Abstract
While it is established that psychosocial stress increases the risk of developing diabetes mellitus (DM), two key knowledge gaps remain: 1) the neurobiological mechanisms that are involved in mediating that risk, and 2) the role, if any, that adiposity plays in that mechanism. We tested the hypotheses that: 1) metabolic activity in the amygdala (AmygA), a key center involved in the neurobiological response to stress, associates with subsequent DM risk, and 2) this association is independent of adiposity. AmygA and adipose tissue volumes were measured, and serial blood assessments for DM were obtained in 232 subjects who underwent combined 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) imaging. Higher baseline AmygA predicted subsequent, new-onset DM, independently of adiposity and other DM risk factors. Furthermore, higher adiposity only increased DM risk in the presence of higher AmygA. In a separate cross-sectional cohort, higher AmygA associated with higher insulin resistance. Accordingly, the current study shows, for the first time, that activity in a stress-responsive neural region predicts the onset of DM. Further, we observed that this neurobiological activity acts independently of, but also synergistically with adiposity to increase DM risk. These findings suggest novel therapeutic targets to help manage and possibly prevent DM.
Keywords: amygdala, diabetes mellitus, positron emission tomography/computed tomography, visceral adiposity
Graphical Abstract
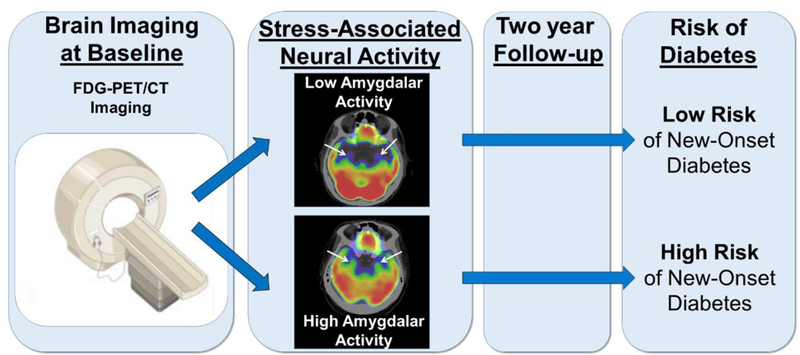
1. Introduction
Diabetes mellitus (DM) represents a rapidly growing threat to global health (Mokdad et al., 2003). The development of DM is closely associated with obesity and, more potently, with excess visceral adipose tissue (VAT) (Neeland et al., 2012). Yet, most obese individuals do not develop DM, underscoring the fact that additional variables contribute to the development of diabetes (Rosen and Spiegelman, 2006). Psychosocial stress represents such a factor (Pouwer et al., 2010). Epidemiologic evidence suggests that stress adversely impacts glycemic control among individuals with pre-existing DM (Chida and Hamer, 2008) and contributes to the development of DM (Kumari et al., 2004; Mooy et al., 2000). However, the mechanistic pathway that links stress to DM remains incompletely defined.
Stress associates with increased adiposity (notably VAT), in part due to its association with adverse health behaviors, such as excess caloric intake and physical inactivity (Adam and Epel, 2007; Kouvonen et al., 2005). Since increased VAT is a potent risk factor for DM, it would be appealing to simply rely on measurements of VAT to gain insights into the risk of DM in the context of stress. However, prior work raised doubts about the importance of adiposity in mediating the risk of developing DM from stress (Mooy et al., 2000). In that cross-sectional study, although life stressors were associated with DM, adjusting for waist-to-hip ratio only marginally attenuated that association. Moreover, radiographic measures of adiposity (e.g., VAT) were not available. Accordingly, it remains unclear to what degree adiposity mediates the relationship between stress and subsequent DM.
Another key question, regarding the association between stress and DM, is which regional brain areas participate in the pathobiological mechanism. Translation of external stressors to their physiological consequences may involve activation of the brain’s salience network, an ensemble of interconnected structures involved in complex functions such as cognition and emotion, among which the amygdala is an important component (Wang et al., 2010). Advanced imaging tools allow objective assessment of signals in brain regions (including the amygdala) that are known to be activated by psychosocial stress and stress conditions. Resting amygdalar metabolic activity (AmygA) can be measured using 18F- fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) (Tawakol et al., 2017). The AmygA signal is reproducible, as indicated by an observed median change of only 2% in a clinically stable population over a three-month period (Hammad et al., 2017). AmygA associates with anxious temperament in animal models (Oler et al., 2010) and perceived stress in humans (Tawakol et al., 2017), and it is upregulated in chronic stress conditions (e.g., post-traumatic stress disorder, anxiety) (Bremner et al., 2005; Whalen et al., 2002). This signal may also provide important insights into the pathobiological consequences of stress, in that increased AmygA has previously been shown to robustly predict incident cardiovascular disease events in humans (Tawakol et al., 2017). Therefore, of the several brain regions potentially involved in the neurobiological response to stress, we prospectively hypothesized that the amygdala plays an important role in the mechanism linking chronic stress to DM.
18F-FDG-PET/CT imaging is uniquely suited for investigating the relationship between the neurobiological response to stress and metabolic disease, since it enables simultaneous measurement of regional brain metabolic activity, using 18F-FDG-PET, and volumetric measures of adipose tissues (e.g., VAT), using CT (Figueroa et al., 2016). Accordingly, we employed these imaging techniques and assessed for the development of new-onset type 2 DM to determine whether: 1) increased AmygA associates with subsequent new-onset DM and 2) this association is independent of adiposity.
2. Materials and Methods
2.1. Overview
The study findings derive from two separate cohorts: 1) a retrospective, longitudinal outcomes study, which evaluated the relationship between AmygA, adipose tissue volumes, and incident type 2 DM, and 2) a cross-sectional biomarker study, which evaluated the relationship between AmygA and biomarkers related to metabolism and inflammation. This study protocol was approved by Partners Human Research Committee/Institutional Review Board and was completed in agreement with the Declaration of Helsinki.
