Abstract
Background
As a consequence of indoor occupations and reduced exposure to sunlight, concerns have been raised that vitamin D deficiency is widespread in developed countries. Vitamin D is known to be associated with increased risks of morbidity and mortality in various diseases.
Objective
To investigate the serum vitamin D status and its relation with life-style factors in pregnant Japanese women.
Methods
Among a cohort for 3,327 pregnant women who participated in an the adjunct study of the Japan Environment and Children's Study during 2011–2013, in which data were obtained on various life-style factors, including both dietary intake of vitamin D and frequency of UV exposure, this study consisted of 1,592 pregnant women, from whom 2,030 serum samples were drawn in Jan, Apr, Jul, and Oct, and the association between serum 25(OH)D level and life-style factors were analyzed using linear mixed models.
Results
Serum 25(OH)D levels were less than 20ng/mL in 1,486 of 2,030 samples (73.2%). There was an obvious seasonal change, with serum 25(OH)D levels of less than 20 ng/mL in 89.8% and 47.8% of samples in spring (April) and autumn (October), respectively. Both the frequency spent under sunlight and dietary intake of vitamin D were significantly associated with serum 25(OH)D level. An increase in sunlight exposure of more than 15 min for 1 to 2 days per week in non-winter, or dietary intake of 2 μg/day of vitamin D resulted in an elevation of 1 ng/mL in serum 25(OH)D levels.
Conclusion
These findings indicate that vitamin D deficiency is very severe in Japanese pregnant women, especially those rarely exposed to sunlight. The benefits of UV rays should also be informed of when its risk is alerted, and clinicians should propose the adequate UV exposure level.
Introduction
Vitamin D is a fat-soluble secosteroid with well-established effects on calcium homeostasis. More recently, vitamin D has also been recognized to interact with a nuclear receptor in various other organs[1] and its deficiency is associated with increased risks of morbidity and mortality in various diseases including cardiovascular, malignant, and autoimmune diseases[2,3]. Accumulating evidence suggests that vitamin D deficiency during pregnancy may cause complications such as preeclampsia[3–5], although its implications and the underlying mechanisms are not fully understood. And it is even hypothesized that vitamin D deficiency in the fetal period leads to an increased risk of allergic diseases, multiple sclerosis, and cardiovascular diseases in later life[2,6–8].
As a consequence of indoor occupations and reduced exposure to sunlight, concerns have been raised that vitamin D deficiency is widespread in developed countries[2,3]. In Japan, because fish is a primary component of the traditional diet, the risk of vitamin D deficiency is rarely discussed. However, studies indicate that younger people consume less fish [9–12], and while females appear to be at higher risk of vitamin D deficiency because they tend to avoid direct sunlight exposure to prevent skin-tanning, may be malnourished from maintaining a lean proportion [13]. The importance of monitoring the vitamin D status in Japan has only recently been demonstrated [14,15].
Accordingly, the present study aimed to examine the serum vitamin D status of pregnant Japanese women and to estimate the impact of lifestyle factors on vitamin D levels in a population-based cohort.
Methods
Study design
This was cross-sectional sub-study comprising pregnant Japanese women enrolled in the adjunct study of the Japan Environment and Children's Study (JECS) to examine the effects of desert dust exposure on allergic diseases in pregnant women and their children in three areas in Japan; Kyoto (N 35°), Toyama (N 36°), and Tottori (N 35.5°) [16]. The study protocol was approved by ethic comities in Kyoto University, University of Toyama, and Tottori University, and was registered at UMIN000010826[16].
The details of the study design and protocol have been previously reported [16]. In brief, the JECS is a community based national birth cohort study[17,18], and the JECS participants from the above three regions who agreed to participate in the adjunct study were enrolled prior to delivery. Questionnaires on lifestyle factors and diet were sent out twice through the JECS[17,18]. Serum samples were taken three times during pregnancy; during the first trimester, during the second trimester, and at the timing of delivery[17,18].
Serum 25(OH)D levels were measured in blood samples from Jan (winter), April (spring), July (summer) and October (autumn) to evaluate their vitamin D distributions and seasonal changes, and the impact of lifestyle and dietary factors on 25(OH)D levels were estimated.
Measurements
Demographics
Information on various demographic parameters, including age, pre-pregnancy BMI, housing environment, socioeconomic background, smoking habit, and history of allergic and other diseases, was obtained from the majority of the study population during the 1st trimesters of pregnancy. Full details of demographic parameters are described elsewhere[17].
Serum vitamin D levels
Serum samples were stored at -30°C until biochemical analysis of blood was performed on serum samples at SRL laboratories (Tokyo, Japan). 25(OH)D was measured using the 25(OH)D 125I RIA kit (DiaSorin Inc, Minnesota, USA) [19,20]. The measuring range was 6 to 99900000 ng/ml. Measurement values below the limit of quantification (LOQ) were assigned 50% of the LOQ. Serum 25(OH)D concentrations were natural log-transformed before statistical tests were performed.
Estimated dietary vitamin D intake
A validated self-administered Food Frequency Questionnaire (FFQ) [21–23] was administered twice during pregnancy. The first FFQ was used for the main analysis, and the second FFQ for sensitivity analysis. Vitamin D intake was adjusted by the total energy using the energy-density method[24]. We excluded subjects who returned unreasonable range of total energy (less than 50% or more than 150% of predicted values) for the main analysis and we confirmed the robustness of the result by further analysis including the data from excluded subjects.
Estimated vitamin D supplementation
Dietary vitamin D supplementation was evaluated by a self-administered questionnaire on the frequency and the bland name of vitamin supplements. The dose of vitamin D within each reported tablet was searched, and the subject was deemed as ‘supplemented’ if any dose of vitamin D was contained in the tablet.
UV exposure frequency
A self-administered questionnaire was sent to the subject via their mobile phones [16]. Questions included “On a typical day, how often are you exposed to sunlight for more than 15 minutes from 9 am to 3 pm? Please include the time spent exposed to sunlight under trees or under light clouds.—More than 5 days a week—3–4 days a week—1–2 days a week—rarely”, “How often, for leisure purposes only, are you exposed to sunlight for more than 15 minutes from 9 am to 3 pm? Please include the time spent exposed to sunlight under trees or under light clouds.—Almost weekly—2–3 times a month—once a month—rarely”, and “On a typical day, do you protect your hands and neck from UV rays?—Never expose bare skin under direct sunlight, even in winter—Often block UV rays with cream or sunshades in seasons with strong UV rays—Seldom protect against UV rays”.
Other factors
The following potential influencing factors were examined: age, pre-pregnancy BMI, pregnancy trimester, past history of allergic diseases (asthma, allergic rhinitis, and atopic dermatitis), skin type according to self reported reaction to UV light exposure, how important they think of body weight control during pregnancy, intentional avoidance of fish or eggs, employment in agriculture or fishery, frequency of night-shift working, smoking habit, family income and education level. Age and pre-pregnancy BMI were obtained from the physicians’ record, and others were obtained via a self-administered questionnaire[16–18].
Statistical analysis
Average 25(OH)D levels among groups were estimated using the linear mixed model analysis, with intra-individual variation by repeated measurements accounted for. For comparison of variables among more than two groups, p values were adjusted by Dunnett’s method.
