Abstract
We previously showed that an inflammation‐related, molecule leucine‐rich alpha‐2 glycoprotein (LRG) enhances the transforming growth factor (TGF)‐β1‐induced phosphorylation of Smad proteins and is elevated in patients with pancreatic ductal adenocarcinoma (PDAC). As TGF‐β/Smad signaling is considered to play a key role in epithelial‐mesenchymal transition (EMT), we attempted to clarify the mechanism underlying LRG‐related EMT in relation to metastasis in PDAC. We cultured LRG‐overexpressing PDAC cells (Panc1/LRG) and evaluated the morphology, EMT‐related molecules and TGF‐β/Smad signaling pathway in these cells. We also assessed the LRG levels in plasma and resected specimens from patients with PDAC. Inflammatory cytokines induced LRG production in PDAC cells. A spindle‐like shape was visualized more frequently than other shapes in Panc1/LRG with TGF‐β1 exposure. The expression of E‐cadherin in Panc1/LRG was decreased with TGF‐β1 exposure. Invasion increased with TGF‐β1 stimulation of Panc1/LRG. The phosphorylation of smad2 in Panc1/LRG was increased in comparison with parental Panc1 under TGF‐β1 stimulation. In the plasma LRG‐high group, the recurrence rate tended to be higher and the recurrence‐free survival (RFS) tended to be worse in comparison with the plasma LRG‐low group. LRG enhanced EMT induced by TGF‐β signaling, thus indicating that LRG has a significant effect on the metastasis of PDAC.
Keywords: epithelial‐mesenchymal transition, inflammation, leucine‐rich a2‐glycoprotein, metastasis, pancreatic cancer
1. INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal types of cancer,1 with a 5‐year overall survival (OS) rate of only 8%.2 Its frequency is increasing globally, and it now ranks as the 5th leading cause of cancer‐related death.3 However, even small PDAC can cause early distant metastasis, mainly through liver and peritoneal dissemination, after the complete resection of the main tumor.4 The 5‐year OS rate after curative resection without adjuvant chemotherapy was only 10% in the CONKO study.5 However, in the JASPAC study, which noted that the survival time of resectable PDAC patients was longer in comparison with patients with unresectable PDAC, the 5‐year OS rate was approximately 40% after R0 resection with S‐1 adjuvant chemotherapy.6 Accordingly, we have begun performing neo‐adjuvant chemoradiation therapy at our institution, which may improve the OS7, 8, 9, 10; however, the risk of liver metastasis remains, even with good control of local extension, especially in borderline resectable cases of PDAC.11
We previously showed that the existence of PDAC was related to inflammation‐related molecules.12 During our studies on cancer and inflammation, we searched for new inflammation‐related molecules induced by interleukin (IL)‐6 (other than C‐reactive proteins) using a quantitative proteomic approach13 and identified leucine‐rich alpha‐2‐glycoprotein (LRG). LRG, which was first purified from human serum,14, 15 is an inflammatory acute‐phase glycoprotein that mainly exists in the liver and neutrophils.16, 17 Its production is induced by several inflammatory cytokines, including, but not limited to, IL‐1, IL‐6 and IL‐22, and it has been shown to generate transforming growth factor (TGF)‐β1‐related angiogenesis in non‐cancerous regions.18 In addition, we and others have reported that several cancers produce IL‐6 (eg, biliary tract cancer [BTC]11, 19). The levels of serum LRG (considered here as secretory LRG) in PDAC patients were found to be elevated in comparison with healthy volunteers,12 as well as in cancerous regions without cancer‐related inflammation.12 We therefore hypothesized that the levels of inflammatory cytokines are also elevated in PDAC, thereby inducing LRG production. In addition, LRG was shown to induce the TGF‐related phosphorylation of smad2 in cancer.20
Transforming growth factor (TGF)‐β/Smad signaling is considered to play a key role in epithelial‐mesenchymal transition (EMT), which can cause metastasis, invasion and/or chemoresistance. We therefore hypothesized that because LRG levels increase with PDAC progression,12 LRG induces TGF‐related smad2 phosphorylation,20 which results in early distant metastasis, even in cases of R0 resection.11 PDAC‐related LRG elevation may, therefore, induce distant metastasis via TGF‐β1‐related EMT with Smad phosphorylation.
In the present study, we aimed to clarify the mechanism underlying LRG‐related EMT‐induced metastasis in PDAC. We evaluated the relationship of LRG and other inflammatory cytokines in PDAC cells with TGF‐β‐inducing EMT. We also examined the mechanism underlying TGF‐β‐related EMT via Smad phosphorylation. Our data not only showed that the LRG levels could be used to predict metastasis but also suggested the utility of this molecule as a therapeutic target.
2. MATERIALS AND METHODS
2.1. Cell lines
AsPC‐1, BxPC‐3 and Panc1 cells (human PDAC cell lines) were obtained from the European Collection of Authenticated Cell Cultures (ECACC). HUVEC (a human dermal fibroblast cell line) were obtained from the ATCC, and MiaPACA2 and Suit2 cells (human PDAC cell lines) and HepG2 cells (a human hepatocellular carcinoma cell line) were obtained from the Japanese Collection of Research Bioresources (JCRB). MiaPACA2 cells, Panc1 cells and fibroblasts were maintained in DMEM (Wako Pure Chemical Industries, Osaka, Japan). AsPC‐1, BxPC‐3 and Suit2 cells were maintained in RPMI 1640 media (Nacalai Tesque, Kyoto, Japan). HUVEC were grown in MCDB131 culture medium (Chlorella, Tokyo, Japan). All media were supplemented with 10% FBS (Serum Source International, Charlotte, NC, USA) and 100 U/mL penicillin and 100 μg/mL streptomycin (Nacalai Tesque). The cultures were maintained at 37°C in a humidified atmosphere at 5% CO2.
2.2. Recombinant proteins
Recombinant human TGF‐β1 and IL‐1β, tumor necrosis factor (TNF)‐α, IL‐6 and IL‐22 were purchased from PeproTech (Rocky Hill, NJ, USA). Recombinant human LRG was purchased from R&D (Minneapolis, MN, USA).
2.3. Patients and sample collection
Peripheral blood plasma samples were obtained just before and after surgery from 39 patients with PDAC who underwent R0 resection at our hospital between 2007 and 2012. The collection, processing and storage of all blood samples were standardized as follows: blood samples were collected in a Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ, USA), allowed to clot at room temperature for 30 minutes, and then centrifuged at approximately 1300 g for 10 minutes. The serum was removed and immediately divided into 100‐μL and 1‐mL aliquots and stored at −80°C until use. Formalin‐fixed, paraffin‐embedded tissue blocks from these patients were used. The TNM 7th edition (Union for International Cancer Control [UICC]) criteria were used for surgical and pathological staging and to categorize the histologic differentiation.
2.4. Ethics approval
Informed consent was obtained from all patients, and all studies involving human subjects were approved by the Ethical Committee of the Osaka University Hospital (IRB# 17308).
