Abstract
Although there are sex differences in the effects of alcohol on immune responses, it is unclear if sex differences in immune response can influence drinking behavior. Activation of toll-like receptor 3 (TLR3) by polyinosinic:polycytidylic acid (poly(I:C)) produced a rapid proinflammatory response in males that increased alcohol intake over time (Warden et al., 2019). Poly(I:C) produced a delayed and prolonged innate immune response in females. We hypothesized that the timecourse of innate immune activation could regulate drinking behavior in females. Therefore, we chose to test the effect of two time points in the innate immune activation timecourse on every-other-day two-bottle-choice drinking: (1) peak activation; (2) descending limb of activation. Poly(I:C) reduced ethanol consumption when alcohol access occurred during peak activation. Poly(I:C) did not change ethanol consumption when alcohol access occurred on the descending limb of activation. Decreased levels of MyD88-dependent pathway correlated with decreased alcohol intake and increased levels of TRIF-dependent pathway correlated with increased alcohol intake in females. To validate the effects of poly(I:C) were mediated through MyD88, we tested female mice lacking Myd88. Poly(I:C) did not change alcohol intake in Myd88 knockouts, indicating that poly(I:C)-induced changes in alcohol intake are dependent on MyD88 in females. We next determined if the innate immune timecourse also regulated drinking behavior in males. Poly(I:C) reduced ethanol consumption in males when alcohol was presented at peak activation. Therefore, the timecourse of innate immune activation regulates drinking behavior and sex-specific dynamics of innate immune response must be considered when designing therapeutics to treat excessive drinking.
Keywords: alcohol use disorder, toll-like receptors, sex, females, cytokines, poly(I:C), alcohol, neuroimmune, drinking
1. Introduction
Alcohol increases the expression of proinflammatory cytokines that are transported from blood to brain and the release of inflammatory neuromodulators from glial and neuronal cells within the brain (1). We have hypothesized that positive feedback loops of proinflammatory systemic and central nervous system immune signaling promote excessive alcohol drinking. In support of this hypothesis, alcohol craving and consumption are positively correlated with elevated plasma levels of inflammatory cytokines in human alcoholics (2). Moreover, expression of innate immune components are increased in brains of human alcoholics and alcohol-exposed rodents, with expression correlating with total lifetime consumption and age of drinking onset (1). Therefore, understanding the interplay between immune responses and behavior in the progression to alcohol dependence may reveal new therapeutic targets for treatment of excessive drinking (see (3) for review).
Alcohol affects females differently than males. For instance, females progress from use to addiction more rapidly than males and are more vulnerable to developing neurotoxic and medical consequences of chronic drinking (see (4) for review). Animal models reflect many of the sex differences observed in the human population. Female rats, in general, acquire self-administration of alcohol and escalate their drinking more rapidly than males and show greater reinstatement (4–10). Specifically, in two-bottle choice testing procedures, female mice and rats of various strains show greater alcohol intake and preference than males and females do not decrease intake as much as males when alcohol concentrations are increased (6, 7, 11). Estrogenization of females, which confers a male phenotype on a genetically female brain, reduces ethanol intake compared with normal female rats and results in drinking patterns that are indistinguishable from those of normal male rats (8, 12). Together these studies indicate that sex differences in alcohol drinking behavior are partially attributable to biologic differences between males and females.
Males and females also differ in their innate immune responses (13). For example, innate detection of microorganisms and viruses by toll-like receptors (TLRs) differs between sexes (14). TLRs initiate inflammatory responses via two intracellular signaling transduction cascades: (1) Myeloid differentiation response gene 88 (MyD88)-dependent and (2) TIR-domain-containing adapter-inducing interferon β (TRIF)-dependent. Only TLR3 initiates inflammatory responses solely through the TRIF-dependent pathway. Endosomal TLR3 and TLR7 are more highly expressed in females compared with males, potentially due to lack of X-inactivation resulting in higher expression (15, 16). In contrast, TLR4 is more highly expressed in males (15, 17). After immunization or viral challenge, female peripheral blood mononuclear cells have higher transcript abundance of components of both the TRIF-dependent and MyD88-dependent pathways (e.g. Ticam1, Myd88, Irf3) (18, 19). Yet to date, only a handful of studies have directly examined sex differences in the effects of alcohol on inflammatory and immune responses (20–25). These reports show that in the absence of alcohol exposure, inflammatory and immune responses are stronger in females than in males (20–22). Chronic ethanol intake also produces a greater inflammatory response in both prefrontal cortex and peritoneal macrophages in intoxicated females compared with males (23, 25, 26).
These sex-specific innate immune differences may also explain sex-dependent drinking behaviors. Knockout of various chemokines, immune receptors, and TLR pathway components result in sex-specific changes in drinking behavior (27–30). For instance, genetic deletion of the chemokine CCL2 lowers ethanol preference only in female mice (27). Moreover, binge alcohol intoxication selectively increases expression of several cytokines and chemokines in the prefrontal cortex and plasma of female adolescent mice—indicating that females are more vulnerable than males to inflammatory effects of binge ethanol drinking (25). Together these studies support the hypothesis that sex-dependent innate immune responses can regulate drinking behavior (31).
Previous studies indicate a role for TLR signaling in the regulation of alcohol intake, specifically through TLR4 (31–35). For instance, activation of TLR4 with lipopolysaccharide increased alcohol intake, whereas siRNA inhibition of downstream TLR4 mediators decreased alcohol consumption (31, 34). However, despite evidence that TLR4 may be important for alcohol responses, a recent study using multiple genetic and pharmacological manipulations showed that TLR4 is not the critical determinant of excessive drinking (29, 36). Therefore, we hypothesize that another innate immune pathway may be important for regulation of excessive alcohol consumption, specifically TLR3-dependent signaling. In the frontal cortex of human alcoholics and mice subjected to chronic voluntary alcohol consumption TLR3 and its downstream signaling components are increased (37–39). TLR3 transcript abundance also correlated with lifetime alcohol consumption in human alcoholics (38). In a recent study we showed that activation of TLR3 in male mice increased alcohol consumption in a TLR3-dependent manner (40). Moreover, we have shown that inhibition of TRIF-dependent signaling components IKKI and TBK1 reduce ethanol consumption in male mice (37). It is not known if TLR3 regulates drinking behaviors similarly in female mice.
