Abstract
Background and aims:
Constipation is one of the most frequent symptoms encountered in daily clinical practice and is implicated in the development of atherosclerosis, potentially through altered gut microbiota. However, little is known about its association with incident cardiovascular events.
Methods:
In a nationally representative cohort of 3,359,653 U.S. veterans with an estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 between October 1, 2004 and September 30, 2006 (baseline period), with follow-up through 2013, we examined the association of constipation status (absence or presence; defined using diagnostic codes and laxative use) and laxative use (none, one, or ≥2 types of laxatives) with all-cause mortality, incident coronary heart disease (CHD), and incident ischemic stroke.
Results:
Among 3,359,653 patients, 237,855 (7.1%) were identified as having constipation. After multivariable adjustments for demographics, prevalent comorbidities, medications, and socioeconomic status, patients with (versus without) constipation had 12% higher all-cause mortality (hazard ratio [HR], 1.12; 95% CI, 1.11–1.13), 11% higher incidence of CHD (HR, 1.11; 95% CI, 1.08–1.14), and 19% higher incidence of ischemic stroke (HR, 1.19; 95% CI, 1.15–1.22). Patients with one and ≥2 (versus none) types of laxatives experienced a similarly higher risk of all-cause mortality (HRs [95% CI], 1.15 [1.13–1.16] and 1.14 [1.12–1.15], respectively), incident CHD (HRs [95% CI], 1.11 [1.07–1.15] and 1.10 [1.05–1.15], respectively) and incident ischemic stroke (HRs [95% CI], 1.19 [1.14–1.23] and 1.21 [1.16–1.26], respectively).
Conclusions:
Constipation status and laxative use are independently associated with higher risk of all-cause mortality and incident CHD and ischemic stroke.
Keywords: Constipation, Laxatives, Mortality, Coronary heart disease, Stroke
Graphical Abstract
INTRODUCTION
Constipation is one of the most prevalent gastrointestinal disorders commonly encountered in primary care settings, affecting approximately 30% of the general population during their lifetime.1 Although constipation is usually perceived as a benign, often self-limited condition,2 its chronic symptoms impair patients’ quality-of-life and may impose a considerable social and economic burden.3,4 In recent years, a few observational studies have investigated the association between chronic constipation and future clinical outcomes such as mortality and cardiovascular events and offered seemingly conflicting evidence; some suggesting an independent association of chronic constipation with increased risk of cardiovascular mortality,5–7 while one other showing no association with all-cause and cardiovascular mortality.8 To the best of our knowledge, only two previous studies have examined the association of constipation with incident non-fatal cardiovascular events such as myocardial infarction and stroke,5,8 but did not confirm the statistical significance of the association, partly due to low number of outcome events;5 and hence, it remains unknown whether constipation is associated with increased risk of incident cardiovascular events.
In this study, we hypothesized that patients with constipation are at higher risk of all-cause mortality and incident cardiovascular events, and that patients with more extensive laxative use would have a greater risk of such events than those with less severe constipation, independently of known cardiovascular risk factors. To test these hypotheses, we investigated the association of constipation status and laxative use with all-cause mortality, incident coronary heart disease (CHD) and incident ischemic stroke using a large nationally representative cohort of US veterans with estimated glomerular filtration rate (eGFR) of ≥60 mL/min/1.73 m2.
MATERIALS AND METHODS
Cohort definition
We used data from a retrospective cohort study examining risk factors in patients with incident chronic kidney disease (CKD) (Racial and Cardiovascular Risk Anomalies in CKD [RCAV] study).9 Supplemental Fig. 1 shows the algorithm for cohort definition. We used all serum creatinine measurements obtained in clinical settings in all US Department of Veteran Affairs (VA) health care facilities between October 1, 2004 and September 30, 2006 (baseline period) from the national VA Corporate Data Warehouse LabChem data files.10 Overall, 4,447,691 veterans had at least 1 available serum creatinine measurement, representing ~94% of all veterans who received VA health care during this time period.11 The RCAV cohort included 3,582,478 patients with baseline eGFR ≥60 mL/min/1.73m2. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation.12 After exclusion of patients with missing International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) codes (n = 11,311) or with death (n = 126,405) or last encounter (n = 85,109) before October 1, 2006, 3,359,653 patients were included in our final cohort.
Data collection
Predictors and covariates
Constipation was defined as either having ≥2 prescriptions of laxatives of ≥30-day supply each, that were 60–365 days apart during the baseline period based on information obtained from VA Pharmacy dispensation records (Supplemental Table 1);13 or having at least 2 diagnoses for constipation, as identified by the ICD-9-CM(Supplemental Table 2), that were ≥60 days apart. Among the 3,359,653 patients, 237,855 (7.1%) were identified as having constipation. Laxative use was also quantitatively defined according to the number of different types of laxatives prescribed during the baseline period, and stratified into three groups as follows; none (neither laxative use nor diagnosis of constipation; n = 3,121,798, 92.9%), one type of laxative (n = 128,640, 3.8%), or ≥2 types of laxatives (n = 83,848, 2.5%). Patients with diagnosis of constipation but with no laxative use (n = 25,367, 0.8%) were excluded from analyses that examined the associations of laxative use with outcomes.
Sociodemographic characteristics, comorbid conditions, laboratory characteristics, and medication use were obtained as previously described.14–16 Briefly, data on patients’ age, sex, race, marital status (married, single, divorced or widowed), mean per capita income, service connectedness, body mass index, systolic and diastolic blood pressures, comorbid conditions, and medication use was obtained from various national VA research data files.17 Prevalent comorbidities were defined as the presence of relevant ICD-9-CMand Current Procedural Terminology codes recorded from October 1, 2004, to September 30, 2006 (Supplemental Table 2).14,15 Prevalent CHD was defined as the presence of diagnostic codes for coronary artery disease, angina, or myocardial infarction or procedure codes for percutaneous coronary interventions or coronary artery bypass grafting. Bowel disorders were defined as the presence of diagnostic codes for inflammatory bowel disease, irritable bowel syndrome, or diarrhea. We also included select socioeconomic indicators using 2004 county typology codes (housing stress, low education, low employment, and persistent poverty; Supplemental Table 3), in addition to the information derived from VA sources. Information about smoking status was not available in our cohort.
