Abstract
Introduction
Resistance training (RT) and nutritional supplementation seem to have beneficial effects on muscle properties and physical performance in older adults. However, the reported effects of specific RT programmes and supplementation prescriptions vary among studies. The present study aims to examine the acute and residual effects of RT and/or beta-hydroxy-beta-methylbutyrate (HMB) supplementation on muscle mass, muscle strength and physical performance in older women with reduced muscle mass.
Methods and analysis
This is a randomised, double-blind, placebo-controlled trial. Older women fitting the eligibility criteria were recruited in February 2018 from a population-based sample identified via screening conducted in October 2017. In March 2018, 156 participants were randomly allocated to undergo one of four interventions (RT + HMB, RT + placebo, education + HMB and education + placebo) for 12 weeks. Supervised RT consisted of body weight, elastic band, ankle weight and machine-based exercises two times per week at the Tokyo Metropolitan Institute of Gerontology (TMIG). Each participant ingested HMB (1200 mg) or placebo supplements once daily. Sessions of education not associated with sarcopenia treatment were conducted every 2 weeks. Post-intervention follow-up will be conducted for 12 weeks, until September 2018. The study includes assessments conducted in March (baseline), June (post-intervention) and September 2018 (follow-up). The primary outcome is the longitudinal change in muscle mass. Secondary outcomes include the longitudinal changes in muscle strength, physical performance, muscle thickness, muscle quality, blood counts, blood biochemistry, calf circumference, skin viscoelasticity, habitual dietary intake, habitual physical activity levels, functional capacity and health-related quality of life. Intention-to-treat analyses will be conducted.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of the TMIG, Japan. The study is being conducted according to the principles of the Declaration of Helsinki. The findings will be presented at international academic congresses and published in peer-reviewed international journals.
Trial registration number
UMIN000028560; Post-results.
Keywords: sarcopenia, exercise and nutrition, muscle mass and strength, physical performance, RCT
Strengths and limitations of this study.
To the best of our knowledge, this will be the first four-arm trial to examine the effects of resistance training (RT) and/or beta-hydroxy-beta-methylbutyrate supplementation among older adults with reduced muscle mass.
The study will collect information about the residual effects of the intervention over an observation period of 12 weeks.
The trial has a randomised, double-blind, placebo-controlled design, which supports the generalisability of the findings and minimises the risk of selection, performance and detection bias.
In addition to machine-based exercises, the participants will also perform RT using body weight, ankle weights and elastic bands, which will not provide objective information regarding muscle loading but which represents a more suitable prescription for older adults and a more feasible exercise routine to be performed on a daily basis.
All participants will be older women, which limits generalisation of the findings to men.
Introduction
Sarcopenia is a syndrome characterised by progressive and generalised loss of skeletal muscle mass and strength.1 In older adults, sarcopenia is associated with adverse health outcomes such as increased risk of incident falls,2 reduced performance in instrumental or basic activities of daily living, hospitalisation, institutionalisation and mortality.3 4 To reduce the social burden of sarcopenia, it is important to develop prevention and treatment programmes aimed to extend healthy life expectancy, especially in older people with high risk of sarcopenia.
Most sarcopenia prevention or treatment programmes recommend a combination of resistance training (RT) and nutritional supplementation.5–7 According to a meta-analysis of nine randomised control trials (RCTs) on the combined effect of protein supplementation and RT on muscle outcomes, such strategies may be effective for increasing fat-free mass among older adults.8 Both the European Working Group on Sarcopenia in Older People and the International Working Group on Sarcopenia, which serve as international advisory committees on sarcopenia, have argued in favour of leucine and beta-hydroxy-beta-methylbutyrate (HMB) supplementation as complementary strategies for sarcopenia prevention or treatment.9
HMB is synthesised in skeletal muscle via transamination of leucine to alpha-ketoisocaproic acid (KIC) by aminotransferase.10 It is estimated that only 5%–10% of KIC is metabolised by KIC dioxygenase to produce HMB in the liver.11 HMB supplementation is believed to exert beneficial effects on skeletal muscle by increasing protein synthesis through activation of the mammalian target of rapamycin and by decreasing protein catabolism through down-regulation of the ubiquitin proteasome pathway.12 However, there are few studies on the combined effects of RT and HMB supplementation on muscle outcomes in older adults.13 To our knowledge, two RCTs have examined such effects in elderly populations, but the participants were healthy older adults and the RCTs had a two-arm design.14 15 Additionally, the residual effects of RT and/or HMB supplementation on muscle outcomes remain unclear. Thus, it is necessary to investigate the combined effect of RT and HMB supplementation in older populations with muscle wasting, and such studies should be designed as four-arm RCTs (RT-only, HMB supplementation-only, both and none).9
The present study aims to examine the acute and residual effects of RT and/or HMB supplementation on muscle mass, muscle strength and physical performance in older women with reduced muscle mass. We hypothesised that, compared with RT alone, HMB supplementation alone and placebo, combined RT and HMB supplementation would provide higher benefit in terms of improving and maintaining muscle mass, muscle strength and physical performance. These findings will provide new evidence regarding the effectiveness of nonpharmaceutical interventions for sarcopenia and will be useful in the development of sarcopenia prevention and treatment programmes in physically frail populations.
