Abstract
With the recognition that a sentinel fracture leads to a high imminent risk of fracture, we discuss the implications and challenges of using imminent fracture risk in the secondary fracture prevention setting.
Dear Editor
With an estimated 520,000 fragility fractures every year in the United Kingdom, delivering effective and efficient healthcare for this patient group has significant consequences for patients, families, the NHS and society[1]. A fragility fracture is a major risk factor for further fractures[2], and healthcare systems are now beginning to recognise the benefits of secondary fracture prevention[3]. Despite this, less than 50% of patients receive effective secondary fracture prevention after a fragility fracture[4]. This has led to national[5, 6] and international[7–11] initiatives to improve clinical services by implementing fracture liaison services (FLSs). Successful funding of a new FLS is usually influenced by the number of fractures it is expected to prevent in the first few years after an index fracture. The expected number of fractures prevented is in turn determined by the baseline risk of subsequent fracture, the number of patients at high enough fracture risk to warrant anti-osteoporosis medication (AOM) and the degree of fracture risk reduction by AOMs. Underestimating fracture risk in the post-fracture period will lead to fewer expected fractures prevented and lower perceived benefit of the FLS by payers, and importantly also by patients, families, healthcare providers and payers. Tools are available to determine the long-term risk of fracture based on patient factors, including previous fracture [12–15]. Of these, FRAX and QFracture have been incorporated within UK NICE clinical guidance[16], and the FRAX -derived intervention threshold is used to guide recommendations for AOM in the NHS[17].
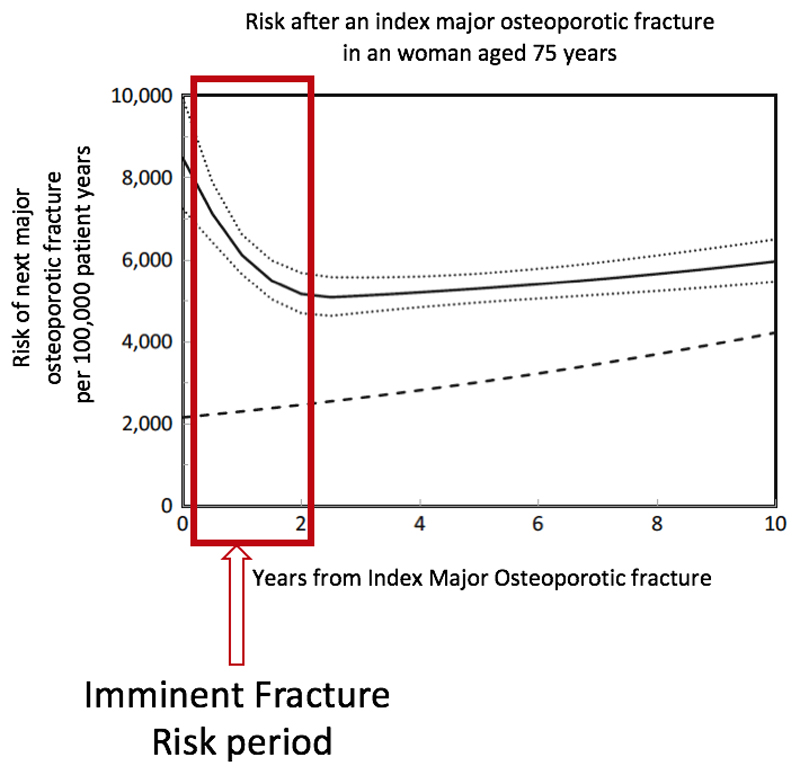
The risk of subsequent fracture is time-dependent, with much higher fracture risk in the first two years after an index fracture, a period that might be termed as “imminent fracture risk” (IFR) (Figure 1) [18–20]. Recency of fracture is one of several determinants of IFR. Whereas FRAX and similar models include previous fracture history, the focus of these algorithms has been on use in primary care, and the recency and site of fracture are not considered both of which significantly influence imminent risk. Linear interpolation of FRAX risk, for example by dividing the 10-year probability by 5 to estimate the 2-year probability (“interpolated-FRAX”), thus necessarily underestimates shorter-term risk immediately following a fracture [21]. This is particularly relevant to the FLS population given that by definition all cases have had a recent fracture[22]. Below we explore the potential benefits and challenges presented by the concept of IFR for assessing the benefits of an FLS service, and potential implications for clinical assessment in the context of immediate secondary prevention.
