Abstract
Metal-catalyzed enantioconvergent cross-coupling reactions of alkyl electrophiles are emerging as a powerful tool in asymmetric synthesis. To date, high enantioselectivity has been limited to couplings of electrophiles that bear a directing group or a proximal p/π orbital. In this report, we demonstrate for the first time that enantioconvergent cross-couplings can be achieved with electrophiles that lack such features; specifically, we establish that a chiral nickel catalyst can accomplish Negishi reactions of racemic α-halosilanes with alkylzinc reagents with good enantioselectivity under simple and mild conditions, thereby providing access to enantioenriched organosilanes, an important class of target molecules.
Keywords: alkylation, asymmetric catalysis, cross-coupling, nickel, silicon
Graphical Abstract

For the first time, enantioconvergent cross-couplings can be achieved with electrophiles that lack a directing group or a proximal p/π orbital. Specifically, a chiral nickel catalyst can accomplish Negishi reactions of racemic α-halosilanes with alkylzinc reagents with good enantioselectivity under simple and mild conditions, thereby providing access to enantioenriched organosilanes, an important class of target molecules.
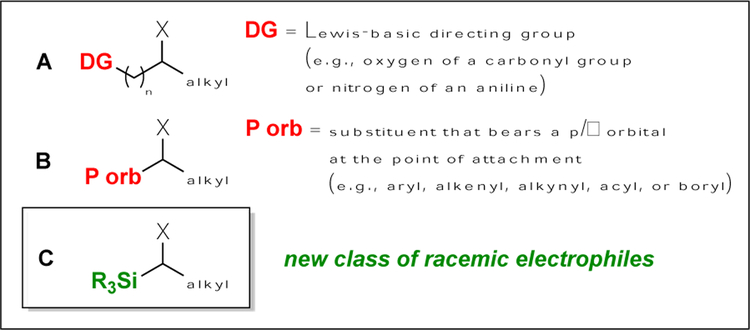
Significant progress has been described in the development of methods for the synthesis of carbon–carbon bonds through enantioconvergent substitution reactions of racemic alkyl electrophiles with carbon nucleophiles.[1–3] To date, high enantioselectivity has only been observed in cross-couplings wherein the electrophile bears either a directing group (A) or a p/π orbital proximal to the leaving group (B) (Figure 1).[4]
Figure 1.
Background: Racemic electrophiles used in enantioconvergent cross-couplings. A and B: Prior work. C: This study.
We have been interested in expanding the scope of enantioconvergent cross-couplings to include electrophiles that lack either of the features illustrated in A and B (Figure 1). An example of such an electrophile is α-halosilane C,[5,6] enantioconvergent cross-coupling of which would provide chiral organosilanes. Chiral organosilanes (e.g., 1[7] and 2[8]) are of interest in fields such as medicinal chemistry, since replacement of carbon with silicon can lead to improved pharmacological properties (e.g., enhanced lipophilicity and potency) without element-specific toxicity due to the presence of silicon;[9] to date, there are limited methods for the direct catalytic asymmetric synthesis of such organosilanes.[10,11] In this report, we establish that a chiral nickel catalyst can achieve the asymmetric synthesis of organosilanes via the cross-coupling of racemic α-halosilanes with alkylzinc reagents under simple and mild conditions [Eq. (1)], thereby demonstrating that enantioconvergent cross-couplings are possible with electrophiles that lack both a directing group and a proximal p/π orbital (Figure 1).