Methods and analysis
Study design, procedure and ethics
In accordance with the Standard Protocol Items: Recommendations for Interventional Trials and Consolidated Standards of Reporting Trials statements, we designed a randomised, double-blind, placebo-controlled trial to be conducted between March and September 2018 at the Tokyo Metropolitan Institute of Gerontology (TMIG), Tokyo, Japan. The study protocol was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) on 7 August 2017
Older women fitting the eligibility criteria were recruited in February 2018, from among a population-based sample identified via a screening conducted in October 2017. After recruitment, a four-arm intervention was conducted between March and June 2018. The participants will be followed until September 2018 to observe the residual effects of the intervention. After the observation period, all interventions will be available to all participants, in agreement with ethical principles. Amendments to the protocol will be disclosed on the UMIN-CTR page of the trial. The study involves assessments conducted in March (baseline), June (post-intervention) and September 2018 (follow-up). Before the baseline assessment, all participants received written and oral information from the researchers (YO, NK and KH) regarding the study purpose, procedures, confidentiality of personal information, possible benefits, possible risks and coping strategy for the risks. Only participants who provided written informed consent proceeded within the trial. The flow chart of the study is shown in figure 1.
Figure 1.
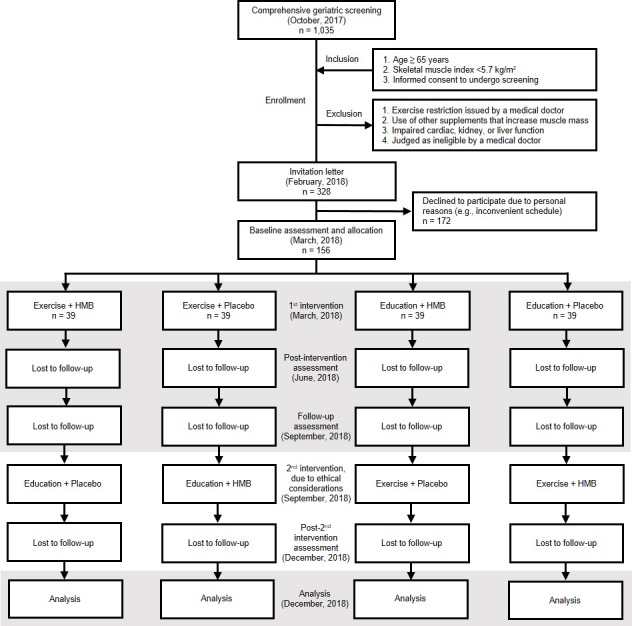
Study flow chart covering participant recruitment and enrolment, group allocation, intervention, observation and data analysis. A second intervention will be provided after the follow-up assessment, in agreement with ethical principles. However, only data from the highlighted fields will be included in the analysis. HMB, beta-hydroxy-beta-methylbutyrate.
Participants
To select the study sample, a population-based comprehensive geriatric survey was conducted as a screening assessment at the TMIG in October 2017. Invitation letters for the screening assessment were sent to 6366 subjects randomly selected from the Basic Resident Register of the Itabashi ward, which is a special ward located in the northwest area of Tokyo. In total, 1035 older women participated in the screening assessment. The inclusion criteria of this study were as follows: (1) age ≥65 years; (2) reduced muscle mass, defined as a skeletal muscle index <5.7 kg/m2, per the sarcopenia diagnosis consensus issued by the Asian Working Group for Sarcopenia16 and (3) informed consent for undergoing screening. The exclusion criteria were as follows: (1) exercise restriction issued by a medical doctor; (2) use of other supplements known to increase muscle mass; (3) impaired cardiac, kidney or liver function and (4) judged as ineligible by a medical doctor. A sample of 328 women fitting the eligibility criteria received invitation letters regarding the study intervention, of whom 156 participated in the baseline assessment, whereas the remaining 172 declined to participate due to personal reasons.
