Abstract
Sleep is beneficial for performance across a range of memory tasks in young adults, but whether memories are similarly consolidated in older adults is less clear. Performance benefits have been observed following sleep in older adults for declarative learning tasks, but this benefit may be reduced for non-declarative, motor skill learning tasks. To date, studies of sleep-dependent consolidation of motor learning in older adults are limited to motor sequence tasks. To examine whether reduced sleep-dependent consolidation in older adults is generalizable to other forms of motor skill learning, we examined performance changes over intervals of sleep and wake in young (n = 62) and older adults (n = 61) using a mirror-tracing task, which assesses visuo-motor adaptation learning. Participants learned the task either in the morning or in evening, and performance was assessed following a 12-h interval containing overnight sleep or daytime wake. Contrary to our prediction, both young adults and older adults exhibited sleep-dependent gains in visuo-motor adaptation. There was a correlation between performance improvement over sleep and percent of the night in non-REM stage 2 sleep. These results indicate that motor skill consolidation remains intact with increasing age although this relationship may be limited to specific forms of motor skill learning.
Keywords: Sleep, Memory consolidation, Motor learning, NREM sleep
Introduction
Sleep is beneficial for memory consolidation, the transfer of memory traces from short-term storage to stable, long-term storage (Stickgold 2005). The benefit of sleep on memory consolidation depends on specific aspects of sleep, such as time in certain sleep stages. However, even in the absence of disease, aging brings a characteristic curtailment of sleep quantity and quality. These changes in sleep are purported to weaken memory consolidation in older adults (Pace-Schott and Spencer 2014a, b), and thus, identifying sleep-dependent memory deficits and the underlying mechanisms is critical in this population.
In young adults, the benefit of sleep on motor skill learning has been amply demonstrated with an array of tasks, including motor sequence learning (Spencer et al. 2007; Fischer et al. 2002), pursuit rotor learning (Maquet et al. 2003a; Fogel et al. 2007), and mirror tracing (Plihal and Born 1997; Tamaki et al. 2008; Javadi et al. 2011). Consolidation of new motor skill memories is greatest over intervals of sleep, possibly due to cortical reactivation (Reis et al. 2009) that is often followed by increased corticothalamic plasticity and communication, via sleep spindles (Fogel et al. 2007; Morin et al. 2008; Peters et al. 2008; Barakat et al. 2013). In older adults, however, although motor skill acquisition (i.e., encoding) is relatively preserved (Spencer et al. 2007; Wilson et al. 2012; Howard and Howard 1989; Fogel et al. 2012), consolidation of such memories is reduced (Spencer et al. 2007; Wilson et al. 2012; Brown et al. 2009; Fogel et al. 2012; Gudberg et al. 2014). For example, we previously compared motor skill acquisition and consolidation in young and older adults using a motor sequencing task (Wilson et al. 2012). Young and older adult groups showed similar levels of learning in the first session, yet only young adults showed sleep-dependent improvements in performance when motor skill was probed 12 h later. Of note, one study (Tucker et al. 2011) suggested consolidation of motor sequence learning over sleep may be preserved with aging, but it is unclear whether benefits in the Sleep group in that study were due to the Sleep group having poorer performance at baseline compared to the Wake group (i.e., leaving more room for change) as opposed to a true benefit of sleep.
Previous investigations of sleep-dependent consolidation of non-declarative, motor skill learning in older adults are limited to motor sequence learning using either the serial reaction time task (Spencer et al. 2007; Wilson et al. 2012), the motor sequencing task (Terpening et al. 2013), or other similar sequencing tasks (Siengsukon and Boyd 2008, 2009). Although it is possible that the failure to find performance benefits following sleep for the motor sequence learning task reflects a general age-related change in consolidation of motor skill learning, it is important to consider that motor sequence learning may be unique. First, the effect of sleep on motor sequence learning is based on reaction time measures because accuracy is typically at ceiling. Given that older adults are slower than young adults, a measurable change may not be detected. Second, studies of neural replay illustrate that task-relevant neural ensembles are reactivated during sleep in older animals, but this replay is disorganized and non-sequential (Gerrard et al. 2008). This may suggest that sleep-dependent processing of tasks that require strings of item-to-item associations may uniquely diminish with age. For these reasons, it is critical to demonstrate this result in another motor skill task. Mirror-tracing, a visuo-motor adaptation task, provides a novel probe of sleep-dependent consolidation of motor skill learning in older adults.
