Abstract
Late Archaean sedimentary rocks contain compelling geochemical evidence for episodic accumulation of dissolved oxygen in the oceans along continental margins before the Great Oxidation Event. However, the extent of this oxygenation remains poorly constrained. Here we present thallium and molybdenum isotope compositions for anoxic organic-rich shales of the 2.5 billion-year-old Mount McRae Shale from Western Australia, which previously yielded geochemical evidence of a transient oxygenation event. During this event, we observe an anti-correlation between thalium and molybdenum isotope data, including two shifts to higher molybdenum and lower thalium isotope compositions. Our data indicate pronounced burial of manganese oxides in sediments elsewhere in the ocean at these times, which requires that water columns above portions of the ocean floor were fully oxygenated: all the way from the air-sea interface to well below the sediment-water interface. Well-oxygenated continental shelves were likely the most important sites of manganese oxide burial and mass-balance modeling results suggest that fully oxygenated water columns were at least a regional-scale feature of early-Earth’s oceans 2.5 billion years ago.
The extent of dissolved O2 accumulation in Earth’s oceans before the Great Oxidation Event remains poorly understood (GOE; ~2.4 to 2.3 Ga1). Multiple lines of geochemical evidence indicate that O2 was produced by cyanobacteria in the surface ocean well before accumulating in the atmosphere during and after the GOE2–8. Models indicate that cyanobacteria in the surface ocean were capable of promoting mild accumulation of dissolved O2 (up to 25 μM9) in shallow waters under a predominately anoxic atmosphere, perhaps extending across large areas of the ocean10. However, it is difficult to test these models because existing geochemical proxies cannot easily be used to assess the breadth and depth of ocean oxygenation.
A case has been made for widespread oxygenation of shallow waters before the GOE in continental margin environments based on carbon isotopes in bulk rock and kerogen from 2.7 Ga carbonate sedimentary rocks2. However, this prior work could not determine whether O2 accumulation was restricted to surface waters or if it extended deeper in the water column, let alone whether oxygenation reached bottom-waters and sediments (i.e., a fully oxygenated water column).
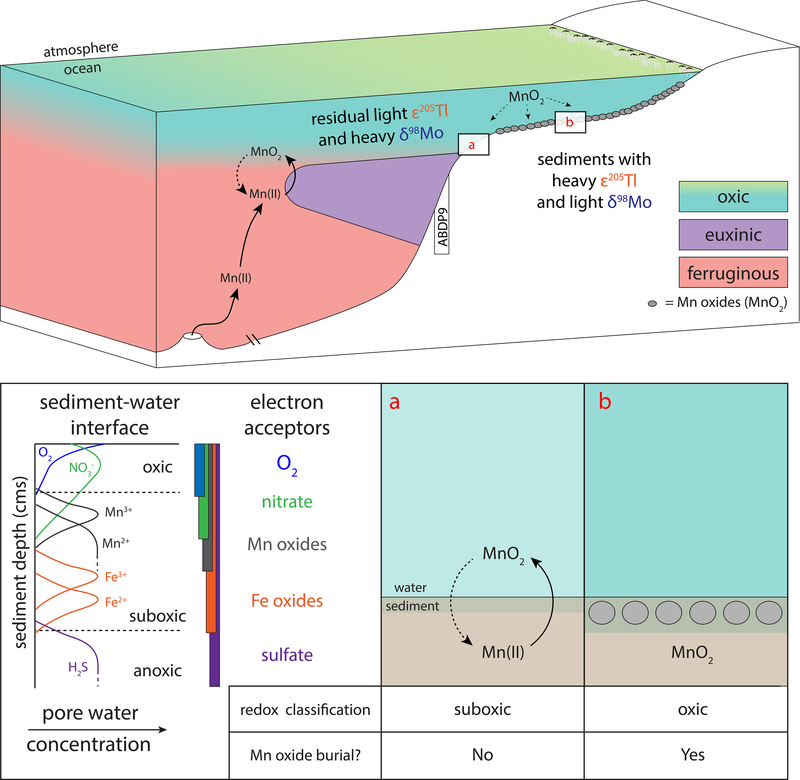
A fully oxygenated water column at 2.6–2.5 Ga was inferred from black shales (upper Nauga Formation, Ghaap Group, South Africa) enriched in Re but not Mo relative to average crustal abundances3. This geochemical signature occurs when O2 is present in pore waters at a depth of up to about one centimeter below the sediment-water interface, when Fe(III) becomes the dominant electron acceptor during organic matter oxidation and sulfide accumulation is low11,12 (Figure 1, panel A). However, this evidence was restricted to a single continental margin (Griqualand West Basin) and could not be extrapolated to the wider oceans.
Figure 1. Illustration of a possible well-oxygenated marine margin before the GOE.
Evidence exists for sufficient O2 accumulation in an ancient water column between 2.6 and 2.5 Ga to weakly oxygenate underlying sediments3 (“suboxic”; Panel A). However, O2 penetration into these sediments was not sufficient to promote Mn oxide burial11,12. If settings capable of burying Mn oxides were present in ancient oceans (“oxic”; Panel B) over a large seafloor area, then seawater Tl and Mo isotope compositions would have decreased and increased, respectively. The Mt. McRae Shale was deposited under locally euxinic conditions37 and should therefore have captured these changes in seawater isotope signatures18,28. Sedimentary redox structure is modified from previous work50.