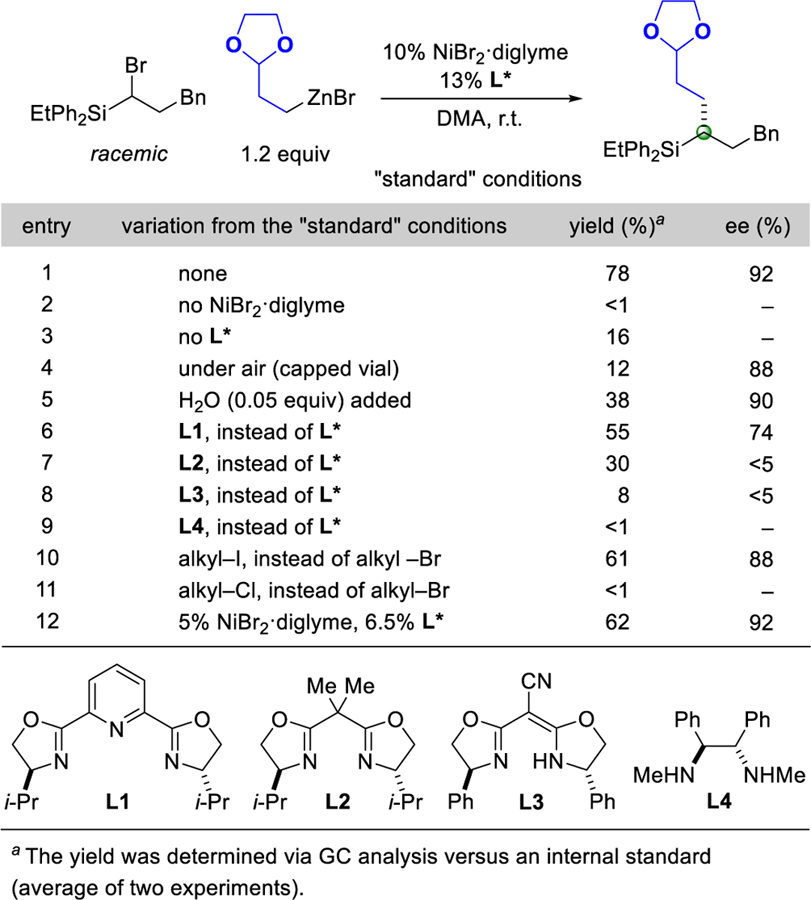
Upon examining a range of reaction parameters, we determined that NiBr2·diglyme and a chiral pybox ligand (L*) can accomplish the enantioconvergent Negishi cross-coupling illustrated in Table 1 in good yield and high ee (78% yield, 92% ee; entry 1). In the absence of NiBr2·diglyme, virtually no carbon–carbon bond formation is observed (entry 2), whereas in the absence of ligand L*, the coupling proceeds in low yield (entry 3). When the reaction is run under an atmosphere of air or in the presence of water, formation of product is inefficient, although the ee is good (entries 4 and 5). Other ligands, including representative examples of classes of ligands that have been useful in other nickel-catalyzed enantioconvergent cross-couplings,1 are less effective than ligand L* (entries 6–9). Although the corresponding alkyl iodide cross-couples with fairly good yield and ee under these conditions (entry 10), use of the alkyl chloride leads to essentially no carbon–carbon bond formation (entry 11). A lower catalyst loading can be employed with only a small loss in yield and no loss in enantioselectivity (entry 12).
Table 1.
Effect of Reaction Parameters.
 |
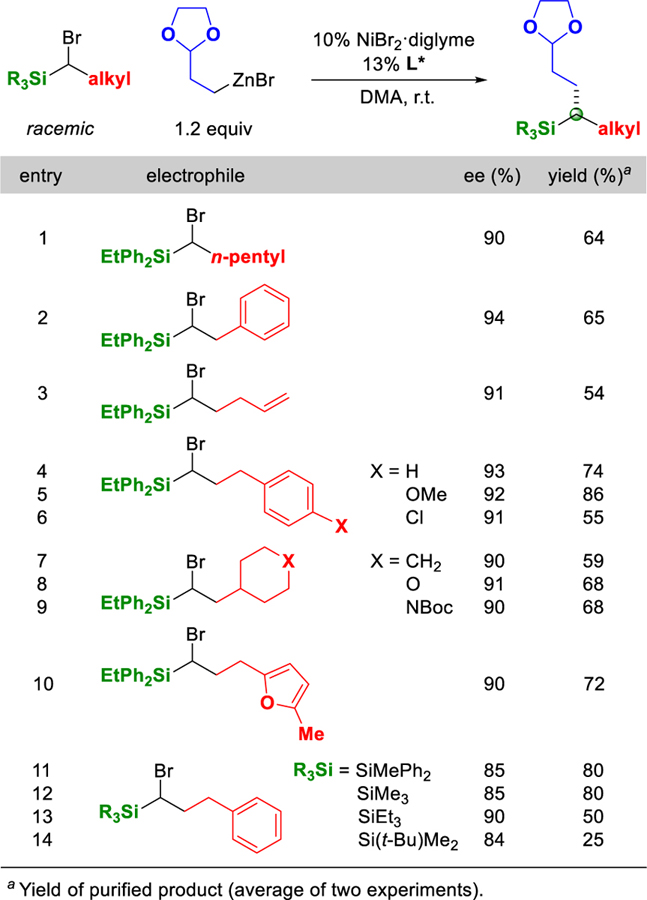
α-Bromosilanes that bear a variety of functional groups serve as suitable electrophiles in these nickel-catalyzed enantioconvergent cross-couplings (Table 2). Thus, an alkene, ether, or an aryl chloride, as well as a saturated or an unsaturated oxygen or nitrogen heterocycle, can be present, with little impact on ee. Although branching in the α position of the alkyl group of the electrophile inhibits cross-coupling, branching in the β position is tolerated (entries 7–9). The enantioconvergent coupling proceeds with an array of substituents on silicon, with lower yields observed as the steric demand of the electrophile increases (entries 11–14).[12]
Table 2.