A uni-variate model was applied for each factor, followed by multi-variate analysis incorporating all variables with p values of <0.1 in the univariate models. Backward elimination method was applied to construct the final model.
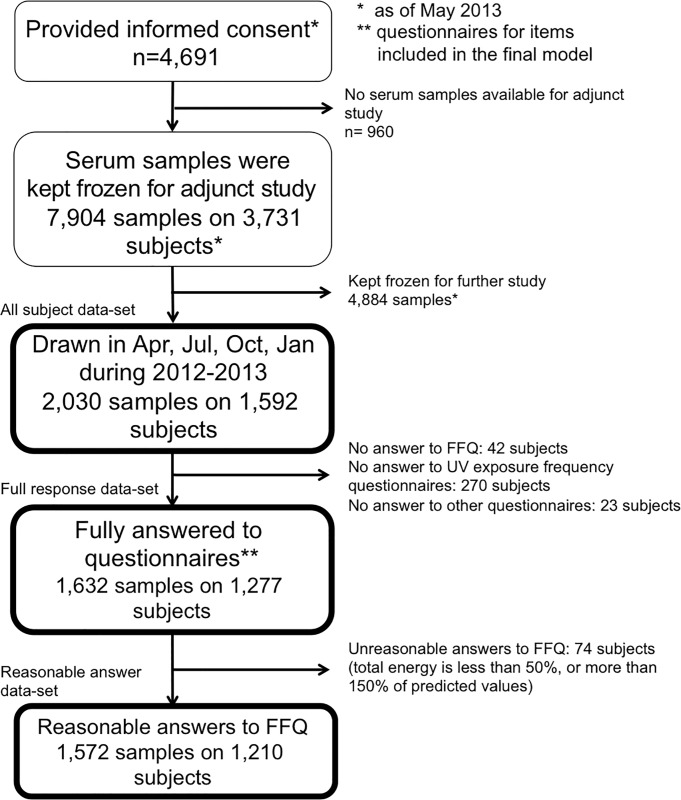
The entire cohort dataset (Fig 1) was used for analysis of seasonal 25(OH)D changes. The reasonable answer dataset, which excluded subjects with total calories on the FFQ of less than 50% and more than 150% of predicted values (Fig 1) was used for the analysis that include dietary intake of vitamin D, and the full answer dataset (Fig 1) was used for sensitivity analysis.
Fig 1. Serum samples analyzed in the sub-study of the Japan Environment and Children’s Study (JECS).
All analyses were performed using SAS software, version 9.3 (SAS Institute), and two-sided P<0.05 were considered statistically significant.
Results
Subjects
Of the 6,340 serum samples from 3,495 pregnant women who participated before May 2013 in the adjunct study, 2,030 samples which were collected in Apr, Jul, Oct, and Jan during 2012 to 2013 from 1,592 pregnant women, were included in this sub-study, as illustrated in Fig 1.
Table 1 summarizes the characteristics of the study cohort. All subjects were pregnant with an age range from teenagers to over 45 years, and various socioeconomic backgrounds. Subject characteristics were similar to those reported by the Japanese government in 2012, except that the proportion of current smokers was lower in the study cohort (1.6–2.7%) than that in the government report (12.8% for women in their 20s and 16.6% for women in their 30s) [25,26]. The proportion of subjects with an education level up to junior high school completion was lower (1.6–3.3%) compared with the government report (6.0%)[27,28]. Overall, the study cohort was considered to be a good representation of pregnant women in Japan.
Table 1. Characteristics of study cohort.
| Entire cohort dataset n = 1,592 |
Reasonable answer dataset n = 1,210 |
|
|---|---|---|
| Age (years) ab | 31.4±4.7 | 31.7±4.6 |
| Height (cm) ab | 158.4±5.3 | 158.6±5.3 |
| BMI before pregnancy ab | 20.8±3.2 | 20.9±2.8 |
| Dietary vitamin D intake (μg/day) ab | 5.5±2.8 | 5.3±2.8 c |
| Use of vitamin D supplements b | n (%) | n (%) |
| Yes | 79 (5.0) | 62 (5.1) |
| No | 1465 (92.0) | 1148 (94.9) |
| No response d | 48 (3.0) | 0 (0) |
| Agree with the importance of BW control | n (%) | n (%) |
| during pregnancy? b Totally agree | 828 (52.0) | 649 (53.6) |
| Agree | 631 (39.6) | 495 (40.9) |
| Cannot decide | 70 (4.4) | 55 (4.6) |
| Disagree | 10 (0.6) | 8 (0.8) |
| Totally disagree | 2 (0.1) | 2 (0.2) |
| No response d | 51 (3.2) | 1 (0.1) |
| Intentionally did not eat eggs | n (%) | n (%) |
| during pregnancy b Yes | 26 (1.6) | 20 (1.7) |
| No | 1518 (95.4) | 1190 (98.4) |
| No response d | 48 (3.0) | 0 (0) |
| Intentionally did not eat fish | n (%) | n (%) |
| during pregnancy b Yes | 36 (2.3) | 26 (2.2) |
| No | 1508 (94.7) | 1184 (97.9) |
| No response d | 48 (3.0) | 0 (0) |
| Usage of sunscreen on neck and hands | n (%) | n (%) |
| Always | 36 (2.3) | 33 (2.7) |
| Only in summer | 767 (48.2) | 706 (58.4) |
| Rarely | 466 (29.3) | 422 (34.9) |
| No response d | 323 (20.3) | 49 (4.1) |
| Frequency of UV exposure in daily life | n (%) | n (%) |
| Rarely | 239 (15.0) | 216 (17.9) |
| Once to twice a week | 343 (21.6) | 314 (26.0) |
| Three to four times a week | 333 (20.9) | 309 (25.5) |
| More than 5 times a week | 407 (25.6) | 371 (30.7) |
| No response d | 270 (17.0) | 0 (0) |
| Frequency of UV exposure at weekends | n (%) | n (%) |
| Rarely | 242 (15.2) | 218 (18.0) |
| Once a month | 57 (3.6) | 53 (4.4) |
| Twice to three times a month | 341 (21.4) | 315 (26.0) |
| Every weekend | 659 (41.4) | 601 (49.7) |