In this study, we tested the hypothesis that activation of TLR3-dependent signaling alters drinking behavior differently in female mice compared with male mice. We activated TLR3-dependent signaling by administering the TLR3 agonist polyinosinic-polycytidylic acid (poly(I:C)) to male and female C57BL/6J mice. We found that females showed no change or decreased consumption of ethanol and a delayed and prolonged immune response to poly(I:C). This was very different from what we found in males, which increased their drinking and showed peak activation of immune responses at 3 hours post-injection (40). We established that the time between TLR3 activation and ethanol access can change drinking behavior in both sexes and that this change in behavior is not correlated with a poly(I:C)-induced sickness response or a change in saccharin preference. We also documented changes in innate immune transcripts after chronic poly(I:C) administration during alcohol intake that may mediate changes in alcohol consumption in females. These results establish that TLR3/TRIF-dependent signaling produces sex-specific effects on alcohol consumption and confirm that sex-dependent innate immune responses can regulate drinking behavior.
2. Materials and Methods
2.1. Mice
Generation of Myd88 (B6.129P2(SJL)-Myd88tm1.1Defr/J, stock #009088) knockout (KO) mice was described previously (41). Mutant strains were purchased from The Jackson Laboratory (Bar Harbor, ME) and were backcrossed onto a C57BL/6J background for 6 generations. Female C57BL/6J mice were purchased from the Jackson Laboratory at 8-10 weeks of age and then bred to maintain our colony. Food and water were available ad libitum. The vivarium was maintained on a 12:12 hour light/dark cycle with lights on at 7:00 a.m. The temperature and humidity of the rooms were kept constant. Behavioral testing began when the mice were at least 2 months old, and mice were weighed every 4 days. All experiments were conducted in isolated behavioral testing rooms. All experiments were approved by the University of Texas at Austin Institutional Animal Care and Use Committee.
2.2. Poly(I:C) administration
To determine the time course of poly(I:C) in females, we injected 5mg/kg poly(I:C) and then sacrificed mice at 3, 24, 48, 72 and 96 hours post injection before qRT-PCR analysis (n=6 per group). For ethanol drinking studies, poly(I:C) was administered intraperitoneally (i.p.) every fourth or fifth day during no alcohol access periods. For the control injection, 0.9% saline (volume matched) was administered to control groups. Single use, sterile needles (27.5 gauge) were used to administer treatment. All injections we made between 8 am and 9 am to animals 8 -16 weeks of age.
2.3. Two-bottle choice every-other-day procedure
Intermittent (every-other-day [EOD]) access to ethanol increases voluntary drinking in rats (42, 43) and mice (44-46). Mice were given EOD access to ethanol (15 or 20%v/v) and water for 24-hour sessions, and water only was offered on off days. The side placement of the ethanol bottles was alternated with each alcohol session. The quantity of ethanol consumed was calculated as g/kg body weight/24 h. Total fluid intake was calculated as g/kg body weight/24h, (n=10/group).
2.4. Preference for saccharin
Mice were tested for saccharin consumption using an every-other-day 2BC protocol in which one bottle contained water and the other contained saccharin solution. Mice were offered saccharin (0.0008%). Saccharin was offered for a series of four poly(I:C) injections (16 days). Bottle positions were changed for each saccharin session (n=10/group).
2.5. Brain Collection
For qRT-PCR experiments brains were quickly harvested and the prefrontal cortex rapidly dissected before being snap frozen in liquid nitrogen. For immunohistochemistry experiments, mice were anesthetized with isoflourane, transcardially perfused with 0.9% saline until cleared of blood, and then perfused with freshly prepared 4% paraformaldehyde (47) in phosphate-buffered saline (PBS). Then the brain was removed and post fixed in 4% PFA at 4 °C for 24 h followed by cryoprotection for 24 h at 4 °C in 20% sucrose. Brains were then placed in a plastic mold containing optimum cutting temperature compound (OCT, VWR, Radnor, PA) and quickly frozen in isopentane on dry ice.
2.6. qRT-PCR
Total RNA was isolated using the MagMAX-96 Total RNA Isolation Kit (Life Technologies, Grand Island, NY). Total RNA was quantified using a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, Grand Island, NY) and assessed for quality using the Agilent TapeStation (Agilent Technologies, Santa Clara, CA). All samples passed quality control measures (RIN >8). Reverse transcription was performed using the Applied Biosystems High Capacity cDNA reverse transcription kit (Applied Biosystems, Grand Island, NY). PCR amplification was performed using TaqMan Universal PCR Master Mix and primer pairs and probes (Thermo Fisher Scientific, Grand Island, NY). Relative quantification of mRNA levels was determined using BIORAD software as previously described (48, 49). Gusb was selected as an endogenous control to normalize target gene mRNA levels. For all qRT-PCR, six animals were used per group, unless otherwise noted.
2.7. Immunohistochemistry
Thirty-micron sections from brain were permeabilized in 0.1% Triton-X-100 and then blocked in 10% donkey serum (Equitech-Bio, Kerrville, TX) for 1 h at room temperature. Sections were then incubated with primary antibodies overnight at 4 °C. Following three washes in PBS, sections were incubated with secondary antibodies for 2 h at RT. Finally, sections were mounted in 0.2% gelatin, dehydrated, and cover slipped with a DAPI (4’,6-diamidino-2-phenylindole)-containing mounting medium (Vector Labs, Burlingame, CA). See Supplemental Table 1 for list of all antibodies used.
2.8. Microscopy
Quantification of immunopositive cells was performed using a Zeiss Axiovert 200 M fluorescent light microscope (Zeiss, Thornwood, NY) equipped with an Axiocam b/w camera. Bilateral images of the PFC (Bregma +2.8 to +2.24) were captured using a 20× objective. For all immunohistochemistry experiments, parameters used for image acquisition were identical across treatments.
2.9. Quantitative Image Analysis
To determine the number of immunopositive cells for each protein type, the 20× images of the prefrontal cortex were separated into quadrants and overlaid with a 10-mm grid. All immunohistochemistry was quantified bilaterally within fixed area frames: PFC (box, 645 μm × 645 μm). Within each fixed area frame, four representative grids were chosen randomly for counting. Total cell counts for each animal were then averaged to give #immunopositive cells/area as previously described (37, 50). Cell counts were performed within each grid by ImageJ plug-in ITCN (http://rsb.info.nih.gov/ij/plugins/itcn.html).
2.10. Statistical analysis
Data are reported as mean ± SEM values, unless otherwise noted. The statistics software program GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) was used to perform 2-way ANOVAs, Pearson correlations and Student’s t-tests. Drinking data were analyzed by repeated-measures 2-way ANOVA followed by Bonferroni post-hoc tests. Transcript abundance data were analyzed by two-way ANOVA followed by Tukey’s HSD post-hoc tests. The Pearson correlation (α = 0.05) was used to evaluate correlations between ethanol consumption and transcript abundance. Grubbs test (α = 0.05) was used to detect potential outliers. Student’s t-tests (two-tailed) were used to analyze raw qRT-PCR data and immunohistochemical data.