Randomisation, allocation concealment and blinding
A baseline assessment was conducted in March 2018. Afterwards, the participants were randomly allocated to four groups in a ratio of 1:1:1:1 based on a computer-generated randomisation number. The allocation was conducted by two researchers from the University of Tsukuba (KW and KT). The allocation keys will be blinded from the researchers at the TMIG (YO, NK and HK), participants, exercise trainer, analysists and assessors until December 2018 in order to maintain allocation concealment. The researchers holding the allocation keys will not have contact with the participants.
Intervention
During the intervention and observational periods, all participants were instructed not to change their habitual dietary intake or physical activity levels.
RT programme
Participants allocated to the RT programme took part in 60 min exercise sessions on two nonconsecutive days per week for 12 weeks. All sessions took place at the TMIG, under the supervision of a well-experienced exercise trainer who was not one of the researchers associated with the study. Each exercise session consisted of a 5 min warm-up exercise, a 50 min RT exercise and a 5 min cool-down exercise. Warm-up and cool-down exercises included stretching activities for the shoulders, trunk, chest, hips and hamstrings. RT consisted of chair-based (weeks 1–12), elastic band (weeks 5–7), ankle weight (weeks 7–12) and machine-based exercises (weeks 9–12). The chair-based exercise included heel raise, toe raise, knee extension, hip adduction using a rubber ball and knee lift exercises from the sitting position, as well as squats. Additionally, heel raise and knee lift exercises were performed progressively from a seated to a standing position, and lateral leg raise exercises were performed while standing behind the chair and holding onto the back of the chair. Elastic band exercises included knee lift, hip adduction and arm rowing exercises. Knee extension exercises in sitting position, as well as heel raise, knee lift and lateral leg raise exercises in standing position also were performed using ankle weights of 0.5, 0.75, 1.0 or 1.5 kg, according to the physical condition of each participant. In the last 4 weeks of the intervention, the participants performed five types of exercise (arm rowing, leg extension, hip lateral rotation, leg press and trunk flexion) using dedicated RT machines (Mizuno, Tokyo, Japan). All exercises were performed as 1–3 sets of 8–10 repetitions with gradual loading. The number of sets and repetitions was increased based on the perceived exertion of each participant. Each movement was performed slowly, in eight counts (increasing the load throughout the first four counts and decreasing it throughout the last four counts). The exercise trainer supervised the participants and provided verbal feedback to ensure sufficient tension was achieved in the target muscle groups. Exercise intensity was maintained at 12–14 points on the Borg Rate of Perceived Exertion scale. This RT programme was based on a programme employed successfully in our previous studies.17–19
HMB and placebo supplementation
All participants were instructed to ingest active (calcium HMB) or placebo products provided by Kyowa, Tokyo, Japan. The active products contain 30 mg of protein, 20 mg of fat, 3500 mg of carbohydrates, 0.2 mg of sodium, 207 mg of calcium and 1200 mg of HMB. Placebo products do not include calcium or HMB, and the missing amounts are provided as carbohydrates. Both products are provided in powder form with the same flavour, appearance and package. The two researchers (KW and KT) maintaining the allocation keys labelled the package with an identification code and sent the products to the researchers at the TMIG (YO, NK and HK). The participants received the blinded product, which they were instructed to mix with 200 mL of water and ingest once a day after any meal. The participants were instructed to record the ingestion of products in a diary.
While there are no clear recommendations regarding HMB supplementation, Wu et al suggested a daily dose of 3000 mg for older adults.20 However, as body weight is lower among Japanese than among western elderly women,17 21 22 we set the active dose at 1200 mg HMB daily based on the positive results we previously obtained in a similar population of sarcopenic women17 taking HMB at a daily dosage similar to that used in other studies (0.03 vs 0.03–0.04 g/kg of body weight).21 22 No adverse events associated with HMB supplementation have been reported.23
Health education programme
Participants allocated to the health education groups took part in six 60 min sessions (once every 2 weeks for 12 weeks) of health education not related to sarcopenia prevention but focused specifically on dementia prevention, prevention of bank transfer fraud, music therapy, nutrition and general health, oral care and social education, each given at the TMIG by an expert in that field.
Assessments
This study includes assessments conducted in March (baseline), June (post-intervention) and September 2018 (follow-up) at the TMIG. All assessors are TMIG employees who are not researchers and are blinded to group allocation.