| No response d | 293 (18.4) | 23 (1.9) |
| Self-reported skin reaction to UV light | n (%) | n (%) |
| Burns easily, never tans | 109 (6.9) | 93 (7.7) |
| Burns easily, tans minimally with difficulty | 521 (32.7) | 472 (39.0) |
| Burns moderately, tans moderately | 465 (29.2) | 431 (35.6) |
| Burns minimally, tans moderately and easily | 122 (7.7) | 110 (9.1) |
| Rarely burns, tans profusely | 6 (0.4) | 5 (0.4) |
| No response d | 369 (23.2) | 99 (8.2) |
| History of allergic diseases | n (%) | n (%) |
| Asthma | 182 (11.4) | 143 (11.8) |
| Allergic rhinitis | 631 (39.6) | 504 (41.7) |
| Atopic dermatitis | 273 (17.2) | 222 (18.4) |
| Works in fishery or agriculture b | n (%) | n (%) |
| Yes | 6 (0.4) | 4 (0.3) |
| No | 1521 (95.5) | 1193 (98.6) |
| No response d | 65 (4.1) | 13 (1.1) |
| Night-shift b | n (%) | n (%) |
| Never | 1400 (87.9) | 1105 (91.3) |
| One to two times a month | 84 (5.3) | 61 (5.0) |
| Three to four times a week | 52 (3.3) | 40 (3.3) |
| More than five times a week | 5 (0.3) | 2 (0.2) |
| No response d | 51 (3.2) | 2 (0.2) |
| Smoking history b | n (%) | n (%) |
| Never smoked | 1009 (65.0) | 784 (64.8) |
| Stopped before pregnancy | 382 (24.0) | 299 (24.7) |
| Stopped during pregnancy | 138 (8.7) | 100 (8.3) |
| Current smoker | 37 (2.3) | 24 (2.0) |
| No response d | 26 (1.6) | 3 (0.3) |
| Partner’s smoking history b | n (%) | n (%) |
| Never smoked | 508 (31.9) | 394 (32.6) |
| Stopped before pregnancy | 409 (25.7) | 320 (26.5) |
| Stopped after pregnancy | 28 (1.8) | 17 (1.4) |
| Current smoker | 608 (38.2) | 467 (38.6) |
| No response d | 39 (2.5) | 12 (1.0) |
| Education level b | n (%) | n (%) |
| Junior high school | 42 (2.6) | 31 (2.6) |
| High school | 369 (23.2) | 268 (22.2) |
| Vocational school | 330 (20.7) | 227 (18.8) |
| College (2 years) | 311 (19.5) | 246 (20.3) |
| High vocational School | 21 (1.3) | 17 (1.4) |
| College (4 years) | 453 (28.5) | 382 (31.6) |
| Graduate school | 39 (2.5) | 36 (3.0) |
| No response d | 27 (1.7) | 3 (0.3) |
| Family annual income b | n (%) | n (%) |
| Under $20,000 | 51 (3.2) | 30 (2.5) |
| $20,000–40,000 | 433 (27.2) | 328 (27.1) |
| $40,000–60,000 | 530 (33.3) | 417 (34.5) |
| $60,000–80,000 | 271 (17.0) | 220 (18.2) |
| $80,000–100,000 | 111 (7.0) | 93 (7.7) |
| Above $100,000 | 82 (5.2) | 64 (5.3) |
| No response d | 114 (7.2) | 58 (4.8) |
a Values are expressed as the mean ± SD.
b Data from Japan Environment and Children's Study (JECS).
c Adjusted by total energy.
d No response corresponds to those who did not return the questionnaire.
Frequency of UV exposure and vitamin D status
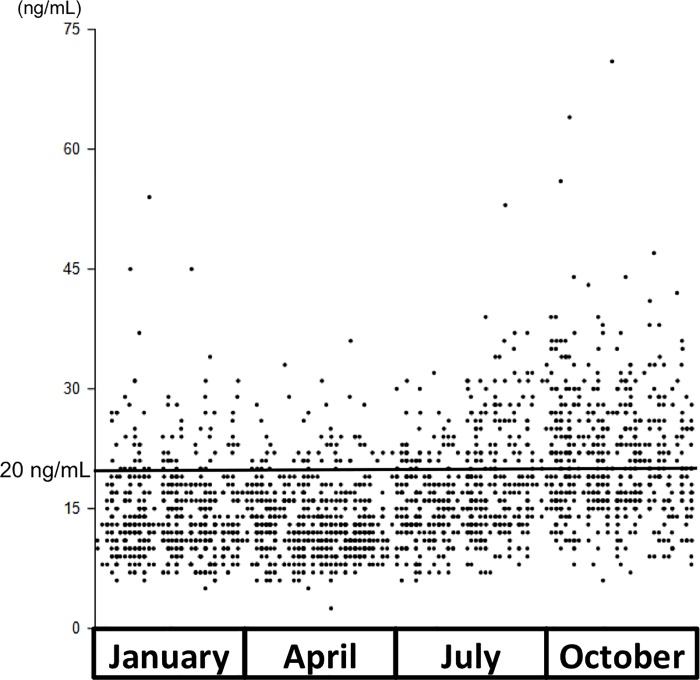
The mean serum 25(OH)D level was 16.7± 6.99 ng/mL (range: <5–71 ng/mL). Vitamin D deficiency, defined as less than 20ng/mL, was present in 73.2% (1,486 of 2,030 samples), and 10.8% (219 of 2,030 samples) had less than 10 ng/mL, which is defined as severe vitamin D deficiency. The distribution showed a clear seasonal change (Fig 2, Table 2), and 87.7% (880 of 1,003 samples) had less than 20ng/mL in winter and spring. This trend was observed even among women who reported sunlight exposure for at least 15 minutes on more than five days a week (Fig 3). However, at the end of summer (October), the mean 25(OH)D level of the group was much higher compared with that of subjects with least exposed to sunlight (Fig 3), and 61.5% (88 of 143 samples) of subjects who reported sunlight exposure for at least 15 minutes on more than five days a week achieved 20 ng/mL, while 34.6% (27 of 78 samples) of subjects with least exposed to sunlight achieved 20 ng/mL.
Fig 2. Seasonal changes in serum 25(OH)D levels.
Table 2. Serum 25(OH) levels (least square means; LS means and 95% confidence intervals; 95%CI) in univariate models.
