Abstract
Objective. Research on chronic low back pain (cLBP) has focused heavily on structural abnormalities with emphasis on diagnostic imaging. However, for many cLBP patients, clinical pain and disability are not clearly associated with identifiable pathology of the spine or associated tissues. Therefore, alternative determinants such as psychological factors and dysfunctional pain modulatory processes have been suggested to be important.
Methods. This observational study examined differences in pain catastrophizing and endogenous pain modulation between 25 cLBP patients and 25 pain-free controls. Associations among pain catastrophizing, endogenous pain modulatory processes, clinical pain reports, and disability were also examined in cLBP patients. Endogenous pain modulation was examined using temporal summation (TS) of mechanical and heat pain stimuli as well as conditioned pain modulation (CPM) with algometry (test stimulus) and the cold pressor task (conditioning stimulus).
Results. Findings demonstrated significantly greater pain catastrophizing as well as greater TS of mechanical and heat pain for cLBP patients compared with controls. CPM was not present in cLBP patients or controls. Among cLBP patients, pain catastrophizing was significantly associated with disability, while TS of mechanical pain was significantly associated with clinical pain severity and disability.
Conclusions. This study suggests that endogenous pain modulatory processes are altered for cLBP patients, particularly TS of mechanical and heat stimuli. Pain catastrophizing and TS of mechanical pain may have important clinical relevance for cLBP, given associations with clinical pain and disability; however, future research is needed to replicate these findings.
Keywords: Chronic Low Back Pain, Pain Catastrophizing, Conditioned Pain Modulation, Temporal Summation, Disability
Introduction
A considerable amount of prior research addressing predictors of chronic low back pain (cLBP) has focused on structural abnormalities of the spine and associated tissues, with emphasis on diagnostic imaging [1–3]. However, the association between clinical symptoms and imaging results has been consistently weak, and up to 85% of cLBP patients cannot be given a precise pathoanatomical diagnosis using these methods [4]. Even when anatomical abnormalities are detected, the significance is unclear, as conditions like disc herniation and facet joint degeneration are found in high percentages of asymptomatic individuals [5]. Because objective measures of disease activity have not consistently been strong predictors of clinical symptoms, it is likely that other “nonanatomical” factors contribute to cLBP presentation. It has been suggested that psychological factors as well as dysfunction in the transmission and modulation of pain signals may be important contributors to cLBP severity and disability [6].
Pain catastrophizing is a psychological factor representing a negative emotional and cognitive response to actual or anticipated pain, and is among the most robust predictors of chronic pain outcomes [7]. Pain catastrophizing has been shown to predict important clinical symptoms such as greater chronic pain severity and related disability [8]. Similarly, in laboratory studies involving healthy volunteers, catastrophizing has been shown to be associated with dysfunctional endogenous pain modulatory processes [8]. Despite demonstrated associations between pain catastrophizing and chronic pain outcomes in other patient populations, the specific influence of pain catastrophizing in cLBP patients is not fully established and should be further investigated. This is because previous systematic reviews have produced conflicting results suggesting that pain catastrophizing may be strongly [9], weakly [10], or not at all [11] related to cLBP outcomes. The mixed nature of the findings from these systematic reviews may be due, at least in part, to the manner in which pain catastrophizing was measured and analyzed. For instance, “cutoff” values have previously been used to group cLBP patients as either high or low for pain catastrophizing [12,13]. cLBP patients with high pain catastrophizing scores demonstrated poorer pain severity and disability outcomes compared with those with lower scores [13], indicating that a “dose-dependent” effect of pain catastrophizing may be present. It is important to note that few studies to date have used this approach for analyzing pain catastrophizing, and the actual cutoff values have varied greatly among those studies that did. Therefore, no cutoff can be recommended, and utilization of cutoff values for analysis of pain catastrophizing does not appear to be empirically justified at present.
In addition to pain catastrophizing, differences in pain sensitivity between cLBP patients and pain-free controls have previously been reported using electrical stimulation [14], heat stimuli [15], mechanical pressure [16], and chemical stimuli [17]. These studies have generally produced consistent results, such that cLBP patients demonstrate decreased pain thresholds compared with controls. Using dynamic quantitative sensory tests of endogenous pain modulation, two recent case-control studies further showed that pain inhibitory capacity was less efficient in cLBP patients [18,19]. However, research addressing the clinical relevance of quantitative sensory tests of pain sensitivity and endogenous pain modulation in relation to cLBP outcomes has produced inconsistent findings. For instance, several previous studies have shown increased pain sensitivity and dysfunctional endogenous pain modulation to be associated with greater clinical pain severity as well as disability in cLBP patients [20,21], while others have found no such associations [22,23]. One possible reason for the incongruent nature of the aforementioned findings could be related to the study design and time course (i.e., cross-sectional vs longitudinal) used to assess the associations of pain sensitivity and endogenous pain modulation with cLBP outcomes. To illustrate, pain sensitivity and endogenous pain modulation were found to be associated with clinical pain severity and disability in the studies incorporating a cross-sectional design [20,21]. Conversely, in the studies that examined these associations using a longitudinal design, endogenous pain modulation was not associated with cLBP outcomes at 4-month [22] or 12-month [23] assessments. Future research that includes cross-sectional and longitudinal design components as part of the same study may be helpful for elucidating the strength of the association between endogenous pain modulation and cLBP outcomes, as well as whether the strength changes over time.
Dysfunctional endogenous pain modulation has been hypothesized as a risk factor for the development and perpetuation of chronic pain [24], and data currently supports this hypothesis [25]. This is important because antidepressant and opioid medications commonly prescribed for the treatment of cLBP have been shown to significantly improve endogenous pain modulation [26,27]; however, it remains to be determined whether these medication-related improvements in endogenous pain modulation translate into positive chronic pain outcomes. The initial conduct of studies aimed at better understanding the impact of dysfunctional pain modulatory processes in the experience of cLBP represents an important foundational step for ultimately informing lines of investigation aimed at addressing whether medications that improve cLBP outcomes do so by exerting effects on endogenous pain modulation.
The current study specifically investigated whether pain catastrophizing and endogenous pain modulation differed between cLBP patients and pain-free controls. It further examined whether pain catastrophizing and endogenous pain modulation were associated with self-reports of clinical pain severity and disability in the cLBP patients. We expanded upon previous research by including a more comprehensive quantitative sensory testing battery designed to assess endogenous pain modulation via temporal summation (TS) of pain and conditioned pain modulation (CPM). TS of pain and CPM are tests that are widely incorporated in studies to invoke neural mechanisms related to pain facilitation and pain inhibition, respectively [28]. We addressed the following hypotheses as part of this study: 1) cLBP patients will report greater pain catastrophizing compared with controls, 2) cLBP patients will demonstrate greater TS of pain and less CPM compared with controls, and 3) pain catastrophizing, TS of pain, and CPM will be associated with clinical pain severity and disability in cLBP patients.
