involved was suggested to be the inhibition of Th17 cell differentiation by the blockade of IL‐6 production from dendritic cells, via the PLCβ–PKC and p38 MAPK pathways (Wei et al., 2013). Accordingly, A2B receptor stimulation in dendritic cells greatly increased the development of experimental autoimmune uveitis (EAU) by enhancing the Th17 autoimmune responses (Chen et al., 2015). In conclusion, adenosine exerts a complex role, acting at A2A and A2B receptors to increase inflammation, EAE and EAU.
Pathological role of adenosine in inflammatory conditions
Inflammation is a hallmark of important diseases such as cardiovascular, metabolic, intestinal and pulmonary disorders (Hotamisligil, 2006; Libby et al., 2011; Barnes, 2016; Karin and Clevers, 2016). As for the detrimental signalling of adenosine in inflammatory conditions, we have to focus on A2B receptors as mediators of the effects of autacoids because this subtype is activated by μM concentrations of adenosine, occurring in inflammation, hypoxia and cell injury (Fredholm, 2007; Bartels et al., 2013). In particular, the expression of A2B receptors is increased in hypoxia in response to hypoxia‐inducible factors (HIFs) (Eckle et al., 2014). Indeed, it has been demonstrated that A2B receptors have a role in the inflammatory environment typically present in asthma, chronic obstructive pulmonary disease (COPD), kidney pathologies and inflammatory bowel diseases (IBD) (Kolachala et al., 2005; Haskó et al., 2008). Likewise, A3 receptors expressed in the human lung, mast cells and in macrophages enriched with lipids, known as foam cells (FC), play a role in the regulation of the inflammation present in pulmonary and atherosclerotic diseases (Hua et al., 2008; Gessi et al., 2010; Borea et al., 2015).
The A2B receptor is present in the intestinal epithelium where it exerts a controversial role in inflammation (Kolachala et al., 2005). Indeed, different data have reported that A2B receptors are able to protect from inflammation development in the intestine (Schingnitz et al., 2010; Hart et al., 2011; Aherne et al., 2015). However, the expression of A2B receptors in non‐immune cells plays an important role in the occurrence of murine colitis. Indeed, colitis is attenuated in A2B receptor KO mice and after of pharmacological blockade of A2B receptors, indicating that A2B receptor antagonism may be an effective treatment for acute inflammatory intestinal diseases, such as IBD (Kolachala et al., 2008a,b; Ingersoll et al., 2012).
Adenosine is responsible for the production of VEGF, IL‐6 and IL‐8 through interaction with all four adenosine receptors in inflamed tissues, in which the levels of these autacoids are increased (Cekic and Linden, 2016; Koszałka et al., 2016). However, the A2B and A3 receptor subtypes are mainly involved in the modulatory effects of adenosine on wound healing processes, for example, angiogenesis and fibrosis (Zhou et al., 2011) (Figure 3). The A2B receptor plays a significant role in the chronic phase of wound healing after tissue injury and increases the adverse signalling responsible for the continuous tissue remodelling and fibrosis occurring in chronic inflammatory conditions (Feoktistov et al., 2009). Pulmonary fibrosis is a harmful lung disease with limited therapeutic options. Adenosine has a role as a pro‐inflammatory messenger in different chronic pulmonary inflammatory conditions, such as asthma and COPD (Cekic and Linden, 2016). Very recently, it has been reported that increased extracellular adenosine concentrations, by inducing the production of IL‐6 and IL‐17, are associated with the progression of experimental pulmonary fibrosis (Luo et al., 2016). As the A2B receptor promotes Th17 differentiation (Wilson et al., 2011), it has been hypothesized that adenosine may induce IL‐17 expression through its A2B receptor subtype in chronic lung injury, thus contributing to lung fibrosis. Furthermore, adenosine promotes the differentiation of alternatively activated macrophages, a macrophage subtype that has been shown to contribute to pulmonary fibrosis, stimulated by Th2 cytokines (IL‐4 and IL‐13) (Karmouty‐Quintana et al., 2015). In addition, it has been reported that A2B receptors are up‐regulated in lung tissue from idiopathic pulmonary fibrosis patients (Zhou et al., 2010) and that the pharmacological blockade of A2B receptors in mice (Karmouty‐Quintana et al., 2012, 2013b; Sun et al., 2006) or genetic removal of this subtype (Zhou et al., 2011) are associated with decreased fibrosis. Therefore, it is important to verify and understand the association between extracellular adenosine levels and the progression of pulmonary fibrosis to develop new drugs for this pathology based on the structures of adenosine ligands binding to A2B receptors. As for the role of adenosine in asthma and COPD, it is interesting to note that A2B receptor signalling promotes the production of Th2‐type cytokines and the recruitment and degranulation of eosinophils (Belikoff et al., 2012; Karmouty‐Quintana et al., 2013a). Furthermore, it has been known for a long time that the secretion of IL‐4 by human mast cells is mediated through A2B receptors and this increases IgE production by B cells and as a consequence triggers allergic inflammation (Ryzhov et al., 2006, 2008). In addition, there is unequivocal evidence from studies in rodent mast cells that the A3 receptor is involved in the bronchoconstrictor response; however, this has not been verified in humans (Rudich et al., 2012). On the one hand, in primary human lung mast cells, stimulation of A3 receptors increases IgE‐induced degranulation (Feoktistov et al., 2003; Gomez et al., 2011); and on the other, no effect was obtained in human umbilical cord blood‐derived mast cells (Hua et al., 2011) or in the LAD2 human mast cell line (Leung et al., 2014), suggesting that this response depends on the tissue studied (Gomez et al., 2011). Recently, it has been demonstrated that the primary function of the A3 receptor is to prime the human mast cells towards tissue remodelling activity (Rudich et al., 2015). Therefore, A2B and A3 receptor antagonists may also be useful for the treatment of asthma and COPD (Figure 4).
Figure 3.
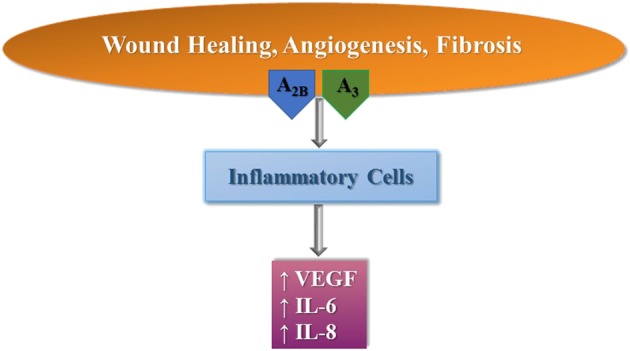
Modulation of wound healing, angiogenesis and fibrosis by A2B and A3 receptors in inflammatory cells.
Figure 4.
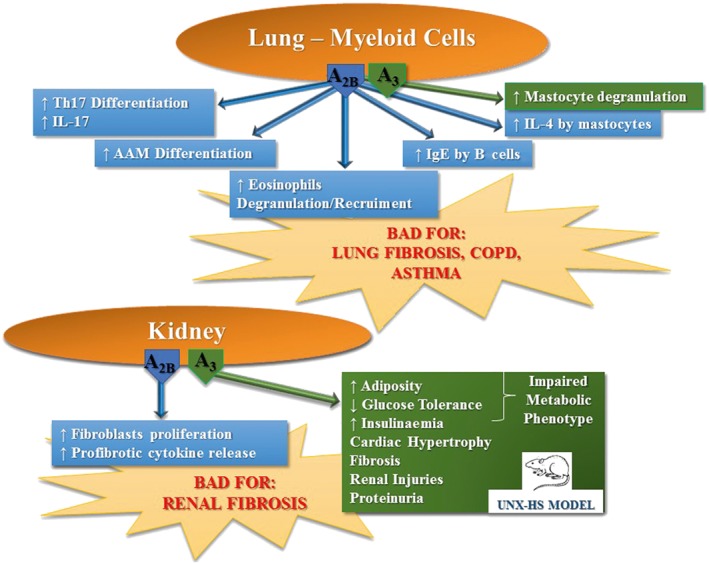
Deleterious effects of adenosine mediated through A2B and A3 receptors expressed in the lung and kidney. AAM, alternatively activated macrophages; HS, high salt; UNX, uninephrectomy.
