Abstract
The biological functions of the Eph/ephrin system have been intensively investigated and well documented so far since its discovery in 1987. Although the Eph/ephrin system has been implicated in pathological settings such as Alzheimer's disease and cancer, the molecular mechanism of the Eph/ephrin system in those diseases is not well understood. Especially in cancer, recent studies have demonstrated that most of Eph and ephrin are up‐ or down‐regulated in various types of cancer, and have been implicated in tumor progression, tumor malignancy, and prognosis. However, they lack consistency and are in controversy. The localization patterns of EphA1 and EphA2 in mouse lungs are very similar, and both knockout mice showed similar phenotypes in the lungs. Ephrin‐A1 that is a membrane‐anchored ligand for EphAs was co‐localized with EphA1 and EphA2 in lung vascular endothelial cells. We recently uncovered the molecular mechanism of ephrin‐A1‐induced lung metastasis by understanding the physiological function of ephrin‐A1 in lungs. This review focuses on the function of EphA1, EphA2, and ephrin‐A1 in tumors and an establishment of pre‐metastatic microenvironment in the lungs.
Keywords: ADAM, Eph, ephrin, metastasis, S100A8
1. INTRODUCTION
The Eph receptor tyrosine kinase was identified from a hepatocellular carcinoma (HCC) cell line in 1987.1 The Eph receptor comprises the largest family in receptor tyrosine kinases and can be divided into two groups based on their structures and receptor‐ligand specificity. EphA (pronounced “eff‐A”) consists of 9 type‐A Eph receptors (EphA1‐8, and EphA10) and 5 type‐B Eph receptors (EphB1‐4, 6, pronounced “eff‐B”).2, 3 Ephrins (pronounced “efrin”) are also divided into two subfamilies, ephrin‐A (ephrin‐A1‐6) and ephrin‐B (ephrin‐B1‐3) based on their structures. Ephrin‐A1, a ligand for the EphA receptors, was identified as an immediate early response gene to TNFα.4 Ephrin‐A1 is a plasma membrane protein anchored by glycosylphosphatidylinositol (GPI) although ephrin‐A1 was originally identified as a soluble factor.4 The EphA receptors transmit their downstream signals mediated by their tyrosine kinase activities to receptor‐expressing cells upon ephrin‐A1 binding by a juxtacrine fashion defined as forward signal, and then ephrin‐As simultaneously transmit their signals to a ligand‐expressing cell termed as reverse signal mediated by the Fyn tyrosine kinase activity5 (Figure 1). A recent study reported that Progranulin is a functional ligand for EphA2.6 Progranulin is ubiquitously expressed and has been implicated in cancer progression.7 The interaction between EphA2 and Progranulin modulates capillary formation in vitro and MAPK signaling pathway.6 While the EphA1/A2 and ephrin‐A1 system plays roles in physiological settings,8, 9, 10 it is implicated in pathological circumustances.11, 12, 13 EphA1 and EphA2 have the highest protein homology (approximately 65% in human) among EphAs. The localization patterns of EphA1 and EphA2 in mouse lungs were almost same. Moreover, both EphA1‐ and EphA2‐deficient mice showed similar phenotypes in the lungs. Collectively, we suppose that EphA1 and EphA2 share their functions in lungs. We described “EphA1 and EphA2” as EphA1/A2 in this review.
Figure 1.
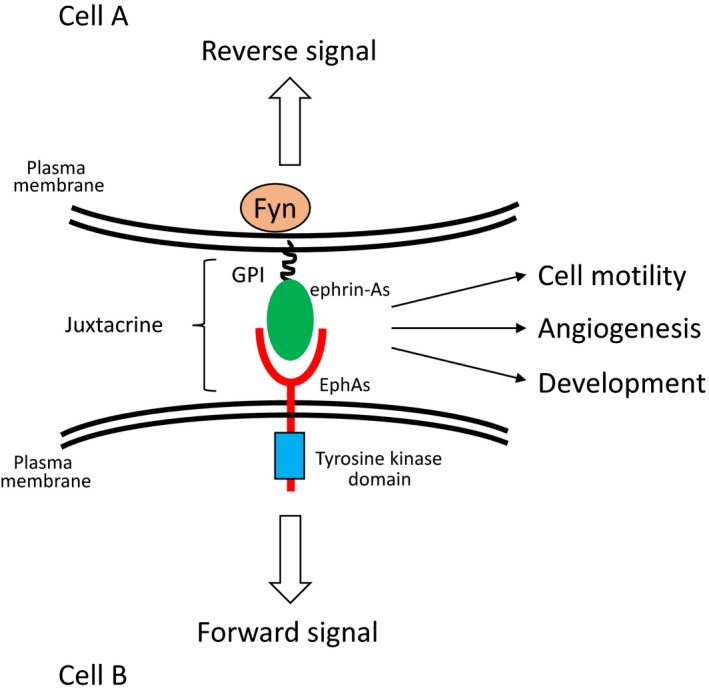
Activation mechanism of the Eph/ephrin system. Ephrin‐As anchored by GPI to the plasma membrane bind to EphAs in adjacent cell. This type of activation mechanism is called juxtacrine. EphAs and ephrin‐As simultaneously transmit their signals into each cell termed as forward and reverse signal, respectively
2. CLINICOPATHOLOGICAL ANALYSIS OF EPHA1/A2 AND EPHRIN‐A1 IN CANCER
Accumulating evidence has showed that EphA1/A2 and ephrin‐A1 are correlated with tumor malignancy and prognosis. EphA1/A2 and ephrin‐A1 are over‐expressed or down‐regulated in various types of cancer.14 For instance, there is a complementary expression of ephrin‐A1 and EphA2 in human breast cancer cell lines. Ephrin‐A1 was down‐regulated in MDA‐MB‐231, an invasive breast cancer cells as compared to MCF‐10A, a benign breast cancer cell. On the other hand, EphA2 was overexpressed in MA‐MB‐231 and down‐regulated in MCF‐10A.15 These expression profiles were also observed in glioma,16 and expression levels of EphA2 and ephrin‐A1 in tumor tissues would be a potential diagnostic and prognostic marker. Some studies have demonstrated that higher ephrin‐A1 expression is positively correlated with worse prognosis in liver and colorectal cancer.17, 18 However, among stage I non‐small cell lung cancer patients, higher expression levels of EphA2 and ephrin‐A1 improved their prognosis.19 In advanced gastric cancer, analysis of mRNA expression showed that EPHA1‐EPHA4 were overexpressed in most of patients, and high EphA1 and EphA2 were significantly associated with poor prognosis20 In human glioblastoma multiforme (hGBM), comparison between EphA2low and EphA2high populations indicated that the EphA2high population has an ability to maintain self‐renewal property and tumorigenicity. In an orthotropic murine xenograft model, mice with tumors of high EphA2 expression exhibited shorter survival than those of low EphA2 expression. Moreover, down regulation of EphA2 expression in hGBM by Fc‐ephrin‐A1 stimulation resulted in loss of self‐renewal ability and a decreased proliferating activity in vitro and tumor growth in vivo.21 Overexpression of EphA3 have showed similar results as observed in EphA2‐overexpressing hGBM. Tumors with high EphA3 expression also showed more aggressive and undifferentiated phenotypes.22 These data suggest that EphA2 and EphA3 seem to be required for the maintenance of self‐renewal ability in hGBM. Moreover, ephrin‐A1 seems to be a key molecule to decrease self‐renewal property of hGBM and prolong survival of cancer patients. However, there is no study to support that high ephrin‐A1 expression in hGBM shows much better clinical outcome.
