Abstract
Osteoarthritis (OA) is a naturally occurring, irreversible disorder and a major health burden. The disease is multifactorial, involving both physiological and mechanical processes, but calcium crystals have been associated intimately with its pathogenesis. This study tested the hypothesis that these crystals have a detrimental effect on the differentiation of osteoclasts and bone homeostasis. This study employed an osteoblast-osteoclast coculture system that resembles in vivo osteoblast-dependent osteoclast differentiation along with Ca2+-phosphate-coated culture dishes. The calcium-containing crystals upregulated the expression of RANKL and increased the differentiation of osteoclasts significantly as a result. On the other hand, osteoblast differentiation was unaffected. MicroRNA profiling showed that dual-specificity phosphatases 1 (DUSP1) was associated with the increased RANKL expression. DUSP1 belongs to a family of MAPK phosphatases and is known to inactivate all three groups of MAPKs, p38, JNK, and ERK. Furthermore, knockdown of DUSP1 gene expression suggested that RANKL expression increases significantly in the absence of DUSP1 regulation. Microarray analysis of the DUSP1 mRNA levels in patients with pathological bone diseases also showed that the downregulated DUSP1 expression leads to increased expression of RANKL and consequently to the destruction of the bone observed in these patients. These findings suggest that calcium-containing crystals may play a crucial role in promoting RANKL-induced osteoclastogenesis via DUSP1.
Keywords: DUSP1, osteoarthritis, osteoclastogenesis, RANKL
Osteoarthritis (OA) is the most common joint disorder in humans (Fuerst et al., 2009). The disease is slowly progressive and involves all components of the joint, including the bone, cartilage, meniscus, and synovium (Macmullan and McCarthy, 2010). The high prevalence and related physical impairment make OA a leading cause of disability worldwide (McCarthy et al., 2009). Thus far, multiple predisposing factors have been identified, including genetic predisposition, joint trauma, obesity, sex, and hormonal status (Brandt et al., 1998). However, our understanding of the pathogenesis of the condition is limited and there is no pharmacological therapy yet to reverse or retard the consequences of OA (Qvist et al., 2008).
Calcification of the articular cartilage is a well-recognized feature of OA and current evidence suggests that it contributes directly to joint degeneration (MacMullan et al., 2010). Calcium pyrophosphate dehydrate (CPP) and basic calcium phosphate (BCP) are the two most common forms of calcium crystals found in the articular cartilage (Molloy and McCarthy, 2006). The presence of these crystals is associated with a number of clinical manifestations (MacMullan et al., 2010). Both types of crystals are found in OA. Individual crystals are typically less than 1 μm in size and they aggregate in the OA synovial fluid to form clumps that are approximately 5 to 20 μm (McCarthy et al., 2011).
In the present study, we hypothesized that these crystals may have a detrimental effect on the differentiation and survival of osteoblasts and osteoclasts. Recent studies have frequently used the term “coupling of bone and cartilage” to describe the crosstalk between the articular cartilage and subchondral bone in the synovial joints through increased vascularization and development of microcracks in the bone matrix (Burr and Radin, 2003; Lajeunesse and Reboul, 2003) and the cellular and molecular interactions between osteoblasts and chondrocytes that affects the initiation and progression of OA (Karsdal et al., 2008; Mansell et al., 2007; Sharma et al., 2013). Thus far, the pathogenic role of crystals in the degradation of the extracellular matrix and subchondral bone remodeling have been studied (Corr et al., 2017) but only few studies have examined their effects on osteoclasts and bone remodeling (Stack and McCarthy, 2016).
