Abstract
Androgens act in almost all tissues throughout the lifetime and have important roles in skeletal muscles. The levels of androgens increase during puberty and remain sustained at high levels in adulthood. Because androgens have an anabolic effect on skeletal muscles and muscle stem cells, these increased levels of androgens after puberty should lead to spontaneous muscle hypertrophy and hyperplasia in adulthood. However, the maintenance of muscle volume, myonuclei number per myofiber, and quiescent state of satellite cells in adulthood despite the high levels of androgens produces paradoxical outcomes. Our recent study revealed that the physiological increase of androgens at puberty initiates the transition of muscle stem cells from proliferation to quiescence by the androgen-Mindbomb1-Notch signaling axis. This newly discovered androgen action on skeletal muscles underscores the physiological importance of androgens on muscle homeostasis throughout life. This review will provide an overview of the new androgen action on skeletal muscles and discuss the paradoxical effects of androgens suggested in previous studies.
Keywords: androgen, Mindbomb1, muscle stem cells, Notch, skeletal muscle
INTRODUCTION
Skeletal muscles composed of multinucleated, post-mitotic fibers account for approximately 40% of the total body weight (Frontera and Ochala, 2015). Founder muscle stem cells derived from mesodermal cells of the dermomyotome express the paired-box/homeodomain transcription factors Pax3 and Pax7, expand their populations, and undergo myogenic commitment through the sequential expression of Myogenic Regulatory Factors (MRFs), Myf5, Mrf4, Myod, and Myogenin (Tajbakhsh, 2009). The committed embryonic and foetal myocytes fuse to generate multinucleated myofibers during primary and secondary myogenesis (Chal and Pourquie, 2017). During the perinatal period, muscle stem cells continue to proliferate extensively and incorporate into pre-existing myofibers, which result in muscle growth. At puberty, however, juvenile muscle stem cells cease their proliferation, and a portion of the muscle stem cells becomes adult-type quiescent muscle stem cells. The quiescent muscle stem cells then reside between the basal lamina of myofibers and the sarcolemma of skeletal muscles under homeostatic condition and retain their stemness (Yin et al., 2013).
In skeletal muscles, androgens have been widely used for clinical and therapeutic purposes. Clinically, testosterone administration to hypogonadal men or eugonadal old men induces muscle growth associated with a decreased fat mass and increased muscle strength (Bhasin et al., 1996; 2001; 2005; Brodsky et al., 1996; Katznelson et al., 1996; Sih et al., 1997; Storer et al., 2003). When androgen is supplemented to aged people, age-associated muscle atrophy is ameliorated (Basualto-Alarcon et al., 2014; Janssen et al., 2000), suggesting that androgen treatment can rescue age-associated muscle atrophy (Bhasin et al., 2005; Serra et al., 2013; Sih et al., 1997; Urban et al., 1995; Wilkinson et al., 2018). Consequently, these therapeutic and clinical effects of testosterone have drawn the attention of many researchers who have identified signaling pathways operating within fibers and muscle stem cells that contribute to muscle hypertrophy and muscle stem cell proliferation. However, paradoxical effects of androgens exist in skeletal muscles, which appear to be due to the differential dose effects of androgens on muscle stem cells. Moreover, several studies using mouse genetic models have reported opposing views on the previous studies on androgen-sensitive sexually dimorphic perineal muscles. As such, in this review, we will discuss the bona fide physiological role of androgens on skeletal muscles during puberty and underscore the paradoxical anabolic effects of androgens reported in previous studies.