In addition, more recently, it has been demonstrated that A2B receptor blockade in hypoxia significantly decreased renal fibroblast proliferation and activation with a consequent reduction of profibrotic cytokine release, thus preventing the generation and development of renal fibrosis, the outcome of all types of chronic kidney disease (Tang et al., 2015). Indeed, in a novel mouse model of renal and cardiovascular disease induced through uninephrectomy and chronic high salt intake, the inhibition of A3 receptor signalling prevented the development of hypertension and attenuated the cardiac hypertrophy and fibrosis together with renal injuries and proteinuria (Yang et al., 2016) (Figure 4). This finding accords with that of a previously reported study showing that A3 receptor antagonism reduces the progression of renal fibrosis (Lee et al., 2013). Overall, these findings indicate that A2B and A3 receptor antagonists could be an interesting new therapeutic strategy for the development of new drugs to prevent tissue remodelling after tissue ischaemia and, consequently, for the treatment of fibrotic diseases including heart failure. Interestingly, it has been shown in a rat model that the blockade of A2B receptors, beginning 1 week after myocardial infarction, reduces the pathology of this condition (Zhang et al., 2014).
Many cardiovascular diseases have atherosclerosis as an aetiological factor. In particular, endothelial dysfunction with the accumulation and oxidation of LDL leads to an increase in foam cells (Eisenstein et al., 2015). The role of adenosine receptors in the development of atherosclerosis has been investigated. Adenosine has been shown to increase the accumulation of HIF‐1α through the stimulation of all of its receptors. This is important because hypoxia is characterized by atherosclerotic plaques and HIF‐1 promotes intraplaque angiogenesis and the development of FCs (Gessi et al., 2010). The signalling mediating this effect on HIF‐1α induced by stimulation of A1, A2A and A2B receptors involves ERK1/2, p38 MAPK and Akt phosphorylation, while only ERK1/2 is involved in the activation induced by A3 receptors. In addition, A2B and A3 receptors stimulate VEGF production in an HIF‐1α‐dependent manner. Finally, adenosine‐stimulated FC formation was decreased by the pharmacological blockade of A3 and A2B receptors and by the silencing of HIF‐1α. This study indicated for the first time that A3 and A2B receptor antagonists may be useful for preventing the development of atherosclerotic plaques (Gessi et al., 2010). In addition, A2B receptor antagonists have been found to inhibit fatty liver formation in mice post alcohol assumption (Peng et al., 2009). However, in contrast, in a different study, using an in vivo mouse model of atherosclerosis, the role of A2B receptors in plaque formation was found to be protective (Koupenova et al., 2012).
Pathological role of adenosine in diabetes
It is well recognized that adenosine regulates insulin secretion, glucose homeostasis and lipid metabolism, by stimulation its receptors. The stimulation of A1 and A2A receptor subtypes seems to promote an antidiabetic phenotype even though recently it has been shown that blockade of A1 receptor activation offers protection from age‐related oxidative stress and secretion of pro‐inflammatory cytokines, thus improving insulin release and effect (Yang et al., 2015). As for the A2B receptor, its role is still controversial with a number of studies supporting a role for its agonists as a therapy for diabetes (Johnston‐Cox et al., 2012; 2014; Peleli et al., 2015). However, this protective effect is challenged by a series of studies reporting the beneficial effects of A2B receptor antagonists. It was firstly reported that A2B receptor antagonists behave as hypoglycaemic agents in rat models of hepatic glucose production induced by adenosine (Harada et al., 2001a,b). Furthermore, it was found that A2B receptor activation increases glucose production by affecting glycogenolysis and gluconeogenesis in the rat liver (Yasuda et al., 2003). Following this line, A2B receptor antagonists were shown to counteract the reduction in insulin levels induced by a non‐selective adenosine receptor agonist in pancreatic cells and plasma from rats, even though this effect is not mediated through A2B receptor activation (Rüsing et al., 2006). A2B receptor antagonists were also demonstrated to reduce the levels of IL‐6 and other cytokines affecting glucose and fat metabolism in a diabetic mouse model, thus improving insulin resistance (Figler et al., 2011). Furthermore, A2B receptor blockade reduced the activation of the pro‐inflammatory caspase‐1 in rat retinal cells incubated in hyperglycaemic conditions (Trueblood et al., 2011; Vindeirinho et al., 2016). Interestingly, it has been found that high glucose levels and experimental diabetes increase the concentrations of adenosine in plasma by reducing the equilibrative NT in proximal tubule cells. This increase correlated with a marker of renal fibrosis in diabetic rats. Furthermore, the expression of profibrotic cell activation markers α‐smooth muscle actin and fibronectin was increased by stimulation of A3 receptors (Kretschmar et al., 2016) (Figure 5).
Figure 5.
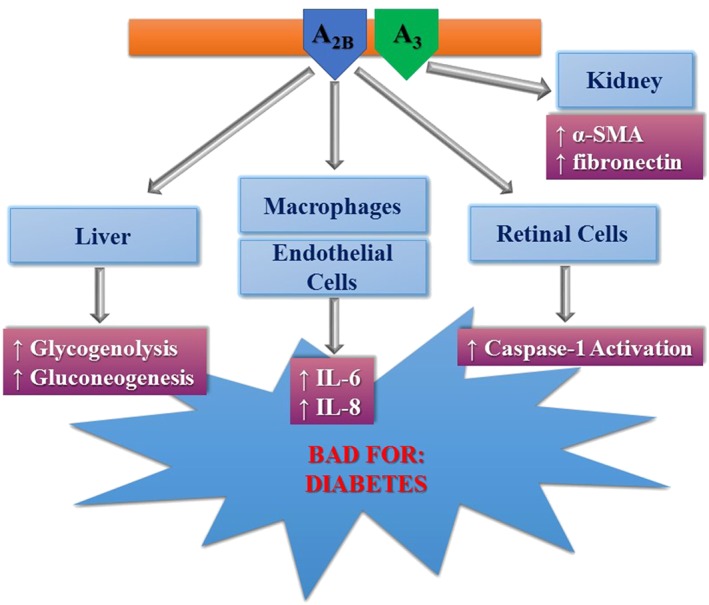
Deleterious effects of adenosine mediated through A2B and A3 receptors in diabetes. α‐SMA, α‐smooth muscle actin.
Therefore, before establishing a role for A2B receptor agonists or A2B/A3 receptor antagonists in diabetic therapy, when looking at the evidence it is important to accurately examine the experimental conditions associated with glucose and insulin regulation including the method used for inhibiting the receptors (pharmacological vs. genetic) and the cell types or model system used (Antonioli et al., 2015; Merighi et al., 2015).
Pathological role of adenosine in cancer
Adenosine plays a role in promoting cancer development by evoking immunosuppressive effects and directly affecting the growth, metastasis and angiogenesis of tumour cells (Antonioli et al., 2014; Allard et al., 2016; Di Virgilio and Adinolfi, 2016; Ohta, 2016) (Figure 6). There is a strong relationship between cancer, hypoxia and adenosine metabolism resulting in increased levels of the autacoids in hypoxic tumours. This effect is essentially a consequence of specific alterations in the enzymes involved in adenosine production, for example, the overexpression of CD73 and the down‐regulation of adenosine kinase (ADK) are both increased by hypoxia (Ohta, 2016). Indeed, clinical studies have reported that the expression of CD73 is associated with a poor prognosis in different types of cancer (Antonioli et al., 2016), including breast (Loi et al., 2013), ovarian (Turcotte et al., 2015), prostate (Leclerc et al., 2016), brain (Quezada et al., 2013) and leukaemia (Coustan‐Smith et al., 2011; Serra et al., 2011). It has been reported that antibody‐ or genetic silencing‐mediated inactivation of CD73 inhibited breast, prostate, fibrosarcoma and melanoma tumour growth (Zhi et al., 2007; Stagg et al., 2010; 2011; 2012; Terp et al., 2013). The effects of CD73 on cancer development have been attributed to the immunosuppressive effects of A2A and A2B receptor stimulation (Beavis et al., 2013; Young et al., 2016). In particular, adenosine induces an immune‐tolerant micro‐environment around tumours affecting the functions of immune and inflammatory cells like T‐ and natural killer (NK) cells, macrophages and dendritic and myeloid‐derived suppressor cells (MDSC). Treg cells express high levels of CD39 resulting in an increased production of adenosine, which then inhibits the antitumour immune response; this effect of adenosine is mediated through inhibition of NK effector lymphocytes that lose their ability to recognize neoplastic cells. This effect was initially attributed to A3 receptor activation, but is now thought to be mediated by A2A receptors (MacKenzie et al., 1994; Ohta et al., 2012; Antonioli et al., 2013). As for tumour‐associated macrophages, stimulation of A2A and A2B receptors induces an enhanced expression of the M2 phenotype, which contributes to the angiogenesis, proliferation and metastasis of tumour cells (Csóka et al., 2012). In addition, A2B receptors mediate the increased production of VEGF by dendritic cells; VEGF is essential for tumour angiogenesis and growth, and favours the activity of MDSC that are able to suppress immune surveillance (Ryzhov et al., 2011). Overall, these results have led to the development of phase I clinical trials that are now underway to consider the safety and the efficacy of CD73 and A2A receptor inhibitors in cancer patients (Allard et al., 2016).