2.2. Study Sample
Outcomes study subjects (N=232) were identified from 2143 patients who had undergone clinical 18F- FDG-PET/CT imaging (mostly for cancer screening) at the Massachusetts General Hospital (Boston, MA, USA) from 2005-2008 (Figure 1A). Inclusion criteria were: 1) age > 30 years, 2) absence of known cardiovascular, inflammatory, or autoimmune disease at the time of imaging, 3) absence of prior malignancy or remission from cancer for at least one year prior to imaging and throughout follow-up, and 4) availability of at least three clinical notes in the medical records (spanning ≥ one year). This study’s cohort represents a subset of the 293 subjects who participated in a prior study of AmygA and cardiovascular events (Tawakol et al., 2017). Of those, we excluded 25 individuals who had DM at baseline and 36 individuals who lacked adequate data for adjudication of subsequent type 2 DM. Accordingly, 232 individuals were included in the current analysis to test the relationship between AmygA and subsequent type 2 DM.
Figure 1. (A) Outcomes study subject selection. (B) Biomarker study subject selection.
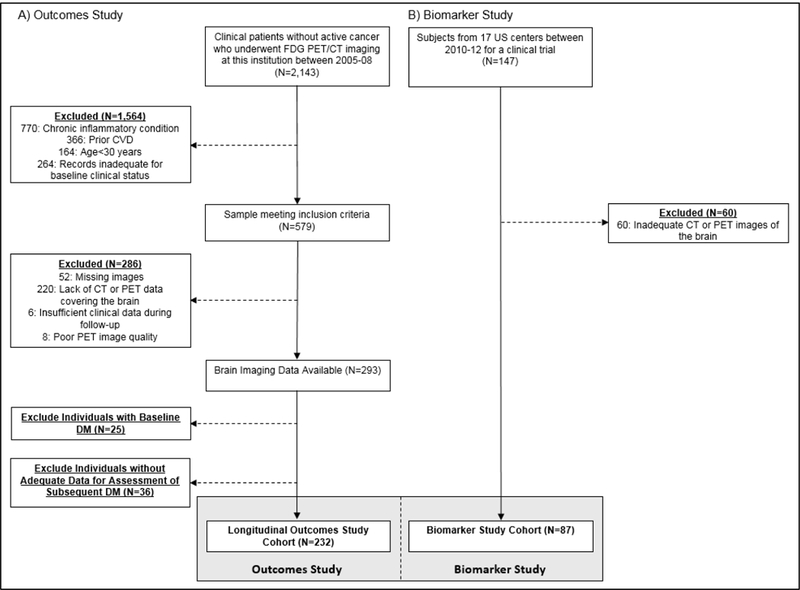
Abbreviations: CVD: cardiovascular disease, CT: computed tomography, DM: diabetes mellitus, FDG: fluorodeoxyglucose, LDL: low density lipoprotein, PET: positron emission tomography.
Biomarker study subjects (N=87) were derived from a study of 147 individuals who participated in an 18F-FDG-PET/CT imaging trial from 2010-2012 at seventeen United States centers (www.clinicaltrials.gov, NCT01258907) (Figure 1B). Only the baseline cross-sectional data from that clinical trial are included in this study; that trial tested the impact of a drug among individuals with known or at high risk for cardiovascular disease and/or metabolic disease. Eligible subjects were between 35 and 80 years old with at least one of the following: 1) stable clinically or objectively diagnosed atherosclerotic disease, or 2) clinically diagnosed type 2 DM by American Diabetes Association (ADA) criteria (American Diabetes Association, 2016). Other inclusion and exclusion criteria have been previously reported (Lehrer-Graiwer et al., 2015). All trial subjects who had the brain within the imaging field of view were included in the current study.
2.3. Measurements
2.3.1. 18F-FDG-PET/CT Imaging Protocol
18F-FDG was administered intravenously at a dose of ~370 MBq following an overnight fast. Whole-body imaging was performed approximately one hour later using an integrated scanner (e.g., Biograph 64 Siemens Healthcare, Erlangen, Germany). Non-gated, non-contrast-enhanced CT was performed for attenuation correction. The imaging parameters are in accordance with the specifications of the Society of Nuclear Medicine Procedure Guideline for FDG PET Brain Imaging (Waxman et al., 2009).
2.3.2. Measurement of Regional Brain 18F-FDG Uptake
Resting AmygA was measured as previously described (Tawakol et al., 2017) by one blinded radiologist using a dedicated offline workstation (Leonardo TrueD, Siemens Healthcare, Forchheim, Germany) to fuse PET and CT datasets. The analysis leveraged approaches that have been used to evaluate the relationship of AmygA to temperament (Oler et al., 2010), stress-related disorders (Bremner et al., 2005; Whalen et al., 2002), and perceived stress (Tawakol et al., 2017). The amygdala, part of the limbic system located dorso-medially in the temporal lobe that forms the ventral superior and medial walls of the inferior horn of the lateral ventricle, was localized on the CT images by anatomic landmarks, as previously described (Tawakol et al., 2017). 18F-FDG uptake was determined by placing circular (approximately 15 mm radius) regions of interest (ROIs) in the right and left amygdalae and measuring the mean tracer accumulation, quantified by the standardized uptake value (SUV), in each ROI. For the pre-specified endpoints, AmygA was defined as the mean amygdalar SUV corrected for background activity (i.e., mean temporal lobe SUV) (Britz-Cunningham et al., 2008). We pre-specified that AmygA was to be assessed both as a continuous variable and as a dichotomous variable (by median value). In order to evaluate the effect of amygdalar laterality and correction for alternative background tissues, posthoc assessments of AmygA were performed, wherein the right and left amygdalae were assessed separately, cerebellar background activity was used to correct for background activity (Britz-Cunningham et al., 2008), and AmygA was divided into tertiles. Cerebral and cerebellar tissues were chosen for background correction because of their high but stable steady-state glucose metabolism.
2.3.4. Measurement of Adipose Tissue Volumes
Adipose tissue volumes were measured, using the same offline imaging workstation, by a blinded investigator from simultaneously acquired CT images. Adipose tissue was identified using a threshold between −195 and −45 Hounsfield units. The abdominal muscular wall was used as a boundary to separate VAT and subcutaneous adipose tissue (SAT). The volumes were measured at the level of the umbilicus and expressed as cubic centimeters (cm3) (Maurovich-Horvat et al., 2007).