2.5. Quantification of plasma leucine‐rich alpha‐2 glycoprotein (ELISA)
The plasma LRG levels were determined using an ELISA, as previously described.21
2.6. Immunohistochemical staining
Sections were prepared from the abovementioned resected specimens (4 μm). Immunohistochemical (IHC) staining for LRG was performed using a rabbit anti‐LRG monoclonal antibody (1:250, ab178698; Abcam, Chicago, IL, USA), a rabbit anti‐Smad4 monoclonal antibody (1:200, ab40759; Abcam), a rabbit anti‐Smad2 polyclonal antibody (1:100, ab53100; Abcam), a mouse anti‐E‐cadherin polyclonal antibody (610181, 1:200; GE Healthcare Biosciences, Piscataway, NJ, USA) and a mouse anti‐vimentin monoclonal antibody (V6630, 1:200; Sigma‐Aldrich, St. Louis, MO, USA) overnight at 4°C, with visualization using Envision ChemMate (Dako, Glostrup, Denmark), according to the manufacturer's protocol. Three independent gastroenterological oncologists (HW, SK and TO), who were blinded to the histologic data, analyzed the stained sections, which were also photographed using a light microscope (DM2500 with the Leica Application Sweat software program [version 3.80]; Leica Microsystems GmbH, Wetzlar, Germany).
2.7. Real‐time RT‐PCR
Total RNA was isolated from the indicated cells using an RNeasy Mini Kit (Qiagen, Tokyo, Japan) according to the manufacturer's protocol. First, 100 ng of RNA was reverse transcribed using a QuantiTect Reverse Transcription Kit (Qiagen). For a quantitative RT‐PCR, standard curves for mLRG, plasminogen activator inhibitor‐1 (PAI‐1) and LRG were generated from serial dilutions of positively expressing cDNA. The relative quantification of the PCR products was performed using an ABI prism 7700 (Applied Biosystems, Darmstadt, Germany) and the comparative threshold cycle (CT) method. The target gene expression was normalized to that of β‐actin in each sample. The following primers were used for the RT‐PCR: human PAI‐1 forward 5′‐AAGAACCCACGGAAATGTTG‐3′, reverse 5′‐GAGGAAGGCACAGCAAAGTC‐3′, human LRG forward 5′‐TTTACAGGTGAAACTCGGGG‐3′, reverse 5′‐ACCCCAAGCTAAGTGGGACT‐3′, human β‐actin forward 5′‐AGCCTCGCCTTTGCCGA‐3′, reverse 5′‐CTGGTGCCTGGGGCG‐3′. Each reaction was performed in triplicate. The variation within samples was <10%.
2.8. Western blotting
Whole‐cell protein extract was prepared from Panc1 or HepG2 cells in RIPA buffer (10 mmol/L Tris‐HCl (pH 7.5), 150 mmol/L NaCl, 1% NP40, .1% SDS, .5% sodium deoxycholate, 1% protease inhibitor cocktail [Nacalai Tesque] and 1% phosphatase inhibitor cocktail [Nacalai Tesque]). The extracted proteins were resolved on SDS‐PAGE and transferred to an Immobilon‐P Transfer Membrane (Millipore, Bedford, MA, USA). The following antibodies were used: anti‐phospho‐Smad1 (Ser463/465)/Smad5 (Ser463/465)/Smad8 (Ser426/428) (41D10, 1:1000; Cell Signaling Technology, Danvers, MA, USA), anti‐Smad1 (D59D7, 1:1000; Cell Signaling Technology), anti‐phospho‐Smad2 (Ser465/467) (D27F4, 1:1000; Cell Signaling Technology), anti‐Smad2 (D43B4, 1:1000; Cell Signaling Technology), anti‐phospho‐NF‐κB (Ser536) (93H1, 1:1000; Cell Signaling Technology), anti‐NF‐κB (C22B4, 1:1000; Cell Signaling Technology), anti‐phospho‐STAT3 (Try705) (M9C6, 1:1000; Cell Signaling Technology), anti‐STAT3 (D1B2J 1:1000; Cell Signaling Technology), anti‐E‐cadherin (610181, 1:1000; GE Healthcare Biosciences), anti‐vimentin (V6630, 1:2000; Sigma‐Aldrich), anti‐ALK5 (TGF‐β1 receptor kinase), (SC‐20072, 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti‐GAPDH (sc‐4775, 1:2000; Santa Cruz Biotechnology). This was followed by treatment with 1:5000 diluted donkey anti‐rabbit HRP‐conjugated secondary antibodies (GE Healthcare Biosciences) and visualization using the western lightning ECL reagent (Perkin‐Elmer, Boston, MA, USA).
2.9. Generation of cell lines with the stable expression of human leucine‐rich alpha‐2‐glycoprotein
To generate cell lines with the stable expression of human LRG (hLRG), Panc1 cells with a TGF‐β1 receptor were transfected with the pcDNA3.1 LRG expression vector, as described previously.20 Transfected cells were selected using 1000 μg/mL of geneticin (Invitrogen, Carlsbad, CA, USA). Clones were maintained in 250 μg/mL of geneticin to ensure the stability of the expression.
2.10. siRNA transfection
siRNA treatment was performed with Dharmacon ON‐TARGETplus SMART pool siRNA probes (Dharmacon, Lafayette, CO, USA) using the ON‐TARGETplus siCONTROL Nontargeting Pool (siCONTROL, D‐001810‐10; Dharmacon) as a control. ON‐TARGETplus SMART pool siRNA for hLRG (siLRG, L‐015179‐01‐0010; Dharmacon) was used. The cells were transfected with siRNA using Lipofectamine 2000 Reagent (Invitrogen) according to the manufacturer's instructions.
2.11. Immunocytochemistry
Panc1 cells were grown on coverslips and fixed with PBS (pH 7.4) containing 4% paraformaldehyde at 4°C for 15 minutes. After being washed in PBS 3 times (for 5 minutes each), the cells were permeabilized in PBS containing .1% Triton X‐100 at room temperature for 15 minutes. The cells were then washed again in PBS 3 times (for 5 minutes each) and blocked in blocking buffer (PBS containing 3% BSA) at room temperature for 60 minutes. They were then incubated with anti‐E‐cadherin polyclonal antibody at a dilution of 1:2000 in the blocking buffer at 4°C overnight, washed in PBS 3 times (for 5 minutes each) and incubated with cyanine 3‐conjugated goat anti‐rabbit immunoglobulin G (Jackson ImmunoResearch, West Grove, PA, USA).
2.12. Invasion assays
Invasion assays were performed with invasion chambers loaded with Matrigel, according to the manufacturer's instructions (Biocoat Matrigel Invasion Chamber; Collaborative Biomedical Products; Becton Dickinson), as described previously.22 In brief, 5 × 104 cells were overlaid onto the Matrigel matrix on a membrane with 8‐μm pores (upper chamber: FBS .5%, lower chamber: FBS 10%). After 48 hours, the cells that had invaded the undersurface of the membrane were fixed with methanol and stained with thiazine and eosinate. Five microscopic fields were randomly selected for cell counting.
2.13. Cell viability assays
Cell viability was assessed using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT; Sigma‐Aldrich) assay, as described previously.23 Each cell line was seeded onto a 96‐well plate (3 × 103 cells/well) and incubated for 48 and 72 hours. Cell viability was then evaluated based on the absorbance using MTT solution.
2.14. Statistical analyses
Statistical analyses were performed using the JMP Pro 13.2.1 software program (SAS Institute, Cary, NC, USA). The OS, recurrence‐free survival (RFS), and cumulative distant metastasis rates were evaluated using the Kaplan‐Meier method and assessed using the log‐rank test. All parameters found to be significant in univariate analyses using the Cox proportional hazard model were included in a multivariate survival analysis. Unpaired Student's t tests were performed to test the significance of differences between 2 groups. P‐values of <.05 were considered to indicate statistical significance.