Figure 6.
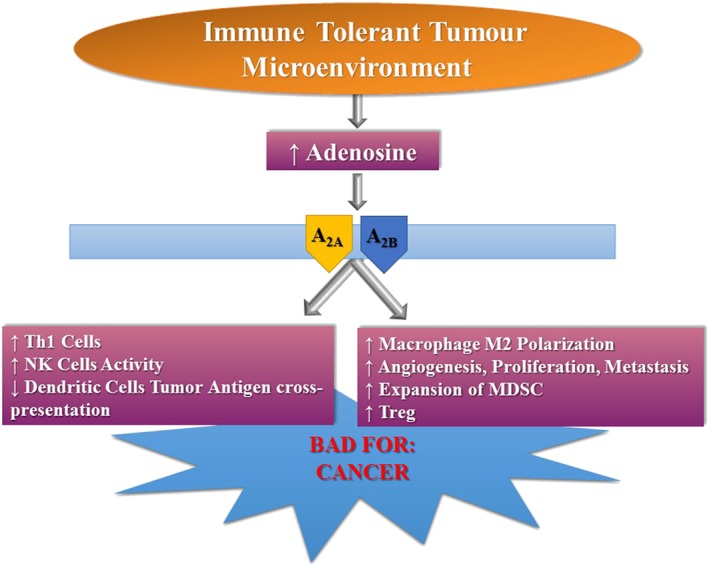
Deleterious effects of adenosine mediated through A2A and A2B receptors in cancer.
The adenosine machinery also mediates its effects directly in tumour cells by affecting their proliferation and cell death through recruitment of different receptors. In particular, opposite effects on cell growth and motility have been observed, with A1, A2A and A2B receptors being promoters whilst A3 receptors are inhibitors of cell proliferation (Merighi et al., 2002; 2005; Gessi et al., 2011a; Antonioli et al., 2013; Borea et al., 2015; Ledderose et al., 2016). Indeed, it is important to note that clinical trials are undergoing for A3 receptor agonists, based on their antiproliferative effects, representing an opportunity for new anticancer drugs (Borea et al., 2016).
Nevertheless, it has been reported that A2B receptors have a prometastatic and prosurvival effect and that stimulation of A1, A2A and A3 receptors increases melanoma growth, neovascularization, angiogenesis and macrophage infiltration in CD73 KO mice (Linden, 2013; Ntantie et al., 2013; Koszałka et al., 2016) (Figure 5).
Interestingly, an overexpression of A3 receptors has been reported in different tumours including colon, breast, hepatocellular and mesothelioma (Madi et al., 2004; Gessi et al., 2004; 2011b; Varani et al., 2011; Borea et al., 2015). Furthermore, an up‐regulation of A2B receptors has also been observed in colorectal cancer (Ma et al., 2010). Therefore, we can conclude that the important effects adenosine exerts on the progression and development cancer depend on the subtype of adenosine receptor expressed in each tumour.
The negative actions of long‐lasting increases in the extracellular adenosine concentration lead to overactivation of membrane receptors, thus opening the way for the clinical development of specific antagonists. First of all, starting with the CNS injuries, A2A selective antagonists may have a role in the therapy of ischaemia, where a careful attention to administration time and to the dose of A2A receptor antagonist has to be considered for a novel therapeutic strategy. PD and other neurodegenerative diseases such as HD and AD, major depression, schizophrenia, epilepsy, acute and chronic stress, RLS and memory fear are pathologies for which A2A selective inhibitors could became new drugs. Even though istradefylline, in combination with L‐DOPA, is now approved in Japan for reducing the “off time” in PD, to date no disease‐modifying treatment is available. Therefore, the research to identify new therapeutics for PD should be increased and the reasons why the translation of basic research to disease‐modifying therapies has been unsuccessful so far should be investigated. The new elegant evidence regarding the modulation of heterotetrameric structure of the A2A/D2 receptor complex and the similarity between A2A receptor agonist and antagonist effect on the affinity and intrinsic efficacy of D2 receptor ligands offers a novel model that can provide new clues about how to adjust the clinical use of these drugs (Ferré et al., 2016).
In MS, an autoimmune disease of central origin, both A2A and A2B receptor antagonists attenuated the increased inflammation and then the development of its pathology. Hence, the blockade of these receptors might be a new strategy for the development of new drugs for MS therapy.
Understanding the effect of A2B receptor modulation in intestinal inflammation, glucose and lipid regulation is necessary to clarify whether this subtype is protective or injurious in both colitis, diabetes and atherosclerosis, respectively, with the aim of adequately recognizing its therapeutic potential.
Furthermore, A2B and A3 receptor antagonists may represent a starting point for the development of novel drugs to hamper remodelling occurring after tissue ischaemia and for the therapy of consequent fibrotic diseases arising in the lung, kidney and heart.
The pioneering research by Sitkowsky and co‐workers on tumours and hypoxia also opens the way for the use of A2A receptor antagonists in the therapy of cancer. Due to their immunosuppressive effects and tumour tolerant behaviour, now both A2A an A2B receptors are considered important and promising targets for the development of a novel class of antitumoural drugs able to weaken the hypoxia‐adenosine mediated signalling pathways. It is important to note that existing A2A receptor antagonists tested in human clinical studies for PD have already demonstrated their safety profile. However, in the future, it will be important to consider the development of new A2A receptor blockers unable to cross the BBB, thus avoiding the occurrence of potential neurological side effects in cancer patients with non‐cerebral tumours (Hatfield and Sitkovsky, 2016). This task may be supported by important advances in the structure‐based design of A2A receptor antagonists that take advantage of the recently revealed molecular basis of this adenosine subtype (Carpenter et al., 2016; Ye et al., 2016; Jazayeri et al., 2017).
Conclusions and perspectives
It is well known that adenosine is beneficial, adopting the role of guardian angel when present at physiological levels or when increased in acute situations (Borea et al., 2016). However, we have to remember that there are instances in which its chronic overproduction is pathological. In this review, we have presented evidence for its bad side, particularly in association with ischaemia, neurodegenerative diseases, fibrosis, hepatic steatosis, colitis, asthma, diabetes and cancer, while focusing on the potentiality of this system as a new therapeutic weapon in the battle against important unmet diseases. Indeed, A2A, A2B and A3 receptor antagonists may find a specific/unique place in different clinical applications. Overall, this is an exciting period for scientists involved in the adenosinergic field seeking to harness the information for new therapeutic applications.
Conflict of interest
The authors declare no conflicts of interest.
Borea, P. A. , Gessi, S. , Merighi, S. , Vincenzi, F. , and Varani, K. (2017) Pathological overproduction: the bad side of adenosine. British Journal of Pharmacology, 174: 1945–1960. doi: 10.1111/bph.13763.
Contributor Information
Pier Andrea Borea, Email: bpa@unife.it.
Stefania Gessi, Email: gss@unife.it.
Stefania Merighi, Email: mhs@unife.it.