Methods
Patients and Controls
This study’s final sample was composed of 25 cLBP patients who were recruited from the Pain Treatment Clinic within the University of Alabama at Birmingham (UAB), Department of Anesthesiology. An additional 25 pain-free controls were recruited from the surrounding community and matched to the cLBP patients by sex, race, and age. All participants were between the ages of 45 to 90 years and self-identified as either non-Hispanic Black or non-Hispanic White. Each of these 50 total participants completed the study in its entirety. All study protocols were approved by the UAB Institutional Review Board and carried out in accordance with guidelines for the ethical conduct of research. Study sessions were completed at the UAB Biobehavioral Pain Research Laboratory (B.R.G., Principal Investigator). Participants were compensated a total of $100 cash ($50 following each study session) for their involvement in the study.
Overview of Study Design
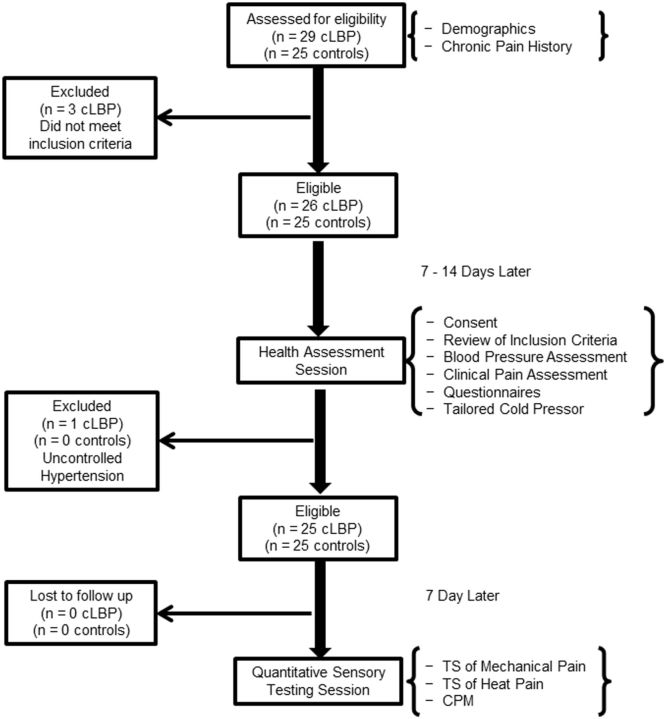
A flow diagram depicting matriculation through the study is presented in Figure 1. cLBP patients and controls who were interested in being part of this study were assessed for eligibility during an initial telephone screening and a subsequent health assessment session, during which they completed measures of clinical pain intensity, perceived disability, pain catastrophizing, depressive symptoms, and a tailored cold pressor task (see below). Approximately 1 week after the health assessment session, participants returned to the laboratory and completed a quantitative sensory testing battery, which included TS of mechanical and heat pain as well as assessment of CPM.
Figure 1.
Overall protocol timeline and matriculation of study participants.
Telephone Screening of Inclusion/Exclusion Criteria
All of the cLBP patients and controls completed screening via telephone to determine eligibility for study inclusion. In accordance with the recently developed research standards for cLBP proposed by the Research Task Force of the NIH Pain Consortium [29], cLBP patients were included in this study only if they reported a back pain problem that had persisted for at least 3 months and had resulted in pain on at least half the days in the past 6 months. Furthermore, cLBP patients were included only if they denied any type of surgery on the low back within the past year. Low back pain had to be the primary pain complaint reported for all cLBP patients. Controls were included in the study only if they denied any ongoing chronic pain problems and also denied any episodes of acute pain within the 2 weeks prior to study participation. Additional inclusion criteria that needed to be met for all study participants were as follows: no evidence of uncontrolled hypertension (i.e., resting blood pressure > 150/95) assessed via sphygmomanometer, no circulatory disorders, no history of cardiac events, no history of stroke or seizure, no history of metabolic disease or neuropathy, no history of cancer, and not currently pregnant. A total of 29 cLBP patients were screened for study eligibility, and 25 were enrolled. The four cLBP patients who did not meet study inclusion criteria did not appreciably differ from the cLBP patients who were enrolled. Of the 25 controls screened for study eligibility, all were enrolled.
In addition to data addressing inclusion/exclusion criteria, the following demographic and socioeconomic data were also obtained as part of the telephone screening: self-reported age, sex, race, marital status, education, and employment status. Information pertaining to acute and chronic painful experiences was collected to confirm status as either a cLBP patient or pain-free control. Individuals who met initial study inclusion criteria presented approximately 1 to 2 weeks later for a health assessment session involving a more thorough examination of inclusion/exclusion criteria.
Health Assessment Session
Clinical Pain Assessment and Related Factors
For all participants, height and weight were measured for the calculation of body mass index (BMI). Standardized self-report questionnaires were then completed for the assessment of pain catastrophizing and depressive symptoms. Also as part of the health assessment session, cLBP patients provided information regarding the current use of opioid pain medication and whether they were receiving social security disability benefits. cLBP patients then used a 0–10 numeric rating scale (0 = no pain, 10 = worst pain imaginable) to rate the average severity of clinical pain experienced in the low back over the past 7 days. This was followed by completion of a questionnaire for determination of self-reported disability due to chronic low back pain.
Tailored Cold Pressor
At the very end of the Health Assessment Session, a tailored cold pressor task was completed to determine the intensity of the conditioning stimulus that would be used in the CPM protocol to be completed during the subsequent quantitative sensory testing session. The use of a tailored cold pressor for CPM assessment is consistent with procedures previously published by our research team [30,31]. This was done by asking participants to fully immerse their nondominant hand to the wrist three consecutive times in cold water maintained at 16°C, 12°C, and 8°C. The water temperatures were maintained (± 0.05°C) by a refrigeration unit (Neslab, Portsmouth, NH, USA), and water was constantly circulated to prevent local warming around the submerged hand [32]. Ratings of pain intensity and unpleasantness were obtained at 30 and 60 seconds after immersion. Participants were provided with audio recorded instructions regarding how to rate the intensity of the pain produced by the cold water on a 0–100 numeric rating scale, such that 0 = no pain and 100 = the most intense pain imaginable [33]. The maximum duration of each hand immersion was 60 seconds and there was a 5-minute rest period between each immersion. Following the tailoring procedure, the cold water temperature that produced a moderately intense pain rating of ∼50 (range: 40–60) on the 0–100 numeric rating scale was selected for each participant as the conditioning stimulus for CPM assessment during the subsequent quantitative sensory testing session.