Outcomes
The co-primary outcomes of interest were all-cause mortality, incident CHD, and incident ischemic stroke. All-cause mortality was ascertained by the VA Vital Status Files, which contain dates of death until July 26, 2013 from all available sources in the VA system with sensitivity and specificity of 98.3% and 99.8%, respectively, as compared with the US National Death Index as gold standard.18 Incident CHD was defined as the composite of a first occurrence of an acute myocardial infarction, coronary artery bypass grafting, or percutaneous coronary intervention; incident ischemic stroke was defined as the first occurrence of an ischemic stroke after October 1, 2006 in patients without such diagnoses (i.e., prevalent cases) prior to this date (Supplemental Table 2).
Statistical analysis
Data are presented as number (percent) for categorical variables and mean±SD for continuous variables with a normal distribution or median (IQR) for those with a skewed distribution. The start of follow-up was October 1, 2006 to avoid immortal time bias, and patients were followed up until death or were censored at the date of the last VA encounter, or on July 26, 2013. The associations of constipation status and laxative use with outcomes were assessed with the Kaplan-Meier method and log-rank tests, and using Cox proportional hazards models. The proportionality assumption was tested by plotting log [-log (survival rate)] against log (survival time) and by scaled Schoenfeld residuals, which showed no violations. All associations were examined in unadjusted and multivariable adjusted models. Models were incrementally adjusted for the following confounders based on theoretical considerations: model 1, age-adjusted; model 2, age plus sex, race, and baseline eGFR; model 3, model 2 variables plus prevalent comorbidities (diabetes mellitus, hypertension, CHD, congestive heart failure, cerebrovascular disease, peripheral vascular disease, peptic ulcer disease, rheumatic disease, malignancy, dementia, Parkinson’s disease, depression, liver disease, chronic lung disease, HIV/AIDS, and bowel disorders) and Deyo-modified Charlson comorbidity index;19 model 4, model 3 variables plus baseline body mass index, systolic and diastolic blood pressure, and total cholesterol; and model 5, model 4 variables plus socioeconomic parameters (mean per capita income, marital status, service connectedness, housing stress, low education, low employment, and persistent poverty), indicators of sickness (number of VA healthcare encounters and cumulative length of hospitalization) and quality of care (receipt of influenza vaccination[s]), each patient’s VA healthcare region, and use of angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers, calcium channel blockers, diuretics, statins, antidepressants, non-opioid analgesics, opioids, antihistamines, anticholinergics, antiarrhythmics, anticoagulants, antipsychotics, anti-Parkinson drugs, antacids, anticonvulsants, alkylating agents, and oral iron supplements at baseline.
We additionally performed several sensitivity analyses. The associations of constipation status with all outcomes were examined in subgroups divided by baseline age, sex, race, prevalent diabetes mellitus, hypertension, CHD, congestive heart failure, opioid use with or without malignancy, eGFR, and income level. Analyses were repeated in a propensity score-matched cohort to account for potential differences arising from dissimilarities in clinical and demographic characteristics between patients with and without constipation. Propensity scores for the likelihood of presence versus absence of constipation were calculated by logistic regression using all variables included in multivariable models. We then matched patients with constipation to comparable patients without constipation using a 1:1 nearest-neighbor matching without replacement. As death and incident CHD/stroke can be competing events, competing risk regressions were also performed using fully adjusted models in the overall cohort, as well as in the propensity-matched cohort. The main analysis was repeated after excluding patients who had outcomes within 12 months after the start of follow-up to reduce the potential for reverse causation. We also repeated our main analysis using a more stringent definition of constipation (i.e., either having ≥80% of days covered by any types of laxatives during the baseline period; or having at least 2 diagnoses for constipation that were >60 days apart). Furthermore, to provide inferences about the severity of constipation, we regarded patients with diagnosis of constipation but with no laxative use (n = 25,367, 0.8%) as those having less severe constipation, and added them to the laxative use category. Subsequently, we repeated the main analysis using the new category of constipation (i.e., none, less severe constipation, one, or ≥2 types of laxatives). Of the variables included in multivariable adjusted models, data points were missing for race (9.4%), body mass index (4.0%), blood pressure (1.2%), total cholesterol (22.3%), per capita income (6.6%), marital status (4.3%), and socioeconomic indicators (4.0%). Of the 3,359,653 patients, 2,042,057 (60.8%) had complete data available for the fully adjusted multivariable model. Missing values were not imputed in primary analyses but were substituted by multiple imputation procedures using the STATA “mi” set of commands in sensitivity analyses.
Because of the large sample size, the significance of differences in the overall cohort was established based on considerations of biologically or clinically meaningful differences (even very small differences are rendered as “statistically significant” in such a situation). In the propensity-matched cohort, differences between variables were examined by calculating standardized differences, and values <0.1 were considered acceptable for the matching. All of the analyses were performed with Stata/MP version 14 (Stata Corporation, College Station, TX) and SAS version 9.4 (SAS Institute Inc., Cary, NC). The study was approved by the institutional review boards at the Memphis and Long Beach VA medical centers, with exemption from informed consent.
