Abstract
Inflammation plays a crucial role in the pathogenesis of cancer with tumor necrosis factor‐α (TNF‐α) as a key mediator. Recently, spermatogenesis‐associated protein 2 (SPATA2) was identified as a TNF receptor modulator which is required for TNF‐induced inflammation and apoptosis. The available data on TNF‐α in ovarian cancer (OC) are inconsistent, and SPATA2 is completely uncharacterized in tumorigenesis. We analyzed expression of SPATA2 and TNFA by quantitative real‐time polymerase chain reaction in tissues of 171 patients with low‐grade serous (LGSOC), high‐grade serous (HGSOC), endometrioid and clear cell OC compared with 28 non‐malignant control tissues. We stimulated OC cells (OVCAR3) with pro‐inflammatory (TNF‐α, interleukin [IL]‐1β) and mitogenic stimuli (IL‐6, lysophosphatidic acid) to establish a direct effect between inflammatory signaling and SPATA2. Pro‐inflammatory, but not mitogenic stimuli, potently induced SPATA2 expression in OC cells. Expression of TNFA and SPATA2 was higher in OC compared with control tissues (P = 0.010 and P = 0.001, respectively) and correlated with each other (P = 0.034, r s = 0.198). When compared with grade 1 cancers, SPATA2 was expressed higher in grade 2 and 3 tumors (P = 0.011) as well as in HGSOC compared with LGSOC (P = 0.024). Multivariate survival analyses revealed that OC with high SPATA2 expression were associated with reduced progression‐free survival (P = 0.048) and overall survival (P < 0.001). In conclusion, SPATA2 expression is regulated by TNF‐α and IL‐1β and is found to independently affect clinical outcome in OC patients. These data implicate a role of SPATA2 in tumorigenesis which warrants further investigation in gynecological malignancies.
Keywords: inflammation, ovarian cancer, spermatogenesis‐associated protein 2, tumor necrosis factor‐α, tumorigenesis
Abbreviations
- CI
confidence interval
- CYLD
cylindromatosis
- FIGO
Fédération Internationale de Gynécologie et d'Obstétrique
- FSH
follicle‐stimulating hormone
- HGSOC
high‐grade serous ovarian cancer
- HOIP
HOIL‐1L interacting protein
- HR
hazard ratio
- IFN
interferon
- IL
interleukin
- LGSOC
low‐grade serous ovarian cancer
- LPA
lysophosphatidic acid
- MAPK
mitogen‐activated protein kinase
- NF
nuclear factor
- OC
ovarian cancer
- OS
overall survival
- PARP
poly (ADP‐ribose) polymerase
- PCR
polymerase chain reaction
- PFS
progression‐free survival
- SPATA2
spermatogenesis‐associated protein 2
- TBP
TATA box‐binding protein
- TNF‐R
TNF receptor
- TNFR1
TNF receptor 1
- TNF
tumor necrosis factor
1. INTRODUCTION
Ovarian cancer is one of the most common cancers amongst women in Europe and the striking cause of death in gynecological cancer entities.1 In recent years, multiple treatment modalities have emerged including surgical therapy, chemotherapy, antiangiogenic agents and PARP inhibitors. Compared with other tumor entities, immunotherapy has not been established and prognosis remains devastating.2 These observations highlight the necessity for a better understanding of disease pathogenesis.
The link between inflammation and cancer, which is termed “cancer‐related inflammation”, has been increasingly emerging over the last decade.3, 4 It is conceived that malignant processes are fueled by a “smoldering” inflammation in the tumor microenvironment that has many tumor initiating and promoting effects.5 Cancer‐related inflammatory events have been shown to play a crucial role in the pathogenesis of OC.4, 6 More specifically, OC is characterized by a pro‐inflammatory network that acts on the tumor microenvironment thereby affecting not only tumor growth but also leukocyte infiltration and neoangiogenesis in peritoneal tumor deposits.7 Urinary neopterin, a marker for IFN‐induced macrophage activation, overwhelming reflects inflammation and has been shown to be a potent prognostic factor in OC.8 The use of non‐steroidal anti‐inflammatory drugs was recently associated with improved survival among 4117 patients with serous tumor histology corroborating the overwhelming inflammatory conditions affecting OC biology and potentially highlighting anti‐inflammatory therapies as treatment options especially for HGSOC patients.9 TNF‐α, a key mediator in acute and chronic inflammation, is expressed in the OC microenvironment and seems to promote tumor progression by the induction of cytokines, proangiogenic factors and metalloproteinases.6 Moreover, TNF‐α may be implicated in the control of key disease features including cachexia, depression and fatigue, alters energy metabolism and aggravates tumor anemia10 and the TNF‐α receptor repertoire may play a role in cancer immune‐editing through modulation of immune responses.11
Recently, SPATA2 was identified as a novel component of the TNFR1 complex and is required for TNFR1 signaling.12 More specifically, SPATA2 links two subunits of the TNFR1 pathway, namely CYLD and HOIP, to allow recruitment of CYLD to the TNFR1 receptor upon TNF‐α ligation.13, 14 Moreover, SPATA2 acts as an allosteric activator for CYLD attenuating TNF‐induced NF‐κB and MAPK signaling15 suggesting that SPATA2 is required for TNF‐induced apoptosis and necroptosis.13, 14, 15 However, loss of SPATA2 had different effects on the pro‐inflammatory TNF signaling,13, 14, 15, 16 indicating heterogeneous effects on NF‐κB activation by TNF‐α.12
Tumor necrosis factor‐α and downstream‐mediated functions in tumorigenesis are context‐dependent and incompletely understood for OC.17, 18, 19, 20 TNF‐α inhibitors were shown to improve tolerability of dose‐intensive chemotherapy in cancer patients and stabilization of progressing OC.21 Controversially, TNF‐α may reduce tumor size of OC.22 Whether pro‐ or antitumor, TNF‐α seems to be highly relevant in cancer biology but we may have to better understand its downstream cascade to dissect the role of TNF‐α signaling in various conditions.21
Here, we investigate the expression of TNFA and the TNF receptor modulator SPATA2 in OC and found that TNF‐α and IL‐1β induced SPATA2 in OC cells and that increased SPATA2 expression was associated with reduced PFS and OS of OC patients. Our data implicate a role for SPATA2 in the pathogenesis of OC.
