Abstract
Before chromosomes segregate during mitosis in metazoans, they align at the cell equator by a process known as chromosome congression. This is in part mediated by the microtubule minus-end-directed kinetochore motor Dynein, which moves peripheral chromosomes to the poles along astral microtubules, and the plus-end-directed kinetochore motor CENP-E/Kinesin-7 that slides polar chromosomes along spindle microtubules towards the equator. Because different kinetochore motors move chromosomes in opposite directions along anisotropic spindle microtubules, a critical longstanding question has been how chromosomes are guided towards specific locations during mitosis. Here we discuss the exciting possibility that tubulin post-translational modifications, the so-called “tubulin code”, work as a navigation system for kinetochore-based chromosome motility along specific spindle microtubules, supporting congression to the cell equator.
Keywords: tubulin code, post-translational modifications, motors, detyrosination, CENP-E, Dynein
Motor-driven chromosome congression
Maintenance of genomic stability depends on the fidelity of chromosome segregation in mitosis. In metazoans, before chromosomes segregate, they align at the cell equator to form a metaphase plate, a process known as chromosome congression (see Glossary). This journey is initiated right after nuclear envelope breakdown (NEB) at the onset of mitosis, marking the beginning of prometaphase. At this stage, condensing chromosomes are confined within the former nuclear region and are kept away from large cellular organelles by the action of a fenestrated membranous envelope that allows the invasion of this chromosomal region by centrosomal microtubules (Schweizer et al., 2015). Depending on their initial position at NEB, chromosomes may undertake one of at least two possible courses to achieve congression (Bancroft et al., 2015, Barisic et al., 2014). Those chromosomes that are favorably positioned between the two spindle poles at NEB might have immediate access to microtubules emanating from both poles, a process that is facilitated by the organization of chromosomes in a ring-like conformation during early prometaphase (Magidson et al., 2011), and thus become near-simultaneously attached to both sister kinetochores. Upon bi-orientation, these chromosomes complete alignment to the cell equator most likely via microtubule depolymerisation-coupled pulling at the kinetochore (McIntosh et al., 2010, Mitchison et al., 1986). In contrast, a smaller fraction of peripheral chromosomes (typically between 15-20% in human U2OS and HeLa cells (Bancroft et al., 2015, Barisic et al., 2014)) located outside the interpolar region, and mainly exposed to microtubules emanating from a single spindle pole, are unable to bi-orient and their congression largely depends on the coordinated action of motor-proteins (Barisic et al., 2014). In this review we will focus on our comprehension of the motor-driven chromosome congression pathway. An excellent and up-to-date review on the depolymerisation-coupled pulling pathway can be found elsewhere (Auckland and McAinsh, 2015).
Glossary.
Chromosome congression: the process occurring during prometaphase of mitosis that accounts for the alignment of chromosomes to form a metaphase plate at the spindle equator.
Polar ejection forces: Forces resulting from the direct action of polymerizing astral microtubules over chromatin (pushing force), and the action of the microtubule plus-end-directed chromokinesins located on chromosome arms (pulling force), that lead to the random ejection of chromosomes away from mitotic spindle poles.
Syntelic attachments: Erroneous configuration in which both sister kinetochores are attached to microtubules from the same spindle pole. If not corrected, this configuration would contribute for chromosome missegregation during mitosis.
Tubulin acetylation: Enzymatic modification of tubulin through the attachment of one acetyl group to α- and/or β-tubulin. The most widely characterized acetylation event is the one involving K40 of α-tubulin, which faces the microtubule lumen and is mediated by the specific acetyltransferase αTat1. Several tubulin deacetylases (e.g. HDAC6 and Sirt2) have been reported to counteract the activity of αTat1. Acetylation of K40 of α-tubulin is normally associated with stable microtubules. Other acetylation events have been reported, but remain to be functionally characterized.
Tubulin code: Set of tubulin PTMs that generate microtubule diversity in cells and is read by microtubule-associated proteins (MAPs) and motors to account for different microtubule-based functions in space and time.
Tubulin detyrosination: Enzymatic removal of the last tyrosine residue from the α-tubulin c-terminal tail on specific subsets of polymerized microtubules. Tubulin detyrosination is mediated by a yet unidentified tubulin carboxipeptidase and reversed by a specific tubulin tyrosine ligase.
Tubulin modifying enzymes: Enzymes that can introduce post-translational modifications on tubulin, independently of specificity (some enzymes can modify other substrates).
Tubulin polyglutamylation: Enzymatic modification of tubulin through the attachment of polyglutamate side chains to specific glutamate residues in the c-terminal tail of both α- and β-tubulin.
Peripheral chromosomes are first brought to the proximate spindle pole by the minus end-directed kinetochore motor Dynein along astral microtubules (Li et al., 2007, Rieder and Alexander, 1990, Vorozhko et al., 2008, Yang et al., 2007) (Figure 1, key figure). The transport of polar chromosomes is then driven by the plus-end directed motors CENP-E at the kinetochores (Kapoor et al., 2006, Schaar et al., 1997, Wood et al., 1997) and chromokinesins on chromosome arms (Antonio et al., 2000, Funabiki and Murray, 2000, Wandke et al., 2012) (Figure 1). However, how these opposite and spatially distinct activities are coordinated to drive chromosome congression remained a critical unanswered question.
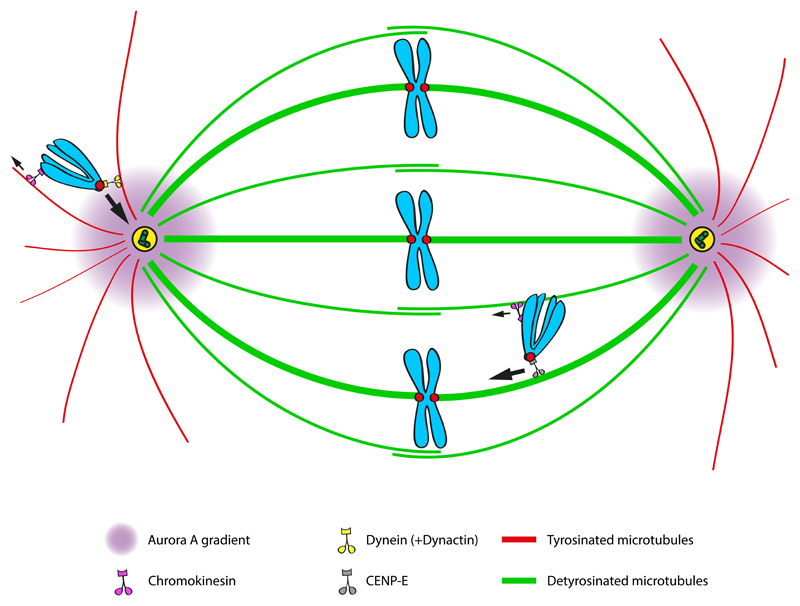
Figure 1. Key figure – Microtubule (de)tyrosination selectively guides kinetochore-based motor proteins required for accurate chromosome congression to the spindle equator.