Quantitative Sensory Testing Session
Approximately 1 week following the health assessment session, participants completed the quantitative sensory testing session. The cLBP patients who reported daily opioid pain medication use were not asked to withhold pain medications on the day of their quantitative sensory testing session, given that temporary withdrawal from these medications could have affected pain perception. Instead, it was determined that opioid medication use would be examined and statistically controlled in data analyses. On the day of the quantitative sensory testing session, all participants underwent a series of controlled sensory stimulation procedures to assess TS of mechanical and heat pain, as well as CPM [34]. TS of mechanical pain was always assessed prior to TS of heat pain, and the TS procedures were always completed prior to CPM. Before commencing this session, participants were again provided with audio recorded instructions regarding how to rate the intensity of the pain produced by the mechanical and heat TS procedures on the 0–100 numeric rating scale.
Temporal Summation (TS) of Mechanical Pain
TS of mechanical pain was assessed using a nylon monofilament (Touchtest Sensory Evaluator 6.65) that was calibrated to bend at 300 g of pressure. Testing sites included the back of the nondominant hand and the ipsilateral trapezius, in randomized order. To assess TS of mechanical pain at each site, participants were instructed to provide a verbal 0–100 rating of pain following a single contact of the monofilament. Then, participants were instructed to provide another 0–100 rating of their greatest pain intensity experienced following a series of 10 contacts, which were provided at a rate of one contact per second. This procedure was repeated twice at each anatomical location. Pain ratings for the single and multiple contacts performed at each anatomical location were averaged across the two trials.
Temporal Summation (TS) of Heat Pain
TS of heat pain was assessed using a Medoc Thermal Sensory Analyzer - II (Medoc, Ltd., Ramat Yishai, Israel) with a 30 × 30–mm diameter thermode. All participants completed an initial practice trial using a minimally painful heat pain stimulus (46°C) to help familiarize them with TS of the heat pain procedure. Following a 30-second rest period, a subsequent trial was completed for the actual assessment of TS of heat pain using a 50°C thermal stimulus. The thermode was placed on the dorsal surface of the nondominant forearm for the practice trial, and replaced onto the volar surface of the forearm for the actual TS of the heat pain trial. For each trial, sequences of 5 consecutive heat pulses of 1-second duration were delivered, with interpulse intervals of 2.5 seconds at 40°C. For TS of the heat pain procedure, participants were asked to rate the intensity of the pain produced by each heat pulse on the 0–100 numeric rating scale.
Conditional Pain Modulation (CPM) Trials
For this study, CPM was tested on the dominant dorsal forearm and ipsilateral trapezius using algometry (test stimulus) and the cold pressor (conditioning stimulus) according to guidelines published by Yarnitsky and colleagues [35]. A handheld algometer (Medoc, Ltd., AlgoMed, Ramat Yishai, Israel) was alternately applied three times at each anatomical location, in counter-balanced fashion, to determine participants’ baseline pressure pain thresholds (PPTs) [36]. Participants indicated when the increasing pressure stimulation first became painful, and PPTs were measured in kilopascals (kPa). Following baseline PPT determination, participants underwent a series of four cold pressor immersions that consisted of placing the nondominant hand, up to the wrist, into the circulating cold water for 1 minute. The cold pressor was maintained at the tailored temperature that produced a moderately painful sensation as determined during the health assessment session. Participants were told they could remove their hand from the water at any time; however, none of the participants prematurely removed their hand prior to the allotted 1 minute for any of the four cold pressor immersions. Approximately 30 seconds after initiation of each cold pressor immersion, while the hand was still immersed, the algometer was used to deliver noxious mechanical stimulation to either the dorsal forearm (two CPM trials) or the ipsilateral trapezius (two CPM trials); the site order was randomized. Participants again indicated when the increasing pressure stimulation first became painful, which represented their conditioned PPTs. There was a 2-minute rest period between each CPM trial.
Measures
Pain Catastrophizing
Pain catastrophizing was assessed using the pain-catastrophizing subscale of the Coping Strategies Questionnaire-Revised (CSQ-R) [37]. The CSQ-R includes six subscales corresponding to cognitive and behavioral strategies of coping with pain. Only the pain catastrophizing subscale was included in the current study. Respondents rate the frequency of their engagement in catastrophizing when experiencing pain on a 7-point scale from 0 (“I never do that”) to 6 (“I always do that”). The revised scoring system suggested by Robinson and colleagues [37] was used in the current study. The CSQ-R pain catastrophizing subscale has demonstrated adequate internal consistency and validity in a variety of clinical populations and healthy subjects.
Clinical Pain. The clinical pain intensity of cLBP patients was measured using the pain intensity item from the Patient-Reported Outcomes Measurement Information System (PROMIS) item bank [38]. cLBP patients were asked, “In the past seven days, how would you rate your low-back pain on average?” Responses were provided using an 11-point Likert scale, such that 0 = no pain and 10 = worst pain imaginable). Evidence has shown that the PROMIS item banks, particularly for pain assessment, offer generally reliable and precise measures [39].
Perceived Disability
The Oswestry Low Back Pain Disability Questionnaire is a low back–specific instrument commonly used to assess disability in cLBP patients [40]. It is composed of 10 items that address perceived disability in 10 areas: pain intensity, ability to lift objects, ability to walk, ability to sit, ability to stand, ability to sleep, sex life, social life, traveling, and ability to complete personal hygiene activities. By using a 6-point Likert scale (0 = no limitation; 5 = severe limitation), the total score is doubled and reported as a percentage of the patient’s perceived pain-related disability, with 0 representing no disability and 100 representing complete disability. This percentage is referred to as the Oswestry Disability Index (ODI), and percentage scores greater than 41% are suggestive of severe disability [41]. For this study, cLBP patients were categorized as either having minimal/moderate disability or severe disability. The validity and internal consistency of the Oswestry Low Back Pain Disability Questionnaire has consistently been reported to be acceptable when used with cLBP patients.
Depressive Symptoms
Depressive symptoms were measured using the Center for Epidemiological Studies Depression Scale (CES-D) [42], which is a 20-item self-report tool that measures symptoms of depression in the past week, including negative mood, guilt/worthlessness, helplessness/hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance. The CES-D has previously been used in research involving psychiatric and nonpsychiatric samples, as well as clinical samples with medical illness [43]. The total score ranges from 0 to 60, and this single total score was used in this study as an estimate of the degree of individuals’ depressive symptomatology. The validity and internal consistency of the CES-D is acceptable when used specifically for people with chronic pain conditions [44].