2. MATERIALS AND METHODS
2.1. Patients and samples
Ovarian tissue samples from 170 patients with OC obtained at primary debulking (patients were 24‐90 years old; median age at diagnosis was 60 years) and control tissues from 28 patients obtained by elective salpingo‐oophorectomy for benign conditions (14 non‐neoplastic tubal tissues [30‐73 years old, median 50 years], 14 non‐neoplastic ovaries [33‐74 years old, median 57 years]) were collected and processed at the Department of Obstetrics and Gynecology of the Medical University of Innsbruck, Austria, between 1989 and 2010 as described recently.23 Written informed consent was obtained from all patients before enrolment. The study was reviewed and approved by the ethics committee of the Medical University of Innsbruck (reference no. 1263/2017) and conducted in accordance with the Declaration of Helsinki. All samples were anonymized before the commencement of the analysis. All patients were monitored within the outpatient follow‐up program of our department. The median observation period was 5.5 years (range, 0.1‐26.1). All patients were of Caucasian race. Clinicopathological features are shown in Table 1.
Table 1.
Association of SPATA2 and TNFA mRNA expression with clinicopathological features in ovarian cancer patients
| Variable | n | SPATA2 mRNA expression (rel. to TBP) | n | TNFA mRNA expression (rel. to TBP) | ||||
|---|---|---|---|---|---|---|---|---|
| Median | IQR | P | Median | IQR | P | |||
| Total | 170 | 105 | ||||||
| Age | ||||||||
| ≤50.0 y | 31 | 0.97 | 0.72‐1.25 | n.s. | 22 | 0.22 | 0.08‐0.44 | n.s. |
| >50.0 y | 139 | 0.98 | 0.78‐1.33 | 83 | 0.22 | 0.10‐0.44 | ||
| FIGO stage | ||||||||
| I | 38 | 0.94 | 0.80‐1.29 | n.s. | 19 | 0.22 | 0.10‐0.44 | n.s. |
| II | 13 | 0.94 | 0.68‐1.36 | 9 | 0.16 | 0.05‐0.90 | ||
| III | 102 | 0.98 | 0.75‐1.27 | 67 | 0.22 | 0.13‐0.37 | ||
| IV | 17 | 1.19 | 0.90‐1.99 | 10 | 0.30 | 0.08‐0.69 | ||
| Tumor grade | ||||||||
| 1 | 12 | 0.77 | 0.70‐0.91 | 0.011 | 8 | 0.23 | 0.18‐0.31 | n.s. |
| 2 | 81 | 1.02 | 0.77‐1.42 | 42 | 0.15 | 0.08‐0.40 | ||
| 3 | 77 | 0.98 | 0.82‐1.29 | 54 | 0.27 | 0.13‐0.91 | ||
| Residual disease after surgery | ||||||||
| No | 78 | 0.98 | 0.77‐1.31 | n.s. | 42 | 0.22 | 0.10‐1.86 | n.s. |
| Yes | 87 | 0.97 | 0.78‐1.33 | 59 | 0.21 | 0.10‐0.65 | ||
| Unknown | 5 | |||||||
| Histology | ||||||||
| HGSOC | 106 | 1.00 | 0.78‐1.41 | 0.020 | 61 | 0.19 | 0.08‐0.47 | n.s. |
| LGSOC | 11 | 0.73 | 0.70‐0.91 | 8 | 0.23 | 0.18‐0.31 | ||
| Endometroid | 43 | 0.97 | 0.81‐1.29 | 28 | 0.22 | 0.10‐0.47 | ||
| Clear cell | 10 | 0.96 | 0.89‐1.08 | 8 | 0.23 | 0.16‐0.40 | ||
Bold values have a significance level of P < 0.05.
The significance level (P) was determined by Mann‐Whitney U‐test or Kruskal‐Wallis test, respectively.
FIGO, Fédération Internationale de Gynécologie et d'Obstétrique; HGSOC, high‐grade serous ovarian cancer; IQR, interquartile range; LGSOC, low‐grade serous ovarian cancer; n.s., not significant; rel., relative.
