Abstract
A patient with Dupuytren’s disease noted progressive disappearance of the contractures of both hands over a 3-year period while taking coenzyme Q10 daily for an unrelated condition. The function and appearance of his hands were restored to almost normal.
Keywords: medical management, orthopaedics, drugs: musculoskeletal and joint diseases
Background
Dupuytren’s disease (DD) is a benign progressive fibroproliferative condition of the palmar and digital fascia of the hands resulting in dysfunction and irreversible flexion deformities of the joints. Interest in the effective treatment for DD remains very active as evidenced by 495 randomised controlled trial (RCT) papers identified in a recent systematic review of the condition. Of those, 20 studies were suitable to be included in the review. The authors were unable to identify a superior form of treatment for the disease and made several recommendations for future studies.1
Histopathologically three consecutive stages of disease are described: proliferative, involutional (contracting) and residual.2 Intervening in the inflammatory stage of the disease process could potentially halt progression of early disease or recurrence of palmar fibromatosis. A recent paper provides an overview of attempts at early disease control describing radiotherapy and pharmacotherapy. Control of myofibroblast activity is mentioned as promising but requiring more evidence. Publications on a wide range of medications including anti-inflammatory drugs (cortisone, interferon and colchicine), antimitotic drugs (fluorouracil and anti-tumor necrosis factor alpha [TNFα] locally), as well as other pharmacological agents (alpha-dihydrotestosterone, tamoxifen and others) are discussed without any specific recommendation by the authors.3
The pathogenesis of disease comprises a complex cascade of molecular and cellular events. Myofibroblasts are derived from dermal fibroblasts in DD subjects and exhibit characteristics typical of smooth muscle cells. Investigators demonstrated the presence of myofibroblasts, macrophages and cytokines in freshly isolated tissue from Dupuytren’s nodules. Several cytokines were isolated of which only TNFα converted normal fibroblasts into myofibroblasts. They applied different approaches to establish TNF blockade in the tissue samples and rated the antitumour necrosis factor agents adalimumab and golimumab as most promising.4 This work was continued in a randomised dose response proof of concept phase 2a clinical trial.5 The effect of locally injected escalating doses of adalimumab was compared with corresponding volumes of placebo in surgical excised tissue 2 weeks after the injections. Down regulation of the myofibroblast phenotype was demonstrated and further trials are planned.
Case presentation
A 52-year-old man presented with unilateral intermittent locking of fingers to a locum general practioner (GP) in 2011 who then diagnosed early right-sided DD. Two years later he attended his own GP again with hand problems. He documented locking of the fingers needing downward pressure to release them and diagnosed early bilateral DD with the right hand affected to a greater extent.
The diagnosis was confirmed when he had a consultation at an orthopaedic clinic. No treatment was offered with his condition viewed as early. Unfortunately, no precise staging was documented nor digital evidence collected. The patient described continued progression of flexion deformities over the following 2 years.
This person was also diagnosed with type 1 diabetes at the age of 9 years. When he attended the diabetes clinic in December 2014, after an absence of 2 years, the patient and his wife expressed concern about two problems: numerous hypoglycaemic events including convulsions, and loss of short-term memory over the previous 18 months. He had erratic glucose readings with no hypoglycaemic awareness. His HbA1c was 11.1%. At the time of presentation, he was on four daily insulin injections, and due to poor memory, often forgot to inject or that he had already taken insulin, resulting in doubling of dosages. He was an ex-smoker, non-drinker without any microvascular diabetic complications or a family history of DD. He had been a manual labourer for 35 years of which the last 10 years was at a stone quarry; he retired from his position early at the age of 50. Other daily medications included ramipril 5 mg, pravastatin 10 mg, fluoxetine 20 mg and vitamin D. Although no formal assessment was done, the patient had obvious cognitive impairment. Baseline laboratory investigations were normal, including thyroid function and vitamin B12. He had been taking pravastatin 10 mg for 8 years as per guidelines with total cholesterol 3.7, high-density lipoprotein cholesterol 1.76, low-density lipoprotein cholesterol 1.83 and triglyceride 0.7 mmol/L. His insulin regime was changed from four daily injections to a two times per day mixed insulin to reduce the risk of errors and allow monitoring of his injections by his family. With statin-induced cognitive impairment as the most likely diagnosis, pravastatin was discontinued and an arbitrary dose of coenzyme Q10 100 mg daily was prescribed for a 3-month period.