3. RESULTS
3.1. Selection of pancreatic ductal adenocarcinoma cell lines
We evaluated 5 pancreatic cancer cell lines (AsPC‐1, BxPC‐3, MiaPACA2, Suit2 and Panc1) and ultimately selected the Panc1 cell line because it expresses EMT markers with wild Smad4. EMT is reportedly induced in BxPC3 and MiaPACA cells by TGF‐β.24 We confirmed the expression of EMT markers (E‐cadherin, N‐cadherin and vimentin) in these cell lines by western blotting (Figure S1). MiaPACA2 cells did not produce E‐cadherin; AsPC‐1, BxPC‐3 and MiaPACA2 cells did not produce N‐cadherin; and BxPC3 cells did not express vimentin. However, Panc1 and Suit2 cells expressed all 3 EMT markers, with Panc1 cells expressing E‐cadherin most clearly among the cell lines. MiaPaCa‐2 cells reportedly do not express TGF‐β receptor II, and AsPc‐1 and BxPc‐3 cells do not express Smad4.25 For the abovementioned reasons, we ultimately selected the Panc1 cell line from the available cell lines for use in the present experiment.
3.2. Production of leucine‐rich alpha‐2 glycoprotein in pancreatic ductal adenocarcinoma cells and the induction of leucine‐rich alpha‐2 glycoprotein stimulated with cytokines
The production of LRG in PDAC cells was determined by measuring the LRG protein in the supernatant of PDAC cells. The production in PDAC cells was very low in comparison with HCC cells (Figure 1A). Thus, LRG was predicted to be produced by inflammatory cytokine stimulation. We then stimulated Panc1 cells using IL‐1β, TNF‐α, IL‐6 or IL‐22, and the levels of phospho‐NF‐κB and phospho‐STAT3 in Hep G2 and Panc1 cells were found to be elevated in comparison with total NF‐κB and STAT3.
Figure 1.
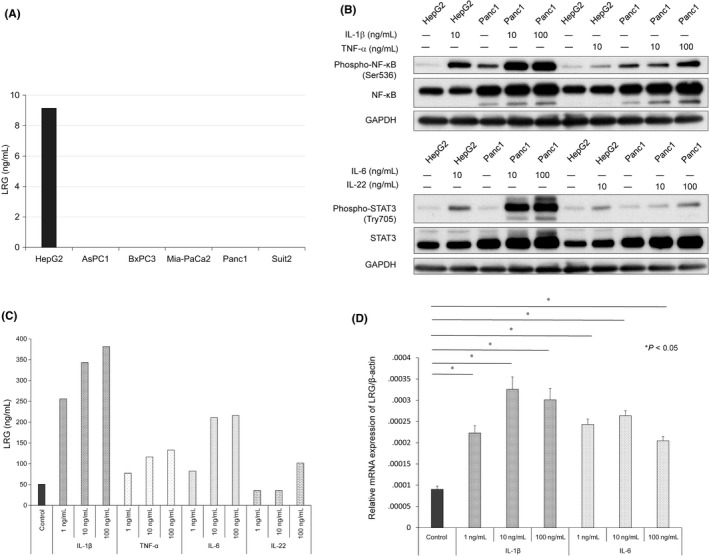
Cytokines induced leucine‐rich alpha‐2‐glycoprotein (LRG) production in Panc1 cells. A, The LRG protein level in the supernatant of pancreatic ductal adenocarcinoma cells and hepatocellular carcinoma cells. In contrast with hepatocellular carcinoma cells, the pancreatic ductal adenocarcinoma cell lines did not produce LRG. B, The phosphorylation of NF‐κB and STAT3 in Hep G2 and Panc1 cells, stimulated by interleukin (IL)‐1β or tumor necrosis factor (TNF)‐α/IL‐6 or IL‐22. Panc1 and HepG2 cells were pre‐treated with the indicated cytokines for 30 min. C, The LRG protein level in culture supernatants of Panc1 cells stimulated for 72 h with increasing doses of IL‐1β, TNF‐α, IL‐6 or IL‐22 (1‐100 ng/mL). D, The relative LRG mRNA expression in Panc1 cells following IL‐1β (1‐100 ng/mL) or IL‐6 (1‐100 ng/mL) exposure for 24 h. *P < .05 in comparison with control
Panc1 cells have been shown to have high sensitivity to IL‐1β and IL‐6 (Figure 1B). Indeed, the production and expression of LRG in Panc1 cells were stimulated by the inflammatory cytokines IL‐1β, TNF‐α, IL‐6 and IL‐22 (Figure 1C). LRG mRNA was expressed in PDAC cells (Figure 1D). Furthermore, among the other PDAC cell lines (AsPC1, BxPC3 and MiaPACA, particularly AsPC1), BxPC3 produced LRG in response to stimulation by inflammatory cytokines (Figure S2A). In addition, AsPC1 and BxPC3 cells showed sensitivity to IL‐1β, TNF‐α, IL‐6 and IL‐22 (Figure S2B,C). In contrast, fibroblasts and HUVEC did not produce LRG, regardless of their sensitivity to IL‐1β, TNF‐α, IL‐6 and IL‐22 (Figure S2A,E,F). The MiaPACA cell line has also been demonstrated to show sensitivity to IL‐1β, TNF‐α, IL‐6 and IL‐22; however, it produced less LRG than the AsPC1 and BxPC3 lines (Figure 2D). Taken together, these findings indicate that stimulation with inflammatory cytokines promoted LRG production in PDAC cells.
Figure 2.
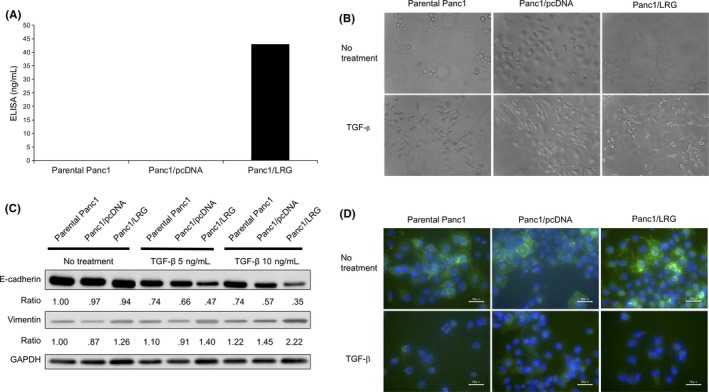
Leucine‐rich alpha‐2‐glycoprotein (LRG) induced epithelial‐mesenchymal transition (EMT) under transforming growth factor (TGF)‐β1 exposure. A, The LRG protein level in the supernatant of parental Panc1, Panc1/pcDNA and LRG‐overexpressing Panc1 cells (Panc1/LRG). Panc1/LRG cells produced LRG. B, Morphological changes in parental Panc1 and LRG‐overexpressing Panc1 cells (Panc1/LRG) with or without TGF‐β1. EMT‐related morphological changes and a spindle‐like shape were more often noted in cells with the overexpression of LRG than in those without. C, The expression of EMT markers E‐cadherin and vimentin in parental Panc1, Panc1/pcDNA and Panc1/LRG cells. The expression of E‐cadherin protein was decreased (approximately 50% of parental Panc1), while that of vimentin was increased (approximately 180% of parental Panc1) in Panc1/LRG cells with TGF‐β1 exposure. D, Immunofluorescence staining for E‐cadherin in parental Panc1, Panc1/pcDNA and Panc1/LRG cells. The E‐cadherin expression was decreased in Panc1/LRG cells with TGF‐β1 exposure
3.3. Epithelial‐mesenchymal transition sensitization for transforming growth factor‐β1 in leucine‐rich alpha‐2 glycoprotein‐expressing pancreatic ductal adenocarcinoma cells
In the PDAC cell experiment using human LRG recombination, recombinant human LRG (rhLRG) enhanced the expression of vimentin mRNA (Figure S3A) and N‐cadherin proteins (Figure S3C), while the expression of E‐cadherin was unaffected (Figure S3B). To investigate the role of LRG in PDAC cells, we constructed continuous LRG‐expressing Panc1 cells (Figure 2A), as Panc1 cells contain a TGF‐β receptor.26 We constructed 9 cell clones that stably expressed LRG (Panc1/LRG, Figure S4) and ultimately selected clone #2 (although we initially selected clones #1 and #2 because of their high LRG production). To investigate EMT, we evaluated the changes in the E‐cadherin expression of these clones on exposure to TGF‐β1 (Figure S5 E‐cadherin protein). Panc1/LRG clone #2 showed the most remarkable change in the expression of E‐cadherin. We therefore selected Panc1/LRG clone #2 for further experiments.