3. ROLES OF THE EPH/EPHRIN SYSTEM IN TUMOR ANGIOGENESIS
It has been reported that EphB4 and ephrin‐B2 determined arterial and venous specification during vasculogenesis by regulating cell adhesion and migration of endothelial cells.23, 24 Moreover, ephrin‐B2 is essential for VEGF‐induced receptor internalization and signalings.25, 26 However, roles of EphB4 and ephrin‐B2 have not been fully understood in pathological settings. In case of colorectal cancer (CRC), expression analysis using clinical samples demonstrated that EphB4 was overexpressed in the plasma membrane of tumor cells but not in normal colon mucosa. Expression of EphB4 was positively associated with TNM stages in CRC, and overexpression of EphB4 resulted in an increase of microvascular density in a xenograft mosue model. ShRNA‐mediated knocked down of EphB4 decreased tumor growth and tumor angiogenesis.27 An inhibition of EphB4/ephrin‐B2 ligation by extracellular domain of EphB4 reduced tumor growth and angiogenesis.28, 29 A recent study demonstrated that genetic deletion of ephrin‐B2 showed more aggressive phenotypes on tumor growth and invasion than those of controls.30 Therefore, roles of EphB4 and ephrin‐B2 in tumor growth and agiogenesis are still controversial. In pathological conditions, EphA1/A2 and ephrin‐A1 have been implicated in tumor angiogenesis that is important for tumor growth to supply O2 and nutrients.31, 32 However, the detailed molecular mechanisms remain to be elucidated. Activating transcription factor 3 (ATF3) is well known to be induced by various stress including hypoxia as often observed in tumor microenvironment.33 We found that EphA1 was up‐regulated in an ATF3‐denpendt manner in NP31, a rat endothelial cell line9 and regulated endothelial tubulogenesis.9, 34 Furthermore, ephrin‐A1‐induced EphA1 activation promoted SDF‐1 secretion and chemotaxis of endothelial progenitor cells to HCC through the SDF‐1/CXCR4 signaling pathway.35, 36 Small interfering RNA (siRNA)‐mediated inhibition of the EphA1/SDF‐1/CXCR4 pathway abolished tube formation in vitro and decreased tumor size and angiogenesis due to an inhibition of endothelial progenitor cell homing to the tumor tissue.36
4. THE SIGNALING CROSSTALK AMONG INTEGRIN, ECM, AND EPHA1
It has been reported that the integrin‐extracellular matrix (ECM) axis contributes to tumor angiogenesis. Integrin αvβ3, an angiogenic marker, is widely expressed in tumors but not in healthy tissues.37 A monoclonal antibody against either integrin αvβ3 or αvβ5 that inhibits the interaction between integrin and ECM attenuated tumor angiogenesis.37 However, double‐knockout of integrin β3 and β5 showed an opposite effect on neovascularization in tumors and enhanced tumor angiogenesis.38 Despite the opposing results, integrins are still attractive target molecules for an inhibition of tumor growth because integrins cooperate with some growth factor receptor signals for cell proliferation.39, 40 Therefore, many researchers devote their intensive works to the development of anti‐integrin drugs.41, 42 EphA1 interacted with fibronectin type I repeat and integrin‐linked kinase (ILK) mediated by the extracellular and intracellular domain of EphA1, respectively.8, 9 The association of EphA1 and fibronectin was partially involved in VEGF‐dependent tube formation in vitro. Although the detailed molecular mechanisms remained unknown, sequestering EphA1 from binding to fibronectin by secretion of Fc‐fused EphA1 diminished tumor burden and VEGF‐induced angiogenesis.9 ECM such as collagen and fibronectin is abundant in tumor surroundings. It has been reported that the integrin‐ECM signaling pathway regulates cell motility and cell shape, which is mediated by Rho family small GTPases.43 We found a linkage among integrin, EphA1, and ECM‐related signaling pathways. EphA1‐induced signaling pathway in tumors inhibited cell spreading mediated by the ECM‐integrin and ECM‐EphA1 pathways.8 EphA1 interacted with integrin β1 without affecting tyrosine phosphorylation state (Figure 2A). ILK is recruited to the SAM domain of EphA1 in response to ephrin‐A1 stimulation and integrin β1 in the presence of integrin‐ECM interactions.8 ECM‐integrin signal maintains cell spreading and adhesion by ILK and Rac1 GTP‐bound form.44 When EphA1 is stimulated with ephrin‐A1, ILK and Rac1 are inactivated, and thereby RhoA/Rock pathway turns to be activated resulting in an inhibition of cell spreading.8 Loss of cell‐cell adhesion and spreading defect could render tumor cell to easily get off from the tumor mass (Figure 2B).