RESULTS
Baseline characteristics overall and in patients categorized by constipation status are shown in Table 1. The mean±SD age at baseline was 59.8±13.9 years; 93.2% were male; 15.5% were African American; and 23.7% were diabetic. The mean eGFR was 83.8±15.5 mL/min/1.73 m2. Compared to patients without constipation, those with constipation were older and more likely to be African American, had a higher prevalence of comorbidities except HIV/AIDS and a poorer socioeconomic status, and were less likely to be married. They also had more frequent healthcare encounters and longer cumulative length of hospitalization during the two-year baseline period. The use of medications and the administration of influenza vaccination(s) were more common in patients with constipation. Baseline characteristics were well balanced between those with and without constipation in the propensity-matched cohort (See Table 1 in [21]).
Table 1.
Baseline patient characteristics, overall and by constipation status
| Characteristics | Overall | Constipation | |
|---|---|---|---|
| No | Yes | ||
| (n = 3,359,653) | (n = 3,121,798) | (n = 237,855) | |
| Mean age (SD), y | 59.8 (13.9) | 59.5 (14.0) | 63.7 (13.0) |
| Mean eGFR (SD), mL/min/1.73 m2 | 83.8 (15.5) | 83.8 (15.5) | 82.8 (15.8) |
| Male, n (%) | 3,131,534 (93.2) | 2,910,065 (93.2) | 221,469 (93.1) |
| African American, n (%) | 519,841 (15.5) | 472,065 (15.1) | 47,776 (20.1) |
| Hypertension, n (%) | 2,004,010 (59.7) | 1,834,032 (58.8) | 169,978 (71.5) |
| Diabetes mellitus, n (%) | 796,883 (23.7) | 720,066 (23.1) | 76,817 (32.3) |
| CHD, n (%) | 380,854 (11.3) | 337,918 (10.8) | 42,936 (18.1) |
| CHF, n (%) | 134,426 (4.0) | 111,351 (3.6) | 23,075 (9.7) |
| CVD, n (%) | 198,031 (6.0) | 169,129 (5.4) | 28,902 (12.2) |
| PAD, n (%) | 179,007 (5.3) | 153,564 (4.9) | 25,443 (10.7) |
| Chronic lung disease, n (%) | 595,482 (17.7) | 523,917 (16.8) | 71,565 (30.1) |
| Dementia, n (%) | 24,603 (0.7) | 19,457 (0.6) | 5,146 (2.2) |
| Parkinson’s disease, n (%) | 24,913 (0.7) | 19,983 (0.6) | 4,930 (2.1) |
| Rheumatologic disease, n (%) | 46,671 (1.4) | 41,168 (1.3) | 5,503 (2.3) |
| Peptic ulcer disease, n (%) | 61,653 (1.8) | 52,703 (1.7) | 8,950 (3.8) |
| Liver disease, n (%) | 11,540 (0.3) | 7,877 (0.3) | 3,663 (1.5) |
| Malignancies, n (%) | 315,383 (9.4) | 275,038 (8.8) | 40,345 (17.0) |
| HIV/AIDS, n (%) | 20,154 (0.6) | 18,673 (0.6) | 1,481 (0.6) |
| Depression, n (%) | 738,164 (22.0) | 643,100 (20.6) | 95,064 (40.0) |
| Bowel disorders, n (%) | 139,152 (4.1) | 117,676 (3.8) | 21,476 (9.0) |
| Charlson comorbidity index score (IQR) | 1 (0, 1) | 0 (0, 1) | 1 (0, 3) |
| Median per capita income (IQR), $ | 23,075 (11,791–36,135) | 23,352 (11,916–37,177) | 19,376 (10,764–30,750) |
| Married, n (%) | 1,809,593 (53.9) | 1,695,127 (54.3) | 114,466 (48.1) |
| Service connected, n (%) | 1,397,265 (41.6) | 1,278,296 (41.0) | 118,969 (50.0) |
| Mean BMI (SD), kg/m2 | 29.2 (5.7) | 29.2 (5.6) | 29.4 (6.2) |
| Mean systolic BP (SD), mmHg | 135.5 (19.0) | 135.6 (19.0) | 134.2 (19.7) |
| Mean diastolic BP (SD), mmHg | 77.2 (11.7) | 77.4 (11.7) | 75.4 (11.9) |