If fully oxygenated water columns on continental margins were a widespread feature of pre-GOE oceans, then Mn oxide burial in sediments beneath these settings would also have been widespread. In the modern ocean, O2 in marine bottom waters and sediments readily oxidizes dissolved Mn(II) and Mn(III) to insoluble Mn(IV)-bearing minerals that precipitate out of solution13,14. In contrast, Mn oxides do not form under anoxic conditions, nor are they buried in anoxic marine sediments. Even if formed under O2-rich conditions, Mn oxides undergo reductive dissolution shortly after being exposed to anoxic conditions within the water column or sediments13–15. Manganese oxides are highly unstable when O2 is absent because in such conditions they are an efficient electron acceptor16. Therefore, appreciable Mn oxide burial today only occurs where water columns are fully oxygenated and O2 persistently penetrates sediment pore waters14. In Earth’s past, Mn oxides should have also been buried where O2 penetrated deeply into marine sediments. This could have occurred under more oxidizing conditions than those identified by Kendall et al. (2010) in the Nauga Formation shales, where Re abundances are elevated but Mo abundances are negligible. Specifically, Mn oxide burial requires penetration of O2 well beyond one centimeter below the sediment water interface and occurs before Fe(III) becomes the primary electron acceptor during organic carbon oxidation14,16 (e.g., Figure 1, Panel B).
Here, we pair Tl and Mo isotope data from the late Archaean (~2.5 Ga) black shales of the Mt. McRae Shale (Hamersley Basin, Western Australia) to track the extent of marine Mn oxide burial before the GOE. The isotopic cycling of both Tl and Mo in the ocean is directly linked to global Mn oxide burial fluxes17,18. Therefore, their paired application is a powerful way to infer the extent of fully oxygenated water columns at a regional-to-global scale, in contrast to other proxies (e.g., Re vs Mo enrichments and11 sedimentary Fe speciation19) that focus only on redox conditions in the local water column.
Pairing Tl and Mo isotopes to track paleoredox conditions
The use of Tl isotopes as a paleoredox proxy is relatively new17,20,21, but builds on extensive prior study of Tl isotope systematics. The Tl isotope composition of modern seawater [reported in epsilon notation: ε205Tl, where ε205Tl = (205/203Tlsample/205/203TlNIST-997 – 1) • 104] is homogenous and lighter than that of bulk continental crust (ε205Tlseawater = −6.0 ± 0.3[18,22], compared to ε205Tlbulk-crust = −2.1 ± 0.3[23]). The light ε205Tl in modern seawater is a result of preferential removal of isotopically heavy Tl from seawater by Mn oxides in well-oxygenated marine sediments24. Importantly, the contemporaneous seawater ε205Tl signature is captured and preserved in sediments from anoxic and sulfidic (i.e. euxinic) basins18. Therefore, Tl isotope studies of sedimentary rocks deposited under euxinic conditions provide a means to track ancient seawater ε205Tl signatures, which should vary in response to changes in Mn oxide burial fluxes. In support of this application, two recent studies used Tl isotope compositions in Mesozoic shales deposited in locally euxinic conditions to track changes in Mn oxide burial fluxes before, during, and after Oceanic Anoxic Events, documenting episodes of significant marine deoxygenation20,21.
Molybdenum isotopes are a more established proxy also shown to be sensitive to marine Mn oxide burial25. The modern seawater Mo isotope composition [reported in delta notation: δ98Mo, where δ98Mo = ((98/95Mosample/98/95MoNIST-SRM-3134 – 1) • 103) + 0.25‰[26]] is heavier than that of bulk continental crust (δ98Moseawater = 2.34 ± 0.10‰[26], compared to δ98Mobulk-crust = 0.47 ± 0.12‰[27]). This heavy seawater δ98Mo composition is due largely to preferential removal of lighter-mass Mo isotopes by adsorption to Mn oxides in well-oxygenated marine sediments25. Similar to Tl, this heavy seawater δ98Mo value is captured in strongly euxinic settings where Mo removal from bottom waters is nearly quantitative28.
It is advantageous to measure Tl isotopes in addition to Mo isotopes because Mo isotope interpretation is complicated by alternative fractionation pathways that do not affect Tl isotopes. For example, processes that occur during continental weathering29 and riverine transport30 can remove isotopically light Mo (but see reference 31), but do not cause measurable Tl isotope fractionation23. Weakly sulfidic marine sediments also incorporate lighter-mass Mo isotopes28 but impart no known isotopic effect on Tl18. Iron oxides can remove isotopically light Mo and drive seawater to heavy δ98Mo values32, but are unlikely to fractionate Tl isotopes because Fe oxides lack the ability to oxidize Tl(I) to Tl(III), which is what drives isotopic fractionation during sorption to Mn oxides24,33. This study is the first to pair both proxies in the same sample set.
Anti-correlation of Mo and Tl isotopes in the Mt. McRae Shale
We focus on the 2.5 Ga Mt. McRae Shale from Western Australia in drill core ABDP9 because black shales from the upper part of this formation host convincing evidence for a widespread oxygenation episode predating the GOE5,6,34–40. These rocks are an ideal archive for tracking fluctuations in seawater Tl and Mo isotope compositions at 2.5 Ga because they were deposited in locally euxinic conditions37 that favor preservation of seawater δ98Mo and ε205Tl values (Figure 1; see supplement for more information about the Mt. McRae Shale).