3. Results
3.1. C57BL/6J females have an extended timecourse for innate immune activation after poly(I:C) in prefrontal cortex
Male and female mammals differ in their innate immune responses, suggesting sex-dependent differences in immune activation could directly regulate behavior (51–53). Therefore, we hypothesized there was a sex-dependent difference in innate immune activation after poly(I:C) in C57BL/6J females, which would be reflected in either the transcript abundance or timecourse for immune activation. To capture changes over time, we measured a time course of poly(I:C) effects on prefrontal cortical transcripts for TRIF- and MyD88-dependent pathway members and for select cytokines and chemokines implicated in behavioral responses to ethanol (see review (54). In females, poly(I:C) (5mg/kg) dynamically changed transcript levels across time. There were two distinct patterns of change in transcript levels: (1) peak abundance occurring between 24-48 hours post-injection; (2) an initial decrease in abundance at 3 hours post-injection followed by a return to baseline (Fig 1, Supplemental Fig 1). Unlike in studies performed in males, toll-like receptors and signaling components (Tlr2, Tlr3, Tlr4, Ticam1, Myd88, Ikkβ) showed no change in abundance at 3 hours post-injection (38, 40, 55). Instead there was a large increase at 24-48 hours post-injection that dissipated by 96 hours post-injection [Ftreatment × time(4,50)=49.85, (p<0.0001) for Tlr2; 33.25, (p<0.0001) for Tlr3; 15.32, (p<0.0001) for Tlr4; 17.13, (p<0.0001) for Ticam1; 31.05; (p<0.0001) for Myd88; 17.53, (p<0.0001) for Ikkβ]. Similarly peak abundance for proinflammatory mediators such as Il6, Il1β, Ifnb, Ccl2, and Ccl5, occurred at 24-48 hours post-injection [Ftreatment × time(4,50)=25.99, (p<0.0001) for Il6; 66.55, (p<0.0001) for Il1β; 11.62, (p<0.0001) for Ifnb; 75.87, (p<0.0001) for Ccl2; 74.32; (p<0.0001) for Ccl5]. In contrast, TRIF-dependent pathway components Irf3 and Ikkε showed an initial decrease 3 hours post-injection before returning to baseline [Ftreatment × time(4,50)=22.97, (p<0.0001) for Irf3; 6.06 (p=0.0005) for Ikkε]. These findings suggest that activation of TLR3 signaling in females produced a delayed and prolonged innate immune response.
Figure 1: Poly(I:C) produces delayed and prolonged innate immune activation in prefrontal cortex of female C57BL/6J mice.

Heat map showing transcript abundance at five time points after poly(I:C) (5mg/kg) injection. The transcript levels are presented using fold-change values (Log2 format) normalized to endogenous control. The red and green colors indicate high and low expression, respectively. The scale representing the relative signal intensity values is shown on the right. Hierarchical clustering was used in the data analysis. (n=6 per group).
3.2. Alcohol access at peak innate immune response decreases alcohol intake in females
Since females have a prolonged innate immune response, we hypothesized that time-dependent activation of the innate immune response could regulate drinking behavior. Therefore, we chose to test the effect of two time points in the innate immune activation timecourse on drinking behavior: (1) peak activation and (2) descending limb of activation after poly(I:C). To determine how alcohol access at peak innate immune activation affects drinking behavior, we administered poly(I:C) (5mg/kg, i.p.) every four days during EOD drinking (Fig 2A) . Poly(I:C) on a 4-day injection schedule significantly decreased ethanol intake [Fig 2B, Ftreatment × time(9,171)=1.885, p=0.057; Ftreatment(1,19)=24.83, p<0.0001, Ftime(9,171)=23.93, p<0.0001] and preference [Fig 2C, Ftreatment × time(9,171)=1.824, p=0.06; Ftreatment(1,19)=11.62, p=0.003, Ftime(9,171)=14.77, p<0.0001]. There was no main effect of poly(I:C) on total fluid intake [Fig. 2D, Ftreatment × time(9,171)=2.879, p=0.003; Ftreatment(1,19)=0.397, p=0.53, Ftime(9,171)=4.29, p<0.0001]. The interaction between time and treatment on total fluid intake was attributed to variability in fluid intake across time. Together this suggested that at peak innate immune activation, poly(I:C) decreases alcohol intake.
Figure 2: Poly(I:C) decreases alcohol intake when alcohol access occurs at peak innate immune activation in females.

C57BL/6J female mice were injected with saline or poly(I:C) (5mg/kg) every fourth day for a total of ten injections during two-bottle choice, every-other-day drinking (EOD,15%v/v). (A) 4-day injection schedule; (B) Ethanol (EtOH) intake (g/kg/24 h); (C) preference for EtOH; (D) total fluid intake. Arrows indicate days when animals received injection; (n=10 per group).
3.3. Increasing time between TLR3 activation and alcohol access prevents poly(I:C)-induced decreases in alcohol intake in females
To determine how alcohol access at the descending limb of innate immune activation affects drinking behavior we administered poly(I:C) (5mg/kg, i.p.) every five days during EOD drinking, with two days of “water only” between poly(I:C) injection and alcohol access (Fig 3A). In this experiment, poly(I:C) did not decrease ethanol consumption [Fig 3B, Ftreatment × time(9,144)=1.256, p=0.26; Ftreatment(1,16)=2.02, p=0.17; Ftime(9,144)= 15.47, p<0.0001] or preference [Fig 3C, Ftreatment × time(9,144)=1.29, p=0.26; Ftreatment(1, 16)=0.19, p=0.66; Ftime(9,144)= 18.95, p<0.0001]. Although there was a trend towards increased total fluid intake with poly(I:C) treatment, this effect was not statistically significant [Fig 3D, Ftreatment × time(9,144)=0.69, p=0.71; Ftreatment(1,16)=4.60, p=0.053; Ftime(9,144)= 3.57, p<0.001]. Because ethanol preference was nearly 0.8 at 15% ethanol, we reran the experiment with 20% ethanol to decrease ethanol preference and avoid a potential ceiling effect. Switching to a higher percentage ethanol dropped preference as expected, but there was still no effect of poly(I:C) on ethanol consumption or preference (Supplemental Fig 2).
Figure 3: Poly(I:C) prevents reductions in alcohol intake when alcohol access occurs after peak innate immune activation in females.

C57BL/6J female mice were injected with saline or poly(I:C) (5mg/kg) every fifth day for a total of ten injections during two-bottle choice, every-other-day drinking (EOD,15%v/v). (A) 5-day injection schedule; (B) Ethanol (EtOH) intake (g/kg/24 h); (C) preference for EtOH; (D) total fluid intake. Arrows indicate days when animals received injections; (n=10 per group).
