Abstract
Racial disparities in living donor kidney transplantation (LDKT) persist but the most effective target to eliminate these disparities remains unknown. One potential target could be delays during completion of the live donor evaluation process. We studied racial differences in progression through the evaluation process for 247 African American (AA) and 664 non-AA living donor candidates at our center between January 2011-March 2015. AA candidates were more likely to be obese (38% vs. 22%: p<0.001), biologically-related (66% vs. 44%: p<0.001), and live ≤50 miles from the center (64% vs. 37%: p<0.001) than non-AAs. Even after adjusting for these differences, AAs were less likely to progress from referral to donation (aHR for AA versus non-AA: 0.260.47 0.83; p=0.01). We then assessed racial differences in completion of each step of the evaluation process and found disparities in progression from medical screening to in-person evaluation (aHR: 0.410.620.94; p=0.02) and from clearance to donation (aHR: 0.28 0.510.91; p=0.02), compared with from referral to medical screening (aHR: 0.781.021.33; p=0.95) and from in-person evaluation to clearance (aHR: 0.59 0.931.44; p=0.54). Delays may be a manifestation of the transplant candidate’s social network, thus, targeted efforts to optimize networks for identification of donor candidates may help address LDKT disparities.
Keywords: Living Donation, Listed Candidates, Donor Candidates
INTRODUCTION
Racial disparities in achieving living donor kidney transplantation (LDKT) have persisted over the last 30 years, but the most effective target to eliminate these disparities remains unknown. Living kidney donation rates have decreased 7% over the past decade, and this decline has been even more pronounced among racial/ethnic minorities (1). Compared to 14% in 2004, only 10% of living kidney donations in 2016 were from African American (AA) donor candidates (1). Several previous studies addressing the racial disparity in living kidney donation have focused on barriers prior to starting the donor evaluation process (2–11). These include unmet educational needs about the living donation process, difficulties finding willing and clinically suitable potential donors, medical mistrust, and suboptimal access to healthcare (2–11). However, less is known about barriers that may influence disparities after patients and living donor candidates are referred to the transplant center to begin the evaluation process.
The living donor evaluation is a complex multistep screening process that requires medical clearance, multiple interactions with the transplant center and sometimes even long distance travel. Thus, one potential source of racial disparities in LDKT could be delays during completion of the living donor evaluation process. Previous single-center studies have reported that AA donor candidates may have a lower likelihood of clinical suitability for donor nephrectomy (4, 12). Additionally, AA donor candidates may be more likely to be deemed medically ineligible (4, 12, 13), lost to follow-up (14), and decide against donation (12, 13). However, it is unclear if additional delays or barriers are experienced by AA donor candidates at specific steps of the living donor evaluation process.
To better understand the process following living donor candidate referral, and thereby gain potential insights into mechanisms underlying racial disparities in LDKT, we studied time to kidney donation for AA and non-AA donor candidates referred to our center. We further studied the progression of the living donor candidates through each step of the evaluation process to identify delays.
METHODS
Study Population
We studied 911 living donor candidates that came forward on behalf of adult kidney transplant candidates at our center between January 2, 2011 and March 16, 2015. We used electronic medical records to obtain donor demographic information (age, sex, race, blood group, and body mass index) and donor candidate outcomes (date the donor candidate reached each step in the evaluation process, became ineligible, or donated). This study was approved by the Johns Hopkins University Institutional Review Board.
Distance to Transplant Center
Distances between donor candidates and transplant centers were calculated with the latitude and longitude of the geographic centroid of the donor candidate ZIP code and the latitude and longitude of the transplant center ascertained by Google maps search. ZIP code locations were obtained from the zipcode package available through R, which contains a database of latitudes and longitudes for US ZIP codes from the CivicSpace database (August 2004) and augmented with data from federalgovernmentzipcodes.us (Jan 22, 2012). As previously described (15), to account for the curve of the earth the distance in miles was calculated using an arc-distance equation with the latitudes and longitudes expressed in radians as:
Distance was categorized a priori to separate major metropolitan areas known to refer patients to our center (0–50 Baltimore, DC; 51–300 New York City, Philadelphia, Richmond; 301–1000 Raleigh, Atlanta, Orlando; >1000 Dallas, Los Angeles).
Donor Candidate Socioeconomic Status
We linked our donor candidate data to U.S. Census data using each donor candidate’s ZIP code to estimate a socioeconomic status (SES) index. We used the Agency for Healthcare Research and Quality SES index with possible values standardized to range from 0 to 100 (14). A higher index value corresponds to higher SES and a lower index value corresponds to lower SES (16). The index was calculated according to the formula:
Living Donor Candidate Evaluation Process
At our center, the living donor candidate evaluation includes 5 key steps:
Donor referral and cross-match – Interested living donor candidates contact the transplant office or complete an online kidney donor questionnaire. Living donor candidates are then asked to obtain blood work for blood type confirmation and tissue typing.
Medical screening- Living donor candidates undergo comprehensive testing such as 24-hour urine collection, urinalysis, blood work, a stool test for occult blood, Tuberculosis testing, current mammogram, and colonoscopy.