ANDROGEN ACTIONS ON SKELETAL MUSCLES
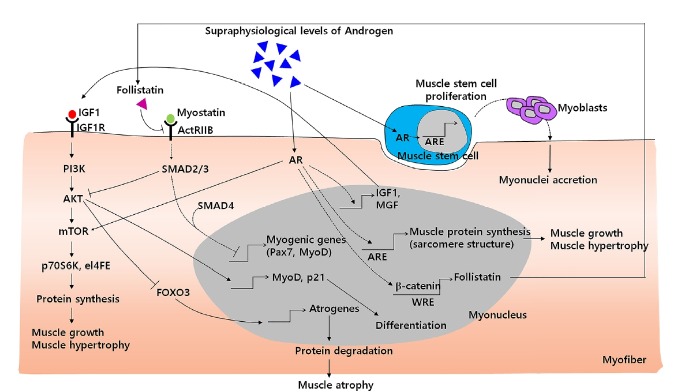
The anabolic effects of androgens occur in myofibers and directly regulate muscle protein synthesis which leads to muscle hypertrophy. Recent studies have shown that androgen-mediated activation of the IGF-1, PI3K/Akt and follistatin pathways stimulates protein synthesis, inhibits protein degradation and consequently increases skeletal muscle mass. Moreover, several studies have revealed that androgens can offset the catabolic effects of both the myostatin and glucocorticoid receptors (Chen et al., 2005; Dubois et al., 2012). In addition to myofibers, androgens also promote muscle stem cell proliferation (Fig. 1)(Fu et al., 2012; Joubert and Tobin, 1989; 1995; Sinha-Hikim et al., 2004). However, there appears to be some discrepancies according to other studies that reported no direct action of androgens in muscle stem cell proliferation in vitro (Chen et al., 2008; Doumit et al., 1996). In addition to the anabolic effects of androgens on muscle hypertrophy, whether androgens activate muscle stem cells still remains controversial.
Fig. 1. A schematic diagram of androgen signaling pathways associated with muscle hypertrophy and hyperplasia in skeletal muscles.
Upon supraphysiological levels of androgen treatment, androgens activate anabolic effects via the IGF-1, PI3K/Akt and follistatin pathways and counteract the catabolic effects by inhibiting the myostatin/SMAD and Foxo3 pathways. Besides the crosstalk between androgens and other signaling pathways in skeletal muscles, androgens also can induce the expressions of IGF1, follistatin and sarcomeric proteins which lead to muscle protein synthesis and hypertrophy. Although the role of androgens in muscle stem cells is still debated, there are some studies that have reported that androgens also can activate muscle stem cells to proliferate and stimulate myonuclei accretion in pre-existing myofibers via muscle stem cell recruitment.
The most unexpected and surprising results would be from studies using genetic mouse models including global androgen receptor knockout (ARKO), muscle stem cell-specific (MyoD-iCre;AR f/y ) and muscle-specific (MCK-Cre; Ar f/y or HSA-Cre;AR f/y) mice. Either global or tissue-specific deletion of AR results in severe loss of perineal muscles but not limb muscles although there is a slight alteration in muscle strength and fiber-type composition (Chambon et al., 2010; Dubois et al., 2014; MacLean et al., 2008; Ophoff et al., 2009). The perineal muscles, such as bulbocavernosus (BC) and levator ani (LA) muscles, are derived from the ventral muscle mass at the ventrocaudal edge, which migrates toward the cloaca, particularly the genital tubercle, and forms the perineal muscles (Buckingham et al., 2003; Valasek et al., 2005). Although both limb and perineal muscles express AR, its expression in perineal muscle is about 3- to 4-fold higher than in skeletal muscles, implying that perineal muscles are more sensitive to androgens (Dube et al., 1976; MacLean et al., 2008).
The perineal muscles are sexually dimorphic; perineal muscles only exist in males and naturally degenerate in females due to the lack of androgens (Cihak et al., 1970; Tobin and Joubert, 1988). The administration of testosterone to pre-pubertal female rats results in the masculinization of LA muscles associated with muscle fiber hypertrophy and an increase in myonuclei numbers (Gori et al., 1969; Joubert and Tobin, 1989). The gradual increase of testosterone in males markedly increases muscle stem cells in LA muscles (Joubert et al., 1994; Tobin and Joubert, 1991; Tobin et al., 1993), suggesting that pubertal testosterone regulates muscle hypertrophy and increases myonuclei numbers via muscle stem cell activation in LA muscles (Fig. 2B)(Joubert and Tobin, 1989, 1995; Joubert et al., 1994; Tobin et al., 1993). Although perineal muscles have been regarded as an ideal system to study the action of androgens, accumulated discrepancies have consequently raised questions about the validity of LA muscles as a physiological model of skeletal muscles. Consequently, the effects of androgens in general skeletal muscles should be precisely re-evaluated using limb muscles.