The mirror-tracing task has been used previously to examine sleep-dependent consolidation of motor skill learning in young adults. Similar to motor sequence learning (Walker et al. 2002), visuo-motor adaptation performance enhancements across the night have been linked with non-REM stage 2 (N2). For example, in split-night studies, in which the participant undergoes experimental sleep deprivation during the first or second half of the night, mirror-tracing improvement is enhanced to a greater extent following the second half of the night, which is rich in N2 when compared to the first half of the night (Plihal and Born 1997). Moreover, gains in mirror-tracing performance have been linked with one of the predominant frequency ranges of N2 (Tamaki et al. 2008) (fast spindles: 13–16 Hz), which are thought to reflect enhanced cortical-parietal communication. Given that fast spindles occur predominantly during the second half of the night (Tamaki et al. 2008), these activity bursts may be a causal mechanism in improving consolidation of visuo-motor adaptation learning. On the other hand, another mechanism potentially responsible for visuo-motor adaptation is REM sleep, which also dominates the second half of the night. REM sleep has similarly been implicated in complex motor learning (Smith 1995) and has also been correlated with consolidation of a visuo-motor adaptation (Schredl and Erlacher 2007). Thus, it remains unclear whether N2, REM, or both contribute to visuo-motor adaption in young adults. Further, it is unknown whether the relationship between sleep and consolidation of visuo-motor adaptation learning is uniform across the lifespan or whether sleep-dependent gains are lost with increasing age, similar to what is seen with the motor sequencing task (Wilson et al. 2012; Brown et al. 2009; Fogel et al. 2012).
Therefore, the current study compared consolidation of visuo-motor adaptation learning over equivalent periods of sleep and wake in both young and older adults. We hypothesized young adults would show greater offline improvement in visuo-motor skill following an interval containing sleep when compared to an equivalent interval spent awake. Moreover, we posited that sleep-dependent gains would be positively correlated with %N2 (Walker et al. 2002; Smith and MacNeill 1994). On the other hand, we hypothesized that older adults who had a period of sleep between learning and re-test would show greater consolida-tion than those who remained awake, but not statistically significantly so, consistent with previous reports on deficits in sleep-dependent consolidation of motor skill learning in older adults (Spencer et al. 2007; Wilson et al. 2012).
Methods
Participants
Participants were 62 young (M = 21.19 years, SD = 2.8, 67 % female) and 61 older (M = 63.89 years, SD = 7.7; 75 % female) adults. Thirty-one young adult and 31 older adult participants were pseudo-randomly assigned to the Sleep group (described below), while 31 young adults and 30 older adults were assigned to the Wake group. Young adults were recruited from the surrounding college community and were compensated with course credit or monetary payment. Community-dwelling older adults were recruited via advertisements and received payment for participation. All older adults had a Mini-Mental Status Examination (MMSE) score >26, indicating normal cognitive functioning. Exclusion criteria included: history of neurological or sleep disorders, habitual sleep of <6 h/night, and use of sleep-affecting medications. Participants were also required to have normal or corrected-to-normal vision.
Task
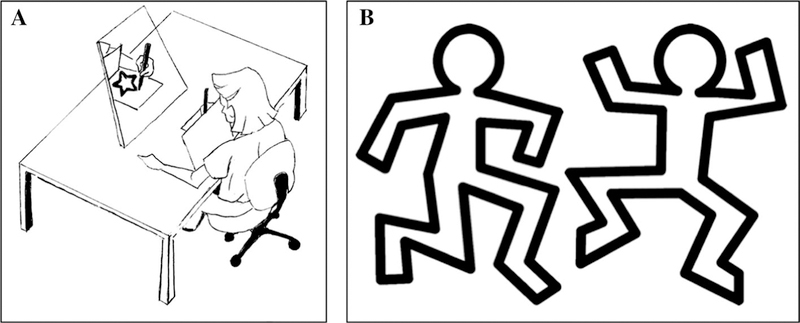
The task was a mirror-tracing task modified from that described previously (Plihal and Born 1997). The task required that participants trace a figure (approximately 10 × 16 cm) on an iPad screen using a stylus (.45 cm tip width). Participants were told to stay within the lines (.8 cm thickness) of the presented figure. A screen mounted over the hand and iPad occluded direct visual feedback, but the workspace was visible through a mirror that was mounted behind the workspace, providing inverted visual feedback (Fig. 1a).
Fig. 1.
a Mirror-tracing task. b Two figures used during the learning phase
There were two sessions. In Session 1, participants completed the learning phase. Participants were first oriented to the task by repeatedly tracing a star. Tracing continued until they reached a criteria of <10 bouts outside of the line during the trial. Verbal feedback was provided during these orientation trials. Subsequently, participants traced six figures, each the outline of a cartoon figure in different poses (Fig. 1b), but identical in parameter and number of corners. The speed and position of the stylus while tracing the figure were recorded through an automated in-house iPad application. In Session 2, the re-test phase, participants re-oriented themselves to the paradigm by tracing the star once. Subsequently, six figures were traced. Importantly, as opposed to Plihal and Born (1997) who used the same six figures in Session 1 and Session 2, six new figures were used in Session 2 so that performance changes reflected learning of the skill of mirror tracing and not a specific sequence of strokes that compose a figure. Speed and accuracy were again recorded to enable comparison with these parameters in the learning phase. All participants traced the same figures and in the same order in both sessions.