| LS means | 95% CI | Adjusted P value | ||||
|---|---|---|---|---|---|---|
| Season | ||||||
| Winter (Jan) | 13.5 | 13.1 | − | 13.9 | ref | |
| Spring (Apr) | 12.7 | 12.3 | − | 13.1 | .006 | |
| Summer (Jul) | 16.3 | 15.9 | − | 16.8 | < .0001 | |
| Autumn (Oct) | 20.2 | 19.6 | − | 20.7 | < .0001 | |
| Residential location | ||||||
| Kyoto (N35) | 15.1 | 14.6 | − | 15.6 | ref | |
| Toyama (N36) | 16.1 | 15.7 | − | 16.5 | .008 | |
| Tottori (N35.5) | 14.4 | 13.8 | − | 15.0 | .105 | |
| Frequency of UV exposure in daily life | < .0001 a | |||||
| Rarely | 13.8 | 13.2 | − | 14.4 | ref | |
| Once a week | 14.8 | 14.2 | − | 15.4 | .062 | |
| Two to three times a week | 15.5 | 14.9 | − | 16.1 | .001 | |
| More than four times a week | 16.8 | 16.2 | − | 17.4 | < .0001 | |
| Frequency of UV exposure at weekends | < .0001 a | |||||
| Rarely | 14.0 | 13.3 | − | 14.6 | ref | |
| Once a month | 15.7 | 14.3 | − | 17.3 | .082 | |
| Two to three times a month | 15.1 | 14.5 | − | 15.7 | .038 | |
| Every week | 16.0 | 15.6 | − | 16.5 | < .0001 | |
| Usage of sunscreen on neck and hands | .188 a | |||||
| Always | 13.9 | 12.3 | − | 15.8 | ref | |
| Only in summer | 15.3 | 14.9 | − | 15.7 | .186 | |
| Rarely | 15.6 | 15.1 | − | 16.1 | .106 | |
| Self-reported skin reaction to UV exposure | .293a | |||||
| Burns easily, never tans | 14.5 | 13.5 | − | 15.6 | .203 | |
| Burns easily, tans minimally with difficulty | 15.7 | 15.2 | − | 16.2 | ref | |
| Burns moderately, tans moderately | 15.2 | 14.7 | − | 15.7 | .996 | |
| Burns minimally, tans moderately and easily | 15.8 | 14.8 | − | 16.9 | .999 | |
| Rarely burns, tans profusely | 15.9 | 11.9 | − | 21.2 | .057 | |
| Dietary vitamin D intake bb | < .0001 a | |||||
| 1st quartile (–3.8μg/day) | 14.6 | 14.0 | − | 15.1 | ref | |
| 2nd quartile (3.8–5.2μg/day) | 15.0 | 14.5 | − | 15.6 | .486 | |
| 3rd quartile (5.2–6.9μg/day) | 15.7 | 15.1 | − | 16.3 | .019 | |
| 4th quartile (6.9 –μg/day) | 17.0 | 16.4 | − | 17.6 | < .0001 | |
| Use of vitamin D supplements | ||||||
| No | 15.3 | 15.0 | − | 15.6 | ref | |
| Yes | 19.4 | 17.9 | − | 21.0 | < .0001 | |
| Pregnant trimester | ||||||
| 1st trimester | 14.5 | 14.0 | − | 15.1 | < .0001 | |
| 2nd trimester | 16.2 | 15.8 | − | 16.7 | ref | |
| At delivery | 15.0 | 14.6 | − | 15.4 | < .0001 | |
| Age | .032 a | |||||
| <25 years | 14.1 | 13.1 | − | 15.1 | .032 | |
| 25–35 years | 15.4 | 15.1 | − | 16.2 | ref | |
| >35 years | 15.7 | 15.1 | − | 15.8 | .759 | |
| BMI before pregnancy | .519 | |||||
| <18.5 | 15.1 | 14.4 | − | 15.7 | .439 | |
| 18.5–25 | 15.5 | 15.2 | − | 15.8 | ref | |
| >25 | 15.4 | 14.5 | − | 16.4 | .974 | |
| History of asthma | ||||||
| No | 15.4 | 15.1 | − | 15.7 | ref | |
| Yes | 15.4 | 14.6 | − | 16.3 | .974 | |
| History of allergic rhinitis | ||||||
| No | 15.4 | 15.1 | − | 15.8 | ref | |
| Yes | 15.4 | 14.9 | − | 15.8 | .860 | |
| History of atopic dermatitis | ||||||
| No | 15.4 | 15.1 | − | 15.7 | ref | |
| Yes | 15.4 | 14.7 | − | 16.1 | .905 | |
| Works in fishery or agriculture | ||||||
| Yes | 18.9 | 14.0 | − | 25.5 | .198 | |
| No | 15.5 | 15.2 | − | 15.8 | ref | |
| Night-shift | .428 a | |||||
| Never | 15.6 | 15.3 | − | 15.9 | .514 | |
| One to three times a month | 14.8 | 13.7 | − | 16.0 | ref | |
| One to four times a week | 14.7 | 13.3 | − | 16.3 | .999 | |
| More than five times a week | 17.1 | 12.1 | − | 24.1 | .782 | |
| Family income | .1194 a | |||||
| Under $20,000 | 15.0 | 13.7 | − | 16.5 | .9996 | |
| $20,000–40,000 | 15.2 | 14.7 | − | 15.7 | ref | |
| $40,000–60,000 | 15.7 | 15.3 | − | 16.2 | .5378 | |
| $60,000–80,000 | 15.6 | 15.0 | − | 16.3 | .8372 | |
| $80,000–100,000 | 15.6 | 14.6 | − | 16.6 | .9588 | |
| Above $100,000 | 16.0 | 14.9 | − | 17.2 | .6913 | |
| Smoking status | .015 a | |||||
| Never smoked | 15.6 | 15.3 | − | 16.0 | ref | |
| Stopped before pregnancy | 15.6 | 15.1 | − | 16.2 | 1.00 | |
| Stopped during pregnancy | 14.3 | 13.4 | − | 15.2 | .018 | |
| Current smoker | 14.0 | 12.5 | − | 15.8 | .204 | |
| Smoking status of subjects’ partners | .019 a | |||||
| Never smoked | 15.7 | 15.2 | − | 16.2 | ref | |
| Stopped before pregnancy | 15.8 | 15.3 | − | 16.4 | .971 | |
| Stopped after pregnancy | 17.0 | 14.8 | − | 19.6 | .567 | |
| Current smoker | 14.9 | 14.5 | − | 15.4 | .068 | |
Entire cohort dataset (excluding non-responders for each question).
All values are from univariate linear mixed models, with 25(OH)D natural log-transformed.
LS means and 95%CIs are shown as exponentials of log-transformed 25(OH)D.
For variables with more than three groups, p values are adjusted by Dunnett.
a P values for trend.
b Reasonable answer dataset, total energy adjusted by residual method.
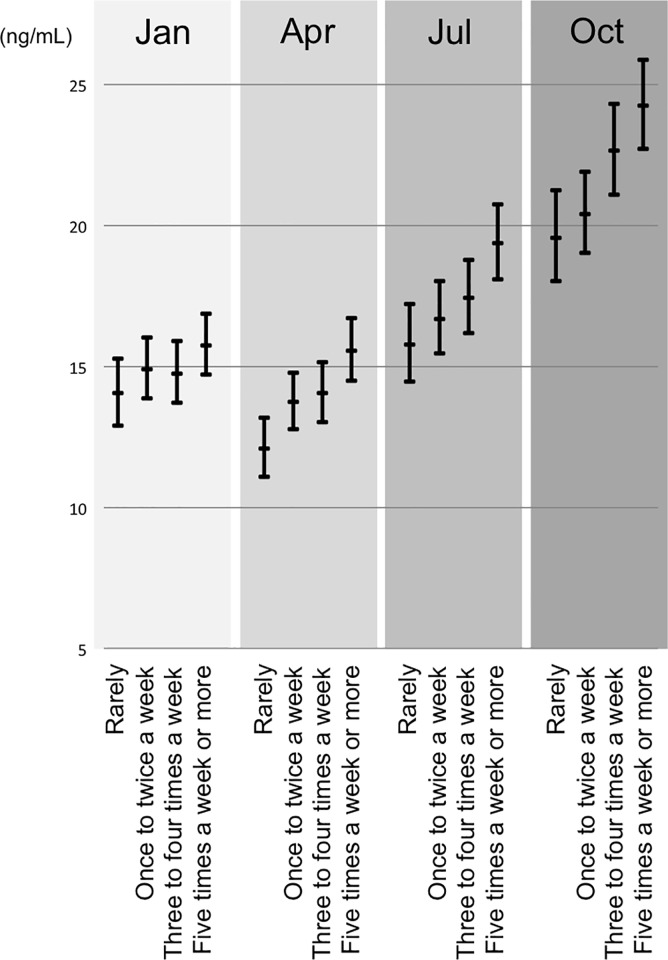
Fig 3. Serum 25(OH)D levels during each month in relation to the frequency of sunlight exposure.