To test this hypothesis, amorphous calcium phosphate (ACP) arrays were first prepared on a 60 mm culture dish, as described previously (Kim et al., 2010). ACP arrays were fabricated on the surface of the dish to mimic the crystals observed in OA patients. As illustrated in Fig. 1(A), murine bone marrow-derived macrophages (BMMs) were cocultured with murine calvarial osteoblasts, as described by Ryu et al. (Ryu et al., 2006) either with or without a Ca2+-phosphate coating. Early studies of cocultures found that a direct cell-to-cell interaction was required for osteoclast generation, while the following studies showed that RANKL expressed on osteoblasts and RANK present on the osteoclast precursors were responsible for the cell surface molecular interaction for osteoclastic differentiation (Suda et al., 1999). The cocultures were treated with prostaglandin E2 (10−6 M) and vitamin D3 (10−8 M) to stimulate osteoblast differentiation and induce cell surface RANKL expression to facilitate the osteoclastic differentiation of BMMs, which resemble the actual osteoblast-dependent osteoclast differentiation in vivo. BMMs were stained for tartrate-resistant acid phosphatase (TRAP) on days 3 and 6 of coculture according to the manufacturer’s instruction (Sigma-Aldrich, USA). TRAP staining from Fig. 1B highlights that osteoclast differentiation in the coculture system was enhanced predominantly by a Ca2+-phosphate coating. The marked increase in the osteoclast number and size were evident in the coated dish compared to that of the non-coated control (Fig. 1B). These results strongly suggest that Ca2+-phosphate crystals can induce accelerated osteoclast differentiation via osteoblasts.
Fig. 1. (A) Diagram of the experimental design employing an osteoblast-osteoclast coculture system.
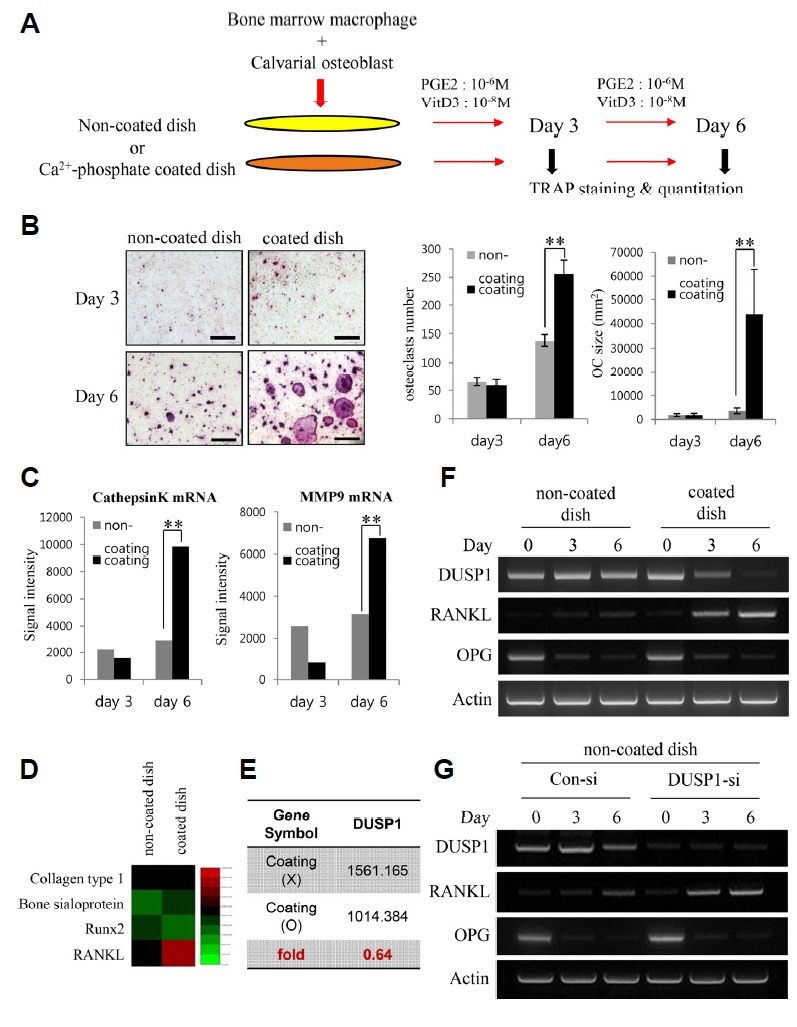
The cells were cultured for 3 or 6 days in the presence or absence of calcium-containing crystals on the surface of the wells. Prostaglandin E2 (PGE2) and vitamin D3 (VtD3) were added to stimulate osteoblast differentiation. The aim of the coculture was for cell surface RANKL on osteoblasts to support osteoclastic differentiation of BMMs. (B) TRAP staining of cells cultured in the non-coated and coated dish on days 3 and 6. There is a noticeable increase in the TRAP expression and number of multinucleated cells on day 6 of the coated dish. The number and average size (mm2) of TRAP-positive multinucleated osteoclasts were quantified. The data represent the means ± SD (**p < 0.01). (C) Quantification of Cathepsin K and MMP9 mRNA expression by qPCR. (D) Comparison of microarray heat map of genes associated with the differentiation of osteoblasts – Collagen type 1, Bone sialoprotein, and Runx2 – and osteoclasts – RANKL – between the uncoated and coated dish. The green and red colors indicate low and high expression, respectively. (E) Average signal value of DUSP1 gene expression in the uncoated and coated dishes evaluated from the microarray results. (F) RT-PCR analysis of DUSP1, RANKL, and OPG mRNA levels in the two dishes. DUSP1 levels were consistent in the non-coated dish. In the coated dish, the decrease in DUSP1 levels was associated with an increase in RANKL intensity. (G) RT-PCR analysis of the efficacy of DUSP1 knockdown and RANKL mRNA levels. Expression of RANKL increases in response to DUSP1 knockdown in the non-coated dish.