2.3.5. Assessment of Glucose Homeostasis and Determination of Incident Diabetes
The development of subsequent incident type 2 DM was determined using available clinical records by an investigator who was blinded to imaging data. Because the longitudinal outcomes study was retrospective, the frequency of subject follow-up and testing was based on individual clinician practices. Available fasting glucose (FBG) and glycohemoglobin (HbA1c) levels were assessed for two years before and up to five years after baseline imaging. Type 2 DM was adjudicated upon identification of a primary and a confirmatory measure of abnormal glucose homeostasis [i.e., two discrete measurements of either HbAlc ≥ 6.5% (48 mmol/mol) or FBG ≥ 126 mg/dL] in accordance with ADA criteria (American Diabetes Association., 2016). Similarly, all individuals who did not develop DM were evaluated for prediabetes at the two and five-year endpoints using ADA criteria [i.e., one measurement of HbA1c 5.7-6.4% (39-46 mmol/mol) or FBG 100-125 mg/dL] (American Diabetes Association., 2016). The onset of incident type 2 DM was defined as the date of the initial measure of abnormal glycemia within two years of index imaging to maximize the number of subjects with complete follow-up. Secondary analyses were performed for presence of DM at one, three or five years after index imaging.
2.3.6. Biomarker Measurements
For the biomarker study, assays were performed using the HumanMAP1.6 multiplex panel from Rules Based Medicine. In addition, interleukin (IL)-6 and tissue necrosis factor (TNF) alpha immunoassays were performed using Quantikine R&D High Sensitivity ELISA, and IL-10 immunoassays were performed using kits from MSA at Pacific Biomarkers. These assays were conducted at a central core laboratory.
2.4. Statistical Analyses
Statistical analyses were performed using SPSS (IBM Corp, Version 24). Continuous variables are given as mean ± standard deviation (SD) or median ± interquartile range (IQR) when not normally distributed. Associations were evaluated using Pearson and Spearman correlations for normally and non-normally distributed variables, respectively. Cox proportional hazards regression was used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Kaplan-Meier estimates of DM-free survival were assessed to compare event-free survival in subjects stratified by AmygA and adiposity. For multivariable models, important covariates were selected a priori and included age, sex, race, measures of adiposity, AmygA, baseline FBG, cardiovascular risk factors, history of pre-diabetes, and statin use. Time between imaging and date of event or last follow-up was entered into models where appropriate. Missing data were dropped from relevant sub-analyses; imputation was not employed. All tests were two-sided with statistical significance defined as p<0.05.
3. Results
3.1. Descriptives
Baseline characteristics, including pre-existing psychiatric conditions, are provided in Table 1. Two hundred thirty-two (232) individuals without baseline DM were followed longitudinally and provided adequate follow-up data to assess the development of incident DM (Figure 1A). Twenty-one (21) (9.1%) and 30 (12.9%) of these patients developed type 2 DM over two and five years, respectively (running total). At baseline, 114 individuals (49.1%) met criteria for pre-diabetes within the two years preceding index imaging. A total of 119 individuals (51.3%), 18 of which did not have pre-diabetes at baseline, had evidence of pre-diabetes within two years of follow-up, and a total of 129 individuals (55.6%), of which 36 did not have pre-diabetes at baseline, had evidence of pre-diabetes within five years of follow-up. Glycemic data were not available for the full five years for 86 subjects.
Table 1.
Baseline characteristics of outcomes study subjects.
| Characteristics | Full Cohort (N=232) |
Subgroups Tyipe by Subsequent Development of 2 DM over 5 Years |
||
|---|---|---|---|---|
| No DM (N=202) |
DM (N=30) |
p-value (DM vs. No DM) |
||
| Age (years), median (IQR) | 55.0 (44.0-64.0) | 53.8 (43.0, 63.0) | 57.5 (47.5, 64.8) | 0.16 |
| Male, N (%) | 98 (42.2) | 79 (39) | 19 (63) | 0.02 |
| Caucasian, N (%) | 207 (89.2) | 180 (89) | 27 (90) | 1.00 |
| Current smoker, N (%) | 19 (8.2) | 15 (7) | 4 (13) | 0.28 |
| Hypertension, N (%) | 71 (30.6) | 62 (31) | 9 (30) | 1.00 |
| Hyperlipidemia, N (%) | 60 (25.9) | 51 (25) | 9 (30) | 0.66 |
| Total cholesterol (mg/dL), Mean (SD) | 194 (47) | 195 (46) | 189 (48) | 0.63 |
| Baseline Blood Glucose (mg/dL), median (IQR) | 95 (88, 104) | 94 (88, 103) | 102 (94, 119) | 0.01 |
| LDL (mg/dL), Mean (SD) | 113 (38) | 114 (38) | 109 (40) | 0.62 |
| Statin therapy, N (%) | 39 (17) | 31 (15) | 8 (27) | 0.12 |
| FRS, Mean (SD) | 5.3 (6.2) | 5.1 (6.3) | 6.7 (5.1) | 0.30 |
| BMI (kg/m2), median (IQR) | 26.4 (23.3, 30.7) | 27.0 (22.8, 30.7) | 28.6 (25.6, 31.6) | 0.04 |
| Visceral Adipose Tissue Volume (cm3), median (IQR) | 47.4 (26.3, 72.5) | 46.8 (26.2, 68.1) | 69.8 (26.9, 103.0) | 0.03 |
| History of cancer, N (%) | 205 (88) | 177 (88) | 28 (93) | 0.54 |
| Prior chemotherapy, N (%) | 191 (82) | 164 (81) | 27 (90) | 0.31 |
| History of Depression, N (%) | 13 (6) | 12 (6) | 1 (3) | 0.58 |
| History of Anxiety, N (%) | 13 (6) | 12 (6) | 1 (3) | 0.58 |
| History of Other Psychiatric Disorder, N (%)* | 3 (1) | 3 (1) | 0 (0) | 0.51 |
| Anti-Depressant Medication Use, N (%) | 22 (10) | 22 (11) | 0 (0) | 0.09 |
Values are mean (SD), median (IQR), or N (%).
Other psychiatric disorders include post-traumatic stress disorder (N=2) and obsessive-compulsive disorder (N=1).