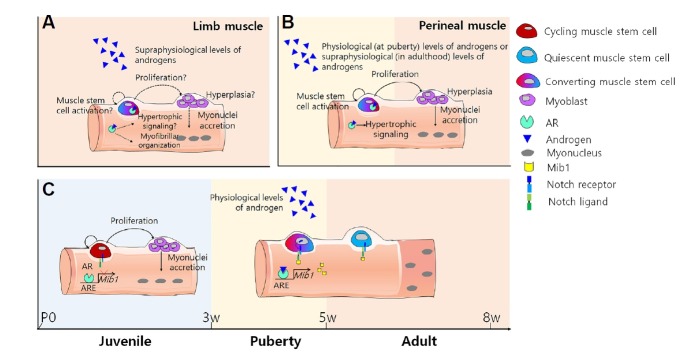
Fig. 2. Different androgen actions on limb and perineal muscles.
(A) Action of supra-physiological levels of androgen on limb muscles in adults. Androgens can induce limb muscle hypertrophy. Although myofiber hyperplasia via androgens is still controversial, some reports have suggested that androgens can induce hyperplasia by the activation of muscle stem cells. (B) Physiological or supra-physiological androgen action on perineal muscles at puberty or adulthood, respectively. During puberty, increased levels of androgens can induce muscle hypertrophy and hyperplasia. Moreover, in adults, androgens can activate and increase muscle stem cell numbers in a dose-dependent manner. (C) Schematic diagram of the conversion of proliferative juvenile muscle stem cells into quiescent adult muscle stem cells via the androgen-Mib1-Notch signaling axis in limb muscles during puberty (modified from Kim et al., 2016).
CONTROVERSIAL EFFECTS OF ANDROGENS ON MUSCLE STEM CELLS
Most studies that have shown androgen-induced muscle stem cell proliferation have used supraphysiologic doses of androgens. When testosterone with graded doses was used, there was no change in the serum total testosterone concentration up to 125 mg and no change in the limb muscle mass as well as in the numbers of muscle stem cells. However, when higher doses of testosterone were used (300- and 600-mg), the serum total testosterone concentration increased by 2- to 4-folds, and muscle stem cell numbers increased (Bhasin, 2003; Sinha-Hikim et al., 2002; 2003), indicating that at least a high dose of testosterone is required to activate muscle stem cells. Even in animal models, testosterone supplementation with a higher dose of testosterone resulted in about a 10-fold increase in the serum total testosterone concentration and muscle stem cell proliferation in both young and old limb muscles (Serra et al., 2013). Moreover, AR expression in isolated muscle stem cells increases when incubated with supraphysiological testosterone and/or DHT concentrations, indicating that the proliferation of muscle stem cells is promoted by androgens in a dose-dependent manner (Doumit et al., 1996; Sinha-Hikim et al., 2004). The increase in AR expression in muscle stem cells might enable muscle stem cells to sensitively respond to AR signaling and would be crucial to enhance the proliferation of muscle stem cells.
If androgens stimulate muscle stem cell proliferation in a dose-dependent manner, muscle stem cells should spontaneously proliferate until sustained high levels of endogenous androgens begin to decrease with age. Contrary to this assumption, muscle stem cells no longer proliferate after puberty and are maintained in a quiescent state until required for regeneration upon injury. As such that, we can speculate that muscle stem cells in vivo become quiescence upon the surge of androgens at puberty, while muscle stem cells in vitro acquire proliferation potency upon supraphysiological levels of androgen treatment in adults (Fig. 2A).
A NOVEL ANDROGEN FUNCTION ON SKELETAL MUSCLES AT PUBERTY
Based on previous studies, muscle hypertrophy and muscle stem cell proliferation should continuously occur in adulthood due to the high levels of androgens after puberty. However, intriguingly, in adulthood, the skeletal muscles reach maturity, and the proliferative juvenile muscle stem cells become quiescence (Allen et al., 1979; White et al., 2010). The lineage tracing of Pax7-descendant cells with X-gal labeling has revealed that the incorporation of X-gal+ cells into myofibers sharply declines at the onset of puberty (postnatal day (P) 21 in mouse (Piekarski et al., 2017; Richman et al., 2001; Sangiao-Alvarellos et al., 2013)) and ceases at P31 (Lepper et al., 2009). Moreover, muscle stem cell kinetics during postnatal growth has shown that myonuclei accretion ceases, and the number of muscle stem cells becomes steady state at P21 (Bachman et al., 2018; White et al., 2010), indicating that endogenously elevated androgens at puberty might have a role in muscle stem cell conversion rather than anabolism.