Scope with Respect to the Electrophile.
 |
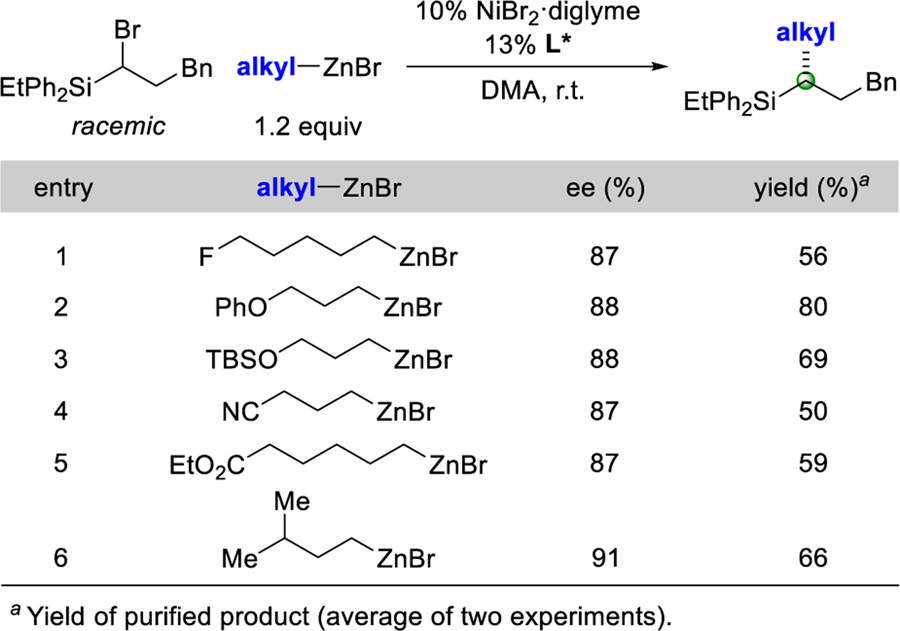
Organozinc reagents that include various functional groups, such as an alkyl fluoride, an ether, a nitrile, and an ester, can be employed as nucleophiles in these nickel-catalyzed enantioconvergent cross-couplings (entries 1–5 of Table 3). The cross-coupling is sensitive to steric effects–while branching at the γ position is tolerated (entry 6), little carbon–carbon bond formation occurs if there is branching at the α or the β position. On a gram scale, the coupling illustrated in entry 2 proceeds in 88% ee and 89% yield (1.66 g of product).[13]
Table 3.
Scope with Respect to the Nucleophile.
 |
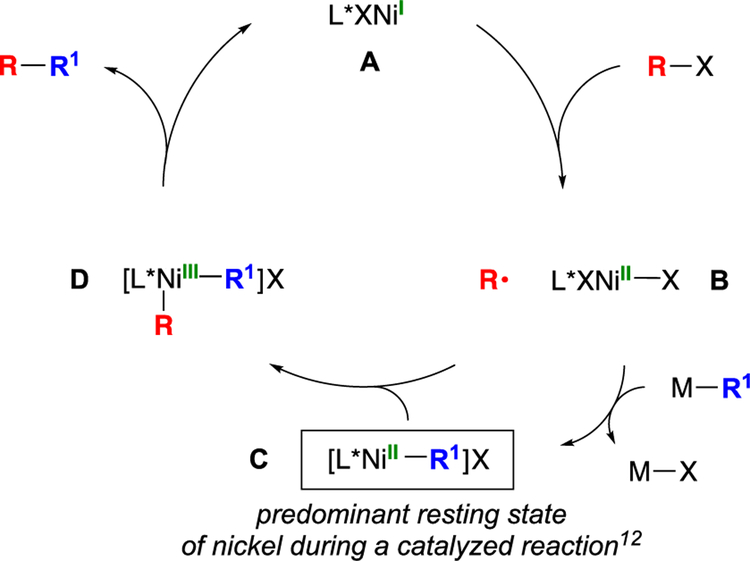
Our working hypothesis is that this process may be following a pathway analogous to that elucidated for nickel/pybox-catalyzed enantioconvergent Negishi arylations of propargylic halides, wherein nickel complex C is the predominant resting state of nickel during catalysis, and complexes A, B, and D do not accumulate (Figure 2).[14] Consistent with this suggestion, ESI–MS analysis of a cross-coupling (the model reaction in Table 1) at partial conversion reveals a strong signal at m/z = 488.2, consistent with the presence of [L*Ni–R1]+ (C in Figure 2; R1 = 2-(1,3-dioxolan-2-yl)ethyl; exact mass: 488.2). Similarly, the EPR spectrum of a reaction at partial conversion indicates that odd-electron nickel intermediates such as A or D do not accumulate to a significant (>2%) extent.