Abbreviations: BMI= body mass index, FRS= Framingham Risk Score, LDL= low density lipoprotein.
3.2. Amygdalar Activity Independently Predicts Incident Diabetes after Multivariable Adjustments
Baseline AmygA, as a continuous variable, associated with incident type 2 DM risk in univariable analysis (HR [95% CI]: 1.46 [1.08, 1.97], p=0.01, Table 2, Supplemental Table A1). This remained significant after adjusting for baseline blood glucose concentrations (1.35 [1.02, 1.77], p=0.03), age (1.43 [1.04, 1.96], p=0.03), or other well-recognized predictors of DM (viz., history of pre-diabetes, sex and baseline VAT). Additionally, AmygA predicted DM in the subset of 118 individuals without a history of impaired glucose tolerance (2.66 [1.23, 5.79], p=0.01) and in the subset of 178 individuals without exposure to systemic corticosteroids within two years prior to imaging (1.43 [1.03, 1.99], p=0.03).
Table 2.
Clinical predictors of incident type 2 diabetes.
| Predictor | Covariates | HR | 95% CI | p |
|---|---|---|---|---|
| Age | None | 1.02 | 0.98, 1.05 | 0.38 |
| Sex | None | 2.29 | 0.95, 5.53 | 0.07 |
| Caucasian | None | 1.16 | 0.27, 4.97 | 0.84 |
| Hypertension | None | 0.89 | 0.35, 2.30 | 0.81 |
| Baseline Glucose | None | 1.87 | 1.59, 2.22 | <0.001 |
| Statin | None | 1.96 | 0.76, 5.05 | 0.16 |
| BMI | None | 1.27 | 0.85, 1.92 | 0.24 |
| Baseline VAT | None | 1.47 | 0.94, 2.31 | 0.09 |
| Baseline SAT | None | 0.83 | 0.50, 1.38 | 0.46 |
| Baseline VAT: SAT | None | 1.27 | 0.83, 1.97 | 0.27 |
| Total Adipose Tissue | None | 1.05 | 0.66, 1.68 | 0.84 |
| AmygA | None | 1.46 | 1.08, 1.97 | 0.01 |
| AmygA | Age | 1.43 | 1.04, 1.96 | 0.03 |
| AmygA | Baseline glucose | 1.35 | 1.02, 1.77 | 0.03 |
| AmygA | BMI, age, race, history of pre-diabetes | 1.40 | 1.01, 1.95 | 0.04 |
| AmygA | BMI, age, race, history of pre-diabetes, statin use | 1.40 | 1.01, 2.95 | 0.04 |
| AmygA | BMI | 1.46 | 1.08, 1.98 | 0.01 |
| AmygA | VAT | 1.44 | 1.01, 2.06 | 0.046 |
In a post-hoc analysis, we additionally observed an association between baseline AmygA and the timing of incident type 2 DM. Individuals who developed DM sooner had higher AmygA than those who later- or never developed DM (p=0.02, Supplemental Figure A1). Additionally, AmygA predicted type 2 DM, whether events were assessed at one, two, three, or five years after index imaging (Supplemental Table A2).
The association between AmygA and type 2 DM became especially robust when AmygA was dichotomized as: < vs. ≥ sample median. Individuals with higher AmygA had an approximately 5-fold increased risk for incident DM (4.91 [1.65, 14.60], p=0.004, Figure 2, Figure 3A, Table 3, Supplemental Figure A2). That risk remained significant in multivariable models that adjusted for traditional risk factors for DM, baseline glycemic variables, or measures of adiposity (Table 3).
Figure 2. Imaging of amygdalar activity and adiposity in individuals with vs. without subsequent type 2 diabetes.
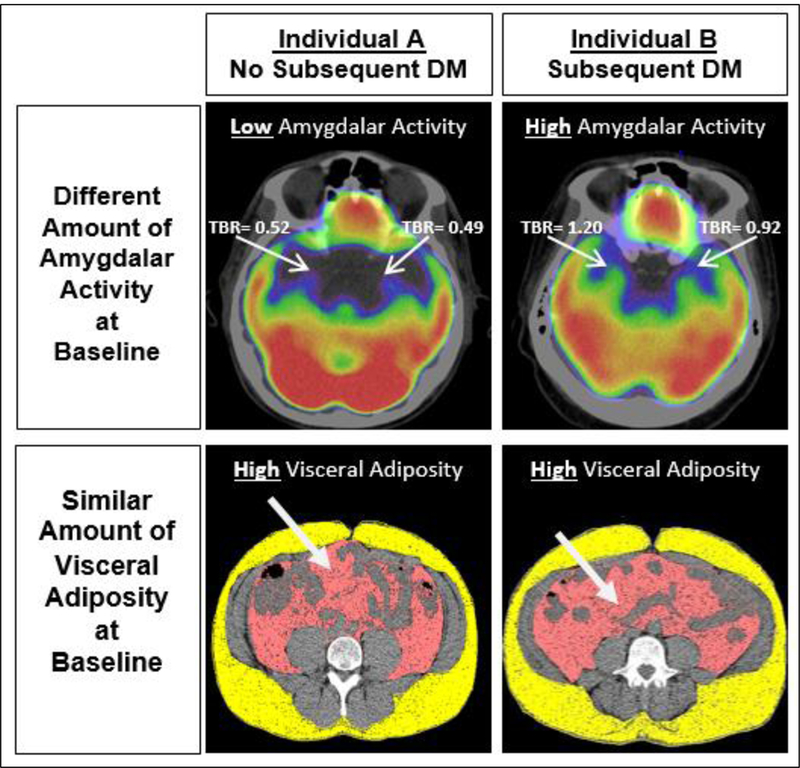
Axial views of amygdala (upper right and left 18F-FDG-PET/CT images) and adipose tissues are shown (lower right and left CT images) from two individuals. Both individuals were obese and had similar amounts of visceral adipose tissue. However, AmygA was increased in the individual who developed DM (right) compared to the individual who did not (left). Abbreviations: DM: diabetes mellitus, TBR: target-to-background ratio.
Figure 3. Kaplan-Meier curves showing type 2 diabetes-free survival as a function of amygdalar activity alone (A) and as a function of both amygdalar activity and (B) body mass index, (C) visceral adipose tissue, and (D) subsequent change in visceral adipose tissue.
