Abstract
Streptococcus suis is a Gram-positive cocci bacterium that are found mainly in pigs and can be transmitted to human through pigs or pork exposure. The disease is mainly found among occupations involving swine contact in western countries whereas in Asia the disease is usually contracted through raw pork consumption. In this case report, we present a case of a middle-aged Thai man who acquired the infection from raw pork consumption. He presented with endogenous endophthalmitis with infective spondylodiscitis, sepsis and meningitis and later developed blindness of the right eye and permanent bilateral hearing loss disseminated from S. suis infection. Our report suggests that S. suis infection be considered as a causative factor in patient presenting with established clinical symptoms and predisposing factors. Cultural habit of eating raw pork should be taken into account especially in Asian countries.
Keywords: meningitis, tropical medicine (infectious disease), drugs: infectious diseases, public health
Background
Streptococcus suis is an emerging zoonosis that is mainly found as normal flora in pigs.1 The pathogen can be transmitted to human through exposure of pigs or consumption of raw pork2 causing a number of serious infections.3 The most common clinical manifestations found were meningitis (68%), followed by sepsis (25.0%) and arthritis (12.9%) whereas infective endocarditis (12.4%), endopthalmitis (4.6%) and spondylodiscitis (3.7%) were uncommon.4 Sensorineural hearing loss (SNHL) (39%) was the most common complication from S. suis meningitis in which the disease is usually irreversible despite effective treatment.4 5
The disease is usually found prevalent among farmers, butchers and abattoir workers involving swine contact in western countries.6 7 However, this was not always the case in Asian countries where pig-related occupation infection is less than half of the reported cases.8 9 According to a recent systematic review and meta-analysis, raw pork consumption, exposure to pigs or raw pork, pig-related occupation and male sex are the significant risk factors of the infection.10 This suggests that traditional food habit involving raw pork consumption probably plays an essential role in the disease infection in the Asian region.
Since the first S. suis infection in human related to meningitis and sepsis was reported in Denmark in 1968,11 the disease has caused wide spread infection globally with more than 1,500 reported cases as of 2012.12 In Thailand, S. suis infection is an important health problem with more than 500 cases reported up to 201710 and possibly the second causative agent of adult streptococcal meningitis.13
We therefore present a patient with endogenous endophthalmitis which is an uncommon clinical presentation, who later developed blindness of the right eye and permanent bilateral SNHL disseminated from S. suis infection.
Case presentation
A 48-year-old educated, Thai male was admitted to Chiang Mai University Hospital (CMUH) with endogenous endopthalmitis infecting his right eye. He is a degree graduate and works as an airport security administration officer. He consumes two to three bottles of beer daily and often consumes raw fermented pork or ‘naem’ (sour pork). He has a medical history of gout and spondylodiscitis. He refused taking any other medications except analgesics for his back pain. He did not travel to anywhere within the region and had no pig farming exposure prior to admission at the hospital.
Upon detailed consultation, the patient narrated that he often experienced lack of sleep due to work stress, fatigue and dizziness for the past few months before admission. Six days before admission, the patient reported to have consumed a northern-Thai style raw pork dish called ‘larb dib’ at Songkran (the Thai new year) party at his office. The pork and ingredients were bought from wet markets in Sankampaeng and Saraphi Districts, Chiang Mai Province and cooked by his colleagues. According to the patient, he was the only one who fell sick after the party. Two days later, he developed a low-grade fever, neck, joint and waist pain and blurred vision at his right eye along with a decrease in hearing ability at his right ear. The symptoms got worse and he visited a local hospital where he underwent an X-ray for waist and neck bone. He received an unknown intravenous antibiotic for joint pain, then later progressed to have nausea, vomiting, hearing loss and redness of his right eye. He was then referred to CMUH.
At admission, the body temperature was 37.5°C with 24 hours peak temperature of 37.8°C, pulse rate of 90 beats per minute and a blood pressure of 130/80 mm Hg. His respiratory rate was 20 breaths per minute. A physical examination identified chemosis and periorbital swelling at the right eye and C-spine tenderness.