Evaluation- Donor candidates are seen by the nephrologist, surgeon, psychologist, social worker, and nurse coordinator during a one day visit and undergo diagnostic testing (3D CAT scan, EKG, Chest X-ray).
Clearance- The transplant team meets to discuss the medical results weekly and deem donor candidates appropriate for surgery.
Transplant- A date is scheduled for donation.
Donor Candidate Acceptance Criteria
At our center, potential donors must have a Body Mass Index (BMI) under 35 kg/m2. Potential donors with hypertension that are considered for donation must be >50 years old, non-AA, and on one blood pressure medication or on less than the maximum dose of a combination pill.
Outcome Ascertainment for Donor candidates
We evaluated time from donor candidate referral to donation, censoring for donor-related ineligibility, kidney transplant candidate-related ineligibility, and end of study date as censored observations. Administrative censorship, as used in the context of this study, provides the number of potential live donors that are still being evaluated in the process. Furthermore, we estimated time from donor candidate referral to medical screening, medical screening to evaluation, evaluation to clearance, and clearance to transplant by donor candidate race, censoring for the same events.
Reasons for Non-donation
The reasons for non-donation among donor candidates at our center were categorized as donor-related reasons (medical, financial, personal, social, or alternate donor candidate selected). For donor-related reasons of non-donation, financial, personal, and social were categorized as “other” in Table 3 and Table 4. The reasons for non-donation among transplant candidates were categorized as transplant candidate-related reasons (transplant candidate medical ineligibility, received a deceased donor transplant, death, moved, and transplantation at another center). For transplant candidate reasons of non-donation, candidate death and candidate moved were categorized as “other” in Table 3 and Table 4. Donor candidates can be deemed ineligible due to social or personal reasons, which, at our center, include donor candidate decided not to go forward in the process or the live donor decision committee decided against proceeding with the donor candidate due to social reasons. For medical reasons of non-donation, we combined various issues pertaining to the kidney in a category named kidney abnormalities (anatomy, proteinuria, cysts, pyelonephritis, atrophic left kidney, urological history, polycystic disease, kidney stones, low GFR, or low creatinine clearance). The “other” category for medical reasons for non-donation included pregnancy and infectious diseases.
Table 3.
Outcomes and reasons for non-donation among donor candidates referred by race.
| N (%) | Overall | AA | Non-AA |
|---|---|---|---|
| N=911 | N=247 | N=664 | |
| Still in the process1 | 173 (18) | 46 (19) | 96(14) |
| Donated | 100 (10) | 17 (7) | 83 (13) |
| Reason for Non-donation | 709 (72)2 | 1843 (74) | 4854 (73) |
| Candidate-related reasons | |||
| No longer a Candidate | 114 (17) | 31 (17) | 73 (16) |
| Received DD Transplant | 72 (11) | 13 (7) | 50 (11) |
| Transplant at another facility | 50 (7) | 5 (3) | 44 (10) |
| Other related issue5 | 16 (2) | 2 (1) | 12 (3) |
| Donor-related reasons | |||
| Other candidate selected | 50 (7) | 7 (4) | 41 (9) |
| Personal6 | 113 (17) | 29 (16) | 81 (17) |
| Social7 | 64 (10) | 17 (10) | 39 (8) |
| Medical | 199 (29) | 74 (42) | 120 (26) |
| HTN | 63 (32) | 42 (45) | 34 (26) |
| BMI | 53 (26) | 30 (32) | 33 (25) |
| Kidney abnormalities8 | 41 (21) | 10 (11) | 32 (25) |
| Medical History | 25 (13) | 6 (7) | 18 (14) |
| Diabetes | 11 (5) | 5 (5) | 5 (4) |
| Other medical reason9 | 6 (3) | 0 (0) | 7 (6) |
BMI, body-mass index; HTN, hypertension; DD, deceased donor
Administrative censorship, as used in the context of this study, provides the number of potential live donors that are still being evaluated in the process.
4% were missing a reason for non-donation
3% were missing a reason for non-donation
5% were missing a reason for non-donation
Other related issue: candidate death and candidate moved
Personal reasons defined by donor candidate decided not to go forward in the process
Social reasons defined by the transplant team decided against proceeding with the donor candidate due to social reasons.
Kidney abnormalities= anatomy, proteinuria, cysts, pyelonephritis, atrophic left kidney, urological history, polycystic disease, kidney Stones, insufficient kidney function (low GFR/creatinine clearance)
Other medical reason= pregnant, unknown, infectious disease
Table 4.