Body composition
Body composition assessments involve measuring the following muscle mass indices: whole-body fat-free mass; upper-extremity and lower-extremity lean mass; appendicular lean mass, as the sum of the upper- and lower-extremity lean mass; and skeletal mass index, as the appendicular lean mass divided by the height squared (kg/m2).24 The body weight, fat mass and per cent fat are also obtained. Body composition measurements are conducted using the InBody720 device (Biospace, Seoul, Korea), which was validated against dual-energy X-ray absorptiometry systems for estimating appendicular lean mass in community-dwelling older populations,25 providing minimal within-day coefficients of variation for all six frequencies (0%–1.9%).26
Muscle strength
Knee extensor strength
Peak isometric knee extensor strength in the dominant leg is measured in a sitting position. The assessor places the hand-held dynamometer (µTas F-1; ANIMA, Tokyo, Japan) on the skin above the anterior ankle, at a level 5 cm above the tip of the lateral malleolus. The participants are instructed to extend the knee with maximum power starting from a knee joint angle of 90°.27 After a few practice repetitions, the participants are measured twice, and the best result is retained.
Hip adductor strength
Peak isometric hip adduction strength is also measured in a sitting position. The assessor places the hand-held dynamometer (µTas F-1; ANIMA) at 3 cm proximal to the medial knee fissure and instructs the participant to exert maximal hip adduction force while compressing a 12 cm rod equipped with a dynamometer, keeping both knee joints at 90°.28 After a few practice repetitions, the participants are measured twice, and the best result is retained.
Handgrip strength
Handgrip strength is measured using a hand-held Smedley-type dynamometer. The participants are instructed to stand naturally, grip the device with their dominant hand and squeeze as hard as possible.29 The best result of two trials is retained.
Physical performance
Usual and maximal gait speed
Using a stopwatch, the usual and maximal gait speed are measured as the time taken to walk 5 m (between markers set at 3 m and 8 m of an 11 m walking path)29 at the usual or maximal speed, respectively. Usual gait speed is measured once. Maximal gait speed is measured twice, and the best result is retained.
Timed up-and-go
This test measures the time taken to stand up from the chair, walk to and around a marker placed 3 m away, return to the chair and sit back down.30 The participants are instructed to perform these movements as quickly as possible. The test is performed twice, and the best result is retained.
Five-repetition sit-to-stand
This test measures the time taken to stand up from the chair until full knee and hip extension, sit back down, and repeat this movement five times as quickly as possible.31 The participants are instructed to fold their arms across the chest, stand-up completely and make firm contact when sitting. After a few practice repetitions, the test is performed twice, and the best result is retained.
Muscle thickness and quality
Muscle thickness is measured using a B-mode ultrasound device (Mysono U6; Samsung Medison, Seoul, Korea). The participants are instructed to lie on the back in a bed and extend the hip and knee until fully relaxed. The assessor performs a transverse scan by placing a linear probe (5–12 MHz) on the skin surface at the midpoint between the lateral epicondyle and the ipsilateral greater trochanter of the femur, perpendicular to the longitudinal axis of the quadriceps femoris muscle. To measure the thickness of the rectus femoris and vastus intermedius, an electronic calliper is used. Quadriceps femoris thickness is defined as the sum of the thickness of the rectus femoris and that of the vastus intermedius. The muscle quality of the rectus femoris is evaluated on ultrasound images processed using dedicated software (Adobe Photoshop CS6 V. 13.0; Adobe Systems, San Jose, CA, USA) and is expressed as the brightness of the image on a scale from 0 (black) to 256 (white). Muscle echo intensity was shown to correlate significantly with interstitial fibrous tissue in muscle biopsy samples.32 The protocol for assessing muscle thickness and quality is described in detail elsewhere.33
Blood biochemistry
Blood samples are collected from the antecubital vein. Analyses are carried out centrally in one laboratory (Health Sciences Research Institute, Kanagawa, Japan). Enzymatic methods are used to determine creatinine, cholesterol (total, high-density lipoprotein, low-density lipoprotein), triglyceride, blood glucose and glycated haemoglobin levels. Reference methods recommended by the Japan Society of Clinical Chemistry are used to assess aspartate transaminase, alanine transaminase, lactate dehydrogenase and creatine kinase levels. Other assays include the urease method with glutamate dehydrogenase (for blood urea nitrogen), direct colorimetry (for Fe), flow cytometry (for white blood cells), erythrocyte fragility test (for red blood cells), sodium lauryl sulfate method (for haemoglobin), microhematocrit method (for haematocrit), latex agglutination turbidimetry (for cystatin C), nephelometric immunoassay (for high-sensitivity C-reactive protein), immunoradiometric assay (for insulin-like growth factor-1) and chemiluminescence immunoassay (for vitamin B12).
Anthropometric indices
The body mass index (kg/m2) is calculated as the body weight divided by the body height squared. Plastic tape is used to measure the calf circumference in the nondominant leg.