Outcome and follow-up
The patient attended the diabetes clinic biannually—his memory problem resolved, diabetes control improved and hypoglycaemic awareness was restored. During a routine clinic visit in 2018, he told me his Dupuytren contractures were resolving. He attributed this to taking coenzyme Q10 100 mg daily for the previous 3 years. Regaining his memory was such relief that he had decided to continue with the supplement indefinitely.
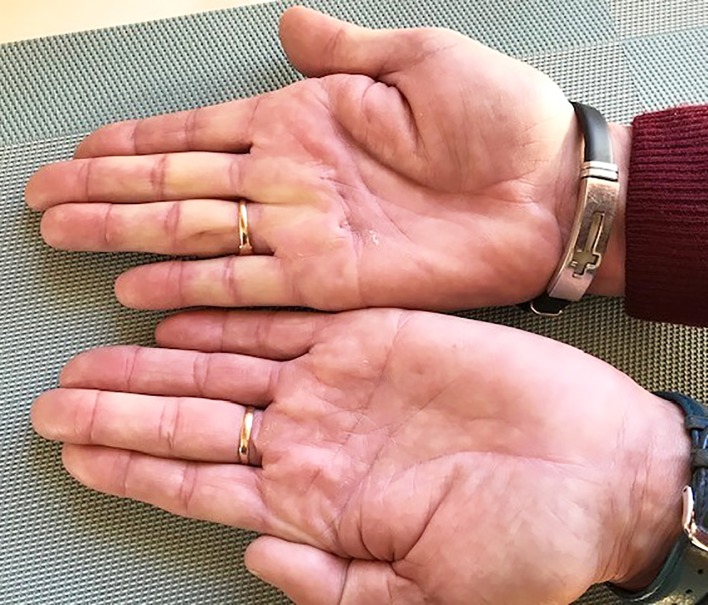
He had documented unilateral DD in 2011 that advanced to bilateral DD in 2013 and even further according to his description. Clinically he now had no contractures, only an area of nodular thickening at the metacarpophalangeal joint of the fourth right finger as seen in figure 1. With hyperextension of the fingers, the nodule appeared more pronounced accompanied by blanching of the proximal three to five digits on the right hand as demonstrated in figure 2.
Figure 1.
Hands at rest after taking coenzyme Q10 for 3 years.
Figure 2.
Hands in hyperextension after taking coenzyme Q10 for 3 years.
Discussion
This patient conducted a fortuitous experiment on himself lasting 3 years, which resulted in almost complete reversal of symptoms and signs of DD. He subsequently self-reported this to me and attributed his success to long term use of coenzyme Q10 100 mg daily. To my knowledge this has not been described in any patient with DD.
However, a study was published in the urological literature on the beneficial effects of coenzyme Q10 in Peyronie’s disease (PD). An RCT of 6 months in 186 patients with chronic early PD was conducted, comparing coenzyme Q10 300 mg daily to placebo. Coenzyme Q10 therapy resulted in statistical significant improvement in International Index of Erectile Function score, plaque size and penile curvature compared with the placebo group who showed a slight deterioration.6
PD is a fibrotic disorder of the tunica albuginea of the corpora cavernosa of the penis. The exact aetiology is uncertain but penile trauma with disordered wound healing in genetically predisposed subjects is suspected. PD is associated with DD and has a similar pathogenesis. Oxidative stress is emphasised as playing a fundamental and early role in PD pathogenesis with references dating back more than 15 years.7 8 Different versions of systemic antioxidant therapy are described in several studies, both singly and in combination. Interpretation of results in general is complicated as PD has a variable course and patients could have been in different phases of disease.9
Coenzyme Q10 is a lipophilic compound essential to the mitochondrial transport system for ATP production; it has both antioxidant and anti-inflammatory activity. It is synthesised in all mammalian cells and is also obtained from the diet. The effects on pro-inflammatory markers was examined in a meta-analysis confirming a decrease in TNFα level, though not in C-reactive protein or interleukin-6.10 The protocol for a systematic review and meta-analysis on the efficacy of coenzyme Q10 supplementation in chronic diseases to reduce proinflammatory cytokines was published in 2017.11
Coenzyme 10 can be used safely as a dietary supplement in doses of 50–1200 mg daily; bioavailability is poor. Different formulations, dosage regimens and duration of treatment complicates comparison of studies. Coenzyme Q10 shares the biosynthetic mevalonate pathway with cholesterol, hence a deficiency could be induced by statin drugs.12
Patient’s perspective.