Molybdenum isotope signatures much heavier than those in igneous crustal rocks were previously found in the Mt. McRae Shale (and in coeval shales from South Africa41) but could not be definitively ascribed to Mn oxide burial elsewhere in the ocean38. If Mn oxides were being buried at this time, ε205Tl should also be fractionated relative to bulk continental crust. An anti-correlation between ε205Tl and δ98Mo is expected because fractionation incurred during Mn oxide adsorption is in opposing directions for the two isotope systems17,18.
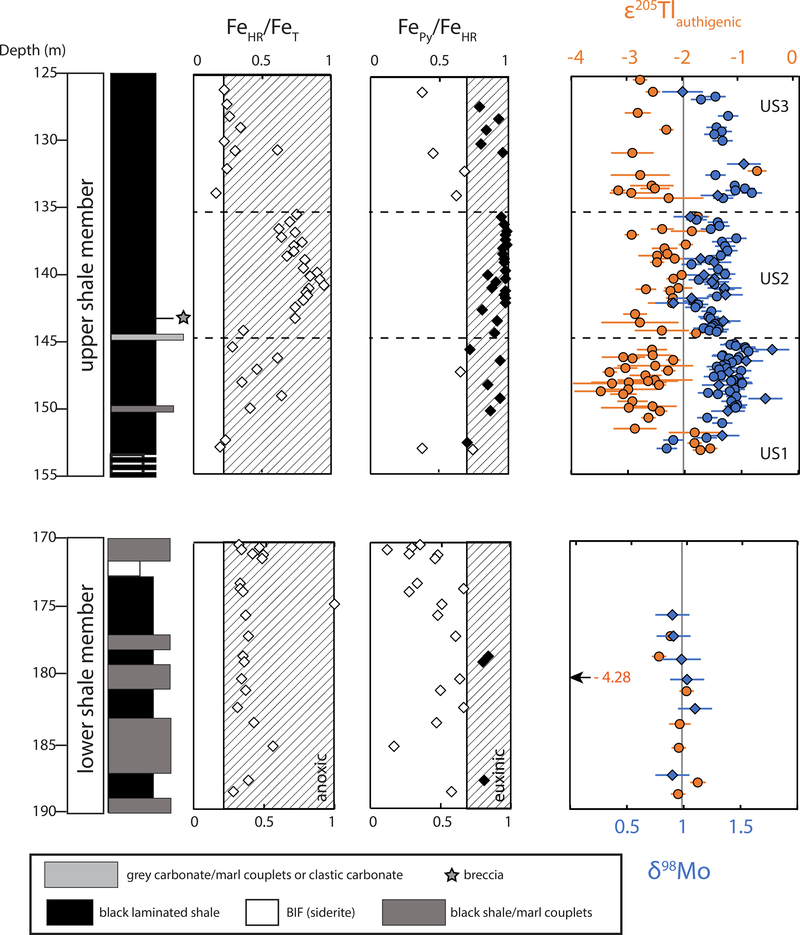
We find that ε205Tl is systematically lighter during two distinct intervals of the euxinic upper shale (US) member in the Mt. McRae Shale: 153.30–144.36 m (US1) and 134.17–126.15 m (US3) (average ε205Tl = −2.65) (Figure 2). δ98Mo exhibits heavier values in these same intervals (average δ98Mo = 1.37‰), revealing the predicted anti-correlation with ε205Tl. Compared to these intervals, Tl and Mo isotope compositions for 144.26–135.58 m (US2) are heavier (average ε205Tl = −2.39, p-value = 0.05, two-tailed unpaired t-test) and lighter (average δ98Mo = 1.23‰, p-value << 0.05), respectively. A cross-plot of shale samples with both isotope measurements from the US reveals a statistically significant anti-correlation (p-value = 0.01). In contrast to the euxinic US, isotope compositions are invariant in the non-euxinic lower shale member (170–190 m core depth, see supplement for discussion of this interval, and interpretation of concentration data).
Figure 2. Geochemical profiles in organic-rich shales from the Mt. McRae Shale (orange = ε205Tl, blue = δ98Mo ).
Hatched boxes represent values indicative of anoxic (FeHR/FeT > 0.22) and euxinic (FePy/FeHR > 0.7) deposition19. Data that exceed both criteria are in black. Diamonds reflect data from previous work37,38 and circles are data from this study. The grey vertical line in the isotope plot represents average isotope compositions from the lower shale member, with the exception of one anomalous Tl isotope value (ε205Tl = −4.28 ± 0.13 at 180.33 m). All error bars represent the 2SD reproducibility of that sample or the external long-term reproducibility of natural reference materials, whichever is greater.
Fully oxygenated regional water columns on continental shelves
Light ε205Tl and heavy δ98Mo in US1 and US3 provide strong evidence for the formation and subsequent burial of Mn oxides elsewhere in the ocean at these times. To drive the observed isotopic trends, water columns needed to have been fully oxygenated over portions of the ocean floor. Most likely, Mn oxide burial at 2.5 Ga occurred in shallow oxygenated shelf environments where O2 produced within the surface ocean by cyanobacteria was capable of being continuously transferred to underlying waters and marine sediments (e.g., the environment illustrated in Figure 1).