Skin viscoelasticity
Skin viscoelasticity is evaluated using the Cutometer dual MPA 580 (Courage +Khazaka electronic GmbH, Cologne, Germany), which measures the elasticity of the upper layer of the skin using negative pressure to induce mechanical deformation; such measurements are useful for quantitative evaluation of age-related changes in skin elasticity.34 35 The displacement of the skin at the aperture of the Cutometer probe is measured optically, and the resistance of the upper skin layer to the applied negative pressure and the ability to return to the original position are displayed as curves in real time. Skin viscoelasticity is assessed for the skin of the cheek. The participants are instructed to refrain from using makeup on the day of the assessment. The test is performed five times with 10 s intervals, and the average result is retained.
Habitual dietary intake and physical activity
Habitual dietary intake is measured using a brief-type self-administered diet history questionnaire, which has been validated for estimating the monthly energy intake and the intake of each nutrient factor in older adults.36 37 These calculations are performed exclusively using software provided by Gender Medical Research, Tokyo, Japan. Habitual physical activity levels are assessed using the International Physical Activity Questionnaire, which has good reliability and validity for estimating daily physical activity levels.38 39 We calculate total scores and the scores for each of the four physical activity domains (leisure time, domestic and gardening, occupational and transport-related physical activity). These evaluations are conducted to determine whether the participants changed their lifestyle patterns during the intervention period.
Functional capacity
Functional capacity is assessed in terms of the instrumental self-maintenance (five items), intellectual activities (four items) and social roles (four items) subscales of the TMIG index of competence. The validity and reliability of the face-to-face assessment of the TMIG index of competence have been demonstrated previously.40 Participants are asked whether or not they are able to perform the function described by each item, to which they may answer either ‘yes’ (able to do so, 1 point) or ‘no’ (unable to do so, 0 point), for a maximum total score of 13 points, with a higher score indicating better functional capacity.
Health-related quality of life
Health-related quality of life (HR-QoL) is assessed using the WHO-Five Well-Being Index, which has good validity for assessing mental condition in older adults and consists of five items representing mood in daily life over the preceding 2 weeks.41 The participants are instructed to rate the frequency of the mood from 0 (never) to 5 (all the time), for a maximum score of 25 points, with a higher score indicating better mental condition.
Assessment of adverse events and adherence
Any RT- or supplement-related adverse events occurring during the intervention period are recorded. The affected participants are free to discontinue the trial or continue other interventions (ie, participants with HMB- or placebo-related side effects may continue to perform RT, whereas participants with RT-related injuries may continue to take HMB or placebo). We assess the adherence to the intervention, including RT participation rate and adherence to the daily use of the assigned supplement (based on the participants’ daily diary of study product intake). If a participant drops out from the programme, the reasons are recorded.
Outcome measures
The primary outcome measure is muscle mass. Secondary outcome measures include muscle strength, physical performance, muscle thickness, muscle quality, blood counts, blood biochemistry, calf circumference, skin viscoelasticity, habitual dietary intake, habitual physical activity levels, functional capacity and HR-QoL.
Sample size
This study is designed to detect a moderate effect size (f = 0.25) for significant interactions between two factors (RT vs education and HMB vs placebo) regarding the longitudinal changes in outcomes. Detecting significant interactions would confirm our hypothesis that, compared with RT alone, HMB supplementation alone and placebo, combined RT and HMB supplementation provides higher benefit regarding muscle mass, muscle strength and physical performance. Setting the two-sided α-error to 0.05 and power to 80%, the minimum sample size required is 31 participants per group. Considering approximately 20% attrition, we aimed to enrol at most 160 participants. The power calculation was conducted using G*Power V.3.1.9.2 for Windows (Heinrich-Heine-Universität Düsseldorf, Düsseldorf, Germany).42
Statistical analysis
To promote the collection of quality data, the collected data will be double-checked by the assessors, and a statistician will check the range of values. Between-group comparisons of baseline characteristics will be conducted using one-way analysis of variance (ANOVA) or the Kruskal-Wallis test for continuous variables, and the Χ 2 test for categorical variables. Continuous data will be expressed as mean and SD or median and IQR. Differences in longitudinal mean changes in main and secondary outcomes from baseline to post-intervention will form the primary focus of the analysis. Two-way ANOVA for the changes in outcomes will be applied to test for interactions between two factors (RT vs education and HMB vs placebo). The main effects of RT (RT + HMB and RT + placebo vs education + HMB and education + placebo) and HMB (RT + HMB and education + HMB vs RT + placebo and education + placebo) will also be tested. Within-group changes will be analysed using paired t-tests, whereas between-group differences in the change in outcomes will be compared using one-way ANOVA. The Scheffe method will be applied for relationships showing significance on one-way ANOVA. For subgroup analysis, the participants will be stratified according to sarcopenia status, as defined based on the Asian Working Group for Sarcopenia criteria. The intention-to-treat principle will be applied in all analyses. Missing data will be treated via multiple imputation, in which the Markov Chain Monte Carlo approach will be applied to generate 20 imputed data sets based on the baseline characteristics and the outcome variables. All statistical analyses will be performed using SPSS V. 25.0 (IBM Corp., Armonk, NY, USA). P Values <0.05 will be considered to indicate significance.