| Mean total cholesterol (SD), mg/dL | 183.7 (41.6) | 184.2 (41.5) | 177.0 (41.7) |
| ACEI/ARB use, n (%) | 744,989 (22.2) | 678,435 (21.7) | 66,554 (28.0) |
| Calcium channel blockers, n (%) | 369,634 (11.0) | 333,625 (10.7) | 36,009 (15.1) |
| Diuretics, n (%) | 499,829 (14.9) | 447,565 (14.3) | 52,264 (22.0) |
| Statin use, n (%) | 490,304 (14.6) | 444,841 (14.3) | 45,463 (19.1) |
| Antidepressants use, n (%) | 597,772 (17.8) | 518,454 (16.6) | 79,318 (33.4) |
| Non-opioid analgesics use, n (%) | 799,129 (23.8) | 702,013 (22.5) | 97,116 (40.8) |
| Opioids use, n (%) | 367,880 (11.0) | 302,346 (9.7) | 65,534 (27.6) |
| Antihistamines, n (%) | 19,549 (0.6) | 15,519 (0.5) | 4,030 (1.7) |
| Anticholinergics, n (%) | 57,888 (1.7) | 47,761 (1.5) | 10,127 (4.3) |
| Antiarrhythmics, n (%) | 16,952 (0.5) | 14,717 (0.5) | 2,235 (0.9) |
| Anticoagulants, n (%) | 113,577 (3.4) | 96,765 (3.1) | 16,812 (7.1) |
| Antipsychotics, n (%) | 153,649 (4.6) | 127,946 (4.1) | 25,703 (10.8) |
| Anti-Parkinson drugs, n (%) | 24,913 (0.7) | 19,983 (0.6) | 4,930 (2.1) |
| Antacids, n (%) | 25,596 (0.8) | 17,267 (0.6) | 8,329 (3.5) |
| Anticonvulsants, n (%) | 245,602 (7.3) | 203,578 (6.5) | 42,024 (17.7) |
| Alkylating agents, n (%) | 744 (0.02) | 569 (0.02) | 175 (0.07) |
| Oral iron supplements, n (%) | 29,785 (0.9) | 21,552 (0.7) | 8,233 (3.5) |
| Influenza vaccination, n (%) | 996,284 (29.7) | 890,280 (28.5) | 106,004 (44.6) |
| Living in area with high housing stress, n (%) | 1,130,132 (33.6) | 1,043,922 (33.4) | 86,210 (36.2) |
| Living in area with low education, n (%) | 348,170 (10.4) | 319,617 (10.2) | 28,553 (12.0) |
| Living in area with low employment, n (%) | 304,968 (9.1) | 280,614 (9.0) | 24,354 (10.2) |
| Living in area of persistent poverty, n (%) | 159,609 (4.8) | 146,292 (4.7) | 13,317 (5.6) |
| Healthcare encounters, n (IQR) | 20 (10, 39) | 19 (10, 35) | 51 (28, 85) |
| Cumulative length of hospitalization (IQR), days | 0 (0, 0) | 0 (0, 0) | 0 (0, 5) |
Data are presented as number (percentage), mean (standard deviation), or median (interquartile range). All p-values except HIV/AIDS (p=0.14) for comparing differences between the absence and presence of constipation were statistically significant at p<0.05. ACEI = angiotensin-converting enzyme inhibitors; AIDS = acquired immunodeficiency syndrome; ARB = angiotensin receptor blockers; BMI = body mass index; BP = blood pressure; CHD = coronary heart disease; CHF = congestive heart failure; CVD = cerebrovascular disease; DM = diabetes mellitus; eGFR = estimated glomerular filtration rate; HIV = human immunodeficiency virus; PAD = peripheral arterial disease.
Mortality
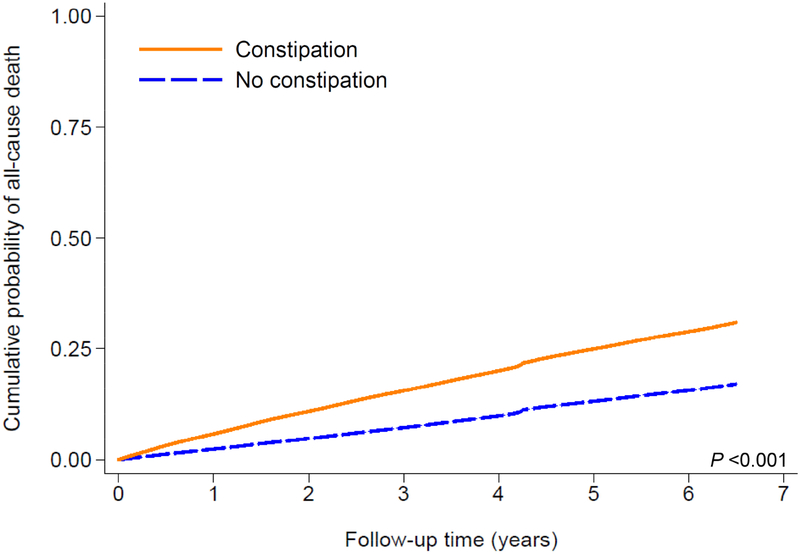
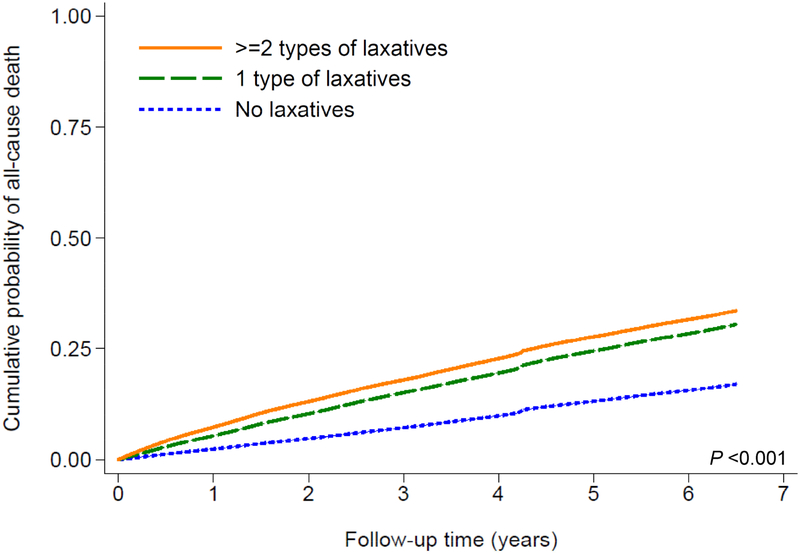
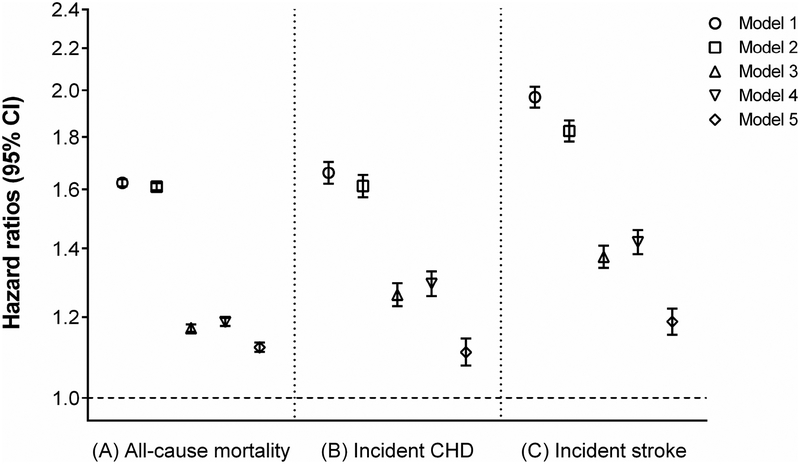
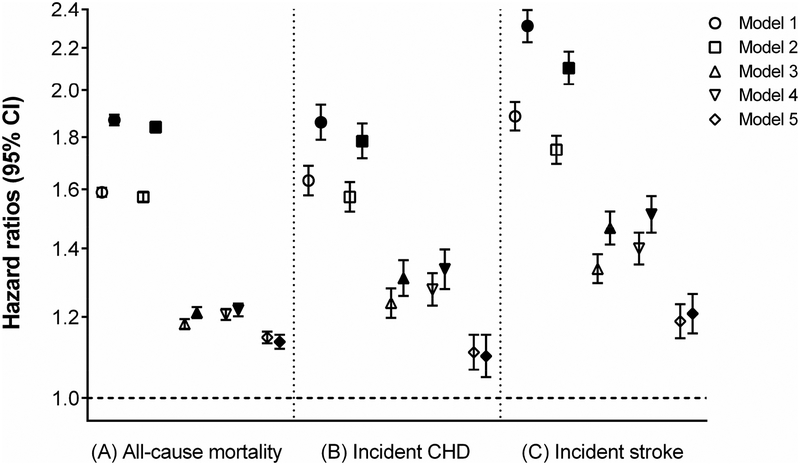
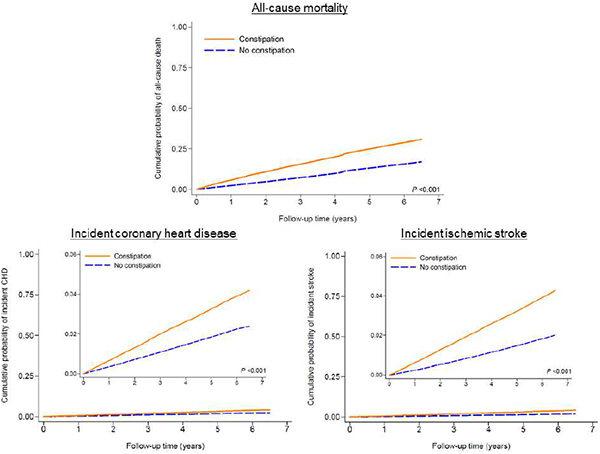