Procedure
Procedures were approved by the University of Massachusetts, Amherst Institutional Review Board. Written informed consent was obtained before the experiment commenced. Participants were randomly assigned to either a Wake group or a Sleep group. The Wake group performed Session 1 in the morning (7–10 A.M.) and Session 2 took place 12 h later (7–10 P.M.), following an interval of day-time wake. The Sleep group performed Session 1 in evening (7–10 P.M.) and Session 2 took place the following morning (7–10 A.M.), following an interval containing overnight sleep. Session 1 took about 1 h to complete and Session 2 about 45 min.
In Session 1, following informed consent procedures, participants completed the Stanford Sleepiness Scale (Hoddes et al. 1973), Epworth Sleepiness Scale (Johns 1994), Pittsburgh Sleep Quality Index (Buysse et al. 1989), Morningness–Eveningness Questionnaire (Horne and Ostberg 1976), and a Wake (Sleep group) or Sleep (Wake group) diary. Participants were oriented to the task by repeatedly tracing a star and, subsequently, completed 6 figure-tracing trials in succession. In each trial, participants completed a figure as quickly and accurately as possible. Participants in the Wake group were then instructed to go about their usual daytime routine, refraining from napping or alcohol intake. Participants in the Sleep group were encouraged to have a normal night’s sleep. A subset of these participants was fitted with polysomnography prior to their habitual bedtime. Twelve hours later, in Session 2, participants completed either a Wake (Wake group) or Sleep (Sleep group) diary and the Stanford Sleepiness Scale before completing the re-test phase (tracing 6 new figures).
Polysomnography
Twenty-three young adults and 15 older adults in the Sleep group opted into the polysomnography (PSG) portion of the study. Over-sleep performance changes did not differ for those in the PSG group and those that opted out (all p value’s >.29). Polysomnography was recorded with the Aura PSG ambulatory system (Grass Technologies; sampled at 200 cycles/s; band-pass filtered from .3–35 Hz). The electrode montage was applied to the participant in their home approximately 1 h before their typical bedtime. The montage included two EOG leads (right and left ocular canthus), two chin EMG leads, and six cortical EEG leads (F3, F4, C3, C4, O1, O2) with each electrode referenced to Cz.
Data analysis
Learning performance
The main measures of interest were error proportion, error time, and drawing time (as in Plihal and Born 1997). Error proportion was the percent of total pixels traced that were outside of the width of the template outline. Although previous studies have used error count (Plihal and Born 1997), error proportion is a more precise measure as it encom-passes both the error count and tracing distance from the figure (i.e., tracing farther outside of the figure outline is a ‘larger’ error because of an increased amount of pixels traced outside of the outline). Error time was defined as total time (in s) the stylus was not on the figure outline. Drawing time was the total time (in s) spent tracing the figures.
Additionally, following initial analyses, a post hoc measurement was created to further explore the impact of sleep on learning. Given that drawing time includes error time, we created an additional measurement, figure time, that encompasses only time spent tracing the figure. This score was calculated by subtracting error time from drawing time.
To assess whether adaptation (i.e., learning) took place during Session 1, we compared the average of error proportion, error time, and drawing time for trials 2–3 with trials 4–6 using paired t tests. t tests were also used to compare performance in the learning phase (trials 2–6 averaged) between Sleep and Wake groups to assess the presence of circadian effects.
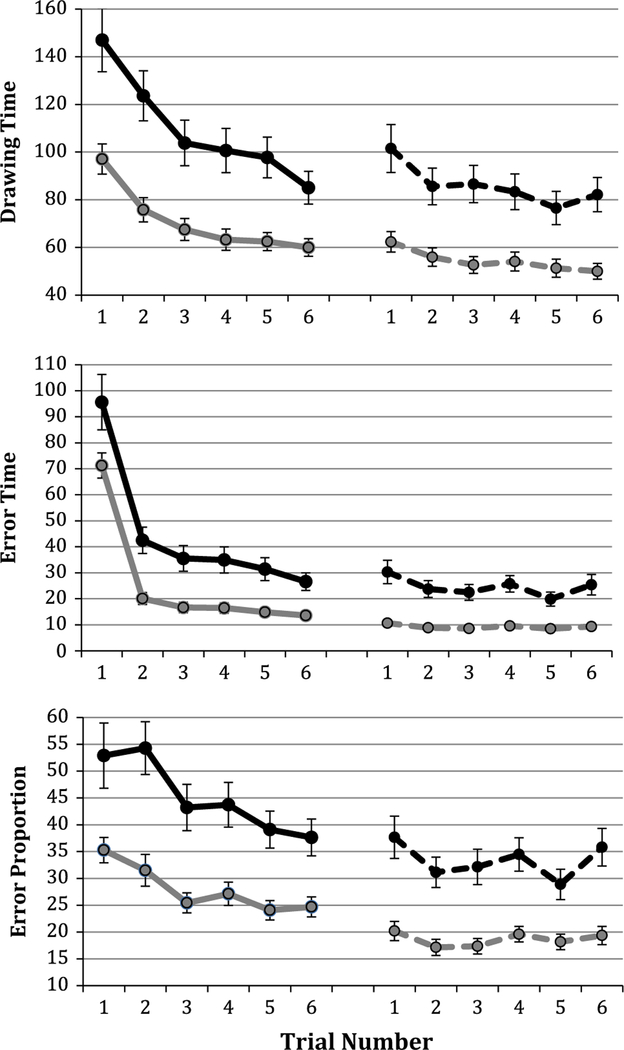
For gauging performance change between Session 1 and Session 2, error proportion, error time, figure time, and drawing time were averaged across figures 2–6 at Session 1 and compared with the average of figures 1–6 at Session 2. This analysis is identical to those in previous work (Plihal and Born 1997; Tamaki et al. 2008), but we chose to exclude figure 1 from the Session 1 average as extensive adaptation occurred in trial 1 (Fig. 2). Given that the same figures were used for all participants in Session 1 and Session 2 and that figures were also the same for all participants and in the same order, any differences driven by the specific shape were equivalent for all participants.