Patient and public involvement
This study will be conducted without participant involvement. Participants will not be invited to comment on the study design, will not be consulted to define relevant outcomes or interpret the results and will not be invited to contribute to the writing or editing of this paper for readability or accuracy.
Ethics and dissemination
The study protocol was approved by the Ethics Committee of the TMIG and will be conducted in agreement with the Declaration of Helsinki. The findings of this study will be presented at international academic congresses and published in peer-reviewed international journals. On publication in a journal, we will also make the findings available on the TMIG homepage.
Discussion
This study addresses the paucity of data regarding the effects of RT and/or HMB supplementation in older women with reduced muscle mass, and the findings will provide insight regarding the potential synergistic effect of RT and HMB supplementation on muscle mass, muscle strength and physical performance. We will also examine the residual effects of such interventions over a 12-week observation period, as such findings may help understand the potential of nonpharmaceutical treatment for sarcopenia.
Literature reports regarding the individual effect of HMB supplementation on muscle properties and physical performance in older people are encouraging.21 22 43 However, to our knowledge, only two studies have evaluated the combined effect of RT and HMB supplementation in older populations.14 15 Specifically, Vukovich et al enrolled 31 adults aged >70 years and reported significant muscle mass gain after 12 weeks of exercise with HMB supplementation but not after exercise with placebo (P for interaction, 0.08).14 On the other hand, Stout et al enrolled 36 adults aged >65 years and reported significant improvement in muscle quality, calculated as muscle strength relative to muscle mass, after 24 weeks of HMB supplementation alone (ie, no exercise).15 It remains unclear whether the combined programme provides higher benefits. These previous studies had a relatively small sample size, which might increase β-error; moreover, the participants were relatively healthy and had almost normal muscle mass, suggesting that they may have benefited less from HMB supplementation. As completely controlled groups were not previously included, the potential synergy between RT and HMB supplementation remains unclear. The present study will address these limitations and serve as the first investigation of the acute and residual, independent and combined effects of RT and HMB supplementation, which will be conducted in a larger sample of older adults with reduced muscle mass. The randomised, double-blind, placebo-controlled design will minimise the risk of selection, performance and detection bias, and will support the generalisability of the results. One limitation is that, in addition to machine-based RT, the intervention involves body weight, elastic band and ankle weight RT, which does not provide objective information regarding muscle loading; on the other hand, such RT programmes are more suitable for older adults and will be more feasible in daily practice. Another limitation is that all participants will be older women, which will preclude generalisation of our findings to men.
Supplementary Material
Acknowledgments
We are grateful to the participants and the staff members of the Tokyo Metropolitan Institute of Gerontology.
Footnotes
Contributors: YO and HK conceived the study, designed the study protocol, participated in the coordination of the study (recruitment and screening) and wrote the manuscript. NK participated in the coordination of the study and helped manage data collection. KW and TK contributed to designing the study protocol, conducted the randomisation and have been maintaining allocation concealment. DM contributed to designing the study protocol and negotiated a contract with the provider of the intervention products. All authors read and approved the final manuscript. HK is the principal investigator in this trial.
Funding: This study is financially supported by Kyowa Co., Ltd., Tokyo, Japan (no grant number) and is being conducted as a joint research effort of the Tokyo Metropolitan Institute of Gerontology, University of Tsukuba, and Kyowa Co., Ltd.
Competing interests: The intervention products (HMB and placebo supplements) were provided by Kyowa Co., Ltd. DM is employed by Kyowa Co., Ltd. The funder is not involved in subject recruitment, intervention, data collection, data analysis or preparation of the manuscript.