Investigations
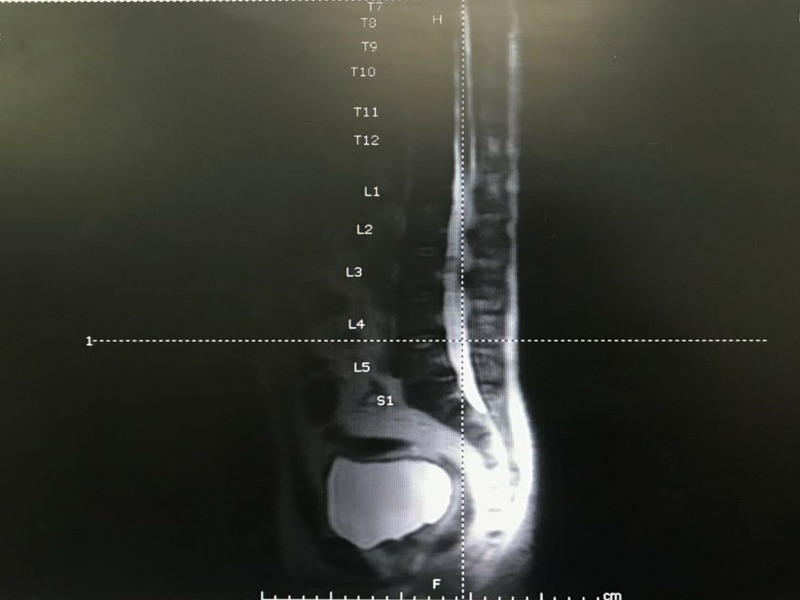
A routine laboratory and microbiological culture were ordered. An MRI of the whole spine was done to rule out infective spondylodiscitis suggested spondylodiscitis at C4/5 level and left side of L5/S2 (figure 1). There was left paracentral disc extrusion with associated disc bulging and osteophyte causing mild spinal cord compression.
Figure 1.
MRI of whole spine.
Assessment of visual acuity (VA) revealed hand movement (HM) at right VA and a VA of 6/16 on the left. There were marked injected conjunctiva, whitish, large keratic precipitate, and hypopyon uveitis in the right eye. The relative afferent pupil defect (RAPD) test showed a positive reverse RAPD. The left eye was normal. The patient was still alert and oriented despite the presence of febrile illness and neck stiffness.
The laboratory results showed no outstanding value except an elevated neutrophil (83.6%; normal range, 40%–75%), and erythrocyte sedimentation rate (65 mm/hour; normal range, 0–15 mm/hour), and a transient increase of alanine aminotransferase (65 U/L; normal range, 0–41 U/L), blood urea nitrogen (21 mg/dL; normal range, 6–20 mg/dL) and hyponatraemia (128 mmol/L; normal range, 136–145 mmol/L). Ultrasound abdomen was performed to rule out liver abscess. A serologic anti-HIV antibody testing was negative. Immunoglobulin and IgG subset analysis were not performed. A lumbar puncture was carried out 3 days after admission showing a turbid colour of the cerebrospinal fluid (CSF), an increased CSF protein level (88 mg/dL; normal range, 15–45 mg/dL) and decreased glucose level (15 mg/dL; normal range, 45–80 mg/dL). The CSF analysis showed white blood cell count 0.131x109/L, red blood cell count 100x1012/L, polymorphonuclear 41%, mononuclear 60%. The Gram staining was negative.
Differential DIAGNOSIS
Three bottles of blood samples and vitreous from the right eye positively revealed S. suis infection after 3 days collections. The Gram strain result of vitreous at the right eye showed moderate quantity of Gram-positive coccobacilli. The minimum inhibitory concentrations were 0.094 µg/mL for both penicillin and ceftriaxone. The repeated microbiological culture was negative 2 days upon treatment initiation. Two days after admission, the right eye globe pathology report showed acute endophthalmitis with perforated cornea and vitreous haemorrhage. The audiogram was done at 5-day post admission suggesting irreversible bilateral SNHL disseminated from S. suis infection. The tympanic membrane was intact and external auditory canal remained normal.
Treatment
Fortified antibiotic ophthalmic solutions (ceftazidime 50 mg/mL at right eye at 1-hour interval and vancomycin 50 mg/mL at right eye at 1-hour interval) and intravenous ceftriaxone (2.0 g at 12-hour interval) and vancomycin (1.0 g at 8-hour interval) were empirically given to treat the infection. Intravenous hydration was administered to treat hypovolaemic hyponatraemia. Ceftriaxone treatment was continued after the microbiological culture confirmed of S. suis infection.
