Abstract
Mucorales can cause cutaneous to deep-seated infections, mainly in the immunocompromised host, resulting in high mortality rates due to late and inefficient treatment. In this study, Galleria mellonella larvae were evaluated as a heterologous invertebrate host to study pathogenicity of clinically relevant mucormycetes (Rhizopus spp., Rhizomucor spp., Lichtheimia spp., Mucor spp.). All tested species were able to infect G. mellonella larvae. Virulence potential was species-specific and correlated to clinical relevance. Survival of infected larvae was dependent on (a) the species (growth speed and spore size), (b) the infection dose, (c) the incubation temperature, (d) oxidative stress tolerance, and (e) iron availability in the growth medium. Moreover, we exploited the G. mellonella system to determine antifungal efficacy of liposomal amphotericin B, posaconazole, isavuconazole, and nystatin-intralipid. Outcome of in vivo treatment was strongly dependent upon the drug applied and the species tested. Nystatin-intralipid exhibited best activity against Mucorales, followed by posaconazole, while limited efficacy was seen for liposomal amphotericin B and isavuconazole. Pharmacokinetic properties of the tested antifungals within this alternative host system partly explain the limited treatment efficacy. In conclusion, G. mellonella represents a useful invertebrate infection model for studying virulence of mucormycetes, while evaluation of treatment response was limited.
Keywords: mucormycosis, Galleria mellonella, antifungal susceptibility, nystatin-intralipid, in vivo model
Introduction
Mucorales are ubiquitously distributed saprophytic fungi that are able to cause severe life-threatening human infections, primarily, in immunocompromised patients.1–3 Invasive infections caused by mucormycetes (mucormycosis) have remarkably increased in recent years, with 500–1000 cases per year in Europe alone.1 Typical risk factors for mucormycosis are: hematological malignancies, stem cell transplantations, diabetic ketoacidosis or iron overload.1Rhizopus, Mucor, Lichtheimia (formerly Absidia), and Rhizomucor are the most common genera isolated from patient material.1,4,5 In Europe, Rhizopus spp. are most prevalent (almost 50% of all mucormycosis cases), with R. arrhizus as the most common species, followed by R. microsporus.1,6Mucor circinelloides and Lichtheimia corymbifera cause, as the second and third most abundant agent, almost 19% of all mucormycosis cases in Europe.6
Severity of the infection caused by members of the order Mucorales, variable antifungal susceptibility, and limited knowledge about pathogenicity mechanisms of this diverse group, make diagnosis, treatment, and prognosis of outcome highly complicated.
Amphotericin B (AMB), especially the liposomal formulation (L-AMB) is the first line treatment,7 and posaconazole (POS) is used as salvage therapy.8,9 Recently, a new triazole, isavuconazole (ISA), was approved for treatment of mucormycosis.10–14 Several studies demonstrated its in vitro and in vivo activity against Mucorales.15–17 Another polyene antifungal, nystatin (NYT), exhibits in vitro activity against Mucorales,18 and in the last years several reports discussed the efficacy of a new formulation, nystatin-intralipid, against Aspergillus spp., Candida spp., and Fusarium spp.19–21 This lipid preparation combines NYT with intralipid, a nontoxic lipid emulsion that is used for parenteral nutrition. Murine studies have shown that this formulation can be systemically administered and is effective against experimental systemic candidiasis and systemic aspergillosis.19,21 So far, no studies were performed to test the efficacy of this novel drug preparation against Mucorales.
Even though three-dimensional cell culture and organoid models are evolving and are useful to study infectious diseases, animal models are still necessary to understand the complex interaction of pathogens and their host. Due to ethical concerns, cost-, and time-efficiency, alternative models that provide comparable data to rodents are of great advantage. The larvae of the greater wax moth, Galleria) mellonella, were shown to be a promising alternative to mammals in determining the virulence potential of fungal pathogens. The larval size enables precise injection of a defined number of pathogens or antimicrobial substance and facilitates collection of tissue or hemolymph. Physiological conditions in mammals, such as 37°C body temperature, can be easily mimicked. Most important, insects possess a cellular and humoral innate immune system which shows similarities to innate immune response of vertebrates.22 Hemocytes, which mediate the cellular immune response, show strong structural and functional similarities with mammalian phagocytes.23,24 Hemocytes are involved in phagocytosis, encapsulation, and melanization, and their number can change upon microbial infections or drug treatment.25–27 Insects recognize pathogens via peptidogylycan recognition proteins, which induces cell signaling through Toll, ImD, and Jak-STAT pathways—pathways similar to the vertebrate—and which result in the expression of antimicrobial peptides.28,29 These similarities explain the strong correlation to data obtained in murine studies.30,31
Furthermore, this model was used for in vivo screening of antimicrobial efficacy25,32 and the determination of pharmacokinetic properties of antibiotics33,34 and antimycotics.35–39 Nevertheless, the lack of standardized protocols, variability in the commercially available G. mellonella strains and the lack of Galleria mutants and transgenic cell lines are limitations that need to be considered. Recently, the first draft genome sequences was reported.40 This, plus the recent characterization of the Galleria immune gene repertoire and transcriptome, will certainly help to promote the further use of G. mellonella larvae as infection model.29
We aimed to adapt the G. mellonella model to assess the virulence potential of mucormycetes and for treatment (L-AMB, POS, ISA, NYT-IL) studies. Correlation to physiological attributes (growth at different temperatures, spore size, and resistance to oxidative stress) and the effect of the composition of the culture medium on virulence was further evaluated. Nutrient composition of culture media influences the growth of filamentous fungi, and was shown to have a potential effect on virulence.41,42 We tested whether the availability of high iron concentrations in the growth medium could have an effect on the virulence potential of mucormycetes.
Methods
Fungal strains and growth conditions
The strains listed in Table S1 were isolated from environmental or clinical specimen and were identified by sequencing internal transcribed spacer (ITS) region of ribosomal RNA gene, according to White et al.43 Isolates were grown on supplemented minimal agar (SUP)44 at 30°C up to 7 days. Sporangiospores were harvested in sterile spore buffer (0.9% NaCl, 0.01% Tween) or insect physiological saline (IPS; 150 mM NaCl, 5 mM KCl, 10 mM EDTA, and 30 mM sodium citrate in 0.1 M Tris–HCl, pH 6.9), washed and filtered through a 40-μm cell-strainer (BD, Heidelberg, Germany). Spore concentrations were determined by hemocytometer. For heat-inactivation, spores were autoclaved for 20 minutes at 121°C. For growth on high iron conditions 1 mM FeSO4 was added to SUP agar.
Determination of conidial size
Freshly harvested sporangiospores of all Mucorales isolates were placed on a cover slip, and their size was determined by measuring diameter of 100 randomly chosen spores in a light microscope (Carl Zeiss; Germany).