Figure 1.
Time dependency of re-fracture after index fracture adapted from Johansson et al.[15]
Solid line: Risk per 100,000; Dotted lines: 95%CI; Dashed line: risk of first major osteoporotic fracture in population
Many definitions of IFR or near-term risk have been used, varying by the sites of the index and subsequent fractures included and the period of interest [18, 23, 24]. Given the time-dependency of subsequent fracture risk, we suggest IFR in the FLS setting could be defined as the risk of any subsequent fragility fracture within two years of the index fracture when the majority of recurrent fractures have occurred[21].
Estimating the IFR within an FLS population is feasible. From observational cohort studies, the rate of subsequent fragility fracture within two years in women varies from 7.6 to 23.2% [19, 21, 25, 26]. Important determinants of the absolute IFR include age, gender, fracture site, bone mineral density and specific comorbidities [18, 21, 23–28].
For IFR to be relevant in the FLS setting, consideration should be given to the therapeutic modalities that can rapidly reduce fracture risk well within the two years after an index fracture. Traditional treatment strategies in the UK have used a stepwise approach driven by cost and tolerability[29] with oral bisphosphonates offered as first-line therapy. Significant fractures occur in the period of IFR despite treatment with oral bisphosphonates as these agents do not provide optimal fracture risk reduction early after initiation, requiring at least 6 to 12 months of adherent therapy before a significant difference in non-vertebral fracture rates is observed [30], with oral agents reaching maximal observed reduction in fracture risk by 36 months[31, 32]. Recently, in head-to-head randomised controlled trials, specific AOMs (teriparatide[33], romosozumab[34] and denosumab[35]- noting the denosumab trial had a randomized open-label alendronate comparator arm) demonstrate both earlier onset of fracture reduction and superiority over oral bisphosphonates within the IFR period of 2 years, and so could be classified as potent AOMs.
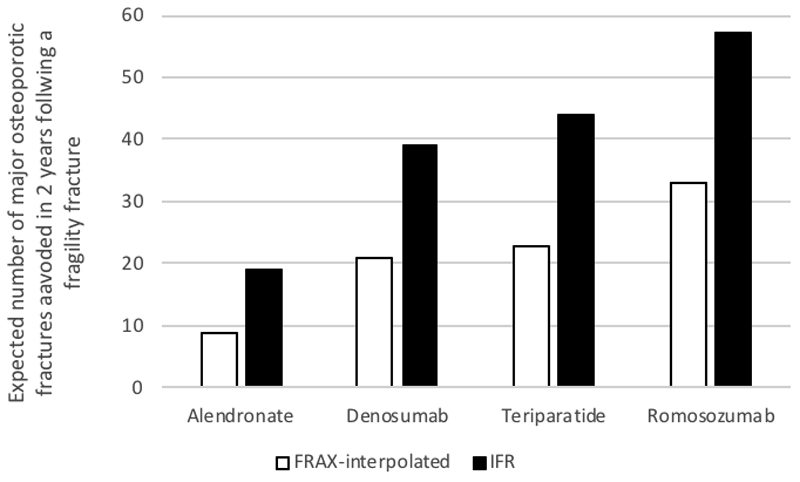
For fracture liaison services, the expected number of fractures prevented is directly related to the expected fracture rate in the IFR period and the risk reduction through the use of quicker acting potent AOMs. For example, for a population represented by 1,000 UK women aged 75 years with a previous fracture and a femoral neck BMD T-score of -2.9, using interpolated-FRAX, the expected number of major osteoporotic fractures within two years is 66 [36]. Using fracture reductions from an indirect treatment comparison at 12 and 24 months for specific fracture sites, based on data from all relevant RCTs identified through a systematic literature review [36], the number of major osteoporotic fracture expected to be prevented within two years varies from 9 to 33 by AOM per 1,000 patients treated immediately following index fracture (Figure 2). When the same model uses IFR instead of interpolated-FRAX, the estimated number of subsequent fractures increases to 107 and the number of fractures avoided almost doubles (mean increase of 91%). Moreover, as IFR applies every time a fracture occurs, potential additional fractures avoided and their consequences can be expected to compound over patients’ lifetimes with its corresponding positive impact on patients’ quality of life and societal costs[37].