Alternative local basin controls or processes in the sedimentary environment where the Mt. McRae Shale was deposited cannot explain the observed isotope trends in the upper shale member. In a modern euxinic basin that is not well-connected to the open ocean (i.e., the Black Sea), Tl isotopes in the local water column and underlying sediments are heavier than the open-ocean signature18. If the Hamersley Basin was also not well-connected to the open-ocean, then ε205Tl of the Mt. McRae Shale may have been higher than the open-ocean signature. This would require even lighter seawater ε205Tl compositions during deposition of US1 and US3, which would then imply an even greater extent of sediment Mn oxide burial elsewhere in the oceans. Furthermore, in modern euxinic settings where Mo is not quantitatively transferred from seawater to sediments, sedimentary δ98Mo compositions are always lighter than the coeval seawater signature28. Hence, if Mo removal from euxinic bottom waters in the Hamersley Basin was not quantitative, then seawater δ98Mo would be even heavier than observed in the Mt. McRae Shale, again implying even more significant sediment Mn oxide burial elsewhere in the ocean.
In theory, the observed Tl and Mo isotope shifts might be alternatively explained by “shuttling” of Tl and Mo bound to oxide minerals formed in oxic surface waters to underlying anoxic waters and/or sediments on Late Archaean continental margins, where these elements could then be captured in euxinic sediments. If so, fully oxygenated water columns would not be required to explain the antithetic shifts in Tl and Mo isotopes recorded in the Mt. McRae Shale. However, this notion is not supported by observations in the modern Cariaco Basin, a modern analog environment where Mn oxides are formed in oxygenated surface waters and subsequently transported to euxinic bottom waters and sediments deeper in the basin42. An oxide shuttle should cause ε205Tl in euxinic sediments to be heavier than in surface oxic seawater24. This is not observed, however. Rather, euxinic sediment ε205Tl in the Cariaco Basin is indistinguishable from overlying seawater (average ε205Tleuxinic = −5.1 ± 1.3; 2SD vs. ε205Tlseawater −5.5 ± 0.7; 2SD)18. One possible explanation for this lack of Tl isotope is that Tl released by Mn oxide dissolution in sulfidic deep waters is first re-mixed and re-homogenized with the dissolved Tl pool prior to Tl capture in pyrite. Regardless, since anoxic sediments in the modern Cariaco Basin do not preserve the Tl isotope effects of oxide adsorption18 even though oxide minerals are shown to be delivered at least transiently to these sediments42, a Mn oxide shuttle in redox-stratified marine basins probably had a minimal impact on the Late Archaean seawater Tl isotope mass-balance.
Although there are proposed pathways of Mn oxide formation that do not require O2, they would not be likely to cause the observed isotopic effects in the upper shale member. Oxidation of reduced Mn in the upper water column by hypothesized Mn-oxidizing phototrophs43 or by UV light44 cannot account for burial at the seafloor because underlying anoxic waters and sediments would recycle Mn back into solution [as Mn(II)]13,14. The high abundance of Fe(II) in deep ferruginous waters of the Archaean oceans1, for example, would readily promote reduction of Mn oxides in anoxic waters. If the water column or sediment pore waters were anoxic, even seasonally15, reductive dissolution of Mn oxides would release sorbed Tl and Mo. Additionally, invoking an O2-free explanation to explain the observed isotopic trends requires neglecting the many independent lines of evidence for a “whiff” of O2 at 2.5 Ga5,6,34–40.
Paleo-seawater ε205Tl and δ98Mo can be estimated directly from the Mt. McRae Shale data and used to reconstruct ocean redox conditions. Measured ε205Tl values serve as a direct estimate for the coeval seawater signature (as low as −3.57 ± 0.48) because euxinic sediments capture the overlying seawater Tl isotope value18. The δ98Mo recorded in euxinic marine sediments is always equal to or lighter than seawater28, and thus represent a lower limit for the coeval seawater signature (as heavy as 1.56 ± 0.10‰). It is possible, indeed likely, that the Tl and Mo isotope compositions of seawater fluctuated during deposition of the US member of the Mt. McRae Shale. Deposition of this interval is estimated to have occurred over ~11 million years34 and the “whiff” of O2 was likely a transient episode of even shorter duration39. To estimate ocean redox conditions during peak Mn oxide burial, we use the lightest ε205Tl and heaviest δ98Mo from the US member. During peak Mn oxide burial, retention of heavier-mass Tl and lighter-mass Mo isotopes would have been maximized, resulting in the lightest ε205Tl and heaviest δ98Mo seawater signatures. Unsurprisingly, the lightest ε205Tl (−3.57 ± 0.48 epsilon units at 148.75 m) and heaviest δ98Mo (1.56 ± 0.10‰ at 145.74 m) occur during US1, an interval that hosts multiple lines of geochemical evidence for an oxygenation episode5,6,34,37–39.
Using the estimated seawater ε205Tl and δ98Mo, ocean redox conditions can be inferred using isotope mass-balance equations as follows:
ε205TlInputs = ε205TlAOC(fAOC) + ε205Tloxic(fTl-oxic) + ε205Tlother(fother)
and
δ98MoInputs = δ98Moeuxinic(feuxinic) + δ98MoSAD(fSAD) + δ98Mooxic(fMo-oxic)
where ε205Tlx and δ98Mox represent the isotopic composition of average oceanic inputs and outputs, and fx denotes the relative removal flux for each output. For Tl, we designate low-T alteration of oceanic crust (fAOC), well-oxygenated Mn oxide-rich sediments (fTl-oxic), and “other” (fother) as the three dominant marine outputs. The “other” output signifies Tl removal with no associated isotopic fractionation (e.g., euxinic basins18). For Mo, similar to recent work, we use euxinic sediments (feuxinic), sediments that are sulfidic at depth (fSAD; where sulfide is limited to sediment pore waters), and well-oxygenated Mn oxide-rich sediments (fMo-oxic) as the three outputs45 (see supplementary material for more detailed information about modeling, including key assumptions).