Radial growth assay
To determine radial growth of colonies, 1 × 102 sporangiospores of each isolate were spotted in a volume of 5 μl on SUP plates in triplicates. Samples were incubated at 30°C and 37°C for up to 24 h, and colony diameter was measured. To determine resistance to oxidative stress, menadione was added to SUP agar in a final concentration of 25 μM, 50 μM, and 100 μM, respectively.
G. mellonella infection studies
Sixth instar larvae of G. mellonella (Biologische Wurmzucht, Langenzersdorf, Austria and SAGIP, Italy), weighing 0.3–0.4 g, were selected for experimental use.45 Larvae, in groups of 20, were injected through the last pro-leg into the hemocoel with 1 × 104, 1 × 105, 1 × 106, and 1 × 107 conidia in a volume of 20 μl as described previously25 and incubated at 30° or 37°C in the dark. Untouched larvae and larvae injected with sterile IPS served as controls. Survival was determined every 24 h over a period of 144 h. To compare virulence potential, larvae were infected with 1 × 106 spores of each species, respectively and incubated at 30°C. For each test group 20 larvae were used, experiments were repeated at least three times. Significance was determined with log-rank (Mantel-Cox) test, utilizing GraphPad Prism 7.00 software. Differences were considered significant at P-values
Histology of larvae
Infected larvae plus control larvae (three per test group) were conserved in formalin for 10 days, embedded in paraffin, cut at 5 μm, and stained with Grocott´s silver stain.46
Antifungal susceptibility testing and in vivo treatment studies
Minimum inhibitory concentration (MIC) of liposomal amphotericin B (L-AMB), posaconazole (POS), isavuconazole (ISA), and nystatin-intralipid (NYT-IL) were determined for all Mucorales according to the EUCAST guidelines 9.2.47 MIC was defined as the lowest concentration that completely inhibited growth.
For in vivo treatment studies, the infected G. mellonella larvae were injected with a single dose of 15 mg/kg of each antifungal drug, respectively, 2 hours post-infection.25,26 The opposite hind pro-leg of the one used for spore administration was used to assure that larvae are not harmed by double-injection via the same pro-leg. Larvae injected twice with IPS (to rule out damage by double injection) and with the drug diluted in IPS (to check for toxicity effects of the respective drugs) served as controls. For each test group 20 larvae were used, and experiments were repeated at least three times. Statistical significance was determined by applying log rank (Mantel-Cox) test, comparing untreated groups with groups that received antifungals.
Bioassay and pharmacokinetics
The single dose pharmacokinetics of AMB, NYT-IL, POS, and ISA were determined following administration of 15 mg antifungal/kg larval weight. Drugs were administered into the hemocoel by injecting 20 μl of appropriate drug concentration, diluted in IPS. Larvae were incubated at 37°C, and hemolymph was collected over 48 h at 1 h, 4 h, 8 h, 24 h, 36 h, and 48 h post-administration.
Agar well diffusion assay was performed as described previously,34 with the following changes: Conidia (1 × 106/ml) of an A. fumigatus isolate were spread on Roswell Park Memorial Institute (RPMI)1640 agar and allowed to dry before equally spaced holes (5 mm in diameter) were punched into the agar with a sterile punch. Hemolymph pooled from five larvae was filled into the agar-holes in triplicates. After incubating the agar plates at 37°C for 48 h, the horizontal and vertical diameters of the inhibition zone was measured. For obtaining standard curves, according to which the concentration of drugs from larval samples could be calculated, dilution series of each antifungal in naïve hemolymph was used. Assays were repeated three times and a mean concentration-time profile of each antifungal was generated. To calculate pharmacokinetic parameters, the maximum concentration (Cmax) the area under the concentration-time curve (AUC) over 24 h (AUC24h), and the elimination half-life (t1/2) was determined by using Graph Pad Prism software.
Hemocyte isolation and determination of hemocyte density
G. mellonella larvae were injected with 15 mg/kg of the respective antifungal drug as described above. Hemocytes were isolated as described in25 from three larvae of each group, 4 h and 24 h post injection of the drug. Larvae were incubated at 37°C. Cell density was determined by enumeration using a hemocytometer. Experiments were repeated three times (each time three larvae were used).
Statistical analysis
All experiments were performed on three independent occasions, at least. Results are expressed as the mean ± standard deviation (SD). Survival rates were evaluated by using Kaplan-Meier survival curves and analyzed with the log rank (Mantel-Cox) test using GraphPad Prism software. Comparisons between groups were performed by one-way analysis of variance (ANOVA), with Bonferroni test with correction for multiple comparisons, or Student test. Differences were considered significant at P ≤ .05.
Results
Species-, dose-, and temperature-dependent killing of G. mellonella larvae by mucormycetes
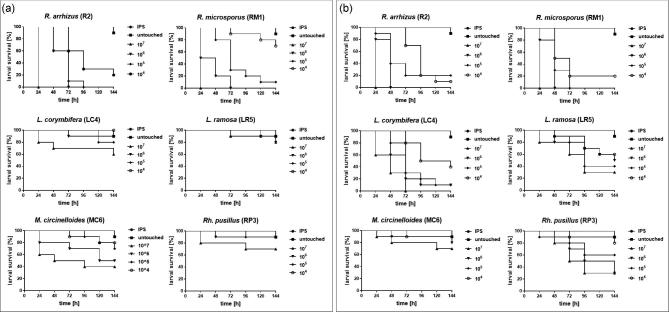
Sequential infection of larvae showed dependency of the killing on inoculum size and incubation temperature (Fig. 1). At both temperatures, Rhizopus spp. were most virulent, killing 100% of all larvae within 24 h at the highest inoculum dose (107 spores per larva). All larvae infected with 106 spores died within 48 h when incubated at 37°C, while it took 72 h to kill all larvae infected with the same inoculum size, but incubated at 30°C.
Figure 1.
Dose- and temperature dependent survival rates of G. mellonella larvae infected with mucormycetes. Larvae were infected with different spore-concentrations (1 × 104, 1 × 105, 1 × 106, 1 × 107) and incubated at either 30°C (a) or 37°C (b), respectively Kaplan-Meyer curves represent the average survival rates of three independent experiments. IPS infected and untouched larvae showthe same survival rate and therefore the curves overlap.
Rhizopus spp. and Lichtheimia spp. were able to cause larval death of at least 30% at a minimal infectious dose of 104 spores at 37°C, while 105 spores of R. pusillus were needed to cause similar outcome.