Schematic representation of the impact of tubulin tyrosination/detyrosination on the regulation of motor proteins responsible for chromosome movements in mitosis. Dynein/Dynactin have a preference for tyrosinated astral microtubules and counteract chromokinesins-mediated PEFs. By bringing chromosomes to the vicinity of the spindle pole-localized Aurora A kinase, Dynein/Dynactic prevents premature stabilization of erroneous end-on attachments and contributes to the local activation of CENP-E. At the pole, CENP-E interacts with detyrosinated microtubules, becoming dominant over dynein to move chromosomes towards the equator. Thus, the levels of microtubule (de)tyrosination provide the directional bias for CENP-E-mediated chromosome transport and regulate the Dynein/CENP-E activity switch that facilitates chromosome congression.
Recent work provided insight into this problem and revealed that, during the initial poleward movement of peripheral chromosomes along astral microtubules, Dynein is the dominant force, overcoming active opposing forces by CENP-E and chromokinesins (Barisic et al., 2014). The use of laser microsurgery to physically separate the chromosome arms from the kinetochore-containing body after CENP-E inhibition allowed the dissection of the respective role of CENP-E and chromokinesins in chromosome transport from the poles towards the equator. Accordingly, it was found that Dynein keeps chromosomes tightly clustered at the poles, while acentric fragments (i.e. without kinetochores) are ejected in random directions away from the pole by chromokinesins (Barisic et al., 2014). This reveals a critical role of CENP-E in defining the directional movement of polar chromosomes exclusively towards the spindle equator (Figure 1). Interestingly, in the absence of stable end-on kinetochore-microtubule attachments, polar-ejection forces (PEFs) by chromokinesins appear to dominate over CENP-E (Iemura and Tanaka, 2015), suggesting that stabilization of kinetochore microtubule attachments is important for the action of CENP-E.
Chromokinesins promote the stabilization of end-on kinetochore-microtubule attachments (Cane et al., 2013, Drpic et al., 2015). Thus, the dominant role of Dynein over chromokinesins and CENP-E prevents the premature stabilization of end-on kinetochore-microtubule attachments, which otherwise could lead to the occurrence of errors (e.g. syntelic attachments) and would compromise CENP-E-mediated sliding of polar chromosomes along spindle microtubules towards the equator (Barisic et al., 2014). The spindle pole-associated Aurora A kinase also promotes the destabilization of end-on kinetochore-microtubule attachments on polar chromosomes, revealing a previously overlooked role for Aurora A in this process (Barisic et al., 2014). This was recently confirmed by two independent studies that demonstrated a role for a spindle-pole Aurora A activity gradient in the correction of erroneous kinetochore-microtubule attachments in mitosis and meiosis I (Chmatal et al., 2015, Ye et al., 2015). Thus, by bringing peripheral chromosomes to the spindle poles, Dynein prevents error formation by counteracting the role of chromokinesins in the stabilization of end-on kinetochore-microtubule attachments, while promoting Aurora A-mediated kinetochore-microtubule destabilization (Barisic and Maiato, 2015) (Figure 1).
Aurora A activity at the poles might also be involved in the molecular switch between Dynein and CENP-E activities on polar chromosomes. Indeed, Aurora A phosphorylation of CENP-E near the spindle poles is required for CENP-E function and was proposed to reduce its affinity for individual microtubules, thus favoring CENP-E interaction with bundled kinetochore-fibers (k-fibers) (Kim et al., 2010). However, it remains elusive how a reduction in microtubule affinity would enable CENP-E to overcome Dynein-mediated poleward forces on polar chromosomes. Taking into account the ability of CENP-E to mediate chromosome congression even in the absence of k-fibers (Cai et al., 2009, Iemura and Tanaka, 2015), it is unlikely that Aurora A-mediated phosphorylation of CENP-E is the sole factor regulating the switch between Dynein and CENP-E activities on polar chromosomes.
Tubulin post-translational modifications in the mitotic spindle
Similar to the “histone code” hypothesis in which epigenetic marks on histone tails expand the information potential of the genetic code (Jenuwein and Allis, 2001), tubulin post-translational modifications (PTMs) constitute the so-called “tubulin code” that was proposed to account for subcellular differentiation of distinct microtubule populations (Janke, 2014, Verhey and Gaertig, 2007). According to this hypothesis, molecular motors and microtubule-associated proteins (MAPs) “read” the tubulin code, as determined by different affinities/preferences for specific tubulin PTMs.
Tubulin PTMs include phosphorylation, polyglutamylation, acetylation and detyrosination (and related Δ2 modification) (Janke, 2014, Verhey and Gaertig, 2007) (Figure 2). Cdk1 is responsible for the phosphorylation of Ser172 on soluble β-tubulin specifically during mitosis (Fourest-Lieuvin et al., 2006), and has been shown to be required for normal cell division in yeast (Caudron et al., 2010). Detyrosination involves the removal of the last tyrosine on the C-terminal tail (CTT) of α-tubulin from polymerized microtubules by a yet unidentified tubulin carboxypeptidase (TCP) (Janke, 2014, Verhey and Gaertig, 2007). The action of a well-established and highly specific tubulin tyrosine ligase (TTL) reverts soluble tubulin into its tyrosinated state (Janke, 2014, Verhey and Gaertig, 2007). Acetylation of α-tubulin on Lys40 in the microtubule lumen is mediated by the acetyl-transferase αTat1 and counteracted by at least two known deacetylases - HDAC6 and Sirt2 - that also play important roles in other cellular processes (Janke, 2014, Verhey and Gaertig, 2007). Polyglutamylation involves the attachment of polyglutamate side chains to glutamate residues in the CTTs of both α- and β-tubulin, preferentially on polymerized microtubules (Regnard et al., 1998), a process that is catalyzed by nine different TTL-like (TTLL) glutamylases, and reversed by six deglutamylases (Janke, 2014, Verhey and Gaertig, 2007). Interestingly, tubulin acetylation, detyrosination and polyglutamylation are specifically enriched on kinetochore and possibly interpolar microtubules in the mitotic spindle (Bobinnec et al., 1998, Gundersen and Bulinski, 1986, Gundersen et al., 1984, Wilson and Forer, 1997) (Figure 3a), raising the attractive possibility that mitotic motors are able to distinguish these microtubule subpopulations from unmodified and more dynamic astral microtubules emanating from the spindle poles.
