Abstract
Objective
Insulin-dependent diabetes can occur with immune checkpoint inhibitor (ICI) therapy. We aimed to characterize the frequency, natural history and potential predictors of ICI-induced diabetes.
Research design and methods
We reviewed 1444 patients treated with ICIs over 6 years at our cancer center, and from the 1163 patients who received programmed cell death protein 1 (PD-1) inhibitors, we identified 21 such cases, 12 of which developed new-onset insulin-dependent diabetes and 9 experienced worsening of pre-existing type 2 diabetes.
Results
ICI-induced diabetes occurred most frequently with pembrolizumab (2.2%) compared with nivolumab (1%) and ipilimumab (0%). The median age was 61 years, and body mass index was 31 kg/m2, which are both higher than expected for spontaneous type 1 diabetes. Other immune-related adverse events occurred in 62%, the most common being immune mediated thyroid disease. New-onset insulin-dependent diabetes developed after a median of four cycles or 5 months; 67% presented with diabetic ketoacidosis and 83% with low or undetectable C-peptide. Autoantibodies were elevated in 5/7 (71%) at the time of new-onset diabetes. Diabetes did not resolve during a median follow-up of 1 year.
Conclusions
PD-1 inhibitors can lead to insulin deficiency presenting as new-onset diabetes or worsening of pre-existing type 2 diabetes, with a frequency of 1.8 %. The underlying mechanism appears similar to spontaneous type 1 diabetes but there is a faster progression to severe insulin deficiency. Better characterization of ICI-induced diabetes will improve patient care and enhance our understanding of immune-mediated diabetes.
Keywords: immune pathogenesis type 1 diabetes, islet autoimmunity, cancer, insulin deficiency, adult diabetes
Significance of this study.
What is already known about this subject?
Diabetes mellitus has been rarely reported in clinical trials of immune checkpoint inhibitors (ICIs) for cancer therapy.
What are the new findings?
We found that ICIs that include programmed cell death protein 1 inhibitors can induce insulin-dependent diabetes, which occurs most frequently with pembrolizumab, can present with diabetic ketoacidosis and does not appear to undergo remission.
How might these results change the focus of research or clinical practice?
These results highlight the importance of monitoring blood glucose and hemoglobin A1c prior to initiating ICIs as well as during follow-up, having a heightened suspicion for the occurrence of diabetes and developing better risk prediction.
Introduction
A balance between immune stimulation and inhibition is essential for homeostasis. In the setting of malignancy, this balance is augmented, allowing tumors to evade immune-mediated cell death.1 Recently, monoclonal antibodies have been developed against immune checkpoints namely cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed cell death protein-ligand 1 (PD-L1). CTLA-4 is expressed on T cells where its role is to downregulate T cell proliferation on B7 engagement early in immune response, primarily in lymph nodes. PD-1 is expressed on activated T cells, including T regulatory cells, B cells, and myeloid cells. Its major role is to limit the activity of T cells in peripheral tissues at the time of an inflammatory response and to limit autoimmunity. In the context of cancer, it binds to its ligands PD-L1 and PD-L2 expressed on tumor cells that causes inhibition of T cell receptor-mediated positive signaling, leading to reduced proliferation, reduced cytokine secretion, and reduced survival of effector T cells. PD-1 is also expressed on regulatory T cells, where it may enhance their proliferation after binding to the ligands. This combined effect suppresses intrinsic immune-mediated antitumor activity.2
The immune checkpoint inhibitors (ICIs) are monoclonal antibodies designed to block these checkpoints, thus resulting in a derepression of cytotoxic T cell function,3 4 in turn leading to enhanced antitumor immune response. Blocking these regulatory molecules, however, also causes breaches in self-tolerance leading to a large spectrum of immune-related adverse events (IRAEs).5 The ICIs include CTLA-4 inhibitors ipilimumab and tremelimumab [not Food and Drug Administration (FDA) approved], PD-1 inhibitors pembrolizumab and nivolumab, and PD-L1 inhibitors atezolizumab and avelumab. Endocrine IRAEs reported with ICIs include hypophysitis, thyroiditis, and in rare cases adrenalitis or diabetes mellitus. The hypophysitis usually affects the anterior pituitary and can lead to central hypothyroidism, hypogonadotrophic hypogonadism and/or secondary adrenal insufficiency. This has been a well-characterized IRAE of the CTLA-4 inhibitor ipilimumab.6 Adrenal insufficiency has also been described in these patients but that is mostly secondary due to hypophysitis or chronic glucocorticoid use either for the cancer or managing other IRAEs. In theory, there is concern for immune-mediated adrenalitis, but that has not been reported very clearly mostly due to lack of comprehensive hormone panel and imaging. The occurrence of immune mediated thyroid disease has been described in more detail, occurring more frequently with PD-1 inhibitor use7 8 as compared with CTLA-4 inhibitor use.
The use of harmonized terminologies to report and describe IRAEs is a major issue in the field at this time. The quality of endocrine IRAE data reporting in clinical trials is suboptimal,9 and important data such as the time of onset, clinical course and possible reversibility of such events are not systematically recorded or reported. New onset of diabetes mellitus has not been reported in clinical trials of CTLA-4 inhibitors and has been reported in <1% of patients in PD-1 inhibitor clinical trials. However, higher rates of up to 1.5% were reported with combined use of CTLA-4 and PD-1 inhibitors recently.10 Clinical trials for PD-L1 inhibitors have not reported new-onset diabetes, but a case of this has been published.11 A recent meta-analysis of clinical trials of ICIs demonstrated 0.2% frequency of insulin-deficient diabetes.12 A recent review of 24 reported cases from multiple centers has synthesized the available information.13 A case series of five patients who developed autoimmune diabetes after starting an anti-PD-1 agent described possible risk factors, including human leukocyte antigen (HLA) association and combined use of checkpoint inhibitors.14 More recently, Stamatouli et al 15 reported on their experience with 24 patients of ICI-induced diabetes showing an association with HLA-DR4 (15). Most of these studies have reported on their experience based on patients referred for concern of ICI-induced diabetes, hence the frequency of this condition has not been well addressed in real world practice. The natural history of the disease and its associated risk factors also need better characterization, and we aimed to address these knowledge gaps.