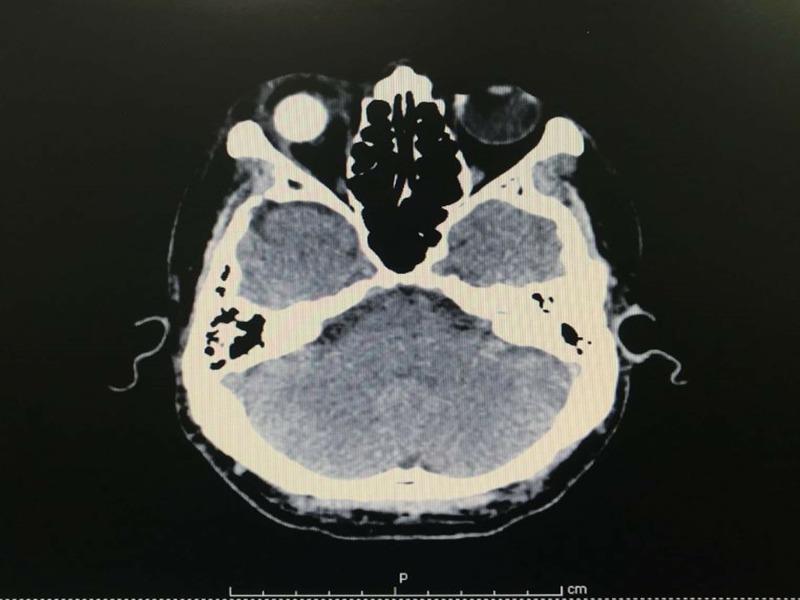
The right vitreous tapping, enucleation was performed. Conjunctival peritomy was done. Tenon was undermined and cut separately from sclera. The residual was cleaned and sutured by vicryl stitches. The optic nerve was cut to around 2 mm under the globe. Mull no. 16 and conformer were placed to fit in orbit. One day later, the patient developed a low-grade fever with a temperature of 38.2°C, and experienced a significant loss of hearing in both ears starting from left to right. The CT brain showed no evidence of brain abscess nor leptomeningeal enhancement. There was heterogeneous enhancing lesion adjacent to anterior aspect of right enucleation material and swelling of right extraocular muscles and right optic nerve which might be due to infectious process or postoperative change (figure 2). The condition remained stable without any complications.
Figure 2.
CT brain with contrast media (CM)
After 15 day of admission, the patient recovered from S. suis meningitis, septicaemia and spondylodiscitis but suffered from loss of hearing and right eye vision. Ceftriaxone 2.0 g at 12-hour interval until 4-week treatment completion and levofloxacin 500 mg 1.5 tablets per oral for 2 months were prescribed for home medication. In addition, the patient was referred to a nearby local hospital for intravenous ceftriaxone injection.
Outcome and follow-up
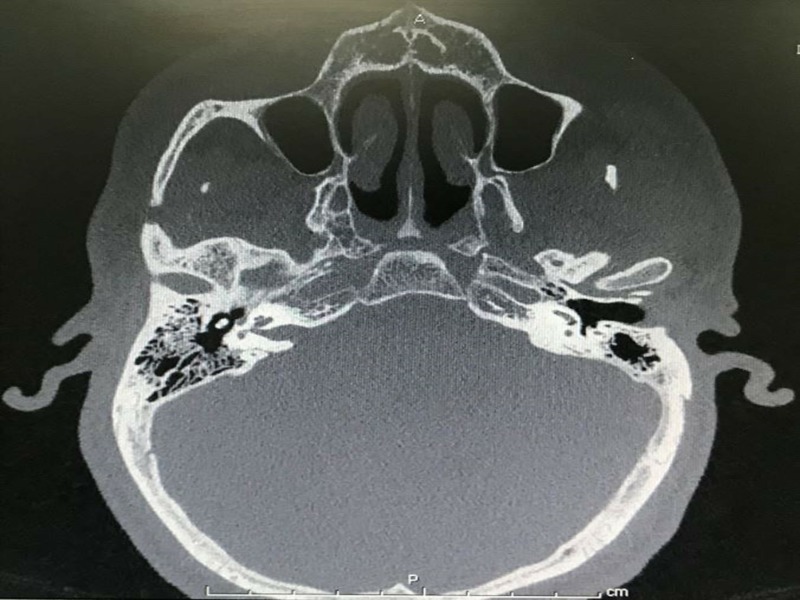
At 3-month follow-up medical examination, the CT scanning of the temporal bone revealed mild to moderate degree of labyrinthitis ossificans involving both vestibules and all semicircular canals of both ears (figure 3). The basal turn of cochlear was more severe on the left side. Both facial and vestibulocochlear nerve were within normal limit. The videonystagmography at 4 months was done showing bilateral death labyrinths. The right cochlear implant was done at 7 months. A lead electrode was fully inserted into the scala tympani. Impedance/neural response imaging responses were detected. The patient was discharged in a stable condition after a 4-day admission.
Figure 3.
CT scan of temporal bone.