The mass-balance model results indicate that well-oxygenated Mn oxide-rich sediments were an important sink for both Tl (fTl-oxic = 6–21%) and Mo (fMo-oxic = 20–34%) at 2.5 Ga. Together, these results suggest that fully oxygenated shelf environments were a common feature on continental margins, at least regionally at 2.5 Ga.
We make no attempt here to convert these fluxes into the areal extent of seafloor because the flux per areal unit into these marine outputs was likely much different in the Archean compared to today. Burial rates of Tl and Mo in modern oxic marine environments that bury Mn oxides are very low, much lower than their burial rates into other modern marine outputs (e.g., burial of Tl during low-T alteration of oceanic crust46 and burial of Mo under strong euxinic conditions45). For this reason, seafloor area calculations using modern Tl and Mo burial rates and our ancient seawater isotope signature estimates would require the majority of the seafloor at 2.5 Ga to have been oxic. Expansive oxic conditions before the GOE is unlikely because many independent lines of evidence support a predominately anoxic global ocean at this time1. Most likely, burial rates of Tl and Mo in 2.5 Ga oxic environments were much higher than today. For example, dissolved Mn concentrations in Archean seawater may have been four orders of magnitude higher than today47, providing a strong potential for high Mn oxide burial rates in oxic environments, and therefore a stronger potential for Tl and Mo adsorption. Furthermore, the burial rate of Mo (and potentially also of Tl) into euxinic environments could have been much lower than today because sulfate concentrations in Archean oceans were very low48. Euxinic conditions in a low-sulfate ocean could have been much weaker than today, lowering the potential for sedimentary retention of Mo. In summary, a smaller area of 2.5 Ga seafloor burying Mn oxides could conceivably drive a more pronounced seawater Tl and Mo isotope signature effect than today but is difficult to estimate precisely.
Our findings provide a new perspective on marine oxygenation at 2.5 Ga, on the eve of the GOE. Multiple lines of geochemical evidence provide strong support for O2 in shallow waters of the Hamersley Basin at 2.5 Ga5,6,34 and the adjoining Griqualand West Basin4,41 (which may have bordered the same ocean basin49). However, because Mn oxide burial requires fully oxygenated water columns at 2.5 Ga, our multi-isotope data supports more oxygenated continental shelves over a greater area than previously recognized using other geochemical datasets. Specifically, the inferred seawater ε205Tl and δ98Mo requires fully oxygenated water columns in shelf environments within the Hamersley Basin and adjoining basins(s), and potentially even large portions of the continental margins worldwide. Our results highlight the significant and expanding role of cyanobacteria as engineers of the Archean biosphere, particularly in the runup to the Great Oxidation Event.
Methods:
Tl isotopes
Tl sample preparation and purification were performed in the NIRVANA Laboratory at Woods Hole Oceanographic Institution (WHOI), as well as in Dr. Jeremy Owens’ Laboratory at Florida State University (FSU) within the National High Magnetic Field Laboratory (NHMFL). Powdered samples from ABDP9 were leached using a method from previous literature17,20, which has been shown to effectively separate authigenic Tl (i.e. Tl bound to pyrite) from detrital Tl. Each fraction was subsequently digested following procedures discussed in that literature. Ion exchange chromatography was completed using previously described techniques51,52. Similar to recent work20, samples were only subjected to one column pass because Tl concentrations were high and thus very little sample mass was processed.
Tl isotopic analyses were conducted at the WHOI Plasma Mass Spectrometry Facility and at the NHMFL in Tallahassee. At both locations a Thermo Neptune MC-ICPMS was used with an Aridus II desolvating nebulizer sample introduction system. Measurements were made in low-resolution mode utilizing sample-standard bracketing relative to NIST 997 Tl in epsilon notation. External normalization to NIST SRM 981 Pb was utilized to monitor instrumental mass bias, similar to previous studies51,52. Since a known quantity of NIST SRM 981 Pb was added to each sample, Tl concentrations could be calculated during MC-ICPMS analysis using the measured 205Tl/208Pb ratios. Tl isotope values are reported in epsilon notation relative to NIST 997 Tl metal. One USGS shale SCO-1 standard was leached, purified, and analyzed with each sample set to monitor accuracy and showed good reproducibility (ε205Tlauthigenic = −2.80 ± 0.13, 2SD, n = 8) compared to a recent study:(−2.92 ± 0.11)(20).
Mo isotopes
All Mo sample digestion, isotope purification, and analysis were completed at the W.M. Keck Foundation Laboratory for Environmental Biogeochemistry at Arizona State University. Quarter cores were cut from ABDP9, powdered, ashed, digested, and concentrations were analyzed using the same techniques employed in previous work34. Enough sample was then taken from the same digested stock solutions to provide 125 ng Mo that was spiked with an optimal amount of calibrated synthetic Mo isotope double-spike (97Mo and 100Mo) before purification via ion exchange chromatography, again utilizing methods from previous studies53,54. The double spike is used for chromatography and instrumental mass fractionation correction.