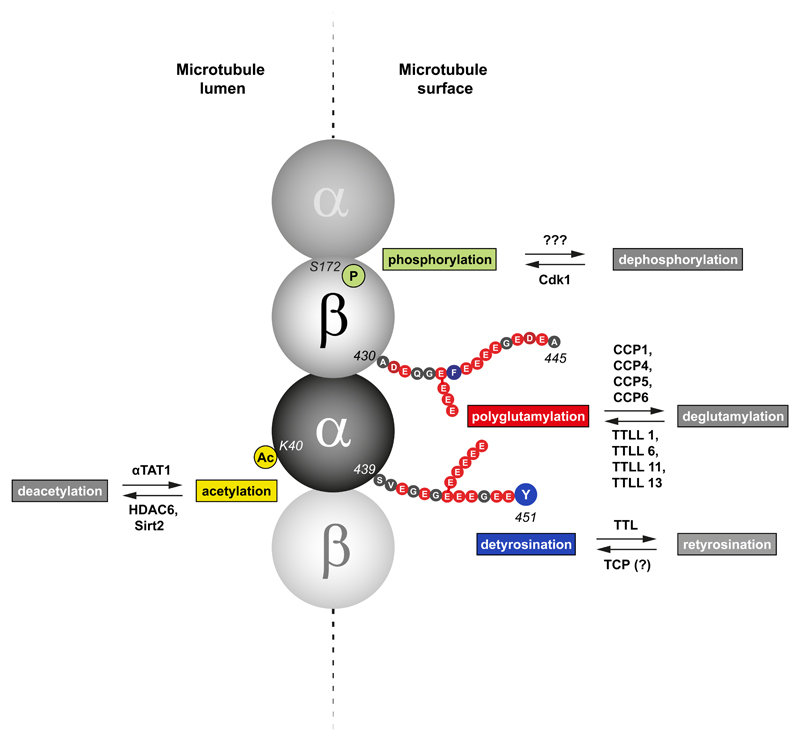
Figure 2. Tubulin PTMs with potential roles in mitosis.
Illustrative model depicting different post-translational modifications of the tubulin dimers within a microtubule (modified from ref. (7)) with potential roles during mitosis. Acetylation of lysine 40 of α–tubulin occurs on the microtubule lumen and is mediated by the tubulin acetyl transferase αTAT1, whereas deacetylation is performed by at least two deacetylases, HDAC6 and Sirt2. Detyrosination of α–tubulin and polyglutamylation of both α– and β–tubulin take place at the outer surface of the microtubule lattice, which is in direct contact with motor proteins. Tubulin detyrosination is driven by a yet-unidentified tubulin carboxypeptidase (TCP), while retyrosination requires action of the tubulin tyrosine ligase (TTL). Polyglutamylation is catalyzed by multiple TTL-like (TTLL) glutamylases and reversed by several deglutamylases. Phosphorylation of Ser172 of β-tubulin occurs specifically during mitosis and is mediated by Cdk1 kinase.
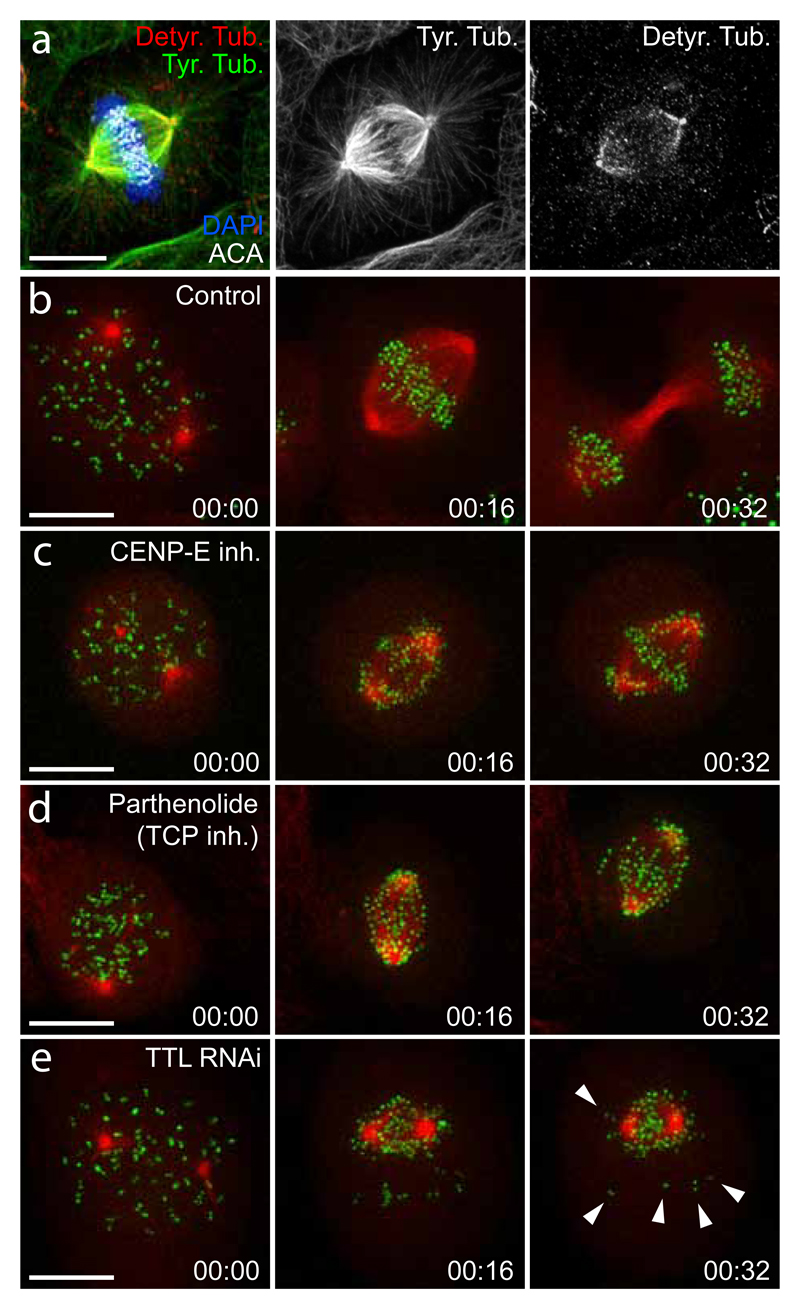
Figure 3. Microtubule detyrosination on a subset of mitotic spindle microtubules is required for accurate chromosome congression.