Figure 2.
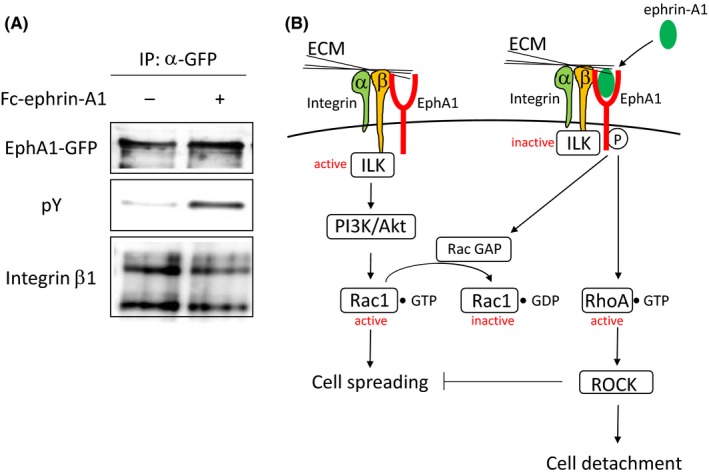
Regulation mechanism of ECM/integrin/EphA1‐mediated cell adhesion. A, Co‐immuno‐precipitation of EphA1 and integrin β1. GFP‐fused EphA1‐expressing HEK293 cells were stimulated with Fc‐ephrin‐A1 (2 μg/mL). EphA1 was co‐immuno‐precipitated with integrin β1 without affecting tyrosine phosphorylation status of EphA1. B, Regulatory signaling pathway of ECM/integrin/EphA1‐mediated cell adhesion. In the absence of ephrin‐A1 stimulation, ILK is in active. ECM/integrin signal provides cell spreading mediated by Rac1 activation. Once EphA1 is stimulated with ephrin‐A1, ILK is inactivated. Subsequently, the Rac1 GTP‐bound form is hydrolyzed by Rac1 GTPase activating protein followed by RhoA activation, which in turn induces cell retraction
SiRNA‐mediated knockdown of ephrin‐A1 attenuated migratory activity of endothelial cells in vitro and tumor angiogenesis in vivo using 4T1, a mammary adenocarcinoma cell.45 Moreover, an intravenous injection of 4T1 cells in which ephrin‐A1 expression was abrogated significantly suppressed a number of metastatic foci in lungs suggesting that ephrin‐A1 contributes to migration and/or regrowth in metastatic microenvironment. Taken together, overexpressed‐ephrin‐A1 also plays an important role in tumor angiogenesis and metastasis. In our knowledge, membrane‐anchored ephrin‐A1 has no ability to induce tyrosine phosphorylation of EphA1/A2 and maintains cell‐cell contacts via association between EphA1/A2 and ephrin‐A1 at cell boundaries. Accordingly, membrane‐anchored ephrin‐A1 needs to be released from the plasma membrane to activate the EphA1/A2 receptor. We identified a disintegrin and metalloproteinase 12 (ADAM12) as a binding partner of EphA1 and EphA2 by the yeast two‐hybrid screening using extracellular domain of EphA1. ADAM12 cleaves membrane‐anchored ephrin‐A1 in trans.46 Cleaved ephrin‐A1 may be capable of activating EphA1/A2 by a paracrine and/or an autocrine fashion. Ephrin‐A1‐induced EphA1 activation showed cell spreading defect as described above. ADAM12‐deficient mice in MMTV‐PyMT mouse breast cancer model showed a delayed tumor progression and spontaneous lung metastasis by unknown mechanisms.47 The other study suggested that ADAM12 localized at the invadepodia and regulated migration and invasion.48 ECM deposition and loss of cell‐cell contacts render tumor cell to metastasize to distant organs. ADAM12 shows matrix metalloproteinase activity toward ECM such as fibronectin.49 Taken together, we suppose that, ADAM12 contributes to degradation of fibronectin and loss of cell‐cell contacts via cleavage of ephrin‐A1 unbound to the receptor in primary tumors (Figure 3).
Figure 3.