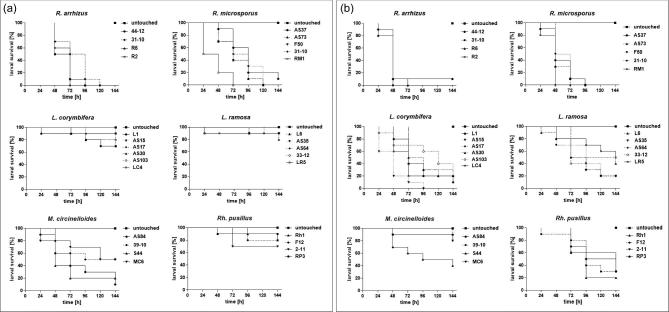
For M. circinelloides even the highest dose, 107, resulted in only 30% dead larvae at 37°C. At 30°C, M. circinelloides exhibited second highest virulence potential followed by Lichtheimia isolates and R. Pusillus, which were associated with high survival rates (70-90%) at all inocula tested. Data obtained were highly reproducible in repetitive experiments (Table S4 and Table S5), resulting in low standard deviations. Comparison of general virulence potential revealed to be species-specific rather than strain-specific (Fig. 2). One isolate (RM1) was shown to significantly cause higher mortality rates compared to the other isolates tested at 30°C, while no significant intra-species variances were detected for the other species (Fig. 2a). Similar results were obtained when larvae were incubated at 37°C (Fig. 2b). Significant difference in survival was detected for one M. circinelloides isolate, S44, which showed significant higher virulence potential compared to AS84 and 30-10 but not MC6. This strain also exhibited lowest survival rates at 30°C, but no statistical difference was detected at 30°C. In the group of L. corymbifera significant lower survival was found for strain AS15 compared to all other strains tested. In all other groups no significant intraspecies variation was observed. No statistically significant difference in survival rates due to the origin (clinical or environmental) of the isolate was observed for both temperatures.
Figure 2.
Intra-species variability in virulence potential of human pathogenic mucormycetes. Larvae were infected with 1 × 106 spores of the respective strain and incubated at either 30°C (a) or 37°C (b), respectively. Survival was monitored in 24 h intervals over a period of 6 days (144 h). Kaplan-Meyer curves represent the average survival rates of three independent experiments.
For all strains tested, death of larvae was due to fungal growth and proliferation, as heat-inactivated spores did not have an effect on larval survival at both temperatures (data not shown).
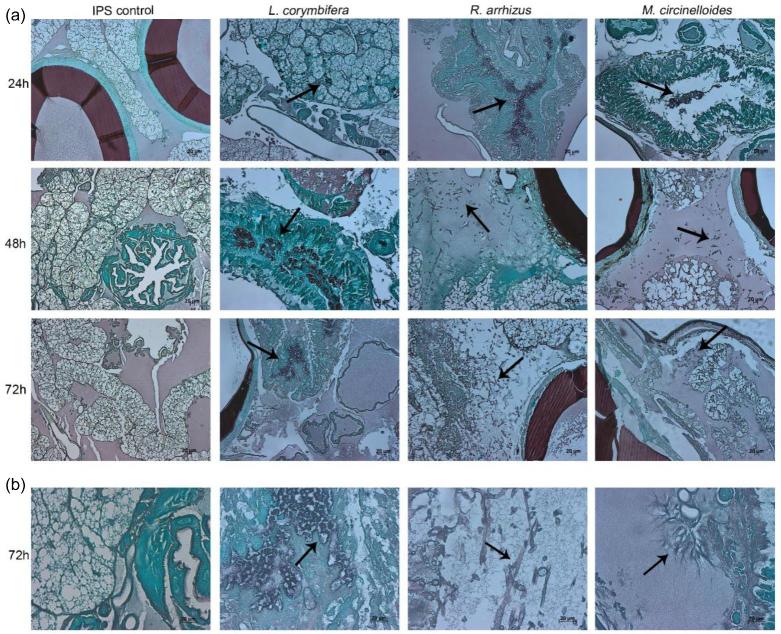
This finding was further supported by histological tissue sections of infected larvae, exhibiting hyphal elements in larvae infected with R. arrhizus and M. circinelloides (Fig. 3).
Figure 3.
Histopathology of Galleria mellonella infected with L. corymbifera AS30, R. arrhizus 44-12 and M. circinelloides AS84. (a) Larvae were infected with IPS (left panel) or 1 × 106 spores of the respective strain. Larvae were fixed in formaldehyde at 24, 48 and 72 h and histological samples stained with Grocott. (b) Enlarged view of tissue sections 72 h post infection. Arrows indicate fungal elements.
Survival data correlate with species-specific features (spore size, in vitro growth rates, and resistance to oxidative stress)
Rhizopus species that caused highest mortality of larvae had the largest spores (5.5–9.1 μm). In contrast, Lichtheimia species or R. pusillus, associated with minor virulence potential especially when larvae were incubated at 30°C, had the smallest spores (2.5–4.2 μm; Table S2).
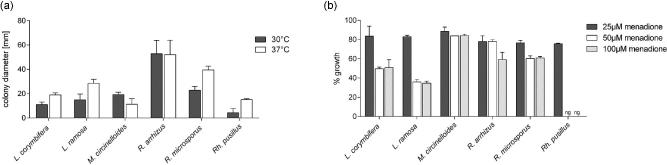
The most virulent species, R. arrhizus, exhibited significantly faster growth compared to all other Mucorales, while less virulent R. pusillus showed significantly slower growth compared to all other Mucorales (Fig. 4a). In vitro growth rates correlated to our observations in histological preparations of infected larvae. High virulent strains (Rhizopus spp.) germinated and grew fast within the larval tissue, while those with low virulence (Lichtheimia spp.) were not able to germinate within the larvae at the same time post-infection.
Figure 4.
Growth rates and oxidative stress tolerance of different mucormycetes. (a) Radial growth of each species at 30 and 37°C. (b) Radial growth (% of untreated controls) under oxidative stress conditions (menadione exposure at concentrations 25 μM, 50 μM, and 100 μM) at 30°C.
Growth rates under oxygen stress (on menadione-containing media) were highest in M. circinelloides, followed by Rhizopus spp. (Fig 4b), which correlates to the high mortality rates caused by these species. Mucor circinelloides was least affected by menadione with growth rates reaching more than 80% of the untreated control for all three menadione concentrations tested.
Iron content of culture medium affects virulence potential in a species dependent manner
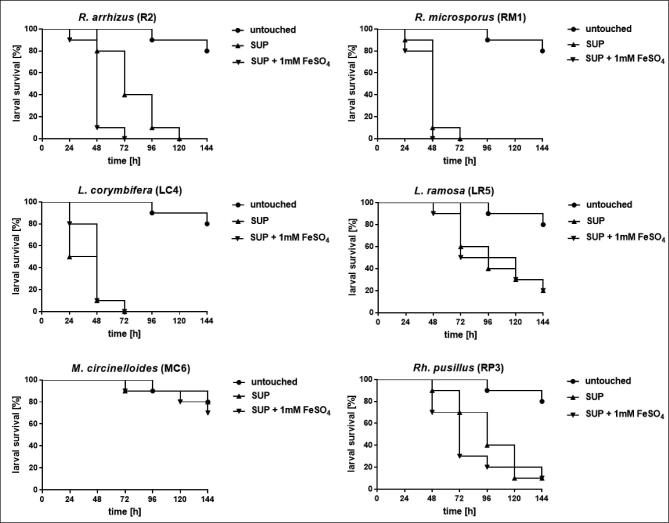
Comparison of virulence of spores grown on SUP agar versus that of spores grown on SUP supplemented with 1 mM FeSO4 showed that virulence potential of R. arrhizus was significantly enhanced when spores were harvest from cultures grown under high iron conditions. For all other species no significant difference was detected (Fig. 5). The same trend was observed for larvae infected with R. arrhizus or L. corymbifera, respectively, and incubated at 30°C (data not shown).