Poly(I:C) can induce a transient sickness response; therefore, we estimated the severity of this response by measuring changes in body weight and water intake (31, 55). Poly(I:C) prevented increases in body weight over time for both injection schedules (Supplemental Fig 3A-B). Additionally, poly(I:C) treatment resulted in a small but significant weight loss for the 4-day injection schedule [Supplemental Fig 3A, Ftreatment × time(9,171)=4.66, p<0.0001; Ftreatment(1,19)=4.6, p=0.045; Ftime(9,171)=10.12, p<0.0001]. No weight loss was observed for the 5-day injection schedule [Supplemental Fig 3B, Ftreatment × time(9,162)=3.07, p=0.002; Ftreatment(1,18)=2.87, p=0.10; Ftime(9,162)=33.67, p<0.0001]. Because we observed poly(I:C)-induced weight loss on the 4-day injection schedule, we next tested if the severity of this sickness behavior was related to changes in alcohol intake. There was no correlation between change in body weight and average alcohol intake in poly(I:C)-treated animals (Supplemental Fig 3C). Taken together, this suggested that changes in alcohol intake due to poly(I:C), for either injection schedule, were not due to a sickness response.
3.4. Time between TLR3 activation and alcohol access produces directional changes in innate immune transcript abundance in females
Since the TLR3/TRIF-dependent pathway regulates escalations in alcohol intake in males (40), we hypothesized that TRIF-dependent pathway transcript abundance would be positively associated with alcohol intake in poly(I:C)-treated females that did not decrease drinking (i.e. 5-day injection schedule). To test this hypothesis, we measured changes in the abundance of transcripts for TRIF-dependent genes, as well as for MyD88-dependent genes and proinflammatory mediators in female mice from both drinking procedures. The values for each transcript, represented as the fold-change from the respective saline group, are shown as a heatmap in Fig 4A. Raw qRT-PCR data for each transcript are available in Supplemental Figure 4. Poly(I:C)-treated females that did not decrease alcohol intake (5-day injection schedule) showed an increase in transcript abundance of TRIF-dependent pathway components (Tlr3, Ticam1, Ikkε, Irf3), interferon signaling components (Ifnb, Ifnar1) and MyD88-dependent pathway components (Tlr2, Tlr4, Myd88, Ikkβ). In contrast, in females that decreased alcohol intake after poly(I:C) (4-day injection schedule), there was no change in mRNA expression of TRIF-dependent pathway components (Tlr3, Ticaml, Ikkε, Irf3). Instead, we observed a decrease in transcript abundance for interferon signaling components (Ifnb, Ifnar1) and MyD88-dependent signaling components (Myd88, Ikkβ).
Figure 4: Innate immune transcript abundance correlates with directional changes in alcohol intake in females.
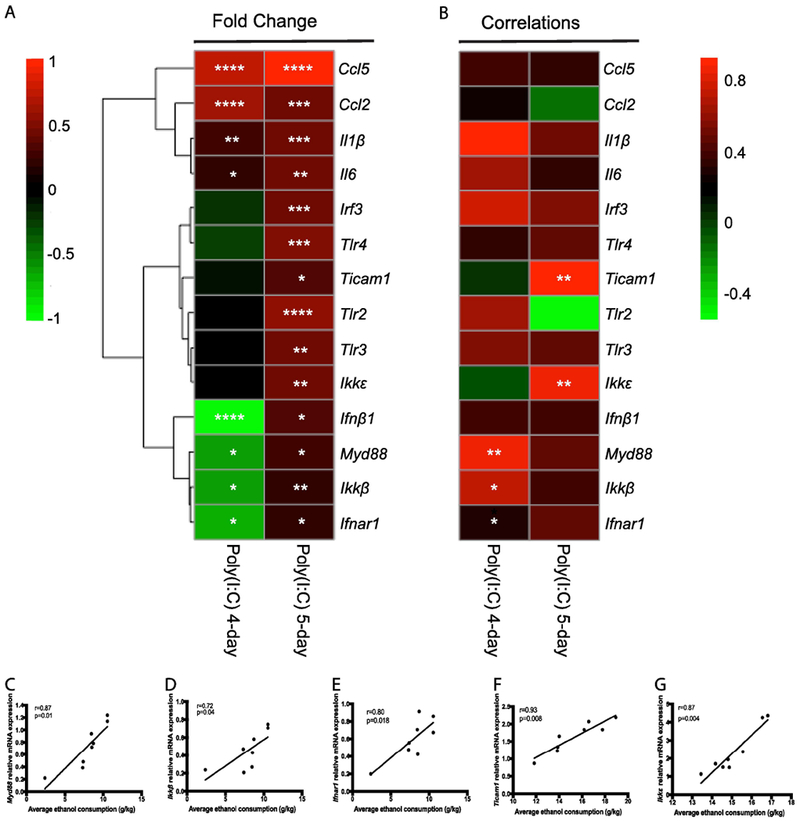
(A) Heat map showing transcript abundance after both the 4-day poly(I:C) injection schedule and 5-day poly(I:C) injection schedule drinking experiments. The transcript levels are presented using fold-change values (Log2 format) normalized to endogenous control and saline-treated controls for each injection schedule. (B) Correlation heatmap of individual transcripts with average ethanol intake. The red and green colors indicate high and low transcript abundance or correlation strength, respectively. For the 4-day injection schedule, there were significant positive correlations between Myd88 (C) and amount of ethanol consumed, between Ikkβ (D) and amount of ethanol consumed and Ifnar1 (E) and amount of ethanol consumed in poly(I:C)-treated mice. For the 5-day injection schedule, there were significant positive correlations between Ticam1 (F) and amount of ethanol consumed and between Ikkε (G) and amount of ethanol consumed in poly(I:C)-treated mice. ****p<0.0001, ***p<0.0002, **p<0.0021, *p<0.05 for Tukey post-hoc tests and correlations.
To identify potential targets that regulate poly(I:C)-induced directional changes in drinking behavior, we examined correlation between transcript abundance and average ethanol consumption for each injection schedule (Fig 4B). Several transcripts were significantly affected by poly(I:C) and alcohol exposure for each injection schedule, but only five transcripts significantly correlated with ethanol consumption overall. In females that decreased drinking (4-day injection schedule), there was a significant positive correlation between Myd88-dependent transcript levels and alcohol intake in poly(I:C)-treated female mice (Fig 4C-D, Myd88: r= 0.87, p=0.004; Ikkβ: r=0.72, p=0.04). Interestingly, type-1 interferon receptor 1 (Ifnar1) was also positively correlated (r=0.80, p=0.0018) with alcohol intake for this injection schedule (Fig 4E). In contrast, in females that did not decrease alcohol intake (5-day injection schedule), there was no significant correlation between Myd88-dependent pathway transcript levels and alcohol intake. However, we observed a significant positive correlation between TRIF-dependent pathway transcript abundance and alcohol intake for this schedule (Fig 4F-G, Ticam1 r= 0.93, p=0.0008; Ikkε r=0.94, p=0.0004). We verified that changes in transcript levels were associated with corresponding changes in proteins that they encode by immunohistochemistry (Supplemental Figures 5-6). Together this suggested that the TRIF-dependent pathway may regulate increased alcohol intake and the MyD88-dependent pathway be involved in suppression of alcohol intake in females.