Fig. 2.
Learning and consolidation curves. 1–6 in the first block represent figures in the learning phase, whereas 1–6 in the second block represent re-test figures. The solid black line represents older adult learning; the dashed black line represents older adult re-test; the solid gray line represents young adult learning; the dashed gray line represents young adult re-test
An improvement score was created to gauge error proportion, error time, and drawing time change between sessions while accounting for baseline differences. Improvement was calculated by subtracting Session 2 performance (i.e., error proportion, error time, figure time, drawing time) from Session 1 performance, normalizing for baseline by dividing by Session 1 performance, and multiplying by 100.
Main effects of each factor on improvement (drawing time, error time, figure time, and error proportion) were examined using a 2 × 2 ANOVA, with between-subject variables condition (Sleep vs Wake) and group (young vs older). The dependent variable was the improvement score.
Two-tailed t tests were used to compare young adult and older adult sleep architecture and quality [sleep stages: %N1, %N2, %SWS, %REM, total sleep time, sleep efficiency (time asleep/time in bed), wake after sleep onset, sleep latency].
Improvement scores were then correlated with sleep parameters with using Pearson’s correlations. Given that our measure of interest, %N2, had overlapping distributions (Kolmogorov–Smirnov test p values: %N2 = .19) between young and older adult groups, these scores were collapsed and analyzed as one after being analyzed separately. All statistical analyses were performed using SPSS version 21 (Armonk, NY: IBM Corp.).
Polysomnography
Polysomnography data were scored using the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Associated Events (Silber et al. 2007) for N1, N2, SWS, and REM using 30-s scoring epochs. Total sleep time, sleep efficiency, wake after sleep onset (WASO), and percent time in each sleep stage were calculated and compared across young and older adult groups.
Results
Participants
Demographic information and group characteristics are presented in Table 1. The young adult group had significantly more years of education than the older adult group [t(56.74) = −2.94, p = .01; degrees of freedom reflecting results adjusted to account for heterogeneity of variance; the older adult group had greater variability] and, as expected, the older adult group exhibited more ‘morningness’ tendencies, as evidenced by a significantly lower Morningness–Eveningness Questionnaire [t(121) = −8.71, p < .001]. Young adults were also significantly more sleepy at Session 1, as shown by higher Stanford Sleepiness Scores [t(121) = 4.02, p < .001].
Table 1.
Participant demographics, questionnaire information, and sleep architecture
| OA (n = 61) | YA (n = 64) | p | |
|---|---|---|---|
| Age (years) | 63.89 ± 7.69 | 21.19 ± 2.79 | – |
| Female (%) | 75.47 | 67.24 | – |
| Education (years) | 15.43 ± 1.81 | 17.09 ± 3.86 | .01* |
| PSQI | 4.43 ± 2.08 | 4.37 ± 2.51 | .89 |
| SSS1 | 1.93 ± 1.13 | 2.67 ± 0.87 | <.001* |
| SSS2 | 2.33 ± 1.08 | 2.33 ± 1.09 | .978 |
| MEQ | 43.58 ± 8.052 | 58.67 ± 8.989 | <.001* |
| ESS | 6.07 ± 3.11 | 6.70 ± 3.94 | .35 |
OA older adults, YA young adults; PSQI Pittsburgh Sleep Quality Index, SSS Stanford Sleepiness Scale at Sessions 1 and 2, MEQ Morningness–Eveningness Questionnaire, ESS Epworth Sleepiness Scale
p value <.05. Values are mean ± SD
Learning phase
To assess initial acquisition, or adaptation to the inverted visual feedback during the learning phase, the average scores for trials 2 and 3 were compared with scores from trials 4, 5, and 6. Consistent with previous reports (Spencer et al. 2007; Wilson et al. 2012; Howard and Howard 1989; Fogel et al. 2012), both older and younger adults improved significantly from the beginning of the learning phase to the end of the learning phase (young adults: drawing time [t(60) = 10.1, p = <.001], error time [t(60) = 15.1, p < .001], error proportion [t(60) = 5.59, p < .001]; older adults: drawing time [t(59) = 8.12, p = <.001], error time [t(59) = 11.3, p < .001], and error proportion [t(59) = 4.78, p < .001]. The shape and trajectory of the learning curves are also notably similar (Fig. 2).