Figure 5.
Impact of iron starvation in preculture conditions on the virulence potential of human-pathogenic mucormycetes. Larvae were infected with spores that were produced by mucormycetes grown under iron starvation (SUP agar) or under high iron conditions (SUP agar supplemented with 1 mM FeSO4). In preexperiments virulence potential of each species was determined, inocula were adapted accordingly as follows: 1 × 105 for Rhizopus spp., 1 × 106 for M. circinelloides and 1 × 107 for Lichtheimia spp and Rh. pusillus. Larvae were incubated at 37°C.
Efficacy and pharmacokinetic profile of L-AMB, POS, ISA, and NYT-IL in the G. mellonella model of mucormycosis
In vitro MIC values of L-AMB, POS, ISA, NYT, and NYT-IL per species are presented in Table S3. In short, MIC range of L-AMB was 0.25 -0.5 μg/ml, demonstrating best potency of all tested drugs, followed by POS (MIC range of 1.0–2.0 μg/ml), NYT-IL (MIC range of 1.0–4.0 μg/ml) and ISA (MIC range of 0.5–8.0 μg/ml). Markedly, a dramatic reduction in MIC values for the intralipid formulation of NYT was observed in Lichtheimia spp., from a median of 8.0 μg/ml to 1.0–2.0 μg/ml.
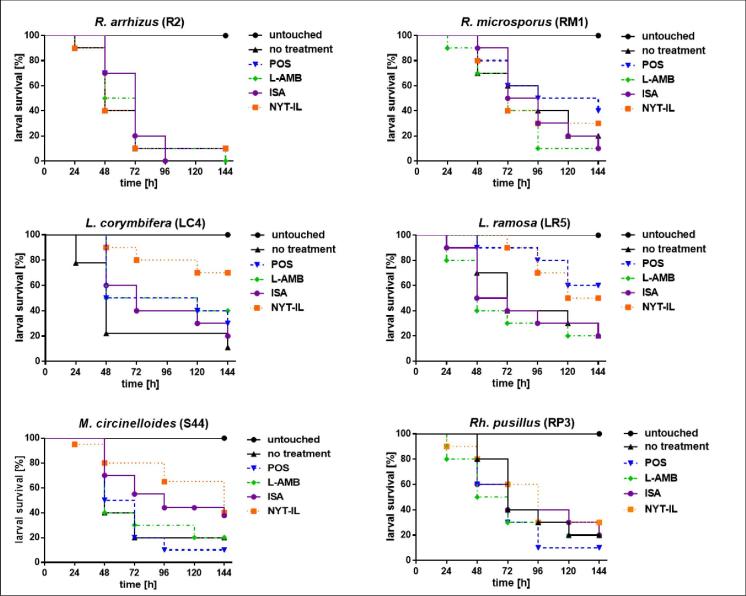
Antifungal activity of tested drugs against mucormycosis induced in G. mellonella is shown in Figure 6. Best in vivo efficacy was detected for NYT-IL. Larvae infected with any of the Mucorales strains demonstrated prolonged survival when receiving NYT-IL treatment, except those infected with R. arrhizus. Best treatment outcome was visible for larvae infected with Lichtheimia spp. with significant improvement of 60% survival at 144 h post-infection for larvae infected with L. corymbifera and 30% for L. ramosa infected larvae. This corresponds to the high in vitro efficacy of NYT-IL against Lichtheimia isolates. Surprisingly, L-AMB, which demonstrated best activity in vitro (Table S3), showed no significant improvement in the survival of infected larvae, except for those infected with L. corymbifera (30% better outcome than in the untreated group after 144 h post-infection).
Figure 6.
Impact of antifungal therapy (POS, L-AMB, ISA, and NYT-IL) on survival of G. mellonella infected with human pathogenic mucormycetes. In pre-experiments virulence potential of each species was determined, inocula were adapted accordingly as follows: 1 × 105 for R. arrhizus, 1 × 104 for R. microsporus, 1 × 106 for Lichtheimia spp., for M. circinelloides and Rh. pusillus. Larvae were incubated at 37°C. Antifungal treatment (15 mg/kg) was applied as a single dose two hours post infection. Survival was monitored in 24 h intervals for up to six days. Survival rates were compared to the untreated infected control.
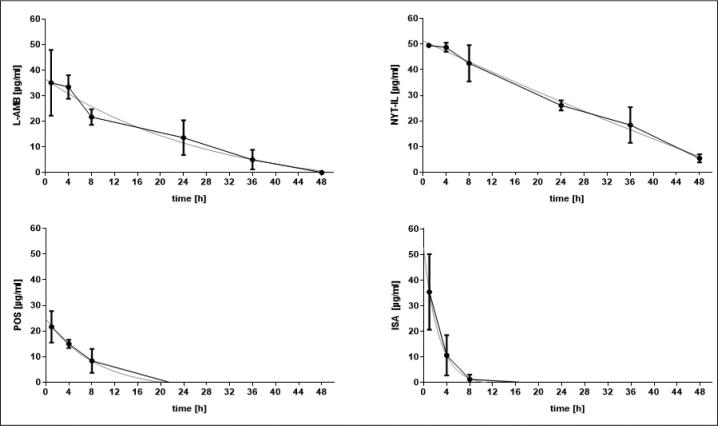
In hemolymph, the polyenes, L-AMB and NYT-IL, were more stable in the larval hemolymph, resulting in higher hemolymph concentrations over the 48 h of sampling compared to the azoles (Fig. 7). Consequently, polyenes exhibited higher t1/2 and AUC values (Table 1). The hemolymph concentrations of the polyenes remained above the quantifiable limit over 48 h (NYT-IL) and 36 h (L-AMB) and remained above in vitro MIC levels at all time- points. According to Andes et al.,48 the Cmax/MIC ratio is considered the PK/PD index determining therapeutic efficacy for the polyenes. It was shown by others49 that L-AMB reached maximal efficacy when Cmax/MIC ratios were above 40. Cmax/MIC ratios, that were based on the median MIC for each species were in the range of 70.2–140.4 for L-AMB, and 12.4–49.5 for NYT-IL. All values were above the threshold of 40, except for NYT-IL against L. ramosa and R. microsporus.
Figure 7.
Concentrations-time curve in haemolyph for L-AMB, NYT-IL, POS and ISA. A single dose (15 mg/kg) of the respective antifungal agent was administered and haemolymph drug-levels were determined using an agar well diffusion assay at intervals up to 48 h. Haemolymph samples were taken 4 h, 8 h, 12 h, 24 h, 36 h, and 48 h after drug administration. Larvae and agar plates were incubated at 37°C.