Outcomes and reasons for non-donation among donor candidates referred by phase.
| N (%) | Ref-Med | Med-Eval | Eval-Clear | Clear-Transplant |
|---|---|---|---|---|
| N=911 | N=333 | N=244 | N=149 | |
| Still in the process1 | 142 (14) | 41 (12) | 19 (8) | 19 (13) |
| Donated | 342 (33) | 178 (54) | 147 (60) | 103 (69) |
| Reason for Non-donation | 555 (53)2 | 114 (34) | 78 (32)3 | 27 (18) |
| Candidate-related reasons | ||||
| No longer a Candidate | 110 (20) | 14 (12) | 2 (4) | 4 (15) |
| Received DD Transplant | 44 (8) | 15 (13) | 10 (21) | 7 (26) |
| Transplant at another facility | 42 (8) | 4 (4) | 4 (8) | 3 (11) |
| Other related issue4 | 43 (8) | 7 (6) | 3 (6) | 3 (11) |
| Donor-related reasons | ||||
| Other candidate selected | 45 (8) | 4 (4) | 3 (6) | 2 (7) |
| Social/Personal5 | 137 (25) | 30 (26) | 6 (12) | 6 (23) |
| Medical | 125 (23) | 40 (35) | 21 (43) | 2 (7) |
| HTN | 39 (31) | 13 (33) | 7 (33) | 0 (0) |
| BMI | 43 (34) | 1 (2) | 0 (0) | 0 (0) |
| Kidney abnormalities6 | 17 (14) | 15 (38) | 10 (48) | 1 (50) |
| Medical History | 18 (14) | 3 (7) | 4 (19) | 0 (0) |
| Diabetes | 6 (5) | 4 (10) | 0 (0) | 0 (0) |
| Other medical reason7 | 2 (2) | 4 (10) | 0 (0) | 1 (50) |
BMI, body-mass index; HTN, hypertension; DD, deceased donor
Administrative censorship, as used in the context of this study, provides the number of potential live donors that are still being evaluated in the process.
2% were missing a reason for non-donation
37% were missing a reason for non-donation; this may be due to a lack of record updating during this step since majority of the donor candidates that did not proceed forward were due to donor-related medical reasons.
Other related issue: candidate death, candidate moved, or do not know.
Social/Personal reasons includes donor candidate decided not to go forward in the process or transplant team decided against proceeding with the donor candidate due to social reasons.
Kidney abnormalities= anatomy, proteinuria, cysts, pyelonephritis, atrophic left kidney, urological history, polycystic disease, kidney Stones, insufficient kidney function (low GFR/creatinine clearance)
Other medical reason= pregnant, unknown, infectious disease
Statistical Analysis
We followed AA and non-AA living donor candidates through the evaluation process, from the date of referral to their end-point (donated, donor-related ineligibility, kidney transplant candidate-related ineligibility, or administratively censored) to investigate differences in the donor candidate experience by race. No Non-Directed Donors, Kidney Paired Exchange Donors, or ABO incompatible donors were included in the study. At the medical screening step, only one donor candidate is chosen to progress through the rest of the process. We conducted a sensitivity analysis comparing characteristics and results of the first donor referred compared to subsequent donors referred on behalf of a kidney transplant candidate and found no differences. Thus, we restricted the analysis to the first donor candidate who came forward on behalf of a transplant candidate. For all models, we adjusted for donor candidate characteristics including race, sex, age, obesity (BMI >30), donor-recipient relationship, and distance from the transplant center.
In a sensitivity analysis, we used a competing risks framework comparing the outcomes of medical ineligibility, reason for non-donation, and progression to the next step (17). Similar results were obtained in the sensitivity analysis using competing risks regression and thus we chose to describe our results using cox regression. For all models, we administratively censored donor candidates on March 16, 2015. Confidence intervals are reported as per the method of Louis and Zeger (18). All analyses were performed using Stata 14.0/MP for Linux (College Station, Texas).
RESULTS
Living Donor Candidates
There were a total of 911 donor candidates referred to our center between January 2, 2011 and March 16, 2015, on behalf of their kidney transplant candidate. Among the 247 (28%) donor candidates who were AA, the median age was 44 years old (IQR: 34–54), 59% were female, 38% were obese, 66% were biologically related to the transplant candidate, 64% lived within 50 miles from the transplant center, and the median SES was 63 (IQR: 56–68, Table 1). Among the 664 (72%) non-AA donor candidates, the median age was 49 years old (IQR: 38–59), 66% were female, 22% were obese, 44% were biologically related to the transplant recipient, 37% lived within 50 miles from the transplant center, and the median SES was 66 (IQR: 60–70). Compared with non-AA donor candidates, AA donor candidates were younger and more likely to be male, obese, biologically related to the transplant candidate, live closer to the transplant center, and have a lower socioeconomic status (Table 1).
Table 1.
Characteristics of donor candidates referred on behalf of kidney transplant candidates on the waitlist at The Johns Hopkins Hospital between 01/2011 and 03/2015.
| AA | Non-AA | p-value | |
|---|---|---|---|
| N=247 | N=664 | ||
| Age, Median (IQR) | 44 (34–54) | 49 (38–59) | <0.001 |
| Female, n (%) | 172 (59) | 480 (66) | 0.049 |
| Blood Type, n (%)1 | 0.004 | ||
| O | 94 (58) | 268 (49) | |
| A | 33 (20) | 196 (36) | |
| B | 26 (16) | 67 (12) | |
| AB | 8 (5) | 20 (4) | |
| BMI, n (%)2 | <0.001 | ||
| <25 | 59 (20) | 294 (40) | |
| 25–29 | 97 (33) | 264 (36) | |
| >30 | 110 (38) | 158 (22) | |
| Relationship to Recipient, n (%)3 | <0.001 | ||
| Biologically Related | 190 (66) | 322 (44) | |
| Spousal/Life Partner | 40 (14) | 144 (20) | |
| Biologically Unrelated | 51 (18) | 247 (34) | |
| Distance to center (miles, n %)4 | <0.001 | ||
| 0–50 | 186 (64) | 271 (37) | |
| 51–300 | 55 (19) | 260 (36) | |
| 301–1000 | 35 (12) | 132 (18) | |
| >1000 | 15 (5) | 68 (9) | |
| Socioeconomic Status Index5 Median (IQR) | 63 (56–68) | 66 (60–70) | <0.001 |
IQR, interquartile range; BMI, body-mass index
ABO Blood Type was missing for 34.6% due to a smaller number of donor candidates qualifying for the tissue typing/blood compatibility step of the evaluation process.