a) Point-scanning confocal immunofluorescence images of U2OS cells stained for DNA [4′,6- diamidino-2-phenylindole (DAPI), blue], anti-centromere antibody (ACA, white), tyrosinated tubulin (green), and detyrosinated tubulin (red). Note that the astral microtubules are immuno-stained exclusively with tyrosinated tubulin antibody, as detyrosination occurs only on more stable kinetochore (and possibly interpolar) microtubules. Scale bar, 10 μm. b-e) Spinning-disk confocal imaging of live U2OS cells stably expressing CENP-A–GFP and mCherry-tubulin. Pole-proximal chromosomes are unable to congress and remain locked at the poles when either CENP-E activity is inhibited (c) or microtubule detyrosination is prevented using the TCP inhibitor parthenolide (d). In contrast, TTL depletion prevents pole-proximal chromosomes to reach the pole causing their ejection in random directions (e). Arrowheads highlight chromosomes that cannot reach the pole, likely due to the Dynein loss of function on now detyrosinated astral microtubules and/or are ejected away from the pole by the enhanced CENP-E activity on detyrosinated astrals. Time, hours:min. Scale bar, 10 μm.
Although several tubulin PTMs coincide with more stable, long-lived microtubules, it remains unclear whether microtubule stability is a cause or consequence of the tubulin PTMs. Although these parameters are difficult to dissociate, several studies showed that altered levels of acetylation or detyrosination did not have any effect on microtubule stability (Khawaja et al., 1988, Maruta et al., 1986, Paturle et al., 1989, Webster et al., 1990), while taxol-stabilized microtubules show increased acetylation (Piperno et al., 1987, Wilson and Forer, 1997). Interestingly, overexpression of αTat1 destabilizes microtubules independently of its acetyltransferase activity (Kalebic et al., 2013a), whereas inhibition of HDAC6 stabilizes microtubules independently of its effect on tubulin acetylation (Zilberman et al., 2009). Overall, these results suggest that tubulin PTMs do not directly account for microtubule stability. Alternatively, the more stable spindle microtubules accumulate certain PTMs, possibly because they have more time to interact with tubulin modifying enzymes.
The tubulin code as a navigation system for chromosomes during mitosis
Previous works have implicated tubulin PTMs in the regulation of Kinesin-1-dependent transport in neurons (Hammond et al., 2010, Konishi and Setou, 2009, Maas et al., 2009, Reed et al., 2006). Moreover, pioneer in vitro reconstitution experiments revealed a subtle, but direct effect of some tubulin PTMs on Kinesin-1, Kinesin-2, Kinesin-13 and Dynein motor activities (Kaul et al., 2014, Sirajuddin et al., 2014). Thus, an exciting possibility would be that tubulin PTMs generate spatial cues that guide distinct mitotic motors along specific spindle microtubules. As so, the regulatory switch between Dynein and CENP-E on polar chromosomes, as well as the bias of CENP-E-mediated chromosome motion exclusively towards the equator, would be determined by distinct preferences of these kinetochore motors for microtubules with different PTMs (Figure 1). If this were the case, experimental perturbation of specific tubulin PTMs should phenocopy the inhibition of the motors that read the code and thus compromise distinct steps involved in the congression of polar chromosomes.
Experimental modification of the post-translational state of spindle microtubules can be achieved by perturbation of the respective tubulin modifying enzymes that account for specific tubulin PTMs. While this task might sound daunting for tubulin polyglutamylation, which is synergistically regulated by multiple glutamylases and deglutamylases, it is relatively straightforward for tubulin acetylation and detyrosination, given that these modifications are regulated by a single and specific enzyme (αTat1 and TTL, respectively). However, strong reduction of tubulin acetylation on mitotic spindles from human culture cells after αTat1 RNAi did not perturb chromosome congression, suggesting that mitotic motors involved in this process are insensitive to microtubule acetylation (Barisic et al., 2015). This is not particularly surprising given that acetylation of α-tubulin on Lys40 occurs inside the microtubule lumen (Janke, 2014, Soppina et al., 2012), and therefore is not in direct contact with mitotic motors travelling along the outer surface of the microtubule lattice. In contrast, experimental attenuation of tubulin detyrosination, either by means of overexpression of TTL, or by treating cells with the TCP inhibitor parthenolide (Fonrose et al., 2007, Whipple et al., 2013), results in few chromosomes locked at the spindle poles, phenocopying the abrogation of CENP-E activity (Barisic et al., 2015) (Figure 3b-d). Strikingly, RNAi-mediated knockdown of TTL forces spindle microtubule detyrosination, including astral microtubules, and polar chromosomes move in all directions, including towards the cell cortex, in a CENP-E-dependent manner (Barisic et al., 2015) (Figure 3e). Overall, these data indicate that CENP-E-mediated congression of polar chromosomes is sensitive to microtubule detyrosination.
But how does microtubule detyrosination regulate CENP-E activity? Parthenolide treatment in living cells displaces CENP-E from detyrosinated microtubules in G2 cells, indicating that the in vivo association of CENP-E with microtubules is strictly regulated by tubulin detyrosination (Barisic et al., 2015). In vitro reconstitution experiments using microtubules enriched either for tyrosinated or detyrosinated tubulin, further revealed that CENP-E is able to perform longer and faster runs, while carrying significantly larger cargo on detyrosinated microtubules (Barisic et al., 2015). Taken together, these data support a model in which CENP-E-dependent transport of polar chromosomes towards the spindle equator requires microtubule tracks that are detyrosinated (Figure 1).