Data Analysis
All data were analyzed using SPSS, version 21 (IBM, Chicago, IL, USA). All participants provided complete demographic and quantitative sensory testing data; however, a small portion of missing data existed for some of the self-report measures (< 5%). The missing data did not systematically vary between cLBP patients and controls, and was deemed to be missing at random. Therefore, a simple data imputation method was completed using the macro for Hot Deck imputation [45]. This data imputation method is well validated and readily accepted in the statistical community, and resulted in complete data for all of the study participants. Descriptive data for the sample are reported separately for cLBP patients and controls either as percentages or as means and standard deviations. Differences in descriptive data between cLBP patients and controls were assessed using Chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables. TS of mechanical and heat pain as well as CPM were tested in cLBP patients and controls using repeated measures ANOVA with Greenhouse-Geisser corrections. Control variables were included in study analyses as warranted. Statistical presentation includes the squared partial eta (ηp2) as a measure of effect size where appropriate, such that ηp2 = 0.01 is considered a small effect, partial ηp2 = 0.06 a medium-sized effect, and ηp2 = 0.14 a large effect. Lastly, associations among study variables were assessed specifically in cLBP patients using partial correlations.
For the partial correlation analysis, endogenous pain modulation data obtained via TS and CPM testing were transformed. TS indices for mechanical pain at the hand and trapezius were obtained by subtracting the pain intensity rating following a single contact with the mechanical stimulus from the pain intensity rating following 10 contacts. For the index representing TS of heat pain, pain intensity ratings for the first heat pulse were subtracted from pain intensity ratings for the fifth heat pulse. CPM indices were created by subtracting baseline PPTs from conditioned PPTs for both the forearm and trapezius. These indices reflect the Δ change scores for TS of pain as well as CPM [35], and they were subsequently included in the partial correlation analysis.
Results
Participant Characteristics
Table 1 presents demographic characteristics and clinical data separately for cLBP patients and controls. Given that controls were matched to cLBP patients according to sex, race, and age, these factors did not significantly differ across the groups. There were slightly more women (56%) than men within each group, and each group was comprised primarily of African American participants (72%). cLBP patients and controls did not differ in BMI, marital status, or education. A significantly greater proportion of cLBP patients than controls reported their employment status as not working (χ2 = 9.74, P = 0.002). Although cLBP patients reported a greater mean level of depressive symptoms than controls, this difference was not statistically significant (F = 1.49, P = 0.229; ηp2 = .031). Among cLBP patients, 72% reported pain duration of at least 1 year, while 36% reported that their low back pain had persisted for longer than 5 years. Approximately half of the cLBP patients were classified as severely disabled according to their Oswestry Disability Index (52%). Similarly, approximately half of the cLBP patients also reported receipt of social security disability benefits (48%). Current use of opioid pain medication was reported by 17% of cLBP patients. The mean level of clinical low back pain reported over the past 7 days was a 6 out of 10.
Table 1.
Descriptive characteristics and clinical data for cLBP and control participants
| cLBP (N = 25) | Controls (N = 25) | |
|---|---|---|
| Demographic characteristics | ||
| Age (SD) years | 57.64 (10.84) | 55.16 (7.86) |
| BMI (SD) (weight/height2) | 29.07 (5.90) | 29.79 (7.39) |
| Sex (% female) | 56% | 56% |
| Race (% African American) | 72% | 72% |
| Marital status (% not married) | 72% | 76% |
| Education (% ≤ high school) | 60% | 36% |
| Employment (% not working) | 76% | 32% |
| Clinical characteristics | ||
| Depressive symptoms (SD) CES-D | 19.12 (11.33) | 15.28 (10.93) |
| Pain catastrophizing (SD) CSQ-R | 2.80 (1.51) | 1.53 (1.46) |
| Pain duration | ||
| 3–6 months | 16% | |
| 6 months–1 year | 12% | |
| 1–5 years | 36% | |
| > 5 years | 36% | |
| Clinical pain (SD) 0–10 NRS | 6.00 (2.51) | |
| Pain medication (% taking opioids) | 16% | |
| Disability (% severely disabled) ODI | 52% | |
| Disability benefits (% yes) | 48% |
Note: BMI = body mass index; CES-D = Center for Epidemiological Studies – Depression Scale; CSQ-R = Coping Strategies Questionnaire – Revised; NRS = numeric rating scale, 0 = no pain, 10 = most intense pain; ODI = Oswestry Disability Index.
In addition to opioid medication use (0 = not currently using opioid pain medications, 1 = currently using opioid pain medications), depressive symptoms and employment status (0 = not currently working, 1 = currently working) were included as control variables in subsequent data analyses. Given the overlap between measures of catastrophizing and negative affect, it is now customary (if not required) that any analysis of pain catastrophizing adjust for depressive symptoms or some general negative affective factor to establish the unique effect of catastrophizing [7]. Similarly, employment status is related to important pain and health-related outcomes in cLBP patients. cLBP patients who are not working tend to self-report poorer mental and physical health as well as greater disability compared with those who are working [46]. Therefore, when examining predictors of cLBP severity and disability, analyses should control for employment status.
Differences in Pain Catastrophizing Between cLBP Patients and Controls
As shown in Figure 2, pain catastrophizing was found to significantly differ between cLBP patients and controls, such that cLBP patients reported significantly greater pain catastrophizing. This difference in pain catastrophizing remained significant even after controlling for employment status, depressive symptoms, and opioid medication use (F = 4.86, P = 0.033; ηp2 = 0.108).
Figure 2.
Difference between chronic low back pain patients and controls on the Coping Strategies Questionnaire-Revised (CSQ-R) pain catastrophizing subscale. The range of pain catastrophizing scores was 0.00 to 6.00 for chronic low back pain patients and 0.00 to 5.83 for controls.
Differences in TS of Mechanical Pain Between cLBP Patients and Controls
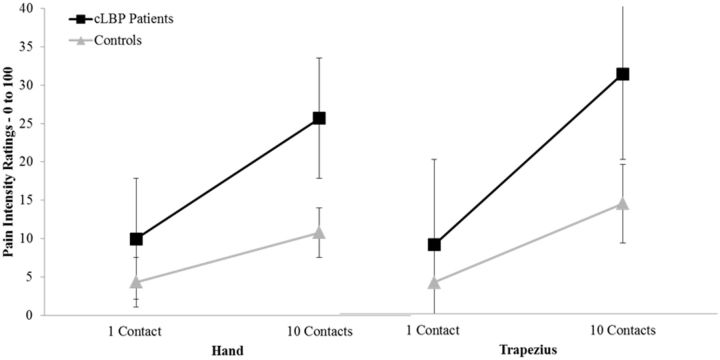
Data for TS of mechanical pain are presented in Table 2. Across the entire sample, pain intensity ratings following 10 contacts were significantly greater than ratings for the first contact at the nondominant hand (F = 9.79, P = 0.003; ηp2 = 0.165) and ipsilateral trapezius (F = 5.15, P = 0.028; ηp2 = 0.099). These results are suggestive of significant TS of mechanical pain at both anatomical testing sites. Importantly, Figure 3A and Figure 3B show that cLBP patients demonstrated significantly greater magnitude (i.e., slope) of TS of mechanical pain at both the hand (F = 4.99, P = 0.031; ηp2 = 0.095) and trapezius (F = 4.14, P = 0.048; ηp2 = 0.075) even after adjusting for control variables.