Linear mixed model with random effect of repeated measurements. Adjusted by location, dietary vitamin D intake, dietary calorie intake, vitamin D supplementation, pregnancy trimester, and if they live with children.
Furthermore, vitamin D levels were evaluated in subjects employed in agriculture/fishery, who work outside during their daily lives. As expected, this group showed higher 25(OH)D levels, especially in autumn (LS mean 33.6 ng/mL, 95%CI; 17.8–63.6), although the number of subjects employed in agriculture/fishery was very few (7 samples on 6 subjects) and a statistical significance was not achieved.
Unexpectedly, the subjects whose self-reported skin type was fair tended to have a lower 25(OH)D levels than other skin types in this relatively homogenous population (Table 2).
An increase in the frequency of sunlight exposure to at least 15 minutes for 1 to 2 days per week resulted in elevations in 25(OH)D levels of approximately 1 ng/mL in non-winter and 0.5 ng/mL in winter (Fig 3).
Vitamin D status and dietary vitamin D intake
The mean dietary intake of vitamin D (energy-adjusted) in Japanese pregnant women was estimated to be 5.5±2.8 μg/day. As few as 22.6% of subjects consumed above 7.0 μg/day, described as the “adequate intake” per day for Japanese pregnant women[29]. Nobody exceeded 100μg/day, described as the “tolerable upper intake” per day in the guideline.
The amount of dietary vitamin D intake was significantly associated with the 25(OH)D level (Tables 2 and 3, Fig 4), and 198 (90.8%) of 218 samples from subjects with above 7.0 μg/day (energy-adjusted) had above 10 ng/mL even in winter and spring, although the majority (174/218, 79.8%) of these subjects did not achieve 20 ng/mL. The average daily intake of vitamin D in women with above 20 ng/mL of 25(OH)D in winter and spring was 6.5μg/day.
Table 3. Serum 25(OH) levels (least square means; LS means and 95% confidence intervals; 95%CI) in multivariate models.
| LS means | 95%CI | p value | |||
|---|---|---|---|---|---|
| Residential location | < .001 | ||||
| Kyoto (N35) | 16.4 | 15.6 | - | 17.2 | ref |
| Toyama (N36) | 17.7 | 16.8 | - | 18.4 | .002 |
| Tottori (N35.5) | 16.0 | 15.1 | - | 16.9 | .570 |
| Season | < .001 | ||||
| Winter (Jan) | 14.8 | 14.1 | - | 15.5 | ref |
| Spring (Apr) | 13.8 | 13.2 | - | 14.6 | .009 |
| Summer (Jul) | 17.3 | 16.4 | - | 18.2 | < .0001 |
| Autumn (Oct) | 21.7 | 20.6 | - | 22.7 | < .0001 |
| Pregnancy trimester | < .0001 | ||||
| 1st trimester | 16.1 | 15.3 | - | 17.0 | < .0001 |
| 2nd trimester | 17.7 | 17.0 | - | 18.6 | Ref |
| At delivery | 16.1 | 15.4 | - | 16.8 | < .0001 |
| Use of vitamin D supplements | |||||
| No | 14.7 | 14.4 | - | 15.0 | Ref |
| Yes | 19.0 | 17.5 | - | 20.5 | < .0001 |
| Dietary vitamin D intake (μg/day) a | < .0001 | ||||
| 1st quartile (– 3.8μg/day) | 15.4 | 14.6 | - | 16.3 | ref |
| 2nd quartile (3.8–5.2μg/day) | 16.4 | 15.5 | - | 17.3 | .052 |
| 3rd quartile (5.2–6.9μg/day) | 16.7 | 15.9 | - | 17.6 | .006 |
| 4th quartile (6.9 – μg/day) | 18.1 | 17.2 | - | 19.1 | < .0001 |
| Frequency of UV exposure in daily life | < .0001 | ||||
| Rarely | 15.1 | 14.3 | - | 16.0 | ref |
| Once a week | 16.2 | 15.4 | - | 17.1 | .017 |
| Two to three times a week | 16.9 | 16.1 | - | 17.9 | .0001 |
| More than four times a week | 18.4 | 17.5 | - | 19.4 | < .0001 |
Reasonable answer dataset
Multivariate linear mixed models, with 25(OH)D natural log-transformed.
LS means and 95%CIs are shown as exponentials of natural log-transformed 25(OH)D.
For variables with more than three groups, the p values are adjusted by Dunnett.
a Adjusted by total energy by residual method.
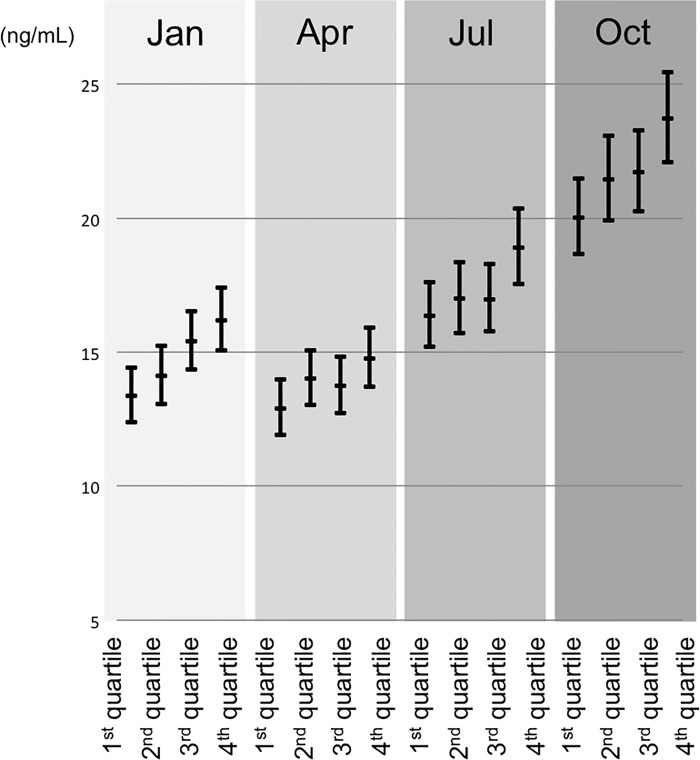
Fig 4. Serum 25(OH)D levels during each month in relation to the daily dietary intake of vitamin D.
Linear mixed model with random effect of repeated measurements. Adjusted by location, frequency of sunlight exposure, dietary calorie intake, vitamin D supplementation, pregnancy trimester, and if they live with children. Average of 1st quartile was 2.5μg/day, and average of 4th quartile was 9.4μg/day.
An increase of 1μg/day dietary vitamin D intake led to an elevation of approximately 0.5 ng/mL in serum 25(OH)D (Fig 4).