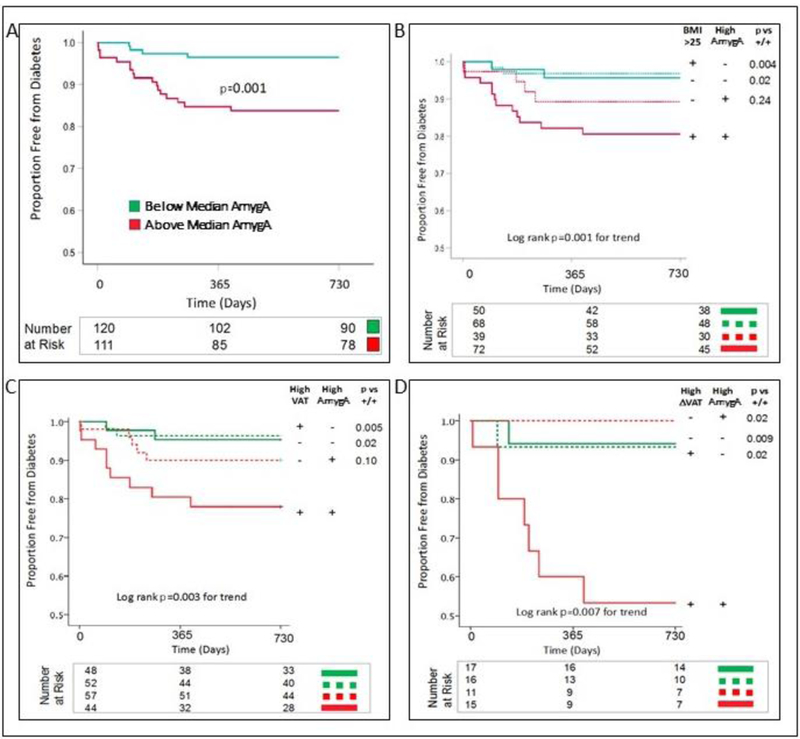
A. Subjects were categorized by baseline AmygA [< (green) or ≥ (red) sample median]. Those with high AmygA had substantially higher incidence of DM. The total number of subjects assessed is 231, as one individual did not provide cerebral background data to calculate the pre-specified AmygA endpoint. B. Subjects were categorized by baseline AmygA [< (green) or ≥ (red) sample median] and baseline BMI [< (dashed) or ≥ (solid) 25 kg/m2]. Those with high AmygA and BMI had substantially higher incidence of DM. The total number of subjects assessed is 229, as one individual did not provide cerebral background data to calculate the pre-specified AmygA endpoint, and two subjects did not provide height data to calculate BMI. C. Subjects were categorized by baseline AmygA [< (green) or ≥ (red) sample median] and baseline VAT [< (dashed) or ≥ (solid) sample median]. Those with high AmygA and VAT had substantially higher incidence of DM. The total number of subjects assessed is 201, as one individual did not provide cerebral background data to calculate the pre-specified AmygA endpoint, and 30 individuals did not provide data for measurement of VAT. D.Subjects were categorized by baseline AmygA [< (green) or ≥ (red) sample median] and subsequent change in VAT [< (dashed) or ≥ (solid) sample median]. Those with high AmygA and change in VAT had substantially higher incidence of DM. Serial VAT measurements were available in 59 individuals. Abbreviations: AmygA: amygdalar activity, BMI: body mass index, DM: diabetes mellitus, VAT: visceral adipose tissue.
Table 3.
Amygdalar activity vs. incident type 2 diabetes after multivariable adjustments.
| Predictor | Covariates | Full Cohort | Subgroup Analysis: Individuals with BMI ≥ 25 kg/m2 at baseline | Subgroup Analysis: Individuals with ≥ median VAT at baseline | |||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Above vs. Below Median Amygdalar Activity |
None | 4.91 (1.65, 14.60) | 0.004 | 6.37 (1.44, 28.24) | 0.02 | 6.57 (1.42, 30.43) | 0.02 |
| Age | 4.78 (1.60, 14.26) | 0.005 | 6.20 (1.39, 27.54) | 0.02 | 6.18 (1.35, 28.24) | 0.02 | |
| Sex | 5.69 (1.90, 17.01) | 0.002 | 8.35 (1.88, 37.12) | 0.005 | 6.90 (1.49, 31.90) | 0.01 | |
| Baseline Fasting Blood Glucose | 3.82 (1.64, 8.91) | 0.002 | 6.81 (1.52, 30.58) | 0.01 | 4.98 (1.08, 23.08) | 0.04 | |
| Maximum Blood Glucose 2 years prior to imaging | 4.52 (1.51, 13.54) | 0.007 | 6.04 (1.35, 26.98) | 0.02 | 6.87 (1.47, 32.20) | 0.01 | |
| Average Blood Glucose over 2 years prior to imaging | 4.01 (1.33, 12.10) | 0.01 | 4.86 (1.08, 21.95) | 0.04 | 4.90 (1.02, 23.59) | 0.048 | |
| History of Pre-Diabetes | 4.01 (1.33, 12.07) | 0.01 | 5.36 (1.18, 24.34) | 0.03 | 5.13 (1.05, 24.93) | 0.04 | |
| DM Risk Factorsa | 4.95 (1.67, 14.79) | 0.004 | 6.68 (1.51, 29.62) | 0.01 | 6.38 (1.38, 29.53) | 0.02 | |
| Cardiac Risk Factorsb | 5.91 (1.97, 17.70) | 0.002 | 9.91 (1.47, 30.58) | 0.004 | 6.70 (1.47, 30.58) | 0.01 | |
| Statin Use | 5.01 (1.69, 14.90) | 0.004 | 6.52 (1.47, 28.91) | 0.004 | 7.41 (1.59, 14.90) | 0.01 | |
| BMI | 4.87 (1.64, 14.52) | 0.004 | 6.67 (1.50, 29.64) | 0.01 | 6.45 (1.36, 34.56) | 0.02 | |
| VAT | 4.10 (1.35, 12.46) | 0.01 | 5.37 (1.18, 24.59) | 0.03 | 5.98 (1.29, 27.79) | 0.02 | |
| SAT | 4.00 (1.32, 12.15) | 0.01 | 5.11 (1.12, 23.24) | 0.04 | 7.41 (1.57, 34.96) | 0.01 | |
| VAT:SAT | 3.97 (1.31, 12.09) | 0.02 | 5.05 (1.10, 23.08) | 0.04 | 6.78 (1.46, 31.40) | 0.01 | |
| Total Adiposity | 4.03 (1.33, 12.25) | 0.01 | 5.30 (1.16, 24.19) | 0.03 | 6.68 (1.42, 31.49) | 0.02 | |
| History of Prior Cancer | 4.84 (1.63, 14.39) | 0.005 | 6.43 (1.45, 28.51) | 0.01 | 6.47 (1.39, 30.08) | 0.02 | |
| History of Depression/Anxiety | 4.44 (1.48, 13.27) | 0.008 | 5.68 (1.27, 25.37) | 0.02 | 5.76 (1.22, 27.12) | 0.04 | |
| History of Corticosteroid Use | 4.91 (1.65, 14.61) | 0.004 | 6.79 (1.64, 32.33) | 0.009 | 6.53 (1.41, 30.25) | 0.02 | |
231 individuals were included in the outcomes cohort analysis, as one individual did not provide background cerebral data to calculate the pre-specified amygdalar activity endpoint.