BMI missing for 10% of donor candidates.
Relationship to Recipient missing for 3% of donor candidates.
Distance was categorized a priori by cities closest to our center (0–50 Baltimore, DC; 51–300 New York City, Philadelphia, Richmond; 301–1000 Raleigh, Atlanta, Orlando; >1000 Dallas, Los Angeles).
The socioeconomic status is an index created by the Agency for Healthcare Research and Quality.
Living Donor Evaluation and Clearance
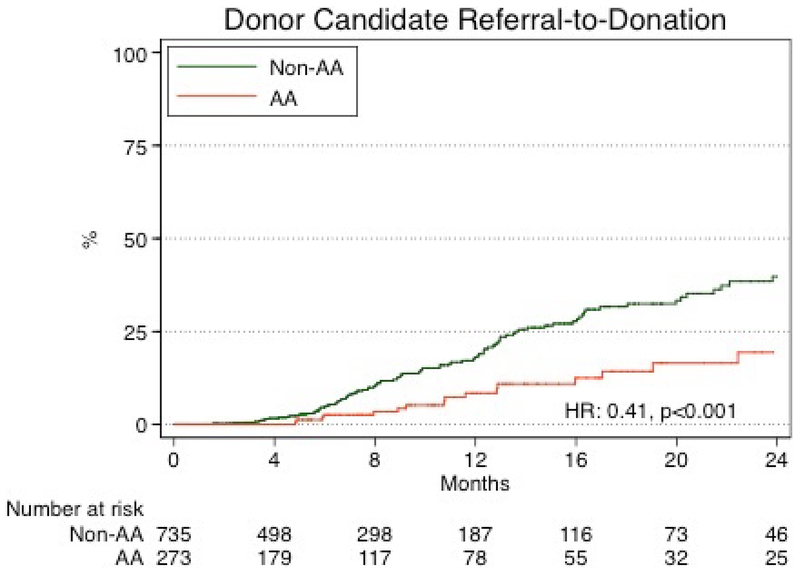
The 1-year and 2-year cumulative incidence of donation was 14% and 30% respectively. The 1-year and 2-year cumulative incidence of donation among AA donor candidates was 8.0% and 20%, respectively, in comparison to, 17% and 36% for non-AA donor candidates (Figure 2). In an unadjusted analysis, AA donor candidates were less likely to progress from referral to donation (HR: 0.27 0.410.79, p<0.001). After adjustment for donor candidate characteristics, AA donor candidates remained less likely to progress from referral to donation (aHR: 0.26 0.47 0.83; p=0.01, Table 2).
Figure 2. Time from donor candidate referral to donation by race.
The 1-year and 2-year cumulative incidence of donation was 14% and 30% respectively. The 1-year and 2-year cumulative incidence of donation among AA donor candidates was 8.0% and 20%, respectively, in comparison to, 17% and 36% for non-AA donor candidates.
Table 2.
Donor candidate characteristics associated with rates of progression through each step of the living donor evaluation process.
| Overall | Referral-Medical Screening | Medical Screening-Evaluation | Evaluation-Clearance | Clearance-Donation | |
|---|---|---|---|---|---|
| N=911 | N=910 | N=333 | N=238 | N=146 | |
| Donor Candidate Characteristics | |||||
| AA | 0.26 0.470.83 | 0.851.101.43 | 0.410.620.94 | 0.59 0.931.44 | 0.28 0.510.91 |
| Female | 0.47 0.650.97 | 0.921.161.46 | 0.660.921.26 | 0.58 0.82 1.17 | 0.47 0.73 1.10 |
| BMI ≥30 | 0.42 0.72 1.23 | 0.811.051.36 | 0.560.841.22 | 0.41 0.65 1.04 | 0.82 1.43 2.47 |
| Age | |||||
| 18–44 | Reference | Reference | Reference | Reference | Reference |
| 45–64 | 0.89 1.39 2.16 | 0.851.071.36 | 0.921.281.80 | 0.79 1.14 1.65 | 0.76 1.16 1.77 |
| 65–100 | 0.61 1.27 2.67 | 0.771.121.65 | 0.851.442.43 | 0.45 0.84 1.58 | 0.42 0.94 2.05 |
| Relationship to Recipient | |||||
| Related | Reference | Reference | Reference | Reference | Reference |
| Spousal | 0.42 0.76 1.36 | 0.841.121.52 | 0.741.121.71 | 0.57 0.90 1.41 | 0.39 0.72 1.30 |
| Other Unrelated | 0.58 0.93 1.48 | 0.650.851.09 | 0.831.191.72 | 0.57 0.86 1.28 | 0.65 1.02 1.62 |
| Distance to center | |||||
| 0–50 | Reference | Reference | Reference | Reference | Reference |
| 51–300 | 0.34 0.570.94 | 0.911.171.52 | 0.58 0.83 1.19 | 0.49 0.76 1.15 | 0.36 0.61 1.03 |
| 301–1000 | 0.360.631.10 | 0.831.141.56 | 0.470.731.45 | 0.59 0.95 1.53 | 0.31 0.56 1.01 |
| >1000 | 0.30 0.63 1.31 | 0.931.392.07 | 0.44 0.75 1.30 | 0.77 1.37 2.39 | 0.23 0.48 0.99 |
Bold- statistically significant p<0.05
Reasons for non-donation
Among 247 AA donor candidates, 46 (19%) were still in the process, 17 (7%) donated, and 184 (74%) did not donate (Table 3). The transplant candidate-related reasons for non-donation were as follows: the transplant candidate received a deceased donor transplant (7%), was no longer a candidate (17%), received a transplant at another facility (3%), and other transplant candidate related issue (1%). The donor-related reasons for non-donation were medical (42%), social (10%), personal (16%), or other donor selected (4%). The top medical reasons for non-donation among AA donor candidates were hypertension (45%), obesity (32%), and kidney abnormalities (11%).