Table 2.
Temporal summation (TS) of mechanical and heat pain according to ratings of pain intensity on the 0–100 numeric rating scale
| cLBP Patients Mean (SD) | Controls Mean (SD) | |
|---|---|---|
| TS of mechanical pain | ||
| Hand | ||
| 1 Contact | 9.96 (16.07) | 4.32 (5.13) |
| 10 Contacts | 25.68 (24.63) | 10.80 (10.92) |
| Trapezius | ||
| 1 Contact | 9.02 (13.88) | 4.12 (3.77) |
| 10 Contacts | 31.24 (29.92) | 14.38 (15.09) |
| TS of heat pain—50°C | ||
| Forearm | ||
| First Pulse | 52.24 (30.32) | 50.28 (27.92) |
| Fifth Pulse | 61.00 (33.87) | 51.72 (28.38) |
Note: 1 Contact = pain intensity rating in response to first contact with mechanical stimuli, 10 contact = pain intensity rating in response to 10 contacts with mechanical stimuli; first pulse = pain intensity rating for first heat pulse, fifth pulse = pain intensity rating for fifth heat pulse.
Figure 3.
Differences in temporal summation of mechanical pain at the hand (3A) and the trapezius (3B) between chronic low back pain patients and controls.
Differences in TS of Heat Pain Between cLBP Patients and Controls
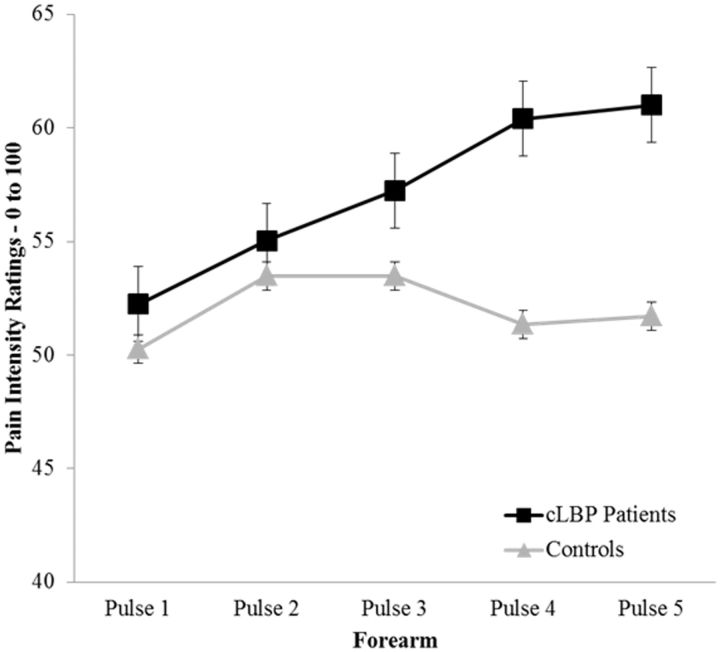
For TS of heat pain, the pain intensity rating elicited by the first heat pulse was compared with the rating elicited by the fifth heat pulse for the 50°C stimulus assessed at the nondominant volar forearm. Data representing TS of heat pain are presented in Table 2. Adjusted results show that cLBP patients demonstrated significant TS of heat pain while controls did not (F = 3.28, P = 0.049; ηp2 = .061), and the magnitude (i.e., slope) of TS of heat pain was greater for cLBP patients compared with controls (Figure 4).
Figure 4.
Difference in temporal summation of heat pain—500C between chronic low back pain patients and controls.
Differences in CPM Between cLBP Patients and Controls
For analysis of CPM at the dominant forearm and ipsilateral trapezius, baseline PPTs were compared with the conditioned PPTs, as shown in Table 3. Across the entire sample, there was no evidence of significant CPM at either the forearm (F = 3.13, P = 0.070; ηp2 = 0.055) or the trapezius (F = 1.35, P = 0.251; ηp2 = 0.029). Similarly, there was no evidence of significant differences in the magnitude of CPM between cLBP patients and controls at the forearm (F = 0.56, P = 0.457; ηp2 = 0.012) or trapezius (F = 0.91, P = 0.346; ηp2 = 0.019). The temperature of the water used to test CPM did not significantly differ between cLBP patients and controls (χ2 = 0.35, P = 0.841). Taken together, this evidence suggests an overall lack of endogenous pain inhibition for both the cLBP patients and the controls.
Table 3.
Baseline and conditioned pressure pain thresholds (PPTs) to determine conditioned pain modulation (CPM) at the forearm and trapezius
| cLBP Patients Mean (SD) | Controls Mean (SD) | |
|---|---|---|
| Forearm | ||
| Baseline PPTs | 369.70 (217.94) | 393.16 (180.87) |
| Conditioned PPTs | 402.97 (209.65) | 449.88 (213.29) |
| Trapezius | ||
| Baseline PPTs | 340.80 (196.27) | 412.98 (212.67) |
| Conditioned PPTs | 398.40 (230.01) | 525.40 (246.71) |
Note: All PPT scores are presented as kilopascals (kPa).
Associations Among Pain Catastrophizing, Endogenous Pain Modulation, Clinical Pain, and cLBP Disability
A partial correlation analysis controlling for employment status, depressive symptoms, and opioid medication use was carried out with cLBP patients only, and the results are presented in Table 4. Greater pain catastrophizing was shown to be significantly associated with more severe cLBP disability (r = 0.42, P = 0.045), but not clinical pain reports. Greater TS of mechanical pain at the hand was significantly associated with greater clinical pain severity (r = 0.41, P = 0.047) and severity of cLBP disability (r = 0.45, P = 0.031). Similarly, greater TS of mechanical pain at the trapezius was also significantly associated with greater clinical pain severity (r = 0.49, P = 0.018) and severity of cLBP disability (r = 0.48, P = 0.021). Pain catastrophizing was not significantly associated with TS of mechanical pain, TS of heat pain, or CPM for any of the body sites tested (Table 4).
Table 4.
Partial correlations among pain catastrophizing, endogenous pain modulatory processes, clinical pain severity, and cLBP disability
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| 1. Clinical pain severity | ___ | ||||||
| 2. ODI | 0.75** | ___ | |||||
| 3. TS mechanical—hand | 0.42* | 0.45* | ___ | ||||
| 4. TS mechanical—trapezius | 0.49* | 0.48* | 0.89** | ___ | |||
| 5. TS heat—50°C | −0.05 | 0.03 | 0.20 | 0.02 | ___ | ||
| 6. CPM—forearm | −0.11 | −0.16 | −0.18 | 0.01 | −0.17 | ___ | |
| 7. CPM—trapezius | −0.29 | −0.02 | −0.21 | −0.15 | −0.12 | 0.23 | ___ |
| 8. Pain catastrophizing | 0.34 | 0.42* | 0.18 | 0.06 | 0.02 | −0.11 | 0.04 |
*= P < 0.05.