Discussion
S. suis is usually an occupational related disease affecting farmers, abattoir workers and butchers in western countries.6 7 The first case of endopthalmitis secondary to S. suis infection was reported in 1978 by McLendon et al 14 in which a Caucasian man from the UK who worked at a pork pie factory developing deafness at the right ear and bilateral endophthalmitis was described.14
In our case, the patient was an office worker without any occupational exposure to pigs or raw pork. The disease transmission was presumably from raw pork consumption or ‘Larb Dib’ he ate 6 days predated the admission. ‘Larb’ is a northern Thai dish commonly eaten in raw form in northern Thailand. This emphasises the significant role of raw pork eating in S. suis infection. His physical weakness from sleep deprivation and underlying conditions were probably contributing factors resulting in a more susceptible condition to infection. His medical history of regular alcohol drinking was not surprising as raw pork is usually consumed together with alcohol drinks among Thai people especially northern Thai men. Consistently, a considerable large number of alcohol consumption was also noted in studies from Thailand.13 15 16
The patient established acute endopthalmitis and infective spondylodiscities in addition to meningitis and septicaemia which are common clinical manifestations of S. suis. He developed the symptom shortly after consuming raw pork together with back and joint pain implicating infective spondylodiscites. However, the cause of his illness was not identified until nearly a week after the onset when fortified antibiotic ophthalmic solutions were given. The urgent enucleation due to endopthalmitis with perforated cornea at his right eye was performed and, right blindness resulted.
According to a systematic review, the disease transmission from skin abrasion is believed to be the main route of pathogen entry, however this was noted only in some studies.10 The presence of fever prior to the ocular symptom suggested that endopthalmitis could be disseminated from S. suis septicaemia in this patient.
The property of steroids in reducing inflammatory reactions at subarachnoid space which is a major process causing brain injury and neuronal dysfunction in acute bacterial meningitis has provided the rational of adjunctive corticosteroids use.17 18 However, the benefit of steroids in reducing hearing loss has been established in paediatric meningitis but has yet to be clearly confirmed among adult population.19 As S.suis infection is mainly found in adults and its effects in this population remains uncertain, using adjunctive dexamethasone may not have reversed the sequelae. In this patient, dexamethasone was not administered. The diagnosis was delayed in the primary setting and permanent SNHL as a consequence of S. suis meningitis seemed to be existed before admission. In our views, it still warrants the use of steroids to benefit the patient from its anti-inflammatory property in order to reduce inflammation. A comprehensive counselling session to emphasise on the risk of acquiring S.suis infection from raw or partially cooked pork including sour pork or ‘Naem’ in raw form should have been mandated.
The high fatality rate in the China outbreak in 2005 involving 215 patients and 38 deaths has emphasised the importance of this zoonosis infection.20 In this outbreak, the main cause of the widespread of infection was due to backyard slaughtering of sick pigs whereas the deep-rooted behaviour of raw pork consumption seemed to be the predominating cause of the infection in Thailand. Apart from laboratory confirmation, the virulence factors of S.suis isolates from the outbreak including mrp, sly and ef were also related to strains in Europe which are considered to be more virulent than strains identified in North America.21
Regardless of the rare incidence of endopthalmitis, physicians should be more aware of the clinical symptoms especially in patient with suggestive clinical presentations and predisposing risk factors of S. suis infection. In addition, S.suis could also be the causative pathogen causing septicaemia among recent travellers to the Southeast Asia region or endemic areas presenting with fever. A detailed patient interview and history taking to indicate the timelines, relevant exposures and all relevant information are essential for a clear clinical picture. In the absence of available S. suis vaccine, early and adequate treatment is crucial to alleviate the disease progress and clinical complications as well as long term neurological sequelae particularly deafness which usually occurs among most S. suis meningitis survivors.
Learning points.
Streptococcus suis is not uncommon in Asia population where there is an association between traditional eating habits and raw pork consumption, whereas farmers and abattoirs workers in western countries are certainly at high risk of S. suis infections.
S. suis infection should be considered in patients presenting with established clinical symptoms and predisposing factors.
Early detection and appropriate treatment are important to improve symptom outcomes and complications.
Public health awareness programme is essential especially to those areas where traditional cultural habit of raw pork consumption is still practiced.
There is limited data concerning S. suis epidemiology and molecular characteristics. Further research should be carried out to understand the disease epidemiology, clinical manifestations and treatment regimens.
Acknowledgments
We would like to thank the patient described for allowing us to share his details, and Dr Vengadesh Letchumanan of Monash University—Malaysia Campus, Novel Bacteria and Drug Discovery Research Group, Microbiome and Bioresource Research Strength, Jeffrey Cheah School of Medicine and Health Sciences for his help in English proof reading.