The morphology of the LRG‐expressing Panc1 (Panc1/LRG) cells was similar to that of the Panc1 parent cells without any stimulation. A spindle‐like shape was visualized more often than other shapes in Panc1/LRG with TGF‐β1 exposure (Figure 2B). Furthermore, the expression of E‐cadherin decreased while that of vimentin increased after TGF‐β1 exposure in Panc1/LRG than in parental Panc1 cells (Figure 2C). We confirmed the changes in the E‐cadherin levels by immunocytochemistry, and a greater decrease in the E‐cadherin expression was observed in Panc1/LRG cells exposed to TGF‐β1 in comparison with parental Panc1 and Panc1/pcDNA cells (Figure 2D). In contrast, the knockdown of LRG by siRNA (siLRG) resulted in the elevation of E‐cadherin (Figure S6A,B). In an invasion assay, Panc1/LRG cells showed a greater invasion ability than the parental Panc1 and Panc1/pcDNA cells under exposure to TGF‐β1 (Figure 3). The decrease in the expression of changing spindle‐like, E‐cadherin and invasion ability was canceled by the knockdown of LRG (siLRG) (Figure S6C,D). We performed cell viability assays of parental Panc1, Panc1/pcDNA and LRG‐overexpressing Panc1 (Panc1/LRG) cells. The cell viability of these lines was similar (Figure S7). We performed western blotting to assess the phosphorylation of Smad1/5/8 and Smad2/3. The expression of phosphorylated Smad2 under TGF‐β1 exposure increased in Panc1/LRG cells in comparison with parental Panc1 cells; however, Panc1/LRG cells and parental Panc1 cells expressed similar levels of phosphorylated Smad1/5/8 (Figure 4A,B). In addition, we evaluated the level of PAI‐1, which is a transcriptional target gene of Smad2/3.20 Thereafter, we performed quantitative RT‐PCR to evaluate the PAI‐1 expression of Panc1/LRG cells under TGF‐β1 stimulation. TGF stimulation increased the PAI‐1 expression in Panc1/LRG cells to a greater extent than it did in parental Panc1 and Panc1/pcDNA cells (Figure 4C). In addition, the expression of the TGF‐β type I receptor (ALK5) in Panc1/LRG was increased in comparison with parental Panc1 and Panc1/pcDNA cells (Figure 4D). Taken together, these findings suggest that LRG‐expressing Panc1 cells were sensitized to TGF‐induced EMT via the increased expression of the TGF‐β type I receptor and the phosphorylation of Smad2.
Figure 3.
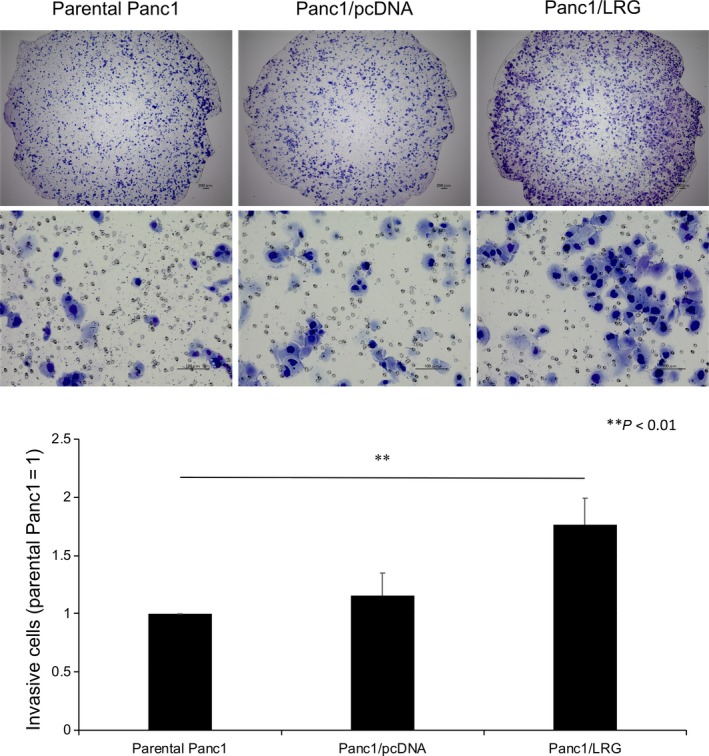
The overexpression of leucine‐rich alpha‐2‐glycoprotein (LRG) in Panc1 cells promoted invasion with transforming growth factor (TGF)‐β1 treatment. A 48‐h invasion assay revealed that Panc1/LRG cells had a stronger invasion ability with TGF‐β1 exposure than parental Panc1 and Panc1/pcDNA cells (P < .05). The upper panel shows a representative picture (upper: low magnification, middle: high magnification). Cells were stained for 48 h after plating. The lower panel shows the number of invading cells. The number of invading Panc1/LRG cells was approximately 180% that of the number of invading parental Panc1 cells
Figure 4.