Abbreviations: BMI: body mass index, CI: confidence interval, HR: hazard ratio, SAT: subcutaneous adipose tissue, VAT: visceral adipose tissue.
Age, race, history of pre-diabetes, and BMI entered as cofactors.
Age, smoking, hypertension, dyslipidemia, statin use and family history entered as cofactors.
Notably, high AmygA significantly increased risk for DM even among the sub-groups of individuals at increased risk for type 2 DM due to greater adiposity (Table 3) as assessed as: A) baseline BMI ≥ 25 kg/m2 (6.37 [1.44, 28.23], p=0.02) or B) ≥ median baseline VAT (6.57 [1.42, 30.43], p=0.02). Those associations remained significant in multivariable analyses. Additionally, high AmygA predicted incident DM in individuals who were not obese (BMI <30 kg/m2) at baseline (6.59 [1.45, 30.10], p=0.02). This finding remained significant after adjusting for baseline VAT (6.61 [1.44, 30.36], p=0.02, Supplemental Figure A3).
3.3. Synergy between Amygdalar Activity and Adiposity in Determining Diabetes Risk
We next explored the potential synergy between AmygA and adiposity on type 2 DM risk. For these analyses, AmygA was again dichotomized by the median value. Adiposity measures were dichotomized similarly. This two-by-two factorial analysis placed individuals into one of four groups: A) low adiposity, low AmygA, B) low adiposity, high AmygA, C) high adiposity, low AmygA, and D) high adiposity, high AmygA. The analysis was repeated three times, each using a different measure of adiposity: A) BMI (<25 or ≥25 kg/m2), B) baseline VAT (< or ≥ sample median), and C) change in VAT (< or ≥ sample median). Across each of these analyses (Figure 3B-D), low AmygA associated with a low risk of DM, regardless of adiposity. Conversely, the greatest DM risk was observed in individuals with both high AmygA and high adiposity, suggesting that high AmygA and high adiposity may act synergistically to increase type 2 DM risk.
3.4. Validation in a Separate Cohort
We tested the relationship between AmygA and metabolic parameters in a separate biomarker study of 87 prospectively recruited individuals (characteristics in Supplemental Table A2). Subjects were designated as having “higher” vs. “lower” AmygA (highest tertile vs. pooled middle and lower tertiles). Higher AmygA associated with higher levels of several inflammatory biomarkers: CCL5 (p=0.04), CXCL5 (p=0.03), and ENRAGE (neutrophil-derived extracellular newly identified receptor for advanced glycosylation end products) (p=0.02). Furthermore, higher AmygA associated with greater insulin resistance, measured as homeostatic model assessment of insulin resistance (HOMA-IR) (p=0.02). Higher AmygA also associated with lower levels of the beneficial adipokine, adiponectin (p=0.005). These findings are shown in Supplemental Figure A4 and Supplemental Table A4.
4. Discussion
This study employed simultaneous imaging of brain and adipose tissues and leveraged longitudinal clinical assessments to demonstrate that increased neurobiological activity related to stress (i.e., AmygA) confers a substantially increased risk of developing type 2 DM in the future. The association between higher AmygA and DM risk remained robust after accounting for type 2 DM risk factors, including glucose measures, demographic data, and imaging measures of adiposity. Lower AmygA associated with a low risk of DM regardless of adiposity. Conversely, individuals with both high AmygA and high adiposity had a markedly increased risk of type 2 DM, suggesting that increased AmygA may combine with adiposity via a “two-hit” mechanism to promote DM. Accordingly, these findings identify the amygdala, a neural region involved in the neurobiology of stress, as a previously underappreciated, yet potentially important participant in the mechanisms that provoke type 2 DM in humans.
4.1. Mechanistic Insights
It has been hypothesized for millennia that psychological stress associates with physical maladies. Recently, stress has gained attention as a risk factor for metabolic disease (Kumari et al., 2004; Mooy et al., 2000). Clinical guidelines currently acknowledge that psychosocial stress may complicate DM care (Chida and Hamer, 2008). Our results provide novel evidence implicating the role of increased AmygA in predicting incident type 2 DM. Although it has been reported that individuals with overt DM are prone to have structural abnormalities of the amygdala (den Heijer et al., 2003), the role of AmygA in potentiating future DM risk was previously unknown. The current study, for the first time in animals or humans, identifies the amygdala as a brain region that may link stressors to the subsequent development of type 2 DM. From these observations, we hypothesize that the amygdala may be a pathobiological contributor to metabolic disorders. Stimulation of the amygdala activates both the hypothalamic-pituitary-adrenal axis (HPAA) and the sympathetic nervous system (the latter through the amygdala’s projections to the brainstem) (Drevets et al., 2002; LeDoux et al., 1988). Importantly, sympathetic efferents to the bone marrow stimulate turnover and release of immune cells, which contribute to systemic inflammation and modify the metabolic milieu. In animal studies, chronic stress promotes systemic inflammation (Heidt et al., 2014) and increases VAT (Bartolomucci et al., 2009), which both lead to greater insulin resistance (Karagiannides et al., 2014; Uchida et al., 2012). We observed associations between AmygA and markers of inflammation and metabolic impairment. Together these factors could combine to promote the development of type 2 DM (Supplemental Figure A5).