References
- Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L et al. (2015). Epithelial‐specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol 8: 1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015c). The concise guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allard B, Beavis PA, Darcy PK, Stagg J (2016). Immunosuppressive activities of adenosine in cancer. Curr Opin Pharmacol 29: 7–16. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Blandizzi C, Csóka B, Pacher P, Haskó G (2015). Adenosine signalling in diabetes mellitus–pathophysiology and therapeutic considerations. Nat Rev Endocrinol 11: 228–241. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Blandizzi C, Pacher P, Haskó G (2013). Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 13: 842–857. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Haskó G, Fornai M, Colucci R, Blandizzi C (2014). Adenosine pathway and cancer: where do we go from here? Expert Opin Ther Targets 18: 973–977. [DOI] [PubMed] [Google Scholar]
- Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G (2016). Anti‐CD73 in cancer immunotherapy: awakening new opportunities. Trends Canc 2: 95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentero MT, Pinna A, Ferre S, Lanciego JL, Muller CE, Franco R (2011). Past, present and future of A(2A) adenosine receptor antagonists in the therapy of Parkinson's disease. Pharmacol Ther 132: 280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascherio A, Weisskopf MG, O'Reilly EJ, McCullough ML, Calle EE, Rodriguez C et al. (2004). Coffee consumption, gender, and Parkinson's disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am J Epidemiol 160: 977–984. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE et al. (2001). Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 50: 56–63. [DOI] [PubMed] [Google Scholar]
- Azdad K, Gall D, Woods AS, Ledent C, Ferré S, Schiffmann SN (2009). Dopamine D2 and adenosine A2A receptors regulate NMDA‐mediated excitation in accumbens neurons through A2A‐D2 receptor heteromerization. Neuropsychopharmacology 34: 972–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ (2016). Kinases as novel therapeutic targets in asthma and chronic obstructive pulmonary disease. Pharmacol Rev 68: 788–815. [DOI] [PubMed] [Google Scholar]
- Bartels K, Grenz A, Eltzschig HK (2013). Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci U S A 110: 18351–18352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalha VL, Pego JM, Fontinha BM, Costenla AR, Valadas JS, Baqi Y et al. (2013). Adenosine A(2A) receptor blockade reverts hippocampal stress‐induced deficits and restores corticosterone circadian oscillation. Mol Psychiatry 18: 320–331. [DOI] [PubMed] [Google Scholar]
- Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C et al. (2013). Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A 110: 14711–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belikoff BG, Vaickus LJ, Sitkovsky M, Remick DG (2012). A2B adenosine receptor expression by myeloid cells is proinflammatory in murine allergic‐airway inflammation. J Immunol 189: 3707–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventura J, Navarro G, Casadó‐Anguera V, Azdad K, Rea W, Moreno E et al. (2015). Allosteric interactions between agonists and antagonists within the adenosine A2A receptor‐dopamine D2 receptor heterotetramer. Proc Natl Acad Sci U S A 112: E3609–E3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borea PA, Gessi S, Merighi S, Varani K (2016). Adenosine as a multi‐signalling guardian angel in human diseases: when, where and how does it exert its protective effects? Trends Pharmacol Sci 37: 419–434. [DOI] [PubMed] [Google Scholar]
- Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S et al. (2015). The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67: 74–102. [DOI] [PubMed] [Google Scholar]
- Borroto‐Escuela DO, Romero‐Fernandez W, Garriga P, Ciruela F, Narvaez M, Tarakanov AO et al. (2013). G protein‐coupled receptor heterodimerization in the brain. Methods Enzymol 521: 281–294. [DOI] [PubMed] [Google Scholar]
- Borroto‐Escuela DO, Romero‐Fernandez W, Tarakanov AO, Gomez‐Soler M, Corrales F, Marcellino D et al. (2010). Characterization of the A2AR–D2R interface: focus on the role of the C‐terminal tail and the transmembrane helices. Biochem Biophys Res Commun 402: 801–807. [DOI] [PubMed] [Google Scholar]
- Borroto‐Escuela DO, Tarakanov AO, Guidolin D, Ciruela F, Agnati LF, Fuxe K (2011). Moonlighting characteristics of G protein‐coupled receptors: focus on receptor heteromers and relevance for neurodegeneration. IUBMB Life 63: 463–472. [DOI] [PubMed] [Google Scholar]
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR et al. (2003). Adenosine A2A‐dopamine D2 receptor–receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278: 46741–46749. [DOI] [PubMed] [Google Scholar]
- Canas PM, Porciúncula LO, Cunha GMA, Silva CG, Machado NJ, Oliveira JMA et al. (2009). Adenosine A2A receptor blockade prevents synaptotoxicity and memory dysfunction caused by beta‐amyloid peptides via p38 mitogen‐activated protein kinase pathway. J Neurosci 29: 14741–14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Cirrito JR, Lin X, Wang L, Verges DK, Dickson A et al. (2009). Caffeine suppresses amyloid‐beta levels in plasma and brain of Alzheimer's disease transgenic mice. J Alzheimers Dis 17: 681–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Loewenstein DA, Lin X, Zhang C, Wang L, Duara R et al. (2012). High blood caffeine levels in MCI linked to lack of progression to dementia. J Alzheimers Dis 30: 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter B, Nehmé R, Warne T, Leslie AG, Tate CG (2016). Structure of the adenosine A(2A) receptor bound to an engineered G protein. Nature 536: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Kachroo A, Schintu N, Xu K, Schwarzschild MA, Wardas J et al. (2009). Inactivation of neuronal forebrain A receptors protects dopaminergic neurons in a mouse model of Parkinson's disease. J Neurochem 111: 1478–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic C, Linden J (2016). Purinergic regulation of the immune system. Nat Rev Immunol 16: 177–192. [DOI] [PubMed] [Google Scholar]
- Chen GQ, Chen YY, Wang XS, Wu SZ, Yang HM, Xu HQ et al. (2010). Chronic caffeine treatment attenuates experimental autoimmune encephalomyelitis induced by guinea pig spinal cord homogenates in Wistar rats. Brain Res 1309: 116–125. [DOI] [PubMed] [Google Scholar]
- Chen JF, Eltzschig HK, Fredholm BB (2013). Adenosine receptors as drug targets — what are the challenges? Nat Rev Drug Discov 12: 265–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D et al. (1999). A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci 19: 9192–9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Liang D, Zuo A, Shao H, Kaplan HJ, Sun D (2015). An A2B adenosine receptor agonist promotes Th17 autoimmune responses in experimental autoimmune uveitis (EAU) via dendritic cell activation. PLoS One 10: e0132348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Pedata F (2008). Modulation of ischemic brain injury and neuroinflammation by adenosine A2A receptors. Curr Pharm Des 14: 1490–1499. [DOI] [PubMed] [Google Scholar]
- Chen JF, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P et al. (2007). Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol 83: 310–331. [DOI] [PubMed] [Google Scholar]
- Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M et al. (2001). Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci 21: RC143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gawryluk JW, Wagener JF, Ghribi O, Geiger JD (2008a). Caffeine blocks disruption of blood–brain barrier in a rabbit model of Alzheimer's disease. J Neuroinflammation 5: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Lan X, Roche I, Liu R, Geiger JD (2008b). Caffeine protects against MPTP‐induced blood–brain barrier dysfunction in mouse striatum. J Neurochem 107: 1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M et al. (2006). Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1–A2A receptor heteromers. J Neurosci 26: 2080–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colino‐Oliveira M, Rombo DM, Dias RB, Ribeiro JA, Sebastião AM (2016). BDNF‐induced presynaptic facilitation of GABAergic transmission in the hippocampus of young adults is dependent of TrkB and adenosine A2A receptors. Purinergic Signal 12: 283–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coustan‐Smith E, Song G, Clark C, Key L, Liu P, Mehrpooya M et al. (2011). New markers for minimal residual disease detection in acute lymphoblastic leukemia. Blood 117: 6267–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ et al. (2012). Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J 26: 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA (2005). Neuroprotection by adenosine in the brain: from A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal 1: 111–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA (2016). How does adenosine control neuronal dysfunction and neurodegeneration? J Neurochem . doi: 10.1111/jnc.13724. [DOI] [PubMed] [Google Scholar]