Methods
Subject identification and case definition
After receiving approval from the Mayo Clinic Institutional Review Board, we reviewed our cancer center database of patients treated with a CTLA-4 inhibitor (ipilimumab) or PD-1 inhibitor (pembrolizumab and nivolumab) from January 2012 to December 2017. During this time period, a total of 1444 patients with cancer received therapy with one of these ICIs, of which 281 received ipilimumab, 774 received pembrolizumab and 389 received nivolumab. From this list, medical charts were reviewed to identify cases concerning for ICI-induced insulin-dependent diabetes based on the following criteria:
New diagnosis of insulin-dependent diabetes or hyperglycemic crisis.
Worsening of prediabetes or type 2 diabetes without another attributable reason, defined as an increase in hemoglobin A1c (HbA1c) value by 10% in 6 months, clinical need for a second antihyperglycemic agent or insulin, diabetic ketoacidosis (DKA), or new-onset ketonuria or ketonemia.
We chose duration of 6 months to ensure that we saw a pattern of persistent worsening. We chose an increase in HbA1c of 10% since we believe this represents a significant change. We excluded patients with pre-existing type 1 diabetes and those with type 2 diabetes on insulin pump prior to ICI initiation but did not identify any such patients during subject identification. We collected data for baseline variables at the start of ICI therapy, diabetes-related clinical and laboratory variables at the time of diagnosis and also at the most recent follow-up.
Laboratory testing
All laboratory testing was performed at the Mayo Medical Laboratory, Rochester, Minnesota. Autoantibodies associated with type 1 diabetes that were measured included antibodies against glutamic acid decarboxylase 65 (GAD65), islet antigen 2, zinc transporter 8 and insulin autoantibodies.
Results
Frequency of ICI-induced diabetes
From a total of 1444 ICI-treated patients with cancer, 21 patients (1.4%) met criteria for ICI-induced diabetes, of which 12 (0.8%) had new-onset insulin-dependent diabetes and 9 (0.6%) had unexplained worsening of pre-existing type 2 diabetes. There were no cases of ICI-induced diabetes in the 281 patients treated with CTLA-4 inhibitor ipilimumab alone, 25 of whom had pre-exisiting type 2 diabetes. Among the 1163 patients treated with a PD-1 inhibitor, 21 (1.8%) (17 on pembrolizumab and 4 on nivolumab) met criteria for ICI-induced diabetes, of which 12 (1%) had new-onset insulin-dependent diabetes and 9 (0.8%) experienced worsening of pre-existing type 2 diabetes. In this cohort, 96 had pre-existing prediabetes or type 2 diabetes, out of which 9 (9.3%) had worsening of glycemic control after ICI initiation. Among the 774 patients treated with pembrolizumb, 17 (2.2%) met criteria for ICI-induced diabetes, of which 11 (1.4%) had new-onset insulin-dependent diabetes and 6 (0.8%) had worsening of pre-existing type 2 diabetes. In this cohort, 4 had prediabetes and 52 had type 2 diabetes before ICI initiation, and 6/56 (10.7%) had worsening of glycemic control after ICI initiation. Among the 389 patients treated with nivolumab, 4 (1%) met criteria for ICI-induced diabetes, of which 1 (0.2%) had new-onset insulin-dependent diabetes and 3 (0.8%) had worsening of pre-existing type 2 diabetes. In this cohort, 2 had prediabetes and 38 had type 2 diabetes before ICI initiation, and 3/40 (7.5%) had worsening of glycemic control after ICI initiation.
Baseline characteristics at ICI initiation
The patients had a median age at ICI initiation of 61.3 years, 57% were male, 95% were Caucasian, and median body mass index (BMI) was 31.4 kg/m2. Fourteen percent had a personal history of autoimmunity (all with Hashimoto’s thyroiditis) and 24% had a family history of autoimmunity. Information regarding family history was only available for 15 patients. ICIs were prescribed for various malignancies, with the most common being melanoma (45%) and lung cancer (25%). Eighty-one per cent of the patients were treated with pembrolizumab and the rest with nivolumab; two patients had received ipilimumab prior to therapy with pembrolizumab. Those that developed new-onset insulin-dependent diabetes had a median age at ICI initiation of 68 years, 50% were male, all were Caucasian with a median BMI of 32.2 kg/m2. Those that had worsening of pre-existing type 2 diabetes had a median age at ICI initiation of 57 years, 67% were male, 89% were Caucasian with a median BMI of 27 kg/m2. Other baseline characteristics are mentioned in table 1 and online supplement table S1.
Table 1.