In addition, gene expression of the common markers of osteoclast activity, cathepsin K, and matrix metallopeptidase 9 (MMP9) was assessed (Fig. 1C). In keeping with the earlier results, it was found that the expression of those two genes were increased notably in osteoclastic cells cultured in the coated dish on day 6. In an attempt to identify the molecular mechanisms underlying the augmentation of osteoclastogenesis by the crystals in the coculture, the altered expression of genes between the non-coated and coated conditions were analyzed using MouseWG-6 v2.0 Expression BeadChip (Illumina). Microarray results from Fig. 1D revealed that the gene expression of some common markers of osteoblastic activity, such as collagen type 1, bone sialoprotein, and Runx2, did not show a significant difference between the two dishes on day 6 of coculture. Conversely, gene expression of RANKL, which plays an essential role in the commitment of precursors to osteoclastic differentiation (Boyle et al., 2003; Suda et al., 1999; Teitelbaum and Ross, 2003), was upregulated by 140% from 237.1 to 326.9, suggesting that a Ca2+-phosphate coating does not significantly alter osteoblast differentiation but enhances osteoclast differentiation.
The microarray results from cultures of osteoblasts grown on either non-coated or coated dishes for six days were next examined to determine the gene responsible for the increase in RANKL expression. Microarray analysis identified the genes that showed a greater than 1.35-fold difference in expression between the two dishes. Among the 167 genes, two genes reported the greatest difference and dual-specificity phosphatases 1 (DUSP1) was selected as the most relevant after pathway analysis using PANTHER (Mi et al., 2013). DUSPs are cysteine-based enzymes that can remove phosphate groups from phosphor-serine/threonine residues (Patterson et al., 2009) and play important roles in MAPK signaling pathway in the development and immune response (Nunes-Xavier et al., 2011). Among them, DUSP1 is a nuclear phosphatase and its major substrates are JNK, p38, and ERK1/2 (Camps et al., 2000). The data showed that the gene expression of DUSP1 was downregulated to 0.64 in the coated dish compared to the non-coated dish (Fig. 1E). This suggested a role for DUSP1 to regulate RANKL expression to mediate osteoclast differentiation. To test this idea, the expression level of DUSP1 mRNA in the coculture was first observed (Fig. 1F). In the non-coated dish, DUSP1 mRNA expression was consistent and RANKL was increased slightly during six days of coculture, whereas the levels of DUSP1 decreased markedly with increasing RANKL levels during osteoclastogenesis in the coated-dish. Furthermore, DUSP1-knockdown osteoblasts were prepared and cocultured in the non-coated dish to validate the negative effects of DUSP1 on RANKL expression (Fig. 1G). The RT-PCR result revealed the DUSP1-knockdown-associated increase in RANKL expression in the DUSP -siRNA coculture, whereas minimal RANKL expression was observed in the control si-RNA transfected cells. Together, the data indicate that DUSP1 plays a role in the downregulation of RANKL expression.