Before taking coenzyme Q10 I had great difficulty changing gears when driving. I had to give up work due to my hands seizing, the pain was very bad. Now I am pain free and have full use of my hands.
Learning points.
A patient’s observations can contribute to medical information.
Early Dupuytren’s disease may prove to be reversible with long-term safe use of coenzyme Q10.
Considerable time is required for changes to take effect in chronic inflammatory conditions.
Photographs on a patient’s digital device could be used to assist in easy record keeping.
Footnotes
Contributors: WL examined and treated this patient.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Soreide E, Murad MH, Denbeigh JM, et al. . Treatment of dupuytren’s contracture. Bone Joint J 2018;100-B:1138–45. 10.1302/0301-620X.100B9.BJJ-2017-1194.R2 [DOI] [PubMed] [Google Scholar]
- 2. LUCK JV. Dupuytren’s contracture; a new concept of the pathogenesis correlated with surgical management. J Bone Joint Surg Am 1959;41-A:635–64. [PubMed] [Google Scholar]
- 3. Werker PMN, Degreef I. Alternative and adjunctive treatments for dupuytren disease. Hand Clin 2018;34:367–75. 10.1016/j.hcl.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 4. Verjee LS, Verhoekx JS, Chan JK, et al. . Unraveling the signaling pathways promoting fibrosis in dupuytren’s disease reveals TNF as a therapeutic target. Proc Natl Acad Sci U S A 2013;110:E928–37. 10.1073/pnas.1301100110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nanchahal J, Ball C, Davidson D, et al. . Anti-tumour necrosis factor therapy for dupuytren’s disease: a randomised dose response proof of concept phase 2a clinical trial. EBioMedicine 2018;33:282–8. 10.1016/j.ebiom.2018.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Safarinejad MR. Safety and efficacy of coenzyme Q10 supplementation in early chronic Peyronie’s disease: a double-blind, placebo-controlled randomized study. Int J Impot Res 2010;22:298–309. 10.1038/ijir.2010.20 [DOI] [PubMed] [Google Scholar]
- 7. Paulis G, Romano G, Paulis L, et al. . Recent pathophysiological aspects of peyronie’s disease: role of free radicals, rationale, and therapeutic implications for antioxidant treatment-literature review. Adv Urol 2017;2017:1–17. 10.1155/2017/4653512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bilgutay AN, Pastuszak AW. Peyronie’s disease: a review of etiology, diagnosis, and management. Curr Sex Health Rep 2015;7:117–31. 10.1007/s11930-015-0045-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paulis G, Paulis A, Romano G, et al. . Rationale of combination therapy with antioxidants in medical management of Peyronie’s disease: results of clinical application. Res Rep Urol 2017;9:129–39. 10.2147/RRU.S141748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhai J, Bo Y, Lu Y, et al. . Effects of co-enzyme q10 on markers of inflammation: a systematic review and meta-analysis. PloS one 2017;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farsi F, Heshmati J, Janani L, et al. . Can coenzyme Q10 supplementation effectively reduce human tumour necrosis factor-α and interleukin-6 levels in chronic diseases? Protocol for a systematic review and meta-analysis of randomised controlled trials. BMJ Open 2017;7:e016841 10.1136/bmjopen-2017-016841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hernández-Camacho JD, Bernier M, López-Lluch G, et al. . Coenzyme Q10 supplementation in aging and disease. Front Physiol 2018;9:44 10.3389/fphys.2018.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]