Figure 2.
Expected number of major osteoporotic fractures prevented per 1,000 patients treated immediately following index fracture by different anti-osteoporosis medications using interpolated-FRAX vs. IFR after an index fracture[32]
While IFR has clear implications for the planning and justification of FLS services; it also leads to the equally clear message that potent AOMs should be considered promptly following a fragility fracture. The use of potent AOMs in an IFR approach to risk assessment is in line with treat-to-target strategies recommending potent AOMs used first followed by maintenance therapy with bisphosphonates afterwards [38–41]. However, the route to the incorporation of IFR in clinical assessment pathways, as opposed to its use in service planning, is yet to be defined. For example, the specific threshold required for a patient to be recommended potent AOMs might be based on an IFR threshold reached as well as age, gender, bone mineral density, fracture type, number and recency. In certain situations, the skeletal site of the fracture and the number of previous fractures may suffice. Alternatively, since IFR markedly influences FRAX 10 year fracture probability, a threshold based on a modified form of this metric might offer a further way forward.
There are many apparent challenges of implementing an IFR approach for UK FLSs. For it to be effective, eligible patients need to be identified, investigated, initiated and adhering to AOM soon after the index fracture. Results from the 2017 UK national audit of FLSs demonstrated that only 6% of submitted patients had a sentinal vertebral fracture, 41% of patients were monitored within 16 weeks of index fracture, and 31% had initiated therapy[5]. Improving detection of vertebral fractures is likely to require integration with radiology systems[42]; reducing the time to treatment is likely to need integration of FLS directly into existing orthopaedic pathways, minimising additional clinical workup[43]. While the benefits of potent AOMs in the setting of a recent fracture have not been formally tested, subgroup analyses from studies stratified by recency of fracture have been encouraging[34, 44]. Further, there are no comparative data with intravenous bisphosphonates and the potent AOMs listed above. The data informing IFR calculations and potential benefits mainly come from non-UK sources, where fracture risk may differ due to genetic and environmental factors. The benefits need to be weighed against costs, potential side-effects and the ability of the NHS to rapidly identify, investigate and initiate therapy in the real-world setting. From a payer perspective, work is urgently needed to simulate the impacts of incorporating an IFR approach into secondary fracture prevention on the clinical and cost-effectiveness of the intervention in a real-world FLS population, considering differences in age, gender, fracture site and type of AOM administered. This is particularly relevant for FLSs, whose current benefits are usually calculated based on a uniform interpolation of fracture risk using 10-year values and generic alendronate. Consideration of prior fracture location and recency in future versions of FRAX may thus offer a further opportunity to assess these impacts, especially given the global priority for establishing the benefits for sustainable resourcing of effective FLSs[42].
In summary, we recommend the IFR approach to address the need for accurate estimation of the expected number of fractures prevented in the FLS setting and guide recommendation of specific, potent AOMs that demonstrate a rapid onset of fracture protection and superiority over oral bisphosphonates.
Footnotes
Disclosure of potential conflicts of interests
The concept for this paper arose from a meeting that was funded by UCB Pharma, with no further funding provided. M Charokopou, E Toth, K Donnelly and C Libanati are employees of UCB. Outside of the submitted work: MK Javaid has received research grants and speaker honorarium from UCB, Lilly UK and Amgen; R Pinedo-Villanueva has been co-applicant in research grants from Amgen; C Cooper has received lecture fees and honoraria from Amgen, Danone, Eli Lilly, GSK, Medtronic, Merck, Nestlé, Novartis, Pfizer, Roche, Servier, Shire, Takeda and UCB; D Prieto-Alhambra has received research grants from Servier, Amgen and UCB and his department has received speaker and consultancy fees from Amgen and UCB respectively.