To complement the PCR experiments, DUSP1-knockdown osteoblasts were cocultured with osteoclasts to provide a quantitative estimate. A coculture in a coated dish was used as the control for osteoclastogenesis. TRAP staining showed that the knockdown of DUSP1 in the non-coated dish results in increased formation of large osteoclasts and the staining intensity was almost as intense as that in the control coated dish on day 6 (Fig. 2A). Osteoclastogenesis was next evaluated by counting the number of TRAP-positive multinucleated cells (three or more nuclei each cell) under a microscope using semiautomatic software (NIS-Elements AR 4.30.01). After knockdown, a significant increase in the number of TRAP-positive, multinucleated osteoclasts was observed on day 6 of coculture. Indeed, these results show that the knockdown of DUSP1 results in increased osteoclast formation.
Fig. 2. (A) TRAP staining of osteoclastic cells on day 6 of coculture.
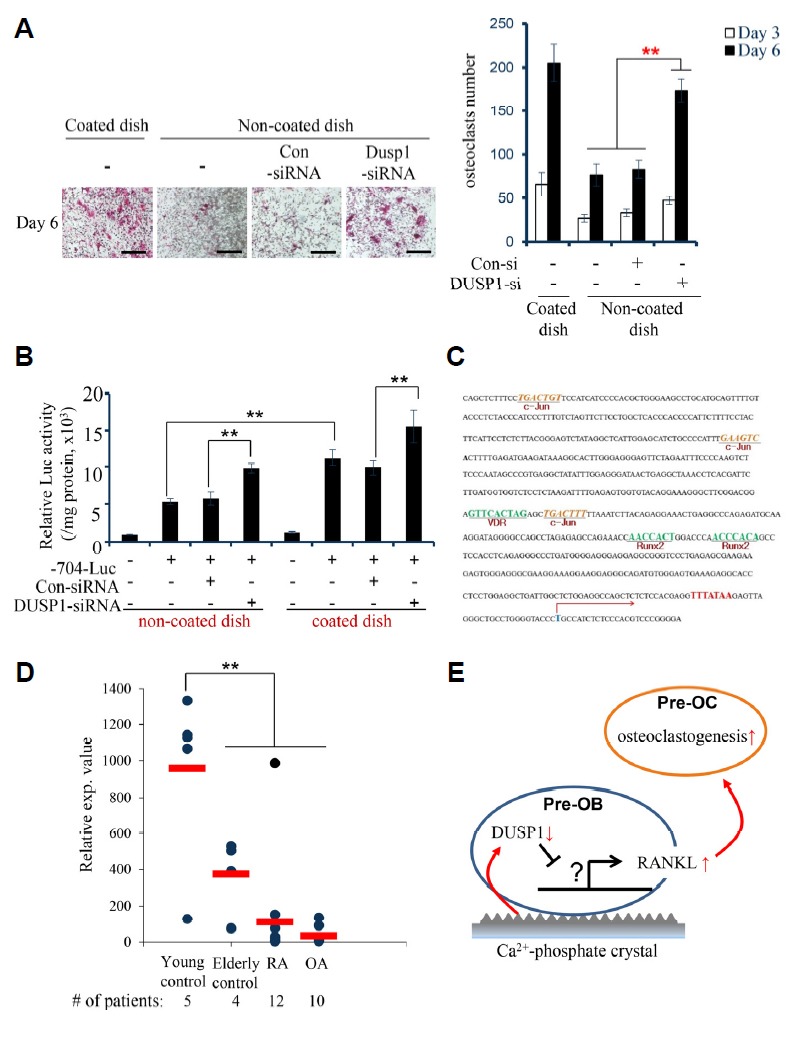
Calvarial osteoblasts were transfected with either control siRNA or DUSP1 siRNA. The number of TRAP-positive, multinucleated cells were counted on days 3 and 6. The data represent the means ± SD (**p < 0.01). Scale bar, 200 μm. (B) BMMs were transiently transfected with the luciferase reporter constructs containing the −704 to +111 (−704-luc) region of the murine RANKL promoter for the analysis of the luciferase activity in two dishes before and after DUSP1 knockdown. (C) The murine RANKL promoter was analyzed for the identification of putative transcription factor binding sites using the PROMO based on Ver. 8.3 of TRANSFAC. (D) qRT-PCR validation of the differences in the relative gene expression of DUSP1 identified in a microarray analysis from synovial membrane samples of normal controls, rheumatoid arthritis (RA) patients, and osteoarthritis (OA) patients. (E) Schematic representation of the proposed mechanism of RANKL-mediated osteoclastogenesis by Ca2+-phosphate crystals. Decreased level of DUSP1 in pre-osteoblasts (pre-OB) by the crystals lead to the increased expression of RANKL, thereby enhancing osteoclastogenesis among pre-osteoclasts (pre-OC).