Other factors associated with 25(OH)D levels
In the univariate linear mixed models with random effect of repeated measurements, the following factors were found to be significantly associated with 25(OH)D level (Table 2); residential location, frequency of UV exposure in daily life, frequency of UV exposure at weekends, month of blood sample collection, pregnancy trimester of blood sample, use of vitamin D supplements, dietary intake of vitamin D, dietary calorie intake, living with children, and smoking habits of subjects and their partners.
In the multivariate model incorporating all these variables, the following were consecutively excluded; frequency of UV exposure at weekends, age, smoking of partner, and smoking. The final model included the following factors; month of blood collection, residential location, pregnancy trimester of blood collection, use of vitamin D supplements, frequency of UV exposure in daily life, and dietary intake of vitamin D (Table 3).
Vitamin D supplementation, multivitamin tablets or calcium tablets, was reported by very few pregnant women (5.1%). Most tablets contained 2.5–5.0 μg/day of vitamin D, and 15μg/day at most (one case). However, their serum increase of 25(OH)D level were as much as 4.5 ng/mL (Table 3).
Contrary to previous reports from other countries, a univariate model showed that age was negatively associated with 25(OH)D levels in Japanese pregnant women (Table 2). This tendency was lost in the multivariate model or a model including covariates of dietary intake of vitamin D, UV exposure frequency, and living with children. Therefore it is suggested that this was the consequence of confounding effects of less exposure to sunlight and lower dietary consumption of vitamin D among younger populations in Japan[9,30].
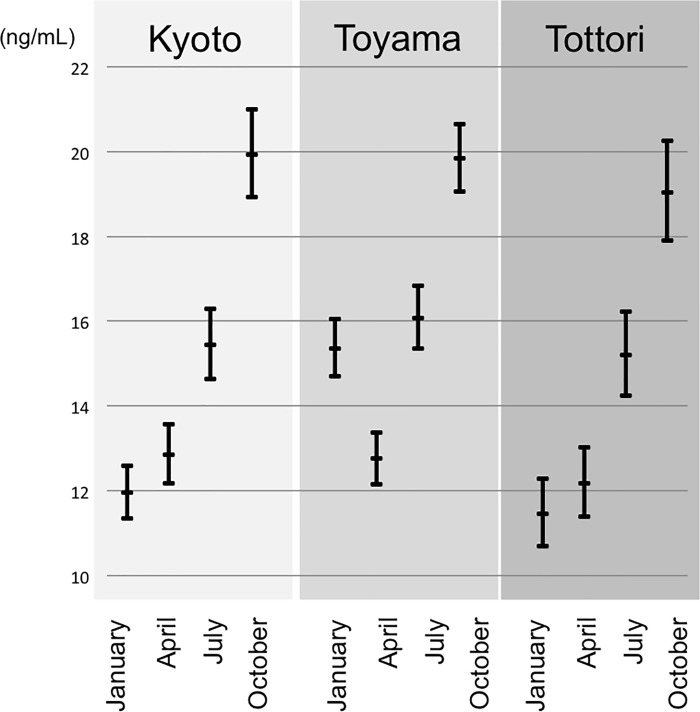
Unexpectedly, even after the adjustment for lifestyle and dietary variables, pregnant women living in Toyama, the northern-most of the three locations, exhibited significantly higher 25(OH)D levels than in the other two locations (Table 3). By examining other characteristics, it was found that only samples taken in winter (January) in Toyama showed higher 25(OH)D levels than in the other two locations (Fig 5). Because Toyama is famous for cold yellowtail and other seafood products in winter, these women may have had a fish-rich diet in winter, although this was not sufficiently reflected in the answers to the questionnaire on “typical” diet. Furthermore, Toyama had higher snowfall than the other two locations in January 2013 (Toyama: 4cm/day, Tottori: 0.8cm/day, Kyoto: 0cm/day) and some sunlight hours (Toyama: 79 hours /month, Tottori 73 hours /month, Kyoto: 124 hours /month). Reflection of UV rays from snow on the ground may have increased UV exposures. This may be a strategy for maintaining adequate serum 25(OH)D levels for people living in northern, snowy areas, and should be confirmed by further studies.
Fig 5. Serum 25(OH)D levels in Kyoto, Toyama, and Tottori in relation to the sampled months.
Linear mixed model with random effect of repeated measurements. Adjusted by location, frequency of sunlight exposure, dietary vitamin D intake, dietary calorie intake, vitamin D supplementation, pregnancy trimester, and if they live with children.
Discussion
In this study, we showed that Japanese pregnant women are in severe vitamin D deficiency status (10.8% are <10 ng/mL, 73.2% are <20 ng/mL). This was expected from their lifestyles, and consistent with previous urban Japanese studies and other recent Asian studies in which it is reported that lighter skin tones are culturally preferred [14,15,31–33]. The thresholds for 25(OH)D levels (10 ng/mL for severe deficiency, and 20 ng/mL for deficiency) were derived from non-pregnant populations and an optimal serum level during pregnancy has not been established. However, it is at least suggested that Japanese pregnant women have lower vitamin D levels compared with a century ago, at which time the majority of the populations engaged in agriculture or fishery, spending many hours outside every day; among subjects who reported themselves being exposed to sunlight at least 15 minutes on more than 5 days a week without UV protection in neck and hands and consumed more than 7.0 μg/day of dietary vitamin D, 49 of 50 subjects (98.0%) showed 25(OH)D above 10 ng/mL with the mean 25(OH)D level of 20.2 ng/mL throughout the year. Because vitamin D has functions in various organs, deficiency can cause or contribute to a variety of diseases [34]. The association between vitamin D deficiency and specific morbidities, especially diseases that is increasing in these decades, should be further investigated.
In this study, the average daily vitamin D intake among women with 25(OH)D levels above 20 ng/mL in winter and spring was 6.3 μg/day, which is similar to reports in Norwegian pregnant women (7.0 μg/day) [35]. Although the Japanese Guideline for Nutrition suggest 7.0 μg/day, and this appears reasonable for Japanese pregnant women based on the results of our study, it is also important to note that 25(OH)D levels above 20 ng/mL were not achieved only by diet for majority of the women in winter and spring. This leads to the proposal that dietary intake of 7.0 μg/day is necessary, but not sufficient to maintain adequate 25(OH)D levels at least 20 ng/mL in Japan.
Although vitamin D supplementation was reported by few pregnant women (5.1%) and most consumed only 100 to 200 IU/day of vitamin D in our study, the serum 25(OH)D levels increased by as much as 4 ng/mL in supplemented women. This figure is consistent with the previous report that showed 100IU of vitamin D increased the 25(OH)D level by 2 to 3 ng/mL in subjects with serum 25(OH)D levels of less than 15 ng/mL[34].