**= P < 0.01.
Note: Partial correlations for cLBP only controlling for employment status and depressive symptoms; ODI = Oswestry Disability Index coded 0 = minimally/moderately disabled, 1 = severely disabled.
Discussion
In this study, it was observed that cLBP patients reported greater pain catastrophizing and demonstrated some evidence suggestive of dysfunctional endogenous pain modulation in comparison to controls. Specifically, TS of mechanical and heat pain were significantly greater in cLBP patients compared with controls. There was no evidence of significant CPM within either the cLBP patients or controls; therefore, they did not differ from one another in regard to CPM. Pain catastrophizing was significantly associated with more severe disability, but not clinical pain severity, per the self-report of cLBP patients. TS of mechanical pain was significantly associated with greater clinical pain severity as well as more severe disability. Significant associations remained even after controlling for employment status, depressive symptoms, and opioid medication use. Taken together, our results lend support to the idea that psychological factors and dysfunctional endogenous pain modulation may be important contributors to cLBP severity and disability.
Pain catastrophizing has emerged as a potent predictor of a variety of pain-related outcomes, both in chronic pain patients and pain-free samples [7]. The literature generally points to consistent associations between pain catastrophizing and measures of clinical pain severity, pain-related activity interference, disability, and negative mood, as well as alterations in social support [8]. Moreover, pain catastrophizing has been linked to increased behavioral expressions of pain and an array of illness behaviors such as more frequent visits to health care professionals [8]. In the current study, we found that pain catastrophizing was significantly associated with perceived disability in cLBP patients. Pain catastrophizing is considered an important component of the fear-avoidance model of chronic pain [47]. The fear-avoidance model theoretically describes how psychological factors affect the experience of pain and the development of chronic pain and disability. Within this model, it is theorized that negative beliefs about pain and/or negative illness information produce a catastrophizing response in which patients imagine the worst possible outcome. This leads to fear of activity and avoidance that in turn causes disuse, disability, and resultant distress, thereby reinforcing pain catastrophizing in a deleterious cycle [48]. It may be that cLBP patients with high pain catastrophizing are more likely to avoid physical activity in an effort to prevent worsened pain severity, which could result in increased perceptions of disability. Our efforts to further characterize the role of pain catastrophizing in cLBP outcomes is important given that catastrophic cognitions and emotions are often important treatment targets of psychological approaches to chronic pain management. During multidisciplinary pain treatment, early changes in pain catastrophizing have been linked with later improvements in pain severity [49,50], and it has further been shown that reductions in pain catastrophizing during cognitive-behavioral or other pain treatment may mediate positive treatment outcomes [51,52].
Pain catastrophizing was not significantly associated with any of the endogenous pain modulatory processes that assessed TS of pain and CPM. This may possibly be explained by the fact that pain catastrophizing was measured as a “dispositional” construct in this study, and not a “situational” one. It has been argued that dispositional pain catastrophizing is more related to the historical recall of pain in everyday life, while situational pain catastrophizing refers to the measurement of catastrophizing immediately following a painful event or stimulus [53]. In the laboratory, situational measures of pain catastrophizing have been shown to be more strongly associated with quantitative sensory tests of pain compared with dispositional measures [53,54]. Similar to our findings for endogenous pain modulation, pain catastrophizing was also not significantly associated with clinical pain severity; however, the magnitude of this relationship (r = 0.34) was medium in size according to established guidelines. It seems likely that a larger sample size of cLBP patients was needed to provide adequate statistical power for the detection of a significant association between pain catastrophizing and reports of clinical pain severity in this study.
In addition to pain catastrophizing, TS of pain for heat and mechanical stimuli was significantly greater for cLBP patients compared with controls. For both heat and mechanical stimuli, cLBP patients reported significantly greater pain in response to the final stimulus application compared with the first despite the stimulus intensity remaining the same across each respective procedure. TS of pain has been a widely incorporated test of endogenous pain modulation in previous studies [28]; however, only a few studies to date have examined TS of pain in cLBP patients. The enhanced endogenous pain facilitation (i.e., TS of pain) found in our study may be indicative of central nervous system (CNS) sensitization in cLBP patients [55]. This is because endogenous pain facilitatory pathways are particularly important in initiating and maintaining CNS sensitization [56]. It has been reported that cLBP patients report decreased pain thresholds, increased pain responses, and prolonged duration of pain during and after experimental stimuli consistent with CNS sensitization [57].
That TS of mechanical pain, but not TS of heat pain, was significantly associated with clinical pain severity and disability in cLBP patients may be related to differences in clinical relevance between these two stimulus modalities. Increased mechanical stress due to overuse, repetitive movement, and/or hypermobility can cause tissue damage and inflammation [6], which may contribute to the development and maintenance of cLBP. Clinical pain experiences for cLBP patients are often mechanically evoked (e.g., trunk flexion, postural perturbations) [58]. Therefore, the mechanical stimuli used to test TS of pain may be more clinically relevant than other modalities such as heat. Additional research addressing this topic is needed, however, in light of a recent systematic review and meta-analysis that reported no difference between mechanical and heat stimuli for predicting cLBP severity and disability [59]. Should future research replicate our finding that TS of mechanical pain is associated with clinical pain severity and disability in cLBP patients, important clinical applications for TS of mechanical pain may become apparent. Unlike TS of heat pain assessment, which requires costly equipment and methodologies that are technically elaborate and time-consuming, we have previously suggested that TS of mechanical pain assessment with a nylon monofilament is relatively brief, inexpensive, and requires minimal technical expertise [60]. Therefore, the simple procedure for assessing TS of mechanical pain in this study could one day be easily included in clinical practice to help approximate the clinical pain severity and disability of cLBP patients.
Our hypothesis that cLBP patients would demonstrate less efficient CPM compared with controls was only partially supported. The concurrent cold pressor pain had no significant conditioning effect on pressure pain thresholds for cLBP patients, which suggests a lack of endogenous pain inhibition (i.e., CPM). Lack of CPM in cLBP patients was expected and consistent with our second hypothesis. Contrary to what was hypothesized, however, the control group also demonstrated lack of a CPM effect. Our findings regarding CPM are in contrast to a recently published study that did detect the presence of CPM in cLBP patients and controls [32]. In that study, CPM effects were longer lasting for controls compared with cLBP patients; however, it was suggested that CPM was still partially functioning in that sample of cLBP patients. One possible contributory factor related to the lack of CPM in our study is that 70% of cLBP patients and 70% of controls self-reported their ethnic/racial background as non-Hispanic African American. Previous research involving both clinical and healthy populations has shown that non-Hispanic African Americans demonstrate diminished CPM compared with non-Hispanic Caucasians [61,62]. Given that our sample was predominantly comprised of non-Hispanic African Americans, possible racial differences in endogenous pain inhibition might help explain the lack of CPM observed in both cLBP patients and controls in this study.