In order to analyze the transcriptional activity of the RANKL gene promoter in response to the knockdown of DUSP1, the 5′-flanking region (704 bp) of the murine RANKL gene was next isolated and sequenced using a previously described technique (O’Brien et al., 2002). Fragments spanning −704 to +111 (704-luc), relative to the transcription start-site, were excised from the TA-cloning vector with EcoRI, blunt-ended, and ligated into the Sma I site of the pGL3-Basic luciferase reporter vector (Promega, USA). As a control, the activity of −704-luc was measured in both coated and non-coated dishes. As shown in Fig. 2B, the RANKL promoter activity was higher in the coated dish than in the non-coated. Therefore, as postulated earlier, it is conceivable that the Ca2+-phosphate coating may facilitate osteoclastogenesis. In addition, the RANKL promoter activity was stimulated markedly after DUSP1 knockdown in both dishes. These results suggest that the Ca2+-phosphate coating, an extracellular factor, can affect the RANKL promoter activity within pre-osteoblasts and upregulate RANKL expression via the pathways involving DUSP1.
To determine the inverse relationship between DUSP1 and RANKL expression, the transcription factor binding sites in the RANKL promoter region were examined using an in silico tool. The 5′-flanking region of the RANKL promoter was examined by PROMO-based on Ver. 8.3 of TRANSFAC. Interestingly, the nucleotide sequence revealed multiple binding sites for c-Jun as well as Runx2 and VDR, which are well-known factors responsible for osteoblast differentiation (Fig. 2C). c-Jun is one of the components of AP-1, a dimeric transcription factor known as some of the best characterized substrates of c-Jun N-terminal kinases (JNKs) family of mitogen-activated protein kinases (MAPKs; David et al., 2002). Multiple reports have demonstrated that RANKL binding to its receptor RANK results in the recruitment of TNF-receptor associated factor (TRAF) family of proteins, such as TRAF6, which potently activates JNK pathways (Kobayashi et al., 2001; Suda et al., 1999; Takayanagi et al., 2000; Wong et al., 1998). The activation of JNK results in the phosphorylation of c-Jun in osteoclasts (David et al., 2002; Ikeda et al., 2004) and indirect modulation of NF-κB nuclear translocation, enhancing the expression of genes essential for osteoclastogenesis. Furthermore, DUSP1 is known to regulate the dephosphorylation of p-JNK, p-ERK, and p-p38 (Camps et al., 2000) and Yoshitake et al. suggested that DUSP1 may be involved in the suppression of JNK activation, inhibiting the TRAF6 and JNK signaling pathway activated by the RANKL-RANK interaction (Yoshitake et al., 2008). Indeed, the present observation and recent studies describe the DUSP1/JNK pathway as a mechanism responsible for the regulation of osteoclastogenesis via the mediation of RANKL. Whether this pathway directly inhibits RANKL remains to be studied.
The potential association between DUSP1 gene expression and pathologic bone diseases in humans was examined by analyzing publicly available datasets in GEO (accession number GSE12021; Huber et al., 2008). The microarray results were grouped as the young control (n = 5), elderly control (n = 4), rheumatoid arthritis (RA) patients (n = 12), and osteoarthritis (OA) patients (n = 10). In this dataset, DUSP1 mRNA expression in the elderly control was 50% of that of the young control, which implies an aging-dependent decline in the levels of DUSP1 (Fig. 2D). More importantly, in accordance with our results, the DUSP1 expression level was downregulated considerably in RA patients and appeared to be the lowest in OA patients. From this perspective, it is likely that the downregulated levels of DUSP1 allows the enhanced expression of RANKL, which eventually results in the destruction of bone observed in RA and OA patients. Further studies will be needed to examine the precise mechanisms underlying the regulation of osteoclastogenesis.