References
- 1.Kanis J, McCloskey E, Harvey NC, Javaid MK, Borgstrom F. Broken Bones, Broken Lives: A roadmap to solve the fragility fracture crisis in the United Kingdom. Broken Bone, Broken Lives:. International Osteoporosis Foundation; 2018. pp. 1–19. http://share.iofbonehealth.org/EU-6-Material/Reports/IOF_report_UK.pdf. [Google Scholar]
- 2.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 3.Marsh D, Akesson K, Beaton DE, Bogoch ER, Boonen S, Brandi ML, McLellan AR, Mitchell PJ, Sale JE, Wahl DA. Coordinator-based systems for secondary prevention in fragility fracture patients. Osteoporos Int. 2011;22:2051–2065. doi: 10.1007/s00198-011-1642-x. [DOI] [PubMed] [Google Scholar]
- 4.Klop C, Gibson-Smith D, Elders PJ, Welsing PM, Leufkens HG, Harvey NC, Bijlsma JW, van Staa TP, de Vries F. Anti-osteoporosis drug prescribing after hip fracture in the UK: 2000-2010. Osteoporos Int. 2015;26:1919–1928. doi: 10.1007/s00198-015-3098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulton C, Gallagher C, Rai S, Tsang C, Vasilakis N, Javaid MK. In: Royal College of Physicians. Fracture Liaison Service Database (FLS-DB) clinical audit. FLS forward: Identifying high-quality care in the NHS for secondary fracture prevention. Programme FaFFA, editor. Royal Collge of Physicians; London: 2017. pp. 1–70. [Google Scholar]
- 6.Javaid MK, Boulton C, Gallagher C, Judge A, Vasilakis N. In: Fracture Liaison Service Database (FLS-DB) annual report: Leading FLS improvement: secondary fracture prevention in the NHS. Programme FaFFA, editor. Healthcare Quality Improvement Partnership; London: 2017. [Google Scholar]
- 7.Akesson K, Marsh D, Mitchell PJ, McLellan AR, Stenmark J, Pierroz DD, Kyer C, Cooper C. Capture the Fracture: a Best Practice Framework and global campaign to break the fragility fracture cycle. Osteoporos Int. 2013;24:2135–2152. doi: 10.1007/s00198-013-2348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javaid MK, Kyer C, Mitchell PJ, et al. Effective secondary fracture prevention: implementation of a global benchmarking of clinical quality using the IOF Capture the Fracture(R) Best Practice Framework tool. Osteoporosis Int. 2015;26:2573–2578. doi: 10.1007/s00198-015-3192-0. [DOI] [PubMed] [Google Scholar]
- 9.Eisman JA, Bogoch ER, Dell R, Harrington JT, McKinney RE, Jr, McLellan A, Mitchell PJ, Silverman S, Singleton R, Siris E. Making the first fracture the last fracture: ASBMR task force report on secondary fracture prevention. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1698. [DOI] [PubMed] [Google Scholar]
- 10.Lems WF, Dreinhofer KE, Bischoff-Ferrari H, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis. 2017;76:802–810. doi: 10.1136/annrheumdis-2016-210289. [DOI] [PubMed] [Google Scholar]
- 11.Geusens P, Eisman JA, Singer A, Van Den Berg J. Fracture Liaison Service. In: Bilezikian JP, editor. Primer in the Metabolic Bone Diseases and Disorders of Mineral Metabolism. John Wiley & Sons INc; 2019. pp. 405–411. [Google Scholar]
- 12.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporosis Int. 2008;19:385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanis JA, Johnell O, De Laet C, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 14.Collins GS, Mallett S, Altman DG. Predicting risk of osteoporotic and hip fracture in the United Kingdom: prospective independent and external validation of QFractureScores. BMJ. 2011;342:d3651–d3651. doi: 10.1136/bmj.d3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen ND, Frost SA, Center JR, Eisman JA, Nguyen TV. Development of prognostic nomograms for individualizing 5-year and 10-year fracture risks. Osteoporos Int. 2008;19:1431–1444. doi: 10.1007/s00198-008-0588-0. [DOI] [PubMed] [Google Scholar]