2.2. RNA isolation and reverse transcription
Total cellular RNA extraction from tissue samples and in vitro experiments and reverse transcription were performed as previously described.23
2.3. Quantitative real‐time PCR
Primers and probes for TNFA, CYLD and RNF31 were purchased from Applied Biosystems (Hs00174128_m1, Hs01031576_m1, Hs00215938_m1). Primers and probes for SPATA2 (GenBank no. NM_001135773.1) were determined with the assistance of the computer program Primer Express (Life Technologies, Carlsbad, CA, USA): SPATA2 forward primer, 5′‐CCG TGG AAG AAG GAA TTC AGA A‐3′; SPATA2 reverse primer, 5′‐CCA GTA ATG TCG ACT TGA CAT AAT AAA CA‐3′; and SPATA2 TaqMan probe, 5′‐FAM‐CAT CAA GAC CTA CAC GGG CCC TT‐3′‐TAMRA. TBP was used as the reference gene. PCR reactions were performed as previously described.23
2.4. Immunohistochemistry
Immunohistochemistry was performed using an automated immunostainer (BenchMark ULTRA; Ventana Medical Systems, Tucson, AZ, USA). In short, formalin‐fixed, paraffin‐embedded tissue sections were prepared with cell conditioning reagent for antigen retrieval. Anti‐SPATA2 antibody (HPA048581; Sigma‐Aldrich, St Louis, MO, USA) was incubated for 30 minutes at 37°C and for visualization the Ultra View DAB Detection Kit (Ventana Medical Systems) was used as recommended. Slides were counterstained with hematoxylin and bluing reagent. Images were acquired with a Zeiss AxioCam.
2.5. Culture and stimulation of OC cells
OVCAR3, HOC7, SKOV6 and HTB77 human OC cells were purchased from ATCC (Middlesex, UK) and cultured in RPMI supplemented with 10% fetal bovine serum and penicillin/streptomycin. Cells were stimulated with recombinant human IL‐1β (10 ng/mL; Invitrogen, San Diego, CA, USA), TNF‐α (25 ng/mL; Peprotech, Rocky Hill, NJ, USA), IL‐6 (10 ng/mL; Peprotech), LPA (20 μmol/L; Sigma‐Aldrich) and FSH (50 mIE/mL; Fostimon®) for indicated time points.
2.6. Statistical analysis
The non‐parametric Mann‐Whitney U‐test or Kruskal‐Wallis test were applied to test for statistical significance between two groups or more than two groups, respectively. The correlations between SPATA2 and TNFA mRNA expression were assessed by Spearman's rank correlation coefficient analyses. PFS was defined as the time from diagnosis of the primary tumor to the histopathological confirmation of recurrence or metastases, and OS as the time from diagnosis of the primary tumor to death from any cause or to the last clinical inspection. Univariate Kaplan‐Meier analyses and multivariable Cox survival analyses were used to explore the association of TNFA and SPATA2 expression with PFS and OS (the P‐value cut‐off for inclusion to the multivariable Cox analysis was 0.2). For survival analyses, patients were dichotomized into low and high mRNA expression level groups by the optimal cut‐off expression value calculated by Youden's index.24 Experiments with more than two comparisons were tested for statistical significance by one‐way ANOVA. P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS statistical software (version 20.0.0; SPSS, Chicago, IL, USA).
3. RESULTS
3.1. TNFA expression correlates with SPATA2 expression in OC tissue
To investigate a potential role of TNF‐α and SPATA2 in the biology of OC, we measured SPATA2 and TNFA mRNA levels in tumor tissues of 170 OC patients by quantitative PCR and compared it with 24 non‐neoplastic tissues of healthy controls. TNFA expression was elevated in OC tissue compared with non‐malignant tubes or ovaries (P = 0.010; Figure 1A). We further observed higher levels of SPATA2 in OC tissue compared with non‐neoplastic control tissues (P = 0.001; Figure 1B). Performing Spearman's rank correlation coefficient analyses in malignant and non‐malignant samples, we noted a significant correlation between SPATA2 and TNFA expression (P = 0.034, r S = 0.198; Figure 1C). Immunohistochemical analyses identified tumor epithelial cells as the main source of SPATA2 (Figure S1) which was approximately 90% positive (range, 10‐99%). In contrast, tumor stromal cells were negative for SPATA2. In control tissue, non‐malignant ovaries and stromal cells of the fallopian tubes were negative for SPATA2. The epithelium of fallopian tubes was slightly positive for SPATA2 expression which appeared to a lesser extent when compared to OC epithelium (Figure S1).
Figure 1.