To rationalize the physiological role of endogenous androgens on muscle stem cell conversion during puberty in skeletal muscles, muscle stem cell kinetics during puberty was examined. Recent work from our lab proposed that a quiescent muscle stem cell population is established during puberty with Notch activation which has an important role in the maintenance of adult quiescent muscle stem cells in skeletal muscles (Bjornson et al., 2012; Mourikis et al., 2012a; 2012b). When the pubertal process is mimicked by injecting a physiological level of dihydrotestosterone (DHT) into 10-day-old pre-pubertal mice, Notch signaling is activated, and proliferative juvenile muscle stem cells become quiescence. In accordance with muscle stem cell kinetics during puberty, the muscle stem cell pool is depleted at 8 weeks of age when the sex hormone is surgically (orchiectomy at 2 weeks of age), pharmacologically (a Nal-Lys gonadotrophin releasing-hormone antagonist (antide)(Edelstein et al., 1990) treatment at 1 week of age) or genetically (hypogonadal mice (Gnrh1hpg/hpg )(Cattanach et al., 1977)) inhibited. Moreover, the expressions of Mib1, Notch-ligand regulating E3 ubiquitin ligase, and Notch target genes are down-regulated in all sex hormone inhibited myofibers at 4 weeks of age. When Mib1 is specifically deficient in myofibers (MCK-Cre; Mib1f/f ), there is no conversion of proliferative muscle stem cells into quiescent muscle stem cells in adults and even after DHT treatment in pre-pubertal mice, demonstrating that a reserve pool of quiescent muscle stem cells is established by the androgen-Mib1-Notch signaling pathway at puberty (Kim et al., 2016)(Fig. 2C). This finding sheds light on the biological mechanisms of androgens implicated in the regulation and maintenance of quiescent muscle stem cells.
CONCLUSION AND PERSPECTIVES
For a few decades, much effort has been given to understanding the role of androgens on skeletal muscles in adult homeostasis, disease and sarcopenic conditions for clinical purposes rather than for normal development including the pubertal period. Previously, the anabolic effects of androgens on skeletal muscles drew the attention of many researchers to focus mostly on muscle hypertrophy as well as SC proliferation. However, the apparent muscle growth during puberty somehow gets less attention. Recently, the action of androgens on skeletal muscles during puberty was revealed. The appearance of a quiescent adult SC pool through an androgen-Mib1-Notch signaling axis provides further molecular insights into the actions of androgens on skeletal muscles during puberty (Fig. 3).
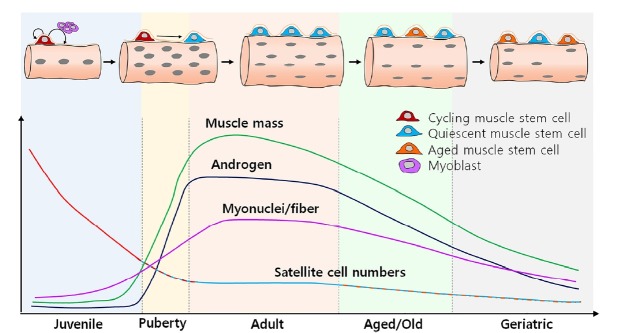
Fig. 3. Dynamics of muscle stem cells and physiological role of androgens in limb muscles.
During puberty, androgens surge via the hypothalamic-pituitary-gonadal axis. These high levels of androgens promote muscle growth and increase the myonuclei number per myofiber leading to an increase in muscle mass during puberty. However, after puberty, muscle mass and myonuclei number per myofiber reach a steady state and remain stable during adulthood. The maintenance of the limb muscle mass during adulthood is generally achieved by the conversion of proliferative juvenile muscle stem cells into quiescent adult muscle stem cells during the pubertal period. Intriguingly, the physiological increase of androgens induces the Mib1-Notch signaling axis to initiate muscle stem cell conversion and consequently establishes the pool of quiescent adult muscle stem cells throughout their lifetime. In summary, physiological androgens have a critical role in muscle stem cell conversion and muscle maintenance rather than anabolic effects on limb muscles.
Among the different types of muscles, the androgen-sensitive perineal muscles have been widely studied until genetic dissection in a mouse model revealed critical differences between limb and perineal muscles. Although both perineal and limb muscles express AR, AR in perineal muscles is more sensitive to androgens, demonstrating that perineal muscles have more potential to have an enhanced response to androgens. A recent study on the action of androgens on skeletal muscles revealed that androgens can act on skeletal muscles by myofiber to muscle stem cell communication. Because androgens can regulate various gene expressions, it would be possible that other genes like Mib1, which exist in myofibers, can be induced by androgens and could provide cues to muscle stem cells in a cell-autonomous manner. Further studies are required whether androgen-mediated genes are involved in the functions of muscle stem cells.