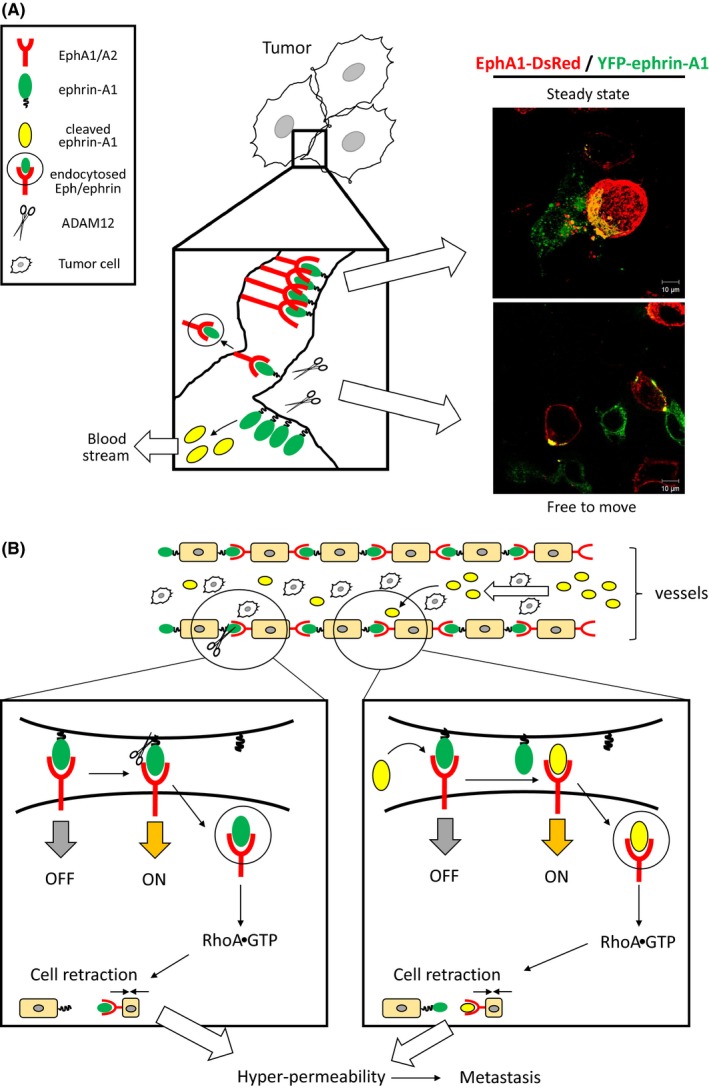
Role of the EphA1/A2 and ephrin‐A1 system in tumors and lung metastasis. A, Cell boundary formation by the Eph/ephrin system. The Eph/ephrin system provides cell‐cell contacts via receptor‐ligand interaction. Co‐culture of EphA1‐DsRed‐ and YFP‐ephrin‐A1‐ expressing cells (E‐cadherin‐negative HEK293 cells) induced boundary formation. The cells kept sitting together on a culture dish (upper panel of pictures). TGFβ‐induced ADAM12 activation leads to endocytosis of the Eph/ephrin complex by its protease activity. In our hypothesis, loss of Eph/ephrin‐mediated cell‐cell contacts render tumor cells free to move for metastasis (lower panel of pictures). Scale Bars: 10 μm. B, An enhancement of lung vascular permeability by breakdown of the Eph/ephrin system. ADAM12 localizes in vascular endothelial cells. Activated ADAM12 may cleave membrane‐anchored ephrin‐A1 joining to boundary formation. The cleavage induces endocytosis and RhoA activation leading to cell retraction. Cleaved ephrin‐A1 competes for pre‐existing Eph/ephrin interactions. Ephrin‐A1‐stimulated vascular endothelial cells are shrunk, and thereby enhances lung vascular permeability
5. REGULATORS OF EPHRIN‐A1 EXPRESSION
Overexpression of ephrin‐A1 has been implicated in poor prognosis and tumor malignancy. The highest risk factor for poor prognosis is whether metastasis occurs. However, the detailed molecular mechanisms in metastasis were largely unknown. Ephrin‐A1 expression had no response to TNFα stimulation in some mouse tumor cells such as Lewis lung carcinoma (LLC) and E0771 although ephrin‐A1 expression was significantly enhanced by TNFα stimulation in HUVECs and F2, vascular endothelial cell lines.50 It has been reported that pattern recognition receptors (PRRs) and their ligands, damage‐associated molecular patterns (DAMPs) regulate ephrin‐A1 expression. It is well known that inflammatory cytokines and DAMPs are abundant in tumor microenvironment and modulate angiogenesis, tumor growth, and immune responses.51 The S100A9/EMMPRIN52 and S100A4/RAGE53, 54 axis have been considered to be involved in the regulation of ephrin‐A1 expression. Lipopolysaccharides (LPS), a gram‐negative bacteria‐derived ligand for the toll‐like receptor 4 (TLR4) has been thought to be a positive regulator of ephrin‐A1 expression. TLR4 is a PRR and essential for the innate immune system. TLR4 is expressed not only in immune cells but also in some tumor cells and regulates tumor microenvironment.55 LPS stimulation up‐regulated ephrin‐A1 expression in LLC.50 Subsequently, we investigated the effect of S100A8 on ephrin‐A1 expression since S100A8 has been proposed as an endogenous ligand for TLR4 and elicits inflammatory actions.56, 57 Knockdown of TLR4 abrogated S100A8‐induced ephrin‐A1 expression.50 Hypoxia as often observed inside of tumors is strongly associated with inflammation in various pathological conditions. Nuclear factor‐kappa B (NF‐κB) is a common downstream target of hypoxia‐ and inflammation‐induced signals, and its expression is up‐regulated by hypoxia inducible factor 1α (HIF1α) and inflammatory cytokines such as TNFα and 100A8.58 It was reported that ephrin‐A1 was up‐regulated in hypoxic conditions.59 Taken together, overexpression of ephrin‐A1 in tumors is mediated through inflammation‐ and hypoxia‐induced NF‐κB activation.