- 16.National Clinical Guideline Centre N. (NICE) NIfHaCE, editor. Osteoporosis: assessing the risk of fragility fracture: NICE clinical guideline 146. 2012 [Google Scholar]
- 17.National Clinical Guideline Centre N. Excellence NIfHaC, editor. Osteoporosis: Quality Standard 149. 2017 [Google Scholar]
- 18.Johansson H, Siggeirsdottir K, Harvey NC, Oden A, Gudnason V, McCloskey E, Sigurdsson G, Kanis JA. Imminent risk of fracture after fracture. Osteoporos Int. 2017;28:775–780. doi: 10.1007/s00198-016-3868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Geel TA, van Helden S, Geusens PP, Winkens B, Dinant GJ. Clinical subsequent fractures cluster in time after first fractures. Ann Rheum Dis. 2009;68:99–102. doi: 10.1136/ard.2008.092775. [DOI] [PubMed] [Google Scholar]
- 20.Roux C, Briot K. Imminent fracture risk. Osteoporos Int. 2017;28:1765–1769. doi: 10.1007/s00198-017-3976-5. [DOI] [PubMed] [Google Scholar]
- 21.Kanis JA, Johansson H, Oden A, et al. Characteristics of recurrent fractures. Osteoporosis Int. 2018;29:1747–1757. doi: 10.1007/s00198-018-4502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roux S, Cabana F, Carrier N, Beaulieu M, April PM, Beaulieu MC, Boire G. The World Health Organization Fracture Risk Assessment Tool (FRAX) underestimates incident and recurrent fractures in consecutive patients with fragility fractures. J Clin Endocrinol Metab. 2014;99:2400–2408. doi: 10.1210/jc.2013-4507. [DOI] [PubMed] [Google Scholar]
- 23.Bynum JPW, Bell JE, Cantu RV, Wang Q, McDonough CM, Carmichael D, Tosteson TD, Tosteson ANA. Second fractures among older adults in the year following hip, shoulder, or wrist fracture. Osteoporos Int. 2016;27:2207–2215. doi: 10.1007/s00198-016-3542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonafede M, Shi N, Barron R, Li X, Crittenden DB, Chandler D. Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch Osteoporos. 2016;11:26. doi: 10.1007/s11657-016-0280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Center JR, Bliuc D, Nguyen TV, Eisman JA. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA. 2007;297:387–394. doi: 10.1001/jama.297.4.387. [DOI] [PubMed] [Google Scholar]
- 26.van Helden S, Cals J, Kessels F, Brink P, Dinant GJ, Geusens P. Risk of new clinical fractures within 2 years following a fracture. Osteoporosis Int. 2006;17:348–354. doi: 10.1007/s00198-005-2026-x. [DOI] [PubMed] [Google Scholar]
- 27.Chapurlat RD, Bauer DC, Nevitt M, Stone K, Cummings SR. Incidence and risk factors for a second hip fracture in elderly women. The Study of Osteoporotic Fractures. Osteoporos Int. 2003;14:130–136. doi: 10.1007/s00198-002-1327-6. [DOI] [PubMed] [Google Scholar]
- 28.Weycker D, Edelsberg J, Barron R, Atwood M, Oster G, Crittenden DB, Grauer A. Predictors of near-term fracture in osteoporotic women aged >/=65 years, based on data from the study of osteoporotic fractures. Osteoporos Int. 2017;28:2565–2571. doi: 10.1007/s00198-017-4103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.NICE. Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. NICE; London: 2008. [Google Scholar]
- 30.Inderjeeth CA, Chan K, Kwan K, Lai M. Time to onset of efficacy in fracture reduction with current anti-osteoporosis treatments. J Bone Miner Metab. 2012;30:493–503. doi: 10.1007/s00774-012-0349-1. [DOI] [PubMed] [Google Scholar]