Perturbation of puberty leads to the inactivation of Notch signaling and consequently the depletion of the adult quiescent muscle stem cell pool due to the loss of quiescence and premature differentiation of muscle stem cells. Moreover, the muscle stem cell depletion and regeneration failure with age suggest a close relationship between muscle stem cell status and age-associated androgen levels. Recent studies have suggested the possibilities that aging with low androgen levels may result in systemic aging (Zhang et al., 2013) and dysfunctional muscle stem cells (Carlson and Conboy, 2007; Conboy et al., 2005). With age, the androgen level declines as well as Notch signaling. Activation of Notch signaling in aged mice results in an improved regenerative potential. In addition, when old mice were exposed to the circulatory system of young mice by parabiotic paring between young and old mice, not only the activation of Notch signaling but also the regenerative capacity of aged SCs is ameliorated (Conboy et al., 2003; 2005). This suggests that systemic factors change with age, particularly androgens, which might contribute to the loss of Notch signaling and impaired function of muscle stem cells in aged muscles. Consequently, the androgen-Mib1-Notch signaling axis may have a significant role in skeletal muscle development, quiescence and maintenance throughout an individual’s lifetime.
ACKNOWLEDGMENTS
We would like to thank J.-S. Kang, Y.-W. Jo, J. Seong and I. Park for their helpful comments during the preparation of the minireview. This work was supported by the National Research Foundation of Korea (NRF-2017R1A2B3007797) funded by the Korea government, and Korea Mouse Pheno-typing Project (NRF-2014M3A9D5A01073930) of the Ministry of Science and ICT through the National Research Foundation.
REFERENCE
- Allen R.E., Merkel R.A., Young R.B. Cellular aspects of muscle growth: myogenic cell proliferation. J Anim Sci. 1979;49:115–127. doi: 10.2527/jas1979.491115x. [DOI] [PubMed] [Google Scholar]
- Bachman J.F., Klose A., Liu W., Paris N.D., Blanc R.S., Schmalz M., Knapp E., Chakkalakal J.V. Prepubertal skeletal muscle growth requires Pax7-expressing satellite cell-derived myonuclear contribution. Development. 2018;145 doi: 10.1242/dev.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basualto-Alarcon C., Varela D., Duran J., Maass R., Estrada M. Sarcopenia and androgens: a link between pathology and treatment. Front Endocrinol. 2014;5:217. doi: 10.3389/fendo.2014.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin S. Testosterone supplementation for aging-associated sarcopenia. J Gerontol A Biol Sci Med Sci. 2003;58:1002–1008. doi: 10.1093/gerona/58.11.m1002. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Storer T.W., Berman N., Callegari C., Clevenger B., Phillips J., Bunnell T.J., Tricker R., Shirazi A., Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Woodhouse L., Casaburi R., Singh A.B., Bhasin D., Berman N., Chen X., Yarasheski K.E., Magliano L., Dzekov C., et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Woodhouse L., Casaburi R., Singh A.B., Mac R.P., Lee M., Yarasheski K.E., Sinha-Hikim I., Dzekov C., Dzekov J., et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- Bjornson C.R., Cheung T.H., Liu L., Tripathi P.V., Steeper K.M., Rando T.A. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky I.G., Balagopal P., Nair K.S. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men-a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D., Relaix F. The formation of skeletal muscle: from somite to limb. J Anat. 2003;202:59–68. doi: 10.1046/j.1469-7580.2003.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M.E., Conboy I.M. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach B.M., Iddon C.A., Charlton H.M., Chiappa S.A., Fink G. Gonadotrophin-releasing hormone deficiency in a mutant mouse with hypogonadism. Nature. 1977;269:338–340. doi: 10.1038/269338a0. [DOI] [PubMed] [Google Scholar]
- Chal J., Pourquie O. Making muscle: skeletal myogenesis in vivo and in vitro. Development. 2017;144:2104–2122. doi: 10.1242/dev.151035. [DOI] [PubMed] [Google Scholar]