Among 664 non-AA donor candidates, 96 (14%) were still in the process, 83 (13%) donated, and 485 (73%) did not donate (Table 3). The transplant candidate-related reasons for non-donation were: the transplant candidate received a deceased donor transplant (11%), was no longer a candidate (16%), received a transplant at another facility (10%), and other transplant candidate related issue (3%). The donor-related reasons for non-donation were medical (26%), social (8%), personal (17%), or other donor selected (9%). The top medical reasons for non-donation among non-AA donor candidates were hypertension (26%), obesity (25%), and kidney abnormalities (25%). Compared with non-AA donor candidates, AA donor candidates were more likely to be deemed medically ineligible for donation due to hypertension and obesity (HTN: 45% AA vs. 26% non-AA, BMI: 32% AA vs. 25% non-AA).
Overall, the reasons for non-donation differed during each step of the donor evaluation process. The reasons for non-donation during referral to medical screening (56% vs. 44%), medical screening to evaluation (65% vs. 35%), and evaluation to clearance (61% vs. 39%) were mostly due to donor-related reasons. Whereas, reasons for non-donation during cleared to donation (63% vs. 37%) were mostly due to candidate-related reasons (Table 4).
Referral to Medical Screening
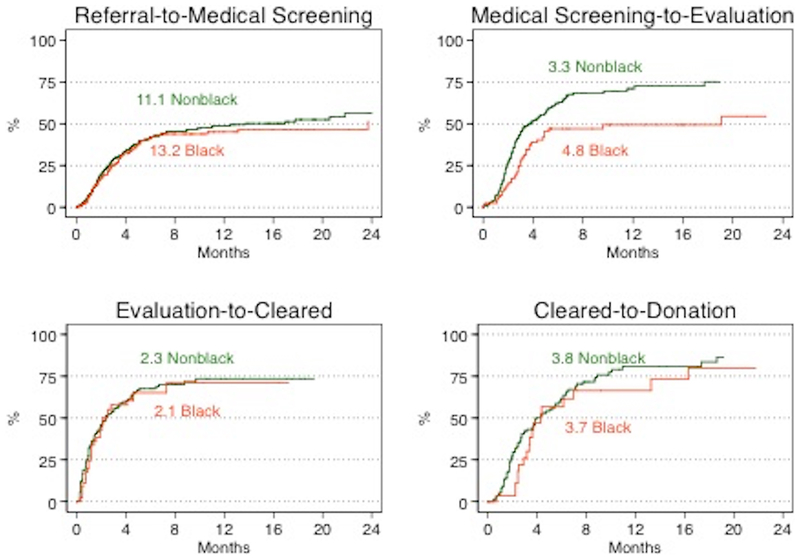
Upon further examination of each step in the donor evaluation process, the median time from referral to medical screening for those who actually donated was 2.2 months (2.5 months AA vs. 2.2 months non-AA) (Figure 1). Among those who did not make it to the next step, the median time from referral to medical screening was 3.4 months (2.6 months AA vs. 3.7 months non-AA). The median time for AA donor candidates compared with non-AA was similar (11.1 months vs. 13.2 months; p=0.6). After adjusting for donor characteristics, AA donor candidates were as likely to progress from referral to medical screening as non-AA donor candidates (aHR: 0.851.101.43, p=0.5, Table 2).
Figure 1. Living donor candidate evaluation process.