A corollary from this model would be that, as opposed to CENP-E, Dynein has a preference for tyrosinated astral microtubules and thus mediate the motion of peripheral chromosomes to the vicinity of the poles, before CENP-E-mediated congression (Figure 1). It has long been known that tubulin CTTs are required for cytoplasmic Dynein processivity in vitro (McKenney et al., 2014, Wang and Sheetz, 2000) and axonemal Dyneins have recently been shown to be sensitive to the presence of tubulin CTTs and specific tubulin PTMs, with functional implications for flagellar motility (Alper et al., 2014, Kubo et al., 2010). Interestingly, the large Dynactin subunit p150, required for cytoplasmic Dynein processivity (McKenney et al., 2014), has a preference for tyrosinated microtubules (Peris et al., 2006), whereas irreversible modification of α-tubulin C-terminal tyrosine interferes with normal Dynein intracellular distribution (Eiserich et al., 1999), suggesting that Dynein/Dynactin function is regulated by the microtubule tyrosination state.
More recently, it was shown that the initiation of Dynein-driven motility in vitro is mediated by its co-factor p150/Dynactin and is strongly enhanced by tyrosinated microtubules (McKenney et al., 2016). Similar findings have been reported in vivo, where p150/Dynactin and tubulin tyrosination were shown to mediate the initiation of retrograde vesicle transport in neurons (Nirschl et al., 2016). This explains why CENP-E inhibition only partially rescues the random polar chromosome ejection after TTL RNAi (Barisic et al., 2015), as well as the mitotic spindle orientation problems observed in TTL-null fibroblasts (Peris et al., 2006), where forced detyrosination of astral microtubules might interfere with Dynein-mediated poleward movement and cortical microtubule interaction. Overall, these studies strengthen our “navigation system” hypothesis that helps explaining the Dynein/CENP-E switch that mediates congression of peripheral chromosomes (Figure 1). In summary, Dynein´s dominance over CENP-E during the initial poleward movement of peripheral chromosomes might be due to its preference for tyrosinated (astral) microtubules. At the poles, CENP-E, possibly activated by Aurora A (which additionally prevents the formation of end-on kinetochore-microtubule attachments), becomes dominant over dynein by selectively sliding polar chromosomes towards the equator along pre-stabilized detyrosinated spindle microtubules. Thus, in addition to regulating the motors, the cell might regulate the tracks in which they move on to guide chromosomes during mitosis. Full validation of this model will require a more complete understanding of how tubulin PTMs cooperate with Dynein co-factors (e.g. Spindly and Rod/Zw10/Zwilch complex) that specifically modulate Dynein functions at the kinetochore.
Concluding remarks
Despite being known for nearly 40 years, a comprehensive understanding of the biological roles of tubulin PTMs is still missing. Importantly, perturbation of tubulin PTMs in living cells and organisms has recently been made possible after the identification of some of the key enzymes that specifically modify tubulin (Barisic et al., 2015, Bosch Grau et al., 2013, Erck et al., 2005, Kalebic et al., 2013b, Peris et al., 2006, Rocha et al., 2014), but their impact on mitosis remains largely unknown (Table 1). This, together with the development of in vitro reconstitution systems (Sirajuddin et al., 2014, Vemu et al., 2014), in which tubulin modifications can be tailor made, and the identification of the full repertoire of tubulin PTMs (Liu et al., 2015), makes it realistic to think that the main challenges for the future (see Outstanding Questions) are within reach, and the field will likely enter an expansion phase in the next few years. One challenging aspect is the present lack of fundamental knowledge on how multiple tubulin PTMs relate to each other and how they are collectively interpreted by the “readers” of the tubulin code. Importantly, certain tubulin PTMs and the respective modifying enzymes have been implicated in tumour growth and correlate with a poor prognosis, metastasis and resistance to chemotherapeutic drugs (Castro-Castro et al., 2012, Froidevaux-Klipfel et al., 2015, Kashiwaya et al., 2010, Kato et al., 2004, Kuroda et al., 2010, Lafanechere et al., 1998, Mialhe et al., 2001, Rocha et al., 2014, Soucek et al., 2006, Wasylyk et al., 2010, Whipple et al., 2010). However, despite their enormous potential, tubulin PTMs have not yet been explored therapeutically, which might represent an opportune area for translational and clinical research in the near future.
Table 1. Summary of known “readers” of the tubulin code with roles in mitosis.
| Protein | Preference for tubulin PTMs | Roles during cell division | References (for tubulin PTMs) |
|---|---|---|---|
| CENP-E/Kinesin-7 | Detyrosination | Polar chromosome congression | (Barisic et al., 2015) |
| CLIP-170 | Tyrosination | Chromosome congression; Stabilization of kinetochore-microtubule attachments | (Peris et al., 2006) |
| Dynein/Dynactin | Tyrosination | Poleward movement of peripheral chromosomes; chromosome congression; spindle pole focusing; spindle orientation; spindle assembly checkpoint inactivation; prevention of erroneous kinetochore-microtubule attachments | (McKenney et al., 2016, Nirschl et al., 2016, Peris et al., 2006) |
| MCAK/Kinesin-13 | Tyrosination | Correction of kinetochore-microtubule attachments; regulation of kinetochore-microtubule dynamics | (Peris et al., 2009, Sirajuddin et al., 2014) |
| Kif2a/Kinesin-13 | Tyrosination | Mitotic spindle assembly and length; Spindle midzone organization | (Peris et al., 2009) |
| Spastin | Polyglutamylation | Abscission during cytokinesis | (Lacroix et al., 2010, Roll-Mecak and Vale, 2008, Valenstein and Roll-Mecak, 2016) |
Supplementary Material
Acknowledgements
Funding support provided by PRECISE and CODECHECK grants from the European Research Council, FLAD Life Science 2020, and the Louis-Jeantet Young Investigator Career Award to H.M. and by a Junior Group Leader start-up research grant from the Danish Cancer Society to M.B.