3.5. Poly(I:C) decreases alcohol intake in a MyD88-dependent manner in females.
Male and female global Myd88 knockouts display different drinking behaviors, with increased alcohol consumption in male knockouts but no change in consumption in female knockouts (29), suggesting sex-dependent differences in how MyD88-dependent pathway may be influencing drinking behavior. The strong positive correlation between poly(I:C)-induced decreases in alcohol intake and MyD88-dependent transcript abundance suggested that decreases in MyD88 may be necessary for poly(I:C)-induced changes in alcohol intake in females. If this were true, then administration of poly(I:C) should not decrease alcohol intake in females on a 4-day injection schedule. To test this hypothesis, we injected female Myd88 knockout mice (−/−) with poly(I:C) (5mg/kg) every four days while they were consuming 15% ethanol in an EOD procedure for a total of 28 days. Poly(I:C) produced no significant effect on ethanol consumption [Fig 5A, Ftreatment × time(6,78)=0.80, p=0.57; Ftreatment(1,13)=0.28, p=0.60; Ftime(6,78)=2.58, p=0.02] or preference [Fig 5B, Ftreatment × time(6,78)=0.38, p=0.88; Ftreatment(1,13)=2.2, p=0.16; Ftime(6,78)=3.27, p=0.006]. Although there was a trend towards decreased total fluid intake with poly(I:C) treatment, this effect was not statistically significant [Fig 5C, Ftreatment × time(6,78)=0.44, p=0.85; Ftreatment(1,13)=4.69, p=0.05; Ftime(6,78)=1.14, p=0.34]. These results indicate that in females, poly(I:C)-mediated decreases in alcohol intake are dependent on MyD88. One limitation of this knockout study is that wildtype controls were not used in conjuction with Myd88 knockout mice, making the null effect more difficult to intrepret. One interpretation of the data is that poly(I:C)-induced decreases in alcohol intake did not replicate, negating the biological interpretation of the Myd88 knockout data. However, we have replicated the poly(I:C)-induced decreases in alcohol intake in a separate study (Supplemental Figure 7), increasing the likelihood that poly(I:C) decreases alcohol intake through mechanisms dependent on MyD88 in females.
Figure 5: Poly(I:C)-induced decreases alcohol intake are dependent on MyD88.
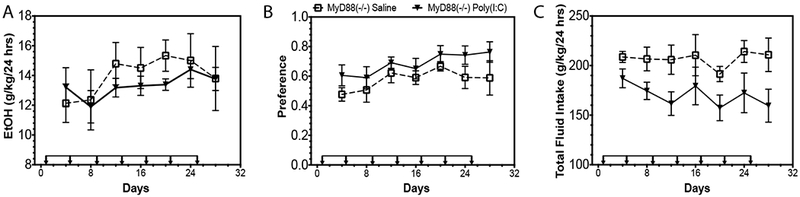
Mutant female mice (Myd88 −/−) were injected with saline or poly(I:C) (5mg/kg) every four days for a total of seven injections during a two-bottle choice every-other-day drinking procedure (EOD, 15%v/v). (A) Ethanol (EtOH) intake (g/kg/24 h), (B) preference for EtOH, and (C) total fluid intake (n=10 per group).
3.6. Poly(I:C) does not alter saccharin consumption in C57BL/6J females
We also studied consumption of saccharin using an EOD procedure (0.008% saccharin) to determine whether altered taste perception could account for poly(I:C)-induced decreases in alcohol intake in females on a 4-day injection schedule. Poly(I:C) did not change saccharin consumption [Fig 6A, Ftreatment × time(3,54)=1.89, p=0.14; Ftreatment(1,18)=0.004, p=0.94; Ftime(3,54)=7.633, p<0.001] or preference [Fig 6B, Ftreatment × time(3,54)=0.07, p=0.97; Ftreatment(1,18)=0.38, p=0.54; Ftime(3,54)=24.59, p<0.001]. Total fluid intake was unchanged by poly(I:C) treatment [Fig 6C, Ftreatment × time(3,54)=2.87, p=0.05; Ftreatment(1,18)=0.13, p=0.71; Ftime(3,54)=15.07, p<0.0001]. These findings indicate that poly(I:C) does not change detection of sweet taste, suggesting that poly(I:C) does not decrease alcohol intake by perturbing saccharin taste perception in females.
Figure 6: Poly(I:C) does not change saccharin taste perception.

C57BL6/J female mice were injected with saline of poly(I:C) (5mg/kg) every four days for a total of four injections during a two-bottle-choice every-other-day saccharin procedure (EOD, 0.008%). (A) Saccharin consumption (g/kg/24 h), (B) preference for saccharin, and (C) total fluid intake (g/kg/24 h) (n=10 per group).
3.7. Alcohol access at peak innate immune response prevents escalation of alcohol intake in males
Presentation of alcohol at peak innate immune activation decreased alcohol intake in females, therefore, we hypothesized that the timecourse of innate immune activation could also regulate drinking behavior in males. If this were true, then males given alcohol access at their peak innate immune response (3 hr post-injection) should decrease alcohol intake. To test this hypothesis, we injected C57BL/6J male mice with poly(I:C) (5mg/kg, i.p) every four days and allowed access to 15% ethanol 3 hours post-injection in an EOD procedure for a total of 32 days. Access to alcohol 3 hours post-injection prevented poly(I:C)-induced escalation of alcohol intake and caused a trend toward decreased alcohol consumption [Fig 7B, Ftreatment × time(7,126)=1.38, p=0.21; Ftreatment(1,18)=3.29, p=0.08; Ftime(7,126)=5.04, p<0.0001]. Access to alcohol 3 hours post-injection did not change ethanol preference for poly(I:C)-treated compared with saline-treated animals [Fig 7C, Ftreatment × time(7,126)=2.39, p=0.02; Ftreatment(1,18)=1.16, p=0.30; Ftime(7,126)=2.23, p=0.04]. Total fluid intake was unchanged by treatment [Fig 7D, Ftreatment × time(7,126)=1.33, p=0.24; Ftreatment(1,18)=3.78, p=0.06; Ftime(7,126)=8.53, p<0.0001]. These results indicate that access to alcohol at peak innate immune activation prevents previously reported poly(I:C)-induced escalation of alcohol intake in males (40).