- 31.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet (London, England) 1996;348:1535–1541. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 32.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 33.Kendler DL, Marin F, Zerbini CAF, et al. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230–240. doi: 10.1016/S0140-6736(17)32137-2. [DOI] [PubMed] [Google Scholar]
- 34.Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, Maddox J, Fan M, Meisner PD, Grauer A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura T, Matsumoto T, Sugimoto T, et al. Clinical Trials Express: fracture risk reduction with denosumab in Japanese postmenopausal women and men with osteoporosis: denosumab fracture intervention randomized placebo controlled trial (DIRECT) J Clin Endocrinol Metab. 2014;99:2599–2607. doi: 10.1210/jc.2013-4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jonsson E, Borgström F, Strom O, Toth E, LibanatI C, Stollenwerk B, Kanis JA, Charokopou M. An Economic Value Framework to assess the cost-effectiveness of fracture prevention treatments in patients with osteoporosis at imminent risk of fracture. Value in Health. 2018;21:S295–S295. [Google Scholar]
- 37.Griffin XL, Parsons N, Achten J, Fernandez M, Costa ML. Recovery of health-related quality of life in a United Kingdom hip fracture population. The Warwick Hip Trauma Evaluation--a prospective cohort study. Bone Joint J. 2015;97-b:372–382. doi: 10.1302/0301-620X.97B3.35738. [DOI] [PubMed] [Google Scholar]
- 38.Lewiecki EM, Cummings SR, Cosman F. Treat-to-target for osteoporosis: is now the time? J Clin Endocrinol Metab. 2013;98:946–953. doi: 10.1210/jc.2012-3680. [DOI] [PubMed] [Google Scholar]
- 39.Cummings SR, Cosman F, Lewiecki EM, et al. Goal-Directed Treatment for Osteoporosis: A Progress Report From the ASBMR-NOF Working Group on Goal-Directed Treatment for Osteoporosis. J Bone Miner Res. 2017;32:3–10. doi: 10.1002/jbmr.3039. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari S, Reginster JY, Brandi ML, Kanis JA, Devogelaer JP, Kaufman JM, Feron JM, Kurth A, Rizzoli R. Unmet needs and current and future approaches for osteoporotic patients at high risk of hip fracture. Arch Osteoporos. 2016;11:37. doi: 10.1007/s11657-016-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kanis JA, Cooper C, Rizzoli R, Reginster JY. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blain H, Masud T, Dargent-Molina P, et al. A comprehensive fracture prevention strategy in older adults: the European Union Geriatric Medicine Society (EUGMS) statement. Aging Clin Exp Res. 2016;28:797–803. doi: 10.1007/s40520-016-0588-4. [DOI] [PubMed] [Google Scholar]
- 43.Senay A, Delisle J, Giroux M, Laflamme GY, Leduc S, Malo M, Nguyen H, Ranger P, Fernandes JC. The impact of a standardized order set for the management of non-hip fragility fractures in a Fracture Liaison Service. Osteoporos Int. 2016;27:3439–3447. doi: 10.1007/s00198-016-3669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geusens P, Marin F, Kendler DL, et al. Effects of Teriparatide Compared with Risedronate on the Risk of Fractures in Subgroups of Postmenopausal Women with Severe Osteoporosis: The VERO Trial. J Bone Miner Res. 2018;33:783–794. doi: 10.1002/jbmr.3384. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson E, Ström O, Spångéus A, Åkesson K, Ljunggren Ö, Borgström F, Banefelt J, Toth E, Libanati C, Charokopou M. Risk of Major Osteoporotic Fracture (Hip, Vertebral, Radius, Humerus [MOF]) After First, Second and Third Fragility Fracture In A Swedish General Population Cohort. Value in Health. 2017;20:A528. [Google Scholar]