Table 1.
Pharmacokinetic data obtained from G. mellonella larvae exposed to a single dose of antifungal agent.
| Galleria mellonella | Humana | ||||||||
| Antifungal agent | Concentration/body weight (mg/kg) | Cmax (μg/ml) |
t1/2 (h) | AUC24 (h μg/ml) | Concentration/body weight (mg/kg) | Concentration/ day (mg) | Cmax (μg/ml) | t1/2 (h) | AUC24 (h μg/ml) |
|---|---|---|---|---|---|---|---|---|---|
| L-AMB | 15 | 35.10 | 20.05 | 495 ± SD 64 | 1572 | n.d. | 206.3 | 32.80 | 5,019.30 |
| NYT-IL | 15 | 49.50 | 165.20 | 878 ± SD 61 | 873 | n.d. | 28.33 | 7.42 | 208.44 |
| ISA | 15 | 35.45 | 1.78 | 102 ± SD 31 | n.d. | 10074 | 1.09 | 123.00 | 39.00 |
| POS | 15 | 21.65 | 6.01 | 161 ± SD 34 | n.d. | 10075 | 1.33 | 19.60 | 11.20 |
Data obtained from the Galleria model were compared to available data obtained from studies carried out in healthy humans and a murine model for NYT-IL. All parameters were calculated over a 24-h period. Cmax, maximum concentrations in hemolymph; t1/2, half-life; AUC, area under the concentration-time curve representing total exposure to the antifungal agent, n.d. not determined.
aData from healthy humans, except for NYT-IL, which were from a murine model.
Application of POS prolonged survival of larvae infected with L. corymbifera (20% improvement compared to the untreated insects at 144 h; P = .1784), L. ramosa (40% improvement at 144 h; P = .09), and R. microsporus (30% improvement at 144 h; P = .0465), while no improvement was detected for larvae infected with R. arrhizus, M. circinelloides and R. pusillus. Isavuconazole was not effective against any of the strains except for larvae infected with M. circinelloides, which showed 20% better survival after 144 h post-infection, although differences did not reach statistical significance.
The hemolymph concentration of POS and ISA reached undetectable levels already at 22 h and 16 h post-injection, respectively, and dropped below the MIC value at 16 h (POS) and 8 h (ISA). For the azoles, the AUC/MIC ratio was determined as the best indicator for therapeutic efficacy, with a predictive positive outcome when levels are greater than 25.48 This criterion was met in our study, since the AUC/MIC ratio for both azoles and all species tested was above 25.
Effect of drug administration on hemocyte density in G. mellonella larvae
Previous work demonstrated that administration of antifungals, for example, caspofungin into larvae primes the larval immune response by elevation in hemocyte density.25 Administration of L-AMB (15 mg/kg) resulted in a significant (P < .05) increase of 27.0%; SD ± 1.4 in hemocyte density 24 h after administration, while in larvae that received ISA (15 mg/kg) the number of hemocytes was reduced by 25.3%, SD ±9.6. No significant difference in hemocyte density was observed in larvae injected with POS or NYT-IL, respectively, nor 4 h post-treatment (Fig. 8).
Figure 8.
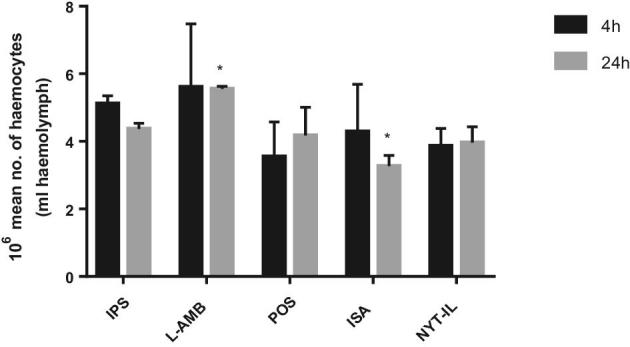
Effect of antifungal drugs on the hemocyte density in G. mellonella. Hemocyte density in larvae injected with IPS or antifungal drug (15 mg/kg) and incubated at 37°C was assessed after 4 h and 24 h. Results are significantly different (* p ≤ 0.05; Students t-test, two tail) to the IPS control.
Discussion
Until now, no standardized experimental settings for Galleria experiments and no “laboratory strain” of G. mellonella larvae is available, which makes comparison of data between laboratories rather difficult. Therefore, a great number of different strains belonging to six different species were compared. The high reproducibility in our experiments indicates that G. mellonella larvae are a suitable complementary in vivo model to study virulence potential and virulence factors of mucormycetes.
Results demonstrated that G. mellonella can be infected in a dose- and temperature-dependent manner, these dependencies were previously reported for Lichtheimia spp. in an ectothermic chicken embryo model.50Rhizopus spp. demonstrated highest virulence potential, a finding that correlates with incidences in humans.1,5,6M. circinelloides was the only mucormycetes tested that caused higher mortality rates at 30°C when compared to 37°C. A detailed study by Kaerger et al.51 revealed higher virulence potential of thermotolerant Rhizopus spp. in the chicken embryo and the Galleria model compared to mesophilic species. The fact that growth speed of mesophilic Rhizopus spp. highly resembled that of thermo-tolerant species at 30°C let us assume that additional mechanisms play an important role in virulence potential. Another study supporting this idea was carried out by Schwartze et al.50, who demonstrated that L. ornata strains, which are known to grow very slow, were fully virulent in the chicken embryo model.
Several studies have described that environmental and clinical fungal strains might differ in their virulence potential.52,53 However, no difference in the pathogenicity due to origin of strains was detected in our study and these findings are in agreement with previously described data for Lichtheimia spp. and Rhizopus spp.50,51 Nevertheless, to statistically evaluate and strengthen these findings, a much larger set of strains would be necessary.
The positive correlation between virulence potential and physiological features of the pathogen, such as spores size, growth speed, and oxidative stress tolerance in Galleria larvae, that we detected, was already discussed before for some Mucorales species.50,54–56 In contrast, Jackson et al. did not find any correlation between faster germination and higher pathogenicity of A. fumigatus color mutants.57 Species that bear higher resistance to oxidative stress might be less affected by host immune response and therefore exhibit higher virulence.57,58 Other stressors besides high temperature and oxidative stress were evaluated by Kaerger et al.51 who could not clearly link virulence potential to resistance toward osmotic or cell wall stress within Rhizopus spp.
The composition of the culture media is known to have a high impact in the growth of filamentous fungi, but no detailed study has been undertaken to check for its effect on virulence in different model systems.41 Virulence potential of spores grown on high iron agar plates was enhanced for R. arrhizus, not for any of the other species tested. Also, for Cunninghamella bertholletiae growth on high iron medium did not enhance virulence.42 This may indicate different systems for iron uptake and iron storage in the heterogeneous group of Mucorales, which so far are not fully understood for the each species.