6. MOLECULAR MECHANISM OF EPHRIN‐A1‐INDUCED LUNG METASTASIS
Interactions between EphA1/A2 and ephrin‐A1 at cell boundaries provide cell‐cell adhesion (Figure 3A). Co‐culture of EphA1‐expressing cells and ephrin‐A1‐expressing HEK293 cells formed large cell aggregations compared to HEK293 cells transfected with mock vector. EphA1/A2 and ephrin‐A1 were expressed and co‐localized in vascular endothelial cells, and both EphA1 and EphA2 knockout mice showed lung hyper‐permeability because of the absence of Eph/ephrin‐mediated cell adhesion in vascular endothelial cells.46 The other group demonstrated that an injection of Fc‐fused ephrin‐A1 (Fc‐ephrin‐A1) caused lung hyper‐permeability followed by loss of the plasma membrane localization of cell adhesion‐related molecules such as ZO‐1 and claudin‐5.60 Moreover, soluble forms of ephrin‐A1 such as Fc‐ephrin‐A1 compete for pre‐existing EphA2/ephrin‐A1 binding at cell boundaries. Stimulation with soluble forms of ephrin‐A1 in HUVECs induced endocytosis of EphA2 followed by degradation of VE‐cadherin. An intravenous injection of Fc‐ephrin‐A1 resulted in a significant reduction of EphA2 at the plasma membrane and enhanced tumor cell recruitment to the lungs.46 EphA2 is known to be degraded by the c‐Cbl‐dependent ubiquitination system in response to ephrin‐A1 stimulation.61 These data suggest that the Eph/ephrin system regulates vascular homeostasis in lungs, and breakdown of the Eph/ephrin‐mediated cell adhesion in lung endothelial cells causes lung hyper‐permeability facilitating lung metastasis. A neutralizing antibody against ephrin‐A1 that inhibits the interaction between EphA1/A2 and ephrin‐A1 successfully inhibited lung metastasis.46 Taken together, tumor‐derived and ADAM12‐cleaved ephrin‐A1 enter into blood stream and compete for pre‐existing Eph/ephrin binding at cell boundaries. Ephrin‐A1‐induced lung hyper‐permeability render tumor cells easily to extravasate into lungs (Figure 3B). The data suggest that soluble ephrin‐A1 would be a metastatic marker and a good candidate as a molecular targeting drug. Moreover, in primary tumors, ADAM12 may contribute to extravasation of tumor cells because ADAM12 cleaves E‐cadherin that is an essential molecule for adherence junction.62 We have demonstrated that ADAM12 cleaves membrane‐anchored ephrin‐A1 at the plasma membrane, and then cell boundaries organized by the Eph/ephrin interaction were disorganized. Tumor cells in which E‐cadherin and ephrin‐A1 are cleaved leave tumor mass and become free to move to distant organs (Figure 3A).
7. PERSPECTIVES
Ephs and ephrins are regarded as promising candidates for drug development. However, Eph and ephrin had been considered as undruggable target molecules because the interactions between Eph and ephrin are not specific and promiscuous. Therefore, there are no drugs against the Eph/ephrin families for medical use so far. Dasatinib is the only drugs for medical use that shows an inhibitory effect on EphA2 activity. Dasatinib, a Bcr‐Abl tyrosine kinase inhibitor that is clinically used for chemotherapy against chronic myeloid leukemia and acute lymphoblastic leukemia. Pretreatment of dasatinib completely blocked ephrin‐A1‐induced tyrosine phosphorylation of EphA2 in vitro.63 However, dasatinib has not been used for any anti‐EphA2 therapy so far. As we demonstrated, the Eph/ephrin system regulates tyrosine kinase activity‐dependent and ‐independent physiological functions. Therefore, we suppose that tyrosine kinase inhibitors may not be always effective on the Eph/ephrin system. Based on this idea, some researchers isolated protein‐protein interaction (PPI) inhibitors by a typical chemical screening and isolated lithocholic acid (LCA) as a PPI inhibitor of EphA2‐ephrin‐A1.64 LCA completely blocked ephrin‐A1‐induced tyrosine phosphorylation of EphA2 without affecting cell viability and other receptor tyrosine kinase such as EGFR and IGFR. However, LCA showed an inhibitory effect on all Eph‐ephrin‐A1 and all EphB‐ephrin‐B1 interactions. This means that LCA is broad spectrum PPI inhibitor for the Eph/ephrin system. In silico optimization of LCA improved the affinity to the Eph receptors. The derivatives called UniPR1331 is orally available and decreased tumor burden and angiogenesis in an orthotropic mouse GBM model.65 Pasquale and her colleagues successfully isolated some antagonistic and agonistic peptides with micromolar affinity that interact with ligand binding pocket of the Eph receptors by phage display.66 Isolated peptides are very specific and bind to the only single Eph receptor. Some modifications in those peptides gave stability in blood and made them possible to be used for SODG93A transgenic mice, an ALS mouse model.67 The effect of antagonistic peptides against EphA4 on ALS therapy is still under investigation. EphA2 agonistic peptides conjugating to paclitaxel markedly decreased tumor growth in prostate cancer and renal cell carcinoma models compared to sole paclitaxel use.68 Therefore, the Eph/ephrin system is currently no longer undruggable target. We suppose that anti‐Eph/ephrin drugs will come soon.
We have demonstrated that soluble form of ephrin‐A1 would be a good therapeutic target and biomarker for metastasis. In our study, soluble form of ephrin‐A1 was increased in serum of tumor‐bearing mouse compared to that of healthy mice46 and also in HCC patients.69 Furthermore, partial ephrin‐A1 peptides were found in human urine derived from kidney disease patients,70 and we found that immuno‐reactive bands around 25 kDa by using anti‐ephrin‐A1 antibody by western blotting. The size of immuno‐reactive bands were almost same as that of membrane‐anchored ephrin‐A1 and commercially available recombinant ephrin‐A1 (19aa‐182aa) (unpublished data). Therefore, we suppose that the immuno‐reactive urinary ephrin‐A1 is nearly intact. Urinalysis is the best investigation method because it is much less harmful and expensive compared to others such as biopsy for cancer patients. Accordingly, urinary ephrin‐A1 would be a good biomarker for some diseases such as cancer and kidney diseases. We need further investigations to use urinary ephrin‐A1 as a biomarker and/or a therapeutic target.
Lastly, we underline basic research in this system. It was reported that C‐C motif chemokine ligand 2 (CCL2, also known to MCP1) is up‐regulated by ephrin‐A1 stimulation in endothelial cells.71 An enhanced expression was observed in hyper‐permeable regions in tumor‐bearing mouse lungs compared to non‐permeable regions. The CCR2/CCL2 axis promoted S100A8/A9 expression leading to up‐regulation of SAA3 mediated by TLR4.72 S100A8/A9 up‐regulated by the CCR2/CCL2 axis induces ephrin‐A1 and SAA3 expression, and then ephrin‐A1 enhanced CCL2 expression. Collectively, the paracrine system by the ephrin‐A1‐CCL2‐S100A8/A9 axis may exist in endothelial cells in the hyper‐permeable regions in tumor‐bearing mouse lungs, and the paracrine system may increase each protein expression levels and establish fertile soil in the pre‐metastatic lungs (Figure 4). An inhibition of the paracrine loop would be a good approach to inhibit lung metastasis.