Figure 7: Poly(I:C) prevents escalation of alcohol intake when alcohol access occurs at peak innate immune activation in males.

C57BL/6J male mice were injected with saline or poly(I:C) (5mg/kg) every fourth day for a total of eight injections during two-bottle choice, every-other-day drinking (EOD,15%v/v). (A) 3hr delay injection schedule; (B) Ethanol (EtOH) intake (g/kg/24 h); (C) preference for EtOH; (D) total fluid intake. Arrows indicate days when animals received injection; (n=10 per group).
4. Discussion
These results, along with our recent study (40), reveal pronounced sex differences in how TLR3 activation regulates drinking behavior. Females have a delayed and prolonged immune response after poly(I:C) in prefrontal cortex. Females decreased drinking when alcohol access occurred at the peak of innate immune activation after poly(I:C). Females did not decrease drinking when alcohol access occurred on the descending limb of innate immune activation. To further support our hypothesis that the time between alcohol access and immune activation regulates behavior, we found that poly(I:C) can prevent escalation of alcohol intake in males when alcohol access occurs at peak innate immune activation. Importantly, these findings support the conclusion that females require different treatment strategies than males due to sex-dependent differences in innate immune signaling.
Several reports have demonstrated clear sex differences in the neurotoxic and inflammatory effects of ethanol. For instance, binge drinking has been associated with sex-specific differences in the frontal, temporal, and cerebellar brain activation during spatial working memory tasks, with females exhibiting poorer sustained attention and working memory performance (56, 57). Animal models confirmed that alcohol preferentially damages the cortex and hippocampus of female compared with male adolescent rats (23). Chronic ethanol intake also causes a greater inflammatory response in both prefrontal cortex and peritoneal macrophages in intoxicated females than in males (23, 25, 26). Moreover, females of many species have sex-dependent expression of innate immune components and launch more robust immune responses than males (13, 58, 59). For instance, TLR3 and TLR7 and its downstream mediators (e.g. IRF5) are more highly expressed in human and mouse female peripheral immune cells (15-17). Immune activation with specific toll-like receptor agonists produce sex-dependent responses with higher expression of TLR7 in female immune cells leading to greater cytokine and interferon production—which is regulated by sex chromosome expression (13). These results indicate that (1) females are more vulnerable to the neurotoxic and cognitive effects of heavy alcohol intake than males, and that (2) inflammatory and immune responses are more marked in females than in males after ethanol or immune stimulation.
Consistent with these findings, we observed sex-dependent differences in immune activation in our study. Following stimulation by poly(I:C), we observed a delayed and prolonged innate immune activation in females, with peak production of proinflammatory mediators (e.g. Il1β, Il6) 24-48 hours post-injection. Recent studies in males demonstrated that poly(I:C) significantly upregulated proinflammatory mediators in brain at much earlier timepoints—between 3-8 hours post-injection (55, 60, 61). To exemplify the immune activation delay between sexes we plotted Il6 mRNA expression across time (Fig 8). The observed delayed immune response for females is consistent with studies on sex-dependent differences in microglial production of proinflammatory cytokines. After binge-drinking, adolescent females display heightened and different cytokine responses compared to males (25). After traumatic brain injury, males have a more immediate production of proinflammatory cytokines (e.g. Il1β, Tnfα) and females have a biphasic and delayed immune response (53). Moreover, at baseline, females show a greater proportion of ramified microglia relative to males alongside heightened basal proinflammatory gene expression (62-64). These findings suggest that females have a lower threshold for activation and launch a delayed but more robust proinflammatory response compared with males.
Figure 8: Males and females have different innate immune activation timecourses.
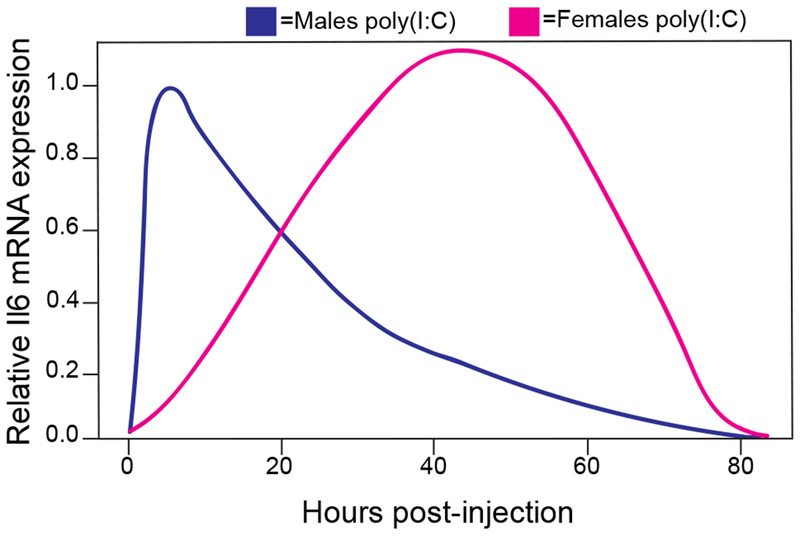
Expression of Il6 mRNA after poly(I:C) (5mg/kg) injection. Note after poly(I:C), male peak innate immune activation occurs between 3-8 hours post-injection and female peak innate immune activation occurs between 24-48 hours post-injection. mRNA was normalized to saline treated animals as well as the endogenous control, Gusb.
Poly(I:C)-injected females given access to alcohol at peak innate immune activation decreased alcohol intake. This decrease in drinking was not accompanied by a decrease in body weight or total fluid intake, suggesting that this result was not due to a general sickness response. A drug can alter taste perception, which can change the taste of ethanol leading to changes in consumption. A reduction in the perception of sweet taste or an increase in aversion to bitter taste can reduce ethanol consumption (65). A previous study linked deletions in genes expressed in taste buds and critical to taste transduction to changes in alcohol consumption, revealing that perception of sweet taste, not bitter aversion, is important for voluntary alcohol consumption (65). Therefore, we measured if poly(I:C) decreased alcohol consumption by altering sweet taste perception. Saccharin consumption was unchanged by poly(I:C) on this injection schedule, suggesting poly(I:C) has an ethanol-specific effect.
We hypothesized that the timing between activation of immune signaling and alcohol access could regulate drinking behavior. Extending the time between poly(I:C) injection and alcohol access to accommodate delayed immune activation in females prevented poly(I:C)-induced decreases in alcohol intake. Supporting our hypothesis, males given access to alcohol during peak innate immune activation decreased alcohol intake. Taken together, these findings indicate that the timing of innate immune activation and alcohol access regulates drinking behavior via sex-dependent differences in immune activation.