Mucormycosis still results in high mortality rates, even with therapeutic interventions.1,59–62 Importantly, NYT-IL, tested for its in vivo efficacy against Mucorales for the first time, was the most effective antifungal agent in G. mellonella. Similarly, NYT-IL was shown to be therapeutically effective in a murine model of candidiasis and aspergillosis, so it might be of interest to perform murine experiments with Mucorales to strengthen the data obtained in the larvae.19,21 Furthermore, in vivo efficacy of NYT-IL is supported by its favorable pharmacokinetics. In the hemolymph, it has the highest stability, reflected in the longest half-life, and the highest AUC.
Despite high in vitro potency, the other drugs did not show significant in vivo efficacy, a phenomenon seen before.36,63 Studies investigating in vivo efficacy of POS in murine models of mucormycosis showed variable results, indicating that efficacy is dependent on the species and the infection model used.64,65–67,68
Antifungals, for example, caspofungin, or other external agents were shown to result in an unspecific antimicrobial immune response which is mediated also by changes in the hemocyte density of the larvae.25,69,70 Increased hemocyte numbers due to L-AMB seem not to be enough to cure the infection or have a positive effect on survival rates. The increase observed here, correlates to a previous study carried out in our laboratory.71 On the other hand, the decrease in hemocyte number caused by ISA might even aggravate the infection process, explaining in part, why no improvement in survival can be detected. NYT-IL did not influence hemocyte numbers; therefore, we can assume that the increase in survival was mediated by inhibiting fungal growth through exerting its antifungal activity and not through an unspecific immune response.
Reasons for the outcome seen in the G. mellonella model are multifactorial. First, in our model system all drugs are only applied once, and a fast clearance may partially explain the low in vivo therapeutic outcome seen in the present study. Several studies demonstrated that concentrations of antibiotics also change rapidly in G. mellonella larvae.33,34 Concentrations of ISA in the hemolymph were lowest of all drugs. This, plus the uncertainty if the esterases necessary to produce the active drug are available in G. mellonella larvae, could explain treatment failure, even though the predictive PK/PD index, AUC/MIC ratio for azoles, was above the threshold value of 25. A recent study investigated the therapeutic effect of azoles and AMB against Madurella mycetomatis in the Galleria model and revealed no treatment effect of azoles, although concentrations above the MIC could be measured in the hemolymph of larvae.36 In contrast, improvement in survival was seen when larvae received AMB or terbinafine, even at concentrations below the MIC. By now, several studies have used the larval model to test the efficacy of antifungal drugs, or novel substances with potential antifungal activity. Altogether, in vivo outcome, correlation to in vitro data and to data obtained in mice varied.
Second, the fungistatic activity against mucormycetes aggravates treatment efficacy.
Further studies that evaluate the concentrations of antifungal drugs within the larval tissue, as well as the tropism of Mucorales in the larvae are needed.
In conclusion, G. mellonella has the potential to serve as a convenient invertebrate animal host model for studying virulence factors in mucormycetes. The evaluation of treatment response in G. mellonella remains challenging until pharmacodynamics in this invertebrate host are better understood.
Supplementary Material
Acknowledgments
We thank Sabrina Foidl and Manuela Sparber for excellent technical assistance in performing Galleria experiments.
Funding
This work was financially supported by the Medical University of Innsbruck (MUI start grant number 19970) and an independent research grant from Gilead Sciences Europe (2012) to U.B., and the “Christian Doppler Forschungsgesellschaft” (CD-Labor Invasive Pilzinfektionen) to C.L.F.
Declaration of interest
C.L.F. has received grant support from the Austrian Science Fund (FWF), MFF Tirol, Astellas Pharma, Gilead Sciences, Pfizer, Schering Plough, and Merck Sharp & Dohme. She has been an advisor/consultant to Gilead Sciences, Merck Sharp & Dohme, Pfizer, and Schering Plough. She has received travel/accommodation expenses from Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough and has been paid for talks on behalf of Gilead Sciences, Merck Sharp & Dohme, Pfizer, Astellas, and Schering Plough. U.B. has received an independent research grant from Gilead Sciences. All other authors have no conflicts of interest to declare.
References
- 1. Roden MM, Zaoutis TE, Buchanan WL et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005; 41: 634–653. [DOI] [PubMed] [Google Scholar]
- 2. De Hoog GS, Guarro J, Gene J, Figueras MJ. Atlas of Clinical Fungi. 2nd edn: Centralbureau voor Schimmelcultures; 2011. [Google Scholar]
- 3. Binder U, Maurer E, Lass-Florl C. Mucormycosis: from the pathogens to the disease. Clinical Microbiology and Infection: The Official Publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014. [DOI] [PubMed] [Google Scholar]
- 4. Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clin Infect Dis. 2012; 54: S8–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanternier F, Dannaoui E, Morizot G et al. A global analysis of mucormycosis in France: the RetroZygo Study (2005–2007). Clin Infect Dis. 2012; 54: S35–43. [DOI] [PubMed] [Google Scholar]
- 6. Skiada A, Pagano L, Groll A et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clinical Microbiol Infect. 2011; 17: 1859–1867. [DOI] [PubMed] [Google Scholar]
- 7. Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. Eurn J Clin Microbiol Iinfect Dis. 2006; 25: 215–229. [DOI] [PubMed] [Google Scholar]
- 8. Almyroudis NG, Sutton DA, Fothergill AW, Rinaldi MG, Kusne S. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob Agents Chemother. 2007; 51: 2587–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rogers TR. Treatment of zygomycosis: current and new options. J Antimicrob Chemother. 2008; 61: i35–40. [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez GM. In vitro activities of isavuconazole against opportunistic filamentous and dimorphic fungi. Med Mycol. 2009; 47: 71–76. [DOI] [PubMed] [Google Scholar]
- 11. Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob Agents Chemother. 2008; 52: 1396–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Warn PA, Sharp A, Denning DW. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J Antimicrob Chemother. 2006; 57: 135–138. [DOI] [PubMed] [Google Scholar]
- 13. Kovanda LL, Maher R, Hope WW. Isavuconazonium sulfate: a new agent for the treatment of invasive aspergillosis and invasive mucormycosis. Expert Rev Clin Pharmacol. 2016; 9: 887–897. [DOI] [PubMed] [Google Scholar]
- 14. Riley TT, Muzny CA, Swiatlo E, Legendre DP. Breaking the mold: a review of mucormycosis and current pharmacological treatment options. Ann Pharmacother. 2016; 50: 747–757. [DOI] [PubMed] [Google Scholar]
- 15. Ervens J, Ghannoum M, Graf B, Schwartz S. Successful isavuconazole salvage therapy in a patient with invasive mucormycosis. Infection. 2014; 42: 429–432. [DOI] [PubMed] [Google Scholar]