- Dai SS, Zhou YG, Li W, An JH, Li P, Yang N et al. (2010). Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci 30: 5802–5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Igna OP, Fett P, Gomes MW, Souza DO, Cunha RA, Lara DR (2007). Caffeine and adenosine A(2a) receptor antagonists prevent beta‐amyloid (25‐35)‐induced cognitive deficits in mice. Exp Neurol 203: 241–245. [DOI] [PubMed] [Google Scholar]
- Dall'Igna OP, Porciúncula LO, Souza DO, Cunha RA, Lara DR (2003). Neuroprotection by caffeine and adenosine A2A receptor blockade of beta‐amyloid neurotoxicity. Br J Pharmacol 138: 1207–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Adinolfi E (2016). Extracellular purines, purinergic receptors and tumor growth. Oncogene . doi: 10.1038/onc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T, Kewley EM, Brodsky KS, Tak E, Bonney S, Gobel M et al. (2014). Identification of hypoxia‐inducible factor HIF‐1A as transcriptional regulator of the A2B adenosine receptor during acute lung injury. J Immunol 192: 1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein A, Patterson S, Ravid K (2015). The many faces of the A2b adenosine receptor in cardiovascular and metabolic diseases. J Cell Physiol 230: 2891–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig HK (2009). Adenosine: an old drug newly discovered. Anesthesiology 111: 904–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Costentin J, Vaugeois JM (2003). Adenosine A2A receptors and depression. Neurology 61: S82–S87. [DOI] [PubMed] [Google Scholar]
- Feoktistov I, Biaggioni I, Cronstein BN (2009). Adenosine receptors in wound healing, fibrosis and angiogenesis. Handb Exp Pharmacol 193: 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I (2003). Mast cell‐mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res 92: 485–492. [DOI] [PubMed] [Google Scholar]
- Ferré S, Bonaventura J, Tomasi D, Navarro G, Moreno E, Cortés A et al. (2016). Allosteric mechanisms within the adenosine A2A–dopamine D2 receptor heterotetramer. Neuropharmacology 104: 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrè S, von Euler G, Johansson B, Fredholm BB, Fuxe K (1991). Stimulation of high‐affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc Natl Acad Sci U S A 88: 7238–7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira DG, Batalha VL, Vicente Miranda H, Coelho JE, Gomes R, Gonçalves FQ et al. (2015). Adenosine A2A receptors modulate α‐synuclein aggregation and toxicity. Cereb Cortex pii: bhv268. [DOI] [PubMed] [Google Scholar]
- Figler RA, Wang G, Srinivasan S, Jung DY, Zhang Z, Pankow JS et al. (2011). Links between insulin resistance, adenosine A2B receptors, and inflammatory markers in mice and humans. Diabetes 60: 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB (2007). Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 14: 1315–1323. [DOI] [PubMed] [Google Scholar]
- Fredholm BB (2014). Adenosine–a physiological or pathophysiological agent? J Mol Med 92: 201–206. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011). International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors–an update. Pharmacol Rev 63: 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Guidolin D, Agnati LF, Borroto‐Escuela DO (2015). Dopamine heteroreceptor complexes as therapeutic targets in Parkinson's disease. Expert Opin Ther Targets 19: 377–398. [DOI] [PubMed] [Google Scholar]
- Gomez G, Zhao W, Schwartz LB (2011). Disparity in FcepsilonRI‐induced degranulation of primary human lung and skin mast cells exposed to adenosine. J Clin Immunol 31: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Phillis JW (1994). CGS 15943, an adenosine A2 receptor antagonist, reduces cerebral ischemic injury in the Mongolian gerbil. Life Sci 55: PL61–PL65. [DOI] [PubMed] [Google Scholar]
- Gessi S, Cattabriga E, Avitabile A, Gafa' R, Lanza G, Cavazzini L et al. (2004). Elevated expression of A3 adenosine receptors in human colorectal cancer is reflected in peripheral blood cells. Clin Cancer Res 10: 5895–5901. [DOI] [PubMed] [Google Scholar]
- Gessi S, Fogli E, Sacchetto V, Merighi S, Varani K, Preti D et al. (2010). Adenosine modulates HIF‐1α, VEGF, IL‐8, and foam cell formation in a human model of hypoxic foam cells. Arterioscler Thromb Vasc Biol 30: 90–97. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Fazzi D, Stefanelli A, Varani K, Borea PA (2011a). Adenosine receptor targeting in health and disease. Expert Opin Investig Drugs 20: 1591–1609. [DOI] [PubMed] [Google Scholar]
- Gessi S, Merighi S, Sacchetto V, Simioni C, Borea PA (2011b). Adenosine receptors and cancer. Biochim Biophys Acta 1808: 1400–1412. [DOI] [PubMed] [Google Scholar]
- Gomes CV, Kaster MP, Tomé AR, Agostinho PM, Cunha RA (2011). Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta 1808: 1380–1399. [DOI] [PubMed] [Google Scholar]
- Gui L, Duan W, Tian H, Li C, Zhu J, Chen JF et al. (2009). Adenosine A2A receptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res 1297: 185–193. [DOI] [PubMed] [Google Scholar]
- Harada H, Asano O, Hoshino Y, Yoshikawa S, Matsukura M, Kabasawa Y et al. (2001a). 2‐Alkynyl‐8‐aryl‐9‐methyladenines as novel adenosine receptor antagonists: their synthesis and structure‐activity relationships toward hepatic glucose production induced via agonism of the A2B receptor. J Med Chem 44: 170–179. [DOI] [PubMed] [Google Scholar]
- Harada H, Asano O, Kawata T, Inoue T, Horizoe T, Yasuda N et al. (2001b). 2‐Alkynyl‐8‐aryladenines possessing an amide moiety: their synthesis and structure–activity relationships of effects on hepatic glucose production induced via agonism of the A2B adenosine receptor. Bioorg Med Chem 9: 2709–2726. [DOI] [PubMed] [Google Scholar]
- Hart ML, Grenz A, Gorzolla IC, Schittenhelm J, Dalton JH, Eltzschig HK (2011). Hypoxia‐inducible factor‐1α‐dependent protection from intestinal ischemia/reperfusion injury involves ecto‐5′‐nucleotidase (CD73) and the A2B adenosine receptor. J Immunol 186: 4367–4374. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Haskó G, Linden J, Cronstein B, Pacher P (2008). Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 7: 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield SM, Sitkovsky M (2016). A2A adenosine receptor antagonists to weaken the hypoxia‐HIF‐1α driven immunosuppression and improve immunotherapies of cancer. Curr Opin Pharmacol 29: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A et al. (2002). Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem 277: 18091–18097. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S et al. (2009). Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348[7‐[2‐[4‐2,4‐difluorophenyl]‐1‐piperazinyl]ethyl]‐2‐(2‐furanyl)‐7H‐pyrazolo[4,3 e][1,2,4]triazolo[1,5‐c]pyrimidin‐5‐[amine] in rodent models of movement disorders and depression. J Pharmacol Exp Ther 330: 294–303. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS (2006). Inflammation and metabolic disorders. Nature 444: 860–867. [DOI] [PubMed] [Google Scholar]
- Hu X, Adebiyi MG, Luo J, Sun K, Le TT, Zhang Y et al. (2016). Sustained elevated adenosine via ADORA2B promotes chronic pain through neuro‐immune interaction. Cell Rep 16: 106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Chason KD, Fredholm BB, Deshpande DA, Penn RB, Tilley SL (2008). Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J Allergy Clin Immunol 122: 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Chason KD, Patel JY, Naselsky WC, Tilley SL (2011). IL‐4 amplifies the pro‐inflammatory effect of adenosine in human mast cells by changing expression levels of adenosine receptors. PLoS One 6: e24947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll SA, Laroui H, Kolachala VL, Wang L, Garg P, Denning TL et al. (2012). A(₂B)AR expression in non‐immune cells plays an important role in the development of murine colitis. Dig Liver Dis 44: 819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri A, Andrews SP, Marshall FH (2017). Structurally enabled discovery of adenosine A2A receptor antagonists. Chem Rev 117: 21–37. [DOI] [PubMed] [Google Scholar]