Baseline characteristics in patients with PD-1 inhibitor-induced diabetes prior to initiation of PD-1 inhibitor
| Baseline characteristics | All cases (n=21) | New-onset insulin-dependent diabetes (n=12) | Worsening of type 2 diabetes (n=9) |
| N (%) or median (IQR) | |||
| Age at ICI initiation (years) | 61.3 (54.5–70) | 67.9 (60.2–71.2) | 57.4 (51–62.9) |
| Male | 12 (57.1) | 6 (50) | 6 (66.7) |
| Race | |||
| White | 20 (95.2) | 12 (100) | 8 (88.9) |
| Black | 1 (4.8) | 0 (0) | 1 (11.1) |
| Body mass index (kg/m2) at ICI initiation | 31.5 (25–36.7) | 32.2 (24.9–37.7) | 27.5 (24.9–35.6) |
| Personal history of autoimmunity | 3 (14.3) | 2 (16.7) | 1 (11.1) |
| Hashimoto’s thyroiditis | 3 (14.3) | 2 (16.7) | 1 (11.1) |
| Family history of autoimmunity (n=15) | 5 (23.8) | 4/11 (33.3) | 1/4 (11.1) |
| Hashimoto’s thyroiditis | 1 (4.8) | 1 (8.3) | – |
| Celiac disease | 1 (4.8) | 1 (8.3) | – |
| Ulcerative colitis | 1 (4.8) | 1 (8.3) | – |
| Psoriasis | 1 (4.8) | – | 1 (11.1) |
| Type 1 diabetes | 1 (4.8) | 1 (8.3) | – |
| Malignancy | |||
| Lung cancer | 5 (25) | 2 (16.7) | 3 (33.3) |
| Melanoma | 9 (45) | 7 (58.3) | 2 (22.2) |
| Breast cancer | 2 (10) | 1 (8.3) | 1 (11.1) |
| Renal cell cancer | 1 (5) | 1 (8.3) | – |
| Multiple myeloma | 1 (5) | 1 (8.3) | – |
| Lymphoma | 1 (5) | – | 1 (11.1) |
| Merkel cell cancer | 1 (5) | 1 (8.3) | – |
| Esophageal cancer | 1 (5) | – | 1 (11.1) |
| Pancreatic cancer | 1 (5) | – | 1 (11.1) |
| RPG (mg/dL) at ICI initiation | 116 (98–154) | 115 (84–140) | 158 (107–254) |
| FPG (mg/dL) at ICI initiation | 108 (99–132) | 102 (93–108) | 132 (114–153) |
| HbA1c (%) at ICI initiation | 8 (6.8–8.6) | – | 8 (6.8–8.6) |
| ICI therapy | |||
| Pembrolizumab | 17 (81) | 11 (91.7) | 6 (66.7) |
| Nivolumab | 4 (19) | 1 (8.3) | 3 (33.3) |
| Other ICI used | 2 (9.5) | 1 (8.3) | 1 (11.1) |
| Ipilimumab | 2 (9.5) | 1 (8.3) | 1 (11.1) |
| Glucocorticoid use | 7 (35) | 4 (33.3) | 3 (33.3) |
FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; RPG, random plasma glucose.
bmjdrc-2018-000591supp001.docx (16KB, docx)
Disease characteristics of new-onset insulin-dependent diabetes
New-onset insulin-dependent diabetes developed in 12 patients after a median of four cycles of ICI or 5 months since initiation of ICI therapy. The HbA1c at presentation was 9.7 (IQR 8.6–10.7)% or 83 (IQR 70–93) mmol/mol. Eight patients (67%) presented with DKA at the time of diagnosis, with one of them having concomitant hyperglycemic hyperosmolar state. C-peptide was tested in six patients, of which five (83%) had a low (reference range 1.1–4.4 ng/mL) or undetectable level. Pancreatic enzymes were tested at the time of diagnosis in seven patients, with increase in four patients (57%) (one had mild elevation and three had elevation more than two times the upper limit of normal). Fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan was performed within 4 months of diagnosis in five patients, of which one (patient 10) demonstrated diffusely increased pancreatic uptake (figure 1) at the initial scan. At least one antibody associated with type 1 diabetes was tested in seven patients, of which five (71%) had at least one positive antibody, with antibody against GAD65 being the most common in 4/7 (57%). Fifty percent of patients developed another IRAE during the follow-up duration, with the most common being thyroiditis (manifested as primary hypothyroidism or thyrotoxicosis followed by primary hypothyroidism) in 42%. Multiple daily injections of insulin (MDI) was initiated and continued for all except one patient that transitioned to insulin pump during median follow-up duration of 13 months since ICI initiation. Other disease characteristics are mentioned in tables 2 and 3.
Figure 1.
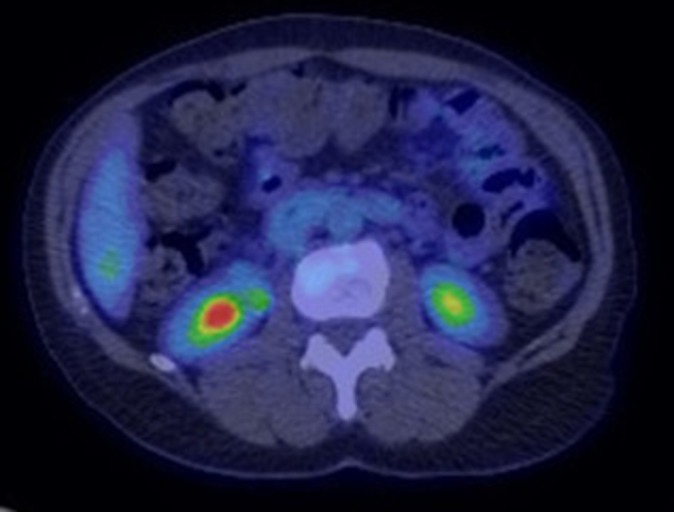
PET scan performed at diagnosis of new-onset insulin-dependent diabetes demonstrating diffusely increased FDG uptake in the pancreas of patient 10. FDG, fluorodeoxyglucose; PET, positron emission tomography.