- Chambon C., Duteil D., Vignaud A., Ferry A., Messaddeq N., Malivindi R., Kato S., Chambon P., Metzger D. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci USA. 2010;107:14327–14332. doi: 10.1073/pnas.1009536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lee N.K., Zajac J.D., MacLean H.E. Generation and analysis of an androgen-responsive myoblast cell line indicates that androgens regulate myotube protein accretion. J Endocrinol Invest. 2008;31:910–918. doi: 10.1007/BF03346441. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zajac J.D., MacLean H.E. Androgen regulation of satellite cell function. J Endocrinol. 2005;186:21–31. doi: 10.1677/joe.1.05976. [DOI] [PubMed] [Google Scholar]
- Cihak R., Gutmann E., Hanzlikova V. Involution and hormone-induced persistence of the M. sphincter (levator) ani in female rats. J Anat. 1970;106:93–110. [PMC free article] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Smythe G.M., Rando T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Doumit M.E., Cook D.R., Merkel R.A. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology. 1996;137:1385–1394. doi: 10.1210/endo.137.4.8625915. [DOI] [PubMed] [Google Scholar]
- Dube J.Y., Lesage R., Tremblay R.R. Androgen and estrogen binding in rat skeletal and perineal muscles. Can J Biochem. 1976;54:50–55. doi: 10.1139/o76-008. [DOI] [PubMed] [Google Scholar]
- Dubois V., Laurent M., Boonen S., Vanderschueren D., Claessens F. Androgens and skeletal muscle: cellular and molecular action mechanisms underlying the anabolic actions. Cell Mol Life Sci. 2012;69:1651–1667. doi: 10.1007/s00018-011-0883-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois V., Laurent M.R., Sinnesael M., Cielen N., Helsen C., Clinckemalie L., Spans L., Gayan-Ramirez G., Deldicque L., Hespel P., et al. A satellite cell-specific knockout of the androgen receptor reveals myostatin as a direct androgen target in skeletal muscle. Faseb J. 2014;28:2979–2994. doi: 10.1096/fj.14-249748. [DOI] [PubMed] [Google Scholar]
- Edelstein M.C., Gordon K., Williams R.F., Danforth D.R., Hodgen G.D. Single dose long-term suppression of testosterone secretion by a gonadotropin-releasing hormone antagonist (Antide) in male monkeys. Contraception. 1990;42:209–214. doi: 10.1016/0010-7824(90)90104-4. [DOI] [PubMed] [Google Scholar]
- Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- Fu R., Liu J., Fan J., Li R., Li D., Yin J., Cui S. Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J Cell Physiol. 2012;227:98–107. doi: 10.1002/jcp.22710. [DOI] [PubMed] [Google Scholar]
- Gori Z., Pellegrino C., Pollera M. The hypertrophy of levator ani muscle of rat induced by testosterone: an electron microscope study. Exp Mol Pathol. 1969;10:199–218. doi: 10.1016/0014-4800(69)90040-9. [DOI] [PubMed] [Google Scholar]
- Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- Joubert Y., Tobin C. Satellite cell proliferation and increase in the number of myonuclei induced by testosterone in the levator ani muscle of the adult female rat. Dev Biol. 1989;131:550–557. doi: 10.1016/s0012-1606(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Joubert Y., Tobin C. Testosterone treatment results in quiescent satellite cells being activated and recruited into cell cycle in rat levator ani muscle. Dev Biol. 1995;169:286–294. doi: 10.1006/dbio.1995.1144. [DOI] [PubMed] [Google Scholar]
- Joubert Y., Tobin C., Lebart M.C. Testosterone-induced masculinization of the rat levator ani muscle during puberty. Dev Biol. 1994;162:104–110. doi: 10.1006/dbio.1994.1070. [DOI] [PubMed] [Google Scholar]
- Katznelson L., Finkelstein J.S., Schoenfeld D.A., Rosenthal D.I., Anderson E.J., Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Han G.C., Seo J.Y., Park I., Park W., Jeong H.W., Lee S.H., Bae S.H., Seong J., Yum M.K., et al. Sex hormones establish a reserve pool of adult muscle stem cells. Nat Cell Biol. 2016;18:930–940. doi: 10.1038/ncb3401. [DOI] [PubMed] [Google Scholar]
- Lepper C., Conway S.J., Fan C.M. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean H.E., Chiu W.S., Notini A.J., Axell A.M., Davey R.A., McManus J.F., Ma C., Plant D.R., Lynch G.S., Zajac J.D. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. Faseb J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- Mourikis P., Gopalakrishnan S., Sambasivan R., Tajbakhsh S. Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells. Development. 2012a;139:4536–4548. doi: 10.1242/dev.084756. [DOI] [PubMed] [Google Scholar]