Ethics approval: Approved by the Ethics Committee of the Tokyo Metropolitan Institute of Gerontology on 15 September 2017.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012;31:652–8. 10.1016/j.clnu.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 3. Hirani V, Blyth F, Naganathan V, et al. Sarcopenia is associated with incident disability, institutionalization, and mortality in community-dwelling older men: the concord health and ageing in men project. J Am Med Dir Assoc 2015;16:607–13. 10.1016/j.jamda.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 4. Bianchi L, Ferrucci L, Cherubini A, et al. The predictive value of the EWGSOP definition of sarcopenia: results from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2016;71:259–64. 10.1093/gerona/glv129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cermak NM, Res PT, de Groot LC, et al. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr 2012;96:1454–64. 10.3945/ajcn.112.037556 [DOI] [PubMed] [Google Scholar]
- 6. Malafarina V, Uriz-Otano F, Iniesta R, et al. Effectiveness of nutritional supplementation on muscle mass in treatment of sarcopenia in old age: a systematic review. J Am Med Dir Assoc 2013;14:10–17. 10.1016/j.jamda.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 7. Liao CD, Tsauo JY, Wu YT, Yt W, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr 2017;106:1078–91. 10.3945/ajcn.116.143594 [DOI] [PubMed] [Google Scholar]
- 8. Finger D, Goltz FR, Umpierre D, et al. Effects of protein supplementation in older adults undergoing resistance training: a systematic review and meta-analysis. Sports Med 2015;45:245–55. 10.1007/s40279-014-0269-4 [DOI] [PubMed] [Google Scholar]
- 9. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–59. 10.1093/ageing/afu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson JM, Fitschen PJ, Campbell B, et al. International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB). J Int Soc Sports Nutr 2013;10:6 10.1186/1550-2783-10-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Koevering M, Nissen S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am J Physiol 1992;262(1 Pt 1):E27–E31. 10.1152/ajpendo.1992.262.1.E27 [DOI] [PubMed] [Google Scholar]
- 12. Fitschen PJ, Wilson GJ, Wilson JM, et al. Efficacy of β-hydroxy-β-methylbutyrate supplementation in elderly and clinical populations. Nutrition 2013;29:29–36. 10.1016/j.nut.2012.05.005 [DOI] [PubMed] [Google Scholar]
- 13. Molfino A, Gioia G, Rossi Fanelli F, et al. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: a systematic review of randomized trials. Amino Acids 2013;45:1273–92. 10.1007/s00726-013-1592-z [DOI] [PubMed] [Google Scholar]
- 14. Vukovich MD, Stubbs NB, Bohlken RM. Body composition in 70-year-old adults responds to dietary beta-hydroxy-beta-methylbutyrate similarly to that of young adults. J Nutr 2001;131:2049–52. 10.1093/jn/131.7.2049 [DOI] [PubMed] [Google Scholar]
- 15. Stout JR, Smith-Ryan AE, Fukuda DH, et al. Effect of calcium β-hydroxy-β-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double-blind pilot trial. Exp Gerontol 2013;48:1303–10. 10.1016/j.exger.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 16. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 17. Kim HK, Suzuki T, Saito K, et al. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 2012;60:16–23. 10.1111/j.1532-5415.2011.03776.x [DOI] [PubMed] [Google Scholar]
- 18. Kim H, Suzuki T, Kim M, et al. Effects of exercise and milk fat globule membrane (MFGM) supplementation on body composition, physical function, and hematological parameters in community-dwelling frail Japanese women: a randomized double blind, placebo-controlled, follow-up trial. PLoS One 2015;10:e0116256 10.1371/journal.pone.0116256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim H, Kim M, Kojima N, et al. Exercise and nutritional supplementation on community-dwelling elderly japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc 2016;17:1011–9. 10.1016/j.jamda.2016.06.016 [DOI] [PubMed] [Google Scholar]
- 20. Wu H, Xia Y, Jiang J, et al. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr 2015;61:168–75. 10.1016/j.archger.2015.06.020 [DOI] [PubMed] [Google Scholar]
- 21. Flakoll P, Sharp R, Baier S, et al. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004;20:445–51. 10.1016/j.nut.2004.01.009 [DOI] [PubMed] [Google Scholar]
- 22. Baier S, Johannsen D, Abumrad N, et al. Year-long changes in protein metabolism in elderly men and women supplemented with a nutrition cocktail of beta-hydroxy-beta-methylbutyrate (HMB), L-arginine, and L-lysine. JPEN J Parenter Enteral Nutr 2009;33:71–82. 10.1177/0148607108322403 [DOI] [PubMed] [Google Scholar]