To assess whether motor adaptation was influenced by time-of-day (circadian) effects, mean scores for the learning phase were compared for the Sleep groups, who learned the task in the evening, and the Wake groups, who learned the task in the morning. There were no significant differences in drawing time, error time, or error proportion (Table 2), although the young adult Wake group performed noticeably better than the Sleep group for drawing time.
Table 2.
Mirror-tracing performance at the learning phase
| Young adults |
Older adults |
|||||
|---|---|---|---|---|---|---|
| Sleep | Wake | p | Sleep | Wake | p | |
| Drawing time (s) | 76.64 ± 24.50 | 65.45 ± 22.96 | .08 | 109.86 ± 51.38 | 109.44 ± 45.12 | .97 |
| Error time (s) | 26.47 ± 8.38 | 24.54 ± 10.56 | .44 | 48.95 ± 29.94 | 39.92 ± 23.78 | .19 |
| Error proportion (%) | 27.72 ± 9.66 | 28.32 ± 10.14 | .81 | 45.63 ± 19.27 | 44.66 ± 20.96 | .85 |
Values are mean ± SD
Intersession change in performance
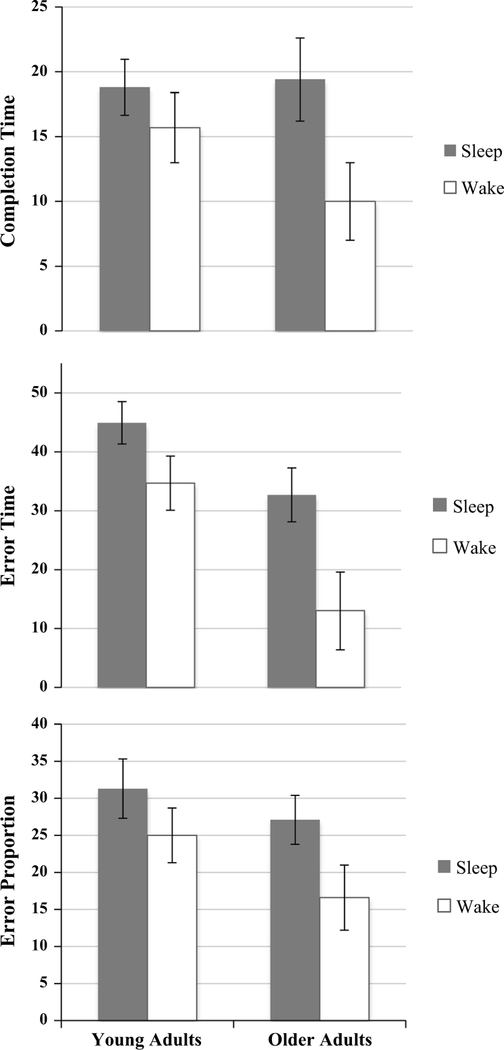
To assess changes in motor skill adaptation following intervals with sleep and wake, an intersession change score, described above, was compared between groups. A 2 × 2 ANCOVA revealed a significant main effect of condition (Sleep vs Wake) for drawing time [F(1,119) = 4.87; p = .03] error time [F(1,119) = 9.11; p = .003], and error proportion [F(1,119) = 4.76; p = .03] with the Sleep group performing better than the Wake group at re-test (Fig. 3). There was a main effect of age group for error time [F(1,119) = 11.8; p = .001], such that young adults outperformed older adults, yet there was no difference for drawing time (p = .36) or error proportion (p = .10). There were no interactions between factors for drawing time (p = .27), error time (p = .33), or error proportion (p = .57).
Fig. 3.
Sleep- and wake-dependent consolidation in young and older adults, calculated using a change score
Next, the post hoc measure of figure time was examined. There was no main effect of condition (p = .22), age group (p = .68), or an interaction between the two factors (p = .45). This indicates that the effect of sleep on drawing time may be representing an improvement in error time. Thus, sleep may not improve speed of drawing per se, but may improve quickness to correct errors.
Sleep
A subset of participants in the Sleep condition (23 young and 15 older adults) underwent overnight PSG. As shown in Table 3, there were no differences in total sleep time, sleep efficiency, or sleep latency between age groups. There was, however, a difference between groups for wake after sleep onset (t(36) = −2.03, p = .05), with young adults spending less time awake during the night. Moreover, sleep architecture differed markedly between age groups. Young adults had significantly lower %N1 (t(36) = −2.14, p = .04) and %N2 (t(36) = −2.26, p = .03) than older adults. On the other hand, young adults had significantly higher %SWS (t(36) = 3.34, p = .002) than older adults (Table 3).
Table 3.