Figure 2.
Outline of a possible mechanism.
An enantioenriched α-bromosilane does not racemize under the standard conditions [Eq. (2)],[15,16] indicating that C–Br bond cleavage is irreversible and that the chiral catalyst is processing both enantiomers of the electrophile in the stereoconvergent coupling of a racemic electrophile (no dynamic kinetic resolution). When an enantioconvergent cross-coupling of a racemic electrophile is stopped at partial conversion, the unreacted electrophile is still racemic, indicating that the chiral catalyst is not discriminating between the enantiomeric electrophiles (no kinetic resolution).[17]

Thus, we have expanded the scope of enantioconvergent cross-couplings beyond electrophiles that bear a directing group or a p/π orbital proximal to the leaving group. Specifically, we have determined that a chiral nickel/pybox catalyst can achieve stereoconvergent cross-couplings of racemic α-bromosilanes with alkylzinc reagents under simple and mild conditions to afford enantioenriched organosilanes, a useful family of target compounds. Our mechanistic observations indicate that the chiral catalyst reacts with both enantiomers of the electrophile, without kinetic resolution, to provide the enantioenriched product via irreversible C–Br bond cleavage. This work sets the stage for substantial enlargement of the range of racemic electrophiles that can be employed in enantioconvergent cross-couplings, free of the need for a directing group or p/π conjugation.
Supplementary Material
Acknowledgements
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences; grant R01–GM062871 to G.C.F. and grant F32–GM116234 to G.M.S.) and the Dow Next Generation Educator Fund (grant to Caltech). We thank Dr. Jun Myun Ahn, Dr. Paul H. Oyala (Caltech EPR Facility), Dr. Mona Shahgholi (Caltech Mass Spectrometry Facility), Dr. Scott C. Virgil (Caltech Center for Catalysis and Chemical Synthesis), Dr. Haolin Yin, and Wanji Zhang for assistance and helpful discussions.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].a) Choi J, Fu GC, Science 2017, 356, eaaf7230 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Fu GC, ACS Cent. Sci 2017, 3, 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].For related reviews, see: a) Bhat V, Welin ER, Guo X, Stoltz BM, Chem. Rev 2017, 117, 4528–4561 [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Iwasaki T, Kambe N, Top. Curr. Chem 2016, 374, 1–36 [DOI] [PubMed] [Google Scholar]; c) Cherney AH, Kadunce NT, Reisman SE, Chem. Rev 2015, 115, 9587–9652 [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Trost BM, Van Vranken DL, Chem. Rev 1996, 96, 395–422. [DOI] [PubMed] [Google Scholar]
- [3].For examples of applications to the synthesis of natural products, see: a) Schmidt T, Kirschning A, Angew. Chem. Int. Ed 2012, 51, 1063–1066; Angew. Chem. 2012, 124, 1087–1091 [DOI] [PubMed] [Google Scholar]; b) Yamada R, Adachi Y, Yokoshima S, Fukuyama T, Angew. Chem. Int. Ed 2016, 55, 6067–6070; Angew. Chem. 2016, 128, 6171–6174. [DOI] [PubMed] [Google Scholar]
- [4].In the case of enantioconvergent arylations of α-trifluoromethyl alkyl halides, it is unclear whether the stereoselectivity is due to the trifluoromethyl group acting as a directing group or as an electron-withdrawing group: Liang Y, Fu GC, J. Am. Chem. Soc 2015, 137, 9523–9526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].We consider generic α-halosilanes to be unactivated alkyl electrophiles, as substitution of a carbon with a silicon does not significantly stabilize an adjacent radical. For example, the bond-dissociation energies for the C–H bonds of Me3CCH2–H (417+5 kJ/mol) and Me3SiCH2–H (415+5 kJ/mol) are essentially identical: Doncaster AM, Walsh R, J. Chem. Soc., Faraday Trans. 1 1976, 72, 2908–2916. [Google Scholar]