Among those who progressed to donation, the median time from referral to donation was 9.2 months (10.2 months AA vs. 8.9 months non-AA). Among those who made it to the next step, the median time from referral to medical screening was 2.2 months (2.5 months AA vs. 2.2 months non-AA), from medical screening to evaluation was 2.4 months (2.8 months AA vs. 2.3 months non-AA), from evaluation to clearance was 1.0 month (1.2 months AA vs. 0.9 months non-AA), and from clearance to transplant was 2.7 months (1.4 months AA vs. 1.0 months non-AA). Among those who were deemed ineligible, the median time from referral to donation was 4.3 months (3.7 months AA vs. 4.6 months non-AA). Among those who did not make it to the next step, the median time from referral to medical screening was 3.4 months (2.6 months AA vs. 3.7 months non-AA), from medical screening to evaluation was 3.9 months (5.3 months AA vs. 3.4 months non-AA), from evaluation to clearance was 2.9 month (4.5 months AA vs. 2.7 months non-AA), and from clearance to transplant was 3.3 months (3.0 months AA vs. 3.7 months non-AA).
Medical Screening to Evaluation
The median time from medical screening to evaluation of those who actually donated was 2.4 months (2.8 months AA vs. 2.3 months non-AA, Figure 1). Among those who did not make it to the next step, the median time from medical screening to evaluation was 3.9 months (5.3 months AA vs. 3.4 months non-AA). Compared to non-AA donor candidates, the median time for AA donor candidates was substantially longer (5.3 months vs. 3.4 months, p=0.01). Even after accounting for differences in donor characteristics, AA donor candidates were much less likely to progress from medical screening to evaluation (aHR: 0.41 0.620.94; p=0.02) (Table 2).
Evaluation to Clearance
From evaluation to clearance, the median time among those who actually donated was 1.0 month (1.2 months AA vs. 0.9 months non-AA, Figure 1). Among those who did not make it to the next step, the median time from evaluation to clearance was 2.9 months (4.5 months AA vs. 2.7 months non-AA). The median time for AA and non-AA donor candidates were similar (2.3 months vs. 2.1 months; p=0.7). Even after accounting for differences in donor characteristics, AA donor candidates were as likely to progress from evaluation to clearance as non-AA donor candidates (aHR: 0.59 0.931.44, p=0.5, Table 2).
Clearance to Donation
The median time from clearance to transplant of those who actually donated was 2.7 months (1.4 months AA vs. 1.0 months non-AA, Figure 1). Among those who did not make it to the next step, the median time from clearance to donation was 3.3 months (3.0 months AA vs. 3.7 months non-AA). The median time for AA and non-AA donor candidates were similar (3.7 months vs. 3.8 months; p=0.4, Figure 3). After accounting for differences in donor characteristics, AA donor candidates were less likely to progress from clearance to donation as non-AA donor candidates (aHR: 0.28 0.51 0.91, p=0.02, Table 2).
Figure 3.
Time through the living donor evaluation process for donor candidates referred on behalf of a kidney transplant candidate by race and phase.
DISCUSSION
In this single-center study of racial differences in living kidney donation, we followed 247 AA and 664 non-AA living donor candidates through the donor referral and evaluation process. Estimated 2-year cumulative incidence of living donation for AA donor candidates was 20% versus 36% for non-AA candidates. Even after accounting for differences in donor characteristics, AA donor candidates were less likely to progress from referral to donation (aHR for AA versus non-AA: 0.260.47 0.83; p=0.01). After studying each step of the living donor evaluation process, we found disparities in progression from medical screening to in-person evaluation (aHR: 0.410.620.94; p=0.02) and from clearance to donation (aHR: 0.28 0.510.91; p=0.02). More AA donor candidates were deemed medically ineligible due to hypertension and obesity (HTN: 45% AA vs. 26% non-AA, BMI: 32% AA vs. 25% non-AA).
Previous studies have reported that AA donor candidates were less likely to be recruited for donation, less likely to have converted from a donor candidate to a donor, less likely to donate, and more likely to decide against donation (12, 13). Extending from this prior literature, the key strength of our study is that we were able to isolate the specific step in the donor evaluation process where the racial differences were most pronounced. We found that AA donor candidates had a longer median time from medical screening to evaluation and from clearance to donation compared to non-AA donor candidates. These findings offer a potential explanation for the previously reported disparities of AA donor candidates being less likely to convert to donors and less likely to donate overall. Current strategies to achieve growth in living donor kidney transplantation do not take this into consideration (5, 10, 19–25). We hypothesized that the disparity in this specific step could be explained by the fact that AA nationally have a higher prevalence of hypertension, obesity, diabetes (26). As such, our study was able to demonstrate that a substantial proportion of AA donor candidates were biologically related. Given well documented familial clustering of diabetes, hypertension, and chronic kidney disease, this heavy reliance on biological relatives might be a nontrivial driver of the observed disparities in access to live donor kidney transplantation (2–5). In addition, our study adjusted for donor candidate distance to transplant center given that time and distance of travel to the center may be a contributing factor to the rate of progression through the living donor evaluation process (27). Unlike previous literature, we were able to demonstrate that a substantial proportion of AA donor candidates lived within 50 miles of our transplant facility (28). Thus, delays may be a manifestation of the transplant candidate’s social network; drawn predominantly from biological relatives, persons living in close proximity, and from persons who are likely to be deemed medically ineligible for live kidney donation. Targeted efforts to optimize networks for identification of donor candidates may help address LDKT disparities.