Sleep characteristics
| Young adults (n = 23) | Older adults (n = 15) | p | |
|---|---|---|---|
| TST (min) | 421.64 ± 62.79 | 399.45 ± 61.26 | .24 |
| Sleep efficiency (%) | 94.16 ± 7.02 | 90.29 ± 7.27 | .11 |
| WASO (min) | 16.05 ± 22.79 | 35.97 ± 39.05 | .05* |
| %N1 | 9.75 ± 3.54 | 12.94 ± 4.99 | .04* |
| %N2 | 50.58 ± 7.51 | 56.70 ± 9.03 | .03* |
| %SWS | 20.78 ± 5.03 | 14.41 ± 6.71 | .002* |
| %REM | 18.90 ± 6.87 | 15.98 ± 2.84 | .07† |
| Sleep latency (min) | 9.82 ± 12.08 | 7.73 ± 7.89 | .56 |
TST total sleep time, WASO wake after sleep onset; %N1 = percent of TST in non-REM sleep stage 1; %N2 = percent of TST in non-REM sleep stage 2; SWS = percent of TST in slow wave sleep; %REM = percent of TST in rapid eye movement sleep
p value <.05
p value <.08. Values are mean ± SD
Sleep and performance
Pearson’s correlations were performed to assess whether task performance following sleep was related to sleep physiology. In the young adult group, there were no significant correlations between performance measures and %N2, %SWS, or %REM (all p’s > .28), yet there was a marginal positive correlation between %N2 and error proportion (r = .38, p = .07). In the older adult group, there was a significant negative correlation between %SWS and drawing time (r = −.67, p = .008) and between %SWS and error time (r = −.59, p = .02). This is likely due to a strong negative correlation between N2 and SWS (r = −.64, p < .001). There was also a trending positive relationship between %N2 and drawing time (r = .49, p = .07) and between %N2 and error time (r = .49, p = .07). In both young and older adult groups, there were no correlations between consolidation measures and %REM.
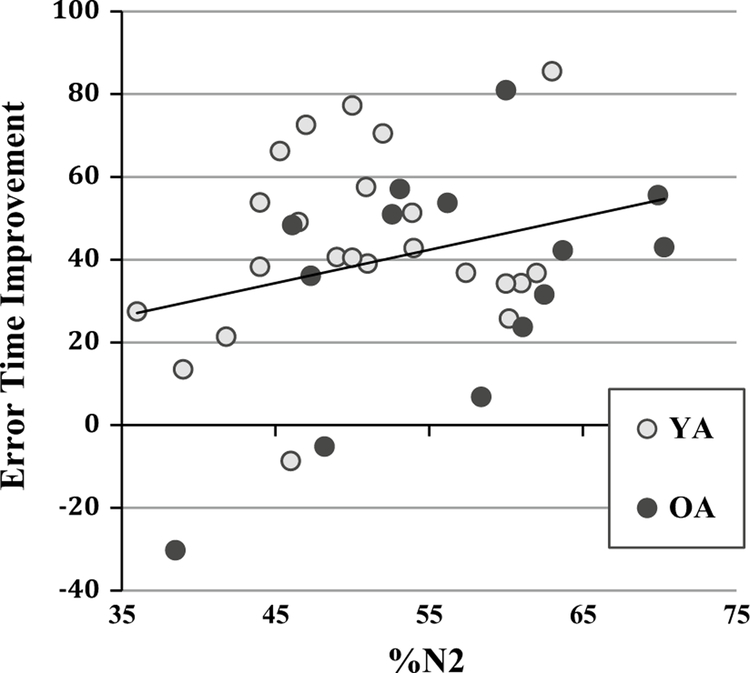
Given that the distributions for sleep parameters statistically overlapped (mentioned above), we combined age groups for additional analyses. As shown in Fig. 4, there was a significant positive relationship between the intersession change score for error time and %N2 (r = .36, p = .03). There was a significant negative relationship between drawing time improvement and %SWS (r = −.43, p = .008) and between error time and %SWS (r = −.42, p = .008). There were no correlations between performance improvement and REM (Table 4).
Fig. 4.
Correlations between error time consolidation and %N2
Table 4.
Correlations between sleep staging and visuo-motor consolidation
| Drawing time |
Error time |
Error proportion |
||||
|---|---|---|---|---|---|---|
| r | p value | r | p value | r | p value | |
| %N2 (n = 38) | .26 | .12 | .36 | .03* | .23 | .17 |
| %SWS (n = 38) | −.43 | .008* | −.42 | .008* | −.27 | .09 |
%N2 = percent of total sleep time in non-REM stage 2 sleep; %SWS = percent of total sleep time in slow wave sleep
p value <.05
Discussion
Prior studies have shown consolidation for motor traces is enhanced following an interval of sleep when compared to an equivalent interval spent awake in young but not in older adults (Spencer et al. 2007; Wilson et al. 2012). To examine whether this deficit is unique to motor sequence learning, we examined sleep-dependent consolidation using a mirror-tracing task, which specifically excluded any sequential component. We found that sleep-dependent consolidation of a visuo-motor adaptation is present in older adults as it is in young adults.