There are two possibilities for how poly(I:C) activates TLR signaling to change alcohol consumption in females: (1) poly(I:C) enters the brain and directly activates TLR3 signaling without involvement of MyD88-dependent signaling; (2) poly(I:C) activates peripheral TLR3, which then leads to induction of inflammogens that can cross the blood brain barrier and activate central neuroimmune receptors. Our recent study demonstrated that synthetic dsRNA can be detected in both prefrontal cortex and liver after peripheral injection of poly(I:C) (5mg/kg), with the greatest increase in dsRNA signal observed in the liver (40). This suggests that poly(I:C) can directly activate central TLR3 but peripheral immune signaling most likely plays a larger role in regulating poly(I:C)-induced changes in drinking behavior. Therefore, we hypothesized that inflammogens from the periphery could activate the MyD88-dependent to produce changes in drinking behavior. Moreover, activation of MyD88-dependent signaling has already been shown to regulate alcohol intake in C57BL6/J males and females, making it a probable pathway to regulate drinking behavior after poly(I:C) (31).
In females that decreased alcohol intake, there was no change in TRIF-dependent pathway mRNA or protein levels. However, we did observe a strong positive correlation between MyD88-dependent pathway levels and ethanol consumption in poly(I:C)-treated mice, implying that decreased MyD88-dependent signaling decreases alcohol consumption in females. Poly(I:C) can downregulate TLR4 signaling via a TLR3-dependent mechanism, implying that the protective effects of poly(I:C) may be mediated by decreased TLR4/MyD88-dependent signaling (66, 67). Therefore, we considered whether TLR3-induced decreases in MyD88-dependent signaling could explain the decrease in alcohol intake that we observed in female mice treated with poly(I:C). Poly(I:C) did not change alcohol intake in Myd88 knockout females, suggesting that poly(I:C) decreases alcohol intake through mechanisms dependent on MyD88.
Poly(I:C)-treated females that escalated drinking similar to saline-treated animals showed increased innate immune transcript abundance for both TLR pathway branches and proinflammatory mediators. Increased abundance of TRIF-dependent pathway transcripts correlated with increased alcohol intake, suggesting that the TRIF-dependent pathway may drive escalations in alcohol intake in females as well. In our recent study, we showed poly(I:C)-induced increases in alcohol intake were, at least partially, dependent on TLR3 in males, supporting a role for TRIF-dependent signaling in excessive drinking behavior in both sexes (40). There is currently no literature on how ethanol treatment itself changes TRIF-dependent signaling in females. However, we hypothesize that chronic alcohol treatment increases expression of TLR3 and TRIF-dependent signaling components (37, 60). Because TLR3 activation resulted in either increased or decreased drinking depending on each sex’s innate immune activation timecourse, we hypothesize that drugs like amlexanox, which inhibits IKKI and TBK1 and reduced ethanol consumption in males, may not be as effective in females due to differences in the TLR3 activation timecourse (37).
One limitation of the current study is that the role of sex steroids as was not addressed in either the context of drinking behavior or immune activation. Studies investigating the role of the normal human menstrual cycle in regulation of alcohol consumption are conflicting and indeterminate (4, 68), thus the precise role that the estrous cycle has on drinking behavior in females remains unknown. Some effects of alcohol are dependent on phase of the estrous cycle. Decreases in alcohol consumption during estrus have been identified in hormone-synchronized female rats (69). A previous microdialysis study showed that alcohol caused the greatest increase in dopamine levels in the medial prefrontal cortex during estrus (70). The sedative effects of alcohol are less pronounced in proestrus and diestrus (71). However, the effect of estrous cycle on alcohol consumption, even in hormone-synchronized rodents, is modest, accounting for (at maximum) only 35% of variation in alcohol intake across the cycle (68, 69). Moreover, multiple studies report that estrous cycle does not substantially impact alcohol intake in naturally cycling rodents (10, 72–74). A recent study showed that hormonal fluctuations had little impact on alcohol intake in models of non-dependent drinking and escalated drinking under free-cycling conditions in which female rodents were single-housed (voluntary two-bottle choice drinking in the home cages) and cohabitated in the same housing room as males (74), These are the same experimental parameters used in our study. Taken together, these results suggest that in rodent models of voluntary alcohol consumption, the estrous cycle is unlikely to significantly impact drinking behavior.
Humans do show strong sex differences in innate immunity, (13). Indeed, receptors for estrogens and estradiol regulate various cells and pathways involved in innate immunity (13). Estradiol has a well-documented biphasic effect on immune function in both preclinical and clinical studies (75, 76), and its pro- or anti-inflammatory effects are dependent upon dose, time and method of testing. For example, high levels of estradiol inhibit NF-kB and decrease proinflammatory signaling (77, 78). In contrast, low doses of estradiol, comparable to normal circulating levels, increase peripheral concentrations of proinflammatory cytokines (79). A previous study demonstrated in primary endometrial epithelial cells that TLR3 expression is cycle-dependent and treatment with 17β-estradiol suppressed cytokine and chemokine production after TLR3 stimulation with poly(I:C)—although this effect was not dependent on TLR3 but rather on estrogen receptor alpha expression (80, 81). Putative response elements and oestrogen response elements are also present in the promoters of several innate immunity genes including those in the TLR3 pathway, suggesting that sex steroids may directly cause dimorphic innate immune responses (13, 18). For instance, the lower level of cell surface TLR4 expression observed on female-derived macrophages following LPS challenge relative to that seen on similarly treated male-derived cells might contribute to the reported sex-based differences in LPS tolerance (17). Moreover, sex steroids may regulate innate immune activity in brain. Production of inflammatory cytokines within the brain following intracerebral injection of LPS is attenuated in ovariectomized animals, which is reversed following exogenous estrogen administration (82). Therefore, it is possible that during different phases of estrous, estrogens may suppress the proinflammatory effect of poly(I:C) leading to a reduction in drinking, accounting for a least a portion of the differences in alcohol intake between the 4- and 5-day poly(I:C) injection schedules. If this were true, then the sex differences in innate immune timecourse could not regulate drinking behaviors in males. However, we observed a trend toward reduced alcohol consumption in males when alcohol access was permitted during peak cytokine activation, suggesting that the innate immune timecourse regulates direction of drinking behavior after TLR3 activation. Future studies will need to address how sex steroids influence the observed poly(I:C) innate immune activation sexual dimorphism as well as the role of sex steroids in poly(I:C)-induced changes in alcohol intake.