4.2. Observed Incident Diabetes Not Solely the Result of Subsequent Gains in Adiposity
This study’s finding that increased AmygA confers DM risk independent of subsequent increases in VAT is particularly notable. This result suggests that the association between AmygA and type 2 DM is unlikely to be mediated solely by behavioral changes (e.g., increased caloric intake and decreased physical activity), which collectively increase adiposity. If altered health behaviors after baseline imaging were indeed the primary reason for the association between AmygA and type 2 DM, then adjusting for an important consequence (i.e., subsequent increases in adiposity) should have attenuated the relationship between AmygA and DM. However, AmygA remained a strong predictor of DM, even after such adjustments, suggesting that these findings extended beyond the effects of altered health behaviors alone. Moreover, in individuals with increases in adiposity after baseline imaging, AmygA remained a particularly potent predictor of type 2 DM risk.
4.3. Limitations
Our study has limitations. Questionnaire-based assessments of stress were not employed in this study, which limits the ability to directly link stress to AmygA in this population; however, we have previously shown that AmygA closely correlated with a measure of perceived stress (Tawakol et al., 2017). Outcomes study subjects were identified from a database of patients who had undergone clinical 18F- FDG-PET/CT screening for cancer, possibly limiting the generalizability of the findings. Because the outcomes study utilized imaging and laboratory data obtained during clinical care, it is subject to the inherent limitations of such a design. However, the biomarker study, which included clinically stable patients without suspected malignancy, yielded confirmatory findings that provide confidence in the outcomes study’s results by demonstrating a similar cross-sectional relationship between increased AmygA and metabolic impairment. Additionally, due to the incomplete coverage of the brain and the limited resolution of clinical whole-body 18F-FDG-PET/CT imaging, we were unable to assess other brain regions involved in stress perception (e.g., the hippocampus and insular cortex). Finally, although it is hypothesized that this neural-metabolic mechanism acts through effects on the HPAA and sympathetic nervous system, direct measurements of these pathways were not performed. Similarly, an evaluation of health behaviors during follow-up was not performed in this retrospective study. Nonetheless, this study’s limitations are counterbalanced by important innovations, including the use of unique hybrid imaging for the simultaneous quantification of AmygA and VAT, to identify a compelling link between neurobiological activity and DM.
4.4. Future perspectives
Future studies should test whether modifying the observed central neural-metabolic mechanism may decrease the burden of metabolic disease. Studies are needed to clarify the hypothesized roles of the HPAA and sympathetic nervous system. Although pharmacotherapies targeting these pathways should be pursued, non-pharmacologic approaches may also be fruitful. For example, stress management and mindfulness-based stress reduction interventions have demonstrated positive effects on DM (Medina et al., 2017; Surwit et al., 2002). Our results suggest value in screening for metabolic impairment in individuals with high stress who are at elevated risk of developing type 2 DM, and who may benefit from stress reduction approaches.
5. Conclusions
This study demonstrates, for the first time, a relationship between amygdalar metabolic activity and subsequent risk of type 2 DM. We observed that high amygdalar activity strongly and independently increases the risk of DM, whereas lower amygdalar activity associates with lower risk of DM, even among overweight and obese individuals. These findings support a “two-hit” model in which adiposity and a neurobiological response to stress combine to precipitate DM, and, furthermore, highlight a path against which novel therapeutic strategies may be applied. Moreover, these results further underscore the need to consider psychosocial stress when assessing individuals at risk for type 2 DM.
Supplementary Material
Highlights:
Stress associates with the development of diabetes, but the mechanism is unknown.
Associations between amygdalar metabolic activity and diabetes were tested.
Elevated amygdalar activity increased the risk of subsequent diabetes.
These results were independent of measures of adiposity and diabetes risk factors.
These findings provide novel insights into the link between stress and diabetes.
Acknowledgments:
This manuscript’s findings were presented in part at the American College of Cardiology Scientific Sessions in March 2017 (Washington, DC) and March 2018 (Orlando, FL) and at the American Heart Association Scientific Sessions in November 2017 (Anaheim, CA).
MTO, AI, JTG, SKG, RKP, and AT contributed to study design. BH and AT performed statistical analysis. AI, BT, and YW provided image analysis and clinical adjudication. AB contributed biomarker data. Expertise was provided by ZAF and AT for PET/CT imaging, SKG and JL for metabolic diseases, KCK and RKP for stress conditions, and LS for neuroimaging. MTO and AT led manuscript preparation and revisions. AT performed leadership duties and is the guarantor of this work. He had full access to the study data and takes responsibility for its integrity and the accuracy of the analysis. All authors participated in preparation and revisions and approved this manuscript for submission. MTO and AI contributed equally and are co-first authors.
Funding: This work was supported by the following United States National Institutes of Health grants: T32HL076136 (MTO), P01HL131478 (ZAF), P30DK040561 (SKG), and R01HL122177 (AT). The funding source had no role in the conduct or reporting of this study.