Figure 4.
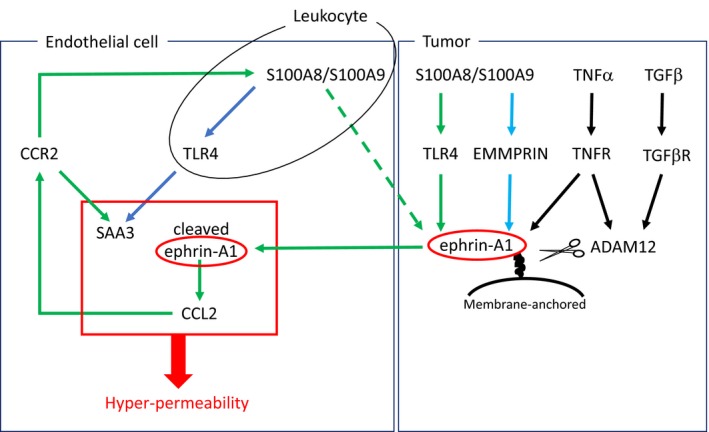
A proposed paracrine loop to establish pre‐metastatic microenvironment in lungs. Ephrin‐A1 expression is enhanced by the TLR4/S100A8 axis. ADAM12 is up‐regulated by inflammatory cytokines such as TNFα and TGF‐β1. Cleaved ephrin‐A1 enters into blood stream and stimulates lung vascular endothelial cells leading to an elevation of CCL2 secretion. The CCR2/CCL2 axis contributes to an increase of SAA3 and S100A8/S100A9 expression. The paracrine loop increases permeability factors enclosed by red box, and then tumor metastasis are facilitated in the lungs. Up‐regulated S100A8/A9 expression in endothelial cells and leukocytes may increase ephrin‐A1 expression mediated by TLR4 and/or EMMPRIN in primary tumors
CONFLICT OF INTEREST
The authors have no conflict of interest.
ACKNOWLEDGMENT
These work was partially supported by JSPS KAKENHI Grant Number JP25870760, the Uehara Memorial Foundation, the Takeda Science Foundation, the Yokoyama zaidan, and Medical Research Institute (MRI), Tokyo Women's Medical University.
Ieguchi K, Maru Y. Roles of EphA1/A2 and ephrin‐A1 in cancer. Cancer Sci. 2019;110:841–848. 10.1111/cas.13942
Contributor Information
Katsuaki Ieguchi, Email: kieguchi@twmu.ac.jp.
Yoshiro Maru, Email: maru.yoshiro@twmu.ac.jp.
REFERENCES
- 1. Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717‐1720. [DOI] [PubMed] [Google Scholar]
- 2. Ieguchi K. Eph as a target in inflammation. Endocr Metab Immune Disord Drug Targets. 2015;15:119‐128. [DOI] [PubMed] [Google Scholar]
- 3. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee. Cell. 1997;90:403‐404. [DOI] [PubMed] [Google Scholar]
- 4. Holzman LB, Marks RM, Dixit VM. A novel immediate‐early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol Cell Biol. 1990;10:5830‐5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davy A, Gale NW, Murray EW, et al. Compartmentalized signaling by GPI‐anchored ephrin‐A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125‐3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neill T, Buraschi S, Goyal A, et al. EphA2 is a functional receptor for the growth factor progranulin. J Cell Biol. 2016;215:687‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monami G, Gonzalez EM, Hellman M, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of ERK1/2 and the formation of a paxillin/FAK/ERK complex. Cancer Res. 2006;66:7103‐7110. [DOI] [PubMed] [Google Scholar]
- 8. Yamazaki T, Masuda J, Omori T, Usui R, Akiyama H, Maru Y. EphA1 interacts with integrin‐linked kinase and regulates cell morphology and motility. J Cell Sci. 2009;122:243‐255. [DOI] [PubMed] [Google Scholar]
- 9. Masuda J, Usui R, Maru Y. Fibronectin type I repeat is a nonactivating ligand for EphA1 and inhibits ATF3‐dependent angiogenesis. J Biol Chem. 2008;283:13148‐13155. [DOI] [PubMed] [Google Scholar]
- 10. Frieden LA, Townsend TA, Vaught DB, et al. Regulation of heart valve morphogenesis by Eph receptor ligand, ephrin‐A1. Dev Dyn. 2010;239:3226‐3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunne PD, Dasgupta S, Blayney JK, et al. EphA2 expression is a key driver of migration and invasion and a poor prognostic marker in colorectal cancer. Clin Cancer Res. 2016;22:230‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naj AC, Jun G, Beecham GW, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late‐onset Alzheimer's disease. Nat Genet. 2011;43:436‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Finney AC, Funk SD, Green JM, et al. EphA2 expression regulates inflammation and fibroproliferative remodeling in atherosclerosis. Circulation. 2017;136:566‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10:165‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fox BP, Kandpal RP. Invasiveness of breast carcinoma cells and transcript profile: Eph receptors and ephrin ligands as molecular markers of potential diagnostic and prognostic application. Biochem Biophys Res Commun. 2004;318:882‐892. [DOI] [PubMed] [Google Scholar]
- 16. Li X, Wang L, Gu JW, et al. Up‐regulation of EphA2 and down‐regulation of EphrinA1 are associated with the aggressive phenotype and poor prognosis of malignant glioma. Tumour Biol. 2010;31:477‐488. [DOI] [PubMed] [Google Scholar]
- 17. Wada H, Yamamoto H, Kim C, et al. Association between ephrin‐A1 mRNA expression and poor prognosis after hepatectomy to treat hepatocellular carcinoma. Int J Oncol. 2014;45:1051‐1058. [DOI] [PubMed] [Google Scholar]
- 18. Yamamoto H, Tei M, Uemura M, et al. Ephrin‐A1 mRNA is associated with poor prognosis of colorectal cancer. Int J Oncol. 2013;42:549‐555. [DOI] [PubMed] [Google Scholar]
- 19. Ishikawa M, Miyahara R, Sonobe M, et al. Higher expression of EphA2 and ephrin‐A1 is related to favorable clinicopathological features in pathological stage I non‐small cell lung carcinoma. Lung Cancer. 2012;76:431‐438. [DOI] [PubMed] [Google Scholar]
- 20. Inokuchi M, Nakagawa M, Baogok N, et al. Prognostic significance of high EphA1‐4 expression levels in locally advanced gastric cancer. Anticancer Res. 2018;38:1685‐1693. [DOI] [PubMed] [Google Scholar]