- Johnston‐Cox H, Eisenstein AS, Koupenova M, Carroll S, Ravid K (2014). The macrophage A2B adenosine receptor regulates tissue insulin sensitivity. PLoS One 9: e98775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston‐Cox H, Koupenova M, Yang D, Corkey B, Gokce N, Farb MG et al. (2012). The A2b adenosine receptor modulates glucose homeostasis and obesity. PLoS One 7: e40584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörg M, May LT, Mak FS, Lee KC, Miller ND, Scammells PJ et al. (2015). Synthesis and pharmacological evaluation of dual acting ligands targeting the adenosine A2A and dopamine D2 receptors for the potential treatment of Parkinson's disease. J Med Chem 58: 718–738. [DOI] [PubMed] [Google Scholar]
- Kachroo A, Schwarzschild MA (2012). Adenosine A2A receptor gene disruption protects in an α‐synuclein model of Parkinson's disease. Ann Neurol 71: 278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Clevers H (2016). Reparative inflammation takes charge of tissue regeneration. Nature 529: 307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty‐Quintana H, Philip K, Acero LF, Chen NY, Weng T, Molina JG et al. (2015). Deletion of ADORA2B from myeloid cells dampens lung fibrosis and pulmonary hypertension. FASEB J 29: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty‐Quintana H, Weng T, Garcia‐Morales LJ, Chen NY, Pedroza M, Zhong H et al. (2013a). Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 49: 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty‐Quintana H, Xia Y, Blackburn MR (2013b). Adenosine signaling during acute and chronic disease states. J Mol Med (Berl) 91: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmouty‐Quintana H, Zhong H, Acero L, Weng T, Melicoff E, West JD et al. (2012). The A2B adenosine receptor modulates pulmonary hypertension associated with interstitial lung disease. FASEB J 26: 2546–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaster MP, Machado NJ, Silva HB, Nunes A, Ardais AP, Santana M et al. (2015). Caffeine acts through neuronal adenosine A2A receptors to prevent mood and memory dysfunction triggered by chronic stress. Proc Natl Acad Sci U S A 112: 7833–7838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D et al. (2005). TNF‐alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell Mol Life Sci 62: 2647–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala V, Ruble B, Vijay‐Kumar M, Wang L, Mwangi S, Figler H et al. (2008a). Blockade of adenosine A2B receptors ameliorates murine colitis. Br J Pharmacol 155: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachala VL, Vijay‐Kumar M, Dalmasso G, Yang D, Linden J, Wang L et al. (2008b). A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 135: 861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszałka P, Gołuńska M, Urban A, Stasiłojć G, Stanisławowski M, Majewski M et al. (2016). Specific activation of A3, A2A and A1 adenosine receptors in CD73‐knockout mice affects B16F10 melanoma growth, neovascularization, angiogenesis and macrophage infiltration. PLoS One 11: e0151420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koupenova M, Johnston‐Cox H, Vezeridis A, Gavras H, Yang D, Zannis V et al. (2012). A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 125: 354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmar C, Oyarzún C, Villablanca C, Jaramillo C, Alarcón S, Perez G et al. (2016). Reduced adenosine uptake and its contribution to signaling that mediates profibrotic activation in renal tubular epithelial cells: implication in diabetic nephropathy. PLoS One 11: e0147430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel U (2016). Purinergic receptors in psychiatric disorders. Neuropharmacology 104: 212–225. [DOI] [PubMed] [Google Scholar]
- Laurent C, Burnouf S, Ferry B, Batalha VL, Coelho JE, Baqi Y et al. (2016). CD73 Expression is an independent prognostic factor in prostate cancer. Clin Cancer Res 22: 158–166. [DOI] [PubMed] [Google Scholar]
- Leclerc BG, Charlebois R, Chouinard G, Allard B, Pommey S, Saad F et al. (2016). CD73 Expression is an independent prognostic factor in prostate cancer. Clin Cancer Res 22: 158–166. [DOI] [PubMed] [Google Scholar]
- Ledderose C, Hefti MM, Chen Y, Bao Y, Seier T, Li L et al. (2016). Adenosine arrests breast cancer cell motility by A3 receptor stimulation. Purinergic Signal . doi: 10.1007/s11302-016-9531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Hwang I, Lee JH, Lee HW, Jeong LS, Ha H (2013). The selective A3AR antagonist LJ‐1888 ameliorates UUO‐induced tubulointerstitial fibrosis. Am J Pathol 183: 1488–1497. [DOI] [PubMed] [Google Scholar]
- Leung CT, Li A, Banerjee J, Gao ZG, Kambayashi T, Jacobson KA et al. (2014). The role of activated adenosine receptors in degranulation of human LAD2 mast cells. Purinergic Signal 10: 465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Silva HB, Real J, Wang YM, Rial D, Li P et al. (2015). Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington's disease models. Neurobiol Dis 79: 70–80. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK (2011). Progress and challenges in translating the biology of atherosclerosis. Nature 473: 317–325. [DOI] [PubMed] [Google Scholar]
- Linden J (2013). Adenosine promotes tumor metastasis. Sci Signal 6: pe20. [DOI] [PubMed] [Google Scholar]
- Loi S, Pommey S, Haibe‐Kains B, Beavis PA, Darcy PK, Smyth MJ et al. (2013). CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 110: 11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz DKV, Lin RC, Jacobson KA (1995). Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol 287: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Le NB, Mills T, Chen NY, Karmouty‐Quintana H, Molina JG et al. (2016). Extracellular adenosine levels are associated with the progression and exacerbation of pulmonary fibrosis. FASEB J 30: 874–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma DF, Kondo T, Nakazawa T, Niu DF, Mochizuki K, Kawasaki T et al. (2010). Hypoxia‐inducible adenosine A2B receptor modulates proliferation of colon carcinoma cells. Hum Pathol 41: 1550–1557. [DOI] [PubMed] [Google Scholar]
- Machado NJ, Simões AP, Silva HB, Ardais AP, Kaster MP, Garção P et al. (2016). Caffeine reverts memory but not mood impairment in a depression‐prone mouse strain with up‐regulated adenosine A2A receptor in hippocampal glutamate synapses. Mol Neurobiol . doi: 10.1007/s12035-016-9774-9. [DOI] [PubMed] [Google Scholar]
- MacKenzie WM, Hoskin DW, Blay J (1994). Adenosine inhibits the adhesion of anti‐CD3‐activated killer lymphocytes to adenocarcinoma cells through an A3 receptor. Cancer Res 54: 3521–3526. [PubMed] [Google Scholar]
- Madi L, Ochaion A, Rath‐Wolfson L, Bar‐Yehuda S, Erlanger A, Ohana G et al. (2004). The A3 adenosine receptor is highly expressed in tumor versus normal cells: potential target for tumor growth inhibition. Clin Cancer Res 10: 4472–4479. [DOI] [PubMed] [Google Scholar]
- Maia L, de Mendonça A (2002). Does caffeine intake protect from Alzheimer's disease? Eur J Neurol 9: 377–382. [DOI] [PubMed] [Google Scholar]
- Martire A, Pepponi R, Domenici MR, Ferrante A, Chiodi V, Popoli P (2013). BDNF prevents NMDA‐induced toxicity in models of Huntington's disease: the effects are genotype specific and adenosine A2A receptor is involved. J Neurochem 125: 225–235. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Agostinho P, Cunha RA, Chen JF (2013). Antagonistic interaction between adenosine A2A receptors and Na+/K+‐ATPase‐α2 controlling glutamate uptake in astrocytes. J Neurosci 33: 18492–184502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matos M, Augusto E, Machado NJ, dos Santos‐Rodrigues A, Cunha RA, Agostinho P (2012b). Astrocytic adenosine A2A receptors control the amyloid‐β peptide‐induced decrease of glutamate uptake. J Alzheimers Dis 31: 555–567. [DOI] [PubMed] [Google Scholar]
- Matos M, Augusto E, Santos‐Rodrigues AD, Schwarzschild MA, Chen JF, Cunha RA et al. (2012a). Adenosine A2A receptors modulate glutamate uptake in cultured astrocytes and gliosomes. Glia 60: 702–716. [DOI] [PubMed] [Google Scholar]
- Melani A, Cipriani S, Vannucchi MG, Nosi D, Donati C, Bruni P et al. (2009). Selective adenosine A2a receptor antagonism reduces JNK activation in oligodendrocytes after cerebral ischaemia. Brain 132: 1480–1495. [DOI] [PubMed] [Google Scholar]