Table 2.
Disease characteristics of patients with PD-1 inhibitor-induced diabetes
| Disease characteristics | All cases (n=21) | New-onset insulin-dependent diabetes (n=12) | Worsening of type 2 diabetes (n=9) |
| N (%) or median (IQR) | |||
| Time from ICI initiation to diagnosis | |||
| Numberof ICI cycles | 4 (2–5.5) | 4 (2–5.8) | 4 (2.5–9) |
| Duration (months) | 4.8 (2–8.2) | 4.9 (2.2–8.1) | 3.9 (1.8–10.3) |
| Weight change (kg) from ICI initiation to diagnosis | −3.3 (–7.1 to +0.2) | −3.4 (–7.3 to -2.4) | 0.3 (-7.6 to +7.6) |
| RPG (mg/dL) at diagnosis | 460 (372–807.5) | 600 (429–971) | 308 (224.3–533.2) |
| FPG (mg/dL) at diagnosis | 285 (206.5–316) | 298 (212.8–331.5) | 230 (199–307.5) |
| HbA1c at diagnosis | 9.9 (8.7–11.1); | 9.7 (8.6–10.7); | 10 (8.8–11.6); |
| (%; mmol/mol) | 85 (72–98) | 83 (70–93) | 86 (73–103) |
| Diabetic ketoacidosis | 8 (38.1)* | 8 (66.7)* | 0 (0) |
| Low C-peptide level (reference 1.1–4.4 ng/mL) | 5/7 (71.4) | 5/6 (83.3) | 0/1 (0) |
| Pancreatic enzymes | |||
| Mildly elevated | 4/14 (28.6) | 1/7 (14.3) | 3/7 (42.8) |
| Moderate to severely elevated (>2 times upper limit of normal) | 4/14 (28.6) | 3/7 (42.8) | 1/7 (14.3) |
| Not elevated | 6/14 (42.8) | 3/7 (42.8) | 3/7 (42.8) |
| Antibody positivity | 5/7 (71.4) | 5/7 (71.4) | Not checked |
| GAD65 (reference ≤0.02 nmol/L) | 4/7 (57.1) | 4/7 (57.1) | |
| IAA (reference ≤0.02 nmol/L) | 2/6 (33.3) | 2/6 (33.3) | |
| IA-2 (reference ≤0.02 nmol/L) | 1/6 (16.7) | 1/6 (16.7) | |
| ZnT8 (reference <15 U/mL) | 0/4 (0) | 0/4 (0) | |
| Increased pancreas FDG uptake | 1/9 (11.1) | 1/5 (20) | 0/4 (0) |
| Other IRAE | 13 (61.9) | 6 (50) | 7 (77.8) |
| Thyroiditis | 9 (69.2) | 5 (41.7) | 4 (44.4) |
| Hypophysitis | 3 (23.1) | 2 (16.7) | 1 (11.1) |
| Dermatitis | 2 (15.4) | 1 (8.3) | 1 (11.1) |
| Ocular | 1 (7.7) | – | 1 (11.1) |
| Hepatitis | 1 (7.7) | – | 1 (11.1) |
| Arthritis | 1 (7.7) | 1 (8.3) | – |
| Diabetes therapy at latest follow-up visit | |||
| MDI±oral agent | 19 (90.5) | 11 (91.7) | 7 (77.8) |
| Basal insulin±oral agent | 1 (4.8) | 0 (0) | 2 (22.2) |
| Insulin pump | 1 (4.8) | 1 (8.3) | 0 (0) |
| Follow-up duration after ICI start (months) | 17.9 (10.4–28.3) | 12.9 (10.4–24.3) | 26 (14.8–30.7) |
| Follow-up duration after diagnosis (months) | 12.5 (5.3–20.7) | 9.7 (3.9–12.9) | 17.2 (9.7–23.7) |
| Deaths (all from cancer) | 4 (19) | 1 (8.3) | 3 (33.3) |
*n=1 had concomitant hyperglycemic hyperosmolar state.
FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; RPG, random plasma glucose.
Table 3.