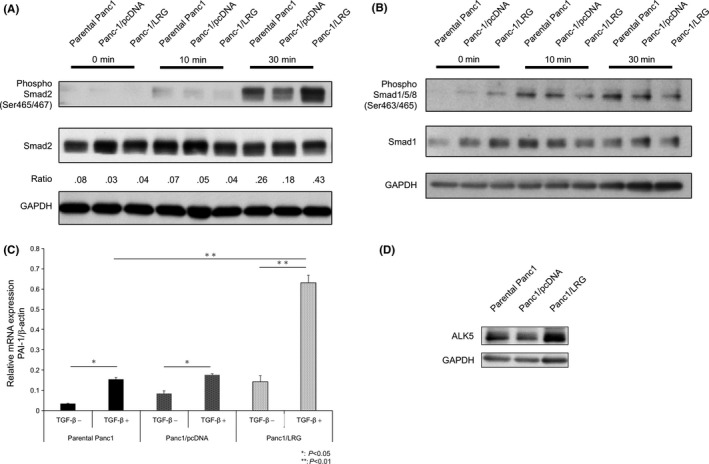
The Smad2 signaling pathway was dominant in leucine‐rich alpha‐2‐glycoprotein (LRG)‐overexpressing Panc1 cells under TGF‐β1 treatment. A, The phosphorylation of smad2 in parental Panc1, Panc1/pcDNA and Panc1/LRG treated with TGF‐β1. After 24 h of serum starvation, cells were treated with or without TGF‐β1 (5.0 ng/mL) for 10 or 30 min. The phosphorylation of smad2 was increased in Panc1/LRG cells exposed to TGF‐β1 (approximately 180% of the parental Panc1 cells). B, The phosphorylation of smad1/5/8 in parental Panc1, Panc1/pcDNA and Panc1/LRG cells treated with TGF‐β1. After 24 h of serum starvation, cells were treated with or without TGF‐β1 (5.0 ng/mL) for 10 or 30 min. The phosphorylation of smad1/5/8 did not change in Panc1/LRG cells exposed to TGF‐β1. C, The plasminogen activator inhibitor‐1 (PAI‐1) mRNA expression with or without TGF‐β1 stimulation (5.0 ng/mL) for 3 h in parental Panc1, Panc1/pcDNA and Panc1/LRG cells. The quantitative real‐time PCR threshold values for the target genes were normalized against the level of β‐actin. The PAI‐1 expression in Panc1/LRG cells was 3‐fold higher than that in the parental Panc1 cells. D, The protein expression of ALK5 (TGF‐β type I receptor kinase) in parental Panc1, Panc1/pcDNA and Panc1/LRG cells. The ALK5 in Panc1/LRG cells was increased in comparison with that in parental Panc1 cells
3.4. Survival of pancreatic ductal adenocarcinoma patients after R0 resection and their leucine‐rich alpha‐2 glycoprotein expression
We examined the preoperative plasma LRG values of PDAC patients (39 cases). The recurrence rate tended to be higher and the RFS was worse in patients with high plasma levels of LRG than in patients with low plasma levels of LRG (Figure 5A‐D), with no marked difference in the baseline characteristics of the patients in these 2 groups (Table 1). In the group without preoperative chemoradiation therapy (22 cases), both the recurrence rate and the distant recurrence rate were significantly increased (Figure 5E‐H), with no marked difference in the baseline characteristics of the patients in the 2 groups (Table 2). However, in the group with preoperative chemoradiation therapy, there were no marked differences in the recurrence rate or the distant recurrence rate (Figure 5I‐L). Regarding immunohistochemical staining for LRG, the median RFS in patients who were strongly positive for LRG was shorter than that in patients who were weakly positive for LRG (.53 years vs 1.04 years) among the patients without preoperative chemoradiation therapy. Taken together, these findings suggest that the production of LRG in plasma influenced the development of distant metastasis in patients with PDAC.
Figure 5.
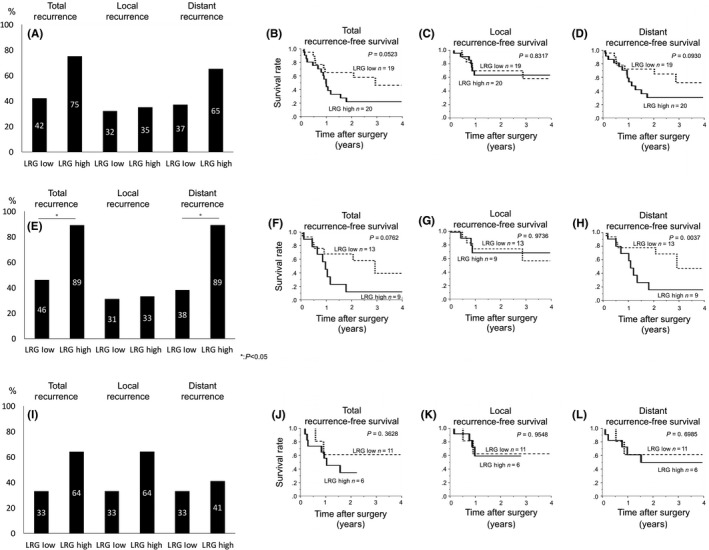
Recurrence pattern analyses in pancreatic cancer patients according to the plasma leucine‐rich alpha‐2‐glycoprotein (LRG) levels in patients treated with or without chemoradiation. A‐D, Total, local and distant recurrence according to the preoperative plasma LRG level (A) and the recurrence‐free survival (RFS) curves for total (B), local (C) and distant recurrence (D). E, F, D and H, Recurrence pattern without preoperative chemoradiation (E) and the RFS curves for total (F), local (G) and distant recurrence (H). I‐L, Recurrence pattern with preoperative chemoradiation (I) and the RFS curves for total (J), local (K) and distant recurrence (L)
Table 1.
Patients’ characteristics regarding plasma LRG
| Variables Data express as number (%) | Preoperative LRG high | Preoperative LRG low | P‐value |
|---|---|---|---|
| Gender | |||
| Male | 55% | 68% | .5231 |
| Female | 45% | 32% | |
| Location of pancreas | |||
| Head | 70% | 68% | .5231 |
| Body and tail | 30% | 32% | |
| Tumor size (mm) | 26 | 23.6 | .1939 |
| Histology | |||
| Well‐differentiated tubular adenocarcinoma | 5% | 5% | 1.0000 |
| Moderately | 85% | 95% | |
| Poorly | 10% | 0% | |
| UICC‐pT | |||
| pT1 | 15% | 16% | 1.0000 |
| pT2 | 15% | 16% | |
| pT3 | 70% | 68% | |
| UICC‐pN1 | 35% | 37% | 1.0000 |
| UICC‐M1 | 0% | 0% | N/A |
| UICC‐pStage | |||
| I | 20% | 26% | .7164 |
| II | 80% | 74% | |
| III | 0% | 0% | |
| IV | 0% | 0% | |
| Positive for microinvasion lymphatic system | 65% | 68% | 1.0000 |
| Positive for microinvasion to venous system | 25% | 42% | |
| Positive for microinvasion to nervous system | 90% | 89% | |
Values are expressed as mean ± SD or number (%). LRG, leucine‐rich alpha‐2‐glycoprotein; N/A, not applicable; UICC, Union for International Cancer Control.
Table 2.
Patients’ characteristics regarding plasma LRG without preoperative chemoradiation therapy
| Variables Data express as number (%) | Preoperative LRG high | Preoperative LRG low | P‐value |
|---|---|---|---|
| Gender | |||
| Male | 55% | 62% | 1.0000 |
| Female | 45% | 38% | |
| Location of pancreas | |||
| Head | 55% | 62% | 1.0000 |
| Body and tail | 45% | 38% | |
| Tumor size (mm) | 31.6 | 23.1 | .0555 |
| Histology | |||
| Well‐differentiated tubular adenocarcinoma | 0% | 8% | .5422 |
| Moderately | 77% | 92% | |
| Poorly | 23% | 0% | |
| UICC‐pT | |||
| pT1 | 11% | 8% | .6161 |
| pT2 | 0% | 15% | |
| pT3 | 89% | 77% | |
| UICC‐pN1 | 56% | 69% | .6656 |
| UICC‐M1 | 0% | 0% | N/A |
| UICC‐pStage | |||
| I | 11% | 23% | .6161 |
| II | 89% | 77% | |
| III | 0% | 0% | |
| IV | 0% | 0% | |
| Positive for microinvasion lymphatic system | 89% | 69% | .3602 |
| Positive for microinvasion to venous system | 56% | 47% | 1.0000 |
| Positive for microinvasion to nervous system | 89% | 92% | 1.0000 |
Values are expressed as mean ± SD or number (%). LRG, leucine‐rich alpha‐2‐glycoprotein; N/A, not applicable; UICC, Union for International Cancer Control.