Our study replicated findings of Plihal and Born (1997), who reported that young adults exhibit sleep-dependent consolidation of a visuo-motor mirror-tracing task. On the other hand, our results are in contrast of those who have reported reduced sleep-dependent consolidation of a motor sequencing task in older adults (Spencer et al. 2007; Wilson et al. 2012). It is therefore worth noting the differences between motor sequencing tasks and the mirror-tracing task. Motor sequencing relies solely on reaction time, which is reduced in older adults (Spencer et al. 2007; Wilson et al. 2012). Mirror tracing provides discreet measures that capture both reaction time (drawing time, error time) and precision (error proportion). There-fore, the measures in the visuo-motor adaptation task may have been better able to capture consolidation. Further-more, motor sequencing requires that the individual performing the task learns a sequence, either implicitly or explicitly. It has been proposed that sequence learning in older adults, not motor learning per se, is reduced relative to young adults (Gerrard et al. 2008). The mirror-tracing task used in the current study intentionally avoided a sequencing element, and therefore we were able to gauge motor learning independently. Overall, these results provide new evidence that older adults exhibit sleep-dependent consolidation of a motor skill learning when sequencing is not present.
We conducted an exploratory analysis to gauge whether sleep-dependent performance is exhibited through increased speed, accuracy, or both. By removing error time from total drawing time, we were able to capture the amount of time spent tracing the figure alone. Interestingly, we did not find a difference in improvement between Sleep and Wake groups, or between young and older adult groups, suggesting error time speed may have been driving the initial differences seen for drawing time. Thus, sleep does not improve speed of drawing, but instead improves how quickly errors can be corrected. Another interpretation of this finding is that faster drawing speed of the figure is compromised for greater accuracy, and all groups improved to roughly the same extent after gaining knowledge from the initial session that slower tracing improves the ability to remain within the lines of the figure.
Sleep changes markedly with age. As predicted, we found characteristic differences in sleep quality and architecture between young and older adults: Older adults had greater WASO, less %SWS, more %N1, more %N2, and marginally less %REM. However, despite these sleep alterations, sleep-dependent consolidation remained intact in older adults. We propose that increased %N2 may have compensated for decreased sleep quality (e.g., greater WASO) and this compensation may render sleep-dependent motor consolidation intact.
This study supports the hypothesis of an active role for sleep in consolidation of motor skill learning, as we found correlations between motor task improvement and sleep physiology. Similar to motor sequence learning (Walker et al. 2002), we found a link between visuo-motor consolidation and %N2 for the error time measure, suggesting visuo-motor adaptation is also reliant on N2 processes and not REM processes, as has been suggested by others (Schredl and Erlacher 2007). These results provide evidence that N2 memory processes remain intact throughout aging. Greater insight could be gained from future work utilizing spindle analyses.
It has been conjectured that age-related changes in sleep-dependent consolidation of motor skill learning are attributed to changes in brain functioning and structural morphology (see King et al. 2013 for review). The striatum, specifically, has been implicated in motor sequence learning. Deficits in striatal functioning and striatal volumetric reductions are seen in normal aging (Fogel et al. 2012, 2014), and it has been hypothesized that these reductions contribute to a lack of sleep-dependent motor consolidation. The striatum has also been linked with mirror-tracing performance, as those with Parkinson’s disease (i.e., bilateral striatal dysfunction) are unable to acquire visuospatial adaptation (Laforce and Doyon 2002). On the other hand, unique from motor sequence learning, the cerebellum has been implicated in visuo-motor adaptation consolidation (see Doyon et al. 2003 for review) and in the sleep-dependent improvement of tasks requiring coordination between visual and motor areas (Miall et al. 2000; Maquet et al. 2003b). Thus, distinctions in the neural basis of consolidation may differentiate the benefit of sleep on these forms of motor skill learning.
Acknowledgments
The authors acknowledge the work of Torey Davis in preparing Fig. 1. This work was supported by National Institutes of Health R01AG040133 awarded to RMC Spencer.