A total of 597,780 patients died overall (crude rate, 30.4 per 1000 patient-years; 95% confidence interval [CI], 30.4–30.6) during a median follow-up of 6.7 years. The follow-up duration was similar in constipated and non-constipated patients. As shown in Fig. 1A, compared to patients without constipation, those with constipation had a higher cumulative incidence of all-cause mortality (log-rank p<0.001). Fig. 2A shows multivariable adjusted mortality rates associated with constipation status. In the age-adjusted model, patients with constipation had 1.62 (95% CI, 1.61–1.64) times higher mortality than those without constipation. Further adjustment for additional covariates substantially attenuated the risk of mortality, but the association remained statistically significant (adjusted HR, 1.12; 95% CI, 1.11–1.13, model 5, Fig. 2A). Compared to patients without laxative use, those with more extensive laxative use showed incrementally higher crude cumulative incidence of death (log-rank p<0.001; Fig. 1B), but the dose-dependency was no longer evident after multivariable adjustment (adjusted HRs [95% CI], 1.15 [1.13–1.16] and 1.14 [1.12–1.15] for one and >2 types of laxatives, respectively, model 5, Fig. 3A).
Figure 1.
Kaplan-Meier cumulative-event curves for all-cause mortality according to (A) constipation status and (B) laxative use in the overall cohort
The lines represent (A) patients without (dashed line) and with (solid line) constipation, and (B) those with no laxatives (dotted line) and with one (dashed line) or ≥2 (solid line) types of laxatives. Both log-rank p values <0.001.
Figure 2.
Hazard ratios and 95% confidence intervals of (A) all-cause mortality, (B) incident CHD, and (C) incident stroke associated with the presence (vs. absence) of constipation in the overall cohort.
Models represent hazard ratios after adjustment for age (model 1); age plus gender, race, and baseline eGFR (model 2); model 2 variables plus comorbidities (diabetes mellitus, hypertension, coronary heart disease, congestive heart failure, cerebrovascular disease, peripheral arterial disease, peptic ulcer disease, rheumatic disease, malignancy, dementia, Parkinson’s disease, depression, liver disease, chronic lung disease, human immunodeficiency virus/acquired immunodeficiency syndrome, and bowel disorders) and Charlson comorbidity index (model 3); model 3 plus baseline body mass index, systolic blood pressure, diastolic blood pressure, and total cholesterol (model 4); model 4 plus socioeconomic parameters (mean per capita income, marital status, service connectedness, housing stress, low education, low employment, persistent poverty), number of VA healthcare encounters, cumulative length of hospitalization, receipt of influenza vaccination(s), each patient’s VA healthcare region, and use of angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, calcium channel blockers, diuretics, statins, antidepressants, non-opioid analgesics, opioids, antihistamines, anticholinergics, antiarrhythmics, anticoagulants, antipsychotics, anti-Parkinson drugs, antacids, anticonvulsants, alkylating agents, and oral iron supplements (model 5). CHD = coronary heart disease; CI = confidence interval; eGFR = estimated glomerular filtration rate; VA = veterans affairs.
Figure 3.
Hazard ratios and 95% confidence intervals of (A) all-cause mortality, (B) incident CHD, and (C) incident stroke associated with laxative use* in the overall cohort.