Isotope ratio measurements were performed on a Thermo Neptune multi-collector ICPMS (MC-ICPMS) in low-resolution mode with an Elemental Scientific Inc. Apex inlet system and using sample-standard bracketing38,55. All measurements were made using the Johnson Matthey Specpure Mo plasma standard (Lot #802309E; RochMo2) as the bracketing standard, and then re-calculated relative to the new international NIST SRM 3134 standard = + 0.25‰(26). Samples and standards were analyzed at a concentration of 25 μg/g Mo, which yielded about three volts of signal on mass 98. Samples were analyzed in triplicate (at least), with the average 2SD sample reproducibility being 0.06‰, and the maximum being 0.11‰. Over the 12-month period of Mo isotope analysis for this study, USGS rock reference material SDO-1 was simultaneously processed with each batch of samples to monitor accuracy and showed good reproducibility (δ98Mo = 1.00 ± 0.09‰ 2SD compared to 1.05 ± 0.14‰ from a previous study56), as did various analytical replicates (Table 1). Lastly, for each analytical run, a series of standards with varying spike-sample ratios was measured. All samples were within the validated spike-sample range for accurate and precise δ98Mo values.
Table 1.
Standard solution δ98Mo values from this study vs. previous work
| Standard | This studya | n | Normalized to NIST + 0.25‰b | Goldberg et al. (2013) |
|---|---|---|---|---|
| Roch-Mo2 | Bracketing std. | −0.09‰ | −0.09 ± 0.05‰ | |
| ICL-Mo | 0.16 ± 0.03‰ | 38 | 0.07 ± 0.03‰ | 0.09 ± 0.05‰ |
| Kyoto-Mo | −0.04 ± 0.05‰ | 39 | −0.13 ± 0.05‰ | −0.12 ± 0.06‰ |
| NIST SRM 3134 | 0.33 ± 0.06‰ | 45 | 0.24 ± 0.06‰ | 0.25‰ (reporting std.) |
| SDO-1 | 1.12 ± 0.05‰ | 45 | 1.03 ± 0.05‰ | 1.05 ± 0.14‰ |
Measured relative to Roch-Mo2
Normalized using δ98MoRoch-Mo2 = −0.09‰ relative to δ98MoNIST+0.25‰56
all reported errors are 2SD of the standard reproducibility
Supplementary Material
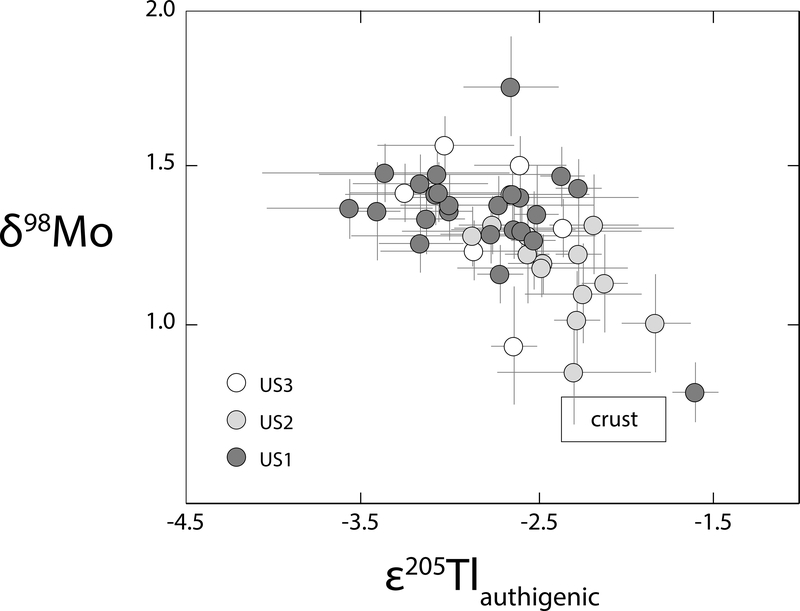
Figure 3. Mo and Tl isotope cross-plot from the upper shale member.
The anti-correlation trend in this plot, which is also apparent in Figure 1, is statistically significant (p-value = 0.01). The brown box indicates current estimates for the isotope compositions of bulk continental crust23,27. All error bars represent the 2SD reproducibility of that sample or the external long-term reproducibility of natural reference materials, whichever is greater.
Acknowledgments:
We would like to thank Wang Zheng and Jurek Blusztajn for their help with instrumental analysis at Arizona State University and Woods Hole Oceanographic Institution, respectively. This research was supported financially by the NSF Frontiers in Earth System Dynamics program (award NSF EAR-1338810), the NSF Chemical Oceanography program (award OCE 1434785), the NASA Exobiology program (award number NNX16AJ60G), and a NSERC Discovery Grant (award number RGPIN-435930). This material is based upon work supported by the National Science Foundation Graduate Research Fellowship Program under Grant No. 026257-001. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Competing interests: The authors declare no competing interests.
Data Availability: All data generated during this study are included in the supplementary information.