Footnotes
Contributors: AR performed conception, designed the work, wrote the first draft of the manuscript and revisions with support from WK and PO. PO guided the interpretation of the clinical data and content. LHL, WK and PO critically reviewed the manuscript for intellectual content. All authors met the ICMJE criteria for authorship and approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Higgins R, Gottschalk M. Streptococcal diseases. Iowa State University: Ames, IA, 2005. [Google Scholar]
- 2. Segura M, Calzas C, Grenier D, et al. Initial steps of the pathogenesis of the infection caused by Streptococcus suis: fighting against nonspecific defenses. FEBS Lett 2016;590:3772–99. 10.1002/1873-3468.12364 [DOI] [PubMed] [Google Scholar]
- 3. Segura M, Fittipaldi N, Calzas C, et al. Critical streptococcus suis virulence factors: are they all really critical? Trends Microbiol 2017;25:585–99. 10.1016/j.tim.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 4. Huong VT, Ha N, Huy NT, et al. Epidemiology, clinical manifestations, and outcomes of Streptococcus suis infection in humans. Emerg Infect Dis 2014;20:1105 10.3201/eid2007.131594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tan JH, Yeh BI, Seet CS. Deafness due to haemorrhagic labyrinthitis and a review of relapses in streptococcus suis meningitis. Singapore Med J 2010;51:e30–3. [PubMed] [Google Scholar]
- 6. Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis 1988;10:131–7. 10.1093/clinids/10.1.131 [DOI] [PubMed] [Google Scholar]
- 7. Goyette-Desjardins G, Auger J-P, Xu J, et al. an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerging Microbes &Amp; Infections 2014;3:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. Qjm 1995;88:39–47. [PubMed] [Google Scholar]
- 9. Mai NT, Hoa NT, Nga TV, et al. Streptococcus suis meningitis in adults in Vietnam. Clinical Infectious Diseases 2008;46:659–67. [DOI] [PubMed] [Google Scholar]
- 10. Rayanakorn A, Goh BH, Lee LH, et al. Risk factors for streptococcus suis infection: a systematic review and meta-analysis. Sci Rep 2018;8:13358 10.1038/s41598-018-31598-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perch B, Kristjansen P, Skadhauge K. Group R streptococci pathogenic for man. Two cases of meningitis and one fatal case of sepsis. Acta Pathol Microbiol Scand 1968;74:69–76. [PubMed] [Google Scholar]
- 12. Huong VT, Ha N, Huy NT, et al. Epidemiology, clinical manifestations, and outcomes of streptococcus suis infection in humans. Emerg Infect Dis 2014;20:1105–14. 10.3201/eid2007.131594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suankratay C, Intalapaporn P, Nunthapisud P, et al. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health 2004;35:868–76. [PubMed] [Google Scholar]
- 14. McLendon BF, Bron AJ, Mitchell CJ, et al. Streptococcus suis type II (group R) as a cause of endophthalmitis. Br J Ophthalmol 1978;62:729–31. 10.1136/bjo.62.10.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Navacharoen N, Chantharochavong V, Hanprasertpong C, et al. Hearing and vestibular loss in streptococcus suis infection from swine and traditional raw pork exposure in northern Thailand. J Laryngol Otol 2009;123:857–62. 10.1017/S0022215109004939 [DOI] [PubMed] [Google Scholar]
- 16. Thayawiwat C, Wichaikham O, Painpringam A. Epidemiology of streptococcus suis infection: patients of chiang kham hospital, 2009 - 2011 [Thai]. J of Health Science 2012;21:575. [Google Scholar]
- 17. Syrogiannopoulos GA, Olsen KD, Reisch JS, et al. Dexamethasone in the treatment of experimental haemophilus influenzae type b meningitis. J of infectious diseases 1987;155:213–9. [DOI] [PubMed] [Google Scholar]
- 18. Täuber MG, Brooks-Fournier RA, Sande MA. Experimental models of CNS infections. Contributions to concepts of disease and treatment. Neurol Clin 1986;4:249–64. 10.1016/S0733-8619(18)30997-6 [DOI] [PubMed] [Google Scholar]
- 19. Brouwer MC, McIntyre P, Prasad K, et al. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2015(9):CD004405 10.1002/14651858.CD004405.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu H, Jing H, Chen Z, et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis 2006;12:914–20. 10.3201/eid1206.051194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berthelot-Hérault F, Gottschalk M, Morvan H, et al. Dilemma of virulence of Streptococcus suis: Canadian isolate 89-1591 characterized as a virulent strain using a standardized experimental model in pigs. Can J Vet Res 2005;69:236–40. [PMC free article] [PubMed] [Google Scholar]