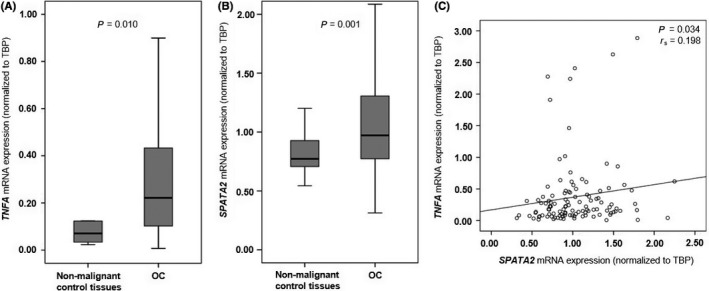
TNFA and SPATA2 expression is elevated in ovarian cancer (OC) tissue compared with non‐neoplastic fallopian tubes. A, TNFA expression in non‐neoplastic control tissues (fallopian tubes, n = 7; ovaries, n = 3) and OC (n = 105). B, SPATA2 expression in non‐neoplastic control tissues (fallopian tubes, n = 14; ovaries, n = 14) and OC (n = 170). C, Linear regression analysis of TNFA (n = 115) and SPATA2 (n = 198) in non‐malignant control tissues and OC. TNFA and SPATA2 mRNA expression values were normalized to TBP expression
3.2. SPATA2 mRNA expression is induced by TNF‐α, IL‐6 and IL‐1 in OC cell lines
Immunohistochemical analyses (Figure S1) identified tumor epithelial cells as the predominant cellular source of SPATA2 in ovarian tumors. We therefore determined the impact of inflammatory or mitogenic signaling on SPATA2 expression in human OC cell lines and stimulated OVCAR3, HOC7, SKOV6 and HTB77 cells with TNF‐α, IL‐1β, IL‐6 and LPA. FSH, which was shown to induce SPATA2,25 served as a positive control. Baseline SPATA2 expression was expressed in all cell lines with the highest levels detected in OVCAR3 cells (P < 0.001; Figure 2A). Notably, RNF31 (HOIP, a member of the LUBAC complex that interacts with SPATA2)26 transcript levels directly correlate with SPATA2 levels in OVCAR3, HOC7, SKOV6 and HTB77 cells (r S = 0.995, P = 0.005; Figure 2B). In OVCAR3 cells, TNF‐α and IL‐1β induced SPATA2 expression more than FSH with the maximal effect after 3 hours of treatment (Figure 2C). LPA and IL‐6 (known to induce OC proliferation)27, 28 did not have an impact on SPATA2 expression (data not shown). Despite directly correlating with SPATA2 levels at baseline, RNF31 could not be induced by TNF‐α and IL‐1β, LPA or IL‐6. In contrast, CYLD, which is also component of the TNF‐R signaling pathway, exhibited similar induction patterns compared with SPATA2. In detail, both SPATA2 and CYLD were induced by TNF‐α and IL‐1β (but not FSH) after 3 hours (Figure 2D). Our data establish a direct effect of pro‐inflammatory cytokines such as TNF‐α and IL‐1β on SPATA2 expression in human OC cells.
Figure 2.
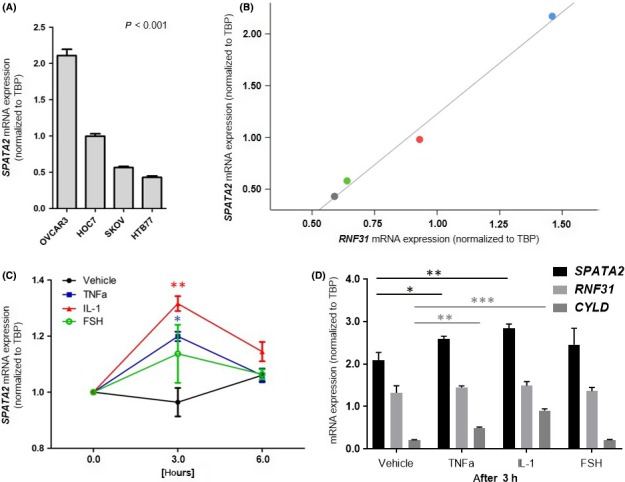
SPATA2 expression is induced by tumor necrosis factor (TNF)‐α and interleukin (IL)‐1β in ovarian cancer cell lines. A, Baseline SPATA2 expression in the human ovarian cancer (OC) cell lines OVCAR3, HOC7, SKOV6 and HTB77. B, Linear regression analysis of RNF31 and SPATA2 in HTB77 (grey point), SKOV6 (green point), HOC7 (red point) and OVCAR3 (blue point) cells. C, OVCAR3 cells were stimulated with TNF‐α, IL‐1β and follicle‐stimulating hormone (FSH) for indicated time points (n = 3). Stars indicate significance levels between vehicle and TNF‐α or IL‐1β, respectively. D, SPATA2,RNF31 and CYLD expression in OVCAR3 cells after stimulation with TNF‐α, IL‐1β and FSH for 3 hours (n = 3). SPATA2,RNF31 and CYLD mRNA expression values were normalized to TBP expression
3.3. Increased SPATA2 expression occurred in higher tumor grades
Next, we explored the association between SPATA2 and TNFA expression with clinicopathological features. As demonstrated in Table 1 and Figure 3, we found that increased SPATA2 expression was associated with higher tumor grade. Specifically, we found higher SPATA2 mRNA levels in tumor grade 2 and 3 compared with tumor grade 1 (Figure 3A) which was in line with higher SPATA2 expression in HGSOC compared with LGSOC (P = 0.024; Figure 3B). In contrast, TNFA expression was not associated with tumor grade and did not differ between histological subtypes (Table 1). SPATA2 and TNFA expression was independent from FIGO stage.
Figure 3.