References:
- 1.Lyons TW, Reinhard CT & Planavsky NJ The rise of oxygen in Earth’s early ocean and atmosphere. Nature 506, 307–315 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Eigenbrode JL & Freeman KH Late Archean rise of aerobic microbial ecosystems. Proc. Natl. Acad. Sci 103, 15759–15764 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendall B et al. Pervasive oxygenation along late Archaean ocean margins. Nat. Geosci 3, 647–652 (2010). [Google Scholar]
- 4.Czaja AD et al. Evidence for free oxygen in the Neoarchean ocean based on coupled iron-molybdenum isotope fractionation. Geochim. Cosmochim. Acta 86, 118–137 (2012). [Google Scholar]
- 5.Kendall B, Brennecka GA, Weyer S & Anbar AD Uranium isotope fractionation suggests oxidative uranium mobilization at 2.50 Ga. Chem. Geol 362, 105–114 (2013). [Google Scholar]
- 6.Stüeken EE, Buick R & Anbar AD Selenium isotopes support free O2 in the latest Archean. Geology 43, 259–262 (2015). [Google Scholar]
- 7.Eickmann B et al. Isotopic evidence for oxygenated Mesoarchaean shallow oceans. Nat. Geosci 11, 133–138 (2018). [Google Scholar]
- 8.Koehler MC, Buick R, Kipp MA, Stüeken EE & Zaloumis J Transient surface ocean oxygenation recorded in the ~2.66-Ga Jeerinah Formation, Australia. Proc. Natl. Acad. Sci 115, 7711–7716 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasting JF Models relating to Proterozoic atmospheric and ocean chemistry In: Schopf J, Klein C (Eds), The Proterozoic Biosphere, A Multidisciplinary Study. Cambridge University Press, 1185–1187 (1992). [Google Scholar]
- 10.Olson SL, Kump LR & Kasting JF Quantifying the areal extent and dissolved oxygen concentrations of Archean oxygen oases. Chem. Geol 362, 35–43 (2013). [Google Scholar]
- 11.Morford JL, Emerson SR, Breckel EJ & Kim SH Diagenesis of oxyanions (V, U, Re, and Mo) in pore waters and sediments from a continental margin. Geochim. Cosmochim. Acta 69, 5021–5032 (2005) [Google Scholar]
- 12.Morford JL, Martin WR & Carney CM, Rhenium geochemical cycling: Insights from continental margins. Chem. Geol 324-325, 73–86 (2012) [Google Scholar]
- 13.Burdige DJ The biogeochemistry of manganese and iron reduction in marine sediments. Earth Sci. Rev 35, 249–284 (1993). [Google Scholar]
- 14.Calvert SE & Pedersen TF Sedimentary Geochemistry of Manganese: Implications for the environment of formation of manganiferous black shales. Econ. Geol 91, 36–47 (1996). [Google Scholar]
- 15.Kristensen E Kristiansen KD & Jensen MH Temporal behavior of Manganese and Iron in a Sandy Coastal Sediment Exposed to Water Column Anoxia. Estuaries 26, 690–699 (2003). [Google Scholar]
- 16.Froelich PN et al. Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: Suboxic diagenesis. Geochim. Cosmochim. Acta 43, 1075–1090 (1979). [Google Scholar]
- 17.Nielsen SG et al. Thallium isotopes in early diagenetic pyrite – A paleoredox proxy? Geochim. Cosmochim. Acta 75, 6690–6704 (2011). [Google Scholar]
- 18.Owens JD, Nielsen SG, Horner TJ, Ostrander CM & Peterson LC Thallium-isotopic compositions of euxinic sediments as a proxy for global manganese-oxide burial. Geochim. Cosmochim. Acta 213, 291–307 (2017). [Google Scholar]
- 19.Raiswell R et al. The iron paleoredox proxies: a guide to pitfalls, problems and proper practice. Am. J. Sci 318, 491–526 (2018). [Google Scholar]
- 20.Ostrander CM, Owens JD & Nielsen SG Constraining the rate of oceanic deoxygenation leading up to a Cretaceous Oceanic Anoxic Event (OAE-2: ~94Ma). Sci. Adv 3, e1701020 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Them TR et al. Thallium isotopes reveal protracted anoxia during the Toarcian (Early Jurassic) associated with volcanism, carbon burial, and mass extinction. Proc. Natl. Acad. Sci doi: 10.1073/pnas.1803478115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen SG et al. Hydrothermal fluid fluxes calculated from the isotopic mass balance of thallium in the ocean crust. Earth and Planet. Sci. Lett 251, 120–133 (2006). [Google Scholar]
- 23.Nielsen SG et al. Thallium isotopic composition of the upper continental crust and rivers – an investigation of the continental sources of dissolved marine thallium. Geochim. Cosmochim. Acta 19, 2007–2019 (2005). [Google Scholar]
- 24.Nielsen SG et al. Towards an understanding of thallium isotope fractionation during adsorption to manganese oxides. Geochim. Cosmochim. Acta 117, 252–265 (2013). [Google Scholar]
- 25.Wasylenki LE et al. The molecular mechanism of Mo isotope fractionation during adsorption to birnessite. Geochim. Cosmochim. Acta 75, 5019–5031 (2011). [Google Scholar]
- 26.Nägler TF et al. Proposal for an international molybdenum isotope measurement standard and data representation. Geostand. Geoanal. Res doi: 10.1111/j.1751-908X.2013.00275.x (2014). [DOI] [Google Scholar]
- 27.Willbold M & Elliot T Molybdenum isotope variations in magmatic rocks. Chem. Geol 449, 253–268 (2017). [Google Scholar]
- 28.Neubert N, Nägler TF & Böttcher ME Sulfidity controls molybdenum isotope fractionation into euxinic sediments: Evidence from the modern Black Sea. Geology 36, 775–778 (2008). [Google Scholar]
- 29.Siebert C et al. Molybdenum isotope fractionation in soils: influence of redox conditions, organic matter, and atmospheric inputs. Geochim. Cosmochim. Acta 162, 1–24 (2015). [Google Scholar]