References
- 1.Alper JD, Decker F, Agana B, Howard J. The motility of axonemal dynein is regulated by the tubulin code. Biophys J. 2014;107:2872–2880. doi: 10.1016/j.bpj.2014.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda AR, Vernos I. Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell. 2000;102:425–435. doi: 10.1016/s0092-8674(00)00048-9. [DOI] [PubMed] [Google Scholar]
- 3.Auckland P, McAinsh AD. Building an integrated model of chromosome congression. J Cell Sci. 2015;128:3363–3374. doi: 10.1242/jcs.169367. [DOI] [PubMed] [Google Scholar]
- 4.Bancroft J, Auckland P, Samora CP, McAinsh AD. Chromosome congression is promoted by CENP-Q- and CENP-E-dependent pathways. J Cell Sci. 2015;128:171–184. doi: 10.1242/jcs.163659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barisic M, Aguiar P, Geley S, Maiato H. Kinetochore motors drive congression of peripheral polar chromosomes by overcoming random arm-ejection forces. Nat Cell Biol. 2014;16:1249–1256. doi: 10.1038/ncb3060. [DOI] [PubMed] [Google Scholar]
- 6.Barisic M, Maiato H. Dynein prevents erroneous kinetochore-microtubule attachments in mitosis. Cell Cycle. 2015;14:3356–3361. doi: 10.1080/15384101.2015.1089369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barisic M, Silva e Sousa R, Tripathy SK, Magiera MM, Zaytsev AV, Pereira AL, et al. Maiato H. Mitosis. Microtubule detyrosination guides chromosomes during mitosis. Science. 2015;348:799–803. doi: 10.1126/science.aaa5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bobinnec Y, Moudjou M, Fouquet JP, Desbruyeres E, Edde B, Bornens M. Glutamylation of centriole and cytoplasmic tubulin in proliferating non-neuronal cells. Cell motility and the cytoskeleton. 1998;39:223–232. doi: 10.1002/(SICI)1097-0169(1998)39:3<223::AID-CM5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Bosch Grau M, Gonzalez Curto G, Rocha C, Magiera MM, Marques Sousa P, Giordano T, et al. Janke C. Tubulin glycylases and glutamylases have distinct functions in stabilization and motility of ependymal cilia. J Cell Biol. 2013;202:441–451. doi: 10.1083/jcb.201305041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai S, O'Connell CB, Khodjakov A, Walczak CE. Chromosome congression in the absence of kinetochore fibres. Nat Cell Biol. 2009;11:832–838. doi: 10.1038/ncb1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cane S, Ye AA, Luks-Morgan SJ, Maresca TJ. Elevated polar ejection forces stabilize kinetochore-microtubule attachments. J Cell Biol. 2013;200:203–218. doi: 10.1083/jcb.201211119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castro-Castro A, Janke C, Montagnac G, Paul-Gilloteaux P, Chavrier P. ATAT1/MEC-17 acetyltransferase and HDAC6 deacetylase control a balance of acetylation of alpha-tubulin and cortactin and regulate MT1-MMP trafficking and breast tumor cell invasion. Eur J Cell Biol. 2012;91:950–960. doi: 10.1016/j.ejcb.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Caudron F, Denarier E, Thibout-Quintana JC, Brocard J, Andrieux A, Fourest-Lieuvin A. Mutation of Ser172 in yeast beta tubulin induces defects in microtubule dynamics and cell division. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013553. e13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chmatal L, Yang K, Schultz RM, Lampson MA. Spatial Regulation of Kinetochore Microtubule Attachments by Destabilization at Spindle Poles in Meiosis I. Curr Biol. 2015;25:1835–1841. doi: 10.1016/j.cub.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drpic D, Pereira AJ, Barisic M, Maresca TJ, Maiato H. Polar Ejection Forces Promote the Conversion from Lateral to End-on Kinetochore-Microtubule Attachments on Mono-oriented Chromosomes. Cell Rep. 2015;13:460–469. doi: 10.1016/j.celrep.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eiserich JP, Estevez AG, Bamberg TV, Ye YZ, Chumley PH, Beckman JS, Freeman BA. Microtubule dysfunction by posttranslational nitrotyrosination of alpha-tubulin: a nitric oxide-dependent mechanism of cellular injury. Proc Natl Acad Sci U S A. 1999;96:6365–6370. doi: 10.1073/pnas.96.11.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erck C, Peris L, Andrieux A, Meissirel C, Gruber AD, Vernet M, et al. Wehland J. A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc Natl Acad Sci U S A. 2005;102:7853–7858. doi: 10.1073/pnas.0409626102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonrose X, Ausseil F, Soleilhac E, Masson V, David B, Pouny I, et al. Lafanechere L. Parthenolide inhibits tubulin carboxypeptidase activity. Cancer Res. 2007;67:3371–3378. doi: 10.1158/0008-5472.CAN-06-3732. [DOI] [PubMed] [Google Scholar]
- 19.Fourest-Lieuvin A, Peris L, Gache V, Garcia-Saez I, Juillan-Binard C, Lantez V, Job D. Microtubule regulation in mitosis: tubulin phosphorylation by the cyclin-dependent kinase Cdk1. Mol Biol Cell. 2006;17:1041–1050. doi: 10.1091/mbc.E05-07-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Froidevaux-Klipfel L, Targa B, Cantaloube I, Ahmed-Zaid H, Pous C, Baillet A. Septin cooperation with tubulin polyglutamylation contributes to cancer cell adaptation to taxanes. Oncotarget. 2015;6:36063–36080. doi: 10.18632/oncotarget.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funabiki H, Murray AW. The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell. 2000;102:411–424. doi: 10.1016/s0092-8674(00)00047-7. [DOI] [PubMed] [Google Scholar]
- 22.Gundersen GG, Bulinski JC. Distribution of tyrosinated and nontyrosinated alpha-tubulin during mitosis. J Cell Biol. 1986;102:1118–1126. doi: 10.1083/jcb.102.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- 24.Hammond JW, Huang CF, Kaech S, Jacobson C, Banker G, Verhey KJ. Posttranslational modifications of tubulin and the polarized transport of kinesin-1 in neurons. Mol Biol Cell. 2010;21:572–583. doi: 10.1091/mbc.E09-01-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iemura K, Tanaka K. Chromokinesin Kid and kinetochore kinesin CENP-E differentially support chromosome congression without end-on attachment to microtubules. Nature communications. 2015;6 doi: 10.1038/ncomms7447. 6447. [DOI] [PubMed] [Google Scholar]