Darker skin is generally a risk factor for a low level of 25(OH)D[34]. However, in our study, comprising subjects of almost uniform ethnicity, the self-reported fair-colored skin had a tendency toward lower 25(OH)D levels. This subpopulation may have avoided sunlight to an extreme due to fear of skin cancer. Among Caucasians, especially those who emigrated to a low latitude area, UV exposure is a definite risk factor for skin cancer development. However, skin cancer mortality is very low in Japanese living in Japan, even in those who were children in the era without UV protection. The skin cancer mortality is 1.2 / 100 thousand Japanese women, while colon cancer mortality is 36.5 / 100 thousand Japanese women in 2017 [36]. The natural skin tone may be adapted to the sunlight in Japan as an evolutionary feature related to island dwelling. Skin production of vitamin D is thought to be accomplished after exposure to moderate sunlight for several (in summer) to several ten (in winter) minutes without causing burns [3,34]. National Institute for Environmental Studies comments that getting exposure to UV ray for several (in summer) to several ten (in winter) minutes in Japan that will never reach 1 MED (Minimal Erythema Dose) for people with skin photo type III (Japanese people), will lead vitamin D synthesis while minimizing its harms [37]. And it provides in real-time the amount of vitamin D synthesized in the body at some locations in Japan on the web [38], based on the logics described by Miyauchi and Nakajima[39]. Individuals should also be informed of the benefits of UV rays when alerted about its risk, with the available information shown above.
Major strengths of this study were a relatively large sample size based on a large population-based birth cohort (from the JECS), and a high response rate for various background questionnaires including both dietary intake of vitamin D and frequency of sunlight exposure, which will contribute to high generalizability. Despite the strengths, this study has some limitations. FFQ and frequency of sunlight exposure were both self-reported, and there may be some mis-categorizations. A uniform questionnaire was used for sunlight exposure throughout a year, which may not be a meaningful measure of differences in sunlight exposure in winter, when it is estimated that at least several ten minutes of sunlight is necessary for vitamin D production in skin, whereas the questionnaire asked the frequency of “at least 15 minute exposure /day” per week. Finally, the present results are applicable to only Japanese pregnant women, as it is known that 25(OH)D levels differ between ethnicities[34].
In conclusion, vitamin D deficiency is very severe in Japanese pregnant women, and lifestyle factors including the frequency of sunlight exposure and dietary intake of vitamin D have a clinically relevant impact on serum levels. This suggests that vitamin D level may be enhanced by changes in lifestyle. Pregnant women should be informed of both the risks and benefits of UV ray. Further investigations are required to establish the impact of vitamin D deficiency on morbidities.
Acknowledgments
Parts of the manuscript were presented as a poster at the European Respiratory Congress 2015.
Japan Environment and Children’s Study;
Kyoto Regional Centre: Ikuo Konishi, Toshio Heike, Takeo Nakayama, Yukuo Konishi, Tohshin Go, Keiko Sato, Miho Egawa, Yoshimitsu Takahashi, Motonobu Watanabe, Reiko Yasumi, Kyoko Hirabayashi, Kaori Konishi, Kumiko Kanatani, Research Coordinators, and members of the JECS Kyoto Management Committee
Toyama Regional Centre: Hidekuni Inadera, Kei Hamazaki, Yuichi Adachi, Akiko Tsuchida, and Research Coordinators
Tottori Regional Centre: Soji Fukumoto, Ryota Teshima, Takao Inoue, Youichi Kurozawa, Tasuku Harada, Susumu Kanzaki, Yoshihiro Maegaki, Kosaku Ohno, Tatsuya Koeda, Kazunari Onishi, and Research Coordinators.
The authors would like to thank all participants of the study who contributed to a very high response rate, and personnel at the hospitals and clinics that collaborated in the JECS study.
Lead author for the group: Takeo Nakayama
nakayama.takeo.4a@kyoto-u.ac.jp
Kumiko T Kanatani confirmed that written permission has been obtained from each person named in the Acknowledgment.
Kumiko T Kanatani had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data availability: Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Epidemiological Research enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Program Office, National Institute for Environmental Studies.
Data Availability
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Epidemiological Research enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Program Office, National Institute for Environmental Studies.
Funding Statement
This work was supported by the Environment Research and Technology Development Fund from the Ministry of the Environment, Japan [5C-1152(2011- 2013) 5-1555(2015-2017)], and JSPS KAKENHI Grant Number 25350044 (2013- 2015), and was conducted as an adjunct study of Japan Environment and Children's Study (JECS). The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Japanese government.
References
- 1.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. The American journal of clinical nutrition. 2008;87(4):1080s–6s. Epub 2008/04/11. 10.1093/ajcn/87.4.1080S . [DOI] [PubMed] [Google Scholar]
- 2.Baggerly CA, Cuomo RE, French CB, Garland CF, Gorham ED, Grant WB, et al. Sunlight and Vitamin D: Necessary for Public Health. Journal of the American College of Nutrition. 2015;34(4):359–65. Epub 2015/06/23. 10.1080/07315724.2015.1039866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. The New England journal of medicine. 2007;357(3):266–81. Epub 2007/07/20. 10.1056/NEJMra070553 . [DOI] [PubMed] [Google Scholar]
- 4.Barebring L, Bullarbo M, Glantz A, Leu Agelii M, Jagner A, Ellis J, et al. Preeclampsia and Blood Pressure Trajectory during Pregnancy in Relation to Vitamin D Status. PloS one. 2016;11(3):e0152198 Epub 2016/03/31. 10.1371/journal.pone.0152198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakhtoura M, Nassar A, Arabi A, Cooper C, Harvey N, Mahfoud Z, et al. Effect of vitamin D replacement on maternal and neonatal outcomes: a randomised controlled trial in pregnant women with hypovitaminosis D. A protocol. BMJ open. 2016;6(3):e010818 Epub 2016/03/10. 10.1136/bmjopen-2015-010818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossein-nezhad A, Holick MF. Optimize dietary intake of vitamin D: an epigenetic perspective. Current opinion in clinical nutrition and metabolic care. 2012;15(6):567–79. Epub 2012/10/19. 10.1097/MCO.0b013e3283594978 . [DOI] [PubMed] [Google Scholar]
- 7.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatric and perinatal epidemiology. 2012;26 Suppl 1:75–90. Epub 2012/07/07. 10.1111/j.1365-3016.2012.01283.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka K, Hitsumoto S, Miyake Y, Okubo H, Sasaki S, Miyatake N, et al. Higher vitamin D intake during pregnancy is associated with reduced risk of dental caries in young Japanese children. Annals of epidemiology. 2015;25(8):620–5. Epub 2015/05/10. 10.1016/j.annepidem.2015.03.020 . [DOI] [PubMed] [Google Scholar]
- 9.Hamazaki K, Natori T, Kurihara S, Murata N, Cui ZG, Kigawa M, et al. Fish consumption and depressive symptoms in undergraduate students: A cross-sectional analysis. European psychiatry : the journal of the Association of European Psychiatrists. 2015;30(8):983–7. Epub 2015/10/27. 10.1016/j.eurpsy.2015.09.010 . [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health LaW. National Health and Nutrition Survey 2003 [cited 2016 Apr. 20]. Available from: http://www.mhlw.go.jp/bunya/kenkou/eiyou-chosa2-01/index.html,.
- 11.Ministry of Health LaW. National Health and Nutrition Survey. 2012.