Disease characteristics and course of individual patients with PD-1 inhibitor induced new-onset insulin-dependent diabetes
| Pt | BMI | ICI | Pre-ICI laboratory data | Presentation | C-peptide (ng/mL), PG (mg/dL) | Doses/ time (months) to presentation |
Antibodies | Other IRAE | Therapy |
| 1 | 25 | Pembrolizumab | FPG 95 mg/dL, RPG 96 mg/dL. | Mild DKA: RPG 580 mg/dL, HbA1c 8.6%, urine ketone 40, and β-OHB 1.3 mmol/L. | 0.9, 129 | 2/2.1 | GAD65 0.06; IAA and IA-2A negative. | Thyroiditis. | MDI. |
| 2 | 35 | Pembrolizumab | FPG 78 mg/dL. | Severe DKA: RPG 971 mg/dL, HbA1c 10.7%, β-OHB 10.8 mmol/L, and lipase 661 IU/L. | <0.1, 147 | 1/2.2 | GAD65 0.21; IAA and IA-2A negative. | Thyroiditis. | MDI → insulin pump. |
| 3 | 38 | Pembrolizumab | FPG 83 mg/dL, RPG 108 mg/dL. | DKA/HHS: RPG 972 mg/dL, HbA1c 7.8%, and β-OHB 3.7 mmol/L. | <0.1, 278 | 1/0.6 | GAD65 15.4, IAA 0.05, IA-2A 0.17; ZnT8A negative. | Thyroiditis. | MDI. |
| 4 | 25 | Pembrolizumab | Not tested. | RPG 600 mg/dL, HbA1c 10.5%, and trace urine ketone. | 0.3, 250 | 3/4.8 | IAA 0.14; GAD 65, IA-2A, ZnT8A negative. | – | Metformin → MDI. |
| 5 | 23 | Nivolumab | FPG tested once 105 mg/dL. | RPG 600 mg/dL. | 4/5.5 | – | Thyroiditis. | MDI. | |
| 6 | 35 | Ipilimumab → Pembrolizumab | FPG 108 mg/dL, RPG tested once 140 mg/dL, HbA1c 5.4%. | RPG 377 mg/dL, FPG 321, and HbA1c 9.7%. | 5.2, 321 | 6/4.9 | – | – | Metformin → MDI. |
| 7 | 31 | Pembrolizumab | FPG 86 mg/dL, RPG tested once 141 mg/dL. | Severe DKA: RPG 644 mg/dL, HbA1c 9.7%, and β-OHB 6.8 mmol/L. | – | 5/7.8 | GAD65 negative. | Thyroiditis and hypophysitis. | MDI. |
| 8 | 33 | Pembrolizumab | FPG tested once 106 mg/dL. | Mild DKA: RPG 460 mg/dL, FPG 311 mg/dL, HbA1c 7.8, urine ketone 750, β-OHB 0.2 mmol/L, and lipase 120 IU/L. | 0.7, 142 | 17/9.7 | GAD65 0.05; IAA, IA-2A, ZnT8A negative. | – | Metformin basal insulin → MDI. |
| 9 | 45 | Pembrolizumab | FPG 96 mg/dL, RPG 114 mg/dL. | Severe DKA: RPG 429 mg/dL, FPG 236 mg/dL, HbA1c 11.3%, urine ketone 750, and β-OHB 8 mmol/L. | – | 16/23.6 | – | Dermatitis, hypophysitis, and arthritis. | MDI. |
| 10 | 25 | Pembrolizumab | FPG tested once 126 mg/dL. | Mild DKA: RPG 426 mg/dL, FPG 335 mg/dL, HbA1c 11.2%, β-OHB 1.9 mmol/L, and increased pancreas FDG uptake. | – | 4/3 | – | – | MDI. |
| 11 | 23 | Pembrolizumab | RPG 84 mg/dL. | Severe DKA: RPG 1389 mg/dL, HbA1c 8.8%, β-OHB 14 mmol/L, lipase 226, and GGT 227. | 0.4, 866 | 2/0.7 | – | – | MDI. |
| 12 | 46 | Pembrolizumab | FPG 99 mg/dL. | Mild ketosis: FPG 448 mg/dL, and HbA1c 10.6 β-OHB 1.2 mmol/L. | – | 4/8.2 | GAD65, IAA, IA-2A, ZnT8A negative. | – | MDI. |
BMI, body mass index; DKA, diabetic ketoacidosis; FPG, fasting plasma glucose; GAD65, glutamic acid decarboxylase 65; GGT, gamma-glutamyl transferase; HHS, hyperglycemic hyperosmolar state; HbA1c, hemoglobin A1c; IA-2, islet antigen 2; IAA, insulin autoantibodies; ICI, immune checkpoint inhibitor; IRAE, immune-related adverse event; MDI, multiple daily injections of insulin; β-OHB, beta-hydroxybutyrate; PD-1, programmed cell death protein 1; RPG, random plasma glucose; ZnT8A, Zinc transporter 8 antibody.
Disease characteristics of worsening type 2 diabetes
There was unexplained worsening of glycemic control in nine patients with pre-existing type 2 diabetes after a median of four cycles of ICI or 4 months since initiation of ICI therapy. The median HbA1c at presentation was 10% or 86 mmol/mol, and median RPG was 308 mg/dL (IQR 224–533). Pancreatic enzymes were tested in seven patients, with increase in four patients (57%) (three had mild elevation and one had elevation more than two times the upper limit of normal). FDG-PET was performed within 4 months of case development in four patients, none of which showed increased pancreatic uptake. None of these patients underwent testing for type 1 diabetes-associated antibodies. C-peptide was normal in the one patient with worsening glycemic control who underwent testing. At the time of worsening of type 2 diabetes, there was initiation of insulin in six patients (MDI initiated in four, basal insulin initiated in two), conversion from basal insulin to MDI in two patients, and increase in total daily dose of MDI by 100 units in one patient. Seventy-eight percent developed another IRAE, with the most common being thyroiditis (manifested as primary hypothyroidism or thyrotoxicosis followed by primary hypothyroidism) in 44%. All patients were on MDI (7/9, 78 %) or basal insulin (2/9, 22%) without any de-escalation in dose during the median follow-up duration of 26 months since ICI initiation. Other disease characteristics are mentioned in tables 2 and 4.
Table 4.