- Mourikis P., Sambasivan R., Castel D., Rocheteau P., Bizzarro V., Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012b;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- Ophoff J., Van Proeyen K., Callewaert F., De Gendt K., De Bock K., Vanden Bosch A., Verhoeven G., Hespel P., Vanderschueren D. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009;150:3558–3566. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- Piekarski D.J., Boivin J.R., Wilbrecht L. Ovarian hormones organize the maturation of inhibitory neurotransmission in the frontal cortex at puberty onset in female mice. Curr Biol. 2017;27:1735–1745 e1733. doi: 10.1016/j.cub.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman C., Kutilek S., Miyakoshi N., Srivastava A.K., Beamer W.G., Donahue L.R., Rosen C.J., Wergedal J.E., Baylink D.J., Mohan S. Postnatal and pubertal skeletal changes contribute predominantly to the differences in peak bone density between C3H/HeJ and C57BL/6J mice. J Bone Miner Res. 2001;16:386–397. doi: 10.1359/jbmr.2001.16.2.386. [DOI] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S., Manfredi-Lozano M., Ruiz-Pino F., Navarro V.M., Sanchez-Garrido M.A., Leon S., Dieguez C., Cordido F., Matagne V., Dissen G.A., et al. Changes in hypothalamic expression of the Lin28/let-7 system and related microRNAs during postnatal maturation and after experimental manipulations of puberty. Endocrinology. 2013;154:942–955. doi: 10.1210/en.2012-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra C., Tangherlini F., Rudy S., Lee D., Toraldo G., Sandor N.L., Zhang A., Jasuja R., Bhasin S. Testosterone improves the regeneration of old and young mouse skeletal muscle. J Gerontol A Biol Sci Med Sci. 2013;68:17–26. doi: 10.1093/gerona/gls083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sih R., Morley J.E., Kaiser F.E., Perry H.M., 3rd, Patrick P., Ross C. Testosterone replacement in older hypogonadal men: a 12-month randomized controlled trial. J Clin Endocrinol Metab. 1997;82:1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I., Artaza J., Woodhouse L., Gonzalez-Cadavid N., Singh A.B., Lee M.I., Storer T.W., Casaburi R., Shen R., Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I., Roth S.M., Lee M.I., Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- Sinha-Hikim I., Taylor W.E., Gonzalez-Cadavid N.F., Zheng W., Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89:5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- Storer T.W., Magliano L., Woodhouse L., Lee M.L., Dzekov C., Dzekov J., Casaburi R., Bhasin S. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478–1485. doi: 10.1210/jc.2002-021231. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J Intern Med. 2009;266:372–389. doi: 10.1111/j.1365-2796.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- Tobin C., Joubert Y. The levator ani of the female rat: a suitable model for studying the effects of testosterone on the development of mammalian muscles. Biol Struct Morphog. 1988;1:28–33. [PubMed] [Google Scholar]
- Tobin C., Joubert Y. Testosterone-induced development of the rat levator ani muscle. Dev Biol. 1991;146:131–138. doi: 10.1016/0012-1606(91)90453-a. [DOI] [PubMed] [Google Scholar]
- Tobin C., Joubert Y., Lebart M.C. Androgen control of myofiber number and size in the rat levator ani muscle. BAM. 1993;3:121–131. [Google Scholar]
- Urban R.J., Bodenburg Y.H., Gilkison C., Foxworth J., Coggan A.R., Wolfe R.R., Ferrando A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. T Am J Physiol. 1995;269:E820–826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- Valasek P., Evans D.J., Maina F., Grim M., Patel K. A dual fate of the hindlimb muscle mass: cloacal/perineal musculature develops from leg muscle cells. Development. 2005;132:447–458. doi: 10.1242/dev.01545. [DOI] [PubMed] [Google Scholar]
- White R.B., Bierinx A.S., Gnocchi V.F., Zammit P.S. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol. 2010;10:21. doi: 10.1186/1471-213X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D.J., Piasecki M., Atherton P.J. The age-related loss of skeletal muscle mass and function: Measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev. 2018;47:123–132. doi: 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Price F., Rudnicki M.A. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Li J., Purkayastha S., Tang Y., Zhang H., Yin Y., Li B., Liu G., Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]