- Melani A, Dettori I, Corti F, Cellai L, Pedata F (2015). Time‐course of protection by the selective A2A receptor antagonist SCH58261 after transient focal cerebral ischemia. Neurol Sci. [DOI] [PubMed] [Google Scholar]
- Melani A, Gianfriddo M, Vannucchi MG, Cipriani S, Baraldi PG, Giovannini MG et al. (2006). The selective A2A receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res 1073‐1074: 470–480. [DOI] [PubMed] [Google Scholar]
- Melani A, Pugliese AM, Pedata F (2014). Adenosine receptors in cerebral ischemia. Int Rev Neurobiol 119: 309–348. [DOI] [PubMed] [Google Scholar]
- Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E et al. (2005). A3 adenosine receptor activation inhibits cell proliferation via phosphatidylinositol 3‐kinase/Akt‐dependent inhibition of the extracellular signal‐regulated kinase 1/2 phosphorylation in A375 human melanoma cells. J Biol Chem 280: 19516–19526. [DOI] [PubMed] [Google Scholar]
- Merighi S, Borea PA, Gessi S (2015). Adenosine receptors and diabetes: focus on the A(2B) adenosine receptor subtype. Pharmacol Res 99: 229–236. [DOI] [PubMed] [Google Scholar]
- Merighi S, Mirandola P, Milani D, Varani K, Gessi S, Klotz KN et al. (2002). Adenosine receptors as mediators of both cell proliferation and cell death of cultured human melanoma cells. J Invest Dermatol 119: 923–933. [DOI] [PubMed] [Google Scholar]
- Mills JH, Kim DG, Krenz A, Chen JF, Bynoe MS (2012). A2A Adenosine receptor signalling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J Immunol 188: 5713–5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, Thompson LF, Mueller C, Waickman AT, Jalkanen S et al. (2008). CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A 105: 9325–9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor TR, Hanff TC (2015). Adenosine signaling in reserpine‐induced depression in rats. Behav Brain Res 286: 184–191. [DOI] [PubMed] [Google Scholar]
- Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E (1998). Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. Neuroreport 9: 3955–3959. [DOI] [PubMed] [Google Scholar]
- Müller CE, Lopes LV, Buée L, Blum D (2016). A2A adenosine receptor deletion is protective in a mouse model of tauopathy. Mol Psychiatry 21: 97–107. [DOI] [PubMed] [Google Scholar]
- Navarro G, Borroto‐Escuela DO, Fuxe K, Franco R (2016). Purinergic signaling in Parkinson's disease. Relevance for treatment. Neuropharmacology 104: 161–168. [DOI] [PubMed] [Google Scholar]
- Ntantie E, Gonyo P, Lorimer EL, Hauser AD, Schuld N, McAllister D et al. (2013). An adenosine‐mediated signaling pathway suppresses prenylation of the GTPase Rap1B and promotes cell scattering. Sci Signal 6: ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel W, Schulz JB (2016). Current and experimental treatments of Parkinson disease: a guide for neuroscientists. J Neurochem. doi: 10.1111/jnc.13750. [DOI] [PubMed] [Google Scholar]
- Ohta A (2016). A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta A, Kini R, Ohta A, Subramanian M, Madasu M, Sitkovsky M (2012). The development and immunosuppressive functions of CD4(+) CD25(+) FoxP3(+) regulatory T cells are under influence of the adenosine–A2A adenosine receptor pathway. Front Immunol 3: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X et al. (2015). Astrocytic adenosine receptor A2A and Gs‐coupled signaling regulate memory. Nat Neurosci 18: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedata F, Dettori I, Coppi E, Melani A, Fusco I, Corradetti R et al. (2016). Purinergic signalling in brain ischemia. Neuropharmacology 104: 105–130. [DOI] [PubMed] [Google Scholar]
- Peleli M, Hezel M, Zollbrecht C, Persson AE, Lundberg JO, Weitzberg E et al. (2015). In adenosine A2B knockouts acute treatment with inorganic nitrate improves glucose disposal, oxidative stress, and AMPK signaling in the liver. Front Physiol 6: 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, Borea PA, Varani K, Wilder T, Yee H, Chiriboga L et al. (2009). Adenosine signaling contributes to ethanol‐induced fatty liver in mice. J Clin Invest 119: 582–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JD, Goldberg JA, Surmeier DJ (2012). Adenosine A2a receptor antagonists attenuate striatal adaptations following dopamine depletion. Neurobiol Dis 45: 409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW (1995). The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res 705: 79–84. [DOI] [PubMed] [Google Scholar]
- Preti D, Baraldi PG, Moorman AR, Borea PA, Varani K (2015). History and perspectives of A2A adenosine receptor antagonists as potential therapeutic agents. Med Res Rev 35: 790–848. [DOI] [PubMed] [Google Scholar]
- Quezada C, Garrido W, Oyarzún C, Fernández K, Segura R, Melo R et al. (2013). 5′‐ectonucleotidase mediates multiple‐drug resistance in glioblastoma multiforme cells. J Cell Physiol 228: 602–608. [DOI] [PubMed] [Google Scholar]
- Quiroz C, Gulyani S, Ruiqian W, Bonaventura J, Cutler R, Pearson V et al. (2016). Adenosine receptors as markers of brain iron deficiency: implications for restless legs syndrome. Neuropharmacology. doi: 10.1016/j.neuropharm.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie K, Carrière I, de Mendonca A, Portet F, Dartigues JF, Rouaud O et al. (2007). The neuroprotective effects of caffeine: a prospective population study (the Three City Study). Neurology 69: 536–545.17679672 [Google Scholar]
- Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH et al. (2000). Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA 283: 2674–2679. [DOI] [PubMed] [Google Scholar]
- Rudich N, Dekel O, Sagi‐Eisenberg R (2015). Down‐regulation of the A3 adenosine receptor in human mast cells upregulates mediators of angiogenesis and remodeling. Mol Immunol 65: 25–33. [DOI] [PubMed] [Google Scholar]
- Rudich N, Ravid K, Sagi‐Eisenberg R (2012). Mast cell adenosine receptors function: a focus on the A3 adenosine receptor and inflammation. Front Immunol 3: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsing D, Müller CE, Verspohl EJ (2006). The impact of adenosine and A(2B) receptors on glucose homoeostasis. J Pharm Pharmacol 58: 1639–1645. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, Goldstein AE, Biaggioni I, Feoktistov I (2006). Cross‐talk between G(s)‐ and G(q)‐coupled pathways in regulation of interleukin‐4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol 70: 727–735. [DOI] [PubMed] [Google Scholar]
- Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I et al. (2011). Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol 187: 6120–6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Blackburn MR, Biaggioni I et al. (2008). Effect of A2B adenosine receptor gene ablation on adenosine‐dependent regulation of proinflammatory cytokines. J Pharmacol Exp Ther 324: 694–700. [DOI] [PubMed] [Google Scholar]
- Sandau US, Colino‐Oliveira M, Jones A, Saleumvong B, Coffman SQ, Liu L et al. (2016). Adenosine Kinase Deficiency in the Brain Results in Maladaptive Synaptic Plasticity. J Neurosci 36: 12117–12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C, Lunet N, Azevedo A, de Mendonça A, Ritchie K, Barros H (2010). Caffeine intake is associated with a lower risk of cognitive decline: a cohort study from Portugal. J Alzheimers Dis 20: S175–S185. [DOI] [PubMed] [Google Scholar]
- Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP et al. (2010). Signaling through the A2B adenosine receptor dampens endotoxin‐induced acute lung injury. J Immunol 184: 5271–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte G, Fredholm BB (2003). Signalling from adenosine receptors to mitogen‐activated protein kinases. Cell Signal 15: 813–827. [DOI] [PubMed] [Google Scholar]
- Sebastiao AM, Ribeiro JA (2009). Adenosine receptors and the central nervous system. Handb Exp Pharmacol 193: 471–534. [DOI] [PubMed] [Google Scholar]
- Serra S, Horenstein AL, Vaisitti T, Brusa D, Rossi D, Laurenti L et al. (2011). CD73‐generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug‐induced cell death. Blood 118: 6141–6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões AP, Machado NJ, Gonçalves N, Kaster MP, Simões AT, Nunes A et al. (2016). Adenosine A2A receptors in the amygdala control synaptic plasticity and contextual fear memory. Neuropsychopharmacology. doi: 10.1038/npp.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP (2009). Caffeine, cognitive failures and health in a non‐working community sample. Hum Psychopharmacol 24: 29–34. [DOI] [PubMed] [Google Scholar]