Disease characteristics and course of individual patients with PD-1 inhibitor-induced worsening of pre-existing type 2 diabetes
| Pt | BMI | ICI | Pre-ICI laboratory data | Presentation | Doses/time (months) to presentation | Other IRAE | Therapy |
| 13 | 33.3 | Nivolumab | FPG 166 mg/dL, HbA1c 8.1%. | HbA1c 10%, FPG 300 mg/dL, RPG 580 mg/dL. | 2/3.9 | Thyroiditis. | TZD and basal insulin. |
| 14 | 24.4 | Nivolumab | FPG 197 mg/dL, RPG 273 mg/dL, HbA1c 8.2% | HbA1c 10%, RPG 378 mg/dL, FPG 311 mg/dL, β-OHB 1.8 mmol/L; methylprednisolone 1000 mg daily for 1 week. | 4/5.7 | Dermatitis; secondary adrenal insufficiency. | MDI. |
| 15 | 25.5 | Pembrolizumab | FPG 104 mg/dL, HbA1c 6.2%. | HbA1c 8.6%, RPG 239 mg/dL, FPG 190 mg/dL. | 12/5 | Thyroiditis. | Basal insulin. |
| 16 | 27.1 | Pembrolizumab | FPG 111 mg/dL, RPG 132 mg/dL, HbA1c 8%. | HbA1c 11.3%, RPG 367 mg/dL, FPG 222 mg/dL. | 5/3.8 | Ocular. | Metformin and MDI. |
| 17 | 37.9 | Pembrolizumab | FPG 121 mg/dL, HbA1c 7.4%. | FPG 208 mg/dL, HbA1c 9%. | 4/1.4 | – | MDI |
| 18 | 45.2 | Pembrolizumab | FPG 140 mg/dL, HbA1c 6.6%. | FPG 230 mg/dL, RPG 249 mg/dL, HbA1c 8.1%, dexamethasone 2 g daily for 5 months. | 3/6.9 | Thyroiditis and hypophysitis. | Metformin and MDI. |
| 19 | 27.5 | Ipilimumab → Pembrolizumab | FPG 117 mg/dL. | FPG 304 mg/dL, HbA1c 11.8%. | 13/12.3 | Hepatitis. | MDI. |
| 20 | 32.3 | Nivolumab | FPG 112 mg/dL, RPG 158 mg/dL, HbA1c 8.8%. | FPG 154 mg/dL, RPG 180 mg/dL, HbA1c 9.5%, GGT 257 IU/L, dexamethasone 8 g daily for 1 week. | 1/1.8 | Thyroiditis. | MDI. |
| 21 | 18.4 | Pembrolizumab | FPG 141 mg/dL, RPG 235 mg/dL, HbA1c 9.2%. | FPG 606 mg/dL, RPG 999 mg/dL, HbA1c 12%. | 3/1.7 | – | MDI. |
BMI, body mass index; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; IA-2, islet antigen 2; IAA, insulin autoantibodies; IRAE, immune-related adverse events; MDI, multiple daily injections of insulin; RPG, random plasma glucose; TZD, Thiazolidinedione.
Discussion
We report the frequency, clinical features and follow-up of new-onset insulin-dependent diabetes and significant unexplained worsening of glycemic control in patients receiving ICIs for various cancers. We confirmed the absence of occurrence of this IRAE in patients receiving CTLA-4 inhibitors and report a slightly increased frequency of this disorder in patients receiving one of the PD-1 inhibitors. Other immune checkpoint manipulation approaches, such as PD-L1 inhibition are now being used and will require investigation for IRAEs including diabetes.
Case identification and frequency
Among the PD-1 inhibitor-treated patients, 12 developed new-onset insulin-dependent diabetes and 9 out of 96 with pre-existing prediabetes or type 2 diabetes had worsening of glycemic control. Those with worsening of pre-existing type 2 diabetes in the absence of other predisposing factors had initiation of insulin therapy in most of these patients and intensification in the rest. This demonstrates a temporal association between the initiation of ICI and development of β cell failure leading to insulin deficiency. This is similar to the rapid onset of hyperglycemia in cases of ICI-induced diabetes reported in literature.13 15 However, as seen in our study, ICI use can also lead to significant worsening of glycemic control in pre-existing type 2 diabetes, and this aspect has not been explored much in the available literature. We used strict criteria to classify these as induced by ICI therapy. However, limited laboratory characterization was available for these nine cases in the medical records, with no testing for antibodies associated with type 1 diabetes and C-peptide testing for only one patient. We also investigated the use of glucocorticoids that occurred in seven patients. It is unlikely that diabetes in these patients was caused by glucocorticoids, as they are not known to cause absolute insulin deficiency. Among the patients who had worsening of pre-existing type 2 diabetes, three were on glucocorticoids but did not have decrease in insulin dose after glucocorticoids were discontinued.
As compared with other studies that have mostly reported on patients referred for concern of diabetes after ICI therapy, we reviewed the medical records of all patients treated with CTLA-4 and PD-1 inhibitors at our institution over a period of 6 years, with at least a 6-month follow-up duration after initiating ICI therapy. This adds to the strength of our study for being able to ascertain the frequency of ICI-induced diabetes. Overall, 1.4% of the entire group developed new-onset insulin-dependent diabetes or significant worsening of type 2 diabetes. There were no cases in the 281 patients treated with CTLA-4 inhibitor ipilimumab. Stamatouli et al 15 and Iyer et al 16 have reported on their experience with ICI-induced diabetes and also did not find any cases with CTLA-4 inhibitor therapy. However, 1.8% of those treated with PD-1 inhibitors developed ICI-induced diabetes, of which 1% had new-onset insulin-dependent diabetes and 0.8% demonstrated worsening of pre-existing type 2 diabetes. In our study, 9/96 (9.3%) patients with pre-existing prediabetes or type 2 diabetes had worsening of glycemic control after starting PD-1 inhibitor therapy. The frequency of new-onset insulin-dependent diabetes of 1% seen in our cohort of PD-1 inhibitor-treated patients is higher than that reported by a recent meta-analysis from available clinical trials.12 This discrepancy between clinical practice and trial data might be due to better characterization of diabetes in clinical practice and longer follow-up after ICI therapy initiation. The difference in frequency of ICI-induced diabetes between the different ICIs may in part be related to the expression of the specific checkpoints that they inhibit. CTLA-4 has been demonstrated to be expressed on pituitary cells. Accordingly, the CTLA-4 inhibitor ipilimumab has been shown to cause hypophysitis with highest frequency among all ICIs.12 However, both in vitro and in vivo studies have demonstrated an association between PD-1 and autoimmune diabetes. Single-nucleotide polymorphisms in the PD-1 gene have been demonstrated to be enriched in individuals with type 1 diabetes (12.2%) compared with controls (6.8%), implicating the PD-1 pathway in the development of immune-mediated diabetes.17 Analysis of peripheral blood mononuclear cells from long-standing type 1 diabetes patients versus healthy controls stimulated with CD3/CD28 to induce proliferation of T-regulatory cells has demonstrated lower expression of PD-1 on the surface of these cells.18 Another study of 22 Japanese type 1 diabetes patients compared with 29 healthy controls demonstrated lower PD-1 expression on CD4 positive T cells.19 These data provide some mechanistic insight into the development of insulin-dependent diabetes with the use of PD-1 inhibitors.