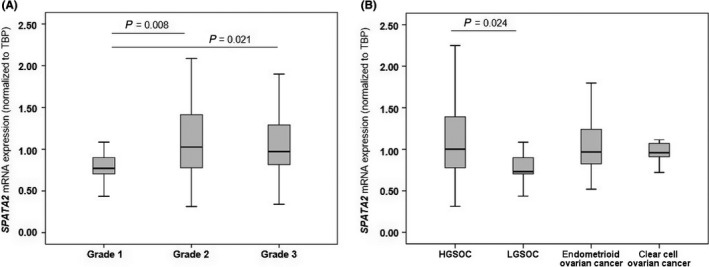
SPATA2 expression according to tumor grades (A) and histological subtypes (B). Expression values were normalized to TBP expression
3.4. High SPATA2 mRNA expression is associated with a poor prognosis
To evaluate SPATA2 and TNFA levels regarding clinical outcome of OC patients, we first determined Youden's index24 which grouped the OC cohort into patients with “high” and “low” SPATA2 and TNFA expression. Univariate survival analyses (Table 2) demonstrated that patients with low SPATA2 expression exhibited a median PFS of 50.5 months (CI, 0.0‐105.1) whereas patients with high SPATA2 expression exhibited a median PFS of only 22.9 months (CI, 14.2‐31.6) (P = 0.073; Figure 4A). This difference in PFS was even more prominent in the subgroup of HGSOC patients (P = 0.008; Figure 4B). A clear association between high SPATA2 expression and impaired OS was revealed. Patients with low SPATA2 expression exhibited a median OS of 105.1 months (CI, 71.4‐138.9) while patients with high SPATA2 expression exhibited a median OS only of 43.4 months (CI, 29.9‐56.9), (P = 0.001; Figure 4C). This was also true for the subgroup analysis of HGSOC (P = 0.002; Figure 4D). Importantly, multivariate analyses identified SPATA2 as an independent prognostic factor for PFS (HR, 1.55; P = 0.048; Table 3) and OS (HR, 2.13; P < 0.001; Table 3). TNFA expression, however, failed to be of prognostic significance either regarding PFS or OS in OC (Table 2, Figure S2A,B).
Table 2.
Univariate survival analysis in ovarian cancer patients
| Variable | No. patients (relapsed/total) | Progression‐free survival | No. patients (died/total) | Overall survival | ||
|---|---|---|---|---|---|---|
| Median, months, 95% CI | P | Median, months, 95% CI | P | |||
| Age | ||||||
| <50 y | 18/31 | 50.3 (2.2‐98.5) | 0.466 | 15/31 | 151.065.6‐236.5) | 0.015 |
| ≥50 y | 78/139 | 24.2 (5.8‐42.6) | 94/139 | 49.6 (28.2‐71.0) | ||
| FIGO stage | ||||||
| I/II | 11/51 | n.r. | <0.001 | 20/51 | n.r. | 0.000 |
| III/IV | 85/119 | 20.0 (14.7‐25.3) | 89/119 | 47.3 (26.6‐68.0) | ||
| Tumor grade | ||||||
| 1/2 | 47/93 | 48.8 (0.0‐101.2) | 0.110 | 53/93 | 100.0 (70.1‐129.9) | 0.012 |
| 3 | 49/77 | 23.6 (12.6‐34.7) | 56/77 | 44.4 (30.4‐58.5) | ||
| Residual disease after surgery | ||||||
| No | 24/79 | n.r. | <0.001 | 30/78 | n.r. | <0.001 |
| Yes | 68/87 | 15.7 (13.2‐18.3) | 76/87 | 35.2 (24.4‐46.1) | ||
| Histology | ||||||
| HGSOC | 69/106 | 23.4 (16.0‐30.9) | 0.008 | 80/106 | 47.1 (27.5‐66.7) | 0.003 |
| Others | 27/64 | n.r. | 29/64 | 132.7 (n.r.) | ||
| SPATA2 mRNA expression | ||||||
| Low | 35/71 | 50.5 (0.0‐105.1) | 0.073 | 67/116 | 105.1 (71.4‐138.9) | 0.001 |
| High | 61/99 | 22.9 (14.2‐31.6) | 42/54 | 43.4 (29.9‐56.9) | ||
| Subgroup: HGSOC | ||||||
| Low | 47/78 | 28.8 (7.1‐50.5) | 0.008 | 52/76 | 58.7 (23.4‐93.9) | 0.002 |
| High | 22/28 | 13.5 (6.9‐20.2) | 28/30 | 35.7 (21.0‐50.4) | ||
| TNFA mRNA expression | ||||||
| Low | 12/27 | n.r. | 0.222 | 68/117 | 68.8 (37.0‐100.7) | 0.434 |
| High | 50/78 | 22.9 (13.9‐31.8) | 42/54 | 69.6 (21.3‐118.0) | ||
| Subgroup: HGSOC | ||||||
| Low | 11/20 | 30.0 (13.8‐46.1) | 0.283 | 10/16 | 49.0 (0.0‐120.4) | 0.171 |
| High | 31/41 | 22.8 (18.7‐27.0) | 38/45 | 41.1 (30.8‐51.5) | ||
Bold values have a significance level of P < 0.05.
The optimal cut‐off points for SPATA2 and TNFA were calculated by Youden‘s index. The significance level (P) was determined by log‐rank test.
CI, confidence interval; FIGO, Fédération Internationale de Gynécologie et d'Obstétrique; HGSOC, high grade serous ovarian cancer; n.r., not reached.
Figure 4.
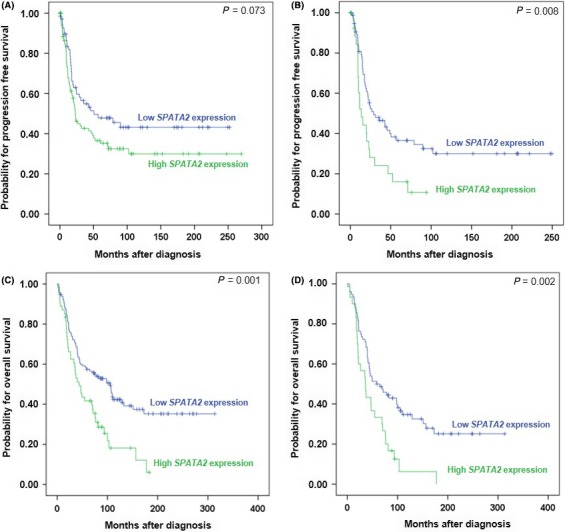
Kaplan‐Meier survival analyses and SPATA2 expression in ovarian cancer (OC) patients. Progression‐free survival according to low and high SPATA2 mRNA expression in (A) OC patients (n = 170) and (B) the subgroup of patients with HGSOC (n = 61). Overall survival according to low and high SPATA2 mRNA expression in (C) OC patients (n = 170) and (D) the subgroup of patients with HGSOC (n = 61)
Table 3.