- 30.Archer C & Vance D The isotopic signature of the global riverine molybdenum flux and anoxia in the ancient oceans. Nat. Geosci 1, 597–600 (2008). [Google Scholar]
- 31.King EK & Pett-Ridge JC Reassessing the dissolved molybdenum isotopic composition of ocean inputs: The effect of chemical weathering and groundwater. Geology, 10.1130/G45124.1 (2018). [DOI] [Google Scholar]
- 32.Goldberg T, Archer C, Vance D & Poulton SW Mo isotope fractionation during adsorption to Fe (oxyhydr)oxides. Geochim. Cosmochim. Acta 73, 6502–6516 (2009). [Google Scholar]
- 33.Peacock CL & Moon EM Oxidative scavenging of thallium by birnessite: Explanation for thallium enrichment and stable isotope fractionation in marine ferromanganese precipitates. Geochim. Cosmochim. Acta 84, 297–313 (2012). [Google Scholar]
- 34.Anbar AD et al. A whiff of oxygen before the great oxidation event? Science 317, 1903–1906 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Kaufman AJ et al. Late Archean biospheric oxygenation and atmospheric evolution. Science 317, 1900–1903 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Garvin J, Buick R, Anbar AD, Arnold GL & Kaufman AJ Isotopic evidence for an aerobic nitrogen cycle in the latest Archean. Science 323, 1045–1048 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Reinhard CT, Raiswell R, Scott C, Anbar AD & Lyons TW A late Archean sulfidic sea stimulated by early oxidative weathering of the continents. Science 326, 713–716 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Duan Y et al. Molybdenum isotope evidence for mild environmental oxygenation before the Great Oxidation Event. Geochim. Cosmochim. Acta 74, 6655–6668 (2010). [Google Scholar]
- 39.Kendall B, Creaser RA, Reinhard CT, Lyons TW & Anbar AD Transient episodes of mild environmental oxygenation and oxidative continental weathering during the late Archean. Sci. Adv 1:e1500777 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gregory DD et al. The chemical conditions of the late Archean Hamersley basin inferred from whole rock and pyrite geochemistry with Δ33S and δ34S isotope analyses. Geochim. Cosmochim. Acta 149, 223–250 (2015). [Google Scholar]
- 41.Wille M et al. Evidence for a gradual rise of oxygen between 2.6 and 2.5 Ga from Mo isotopes and Re-PGE signatures in shales. Geochim. Cosmochim. Acta 71, 2417–2435 (2007). [Google Scholar]
- 42.Algeo TJ and Tribovillard N Environmental analysis of paleoceanographic systems based on molybdenum-uranium covariation. Chem. Geol 268, 211–225 (2009). [Google Scholar]
- 43.Johnson JE et al. Manganese-oxidizing photosynthesis before the rise of cyanobacteria. Proc. Natl. Acad. Sci 110, 11238–11243 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anbar AD & Holland HD The photochemistry of manganese and the origin of banded iron formations. Geochim. Cosmochim. Acta 56, 2595–2603 (1992). [DOI] [PubMed] [Google Scholar]
- 45.Kendall B, Dahl TW & Anbar AD Good golly, why Moly? The stable isotope geochemistry of molybdenum. Rev. Mineral. Geochem 82, 682–732 (2017). [Google Scholar]
- 46.Nielsen SG, Rehkämper M & Prytulak J Investigation and application of thallium isotope fractionation. Rev. Mineral. Geochem 82, 759–798 (2017). [Google Scholar]
- 47.Holland HD The chemical evolution of the atmosphere and oceans (Princeton Univ. Press, Princeton, NJ, 1984). [Google Scholar]
- 48.Habicht KS, Gade M, Thamdrup B, Berg P & Canfield DE Calibration of sulfate levels in the Archean ocean. Science 298, 2372–2374 (2002). [DOI] [PubMed] [Google Scholar]
- 49.De Kock MO, Evans DAD & Beukes NJ Validating the existence of Vaalbara in the Neoarchean. Precambr. Res 174, 145–154 (2009). [Google Scholar]
- 50.Madison AS, Tebo BM, Mucci A, Sundby B & Luther GW III Abundant porewater Mn(III) is a major component of the sedimentary redox system. Science 341, 875–878 (2013). [DOI] [PubMed] [Google Scholar]
References cited only in Methods:
- 51.Rehkämper M & Halliday AN The precise measurement of Tl isotopic compositions by MC-ICPMS: Applications to the analysis of geological materials and meteorites. Geochim. Cosmochim. Acta 63, 935–944 (1999). [Google Scholar]
- 52.Nielsen SG, Rehkämper M, Baker JA & Halliday AN The precise and accurate determination of thallium isotope compositions and concentrations for water samples by MC-ICPMS. Chem. Geol 204, 109–124 (2004). [Google Scholar]
- 53.Siebert C, Nägler TF & Kramers JD Determination of the molybdenum isotope fractionation by double-spike multicollector inductively coupled plasma mass spectrometry. Geochem. Geophys. Geosyst 2:2000GC000124 (2001). [Google Scholar]
- 54.Barling J, Arnold GL & Anbar AD Natural mass-dependent variations in the isotopic composition of molybdenum. Earth and Planet. Sci. Lett 193, 447–457 (2001). [Google Scholar]
- 55.Kendall B, Creaser RA, Gordon GW & Anbar AD Re-Os and Mo isotope systematics of black shales from the Middle Proterozoic Velkerri and Wollogorang Formations, McArthur Basin, northern Australia. Geochim. Cosmochim. Acta 73, 2534–2558 (2009). [Google Scholar]
- 56.Goldberg T et al. Resolution of inter-laboratory discrepancies in Mo isotope data: an intercalibration. J. Anal. At. Spectrom 28, 724–735 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.