- 21. Binda E, Visioli A, Giani F, et al. The EphA2 receptor drives self‐renewal and tumorigenicity in stem‐like tumor‐propagating cells from human glioblastomas. Cancer Cell. 2012;22:765‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Day BW, Stringer BW, Al‐Ejeh F, et al. EphA3 maintains tumorigenicity and is a therapeutic target in glioblastoma multiforme. Cancer Cell. 2013;23:238‐248. [DOI] [PubMed] [Google Scholar]
- 23. Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin‐B2 and its receptor Eph‐B4. Cell. 1998;93:741‐753. [DOI] [PubMed] [Google Scholar]
- 24. Hamada K, Oike Y, Ito Y, et al. Distinct roles of ephrin‐B2 forward and EphB4 reverse signaling in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:190‐197. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Nakayama M, Pitulescu ME, et al. Ephrin‐B2 controls VEGF‐induced angiogenesis and lymphangiogenesis. Nature. 2010;465:483‐486. [DOI] [PubMed] [Google Scholar]
- 26. Sawamiphak S, Seidel S, Essmann CL, et al. Ephrin‐B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature. 2010;465:487‐491. [DOI] [PubMed] [Google Scholar]
- 27. Lv J, Xia Q, Wang J, Shen Q, Zhang J, Zhou X. EphB4 promotes the proliferation, invasion, and angiogenesis of human colorectal cancer. Exp Mol Pathol. 2016;100:402‐408. [DOI] [PubMed] [Google Scholar]
- 28. Martiny‐Baron G, Korff T, Schaffner F, et al. Inhibition of tumor growth and angiogenesis by soluble EphB4. Neoplasia. 2004;6:248‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kertesz N, Krasnoperov V, Reddy R, et al. The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4‐EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood. 2006;107:2330‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Depner C, Zum Buttel H, Bogurcu N, et al. EphrinB2 repression through ZEB2 mediates tumour invasion and anti‐angiogenic resistance. Nat Commun. 2016;7:12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chu M, Zhang C. Inhibition of angiogenesis by leflunomide via targeting the soluble ephrin‐A1/EphA2 system in bladder cancer. Sci Rep. 2018;8:1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brantley‐Sieders DM, Caughron J, Hicks D, Pozzi A, Ruiz JC, Chen J. EphA2 receptor tyrosine kinase regulates endothelial cell migration and vascular assembly through phosphoinositide 3‐kinase‐mediated Rac1 GTPase activation. J Cell Sci. 2004;117:2037‐2049. [DOI] [PubMed] [Google Scholar]
- 33. Petrova V, Annicchiarico‐Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment.Oncogenesis. 2018;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okamoto A, Iwamoto Y, Maru Y. Oxidative stress‐responsive transcription factor ATF3 potentially mediates diabetic angiopathy. Mol Cell Biol. 2006;26:1087‐1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Aasheim HC, Delabie J, Finne EF. Ephrin‐A1 binding to CD4+ T lymphocytes stimulates migration and induces tyrosine phosphorylation of PYK2. Blood. 2005;105:2869‐2876. [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Yu H, Shan Y, et al. EphA1 activation promotes the homing of endothelial progenitor cells to hepatocellular carcinoma for tumor neovascularization through the SDF‐1/CXCR4 signaling pathway. J Exp Clin Cancer Res. 2016;35:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569‐571. [DOI] [PubMed] [Google Scholar]
- 38. Reynolds LE, Wyder L, Lively JC, et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27‐34. [DOI] [PubMed] [Google Scholar]
- 39. Ieguchi K, Fujita M, Ma Z, et al. Direct binding of the EGF‐like domain of neuregulin‐1 to integrins ({alpha}v{beta}3 and {alpha}6{beta}4) is involved in neuregulin‐1/ErbB signaling. J Biol Chem. 2010;285:31388‐31398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saegusa J, Yamaji S, Ieguchi K, et al. The direct binding of insulin‐like growth factor‐1 (IGF‐1) to integrin alphavbeta3 is involved in IGF‐1 signaling. J Biol Chem. 2009;284:24106‐24114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raab‐Westphal S, Marshall JF, Goodman SL. Integrins as therapeutic targets: successes and cancers. Cancers (Basel). 2017;9:110 10.3390/cancers9090110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fujita M, Ieguchi K, Cedano‐Prieto DM, et al. An integrin binding‐defective mutant of insulin‐like growth factor‐1 (R36E/R37E IGF1) acts as a dominant‐negative antagonist of the IGF1 receptor (IGF1R) and suppresses tumorigenesis but still binds to IGF1R. J Biol Chem. 2013;288:19593‐19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murali A, Rajalingam K. Small Rho GTPases in the control of cell shape and mobility. Cell Mol Life Sci. 2014;71:1703‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lawson CD, Burridge K. The on‐off relationship of Rho and Rac during integrin‐mediated adhesion and cell migration. Small GTPases. 2014;5:e27958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brantley‐Sieders DM, Fang WB, Hwang Y, Hicks D, Chen J. Ephrin‐A1 facilitates mammary tumor metastasis through an angiogenesis‐dependent mechanism mediated by EphA receptor and vascular endothelial growth factor in mice. Cancer Res. 2006;66:10315‐10324. [DOI] [PubMed] [Google Scholar]
- 46. Ieguchi K, Tomita T, Omori T, et al. ADAM12‐cleaved ephrin‐A1 contributes to lung metastasis. Oncogene. 2014;33:2179‐2190. [DOI] [PubMed] [Google Scholar]