Several limitations should be considered when evaluating the findings of this study. First, although the inclusion criteria for enrollment of cLBP patients and the breadth of data collected conformed to recently published research standards [29], we did not obtain any information related to potential pathoanatomical underpinnings of cLBP (e.g., disc herniation, stenosis, nonspecific). Thus, different categorizations of cLBP may have been present in this sample, and it is unclear whether this may have impacted tests of endogenous pain modulation. Secondly, opioid pain medication use among cLBP patients is presented in Table 1; however, other nonopioid pain medications were not sufficiently assessed. Only 4 cLBP patients (16%) reported active use of opioid medications, which likely helped minimize the influence of these medications on tests of endogenous pain modulation. Third, although we used highly controlled and standardized quantitative sensory testing procedures to assess endogenous pain modulation, these sessions were not limited to a specific time of day for all participants, but rather were completed at different times of day according to participants’ scheduling needs. This may have introduced some amount of error to the assessment of endogenous pain modulation given that human pain perception has been shown to be affected by diurnal variations [63,64]. Finally, interpretation of the clinical relevance of this study is limited by the cross-sectional nature and temporal aspects of the study design. Because clinical pain severity and disability were measured prior to completion of the QST session, the endogenous pain modulatory variables (e.g., TS and CPM) cannot be used to predict clinical pain severity and disability. Therefore, these associations identified in the current study should be interpreted carefully. As previously mentioned above, future research using cross-sectional and longitudinal designs within the same study may be necessary to further validate the clinical relevance of dysfunctional endogenous pain modulation assessed via tests of TS and CPM in patients with cLBP.
Despite these limitations, this study’s findings provide novel evidence for dysfunctional endogenous pain modulation in cLBP patients compared with controls when dynamic tests of endogenous pain modulation are used. The strongest evidence points to enhanced endogenous pain facilitatory processes when assessed via TS of mechanical and heat stimuli. TS of mechanical pain may have particular clinical relevance for cLBP, given associations with clinical pain severity and disability. Additionally, cLBP patients demonstrated greater pain catastrophizing compared with controls, and the evidence suggests that catastrophizing may be an important psychological factor associated with increased disability in cLBP patients.
References
- 1. Jarvik JG, Deyo RA. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med 2002;137:586–97. [DOI] [PubMed] [Google Scholar]
- 2. Koes BW, van Tulder MW, Thomas S. Diagnosis and treatment of low back pain. Br Med J 2006;332:1430–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Spinal radiographic findings and nonspecific low back pain. A systematic review of observational studies. Spine 1997;22:427–34. [DOI] [PubMed] [Google Scholar]
- 4. Deyo RA, Weinstein JN. Low back pain. N Engl J Med 2001;344:363–70. [DOI] [PubMed] [Google Scholar]
- 5. Brinjikji W, Luetmer PH, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR Am J Neuroradiol 2015;36:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langevin HM, Sherman KJ. Pathophysiological model for chronic low back pain integrating connective tissue and nervous system mechanisms. Med Hypotheses 2007;68:74–80. [DOI] [PubMed] [Google Scholar]
- 7. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- 8. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: A critical review. Expert Rev Neurother 2009;9:745–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wertli MM, Eugster R, Held U, et al. Catastrophizing-a prognostic factor for outcome in patients with low back pain: A systematic review. Spine J 2014;14:2639–57. [DOI] [PubMed] [Google Scholar]
- 10. Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine 2002;27: E109–20. [DOI] [PubMed] [Google Scholar]
- 11. Miles CL, Pincus T, Carnes D, et al. Can we identify how programmes aimed at promoting self-management in musculoskeletal pain work and who benefits? A systematic review of sub-group analysis within RCTs. Eur J Pain 2011;15–775. e1-11. [DOI] [PubMed] [Google Scholar]
- 12. Kovacs FM, Seco J, Royuela A, Corcoll-Reixach J, Pena-Arrebola A; Spanish Back Pain Research Network. The prognostic value of catastrophizing for predicting the clinical evolution of low back pain patients: A study in routine clinical practice within the Spanish National Health Service. Spine J 2012;12:545–55. [DOI] [PubMed] [Google Scholar]
- 13. van der Windt DA, Kuijpers T, Jellema P, van der Heijden GJ, Bouter LM. Do psychological factors predict outcome in both low-back pain and shoulder pain? Ann Rheum Dis 2007;66:313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Flor H, Diers M, Birbaumer N. Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neuroscience Lett 2004;361:147–50. [DOI] [PubMed] [Google Scholar]
- 15. Kleinbohl D, Holzl R, Moltner A, et al. Psychophysical measures of sensitization to tonic heat discriminate chronic pain patients. Pain 1999;81:35–43. [DOI] [PubMed] [Google Scholar]
- 16. Clauw DJ, Williams D, Lauerman W, et al. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine 1999;24:2035–41. [DOI] [PubMed] [Google Scholar]
- 17. O’Neill S, Manniche C, Graven-Nielsen T, Arendt-Nielsen L. Generalized deep-tissue hyperalgesia in patients with chronic low-back pain. Eur J Pain 2007;11:415–20. [DOI] [PubMed] [Google Scholar]
- 18. Correa JB, Costa LO, de Oliveira NT, Sluka KA, Liebano RE. Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: A case-control study. Exp Brain Res 2015;233:2391–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mlekusch S, Neziri AY, Limacher A, et al. Conditioned pain modulation in patients with acute and chronic low back pain. Clin J Pain 2015; doi: 10.1097/AJP.0000000000000238. [DOI] [PubMed]
- 20. George SZ, Wittmer VT, Fillingim RB, Robinson ME. Fear-avoidance beliefs and temporal summation of evoked thermal pain influence self-report of disability in patients with chronic low back pain. J Occup Rehabil 2006;16:95–108. [DOI] [PubMed] [Google Scholar]
- 21. Imamura M, Chen J, Matsubayashi SR, et al. Changes in pressure pain threshold in patients with chronic nonspecific low back pain. Spine 2013;38:2098–107. [DOI] [PubMed] [Google Scholar]
- 22. LeResche L, Turner JA, Saunders K, Shortreed SM, Von Korff M. Psychophysical tests as predictors of back pain chronicity in primary care. J Pain 2013;14:1663–70. [DOI] [PubMed] [Google Scholar]
- 23. Mlekusch S, Schliessbach J, Camara RJ, et al. Do central hypersensitivity and altered pain modulation predict the course of chronic low back and neck pain? Clin J Pain 2013;29:673–80. [DOI] [PubMed] [Google Scholar]