- 12.Otsuka R, Yatsuya H, Tamakoshi K. Descriptive epidemiological study of food intake among Japanese adults: analyses by age, time and birth cohort model. BMC public health. 2014;14:328 Epub 2014/04/10. 10.1186/1471-2458-14-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi F, Takimoto H, Yoshita K, Yoshiike N. Perceived body size and desire for thinness of young Japanese women: a population-based survey. The British journal of nutrition. 2006;96(6):1154–62. Epub 2006/12/22. . [DOI] [PubMed] [Google Scholar]
- 14.Shibata M, Suzuki A, Sekiya T, Sekiguchi S, Asano S, Udagawa Y, et al. High prevalence of hypovitaminosis D in pregnant Japanese women with threatened premature delivery. Journal of bone and mineral metabolism. 2011;29(5):615–20. Epub 2011/03/09. 10.1007/s00774-011-0264-x . [DOI] [PubMed] [Google Scholar]
- 15.Shiraishi M, Haruna M, Matsuzaki M, Murayama R. Demographic and lifestyle factors associated with vitamin D status in pregnant Japanese women. Journal of nutritional science and vitaminology. 2014;60(6):420–8. Epub 2014/01/01. 10.3177/jnsv.60.420 . [DOI] [PubMed] [Google Scholar]
- 16.Kanatani KT, Adachi Y, Sugimoto N, Noma H, Onishi K, Hamazaki K, et al. Birth cohort study on the effects of desert dust exposure on children's health: protocol of an adjunct study of the Japan Environment & Children's Study. BMJ open. 2014;4(6):e004863 Epub 2014/06/25. 10.1136/bmjopen-2014-004863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and children's study (JECS). BMC public health. 2014;14:25 Epub 2014/01/15. 10.1186/1471-2458-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nitta H. Outline of the Japan Environment and Children's Study and the Framework of Genome Analysis. Nihon eiseigaku zasshi Japanese journal of hygiene. 2016;71(1):91–3. Epub 2016/02/03. 10.1265/jjh.71.91 . [DOI] [PubMed] [Google Scholar]
- 19.Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL. Determination of vitamin D status by radioimmunoassay with an 125I-labeled tracer. Clinical chemistry. 1993;39(3):529–33. Epub 1993/03/01. . [PubMed] [Google Scholar]
- 20.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O'Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. Jama. 2016;315(4):362–70. Epub 2016/01/28. 10.1001/jama.2015.18589 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishihara J, Sobue T, Yamamoto S, Yoshimi I, Sasaki S, Kobayashi M, et al. Validity and reproducibility of a self-administered food frequency questionnaire in the JPHC Study Cohort II: study design, participant profile and results in comparison with Cohort I. Journal of epidemiology / Japan Epidemiological Association. 2003;13(1 Suppl):S134–47. Epub 2003/04/19. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa K, Tsubono Y, Nishino Y, Watanabe Y, Ohkubo T, Watanabe T, et al. Validation of a food-frequency questionnaire for cohort studies in rural Japan. Public health nutrition. 2003;6(2):147–57. Epub 2003/04/05. 10.1079/PHN2002411 . [DOI] [PubMed] [Google Scholar]
- 23.Wakai K. A review of food frequency questionnaires developed and validated in Japan. Journal of epidemiology / Japan Epidemiological Association. 2009;19(1):1–11. Epub 2009/01/24. 10.2188/jea.JE20081007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki S, Takahashi T, Iitoi Y, Iwase Y, Kobayashi M, Ishihara J, et al. Food and nutrient intakes assessed with dietary records for the validation study of a self-administered food frequency questionnaire in JPHC Study Cohort I. Journal of epidemiology / Japan Epidemiological Association. 2003;13(1 Suppl):S23–50. Epub 2003/04/19. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Communications MoIAa. Japan Statistical Yearbook 2013. First Editorial Section, Statistical Library, Statistical Research and Training Institute; 2013. [cited 2016 Apr. 19]. Available from: http://www.stat.go.jp/data/nihon/18.htm. [Google Scholar]
- 26.Ministry of Health LaW. The national health and nutrition survey in Japan, 2011. 2011 [cited 2016 Apr.19]. Available from: http://www.mhlw.go.jp/bunya/kenkou/eiyou/dl/h24-houkoku-06.pdf.
- 27.School Health Survey. Available from: http://www.e-stat.go.jp/SG1/estat/eStatTopPortalE.do.
- 28.Statistics Bureau MoIA, and Communications. The Population and Households of Japan 2010. Available from: http://www.stat.go.jp/data/kokusei/2010/pdf/waga3.pdf.
- 29.Ministry of Health LaW. Overview of Dietary Reference Intakes for Japanese [cited 2016 May 1st]. Available from: http://www.mhlw.go.jp/bunya/kenkou/syokuji_kijyun.html, http://www.mhlw.go.jp/stf/seisakunitsuite/bunya/0000061795.html.
- 30.Hasegawa A, Usui C, Kawano H, Sakamoto S, Higuchi M. Characteristics of body composition and resting energy expenditure in lean young women. Journal of nutritional science and vitaminology. 2011;57(1):74–9. Epub 2011/04/23. . [DOI] [PubMed] [Google Scholar]
- 31.Choi R, Kim S, Yoo H, Cho YY, Kim SW, Chung JH, et al. High prevalence of vitamin D deficiency in pregnant Korean women: the first trimester and the winter season as risk factors for vitamin D deficiency. Nutrients. 2015;7(5):3427–48. Epub 2015/05/15. 10.3390/nu7053427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao JP, Zang J, Pei JJ, Xu F, Zhu Y, Liao XP. Low maternal vitamin D status during the second trimester of pregnancy: a cross-sectional study in Wuxi, China. PloS one. 2015;10(2):e0117748 Epub 2015/02/07. 10.1371/journal.pone.0117748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratumvinit B, Wongkrajang P, Wataganara T, Hanyongyuth S, Nimmannit A, Chatsiricharoenkul S, et al. Maternal Vitamin D Status and Its Related Factors in Pregnant Women in Bangkok, Thailand. PloS one. 2015;10(7):e0131126 Epub 2015/07/07. 10.1371/journal.pone.0131126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–55. 10.1016/j.mayocp.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henriksen C, Brunvand L, Stoltenberg C, Trygg K, Haug E, Pedersen JI. Diet and vitamin D status among pregnant Pakistani women in Oslo. European journal of clinical nutrition. 1995;49(3):211–8. Epub 1995/03/01. . [PubMed] [Google Scholar]
- 36.Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan. [cited 2019 Feb 4th]. Available from: https://ganjoho.jp/reg_stat/statistics/dl/index.html#mortality
- 37.Information on Vitamin D synsthesis/Erythermal UV. National Institute for Environmental Studies. [cited 2019 Feb 4th]. Available from: http://db.cger.nies.go.jp/dataset/uv_vitaminD/en/remarks.html
- 38.Information on Vitamin D synsthesis/Erythermal UV. National Institute for Environmental Studies. [cited 2019 Feb 4th]. Available from: http://db.cger.nies.go.jp/dataset/uv_vitaminD/en/radiation.html
- 39.Miyauchi M. and Nakajima H. Determining an effective UV radiation exposure time for vitamin D synthesis in the skin without risk to health: Simplified estimations from UV observation. Photochem. Photobiol. 2016;92:863–9. 10.1111/php.12651 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Epidemiological Research enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr Shoji F. Nakayama, JECS Program Office, National Institute for Environmental Studies.