The results reported here suggest that calcium-containing crystals play a key role in the progression of osteoclastogenesis through the elevated expression of RANKL from osteoblasts. Figure 2E summarizes a possible mechanism of cell-cell signaling that leads to increased osteoclastogenesis. We speculate that the crystals might downregulate DUSP1 gene expression in pre-osteoblasts, which in turn induce RANKL expression via pathways yet to be elucidated. Upregulated RANKL binds to its receptor on pre-osteoclasts, inducing the differentiation of osteoclasts.
This paper reports for the first time using a coculture system that a Ca2+-phosphate coating similar to the crystals found in the articular cartilage of OA patients may stimulate RANKL-mediated osteoclastogenesis. The results provide possible confirmation of the pathogenic role of crystal formation in the development of osteoarthritis. In addition, the present results have shown that the DUSP1 gene is a potential negative regulator of RANKL expression, which appears to be downregulated in both in vitro and in vivo models of osteoarthritis. The mechanism described in this study will expand our understanding of bone-erosive diseases and may provide alternative targets for therapeutic intervention in patients.
ACKNOWLEDGMENTS
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2018R1A5A2023879 and NRF-2016R1C1B 2012891). All authors declare no potential conflicts of interest with respect to the publication of this article.
REFERENCES
- Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- Brandt K., Lohmander L., Doherty M. Osteoarthritis. Oxford: Oxford University Press; 1998. Pathogenesis of osteoarthritis; pp. 70–74. [Google Scholar]
- Burr D.B., Radin E.L. Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin N Am. 2003;29:675–685. doi: 10.1016/s0889-857x(03)00061-9. [DOI] [PubMed] [Google Scholar]
- Camps M., Nichols A., Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- Corr E.M., Cunningham C.C., Helbert L., McCarthy G.M., Dunne A. Osteoarthritis associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res Ther. 2017;19:23. doi: 10.1186/s13075-017-1225-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J.P., Sabapathy K., Hoffmann O., Idarraga M.H., Wagner E.F. JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci. 2002;115:4317–4325. doi: 10.1242/jcs.00082. [DOI] [PubMed] [Google Scholar]
- Fuerst M., Bertrand J., Lammers L., Dreier R., Echtermeyer F., Nitschke Y., Rutsch F., Schäfer F.K., Niggemeyer O., Steinhagen J., et al. Calcification of articular cartilage in human osteoarthritis. Arthritis Rheum. 2009;60:2694–2703. doi: 10.1002/art.24774. [DOI] [PubMed] [Google Scholar]
- Huber R., Hummert C., Gausmann U., Pohlers D., Koczan D., Guthke R. Identification of intra-group, inter-individual, and gene-specific variances in mRNA expression profiles in the rheumatoid arthritis synovial membrane. Arthritis Res Ther. 2008;10:R98. doi: 10.1186/ar2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda F., Nishimura R., Matsubara T., Tanaka S., Inoue J., Reddy S.V., Hata K., Yamashita K., Hiraga T., Watanabe T., et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation. J Clin Invest. 2004;114:475–484. doi: 10.1172/JCI19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal M.A., Leeming D.J., Dam E.B., Henriksen K., Alexandersen P., Pastoureau P., Altman R.D., Christiansen C. Should subchondral bone turnover be targeted when treating osteoarthritis? Osteoarthritis Cartilage. 2008;16:638–646. doi: 10.1016/j.joca.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Kim I., Kim H.J., Kim H.M. Array of amorphous calcium phosphate particles improves cellular activity on a hydrophobic surface. J Biomed Mater Res B (Appl Biomater) 2010;93:113–121. doi: 10.1002/jbm.b.31565. [DOI] [PubMed] [Google Scholar]