*Patients with one (blank symbols) or ≥2 (filled symbols) types of laxatives compared with those with no laxatives (reference). Models represent hazard ratios after adjustment for age (model 1); age plus gender, race, and baseline eGFR (model 2); model 2 variables plus comorbidities (diabetes mellitus, hypertension, coronary heart disease, congestive heart failure, cerebrovascular disease, peripheral arterial disease, peptic ulcer disease, rheumatic disease, malignancy, dementia, Parkinson’s disease, depression, liver disease, chronic lung disease, human immunodeficiency virus/acquired immunodeficiency syndrome, and bowel disorders) and Charlson comorbidity index (model 3); model 3 plus baseline body mass index, systolic blood pressure, diastolic blood pressure, and total cholesterol (model 4); model 4 plus socioeconomic parameters (mean per capita income, marital status, service connectedness, housing stress, low education, low employment, persistent poverty), number of VA healthcare encounters, cumulative length of hospitalization, receipt of influenza vaccination(s), each patient’s VA healthcare region, and use of angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, calcium channel blockers, diuretics, statins, antidepressants, non-opioid analgesics, opioids, antihistamines, anticholinergics, antiarrhythmics, anticoagulants, antipsychotics, anti-Parkinson drugs, antacids, anticonvulsants, alkylating agents, and oral iron supplements (model 5). CHD = coronary heart disease; CI = confidence interval; eGFR = estimated glomerular filtration rate; VA = veterans affairs.
Incident CHD
There were a total of 68,076 patients who experienced an incident CHD (crude rate, 3.93 per 1000 patient-years; 95% CI, 3.90–3.96). The crude cumulative incidence of CHD was higher in patients with constipation than those without (log-rank p<0.001; Supplemental Fig. 2A). Although this association was attenuated after multivariable adjustment, the risk of incident CHD remained higher in patients with constipation (adjusted HR, 1.11; 95% CI, 1.08–1.14; model 5, Fig. 2B). Along with increasing number of different types of laxatives, a higher crude cumulative incidence of CHD was observed (log-rank p<0.001; Supplemental Fig. 2B); however, this dose-dependency was no longer evident after multivariable adjustment (adjusted HRs [95% CI], 1.11 [1.07–1.15] and 1.10 [1.05–1.15] for one and ≥2 [versus none] types of laxatives, respectively, model 5, Fig. 3B).
Incident ischemic stroke
In total, 63,371 patients experienced an incident ischemic stroke (crude rate, 3.44 per 1000 patient-years; 95% CI, 3.42–3.47). Compared to patients without constipation, those with constipation had a higher crude cumulative incidence of ischemic stroke (log-rank p<0.001; Supplemental Fig. 3A). The risk of incident ischemic stroke remained higher in patients with constipation even after multivariable adjustment (adjusted HR, 1.19; 95% CI, 1.15–1.22, model 5, Fig. 2C). The dose-response relationship was observed between laxative use and crude cumulative incidence of ischemic stroke (log-rank p<0.001; Supplemental Fig. 3B), but the risk of ischemic stroke associated with one or ≥2 types of laxatives was almost identical after multivariable adjustment (adjusted HRs [95% CI], 1.19 [1.14–1.23] and 1.21 [1.16–1.26] for one and >2 [versus none] types of laxatives, respectively, model 5, Fig. 3C).
Sensitivity analyses
Findings were similarly observed in selected subgroups (Supplemental Fig. 4) and were robust to various sensitivity analyses accounting for confounding by indication, competing risk, and missing data, excluding events occurred within the first 12 months, and using a more stringent constipation definition (See Table 2 and Fig. 1–4 in[20] and Supplemental Tables 4–7). When using additional category of constipation severity, a risk gradient was observed for incident ischemic stroke (Supplemental Table 8).
DISCUSSION
In this large cohort of US veterans with baseline eGFR ≥60 mL/min/1.73m2, we examined the association of constipation status and laxative use with all-cause mortality, incident CHD, and incident ischemic stroke. We found that patients with constipation had a 12% higher risk of all-cause mortality, and also 11% and 19% higher risk of developing CHD and ischemic stroke, respectively, compared to those without constipation, after adjusting for potential confounders. Furthermore, we found a similarly higher risk of those events in patients with one and ≥2 types of laxatives. Findings were robust to various sensitivity analyses.
These results are similar to some aspects of a few previously published studies.5–7 In a community-based prospective cohort of 93,676 postmenopausal women, Salmoirago et al.5 reported that women with moderate and severe constipation, defined by self-administered questionnaire, were at higher risk of cardiovascular mortality compared to those without constipation. Similarly, in a Japanese population-based prospective cohort of 45,112 participants, Honkura et al.7 demonstrated that constipation, defined as a decreased defecation frequency evaluated using a self-administered questionnaire, was significantly associated with increased risk of cardiovascular mortality, particularly with the risk of ischemic stroke mortality. On the other hand, one recent study by Ma et al.8 reported no association of constipation, defined as stool frequency of up to every 5 days, with the risk of mortality and cardiovascular events; and hence it remains controversial whether constipation is associated with higher risk of such events. We therefore extended these analyses to a very large cohort of >3 million nationwide US veterans, and for the first time demonstrated the independent association of constipation with incident CHD and ischemic stroke, as well as with all-cause mortality.