Our study provides insight on previously reported reasons for racial disparities in living kidney donation. Some studies have reported that AA donor candidates are often lost to follow-up during the evaluation process, which authors assume is a reflection of their willingness to donate. However, survival analytic methods were not used to reach these inferences and thus, the large proportion of AA donor candidates that were lost to follow-up might represent an artifact of the methods used (12–14). Using survival analytic methods provides better estimates, as it separates the number of donor candidates still in the evaluation process from the number of donor candidates who are truly lost to follow-up. Our study found that 19% of AA donor candidates were still in the process compared to 14% of non-AA donor candidates. Furthermore, the progression of a living kidney donor candidate through the evaluation process is inherently linked to the status of the transplant candidate (i.e. transplant candidate medical ineligibility, received a deceased donor transplant, transplant candidate death, moved, or transplantation at another center). Our methods were able to reveal whether the reasons for non-donation were predominantly donor-related or candidate-related for each step of the process. The reasons for non-donation during referral to medical screening (56% donor vs. 44% candidate), medical screening to evaluation (65% donor vs. 35% candidate), and evaluation to clearance (61% donor vs. 39% candidate) were mostly due to donor-related reasons. Whereas the reasons for non-donation from clearance to donation (63% candidate vs. 37% donor) were mostly transplant candidate-related. Further studies should investigate and optimize the donor candidates lost due to candidate-related reasons.
The living donor evaluation process is not standardized across the US and thus our exact evaluation process may not be generalizable to other centers (12–14, 29–31). However, our analytic approach was able to reveal the key features and complexities of the donor evaluation process at our center, which can be used at other transplant centers to identify barriers in their specific process. Our study does reveal the specific steps in the process where the racial disparity is greatest. Additionally, we provide our centers donor acceptance criteria as context for other transplant centers. As such, it is likely that our inferences are relevant to transplant centers nationwide. This information could be incorporated into future studies to better understand barriers at this step and for interventions that are designed towards increasing living donation (5, 10, 11, 24, 32, 33).
In conclusion, AA donor candidates experienced delays during the evaluation process following referral. AA donor candidates were less likely to progress through living donor evaluation process, specifically from medical screening to evaluation and clearance to donation. Delays may be a manifestation of the transplant candidate’s social network; drawn predominantly from biological relatives, persons living in close proximity, and from persons who are likely to be deemed medically ineligible for live kidney donation. Thus, targeted efforts to expand networks for identification of donor candidates may help address LDKT disparities.
ACKNOWLEDGMENTS
The authors were supported by grants K24DK101828 (D.L.S.), R01DK096008 (D.L.S.), F32AG044994 (E.A.K.), K01DK101677 (A.B.M.), and R01DK111966 (A.M.C., K.K., and J.M.K.) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and by grant K01HS024600 (T.S.P) from the Agency for Healthcare Research and Quality. The funders had no role in the design and conduct of the study, interpretation of the data, or preparation of the manuscript.
The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by Clinical Transplantation.
REFERENCES
- 1.Donors Recovered in the U.S. by Donor Type.
- 2.Purnell TS, Hall YN, Boulware LE. Understanding and overcoming barriers to living kidney donation among racial and ethnic minorities in the United States. Adv Chronic Kidney Dis. 2012;19(4):244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purnell TS, Powe NR, Troll MU, Wang NY, Haywood C Jr., LaVeist TA, et al. Measuring and explaining racial and ethnic differences in willingness to donate live kidneys in the United States. Clin Transplant. 2013;27(5):673–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weng FL, Reese PP, Mulgaonkar S, Patel AM. Barriers to living donor kidney transplantation among black or older transplant candidates. Clin J Am Soc Nephrol. 2010;5(12):2338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodrigue JR. The “House Calls” Trial: A Randomized Controlled Trial to Reduce Racial Disparities in Live Donor Kidney Transplantation: Rationale and Design. Contemporary Clinical Trials. 2012;33(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigue JR, Cornell DL, Kaplan B, Howard RJ. Patients’ willingness to talk to others about living kidney donation. Progress in transplantation (Aliso Viejo, Calif). 2008;18(1):25–31. [DOI] [PubMed] [Google Scholar]
- 7.Reese PP, Shea JA, Berns JS, Simon MK, Joffe MM, Bloom RD, et al. Recruitment of live donors by candidates for kidney transplantation. Clin J Am Soc Nephrol. 2008;3(4):1152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Navaneethan SD, Singh S. A systematic review of barriers in access to renal transplantation among African Americans in the United States. Clin Transplant. 2006;20(6):769–75. [DOI] [PubMed] [Google Scholar]
- 9.Barnieh L, McLaughlin K, Manns BJ, Klarenbach S, Yilmaz S, Hemmelgarn BR. Barriers to living kidney donation identified by eligible candidates with end-stage renal disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011;26(2):732–8. [DOI] [PubMed] [Google Scholar]
- 10.Strigo TS. The TALKS study to improve communication, logistical, and financial barriers to live donor kidney transplantation in African Americans: protocol of a randomized clinical trial. BMC Nephrology. 2015;16(160). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garonzik-Wang JM, Berger JC, Ros RL, Kucirka LM, Deshpande NA, Boyarsky BJ, et al. Live donor champion: finding live kidney donors by separating the advocate from the patient. Transplantation. 2012;93(11):1147–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng FL, Dhillon N, Lin Y, Mulgaonkar S, Patel AM. Racial differences in outcomes of the evaluation of potential live kidney donors: a retrospective cohort study. American journal of nephrology. 2012;35(5):409–15. [DOI] [PubMed] [Google Scholar]
- 13.Moore DR, Feurer ID, Zaydfudim V, Hoy H, Zavala EY, Shaffer D, et al. Evaluation of living kidney donors: variables that affect donation. Progress in transplantation (Aliso Viejo, Calif). 2012;22(4):385–92. [DOI] [PubMed] [Google Scholar]
- 14.Lunsford SL, Simpson KS, Chavin KD, Menching KJ, Miles LG, Shilling LM, et al. Racial disparities in living kidney donation: is there a lack of willing donors or an excess of medically unsuitable candidates? Transplantation. 2006;82(7):876–81. [DOI] [PubMed] [Google Scholar]
- 15.Gentry SE, Chow EK, Wickliffe CE, Massie AB, Leighton T, Segev DL. Impact of broader sharing on the transport time for deceased donor livers. Liver transplantation : official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20(10):1237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quality. AfHRa. Chapter 3: Creation of New Race-Ethnicity Codes and SES Indicators for Medicare Beneficiaries U.S. Department of Health & Human Services.