- Kobayashi N., Kadono Y., Naito A., Matsumoto K., Yamamoto T., Tanaka S., Inoue J. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis. EMBO J. 2001;20:1271–1280. doi: 10.1093/emboj/20.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajeunesse D., Reboul P. Subchondral bone in osteoarthritis: a biologic link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol. 2003;15:628–633. doi: 10.1097/00002281-200309000-00018. [DOI] [PubMed] [Google Scholar]
- Macmullan P., McCarthy G.M. The meniscus, calcification and osteoarthritis: a pathologic team. Arthritis Res Ther. 2012;12:116. doi: 10.1186/ar2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMullan P., McMahon G., McCarthy G.M. Detection of basic calcium phosphate crystals in osteoarthritis. Joint Bone Spine. 2011;78:358–363. doi: 10.1016/j.jbspin.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Mansell J.P., Collins C., Bailey A.J. Bone, not cartilage, should be the major focus in osteoarthritis. Nat Clin Pract Rheumatol. 2007;3:306–307. doi: 10.1038/ncprheum0505. [DOI] [PubMed] [Google Scholar]
- McCarthy G.M., Cheung H.S. Point: hydroxyapatite crystal deposition is intimately involved in the pathogenesis and progression of human osteoarthritis. Curr Rheumatol Rep. 2009;11:141–147. doi: 10.1007/s11926-009-0020-6. [DOI] [PubMed] [Google Scholar]
- Mi H., Muruganujan A., Casagrande J.T., Thomas P.D. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy E.S., McCarthy G.M. Calcium crystal deposition diseases: update on pathogenesis and manifestations. Rheum Dis Clin North Am. 2006;32:383–400. doi: 10.1016/j.rdc.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Nunes-Xavier C., Roma-Mateo C., Rios P., Tarrega C., Cejudo-Marin R., Tabernero L., Pulido R. Dual-specificity MAP kinase phosphatases as targets of cancer treatment. Anticancer Agents Med. 2011;11:109–132. doi: 10.2174/187152011794941190. [DOI] [PubMed] [Google Scholar]
- O’ Brien C.A., Kern B., Gubrij I., Karsenty G., Manolagas S.C. Cbfa1 doesn’t regulate RANKL gene activity in stromal/osteoblastic cells. Bone. 2002;30:453–462. doi: 10.1016/s8756-3282(01)00692-5. [DOI] [PubMed] [Google Scholar]
- Patterson K.L., Brummer T., O’Brien P.M., Daly R.J. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Qvist P., Bay-Jensen A.C., Christiansen C., Dam E.B., Pastoureau P., Karsdal M.A. The disease modifying osteoarthritis drug (DMOAD): is it in the horizon? Pharmacol Res. 2008;58:1–7. doi: 10.1016/j.phrs.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Ryu J., Kim H.J., Chang E.J., Huang H., Banno Y., Kim H.H. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.R., Jagga S., Lee S.S., Nam J.S. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. 2013;14:19805–19830. doi: 10.3390/ijms141019805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack J., McCarthy Gm. Basic calcium phosphate crystals and osteoarthritis pathogenesis: novel pathways and potential targets. Curr Opin Rheumatol. 2016;28:122–126. doi: 10.1097/BOR.0000000000000245. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi N., Udagawa N., Jimi E., Gillespie M.T., Martin T.J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- Takayanagi H., Ogasawara K., Hida S., Chiba T., Murata S., Sato K., Akinori T., Yokochi T., Oda H., Tanaka K., et al. T cell-mediated regulation of osteoclastogenesis by signaling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- Wong B.R., Josien R., Lee S.Y., Vologodskaia M., Steinman R.M., Choi Y. The TRAF family of signal transducers mediates NF-κB activation by the TRANCE receptor. J Biol Chem. 1998;273:28355–28359. doi: 10.1074/jbc.273.43.28355. [DOI] [PubMed] [Google Scholar]
- Yoshitake F., Itoh S., Narita H., Ishihara K., Ebisu S. Interleukin-6 directly inhibits osteoclast differentiation by suppressing receptor activator of NF-κB signaling pathways. J Biol Chem. 2008;283:11535–11540. doi: 10.1074/jbc.M607999200. [DOI] [PubMed] [Google Scholar]