- 24. Edwards RR. Individual differences in endogenous pain modulation as a risk factor for chronic pain. Neurology 2005;65:437–43. [DOI] [PubMed] [Google Scholar]
- 25. Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J Pain 2012;13:936–44. [DOI] [PubMed] [Google Scholar]
- 26. Arendt-Nielsen L, Andresen T, Malver LP, et al. A double-blind, placebo-controlled study on the effect of buprenorphine and fentanyl on descending pain modulation: A human experimental study. Clin J Pain 2012;28:623–7. [DOI] [PubMed] [Google Scholar]
- 27. Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;153:1193–8. [DOI] [PubMed] [Google Scholar]
- 28. Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156(suppl 1):S24–S31. [DOI] [PubMed] [Google Scholar]
- 29. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on research standards for chronic low back pain. J Pain 2014;15:569–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bulls HW, Freeman EL, Anderson AJ, et al. Sex differences in experimental measures of pain sensitivity and endogenous pain inhibition. J Pain Res 2015;8:311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodin BR, Anderson AJ, Freeman EL, et al. Intranasal oxytocin administration is associated with enhanced endogenous pain inhibition and reduced negative mood states. Clin J Pain 2014;31:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Edens JL, Gil KM. Experimental induction of pain: Utility in the study of clinical pain. Behav Ther 1995;26:197–216. [Google Scholar]
- 33. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults In: Turk DC, Melzack R, eds. Handbook of Pain Assessment. New York, NY: Guilford Press; 1992:135–51. [Google Scholar]
- 34. Starkweather AR, Heinemann AW, Storey S, et al. Methods to measure peripheral and central pain sensitization using quantitative sensory testing: A focus on individuals with low back pain. Appl Nurs Res 2015; doi: 10.1016/j.apnr.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 35. Yarnitsky D, Arendt-Nielsen L, Bouhassira D, et al. Recommendations on terminology and practice of psychophysical DNIC testing. Eur J Pain 2010;14:339.. [DOI] [PubMed] [Google Scholar]
- 36. Ohrbach R, Gale EN. Pressure pain thresholds in normal muscles: Reliability, measurement effects, and topographic differences. Pain 1989;37:257–63. [DOI] [PubMed] [Google Scholar]
- 37. Robinson ME, Riley JL, 3rd, et al. The Coping Strategies Questionnaire: A large sample, item level factor analysis. Clin J Pain 1997;13:43–9. [DOI] [PubMed] [Google Scholar]
- 38. Cella D, Riley W, Stone A, et al. PROMIS Cooperative Group. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol 2010;63:1179–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Revicki DA, Chen WH, Harnam N, et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain 2009;146:158–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy 1980;66:271–3. [PubMed] [Google Scholar]
- 41. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine 2000;25:2940–52. Discussion 52. [DOI] [PubMed] [Google Scholar]
- 42. Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. [Google Scholar]
- 43. Devins GM, Orme CM, Costello CG, et al. Measuring depressive symptoms in illness populations: Psychometric properties of the Center for Epidemiologic Studies Depression (CES-D) Scale. Psychol Health 1988;2:139–56. [Google Scholar]
- 44. Magni G, Moreschi C, Rigatti-Luchini S, Merskey H. Prospective study on the relationship between depressive symptoms and chronic musculoskeletal pain. Pain 1994;56:289–97. [DOI] [PubMed] [Google Scholar]
- 45. Myers TA. Goodbye listwise deletion: Presenting hotdeck imputation as an easy and effective tool for handling missing data. Commun Methods Meas 2011;5:297–310. [Google Scholar]
- 46. Kuiper JI, Burdorf A, Frings-Dresen MH, et al. Assessing the work-relatedness of nonspecific low-back pain. Scand J Work Environ Health 2005;31:237–43. [PubMed] [Google Scholar]
- 47. Wideman TH, Asmundson GG, Smeets RJ, et al. Rethinking the fear avoidance model: Toward a multidimensional framework of pain-related disability. Pain 2013;154:2262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Linton SJ, Shaw WS. Impact of psychological factors in the experience of pain. Phys Ther 2011;91:700–11. [DOI] [PubMed] [Google Scholar]
- 49. Burns JW, Glenn B, Bruehl S, Harden RN, Lofland K. Cognitive factors influence outcome following multidisciplinary chronic pain treatment: A replication and extension of a cross-lagged panel analysis. Behav Res Ther 2003;41:1163–82. [DOI] [PubMed] [Google Scholar]
- 50. Burns JW, Kubilus A, Bruehl S, Harden RN, Lofland K. Do changes in cognitive factors influence outcome following multidisciplinary treatment for chronic pain? A cross-lagged panel analysis. J Consult Clin Psychol 2003;71:81–91. [DOI] [PubMed] [Google Scholar]
- 51. Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain 2006;7:261–71. [DOI] [PubMed] [Google Scholar]
- 52. Vowles KE, McCracken LM, Eccleston C. Processes of change in treatment for chronic pain: The contributions of pain, acceptance, and catastrophizing. Eur J Pain 2007;11:779–87. [DOI] [PubMed] [Google Scholar]
- 53. Campbell CM, Kronfli T, Buenaver LF, et al. Situational versus dispositional measurement of catastrophizing: Associations with pain responses in multiple samples. J Pain 2010;11:443–53 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and experimental pain sensitivity: Only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain 2005;6:338–9. [DOI] [PubMed] [Google Scholar]
- 55. Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011;152:S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Curr Rheumatol Rep 2011;13:513–20. [DOI] [PubMed] [Google Scholar]
- 58. Griffith LE, Shannon HS, Wells RP, et al. Individual participant data meta-analysis of mechanical workplace risk factors and low back pain. Am J Public Health 2012;102:309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Puta C, Schulz B, Schoeler S, et al. Somatosensory abnormalities for painful and innocuous stimuli at the back and at a site distinct from the region of pain in chronic back pain patients. PLoS One 2013;8:e58885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goodin BR, Bulls HW, Herbert MS, et al. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and older with knee osteoarthritis: Ethnic differences. Psychosom Med. 2014;76:302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Campbell CM, France CR, Robinson ME, et al. Ethnic differences in diffuse noxious inhibitory controls. J Pain 2008;9:759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cruz-Almeida Y, Sibille KT, Goodin BR, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol 2014;66:1800–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aviram J, Shochat T, Pud D. Pain perception in healthy young men is modified by time-of-day and is modality dependent. Pain Med 2015;16:1137–44. [DOI] [PubMed] [Google Scholar]
- 64. Bachmann CG, Nitsche MA, Pfingsten M, et al. Diurnal time course of heat pain perception in healthy humans. Neurosci Lett 2011;489:122–5. [DOI] [PubMed] [Google Scholar]