Multivariate survival analysis in ovarian cancer patients
| Variable | Progression‐free survival | Overall survival | |||
|---|---|---|---|---|---|
| HR of progression (95% CI) | P | HR of death (95% CI) | P | ||
| Age | <50 y ≥ | 1.46 (0.86‐2.47) | 0.162 | 2.26 (1.29‐3.95) | 0.004 |
| FIGO stage | I/II vs III/IV | 2.68 (1.33‐5.42) | 0.006 | 1.33 (0.75‐2.33) | 0.327 |
| Tumor grade | 1/2 vs 3 | 1.21 (0.79‐1.84) | 0.378 | 1.39 (0.94‐2.05) | 0.102 |
| Residual disease after surgery | No vs yes | 2.92 (1.71‐4.98) | <0.001 | 3.02 (1.96‐5.24) | <0.001 |
| Histology | HGSOC vs others | 1.03 (0.62‐1.68) | 0.922 | 0.86 (0.54‐1.37) | 0.519 |
| SPATA2 mRNA expression | Low vs high (< or > optimal cut‐off) | 1.55 (1.00‐2.40) | 0.048 | 2.13 (1.41‐3.24) | <0.001 |
Bold values have a significance level of P < 0.05.
The optimal cut‐off points for SPATA2 were calculated by Youden's index. The significance level (P) was determined by Cox regression.
CI, confidence interval; FIGO, Fédération Internationale de Gynécologie et d'Obstétrique; HGSOC, high grade serous ovarian cancer; HR, hazard ratio; n.r., not reached.
4. DISCUSSION
In this study, we investigated the regulation of TNFA and SPATA2 and the impact on clinical outcome in a Caucasian OC cohort. We found that TNFA and SPATA2 are significantly higher expressed in OC compared with non‐malignant control tissues. Immunohistochemical staining of our cohort and an OC database (human protein atlas) identified OC cells as the main cellular source of SPATA2 expression. Using the OC cell line OVCAR3, we demonstrate that SPATA2 expression was markedly induced by TNF‐α and IL‐1β, indicating that pro‐inflammatory signals induced expression of SPATA2 in OC. Increased SPATA2 expression, which correlated with TNFA expression, was associated with increasing tumor grade, and consequently was higher in HGSOC compared with LGSOC. High SPATA2 (but not TNFA) expression independently reflected poor clinical outcome with regard to PFS and OS in OC patients. This was especially true for the subgroup of HGSOC.
Tumor necrosis factor‐α has been initially discovered as initiator of tumor cell necrosis21 and is now known as a potent pro‐inflammatory cytokine which exerts deleterious effects in chronic inflammation, antimicrobial immunity and autoimmune diseases.29 Contrary to its discovery, TNF‐α failed as an anticancer agent as various studies have clearly demonstrated a tumor‐promoting role for TNF‐α in experimental cancers.21 In OC, TNF‐α and its potential role in disease progression has been described earlier.30 OC cells secrete TNF‐α protein31 which stimulates a constitutive network of other cytokines, angiogenic factors and chemokines that may act in an autocrine/paracrine manner to promote colonization of the peritoneum and neovascularization of developing tumor deposits.32 Furthermore, Charles et al20 demonstrated that chronic production of TNF‐α in the tumor microenvironment increases myeloid cell recruitment and consequently tumor growth in vivo. Previous studies demonstrated an increase of TNF‐α protein and gene expression in human OC compared with non‐malignant controls.18, 30, 33 High ascitic TNF‐α protein levels have previously been found to be associated with poor survival in univariate analyses,34 however, data concerning intra‐tumor TNF‐α expression and clinical outcome are not available. In line with previous data, we demonstrate high levels of TNFA in human OC compared with non‐malignant control tissues.18, 30, 33 However, we were unable to determine a significant prognostic effect of TNFA expression in OC patients. Clinical studies investigating TNF‐α inhibitors (etanercept, infliximab) as a therapeutic option or as supportive treatment to improve chemotherapy tolerability demonstrated biologic activity and safety of TNF blockade in recurrent OC.21 However, the same was true when TNF‐α itself was used in high pharmacological doses combined with chemotherapy to refine the necrotic activity of TNF‐α and to boost antitumor activity.22 Thus, it appears that TNF‐α represents a “double‐dealer” with regard to cancer biology.35 On one hand, TNF‐α through its pro‐inflammatory properties could be an endogenous tumor promoter stimulating cancer cell growth, proliferation, metastasis, angiogenesis and leukocyte infiltration; and on the other hand, TNF‐α could also act as a killer of cancer cells. These divergent observations may be one reason why more recent studies focused on downstream TNF‐α signaling.