We confirmed the immunoreactivity for LRG, Smad4, pSmad2, E‐cadherin and vimentin in resected PDAC specimens (Figure S8A‐E). LRG was strongly stained in 12 cases (34%, Figure S8A), and Smad4 mutations were noted in 15 cases (43%) in our study (Figure S8B). Among the specimens with wild‐type (20 cases) Smad4, pSmad2 was strongly stained in 10 cases (50%, Figure S8C), E‐cadherin was weakly stained in 4 cases (20%, Figure S8D), and vimentin was strongly stained in 13 cases (65%, Figure S8E). We examined the IHC staining for LRG and pSmad2, and evaluated any differences between LRG and pSmad2. According to the results, the group that was strongly positive for LRG and tended to be strongly positive for pSmad2 increased (67% of the cases that were strongly positive for pSmad2 were strongly positive for LRG, while only 30% of the cases that were weakly positive for pSmad2 were weakly positive for pSmad2) (Figure S8F). On categorizing the staining patterns according to strength (Figure S8G), weak staining for E‐cadherin was frequently noted in the LRG‐positive and pSmad2‐strongly positive group (3 of 4 cases, 75%). In addition, strong staining for vimentin was frequently noted in the LRG‐positive and pSmad2‐strongly positive group (Figure S8H). In contrast, among the specimens with a Smad4 mutation, LRG was not relevant for either E‐cadherin or pSmad2 (Figure S8I).
Leucine‐rich alpha‐2‐glycoprotein is produced not only by PDAC cells but also by hepatocytes.17 Thus, we also calculated the rate of decrease in the LRG expression after surgery. We only examined the LRG produced by PDAC cells and excluded any LRG produced by hepatocytes. We measured the plasma LRG levels in PDAC patients before and after surgery (Figure S9A). In most patients, the plasma LRG level was decreased after surgery (Figure S9B). We confirmed the immunoreactivity for LRG in the resected specimen (Figure S8A). Strong positivity for LRG was detected in the patients with high plasma levels of LRG (Figure S9C).
4. DISCUSSION
As hypothesized, we found that the production LRG by PDAC cells was induced by stimulation with inflammatory cytokines. Furthermore, the EMT and invasion induced by TGF‐β1 were enforced by LRG, suggesting a possible mechanism underlying the relationship between LRG and distant metastasis. We and other groups have previously shown that LRG binds to TGF‐β1 and/or its receptors and modulates the downstream pathways of TGF‐β signaling in a Smad1/5‐depedent18, 27 or Smad2‐dependent20 manner. Wang et al18 showed that endoglin plays a critical role in the switch of TGF‐β signaling from the Smad2 to the Smad1/5 pathway. Interestingly, we previously showed that LRG binds to TGF‐β1 and enhances the Smad2 pathway in an endoglin‐independent manner.20, 28 Together with the findings of these previous reports, the results of this study suggest that LRG in association with TGF‐β1 enhances the TGF‐β‐induced EMT via Smad2 signals and increases the expression of the TGF‐β type I receptor, which seems to be associated with increased EMT in TGF‐β1‐sensitive PDAC cells.
Inflammatory change has not been directly seen in PDAC; however, several reports have suggested that inflammatory cytokines may affect the progression of PDAC,12, 29, 30, 31, 32, 33, 34, 35, 36, 37 especially IL‐6. These cytokines also affect the progression of other cancers.38 For example, BTC, a counterpart of PDAC, is commonly studied in this field, and we previously showed that inflammatory cytokines enhance resistance to apoptosis39, 40 and the Smad4 regulation of cancer development41 via the crosstalk between IL‐6 and TGF‐β1 in this entity.42 In PDAC cells, it was reported that IL‐6 was involved in EMT via stat337; however, these studies only showed an “indirect effect” on EMT, including a missing link between IL‐6 signaling and EMT. Our study directly showed that inflammatory cytokines (IL‐6) induced the secretion of LRG and that the secreted LRG subsequently increased the TGF‐related EMT. We therefore consider LRG to be a missing link between inflammation and EMT.
It was reported that LRG was an approximately 50‐kDa glycoprotein that contains repetitive sequences with a leucine‐rich motif.14 and is expressed in the serum,15 neutrophils17 and liver.16 LRG was initially considered a marker of granulocytic differentiation,17, 43 and we previously showed its role as a marker of activity in inflammatory diseases.13, 44, 45 Neutrophils play important roles in inflammation, tissue damage and wound healing, and the TGF‐β1 secreted from platelets induces EMT.46, 47 As neutrophils express LRG, we hypothesized that LRG from neutrophils would directly induce EMT during inflammation as well as act as a marker of inflammation.
Our data showed that cytokines induced the production of LRG in PDAC cells. We and others have studied the expression of LRG by several cells. The LRG expression without stimulation was found to be very low in normal cells and slightly high in the liver,20 while the expression was elevated in gastric cancer cells.48 In the present study, the LRG expression in PDAC was much lower than that in HCC cells. The induction of LRG elevation by cytokines has not been well investigated, and the levels in primary bronchial epithelial cells were only elevated by IL‐4 and TNF‐α.49 We showed that the LRG expression and production were increased in a dose‐dependent manner by exposure to several cytokines, including TNF‐α. Thus, normal cells express LRG very slightly, while cancer cells express LRG slightly more strongly. Furthermore, cancerous cells seem to be able to produce LRG in a dose‐dependent manner after cytokine stimulation.
The IL‐6 loop in pancreatic cancer30, 35 plays a similar role to that in BTC cells39, 40, 42 and affects the surrounding immune cells and/or pancreatic satellite cells.30, 35 Furthermore, the progression of pancreatic cancer induces the secretion of IL‐6.31, 32 IL‐6 loops may, therefore, contribute to the LRG‐related EMT during cancer progression, making LRG a direct marker of the induction of tumor EMT‐type progression. It is generally difficult to detect factors induced by locoregional tumors in serum. However, according to our data, the serum LRG level reflected the local PDAC progression and predicted distant metastasis, suggesting that this marker might directly reflect the effects of treatment on metastasis. Our previous study showed that LRG levels increased with the progression of pancreatic cancer,12 and this study showed that LRG levels decreased following tumor removal, with a lower LRG decrement indicating possible remnant micrometastasis. The bloodstream LRG level may have been elevated not by small local cancer but by small metastasis. Thus, the serum LRG level was useful for detecting micrometastasis rather than small pancreatic cancer.