- [6].The Reisman laboratory has recently described a powerful method for the synthesis of enantioenriched α-aryl allylsilanes through the reductive alkenyl–alkyl cross-coupling of alkenyl halides with activated α-aryl-α-halosilanes (benzylic electrophiles): Hofstra JL, Cherney AH, Ordner CM, Reisman SE, J. Am. Chem. Soc 2018, 140, 139–142. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a pioneering umpolung variant of this reaction, see: Hayashi T, Konishi M, Ito H, Kumada M, J. Am. Chem. Soc 1982, 104, 4962–4963. [Google Scholar]
- [7].Franz AK, Dreyfuss PD, Schreiber SL, J. Am. Chem. Soc 2007, 129, 1020–1021. [DOI] [PubMed] [Google Scholar]
- [8].Fischer C, Zultanski SL, Zhou H, Methot JL, Shah S, Nuthall H, Hughes BL, Smotrov N, Hill A, Szewczak AA, Moxham CM, Bays N, Middleton RE, Munoz B, Shearman MS, Bioorg. Med. Chem. Lett 2012, 22, 3140–3146. [DOI] [PubMed] [Google Scholar]
- [9].For reviews, see: a) Ramesh R, Reddy DS, J. Med. Chem 2018, 61, 3779–3798 [DOI] [PubMed] [Google Scholar]; b) Fujii S, Hasimoto Y, Future Med. Chem 2017, 9, 485–505; [DOI] [PubMed] [Google Scholar]; c) Tacke R, Dörrich S, Top. Med. Chem 2014, 17, 29–60. [Google Scholar]
- [10].For examples of early studies of the catalytic asymmetric synthesis of related chiral silanes (two alkyl substituents on the stereogenic carbon that bears silicon), see: a) 1,4-addition to α,β-unsaturated carbonyl compounds: Hayashi T, Matsumoto Y, Ito Y, J. Am. Chem. Soc 1988, 110, 5579–5581 [Google Scholar]; b) hydrosilylation of olefins: Uozumi Y, Hayashi T, J. Am. Chem. Soc 1991, 113, 9887–9888. [Google Scholar]
- [11].For recent examples of (and leading references to) the catalytic asymmetric synthesis of related chiral silanes (two alkyl substituents on the stereogenic carbon that bears silicon), see: a) hydrosilylation of olefins: Cheng B, Liu W, Lu Z, J. Am. Chem. Soc 2018, 140, 5014–5017 [DOI] [PubMed] [Google Scholar]; b) hydrogenation of vinylsilanes: Wang A, Bernasconi M, Pfaltz A, Adv. Synth. Catal 2017, 359, 2523–2529. [Google Scholar]
- [12].Notes: (a) For the electrophile illustrated in entry 12 of Table 2, when Si is replaced with C, essentially no cross-coupling is observed under our standard conditions.
- [13].When PhZnBr in THF is employed as the nucleophile, under otherwise identical conditions, essentially no cross-coupling is observed.
- [14].Schley ND, Fu GC, J. Am. Chem. Soc 2014, 136, 16588–16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Consistent with the hypothesis that an organic radical is an intermediate in these nickel-catalyzed cross-couplings, the model reaction (Table 1) does not proceed (<1% yield) in the presence of TEMPO (0.4 equiv).
- [16].For an example of isomerization of the electrophile in a nickel-catalyzed enantioconvergent cross-coupling of an alkyl halide, see: Mu X, Shibata Y, Makida Y, Fu GC, Angew. Chem. Int. Ed 2017, 56, 5821–5824; Angew. Chem. 2017, 129, 5915–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].For an example of kinetic resolution of the electrophile in a nickel-catalyzed enantioconvergent cross-coupling of a racemic alkyl halide, see: Lundin PM, Fu GC, J. Am. Chem. Soc 2010, 132, 11027–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.