A number of investigations have disclosed complex and diverging TNF‐R signaling pathways described as a “double‐edged” sword.36 We found that SPATA2, a novel component of the TNFR1 signaling complex, is an independent predictor for adverse PFS and OS in OC patients. SPATA2 is an adaptor for the recruitment of CYLD to the TNF‐R signaling cascade and an activator of CYLD which controls TNF‐induced apoptosis and necroptosis.13, 14, 15 Cells lacking SPATA2 exhibited reduced TNF‐induced cell death due to reduced caspase‐3 suggesting that SPATA2 is required for TNF‐induced cell death. Currently, there are no data on the role of SPATA2 in tumorigenesis and cancer progression. However, CYLD, the co‐factor for SPATA2 is a known tumor suppressor37 shown to inhibit NF‐κB, MAPK and Wnt signaling. On the other hand, CYLD also acts as a mediator of immune activation and inflammation.38, 39 We found that both SPATA2 and CYLD are induced by TNF‐α and IL‐1β in vitro, indicating a similar regulation in OC. CYLD and SPATA2 were shown to synergistically promote TNF‐induced NF‐κB signaling, caspase activation and apoptosis.15 Considering that, OC with high SPATA2 expression levels may also show high rates of apoptosis. In other cancer entities, such as colon carcinoma and breast cancer, high apoptotic rates were associated with increased cellular proliferation and poor prognosis.40, 41, 42 Nonetheless, in various tumor types such as malignant melanoma or breast cancer, downregulation of CYLD is associated with tumor progression.43 However, our data illustrate that in OC high SPATA2 expression is independently associated with worse PFS and OS. As no data on SPATA2 expression and clinical outcome in cancer are currently available, it remains speculative whether CYLD and SPATA2 have always identical biologic functions or whether both could be endowed with additional mutually independent properties.
Nonetheless, it should be emphasized that cancer‐related inflammation plays an exceptional role in OC, and especially in HGSOC, due to its semi‐solid dissemination throughout the abdominal cavity. In this context, OC can hardly be compared with other solid tumors. Our in vitro results showing an induction of SPATA2 expression selectively by pro‐inflammatory cytokines argue for a tight involvement of SPATA2 in the pro‐inflammatory cytokine network within the microenvironment of OC. In this regard, the herein presented clinical results may reflect the tumor‐promoting activity of the tumor‐associated inflammation with its detrimental effect on patients’ prognoses. Thus, SPATA2 fits well into the large row of other pro‐inflammatory factors (such as urinary neopterin,8 90K,44 ascitic TNF‐α and IL‐12),34 which proved to predict adverse clinical outcome in OC.
In conclusion, our study suggests a potential biologic role of SPATA2, possibly as a downstream regulator of TNF‐mediated actions in the pathogenesis and dissemination of OC. Further studies are needed to investigate the exact functional role of SPATA2 in the biology of OC.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Julia Rössler and Annemarie Wiedemair for excellent technical assistance and Georg Goebel from the Department of Medical Statistics, Informatics and Health Economics, Medical University of Innsbruck for his statistical support. The project was supported by the Verein zur Krebsforschung in der Frauenheilkunde, an association which is exclusively financed by donation funds for cancer research in female malignancies.
Wieser V, Tsibulak I, Degasper C, et al. Tumor necrosis factor receptor modulator spermatogenesis‐associated protein 2 is a novel predictor of outcome in ovarian cancer. Cancer Sci. 2019;110:1117–1126. 10.1111/cas.13955
REFERENCES
- 1. Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519‐2529. [DOI] [PubMed] [Google Scholar]
- 2. Baldwin LA, Huang B, Miller RW, et al. Ten‐year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120:612‐618. [DOI] [PubMed] [Google Scholar]
- 3. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer‐related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493‐e503. [DOI] [PubMed] [Google Scholar]
- 4. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454:436‐444. [DOI] [PubMed] [Google Scholar]
- 5. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kulbe H, Chakravarty P, Leinster DA, et al. A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Res. 2012;72:66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balkwill FR, Mantovani A. Cancer‐related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33‐40. [DOI] [PubMed] [Google Scholar]
- 8. Volgger BM, Windbichler GH, Zeimet AG, et al. Long‐term significance of urinary neopterin in ovarian cancer: a study by the Austrian Association for Gynecologic Oncology (AGO). Ann Oncol. 2016;27:1740‐1746. [DOI] [PubMed] [Google Scholar]
- 9. Verdoodt F, Dehlendorff C, Friis S, Kjaer SK. Non‐aspirin NSAID use and ovarian cancer mortality. Gynecol Oncol. 2018;150:331‐337. [DOI] [PubMed] [Google Scholar]