- 23. Nissen S, Sharp RL, Panton L, et al. beta-hydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J Nutr 2000;130:1937–45. 10.1093/jn/130.8.1937 [DOI] [PubMed] [Google Scholar]
- 24. Janssen I, Baumgartner RN, Ross R, et al. Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol 2004;159:413–21. 10.1093/aje/kwh058 [DOI] [PubMed] [Google Scholar]
- 25. Kim M, Shinkai S, Murayama H, et al. Comparison of segmental multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for the assessment of body composition in a community-dwelling older population. Geriatr Gerontol Int 2015;15:1013–22. 10.1111/ggi.12384 [DOI] [PubMed] [Google Scholar]
- 26. Kim M, Kim H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur J Clin Nutr 2013;67:395–400. 10.1038/ejcn.2013.9 [DOI] [PubMed] [Google Scholar]
- 27. Kojima N, Kim H, Saito K, et al. Association of knee-extension strength with instrumental activities of daily living in community-dwelling older adults. Geriatr Gerontol Int 2014;14:674–80. 10.1111/ggi.12158 [DOI] [PubMed] [Google Scholar]
- 28. Kim H, Suzuki T, Yoshida Y, et al. Effectiveness of multidimensional exercises for the treatment of stress urinary incontinence in elderly community-dwelling Japanese women: a randomized, controlled, crossover trial. J Am Geriatr Soc 2007;55:1932–9. 10.1111/j.1532-5415.2007.01447.x [DOI] [PubMed] [Google Scholar]
- 29. Shinkai S, Watanabe S, Kumagai S, et al. Walking speed as a good predictor for the onset of functional dependence in a Japanese rural community population. Age Ageing 2000;29:441–6. 10.1093/ageing/29.5.441 [DOI] [PubMed] [Google Scholar]
- 30. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–8. 10.1111/j.1532-5415.1991.tb01616.x [DOI] [PubMed] [Google Scholar]
- 31. Bohannon RW, Bubela DJ, Magasi SR, et al. Sit-to-stand test: Performance and determinants across the age-span. Isokinet Exerc Sci 2010;18:235–40. 10.3233/IES-2010-0389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pillen S, Tak RO, Zwarts MJ, et al. Skeletal muscle ultrasound: correlation between fibrous tissue and echo intensity. Ultrasound Med Biol 2009;35:443–6. 10.1016/j.ultrasmedbio.2008.09.016 [DOI] [PubMed] [Google Scholar]
- 33. Nishihara K, Kawai H, Hayashi H, et al. Frequency analysis of ultrasonic echo intensities of the skeletal muscle in elderly and young individuals. Clin Interv Aging 2014;9:1471–8. 10.2147/CIA.S67820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Takema Y, Yorimoto Y, Kawai M, et al. Age-related changes in the elastic properties and thickness of human facial skin. Br J Dermatol 1994;131:641–8. 10.1111/j.1365-2133.1994.tb04975.x [DOI] [PubMed] [Google Scholar]
- 35. Ryu HS, Joo YH, Kim SO, et al. Influence of age and regional differences on skin elasticity as measured by the Cutometer. Skin Res Technol 2008;14:354–8. 10.1111/j.1600-0846.2008.00302.x [DOI] [PubMed] [Google Scholar]
- 36. Kobayashi S, Murakami K, Sasaki S, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr 2011;14:1200–11. 10.1017/S1368980011000504 [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi S, Honda S, Murakami K, et al. Both comprehensive and brief self-administered diet history questionnaires satisfactorily rank nutrient intakes in Japanese adults. J Epidemiol 2012;22:151–9. 10.2188/jea.JE20110075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. 10.1249/01.MSS.0000078924.61453.FB [DOI] [PubMed] [Google Scholar]
- 39. Murase N, Katsumura T, Ueda C, et al. Validity and reliability of Japanese version of International Physical Activity Questionnaire. Journal of Health and Welfare Statistics 2002;49:1–9. [Google Scholar]
- 40. Koyano W, Shibata H, Nakazato K, et al. Measurement of competence: reliability and validity of the TMIG Index of Competence. Arch Gerontol Geriatr 1991;13:103–16. 10.1016/0167-4943(91)90053-S [DOI] [PubMed] [Google Scholar]
- 41. Awata S, Bech P, Koizumi Y, et al. Validity and utility of the Japanese version of the WHO-Five Well-Being Index in the context of detecting suicidal ideation in elderly community residents. Int Psychogeriatr 2007;19:77–88. 10.1017/S1041610206004212 [DOI] [PubMed] [Google Scholar]
- 42. Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. 10.3758/BF03193146 [DOI] [PubMed] [Google Scholar]
- 43. Berton L, Bano G, Carraro S, et al. Effect of Oral Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Physical Performance in Healthy Old Women Over 65 Years: An Open Label Randomized Controlled Trial. PLoS One 2015;10:e0141757 10.1371/journal.pone.0141757 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