Footnotes
Declaration of Interests: AT received institutional grants from Genentech, Inc. and Actelion and personal fees from Actelion for research outside the submitted work. AB is employed by Genentech, Inc. The remaining authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam TC, Epel ES, 2007. Stress, eating and the reward system. Physiology & Behavior 91, 449–458. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association, 2016. 2. Classification and Diagnosis of Diabetes. Diabetes Care 39, S13–22. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell’Omo G, Parmigiani S, Palanza P, 2009. Metabolic consequences and vulnerability to diet- induced obesity in male mice under chronic social stress. PLoS One 4, e4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS, 2005. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine 35, 791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz-Cunningham SH, Millstine JW, Gerbaudo VH, 2008. Improved discrimination of benign and malignant lesions on FDG PET/CT, using comparative activity ratios to brain, basal ganglia, or cerebellum. Clinical Nuclear Medicine 33, 681–687. [DOI] [PubMed] [Google Scholar]
- Chida Y, Hamer M, 2008. An association of adverse psychosocial factors with diabetes mellitus: a meta-analytic review of longitudinal cohort studies. Diabetologia 51, 2168–2178. [DOI] [PubMed] [Google Scholar]
- den Heijer T, Vermeer SE, van Dijk EJ, Prins ND, Koudstaal PJ, Hofman A, Breteler MM, 2003. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 46, 1604–1610. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME, 2002. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacology, Biochemistry, and Behavior 71, 431–447. [DOI] [PubMed] [Google Scholar]
- Figueroa AL, Takx RA, MacNabb MH, Abdelbaky A, Lavender ZR, Kaplan RS, Truong QA, Lo J, Ghoshhajra BB, Grinspoon SK, Hoffmann U, Tawakol A, 2016. Relationship Between Measures of Adiposity, Arterial Inflammation, and Subsequent Cardiovascular Events. Circulation Cardiovascular Imaging 9, e004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad B, Osborne MT, Ishai A, Wang Y, Tung B, Baruch A, Klimas M, Shin LM, Fayad Z, Giles JT, Pitman R, Tawakol A, 2017. Abstract 18768: Increased Stress-related Neural Tissue Activity Potentiates Inflammation and Impedes the Anti-inflammatory Impact of Statins. Circulation 136, A18768–A18768. [Google Scholar]
- Heidt T, Sager HB, Courties G, Dutta P, Iwamoto Y, Zaltsman A, von Zur Muhlen C, Bode C, Fricchione GL, Denninger J, Lin CP, Vinegoni C, Libby P, Swirski FK, Weissleder R, Nahrendorf M, 2014. Chronic variable stress activates hematopoietic stem cells. Nat Med 20, 754–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannides I, Golovatscka V, Bakirtzi K, Sideri A, Salas M, Stavrakis D, Polytarchou C, Iliopoulos D, Pothoulakis C, Bradesi S, 2014. Chronic unpredictable stress regulates visceral adipocyte-mediated glucose metabolism and inflammatory circuits in male rats. Physiological Reports 2, e00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouvonen A, Kivimaki M, Cox SJ, Cox T, Vahtera J, 2005. Relationship between work stress and body mass index among 45,810 female and male employees. Psychosomatic Medicine 67, 577–583. [DOI] [PubMed] [Google Scholar]
- Kumari M, Head J, Marmot M, 2004. Prospective study of social and other risk factors for incidence of type 2 diabetes in the Whitehall II study. Arch Intern Med 164, 1873–1880. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ, 1988. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. The Journal of Neuroscience : the official journal of the Society for Neuroscience 8, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer-Graiwer J, Singh P, Abdelbaky A, Vucic E, Korsgren M, Baruch A, Fredrickson J, van Bruggen N, Tang MT, Frendeus B, Rudd JHF, Hsieh F, Ballantyne CM, Ghoshhajra B, Rosenson RS, Koren M, Roth EM, Duprez DA, Fayad ZA, Tawakol AA, 2015. FDG-PET imaging for oxidized LDL in stable atherosclerotic disease: a phase II study of safety, tolerability, and anti-inflammatory activity. JACC Cardiovasc Imaging 8, 493–494. [DOI] [PubMed] [Google Scholar]
- Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U, 2007. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. International Journal of Obesity 31, 500–506. [DOI] [PubMed] [Google Scholar]
- Medina WL, Wilson D, de Salvo V, Vannucchi B, de Souza EL, Lucena L, Sarto HM, Modrego-Alarcon M, Garcia-Campayo J, Demarzo M, 2017. Effects of Mindfulness on Diabetes Mellitus: Rationale and Overview. Current diabetes reviews 13, 141–147. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS, 2003. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289, 76–79. [DOI] [PubMed] [Google Scholar]
- Mooy JM, de Vries H, Grootenhuis PA, Bouter LM, Heine RJ, 2000. Major stressful life events in relation to prevalence of undetected type 2 diabetes: the Hoorn Study. Diabetes Care 23, 197–201. [DOI] [PubMed] [Google Scholar]
- Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA, 2012. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 308, 1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH, 2010. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature 466, 864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwer F, Kupper N, Adriaanse MC, 2010. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med 9, 112–118. [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM, 2006. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surwit RS, van Tilburg MAL, Zucker N, McCaskill CC, Parekh P, Feinglos MN, Edwards CL, Williams P, Lane JD, 2002. Stress Management Improves Long-Term Glycemic Control in Type 2 Diabetes. Diabetes Care 25, 30–34. [DOI] [PubMed] [Google Scholar]
- Tawakol A, Ishai A, Takx RAP, Figueroa AL, Ali A, Kaiser Y, Truong QA, Solomon CJE, Calcagno C, Mani V, Tang CY, Mulder WJM, Murrough JW, Hoffmann U, Nahrendorf M, Shin LM, Fayad ZA, Pitman RK, 2017. Relation between resting amygdalar activity and cardiovascular events: a longitudinal and cohort study. The Lancet 389, 834–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida Y, Takeshita K, Yamamoto K, Kikuchi R, Nakayama T, Nomura M, Cheng XW, Egashira K, Matsushita T, Nakamura H, Murohara T, 2012. Stress augments insulin resistance and prothrombotic state: role of visceral adipose-derived monocyte chemoattractant protein-1. Diabetes 61, 1552–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SS, Yan XB, Hoffman MA, Swaab DF, Zhou JN, 2010. Increased expression level of corticotropin-releasing hormone in the amygdala and in the hypothalamus in rats exposed to chronic unpredictable mild stress. Neuroscience Bulletin 26, 297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman AD, Herholz K, Lewis DL, Herschovitch P, Minoshima S, Ichise M, Drzezga AE, Devous MD, Mountz JM, 2009. Society of Nuclear Medicine Procedure Guideline for FDG PET Brain Imaging Version 1.0. Society of Nuclear Medicine, 1–12. [Google Scholar]
- Whalen PJ, Shin LM, Somerville LH, McLean AA, Kim H, 2002. Functional neuroimaging studies of the amygdala in depression. Seminars in Clinical Neuropsychiatry 7, 234–242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