- 10. Tracey KJ, Wei H, Manogue KR, et al. Cachectin/tumor necrosis factor induces cachexia, anemia, and inflammation. J Exp Med. 1988;167:1211‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swann JB, Vesely MD, Silva A, et al. Demonstration of inflammation‐induced cancer and cancer immunoediting during primary tumorigenesis. Proc Natl Acad Sci U S A. 2008;105:652‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schlicher L, Brauns‐Schubert P, Schubert F, Maurer U. SPATA2: more than a missing link. Cell Death Differ. 2017;24:1142‐1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wagner SA, Satpathy S, Beli P, Choudhary C. SPATA2 links CYLD to the TNF‐alpha receptor signaling complex and modulates the receptor signaling outcomes. EMBO J. 2016;35:1868‐1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kupka S, De Miguel D, Draber P, et al. SPATA2‐Mediated Binding of CYLD to HOIP Enables CYLD Recruitment to Signaling Complexes. Cell Rep. 2016;16:2271‐2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlicher L, Wissler M, Preiss F, et al. SPATA2 promotes CYLD activity and regulates TNF‐induced NF‐kappaB signaling and cell death. EMBO Rep. 2016;17:1485‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elliott PR, Leske D, Hrdinka M, et al. SPATA2 Links CYLD to LUBAC, Activates CYLD, and Controls LUBAC Signaling. Mol Cell. 2016;63:990‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trabert B, Pinto L, Hartge P, et al. Pre‐diagnostic serum levels of inflammation markers and risk of ovarian cancer in the prostate, lung, colorectal and ovarian cancer (PLCO) screening trial. Gynecol Oncol. 2014;135:297‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Szlosarek PW, Grimshaw MJ, Kulbe H, et al. Expression and regulation of tumor necrosis factor alpha in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006;5:382‐390. [DOI] [PubMed] [Google Scholar]
- 19. Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor‐alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355‐10362. [DOI] [PubMed] [Google Scholar]
- 20. Charles KA, Kulbe H, Soper R, et al. The tumor‐promoting actions of TNF‐alpha involve TNFR1 and IL‐17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011‐3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361‐371. [DOI] [PubMed] [Google Scholar]
- 22. Anderson GM, Nakada MT, DeWitte M. Tumor necrosis factor‐alpha in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004;4:314‐320. [DOI] [PubMed] [Google Scholar]
- 23. Goebel G, Berger R, Strasak AM, et al. Elevated mRNA expression of CHAC1 splicing variants is associated with poor outcome for breast and ovarian cancer patients. Br J Cancer. 2012;106:189‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32‐35. [DOI] [PubMed] [Google Scholar]
- 25. Onisto M, Slongo LM, Graziotto R, et al. Evidence for FSH‐dependent upregulation of SPATA2 (spermatogenesis‐associated protein 2). Biochem Biophys Res Commun. 2001;283:86‐92. [DOI] [PubMed] [Google Scholar]
- 26. Schlicher L, Maurer U. SPATA2: new insights into the assembly of the TNFR signaling complex. Cell Cycle. 2017;16:11‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goldsmith ZG, Ha JH, Jayaraman M, Dhanasekaran DN. Lysophosphatidic acid stimulates the proliferation of ovarian cancer cells via the gep Proto‐Oncogene Galpha(12). Genes Cancer. 2011;2:563‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dijkgraaf EM, Welters MJ, Nortier JW, van der Burg SH, Kroep JR. Interleukin‐6/interleukin‐6 receptor pathway as a new therapy target in epithelial ovarian cancer. Curr Pharm Des. 2012;18:3816‐3827. [DOI] [PubMed] [Google Scholar]
- 29. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487‐501. [DOI] [PubMed] [Google Scholar]
- 30. Naylor MS, Stamp GW, Foulkes WD, Eccles D, Balkwill FR. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993;91:2194‐2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor‐alpha as a tumour promoter. Eur J Cancer. 2006;42:745‐750. [DOI] [PubMed] [Google Scholar]
- 32. Kulbe H, Thompson R, Wilson JL, et al. The inflammatory cytokine tumor necrosis factor‐alpha generates an autocrine tumor‐promoting network in epithelial ovarian cancer cells. Cancer Res. 2007;67:585‐592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jammal MP, Da Silva AA, Filho AM, et al. Immunohistochemical staining of tumor necrosis factor‐alpha and interleukin‐10 in benign and malignant ovarian neoplasms. Oncol Lett. 2015;9:979‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zeimet AG, Widschwendter M, Knabbe C, et al. Ascitic interleukin‐12 is an independent prognostic factor in ovarian cancer. J Clin Oncol. 1998;16:1861‐1868. [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29:1275‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aggarwal BB. Signalling pathways of the TNF superfamily: a double‐edged sword. Nat Rev Immunol. 2003;3:745‐756. [DOI] [PubMed] [Google Scholar]
- 37. Bignell GR, Warren W, Seal S, et al. Identification of the familial cylindromatosis tumour‐suppressor gene. Nat Genet. 2000;25:160‐165. [DOI] [PubMed] [Google Scholar]
- 38. Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF‐kappaB activation by TNFR family members. Nature. 2003;424:793‐796. [DOI] [PubMed] [Google Scholar]
- 39. Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF‐kappaB activation and diverse biological processes. Cell Death Differ. 2010;17:25‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu S, Edgerton SM, Moore DH 2nd, Thor AD. Measures of cell turnover (proliferation and apoptosis) and their association with survival in breast cancer. Clin Cancer Res. 2001;7:1716‐1723. [PubMed] [Google Scholar]
- 41. Dawson H, Koelzer VH, Karamitopoulou E, et al. The apoptotic and proliferation rate of tumour budding cells in colorectal cancer outlines a heterogeneous population of cells with various impacts on clinical outcome. Histopathology. 2014;64:577‐584. [DOI] [PubMed] [Google Scholar]
- 42. Alcaide J, Funez R, Rueda A, et al. The role and prognostic value of apoptosis in colorectal carcinoma. BMC Clin Pathol. 2013;13:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hayashi M, Jono H, Shinriki S, et al. Clinical significance of CYLD downregulation in breast cancer. Breast Cancer Res Treat. 2014;143:447‐457. [DOI] [PubMed] [Google Scholar]
- 44. Zeimet AG, Natoli C, Herold M, et al. Circulating immunostimulatory protein 90K and soluble interleukin‐2‐receptor in human ovarian cancer. Int J Cancer. 1996;68:34‐38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