Disease characteristics and natural history of ICI-induced diabetes
In this study, insulin deficiency occurred at a median of 4–5 months after ICI initiation or four cycles of ICI therapy; however, some patients developed it within a few weeks, whereas others developed it much later, especially one patient that presented with severe DKA after 16 cycles or 24 months since ICI initiation (table 3). The few patients that did undergo pretherapy testing in the new-onset diabetes group demonstrated normoglycemia prior to ICI therapy. This progression to insulin deficiency, which was acute and severe in most cases as demonstrated by the frequency of DKA, appears to be more rapid as compared with spontaneous type 1 diabetes, suggesting a faster rate of β cell failure in patients who develop ICI-induced diabetes. The other cases of ICI-induced diabetes reported in the literature have demonstrated a similar rapid progression to hyperglycemia.13 15 With our knowledge of the mechanism of these ICIs, we hypothesize that T cell-mediated destruction of β cells is the underlying cause of ICI-induced diabetes. In this study, we did not evaluate the response of malignancy to ICIs, but some available studies have suggested a possible association between the development of IRAE and better malignancy response to a specific ICI.15 20 21 This would make theoretical sense since ICI-mediated derepression of T cells is responsible for both the antitumor response as well as autoimmune adverse events. Two-thirds of our patients with new-onset insulin-dependent diabetes presented with DKA, suggesting rapid progression to severe insulin deficiency in these patients. All patients were on insulin during the follow-up duration, most being on MDI, and none of them were able to de-escalate their therapy. This suggests that remission of ICI-induced diabetes is unlikely. In literature, remission of such cases is reported rarely, with only 1/21 cases reported to have undergone remission in the experience from MD Anderson Cancer Center.16 In terms of management, some earlier reported cases in the literature were administered glucocorticoids, possibly related to information available from treating other IRAEs. However, those cases did not have improvement in their glycemic control. The patients reported in our study did not receive glucocorticoids for therapy of ICI-induced diabetes.
Potential factors influencing disease occurrence and severity
Even with the retrospective nature of this study and limited information available from review of chart records, we identified some potential predictive factors, which could influence the frequency of occurrence, or the natural history of ICI-induced diabetes. We evaluated the predisposition to autoimmunity by reporting on family history or personal history of autoimmune conditions. In those with new-onset insulin-dependent diabetes, 17% had a personal history of autoimmunity (all with Hashimoto's thyroiditis), and 1/3 had a family history of autoimmunity (table 1 and online supplement table S1). However, because family history was obtained from the medical record in this retrospective study, the rate of positive family history could be under-reported. In the entire cohort, almost two-thirds of our cohort had another IRAE, with the most common being thyroiditis. Hence, the presence of one IRAE may raise the suspicion for occurrence of another. Autoantibodies associated with type 1 diabetes were positive in five of seven patients, all of which had new-onset insulin-dependent diabetes. Unfortunately, these antibodies were not tested prior to initiating ICI therapy; hence, we are not able to comment on their status as a biomarker for predicting the development of ICI-induced diabetes. However, the information available from these antibodies at the time of diagnosis does suggest their role in disease prognostication. Patient 3 who had the most number of positive antibodies had the shortest duration between initiation of ICI and new-onset insulin-dependent diabetes. He also presented with concomitant DKA and hyperglycemic hyperosmolar state. Overall, the frequent occurrence of other IRAEs and antibody positivity in patients with new-onset insulin-dependent diabetes fits with the autoimmune nature of ICI-induced diabetes. Some of the patients with worsening of pre-existing type 2 diabetes did not have optimal glycemic control prior to ICI, highlighting the need to address diabetes control more closely.
New-onset insulin-dependent diabetes in the setting of ICI therapy shares some similarities with spontaneous type 1 diabetes, with the frequent occurrence of DKA and low or undetectable C-peptide levels when tested, which implies lack of endogenous insulin production, most likely due to immune-mediated loss of β cells. However, there are some differences, as noted with the more rapid presentation to insulin deficiency as compared with spontaneous type 1 diabetes. Also, these patients were much older with a median age of 61 years as compared with those with spontaneous type 1 diabetes. The BMI was higher as compared with the usually lean body habitus of patients with spontaneous type 1 diabetes. Hence, in our assessment, age or BMI of the patient are not useful predictors for the development or severity of ICI-induced diabetes. The occurrence of new-onset insulin-dependent diabetes was characterized by a median weight loss of 3 kg when compared with the original weight prior to ICI initiation. Hence, loss of weight might be an indicator for the development of insulin deficiency in the setting of ICI use.