Regarding the limitations associated with this study, the effect of LRG differed between recombinant LRG and continuous LRG‐expressing Panc1. We used recombinant human LRG‐1 (catalog number: 7890‐LR, DCRZ0114011). The source of recombinant human LRG was human embryonic kidney cells, HEK293‐derived Val36‐Gln347, with a C‐terminal 6‐His tag. While the LRG in the continuous LRG‐expressing Panc1 cells in the present study did not have protein tags. It remains possible that such protein tags may inactivate the protein activity. Accordingly, we hypothesized that in continuous LRG‐expressing Panc1, the protein activity and protein conformation are more physiologically appropriate than recombinant human LRG. The LRG blocking effect was insufficiently demonstrated in the present study. As LRG directly contributed to EMT‐type tumor progression, LRG may be a useful therapeutic target. LRG sensitizes TGF receptors, and the LRG level can be assessed from the serum, making it not only a good marker (a marker of progression and for judging the effect of therapy) but also a useful therapeutic target for regulating EMT. Smad4 mutations should also be noted, as 20%‐50% of PDAC have a Smad4 mutation. EMT may be induced by different pathways depending on the presence of a Smad4 mutation. For wild‐type Smad4, TGF‐β1 regulates tumor progression by a tumor cell‐autonomous mechanism,50 as shown in our in vitro study using wild‐type Smad4 PDAC cells. In contrast, for mutant Smad4, TGF‐β reportedly induces EMT not via Smad but by an alternative pathway (eg, the Ras‐MEK‐ERK‐pathway).26 Our in vitro data indicated that LRG enhances TGF‐β‐mediated EMT in wild‐type Smad4 PDAC cells, and these findings are supported by our data using resected specimens (Figure S9C).
In conclusion, LRG enhances TGF‐β, inducing EMT, which is associated with distant metastasis in PDAC. LRG may be useful as a universal marker of tumor progression and a potential therapeutic target for regulating EMT.
CONFLICT OF INTEREST
All authors declare no conflicts of interest in association with the present study.
Supporting information
ACKNOWLEDGMENTS
This work was supported in part by the Practical Project for Rare/Intractable Diseases from Japan Agency for Medical Research and Development (15ek0109045h0002), the JSPS KAKENHI Grant‐in‐Aid for Young Scientists (Start‐up) (15H06918), a Grant‐in‐Aid for Scientific Research (17H04215), and Bristol‐Myers Squibb Foundation Grants, and the present study was supported by a Grant‐in‐Aid for Scientific Research (C) 15K10202.
Otsuru T, Kobayashi S, Wada H, et al. Epithelial‐mesenchymal transition via transforming growth factor beta in pancreatic cancer is potentiated by the inflammatory glycoprotein leucine‐rich alpha‐2 glycoprotein. Cancer Sci. 2019;110:985–996. 10.1111/cas.13918
REFERENCES
- 1. Colvin H. Gastroenterological surgery in Japan: the past, the present and the future. Ann Gastroenterol Surg. 2017;1:5‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 3. Li HY, Cui ZM, Chen J, Guo XZ, Li YY. Pancreatic cancer: diagnosis and treatments. Tumour Biol. 2015;36:1375‐1384. [DOI] [PubMed] [Google Scholar]
- 4. Ishikawa O, Ohigashi H, Imaoka S, et al. Minute carcinoma of the pancreas measuring 1 cm or less in diameter–collective review of Japanese case reports. Hepatogastroenterology. 1999;46:8‐15. [PubMed] [Google Scholar]
- 5. Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long‐term outcomes among patients with resected pancreatic cancer: the CONKO‐001 randomized trial. JAMA. 2013;310:1473‐1481. [DOI] [PubMed] [Google Scholar]
- 6. Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S‐1 versus gemcitabine for resected pancreatic cancer: a phase 3, open‐label, randomised, non‐inferiority trial (JASPAC 01). Lancet. 2016;388:248‐257. [DOI] [PubMed] [Google Scholar]
- 7. Eguchi H, Yamada D, Iwagami Y, et al. Prolonged neoadjuvant therapy for locally advanced pancreatic cancer. Dig Surg. 2017;35:70‐76. [DOI] [PubMed] [Google Scholar]
- 8. Eguchi H, Nagano H, Kobayashi S, et al. A phase I trial of combination therapy using gemcitabine and S‐1 concurrent with full‐dose radiation for resectable pancreatic cancer. Cancer Chemother Pharmacol. 2014;73:309‐315. [DOI] [PubMed] [Google Scholar]
- 9. Eguchi H, Nagano H, Tanemura M, et al. Preoperative chemoradiotherapy, surgery and adjuvant therapy for resectable pancreatic cancer. Hepatogastroenterology. 2013;60:904‐911. [DOI] [PubMed] [Google Scholar]
- 10. Takeda Y, Nakamori S, Eguchi H, et al. Neoadjuvant gemcitabine‐based accelerated hyperfractionation chemoradiotherapy for patients with borderline resectable pancreatic adenocarcinoma. Jpn J Clin Oncol. 2014;44:1172‐1180. [DOI] [PubMed] [Google Scholar]
- 11. Yamada D, Eguchi H, Iwagami Y, et al. Patients treated with preoperative chemoradiation for pancreatic ductal adenocarcinoma have impaired bone density, a predictor of distant metastasis. Ann Surg Oncol. 2017;24:3715‐3724. [DOI] [PubMed] [Google Scholar]
- 12. Furukawa K, Kawamoto K, Eguchi H, et al. Clinicopathological significance of leucine‐rich alpha2‐glycoprotein‐1 in sera of patients with pancreatic cancer. Pancreas. 2015;44:93‐98. [DOI] [PubMed] [Google Scholar]
- 13. Serada S, Fujimoto M, Ogata A, et al. iTRAQ‐based proteomic identification of leucine‐rich alpha‐2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010;69:770‐774. [DOI] [PubMed] [Google Scholar]
- 14. Haupt H, Bohn H. Isolation and characterization of 7S‐beta1‐globuline from human erythrocytes (author's transl). Blut. 1977;35:229‐239. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi N, Takahashi Y, Putnam FW. Periodicity of leucine and tandem repetition of a 24‐amino acid segment in the primary structure of leucine‐rich alpha 2‐glycoprotein of human serum. Proc Natl Acad Sci USA. 1985;82:1906‐1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shirai R, Hirano F, Ohkura N, Ikeda K, Inoue S. Up‐regulation of the expression of leucine‐rich alpha(2)‐glycoprotein in hepatocytes by the mediators of acute‐phase response. Biochem Biophys Res Comm. 2009;382:776‐779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Donnell LC, Druhan LJ, Avalos BR. Molecular characterization and expression analysis of leucine‐rich alpha2‐glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol. 2002;72:478‐485. [PubMed] [Google Scholar]
- 18. Wang X, Abraham S, McKenzie JAG, et al. LRG1 promotes angiogenesis by modulating endothelial TGF‐beta signalling. Nature. 2013;499:306‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandanayake NS, Sinclair J, Andreola F, et al. A combination of serum leucine‐rich alpha‐2‐glycoprotein 1, CA19‐9 and interleukin‐6 differentiate biliary tract cancer from benign biliary strictures. Br J Cancer. 2011;105:1370‐1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takemoto N, Serada S, Fujimoto M, et al. Leucine‐rich alpha‐2‐glycoprotein promotes TGFbeta1‐mediated growth suppression in the Lewis lung carcinoma cell lines. Oncotarget. 2015;6:11009‐11022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fujimoto M, Serada S, Suzuki K, et al. Leucine‐rich alpha2 ‐glycoprotein as a potential biomarker for joint inflammation during anti‐interleukin‐6 biologic therapy in rheumatoid arthritis. Arthritis Rheumatol. 2015;67:2056‐2060. [DOI] [PubMed] [Google Scholar]