Although our observational study cannot conclude a causal relationship, there are several plausible explanations for a mechanistic association between constipation and the risk of incident cardiovascular events. In recent years, there has been a growing interest in the mutual interactions between the intestinal environment and host nutrition, metabolism, physiology, and immune function,21 and emerging evidence has revealed that dysbiosis (abnormal changes in gut microbiota composition) contributes to the pathogenesis of diverse illnesses, such as the metabolic syndrome22 and cardiovascular disease,23 through the processes mediated by altered gut microbiota.24–27 A greater contribution of constipation to the risk of incident CHD and stroke among patients with eGFR <90 (vs. ≥90) mL/min/1.73m2 in our subgroup analysis might partly reflect a pathophysiological interaction of altered gut microbiota accompanied by loss of kidney function.28 Since gastrointestinal motility and environment are closely interrelated and exert reciprocal effects on each other29,30, it seems plausible that constipation, one of the clinical forms of dysbiosis,31,32 can be partially involved in the pathogenesis of atherosclerosis through chronic inflammation partly due to bacterial endotoxins (e.g., lipopolysaccharide) and/or altered gut metabolites (e.g., short chain fatty acids,26 trimethylamine-N-oxide,25 etc.), and consequently contribute to the higher incidence of CHD and ischemic stroke and all-cause mortality. Recently, elevated blood levels of serotonin, a vasoconstrictor which also promotes thrombus formation, have been implicated in the development of atherosclerotic plaques33 and associated with increased risk of atherosclerotic cardiovascular disease.34 Given the fact that serotonin synthesis and release have been increased in individuals with constipation35 and in those using certain laxatives,36 increased serotonin levels could also serve as a potential explanation for the observed association. As another potential underlying mechanism, constipation could be considered as a surrogate for autonomic dysfunction, which has been associated with various known cardiovascular risk factors such as diabetes,37 hypertension,38,39 and depression;40 and indeed, patients with constipation had a higher prevalence of such diseases in our study. Although constipation might merely serve as a systemic indicator of poor health and may thereby contribute to the development of cardiovascular disease, the association of constipation with all outcomes remained statistically significant even after adjusting for several known risk factors, and also after stratification by such factors. Contrary to our expectation, we observed almost identical risk for all outcomes between patients with one and ≥2 types of laxatives after multivariable adjustment. This seemingly counterintuitive observation might be partly explained by the underlying uniform effect of constipation, assuming that the symptoms in patients with more severe constipation who use more laxatives may be treated down to the same level as someone with less severe constipation who uses fewer laxatives. Lastly, albeit still speculative, dehydration due to the use of certain types of laxatives, and/or repeated Valsalva-like breath-holdings as a result of straining at defecation, a well-recognized cause of “defecation syncope”,41 may induce cardiac and cerebral ischemia, and could potentially explain the higher incidence of cardiovascular events. In this context, it seems important to acknowledge risk gradient for incident ischemic stroke among participants with diagnosis of constipation and with vs. without laxative use when using additional category of constipation severity.
Our study results must be interpreted in light of several limitations. Our cohort consisted of predominantly male US veterans, and only 6.8% of the main cohort were women; hence, the results may not apply to women or patients from other geographical areas. Although constipation was defined by unconventional criteria using the ICD-9-CM codes and laxative prescription records during the 2-year baseline period under the (unproven) assumption that the definition could capture those who had relatively chronic symptoms of constipation rather than transient symptoms, we were unable to assess the impact of lifetime duration of constipation and its overall severity on the outcomes. A majority of patients in this cohort were in an outpatient setting; however, it would be difficult to deny the possibility of detection bias because constipation in outpatients may be less likely to be detected in comparison with inpatients. Data on cause-specific mortality were not available in this cohort. Information of actual stool pattern or subjective symptoms of constipation was not available; therefore, patients who indeed had constipation but had no diagnostic codes or laxative prescription records from VA Pharmacy might have been misclassified as absent constipation or having less severe constipation. Indeed, the overall prevalence of constipation in this study was lower than that reported in the general population, suggesting that the current constipation definition may have captured more severe and symptomatic constipation, and hence the results may not apply to those identified by the more common constipation definition of decreased bowel movement frequency or having strain at stool. Nevertheless, such misclassification would tend to bias the true effects toward the null. Several statistical methods were applied to address the effect of confounders, but we cannot eliminate the possibility of unmeasured confounders such as smoking history, functional mobility, nutritional assessment data, and dietary habits.
In conclusion, in this large nationwide cohort of >3 million US veterans, we found that constipation status and laxative use were associated with a higher risk of all-cause mortality, incident CHD, and incident ischemic stroke, independently of known cardiovascular risk factors. Our results raise questions about potential pathophysiologic contribution of constipation to the pathogenesis of atherosclerotic cardiovascular diseases and, given the simplicity of its assessment in everyday clinical practice, may underscore the need for careful observation of cardiovascular complications among patients with constipation. Further studies are warranted to elucidate the underlying mechanisms of the observed association and to determine if treatment of constipation through various interventions (e.g., exercise, high-fiber diet and/or use of probiotics vs. laxatives) can lower the risk of mortality and adverse cardiovascular outcomes.
Supplementary Material
Highlights:
Constipation and laxative use associate with higher risk of mortality
They also associate with higher incidence of coronary heart disease and stroke
Findings are robust to various sensitivity analyses
Acknowledgements:
Support for VA/CMS data is provided by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Health Services Research and Development, VA Information Resource Center (project numbers SDR 02–237 and 98–004). The sponsors had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Financial support:
This study was supported by grant R01DK096920 to Drs. Kovesdy and Kalantar-Zadeh and is the result of work supported with resources and the use of facilities at the Memphis VA Medical Center and the Long Beach VA Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest:
Drs. Kovesdy and Kalantar-Zadeh are employees of the US Department of Veterans affairs. Opinions expressed in this paper are those of the authors’ and do not necessarily represent the opinion of the Department of Veterans Affairs. All authors had access to the data and a role in writing the manuscript.
None of the authors have relevant conflicts of interest.