- 26.Janke C. The tubulin code: molecular components, readout mechanisms, and functions. J Cell Biol. 2014;206:461–472. doi: 10.1083/jcb.201406055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 28.Kalebic N, Martinez C, Perlas E, Hublitz P, Bilbao-Cortes D, Fiedorczuk K, et al. Heppenstall PA. Tubulin acetyltransferase alphaTAT1 destabilizes microtubules independently of its acetylation activity. Mol Cell Biol. 2013a;33:1114–1123. doi: 10.1128/MCB.01044-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalebic N, Sorrentino S, Perlas E, Bolasco G, Martinez C, Heppenstall PA. alphaTAT1 is the major alpha-tubulin acetyltransferase in mice. Nat Commun. 2013b;4 doi: 10.1038/ncomms2962. 1962. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, et al. Khodjakov A. Chromosomes can congress to the metaphase plate before biorientation. Science. 2006;311:388–391. doi: 10.1126/science.1122142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashiwaya K, Nakagawa H, Hosokawa M, Mochizuki Y, Ueda K, Piao L, et al. Nakamura Y. Involvement of the tubulin tyrosine ligase-like family member 4 polyglutamylase in PELP1 polyglutamylation and chromatin remodeling in pancreatic cancer cells. Cancer Res. 2010;70:4024–4033. doi: 10.1158/0008-5472.CAN-09-4444. [DOI] [PubMed] [Google Scholar]
- 32.Kato C, Miyazaki K, Nakagawa A, Ohira M, Nakamura Y, Ozaki T, et al. Nakagawara A. Low expression of human tubulin tyrosine ligase and suppressed tubulin tyrosination/detyrosination cycle are associated with impaired neuronal differentiation in neuroblastomas with poor prognosis. Int J Cancer. 2004;112:365–375. doi: 10.1002/ijc.20431. [DOI] [PubMed] [Google Scholar]
- 33.Kaul N, Soppina V, Verhey KJ. Effects of alpha-tubulin K40 acetylation and detyrosination on kinesin-1 motility in a purified system. Biophys J. 2014;106:2636–2643. doi: 10.1016/j.bpj.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Holland AJ, Lan W, Cleveland DW. Aurora kinases and protein phosphatase 1 mediate chromosome congression through regulation of CENP-E. Cell. 2010;142:444–455. doi: 10.1016/j.cell.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konishi Y, Setou M. Tubulin tyrosination navigates the kinesin-1 motor domain to axons. Nature neuroscience. 2009;12:559–567. doi: 10.1038/nn.2314. [DOI] [PubMed] [Google Scholar]
- 37.Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–445. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 38.Kuroda H, Saito K, Kuroda M, Suzuki Y. Differential expression of glu-tubulin in relation to mammary gland disease. Virchows Arch. 2010;457:477–482. doi: 10.1007/s00428-010-0955-z. [DOI] [PubMed] [Google Scholar]
- 39.Lacroix B, van Dijk J, Gold ND, Guizetti J, Aldrian-Herrada G, Rogowski K, et al. Janke C. Tubulin polyglutamylation stimulates spastin-mediated microtubule severing. J Cell Biol. 2010;189:945–954. doi: 10.1083/jcb.201001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lafanechere L, Courtay-Cahen C, Kawakami T, Jacrot M, Rudiger M, Wehland J, et al. Margolis RL. Suppression of tubulin tyrosine ligase during tumor growth. J Cell Sci. 1998;111(Pt 2):171–181. doi: 10.1242/jcs.111.2.171. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Yu W, Liang Y, Zhu X. Kinetochore dynein generates a poleward pulling force to facilitate congression and full chromosome alignment. Cell Res. 2007;17:701–712. doi: 10.1038/cr.2007.65. [DOI] [PubMed] [Google Scholar]
- 42.Liu N, Xiong Y, Ren Y, Zhang L, He X, Wang X, et al. Zhou J. Proteomic Profiling and Functional Characterization of Multiple Post-Translational Modifications of Tubulin. J Proteome Res. 2015;14:3292–3304. doi: 10.1021/acs.jproteome.5b00308. [DOI] [PubMed] [Google Scholar]
- 43.Maas C, Belgardt D, Lee HK, Heisler FF, Lappe-Siefke C, Magiera MM, et al. Kneussel M. Synaptic activation modifies microtubules underlying transport of postsynaptic cargo. Proc Natl Acad Sci U S A. 2009;106:8731–8736. doi: 10.1073/pnas.0812391106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magidson V, O'Connell CB, Loncarek J, Paul R, Mogilner A, Khodjakov A. The spatial arrangement of chromosomes during prometaphase facilitates spindle assembly. Cell. 2011;146:555–567. doi: 10.1016/j.cell.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maruta H, Greer K, Rosenbaum JL. The acetylation of alpha-tubulin and its relationship to the assembly and disassembly of microtubules. J Cell Biol. 1986;103:571–579. doi: 10.1083/jcb.103.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McIntosh JR, Volkov V, Ataullakhanov FI, Grishchuk EL. Tubulin depolymerization may be an ancient biological motor. J Cell Sci. 2010;123:3425–3434. doi: 10.1242/jcs.067611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKenney RJ, Huynh W, Tanenbaum ME, Bhabha G, Vale RD. Activation of cytoplasmic dynein motility by dynactin-cargo adapter complexes. Science. 2014;345:337–341. doi: 10.1126/science.1254198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKenney RJ, Huynh W, Vale RD, Sirajuddin M. Tyrosination of alpha-tubulin controls the initiation of processive dynein-dynactin motility. EMBO J. 2016 doi: 10.15252/embj.201593071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mialhe A, Lafanechere L, Treilleux I, Peloux N, Dumontet C, Bremond A, et al. Job D. Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 2001;61:5024–5027. [PubMed] [Google Scholar]
- 50.Mitchison T, Evans L, Schulze E, Kirschner M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell. 1986;45:515–527. doi: 10.1016/0092-8674(86)90283-7. [DOI] [PubMed] [Google Scholar]
- 51.Nirschl JJ, Magiera MM, Lazarus JE, Janke C, Holzbaur EL. alpha-Tubulin Tyrosination and CLIP-170 Phosphorylation Regulate the Initiation of Dynein-Driven Transport in Neurons. Cell Rep. 2016;14:2637–2652. doi: 10.1016/j.celrep.2016.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paturle L, Wehland J, Margolis RL, Job D. Complete separation of tyrosinated, detyrosinated, and nontyrosinatable brain tubulin subpopulations using affinity chromatography. Biochemistry. 1989;28:2698–2704. doi: 10.1021/bi00432a050. [DOI] [PubMed] [Google Scholar]