- 16. Perkhofer S, Lechner V, Lass-Florl C. In vitro activity of isavuconazole against Aspergillus species and zygomycetes according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob Agents Chemother. 2009; 53: 1645–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luo G, Gebremariam T, Lee H, Edwards JE Jr., Kovanda L, Ibrahim AS. Isavuconazole therapy protects immunosuppressed mice from mucormycosis. Antimicrobial agents and chemotherapy. 2014;58(4):2450–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh J, Rimek D, Kappe R. In vitro susceptibility of 15 strains of zygomycetes to nine antifungal agents as determined by the NCCLS M38-A microdilution method. Mycoses. 2005; 48: 246–250. [DOI] [PubMed] [Google Scholar]
- 19. Semis R, Mendlovic S, Polacheck I, Segal E. Activity of an Intralipid formulation of nystatin in murine systemic candidiasis. International journal of antimicrobial agents. 2011; 38: 336–340. [DOI] [PubMed] [Google Scholar]
- 20. Semis R, Polacheck I, Segal E. Nystatin-intralipid preparation: characterization and in vitro activity against yeasts and molds. Mycopathologia. 2010; 169: 333–341. [DOI] [PubMed] [Google Scholar]
- 21. Sionov E, Segal E. Treatment of murine systemic aspergillosis with polyene-intralipid admixtures. Med Mycol. 2004; 42: 73–80. [DOI] [PubMed] [Google Scholar]
- 22. Tojo S, Naganuma F, Arakawa K, Yokoo S. Involvement of both granular cells and plasmatocytes in phagocytic reactions in the greater wax moth, Galleria mellonella. J Insect Physiol. 2000; 46: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 23. Bergin D, Reeves EP, Renwick J, Wientjes FB, Kavanagh K. Superoxide production in Galleria mellonella hemocytes: identification of proteins homologous to the NADPH oxidase complex of human neutrophils. Infect Immun. 2005; 73: 4161–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Renwick J, Reeves EP, Wientjes FB, Kavanagh K. Translocation of proteins homologous to human neutrophil p47phox and p67phox to the cell membrane in activated hemocytes of Galleria mellonella. Dev Comp Immunol. 2007; 31: 347–359. [DOI] [PubMed] [Google Scholar]
- 25. Kelly J, Kavanagh K. Caspofungin primes the immune response of the larvae of Galleria mellonella and induces a non-specific antimicrobial response. J Med Microbiol. 2011; 60: 189–196. [DOI] [PubMed] [Google Scholar]
- 26. Scorzoni L, de Lucas MP, Mesa-Arango AC et al. Antifungal efficacy during Candida krusei infection in non-conventional models correlates with the yeast in vitro susceptibility profile. PloS one. 2013; 8: e60047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol. 2002; 32: 1295–1309. [DOI] [PubMed] [Google Scholar]
- 28. Lionakis MS. Drosophila and Galleria insect model hosts: new tools for the study of fungal virulence, pharmacology and immunology. Virulence. 2011; 2: 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogel H, Altincicek B, Glockner G, Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics. 2011;12:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slater JL, Gregson L, Denning DW, Warn PA. Pathogenicity of Aspergillus fumigatus mutants assessed in Galleria mellonella matches that in mice. Med Mycol. 2011; 49: S107–5113. [DOI] [PubMed] [Google Scholar]
- 31. Brennan M, Thomas DY, Whiteway M, Kavanagh K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol. 2002; 34: 153–157. [DOI] [PubMed] [Google Scholar]
- 32. Mesa-Arango AC, Forastiero A, Bernal-Martinez L, Cuenca-Estrella M, Mellado E, Zaragoza O. The non-mammalian host Galleria mellonella can be used to study the virulence of the fungal pathogen Candida tropicalis and the efficacy of antifungal drugs during infection by this pathogenic yeast. Med Mycol. 2013; 51: 461–472. [DOI] [PubMed] [Google Scholar]
- 33. Hill L, Veli N, Coote PJ. Evaluation of Galleria mellonella larvae for measuring the efficacy and pharmacokinetics of antibiotic therapies against Pseudomonas aeruginosa infection. Int J Antimicrob Agents. 2014; 43: 254–261. [DOI] [PubMed] [Google Scholar]
- 34. Thomas RJ, Hamblin KA, Armstrong SJ et al. Galleria mellonella as a model system to test the pharmacokinetics and efficacy of antibiotics against Burkholderia pseudomallei. Int J Antimicrob Agents. 2013; 41: 330–336. [DOI] [PubMed] [Google Scholar]
- 35. Astvad KMT, Meletiadis J, Whalley S, Arendrup MC. Fluconazole pharmacokinetics in Galleria mellonella larvae and performance evaluation of a bioassay compared to liquid chromatography-tandem mass spectrometry for hemolymph specimens. Antimicrobial Agents Chemother. 2017; 61: e00895–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kloezen W, Parel F, Bruggemann R et al. Amphotericin B and terbinafine but not the azoles prolong survival in Galleria mellonella larvae infected with Madurella mycetomatis. Med Mycol. 2017; 56: 469–478. [DOI] [PubMed] [Google Scholar]
- 37. Ames L, Duxbury S, Pawlowska B, Ho HL, Haynes K, Bates S. Galleria mellonella as a host model to study Candida glabrata virulence and antifungal efficacy. Virulence. 2017; 8: 1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alcazar-Fuoli L, Buitrago M, Gomez-Lopez A, Mellado E. An alternative host model of a mixed fungal infection by azole susceptible and resistant Aspergillus spp. strains. Virulence. 2015; 6: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Forastiero A, Bernal-Martinez L, Mellado E, Cendejas E, Gomez-Lopez A. In vivo efficacy of voriconazole and posaconazole therapy in a novel invertebrate model of Aspergillus fumigatus infection. Int J Antimicrob Agents. 2015; 46: 511–517. [DOI] [PubMed] [Google Scholar]
- 40. Lange A, Beier S, Huson DH, Parusel R, Iglauer F, Frick JS. Genome sequence of Galleria mellonella (greater wax moth). Genome Announc. 2018; 6: e01220–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Meletiadis J, Meis JFGM, Mouton JW, Verweij PE. Analysis of growth characteristics of filamentous fungi in different nutrient media. J Clin Microbiol. 2001; 39: 478–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pongas GN, Ben-Ami R, Lewis RE, Walsh TJ, Kontoyiannis DP. Culture medium composition affects the lethality of Cunninghamella bertholletiae in a fly model of mucormycosis. Antimicrol Agents Chemother. 2009; 53: 4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In Immis I., ed., PCR Protocols: A Guide to Methods and Applications, New York: Academic Press, 1990. [Google Scholar]
- 44. Wostemeyer J. Strain-dependent variation in ribosomal DNA arrangement in Absidia glauca. Euro J Biochem. 1985; 146: 443–448. [DOI] [PubMed] [Google Scholar]
- 45. Fallon J, Kelly J, Kavanagh K. Galleria mellonella as a model for fungal pathogenicity testing. Methods Mol Biol. 2012; 845: 469–485. [DOI] [PubMed] [Google Scholar]
- 46. Cowen LE, Singh SD, Kohler JR et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A. 2009; 106: 2818–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Arendrup MCGJ, Cuenca-Estrella M, Meletiadis J, Mounton JW, Lagrou K, Howard SJ and the Subcommittee on Antifungal Susceptibility Testing (AFST) of the ESCMID European Committee for Antimicrobial Susceptibility Testing (EUCAST) EUCAST DEFINITIVE DOCUMENT EDef 9.3: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds 2015.