REFERENCES
- 1.Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology 2013; 144(1):218–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennison C, Prasad M, Lloyd A, Bhattacharyya SK, Dhawan R, Coyne K. The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005;23(5):461–476. [DOI] [PubMed] [Google Scholar]
- 3.Sun SX, Dibonaventura M, Purayidathil FW, Wagner JS, Dabbous O, Mody R. Impact of chronic constipation on health-related quality of life, work productivity, and healthcare resource use: an analysis of the National Health and Wellness Survey. Dig. Dis. Sci 2011;56(9):2688–2695. [DOI] [PubMed] [Google Scholar]
- 4.Guerin A, Carson RT, Lewis B, Yin D, Kaminsky M, Wu E. The economic burden of treatment failure amongst patients with irritable bowel syndrome with constipation or chronic constipation: a retrospective analysis of a Medicaid population. J. Med. Econ 2014;17(8): 577–586. [DOI] [PubMed] [Google Scholar]
- 5.Salmoirago-Blotcher E, Crawford S, Jackson E, Ockene J, Ockene I. Constipation and risk of cardiovascular disease among postmenopausal women. Am. J. Med 2011;124(8):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kubota Y, Iso H, Tamakoshi A. Bowel Movement Frequency, Laxative Use, and Mortality From Coronary Heart Disease and Stroke Among Japanese Men and Women: The Japan Collaborative Cohort (JACC) Study. J. Epidemiol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honkura K, Tomata Y, Sugiyama K, et al. Defecation frequency and cardiovascular disease mortality in Japan: The Ohsaki cohort study. Atherosclerosis 2016;246:251–256. [DOI] [PubMed] [Google Scholar]
- 8.Ma W, Li Y, Heianza Y, et al. Associations of Bowel Movement Frequency with Risk of Cardiovascular Disease and Mortality among US Women. Sci. Rep 2016;6:33005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovesdy CP, Norris KC, Boulware LE, et al. Association of Race With Mortality and Cardiovascular Events in a Large Cohort of US Veterans. Circulation 2015;132(16):1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension 2014;64(5):951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.US Department of Veterans Affairs. Veteran population http://www.va.gov/vetdata/Veteran_Population.asp. Accessed December 15, 2014.
- 12.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.VA Information Resource Center. VIReC Research User Guide: VHA Pharmacy Prescription Data, 2nd ed Hines, IL: US Department of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2008. [Google Scholar]
- 14.Kovesdy CP, Bleyer AJ, Molnar MZ, et al. Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann. Intern. Med 2013;159(4):233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovesdy CP, Lu JL, Molnar MZ, et al. Observational modeling of strict vs conventional blood pressure control in patients with chronic kidney disease. JAMA Intern Med 2014;174(9):1442–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumida K, Molnar MZ, Potukuchi PK, et al. Constipation and Incident CKD. J. Am. Soc. Nephrol 2017;28(4):1248–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Department of Veterans Affairs. VA Information Resource Center http://www.virec.research.va.gov/Resources/Info-About-VA-Data.asp. Accessed December 15. 2014.
- 18.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 20.Sumida K, Molnar MZ, Potukuchi PK, et al. Impact of Constipation on Death and Cardiovascular Events: Data from Propensity-Matched Analysis. Data in Brief 2018. [Google Scholar]
- 21.Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu. Rev. Med 2011;62:361–380. [DOI] [PubMed] [Google Scholar]
- 22.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006;444(7122):1022–1023. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011;472(7341):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang WH, Hazen SL. The contributory role of gut microbiota in cardiovascular disease. J. Clin. Invest 2014;124(10):4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med 2013;368(17):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Org E, Mehrabian M, Lusis AJ. Unraveling the environmental and genetic interactions in atherosclerosis: Central role of the gut microbiota. Atherosclerosis 2015;241(2):387–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drosos I, Tavridou A, Kolios G. New aspects on the metabolic role of intestinal microbiota in the development of atherosclerosis. Metabolism 2015;64(4):476–481. [DOI] [PubMed] [Google Scholar]
- 28.Ramezani A, Raj DS. The Gut Microbiome, Kidney Disease, and Targeted Interventions. J. Am. Soc. Nephrol 2014;25(4):657–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attaluri A, Jackson M, Valestin J, Rao SS. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am. J. Gastroenterol 2010;105(6):1407–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quigley EM. The enteric microbiota in the pathogenesis and management of constipation. Best Pract. Res. Clin. Gastroenterol 2011;25(1):119–126. [DOI] [PubMed] [Google Scholar]
- 31.Parthasarathy G, Chen J, Chen X, et al. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016;150(2):367–379e361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tap J, Derrien M, Tornblom H, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology 2017; 152(1): 111–123e118. [DOI] [PubMed] [Google Scholar]
- 33.Hara K, Hirowatari Y, Yoshika M, Komiyama Y, Tsuka Y, Takahashi H. The ratio of plasma to whole-blood serotonin may be a novel marker of atherosclerotic cardiovascular disease. J. Lab. Clin. Med 2004;144(1):31–37. [DOI] [PubMed] [Google Scholar]
- 34.Vikenes K, Farstad M, Nordrehaug JE. Serotonin is associated with coronary artery disease and cardiac events. Circulation 1999;100(5):483–489. [DOI] [PubMed] [Google Scholar]
- 35.Costedio MM, Coates MD, Brooks EM, et al. Mucosal serotonin signaling is altered in chronic constipation but not in opiate-induced constipation. Am. J. Gastroenterol 2010; 105(5): 1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capasso F, Mascolo N, Autore G, Romano V. Laxatives and the production of autacoids by rat colon. J. Pharm. Pharmacol 1986;38(8):627–629. [DOI] [PubMed] [Google Scholar]
- 37.Vinik AI, Erbas T. Diabetic autonomic neuropathy. Handb. Clin. Neurol 2013;117:279–294. [DOI] [PubMed] [Google Scholar]
- 38.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999;34(4 Pt 2):724–728. [DOI] [PubMed] [Google Scholar]
- 39.Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ. Res 2015;116(6):976–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kop WJ, Stein PK, Tracy RP, Barzilay JI, Schulz R, Gottdiener JS. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom. Med 2010;72(7):626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pstras L, Thomaseth K, Waniewski J, Balzani I, Bellavere F. The Valsalva manoeuvre: physiology and clinical examples. Acta Physiol (Oxf) 2016;217(2):103–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.