- 17.Fine JP G R. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1991;vol. 94(no. 446):pp. 496–509. [Google Scholar]
- 18.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics (Oxford, England). 2009;10(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon EJ, Feinglass J, Carney P, Vera K, Olivero M, Black A, et al. A Website Intervention to Increase Knowledge About Living Kidney Donation and Transplantation Among Hispanic/Latino Dialysis Patients. Progress in transplantation (Aliso Viejo, Calif). 2016;26(1):82–91. [DOI] [PubMed] [Google Scholar]
- 20.Arriola KR, Powell CL, Thompson NJ, Perryman JP, Basu M. Living donor transplant education for African American patients with end-stage renal disease. Progress in transplantation (Aliso Viejo, Calif). 2014;24(4):362–70. [DOI] [PubMed] [Google Scholar]
- 21.Waterman AD, Peipert JD, Hyland SS, McCabe MS, Schenk EA, Liu J. Modifiable patient characteristics and racial disparities in evaluation completion and living donor transplant. Clin J Am Soc Nephrol. 2013;8(6):995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waterman AD. Your Path to Transplant: a randomized controlled trial of a tailored computer education intervention to increase living donor kidney transplant. BMC Nephrology. 2014;15(166). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ephraim PL, Powe NR, Rabb H, Ameling J, Auguste P, Lewis-Boyer L, et al. The providing resources to enhance African American patients’ readiness to make decisions about kidney disease (PREPARED) study: protocol of a randomized controlled trial. BMC Nephrol. 2012;13:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulware LE, Hill-Briggs F, Kraus ES, Melancon JK, Falcone B, Ephraim PL, et al. Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: a randomized controlled trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61(3):476–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigue JR, Paek MJ, Schold JD, Pavlakis M, Mandelbrot DA. Predictors and Moderators of Educational Interventions to Increase the Likelihood of Potential Living Donors for Black Patients Awaiting Kidney Transplantation. Journal of racial and ethnic health disparities. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Center for Health S. Health, United States. Health, United States, 2015: With Special Feature on Racial and Ethnic Health Disparities. Hyattsville, MD: National Center for Health Statistics (US); 2016. [PubMed] [Google Scholar]
- 27.Hays R, Rodrigue JR, Cohen D, Danovitch G, Matas A, Schold J, et al. Financial Neutrality for Living Organ Donors: Reasoning, Rationale, Definitions, and Implementation Strategies. Am J Transplant. 2016;16(7):1973–81. [DOI] [PubMed] [Google Scholar]
- 28.Weng FL, Lee DC, Dhillon N, Tibaldi KN, Davis LA, Patel AM, et al. Characteristics and Evaluation of Geographically Distant vs Geographically Nearby Living Kidney Donors. Transplantation proceedings. 2016;48(6):1934–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe Rudow D, Baliga P. Living Donor Kidney Transplantation: Overcoming Disparities in Live Kidney Donation in the US-Recommendations from a Consensus Conference. Clin J Am Soc Nephrol. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapasia JB, Kong SY, Busque S, Scandling JD, Chertow GM, Tan JC. Living donor evaluation and exclusion: the Stanford experience. Clin Transplant. 2011;25(5):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.KDIGO. KDIGO Clinical Practice guideline on the Evaluation and follow-up care of Living Kidney Donors. [DOI] [PMC free article] [PubMed]
- 32.Kumar K, King EA, Muzaale AD, Konel JM, Bramstedt KA, Massie AB, et al. A Smartphone App for Increasing Live Organ Donation. Am J Transplant. 2016. [DOI] [PubMed] [Google Scholar]
- 33.Gill JDJ, Rose C, Johnston O, Landsberg D, Gill J. The Effect of Race and Income on Living Kidney Donation in the United States. Journal of the American Society of Nephrology. 2013;24(11):1872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]