- 22. Khan S, Ebeling MC, Chauhan N, et al. Ormeloxifene suppresses desmoplasia and enhances sensitivity of gemcitabine in pancreatic cancer. Can Res. 2015;75:2292‐2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eguchi H, Nagano H, Yamamoto H, et al. Augmentation of antitumor activity of 5‐fluorouracil by interferon alpha is associated with up‐regulation of p27Kip1 in human hepatocellular carcinoma cells. Clin Cancer Res. 2000;6:2881‐2890. [PubMed] [Google Scholar]
- 24. Kimura‐Tsuchiya R, Ishikawa T, Kokura S, et al. The inhibitory effect of heat treatment against epithelial‐mesenchymal transition (EMT) in human pancreatic adenocarcinoma cell lines. J Clin Biochem Nutr. 2014;55:56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Takekawa M, Tatebayashi K, Itoh F, Adachi M, Imai K, Saito H. Smad‐dependent GADD45beta expression mediates delayed activation of p38 MAP kinase by TGF‐beta. EMBO J. 2002;21:6473‐6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ellenrieder V, Hendler SF, Boeck W, et al. Transforming growth factor beta1 treatment leads to an epithelial‐mesenchymal transdifferentiation of pancreatic cancer cells requiring extracellular signal‐regulated kinase 2 activation. Can Res. 2001;61:4222‐4228. [PubMed] [Google Scholar]
- 27. Kumagai S, Nakayama H, Fujimoto M, et al. Myeloid cell‐derived LRG attenuates adverse cardiac remodelling after myocardial infarction. Cardiovasc Res. 2016;109:272‐282. [DOI] [PubMed] [Google Scholar]
- 28. Urushima H, Fujimoto M, Mishima T, et al. Leucine‐rich alpha 2 glycoprotein promotes Th17 differentiation and collagen‐induced arthritis in mice through enhancement of TGF‐beta‐Smad2 signaling in naive helper T cells. Arthritis Res Ther. 2017;19:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basso D, Plebani M, Fogar P, et al. Insulin‐like growth factor‐I, interleukin‐1 alpha and beta in pancreatic cancer: role in tumor invasiveness and associated diabetes. Int J Clin Lab Res. 1995;25:40‐43. [DOI] [PubMed] [Google Scholar]
- 30. Saito K, Ishikura H, Kishimoto T, et al. Interleukin‐6 produced by pancreatic carcinoma cells enhances humoral immune responses against tumor cells: a possible event in tumor regression. Int J Cancer. 1998;75:284‐289. [DOI] [PubMed] [Google Scholar]
- 31. Okada S, Okusaka T, Ishii H, et al. Elevated serum interleukin‐6 levels in patients with pancreatic cancer. Jpn J Clin Oncol. 1998;28:12‐15. [DOI] [PubMed] [Google Scholar]
- 32. Kim HW, Lee JC, Paik KH, Kang J, Kim J, Hwang JH. Serum interleukin‐6 is associated with pancreatic ductal adenocarcinoma progression pattern. Medicine. 2017;96:e5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miura T, Mitsunaga S, Ikeda M, et al. Characterization of patients with advanced pancreatic cancer and high serum interleukin‐6 levels. Pancreas. 2015;44:756‐763. [DOI] [PubMed] [Google Scholar]
- 34. Goumas FA, Holmer R, Egberts JH, et al. Inhibition of IL‐6 signaling significantly reduces primary tumor growth and recurrencies in orthotopic xenograft models of pancreatic cancer. Int J Cancer. 2015;137:1035‐1046. [DOI] [PubMed] [Google Scholar]
- 35. Zhang H, Wu H, Guan J, et al. Paracrine SDF‐1alpha signaling mediates the effects of PSCs on GEM chemoresistance through an IL‐6 autocrine loop in pancreatic cancer cells. Oncotarget. 2015;6:3085‐3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holmer R, Goumas FA, Waetzig GH, Rose‐John S, Kalthoff H. Interleukin‐6: a villain in the drama of pancreatic cancer development and progression. Hepatobiliary Pancreat Dis Int. 2014;13:371‐380. [DOI] [PubMed] [Google Scholar]
- 37. Huang C, Yang G, Jiang T, Zhu G, Li H, Qiu Z. The effects and mechanisms of blockage of STAT3 signaling pathway on IL‐6 inducing EMT in human pancreatic cancer cells in vitro. Neoplasma. 2011;58:396‐405. [DOI] [PubMed] [Google Scholar]
- 38. Izano M, Wei EK, Tai C, et al. Chronic inflammation and risk of colorectal and other obesity‐related cancers: the health, aging and body composition study. Int J Cancer. 2016;138:1118‐1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin‐6 contributes to Mcl‐1 up‐regulation and TRAIL resistance via an Akt‐signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054‐2065. [DOI] [PubMed] [Google Scholar]
- 40. Isomoto H, Kobayashi S, Werneburg NW, et al. Interleukin 6 upregulates myeloid cell leukemia‐1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology (Baltimore, MD). 2005;42:1329‐1338. [DOI] [PubMed] [Google Scholar]
- 41. Xu X, Kobayashi S, Qiao W, et al. Induction of intrahepatic cholangiocellular carcinoma by liver‐specific disruption of Smad4 and Pten in mice. J Clin Investig. 2006;116:1843‐1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamada D, Kobayashi S, Wada H, et al. Role of crosstalk between interleukin‐6 and transforming growth factor‐beta 1 in epithelial‐mesenchymal transition and chemoresistance in biliary tract cancer. Eur J Cancer. 2013;49:1725‐1740. [DOI] [PubMed] [Google Scholar]
- 43. Ai J, Druhan LJ, Hunter MG, Loveland MJ, Avalos BR. LRG‐accelerated differentiation defines unique G‐CSFR signaling pathways downstream of PU.1 and C/EBPepsilon that modulate neutrophil activation. J Leukoc Biol. 2008;83:1277‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Serada S, Fujimoto M, Terabe F, et al. Serum leucine‐rich alpha‐2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2169‐2179. [DOI] [PubMed] [Google Scholar]
- 45. Ha YJ, Kang EJ, Lee SW, et al. Usefulness of serum leucine‐rich alpha‐2 glycoprotein as a disease activity biomarker in patients with rheumatoid arthritis. J Korean Med Sci. 2014;29:1199‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen J, Yuan W, Wu L, et al. PDGF‐D promotes cell growth, aggressiveness, angiogenesis and EMT transformation of colorectal cancer by activation of Notch1/Twist1 pathway. Oncotarget. 2017;8:9961‐9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stone RC, Pastar I, Ojeh N, et al. Epithelial‐mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res. 2016;365:495‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamamoto M, Takahashi T, Serada S, et al. Overexpression of leucine‐rich alpha2‐glycoprotein‐1 is a prognostic marker and enhances tumor migration in gastric cancer. Cancer Sci. 2017;108:2052‐2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Honda H, Fujimoto M, Miyamoto S, et al. Sputum leucine‐rich alpha‐2 glycoprotein as a marker of airway inflammation in asthma. PLoS ONE. 2016;11:e0162672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415‐424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