- 53.Peris L, Thery M, Faure J, Saoudi Y, Lafanechere L, Chilton JK, et al. Job D. Tubulin tyrosination is a major factor affecting the recruitment of CAP-Gly proteins at microtubule plus ends. J Cell Biol. 2006;174:839–849. doi: 10.1083/jcb.200512058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peris L, Wagenbach M, Lafanechere L, Brocard J, Moore AT, Kozielski F, et al. Andrieux A. Motor-dependent microtubule disassembly driven by tubulin tyrosination. J Cell Biol. 2009;185:1159–1166. doi: 10.1083/jcb.200902142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Regnard C, Audebert S, Desbruyeres, Denoulet P, Edde B. Tubulin polyglutamylase: partial purification and enzymatic properties. Biochemistry. 1998;37:8395–8404. doi: 10.1021/bi9804131. [DOI] [PubMed] [Google Scholar]
- 58.Rieder CL, Alexander SP. Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J Cell Biol. 1990;110:81–95. doi: 10.1083/jcb.110.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rocha C, Papon L, Cacheux W, Marques Sousa P, Lascano V, Tort O, et al. Janke C. Tubulin glycylases are required for primary cilia, control of cell proliferation and tumor development in colon. EMBO J. 2014;33:2247–2260. doi: 10.15252/embj.201488466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roll-Mecak A, Vale RD. Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature. 2008;451:363–367. doi: 10.1038/nature06482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaar BT, Chan GK, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is essential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schweizer N, Pawar N, Weiss M, Maiato H. An organelle-exclusion envelope assists mitosis and underlies distinct molecular crowding in the spindle region. J Cell Biol. 2015;210:695–704. doi: 10.1083/jcb.201506107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sirajuddin M, Rice LM, Vale RD. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol. 2014;16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soppina V, Herbstman JF, Skiniotis G, Verhey KJ. Luminal localization of alpha-tubulin K40 acetylation by cryo-EM analysis of fab-labeled microtubules. PLoS One. 2012;7:e48204. doi: 10.1371/journal.pone.0048204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soucek K, Kamaid A, Phung AD, Kubala L, Bulinski JC, Harper RW, Eiserich JP. Normal and prostate cancer cells display distinct molecular profiles of alpha-tubulin posttranslational modifications. Prostate. 2006;66:954–965. doi: 10.1002/pros.20416. [DOI] [PubMed] [Google Scholar]
- 66.Valenstein ML, Roll-Mecak A. Graded Control of Microtubule Severing by Tubulin Glutamylation. Cell. 2016;164:911–921. doi: 10.1016/j.cell.2016.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vemu A, Garnham CP, Lee DY, Roll-Mecak A. Generation of differentially modified microtubules using in vitro enzymatic approaches. Methods Enzymol. 2014;540:149–166. doi: 10.1016/B978-0-12-397924-7.00009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 69.Vorozhko VV, Emanuele MJ, Kallio MJ, Stukenberg PT, Gorbsky GJ. Multiple mechanisms of chromosome movement in vertebrate cells mediated through the Ndc80 complex and dynein/dynactin. Chromosoma. 2008;117:169–179. doi: 10.1007/s00412-007-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wandke C, Barisic M, Sigl R, Rauch V, Wolf F, Amaro AC, et al. Geley S. Human chromokinesins promote chromosome congression and spindle microtubule dynamics during mitosis. J Cell Biol. 2012;198:847–863. doi: 10.1083/jcb.201110060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Z, Sheetz MP. The C-terminus of tubulin increases cytoplasmic dynein and kinesin processivity. Biophys J. 2000;78:1955–1964. doi: 10.1016/S0006-3495(00)76743-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wasylyk C, Zambrano A, Zhao C, Brants J, Abecassis J, Schalken JA, et al. Wasylyk B. Tubulin tyrosine ligase like 12 links to prostate cancer through tubulin posttranslational modification and chromosome ploidy. Int J Cancer. 2010;127:2542–2553. doi: 10.1002/ijc.25261. [DOI] [PubMed] [Google Scholar]
- 73.Webster DR, Wehland J, Weber K, Borisy GG. Detyrosination of alpha tubulin does not stabilize microtubules in vivo. J Cell Biol. 1990;111:113–122. doi: 10.1083/jcb.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whipple RA, Matrone MA, Cho EH, Balzer EM, Vitolo MI, Yoon JR, et al. Martin SS. Epithelial-to-mesenchymal transition promotes tubulin detyrosination and microtentacles that enhance endothelial engagement. Cancer Res. 2010;70:8127–8137. doi: 10.1158/0008-5472.CAN-09-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Whipple RA, Vitolo MI, Boggs AE, Charpentier MS, Thompson K, Martin SS. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-kappaB inhibition. Breast Cancer Res. 2013;15:R83. doi: 10.1186/bcr3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson PJ, Forer A. Effects of nanomolar taxol on crane-fly spermatocyte spindles indicate that acetylation of kinetochore microtubules can be used as a marker of poleward tubulin flux. Cell motility and the cytoskeleton. 1997;37:20–32. doi: 10.1002/(SICI)1097-0169(1997)37:1<20::AID-CM3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 77.Wood KW, Sakowicz R, Goldstein LS, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- 78.Yang Z, Tulu US, Wadsworth P, Rieder CL. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr Biol. 2007;17:973–980. doi: 10.1016/j.cub.2007.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ye AA, Deretic J, Hoel CM, Hinman AW, Cimini D, Welburn JP, Maresca TJ. Aurora A Kinase Contributes to a Pole-Based Error Correction Pathway. Curr Biol. 2015;25:1842–1851. doi: 10.1016/j.cub.2015.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zilberman Y, Ballestrem C, Carramusa L, Mazitschek R, Khochbin S, Bershadsky A. Regulation of microtubule dynamics by inhibition of the tubulin deacetylase HDAC6. J Cell Sci. 2009;122:3531–3541. doi: 10.1242/jcs.046813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.