- 47. Frohlich C, Nehammer C, Albrechtsen R, et al. ADAM12 produced by tumor cells rather than stromal cells accelerates breast tumor progression. Mol Cancer Res. 2011;9:1449‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eckert MA, Santiago‐Medina M, Lwin TM, Kim J, Courtneidge SA, Yang J. ADAM12 induction by Twist1 promotes tumor invasion and metastasis via regulation of invadopodia and focal adhesions. J Cell Sci. 2017;130:2036‐2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem. 2004;279:51323‐51330. [DOI] [PubMed] [Google Scholar]
- 50. Ieguchi K, Omori T, Komatsu A, Tomita T, Deguchi A, Maru Y. Ephrin‐A1 expression induced by S100A8 is mediated by the toll‐like receptor 4. Biochem Biophys Res Commun. 2013;440:623‐629. [DOI] [PubMed] [Google Scholar]
- 51. Hernandez C, Huebener P, Schwabe RF. Damage‐associated molecular patterns in cancer: a double‐edged sword. Oncogene. 2016;35:5931‐5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hibino T, Sakaguchi M, Miyamoto S, et al. S100A9 is a novel ligand of EMMPRIN that promotes melanoma metastasis. Cancer Res. 2013;73:172‐183. [DOI] [PubMed] [Google Scholar]
- 53. Boye K, Grotterod I, Aasheim HC, Hovig E, Maelandsmo GM. Activation of NF‐kappaB by extracellular S100A4: analysis of signal transduction mechanisms and identification of target genes. Int J Cancer. 2008;123:1301‐1310. [DOI] [PubMed] [Google Scholar]
- 54. Grotterod I, Maelandsmo GM, Boye K. Signal transduction mechanisms involved in S100A4‐induced activation of the transcription factor NF‐kappaB. BMC Cancer. 2010;10:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li J, Yang F, Wei F, Ren X. The role of toll‐like receptor 4 in tumor microenvironment. Oncotarget. 2017;8:66656‐66667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kawai T, Akira S. The role of pattern‐recognition receptors in innate immunity: update on Toll‐like receptors. Nat Immunol. 2010;11:373‐384. [DOI] [PubMed] [Google Scholar]
- 57. Hiratsuka S, Watanabe A, Sakurai Y, et al. The S100A8‐serum amyloid A3‐TLR4 paracrine cascade establishes a pre‐metastatic phase. Nat Cell Biol. 2008;10:1349‐1355. [DOI] [PubMed] [Google Scholar]
- 58. D'Ignazio L, Bandarra D, Rocha S. NF‐kappaB and HIF crosstalk in immune responses. FEBS J. 2016;283:413‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vihanto MM, Plock J, Erni D, Frey BM, Frey FJ, Huynh‐Do U. Hypoxia up‐regulates expression of Eph receptors and ephrins in mouse skin. FASEB J. 2005;19:1689‐1691. [DOI] [PubMed] [Google Scholar]
- 60. Larson J, Schomberg S, Schroeder W, Carpenter TC. Endothelial EphA receptor stimulation increases lung vascular permeability. Am J Physiol Lung Cell Mol Physiol. 2008;295:L431‐L439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Walker‐Daniels J, Riese DJ 2nd, Kinch MS. c‐Cbl‐dependent EphA2 protein degradation is induced by ligand binding. Mol Cancer Res. 2002;1:79‐87. [PubMed] [Google Scholar]
- 62. Aghababaei M, Hogg K, Perdu S, Robinson WP, Beristain AG. ADAM12‐directed ectodomain shedding of E‐cadherin potentiates trophoblast fusion. Cell Death Differ. 2015;22:1970‐1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chang Q, Jorgensen C, Pawson T, Hedley DW. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giorgio C, Hassan Mohamed I, Flammini L, et al. Lithocholic acid is an Eph‐ephrin ligand interfering with Eph‐kinase activation. PLoS ONE. 2011;6:e18128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Festuccia C, Gravina GL, Giorgio C, et al. UniPR1331, a small molecule targeting Eph/ephrin interaction, prolongs survival in glioblastoma and potentiates the effect of antiangiogenic therapy in mice. Oncotarget. 2018;9:24347‐24363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Riedl SJ, Pasquale EB. Targeting the Eph system with peptides and peptide conjugates. Curr Drug Targets. 2015;16:1031‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Olson EJ, Lechtenberg BC, Zhao C, et al. Modifications of a nanomolar cyclic peptide antagonist for the EphA4 receptor to achieve high plasma stability. ACS Med Chem Lett. 2016;7:841‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang S, Noberini R, Stebbins JL, et al. Targeted delivery of paclitaxel to EphA2‐expressing cancer cells. Clin Cancer Res. 2013;19:128‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cui XD, Lee MJ, Yu GR, et al. EFNA1 ligand and its receptor EphA2: potential biomarkers for hepatocellular carcinoma. Int J Cancer. 2010;126:940‐949. [DOI] [PubMed] [Google Scholar]
- 70. Kistler AD, Serra AL, Siwy J, et al. Urinary proteomic biomarkers for diagnosis and risk stratification of autosomal dominant polycystic kidney disease: a multicentric study. PLoS ONE. 2013;8:e53016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Carpenter TC, Schroeder W, Stenmark KR, Schmidt EP. Eph‐A2 promotes permeability and inflammatory responses to bleomycin‐induced lung injury. Am J Respir Cell Mol Biol. 2012;46:40‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hiratsuka S, Ishibashi S, Tomita T, et al. Primary tumours modulate innate immune signalling to create pre‐metastatic vascular hyperpermeability foci. Nat Commun. 2013;4:1853. [DOI] [PMC free article] [PubMed] [Google Scholar]