- 48. Andes D. Pharmacokinetics and pharmacodynamics of antifungals. Infect Dis Clin North Am. 2006; 20:679–697. [DOI] [PubMed] [Google Scholar]
- 49. Hong Y, Shaw PJ, Nath CE et al. Population pharmacokinetics of liposomal amphotericin B in pediatric patients with malignant diseases. Antimicrobial Agents Chemother. 2006; 50: 935–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schwartze VU, Hoffmann K, Nyilasi I et al. Lichtheimia species exhibit differences in virulence potential. PloS one. 2012; 7: e40908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaerger K, Schwartze VU, Dolatabadi S et al. Adaptation to thermotolerance in Rhizopus coincides with virulence as revealed by avian and invertebrate infection models, phylogeny, physiological and metabolic flexibility. Virulence. 2015; 6: 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aufauvre-Brown A, Brown JS, Holden DW. Comparison of virulence between clinical and environmental isolates of Aspergillus fumigatus. Euro J Clin Microbiol Infect Dis, 1998; 17: 778–780. [DOI] [PubMed] [Google Scholar]
- 53. Fromtling RA, Abruzzo GK, Ruiz A. Virulence and antifungal susceptibility of environmental and clinical isolates of Cryptococcus neoformans from Puerto Rico. Mycopathologia. 1989; 106: 163–166. [DOI] [PubMed] [Google Scholar]
- 54. McCormick A, Loeffler J, Ebel F. Aspergillus fumigatus: contours of an opportunistic human pathogen. Cell Microbiol. 2010; 12: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 55. Li CH, Cervantes M, Springer DJ et al. Sporangiospore size dimorphism is linked to virulence of Mucor circinelloides. PLoS Pathogens. 2011; 7: e1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Petraitis V, Petraitiene R, Antachopoulos C et al. Increased virulence of Cunninghamella bertholletiae in experimental pulmonary mucormycosis: correlation with circulating molecular biomarkers, sporangiospore germination and hyphal metabolism. Med Mycol. 2013; 51: 72–82. [DOI] [PubMed] [Google Scholar]
- 57. Jackson JC, Higgins LA, Lin X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PloS one. 2009; 4: e4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hwang CS, Rhie GE, Oh JH, Huh WK, Yim HS, Kang SO. Copper- and zinc-containing superoxide dismutase (Cu/ZnSOD) is required for the protection of Candida albicans against oxidative stresses and the expression of its full virulence. Microbiology. 2002; 148: 3705–3713. [DOI] [PubMed] [Google Scholar]
- 59. Hammond SP, Baden LR, Marty FM. Mortality in hematologic malignancy and hematopoietic stem cell transplant patients with mucormycosis, 2001 to 2009. Antimicrob Agents Chemother. 2011; 55: 5018–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kontoyiannis DP, Lionakis MS, Lewis RE et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005; 191: 1350–1360. [DOI] [PubMed] [Google Scholar]
- 61. Lewis RE, Kontoyiannis DP. Epidemiology and treatment of mucormycosis. Future Microbiol. 2013; 8: 1163–1175. [DOI] [PubMed] [Google Scholar]
- 62. Cornely OA, Arikan-Akdagli S, Dannaoui E et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014; 20: 5–26. [DOI] [PubMed] [Google Scholar]
- 63. Mircus G, Albert N, Ben-Yaakov D et al. Identification and characterization of a novel family of selective antifungal compounds (CANBEFs) that interfere with fungal protein synthesis. Antimicrob Agents Cchemother. 2015; 59: 5631–5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dannaoui E, Meis JF, Loebenberg D, Verweij PE. Activity of posaconazole in treatment of experimental disseminated zygomycosis. Antimicrob Agents Chemother. 2003; 47: 3647–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Luo G, Gebremariam T, Lee H et al. Efficacy of liposomal amphotericin B and posaconazole in intratracheal models of murine mucormycosis. Antimicrob Agents Chemother. 2013; 57: 3340–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rodriguez MM, Serena C, Marine M, Pastor FJ, Guarro J. Posaconazole combined with amphotericin B, an effective therapy for a murine disseminated infection caused by Rhizopus oryzae. Antimicrob Agents Chemother. 2008; 52: 3786–3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Spreghini E, Orlando F, Giannini D, Barchiesi F. In vitro and in vivo activities of posaconazole against zygomycetes with various degrees of susceptibility. J Antimicrob Chemother. 2010; 65: 2158–2163. [DOI] [PubMed] [Google Scholar]
- 68. Salas V, Pastor FJ, Calvo E et al. In vitro and in vivo activities of posaconazole and amphotericin B in a murine invasive infection by Mucor circinelloides: poor efficacy of posaconazole. Antimicrob Agents Chemother. 2012; 56: 2246–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mowlds P, Coates C, Renwick J, Kavanagh K. Dose-dependent cellular and humoral responses in Galleria mellonella larvae following beta-glucan inoculation. Microbes Infect. 2010; 12: 146–153. [DOI] [PubMed] [Google Scholar]
- 70. Fallon JP, Reeves EP, Kavanagh K. The Aspergillus fumigatus toxin fumagillin suppresses the immune response of Galleria mellonella larvae by inhibiting the action of haemocytes. Microbiology. 2011; 157: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 71. Maurer E, Browne N, Surlis C et al. Galleria mellonella as a host model to study Aspergillus terreus virulence and amphotericin B resistance. Virulence. 2015; 6: 591–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gubbins PO, Amsden JR, McConnell SA, Anaissie EJ. Pharmacokinetics and buccal mucosal concentrations of a 15 milligram per kilogram of body weight total dose of liposomal amphotericin B administered as a single dose (15 mg/kg), weekly dose (7.5 mg/kg), or daily dose (1 mg/kg) in peripheral stem cell transplant patients. Antimicrob Agents Chemother. 2009; 53: 3664–3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Semis R, Nili SS, Munitz A, Zaslavsky Z, Polacheck I, Segal E. Pharmacokinetics, tissue distribution and immunomodulatory effect of intralipid formulation of nystatin in mice. J Antimicrob Chemother. 2012; 67: 1716–1721. [DOI] [PubMed] [Google Scholar]
- 74. Schmitt-Hoffmann A, Roos B, Spickermann J et al. Effect of mild and moderate liver disease on the pharmacokinetics of isavuconazole after intravenous and oral administration of a single dose of the prodrug BAL8557. Antimicrob Agents Chemother. 2009; 53: 4885–4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kersemaekers WM, van Iersel T, Nassander U et al. Pharmacokinetics and safety study of posaconazole intravenous solution administered peripherally to healthy subjects. Antimicrob Agents Chemother. 2015; 59: 1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.