- Soriano A, Ventura R, Molero A, Hoen R, Casadó V, Cortés A et al. (2009). Adenosine A2A receptor‐antagonist/dopamine D2 receptor‐agonist bivalent ligands as pharmacological tools to detect A2A–D2 receptor heteromers. J Med Chem 52: 5590–5602. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg J, Beavis PA, Divisekera U, Liu MC, Möller A, Darcy PK et al. (2012). CD73‐deficient mice are resistant to carcinogenesis. Cancer Res 72: 2190–2196. [DOI] [PubMed] [Google Scholar]
- Stagg J, Divisekera U, Duret H, Sparwasser T, Teng MW, Darcy PK et al. (2011). CD73‐deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res 71: 2892–2900. [DOI] [PubMed] [Google Scholar]
- Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D et al. (2010). Anti‐CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 107: 1547–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG et al. (2006). Role of A2B adenosine receptor signaling in adenosine‐dependent pulmonary inflammation and injury. J Clin Invest 116: 2173–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Jiang X, Zhou Y, Dai Y (2015). Effects of A2BR on the biological behavior of mouse renal fibroblasts during hypoxia. Mol Med Rep 11: 4397–4402. [DOI] [PubMed] [Google Scholar]
- Tarditi A, Camurri A, Varani K, Borea PA, Woodman B, Bates G et al. (2006). Abbracchio, early and transient alteration of adenosine A2A receptor signaling in a mouse model of Huntington disease. Neurobiol Dis 23: 44–53. [DOI] [PubMed] [Google Scholar]
- Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ et al. (2013). Anti‐human CD73 monoclonal antibody inhibits metastasis formation in human breast cancer by inducing clustering and internalization of CD73 expressed on the surface of cancer cells. J Immunol 191: 4165–4173. [DOI] [PubMed] [Google Scholar]
- Trifilieff P, Rives ML, Urizar E, Piskorowski RA, Vishwasrao HD, Castrillon J et al. (2011). Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2– adenosine A2A receptor complexes in the striatum. Biotechniques 51: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueblood KE, Mohr S, Dubyak GR (2011). Purinergic regulation of high‐glucose‐induced caspase‐1 activation in the rat retinal Müller cell line rMC‐1. Am J Physiol Cell Physiol 301: C1213–C1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui S, Schnermann J, Noorbakhsh F, Henry S, Yong VW, Winston BW et al. (2004). A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci 24: 1521–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcotte M, Spring K, Pommey S, Chouinard G, Cousineau I, George J et al. (2015). CD73 is associated with poor prognosis in high‐grade serous ovarian cancer. Cancer Res 75: 4494–4503. [DOI] [PubMed] [Google Scholar]
- Tyebji S, Saavedra A, Canas PM, Pliassova A, Delgado‐García JM, Alberch J et al. (2015). Hyperactivation of D1 and A2A receptors contributes to cognitive dysfunction in Huntington's disease. Neurobiol Dis 74: 41–57. [DOI] [PubMed] [Google Scholar]
- Varani K, Maniero S, Vincenzi F, Targa M, Stefanelli A, Maniscalco P et al. (2011). A₃ receptors are overexpressed in pleura from patients with mesothelioma and reduce cell growth via Akt/nuclear factor‐κB pathway. Am J Respir Crit Care Med 183: 522–530. [DOI] [PubMed] [Google Scholar]
- Varani K, Rigamonti D, Sipione S, Camurri A, Borea PA, Cattabeni F (2001). Aberrant amplification of A(2A) receptor signaling in striatal cells expressing mutant huntingtin. FASEB J 15: 1245–1247. [PubMed] [Google Scholar]
- Varani K, Vincenzi F, Tosi A, Gessi S, Casetta I, Granieri G et al. (2010). A2A adenosine receptor overexpression and functionality, as well as TNF‐alpha levels, correlate with motor symptoms in Parkinson's disease. FASEB J 24: 587–598. [DOI] [PubMed] [Google Scholar]
- Viana da Silva S, Haberl MG, Zhang P, Bethge P, Lemos C, Gonçalves N et al. (2016). Early synaptic deficits in the APP/PS1 mouse model of Alzheimer's disease involve neuronal adenosine A2A receptors. Nat Commun 7: 11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindeirinho J, Santiago AR, Cavadas C, Ambrósio AF, Santos PF (2016). The adenosinergic system in diabetic retinopathy. J Diabetes Res 2016 4270301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Du C, Lv J, Zhao G, Li Z, Wu Z et al. (2013). Blocking A2B adenosine receptor alleviates pathogenesis of experimental autoimmune encephalomyelitis via inhibition of IL‐6 production and Th17 differentiation. J Immunol 190: 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kurtz CC, Black SG, Ross WG, Alam MS, Linden J et al. (2011). The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL‐6. J Immunol 186: 6746–6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods LT, Ajit D, Camden JM, Erb L, Weisman GA (2016). Purinergic receptors as potential therapeutic targets in Alzheimer's disease. Neuropharmacology 104: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Di Luca DG, Orrú M, Xu Y, Chen JF, Schwarzschild MA (2016). Neuroprotection by caffeine in the MPTP model of Parkinson's disease and its dependence on adenosine A2A receptors. Neuroscience 322: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Kobayashi M, Kanda T (2014a). Involvement of adenosine A2A receptors in depression and anxiety. Int Rev Neurobiol 119: 373–393. [DOI] [PubMed] [Google Scholar]
- Yamada K, Kobayashi M, Shiozaki S, Ohta T, Mori A, Jenner P et al. (2014b). Antidepressant activity of the adenosine A2A receptor antagonist, istradefylline (KW‐6002) on learned helplessness in rats. Psychopharmacology (Berl) 231: 2839–2849. [DOI] [PubMed] [Google Scholar]
- Yang T, Gao X, Sandberg M, Zollbrecht C, Zhang XM, Hezel M et al. (2015). Abrogation of adenosine A1 receptor signalling improves metabolic regulation in mice by modulating oxidative stress and inflammatory responses. Diabetologia 58: 1610–1620. [DOI] [PubMed] [Google Scholar]
- Yang T, Zollbrecht C, Winerdal ME, Zhuge Z, Zhang XM, Terrando N et al. (2016). Genetic abrogation of adenosine A3 receptor prevents uninephrectomy and high salt‐induced hypertension. J Am Heart Assoc. doi: 10.1161/JAHA.116.003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda N, Inoue T, Horizoe T, Nagata K, Minami H, Kawata T et al. (2003). Functional characterization of the adenosine receptor contributing to glycogenolysis and gluconeogenesis in rat hepatocytes. Eur J Pharmacol 459: 159–166. [DOI] [PubMed] [Google Scholar]
- Ye L, Van Eps N, Zimmer M, Ernst OP, Prosser RS (2016). Activation of the A2A adenosine G‐protein‐coupled receptor by conformational selection. Nature 533: 265–268. [DOI] [PubMed] [Google Scholar]
- Young A, Ngiow SF, Barkauskas DS, Sult E, Hay C, Blake SJ et al. (2016). Co‐inhibition of CD73 and A2AR adenosine signaling improves anti‐tumor immune responses. Cancer Cell 30: 391–403. [DOI] [PubMed] [Google Scholar]
- Yu L, Huang Z, Mariani J, Wang Y, Moskowitz M, Chen JF (2004). Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med 10: 1081–1087. [DOI] [PubMed] [Google Scholar]
- Yu L, Shen HY, Coelho JE, Araújo IM, Huang QY, Day YJ et al. (2008). Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann Neurol 63: 338–346. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhong H, Everett TH 4th, Wilson E, Chang R, Zeng D et al. (2014). Blockade of A2B adenosine receptor reduces left ventricular dysfunction and ventricular arrhythmias 1 week after myocardial infarction in the rat model. Heart Rhythm 11: 101–109. [DOI] [PubMed] [Google Scholar]
- Zhang M, Li XL, Li H, Wang S, Wang CC, Yue LT et al. (2016). Activation of the adenosine A2A receptor exacerbates experimental autoimmune neuritis in Lewis rats in association with enhanced humoral immunity. J Neuroimmunol 293: 129–136. [DOI] [PubMed] [Google Scholar]
- Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou Z et al. (2007). RNA interference of ecto‐5′‐nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis 24: 439–448. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Murthy JN, Zeng D, Belardinelli L, Blackburn MR (2010). Alterations in adenosine metabolism and signaling in patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. PLoS One 5: e9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Schneider DJ, Morschl E, Song L, Pedroza M, Karmouty‐Quintana H et al. (2011). Distinct roles for the A2B adenosine receptor in acute and chronic stages of bleomycin‐induced lung injury. J Immunol 186: 1097–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H (2000). Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmakol 362: 299–309. [DOI] [PubMed] [Google Scholar]