References
- Barakat M, Carrier J, Debas K, Lungu O, Fogel S, Vandewalle G, Doyon J (2013) Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum Brain Mapp 34:2918–2928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Robertson EM, Press DZ (2009) Sequence skill acquisition and off-line learning in normal aging. PLoS ONE 4:e6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 2:193–213 [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG (2003) Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41:252–262 [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J (2002). Sleep forms memory for finger skills. Proc Nat Acad Sci USA 99:11987–11991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Nader R, Cote KA, Smith CT (2007) Sleep spindles and learning potential. Behav Neurosci 121:1–10 [DOI] [PubMed] [Google Scholar]
- Fogel SM, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, Carrier J (2012) NREM sleep oscillations and brain plasticity in aging. Front Neurol 3:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel SM, Albouy G, Vien C, Popovicci R, King BR, Hoge R, Doyon J (2014) fMRI and sleep correlates of the age-related impairment in motor memory consolidation. Hum Brain Mapp 35:3625–3645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA (2008) Sequence reactivation in the hippocampus is impaired in aged rats. J Neu-rosci 28:7883–7890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudberg C, Wulff K, Johansen-Berg H (2014) Sleep-dependent motor memory consolidation in older adults depends on task demands. Neurobiol Aging 36:1409–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC (1973) Quantification of sleepiness: a new approach. Psychophysiology 10:431–436 [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O (1976) A self-assessment questionnaire to determine morningness–eveningness in human circadian rhythms. Int J Chronobiol 4:97–110 [PubMed] [Google Scholar]
- Howard DV, Howard JH (1989) Age differences in learning serial patterns: direct versus indirectmeasures. Psychol Aging 4:357–364 [DOI] [PubMed] [Google Scholar]
- Javadi AH, Walsh V, Lewis PA (2011) Offline consolidation of procedural skill learning is enhanced by negative emotional content. Exp Brain Res 208:507–517 [DOI] [PubMed] [Google Scholar]
- Johns MW (1994) Sleepiness in different situations measured by the Epworth Sleepiness Scale. Sleep 17:703–710 [DOI] [PubMed] [Google Scholar]
- King BR, Fogel SM, Albouy G, Doyon J (2013) Neural correlates of the age-related changes in motor sequence learning and motor adaptation in older adults. Front Hum Neurosci 7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforce R, Doyon J (2002) Differential role for the striatum and cerebellum in response to novel movements using a motor learning paradigm. Neuropsychologia 40:512–517 [DOI] [PubMed] [Google Scholar]
- Maquet P, Schwartz S, Passingham R, Frith C (2003a) Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci 23:1432–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Schwartz S, Passingham R, Frith C (2003b) Sleep-related consolidation of a visuomotor skill: brain mechanisms as assessed by functional magnetic resonance imaging. J Neurosci 23:1432–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miall RC, Imamizu H, Miyauchi S (2000) Activation of the cerebellum in coordinated eye and hand tracking movements: an fMRI study. Exp Brain Res 135:22–33 [DOI] [PubMed] [Google Scholar]
- Morin A, Doyon J, Dostie V, Barakat M, Tahar AH, Korman M, et al. (2008). Motor sequence learning increases sleep spindles and fast frequencies in post-training sleep. Sleep 31:1149–1156 [PMC free article] [PubMed] [Google Scholar]
- Pace-Schott E, Spencer RMC (2014a) Sleep and cognition in aging and dementia. In: Abel T, Benca R, Meerlo P (eds) Sleep, neuronal plasticity and brain function Springer, New York [Google Scholar]
- Pace-Schott E, Spencer RMC (2014b) Sleep loss in older adults: effects on waking performance and sleep-dependent consolidation with healthy aging and insomnia. In: Bianchi MT (ed) Sleep deprivation and disease: effects on the body, brain and behavior Springer, New York [Google Scholar]
- Peters KR, Ray L, Smith V, Smith C (2008) Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J Sleep Res 17:23–33 [DOI] [PubMed] [Google Scholar]
- Plihal W, Born J (1997) Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci 9:534–547 [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E et al. (2009) Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106:1590–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schredl M, Erlacher D (2007) REM sleep and visuo-motor skill learning: a correlational study. Sleep Hypn 9:52 [Google Scholar]
- Siengsukon CF, Boyd LA (2008) Sleep enhances implicit motor skill learning in individuals poststroke. Top Stroke Rehabil 15:1–12 [DOI] [PubMed] [Google Scholar]
- Siengsukon CF, Boyd LA (2009) Sleep to learn after stroke: implicit and explicit offline motor learning. Neurosci Lett 451:1–5 [DOI] [PubMed] [Google Scholar]
- Silber MH, Ancoli-Israel S, Bonnet MH (2007) The visual scoring of sleep in adults. J Clin Sleep Med 3:121–131 [PubMed] [Google Scholar]
- Smith C (1995) Sleep states and memory processes. Behav Brain Res 69:137–145 [DOI] [PubMed] [Google Scholar]
- Smith C, MacNeill C (1994) Impaired motor memory for a pursuit rotor task following Stage 2 sleep loss in college students. J sleep res 3:206–213 [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, Ivry RB (2007) Age-related decline of sleep-dependent consolidation. Learn Mem 14:480–484 [DOI] [PubMed] [Google Scholar]
- Stickgold R (2005) Sleep-dependent memory consolidation. Nature 437:1272–1278 [DOI] [PubMed] [Google Scholar]
- Tamaki M, Matsuoka T, Nittono H, Hori T (2008) Fast sleep spindle (13–15 Hz) activity correlates with sleep-dependent improvement in visuomotor performance. Sleep 31:204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpening Z, Naismith S, Melehan K, Gittins C, Bolitho S, Lewis SJ (2013) The contribution of nocturnal sleep to the consolidation of motor skill learning in healthy ageing and Parkinson’s disease. J Sleep Res 22:398–405 [DOI] [PubMed] [Google Scholar]
- Tucker M, McKinley S, Stickgold R (2011) Sleep optimizes motor skill in older adults. J Am Geriatr Soc 59:603–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R (2002) Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35:205–211 [DOI] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RMC (2012) Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiol Aging 33:991–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]