We also evaluated for increased FDG uptake in the pancreas on PET scan performed within 4 months of case development, which was done in nine patients. This had limited sensitivity to detect pancreatic inflammation. Mild to moderate elevation in pancreatic enzymes occurred in the setting of ICI therapy, but the clinical use, evaluation and long-term significance of this finding need to be investigated further.
Conclusions
We have identified new-onset insulin-dependent diabetes induced by PD-1 inhibitors with a frequency of 1%. This form of diabetes is characterized by more rapid progression to severe insulin deficiency as compared with spontaneous type 1 diabetes, frequently presents with DKA and does not appear to undergo remission. In addition to new-onset diabetes, PD-1 inhibitors can also worsen glycemic control in pre-existing type 2 diabetes. ICI-induced diabetes requires complex insulin therapy for management and adds significantly to the morbidity of these patients with cancer. With the growing use of these agents for cancer therapy, clinicians are more likely to encounter this condition. This underscores the importance of monitoring blood glucose and HbA1c prior to initiating ICIs as well as during follow-up, having a heightened suspicion for the occurrence of diabetes and developing better risk prediction. Better characterization of ICI-induced diabetes will improve both the diagnosis and management of this condition and also add to our knowledge of immune-mediated diabetes.
Footnotes
Presented at: The initial abstract of this manuscript was presented at Endocrine Society’s 100th Annual Meeting and Expo, Chicago, Illinois, in March 2018, and published in Endocrine Reviews 2018;39(2).
Contributors: AK researched the data, analyzed the data and wrote the manuscript; CH researched the data and wrote the manuscript, MB reviewed/edited the manuscript, YCK developed the research question, supervised all aspects of the project as principal investigator and reviewed/edited the manuscript.
Funding: This work was supported by the Landow gift through the Center for Immunology and Immune Therapies, Mayo Foundation. This publication was made possible by CTSA Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
Disclaimer: Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Mayo Clinic Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 2. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734–6. 10.1126/science.271.5256.1734 [DOI] [PubMed] [Google Scholar]
- 4. Sharma P, Wagner K, Wolchok JD, et al. . Novel cancer immunotherapy agents with survival benefit: recent successes and next steps. Nat Rev Cancer 2011;11:805–12. 10.1038/nrc3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zimmer L, Goldinger SM, Hofmann L, et al. . Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210–25. 10.1016/j.ejca.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 6. Faje AT, Sullivan R, Lawrence D, et al. . Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 2014;99:4078–85. 10.1210/jc.2014-2306 [DOI] [PubMed] [Google Scholar]
- 7. Delivanis DA, Gustafson MP, Bornschlegl S, et al. . Pembrolizumab-induced thyroiditis: comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab 2017;102:2770–80. 10.1210/jc.2017-00448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamauchi I, Sakane Y, Fukuda Y, et al. . Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid 2017;27:894–901. 10.1089/thy.2016.0562 [DOI] [PubMed] [Google Scholar]
- 9. Chen TW, Razak AR, Bedard PL, et al. . A systematic review of immune-related adverse event reporting in clinical trials of immune checkpoint inhibitors. Ann Oncol 2015;26:1824–9. 10.1093/annonc/mdv182 [DOI] [PubMed] [Google Scholar]
- 10. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. . Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hickmott L, De La Peña H, Turner H, et al. . Anti-PD-L1 atezolizumab-induced autoimmune diabetes: a case report and review of the literature. Target Oncol 2017;12:235–41. 10.1007/s11523-017-0480-y [DOI] [PubMed] [Google Scholar]
- 12. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. . Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–82. 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gauci ML, Laly P, Vidal-Trecan T, et al. . Autoimmune diabetes induced by PD-1 inhibitor-retrospective analysis and pathogenesis: a case report and literature review. Cancer Immunol Immunother 2017;66:1399–410. 10.1007/s00262-017-2033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hughes J, Vudattu N, Sznol M, et al. . Precipitation of autoimmune diabetes with anti-PD-1 immunotherapy. Diabetes Care 2015;38:e55–7. 10.2337/dc14-2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stamatouli AM, Quandt Z, Perdigoto AL, et al. . Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67:1471–80. 10.2337/dbi18-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iyer P, Best C, Lavis VR. Checkpoint inhibitor mediated insulin dependent diabetes: a cancer center experience (Abstract). Endocrine Reviews 2018;39. [Google Scholar]
- 17. Nielsen C, Hansen D, Husby S, et al. . Association of a putative regulatory polymorphism in the PD-1 gene with susceptibility to type 1 diabetes. Tissue Antigens 2003;62:492–7. 10.1046/j.1399-0039.2003.00136.x [DOI] [PubMed] [Google Scholar]
- 18. Perri V, Russo B, Crinò A, et al. . Expression of PD-1 molecule on regulatory t lymphocytes in patients with insulin-dependent diabetes mellitus. Int J Mol Sci 2015;16:22584–605. 10.3390/ijms160922584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujisawa R, Haseda F, Tsutsumi C, et al. . Low programmed cell death-1 (PD-1) expression in peripheral CD4(+) T cells in japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol 2015;180:452–7. 10.1111/cei.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freeman-Keller M, Kim Y, Cronin H, et al. . Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–94. 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weber JS, Hodi FS, Wolchok JD, et al. . Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–92. 10.1200/JCO.2015.66.1389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2018-000591supp001.docx (16KB, docx)