5. Conclusions
In summary, we demonstrate a novel role for TLR3-dependent signaling in regulation of escalation of ethanol consumption in both males and females. Our study also highlights the role of the MyD88-dependent pathway in suppression of alcohol intake in females. An implication of this study is that the timecourse of innate immune activation regulates drinking behavior. The ability innate immune activation to control alcohol intake highlights an important question that will be addressed in future studies, namely at what time point during innate immune activation does ethanol become rewarding versus aversive? Our data suggests that some level of proinflammatory response is necessary for escalation of alcohol intake, but too much cytokine response can lead to decreased alcohol consumption. Specific pathways and the balance between them seem to be critical regulators of drinking behavior. Therefore, indiscriminant inhibition of inflammatory pathways may not provide a viable strategy to limit excessive drinking. We conclude that being able to target specific timepoints in innate immune activation—for inhibition of either TRIF- or MyD88-dependent pathways—will permit development of targeted treatment strategies for excessive drinking.
Supplementary Material
Supplemental Figure 2: Poly(I:C) prevents reductions in alcohol intake when alcohol access occurs after peak innate immune activation in females on 20% ethanol. Two-bottle choice every-other-day (20%v/v) drinking in female mice on 5-day injection schedule. Ethanol (EtOH) intake (g/kg/24 h) (A), preference for EtOH (B), and total fluid intake (C) in saline-treated versus poly(I:C)-treated female mice (C57BL/6J, n=10 per group). Data were analyzed by repeated-measures 2-way ANOVA. There was no effect of poly(I:C) on ethanol consumption [(A) Ftreatment × time(8,144)=0.59, p=0.78; Ftreatment(1,18)=1.99, p=0.17; Ftime(8,144)=25.74, p<0.0001] or preference [(B), Ftreatment × time(8,144)=0.18, p=0.99; Ftreatment(1,18)=0.79, p=0.38; Ftime(8,144)=17.1, p<0.0001]. Total fluid intake was unchanged by treatment [(C), Ftreatment × time(8,144)=0.80, p=0.60; Ftreatment(1,18)=0.06, p=0.80; Ftime(8,144)=6.34, p<0.0001].
Supplemental Figure 5: Quantification of TRIF-dependent, MyD88-dependent and interferon proteins in animals on different poly(I:C) injection schedules. Data were analyzed by 2-way ANOVA followed by Tukey HSD post hoc tests (p<0.0001 (****), p<0.0002 (***), p<0.0021 (**), p<0.0332 (*) compared with saline group). Data are represented as mean + SEM, n = 2 per group.
Supplemental Figure 6: Representative images for protein confirmation of mRNA changes in poly(I:C) drinking experiments. 4-day injection schedule: MYD88 (A), IKKB (C), TRIF (E), IKKI (G), IFNB (I). 5-day injection schedule: MYD88 (B), IKKB (D), TRIF (F), IKKI (H), IFNB (J). Scale bar = 100 μm.
Supplemental Figure 1: Raw qRT-PCR data for poly(I:C) timecourse. The levels of transcripts are presented using fold-change values normalized to endogenous control. Data were analyzed by 2-way ANOVA followed by Tukey HSD post hoc tests (p<0.0001 (****), p<0.0002 (***), p<0.0021 (**), p<0.0332 (*) compared to saline group).
Supplemental Figure 3: Lack of correlation between ethanol intake and severity of sickness response after poly(I:C) injections. Body weight (g) during (A) 4-day injection schedule and (B) 5-day injection schedule drinking experiments. (C) Correlation between average alcohol intake (g/kg) for 4-day injection schedule and % body weight change. The severity of sickness was calculated % change in body weight after the first injection of poly(I:C) then averaged across the ten injections. Body weight data was analyzed by repeated-measures 2-way ANOVA followed by Bonferroni post hoc tests (p<0.0332 (*) compared with saline group). Pearson correlation (α=0.05) was used to correlate % weight change to average alcohol intake (g/kg), (Saline r=0.3717, R2=0.1382, p=0.29, poly(I:C) r= −0.1785, R2=0.03185, p=0.59).
Supplemental Figure 4: Raw qRT-PCR data for 4-day and 5-day poly(I:C) schedules after chronic ethanol exposure. The transcript levels of genes are presented as fold-change values normalized to respective endogenous control. Data were analyzed by 2-way ANOVA followed by Tukey HSD post hoc tests (p<0.0001 (****), p<0.0002 (***), p<0.0021 (**), p<0.0332 (*) compared with saline group).
Supplemental Figure 7: Replication experiment for poly(I:C)-induced decreases in alcohol intake in females. C57BL/6J female mice were injected with saline or poly(I:C) (5mg/kg) every fourth day for a total of ten injections during two-bottle choice, every-other-day drinking (EOD,15%v/v) using the same batch of poly(I:C). (A) 4-day injection schedule; (B) Ethanol (EtOH) intake (g/kg/24 h); (C) preference for EtOH; (D) total fluid intake. Arrows indicate days when animals received injection; (n=10 per group).
Supplemental Table 1: Antibody information
Highlights:
-
■
Females have a delayed and prolonged proinflammatory response after poly(I:C).
-
■
Timecourse of cytokine activation regulated alcohol intake in both sexes.
-
■
Increased TRIF-dependent signaling enables escalation of alcohol intake.
-
■
Decreased MyD88-dependent signaling enables suppression of alcohol intake.
-
■
Decreased alcohol intake due to poly(I:C) is dependent on MyD88 in females.
Acknowledgments
Funding: This work was supported by the National Institutes of Health/National Institute of Alcohol Abuse and Alcoholism [U01 AA020926, P01 AA020683, AA013520, AA006399, AA025499]. The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crews FT, Vetreno RP (2016): Mechanisms of neuroimmune gene induction in alcoholism. Psychopharmacology (Berl). 233:1543–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leclercq S, De Saeger C, Delzenne N, de Timary P, Starkel P (2014): Role of inflammatory pathways, blood mononuclear cells, and gut-derived bacterial products in alcohol dependence. Biol Psychiatry. 76:725–733. [DOI] [PubMed] [Google Scholar]
- 3.Montesinos J, Alfonso-Loeches S, Guerri C (2016): Impact of the Innate Immune Response in the Actions of Ethanol on the Central Nervous System. Alcohol Clin Exp Res 40:2260–2270. [DOI] [PubMed] [Google Scholar]
- 4.Becker JB, McClellan ML, Reed BG (2017): Sex differences, gender and addiction. J Neurosci Res 95:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hutchins JB, Allen DL, Cole-Harding LS, Wilson JR (1981): Behavioral and physiological measures for studying ethanol dependence in mice. Pharmacol Biochem Behav 15:55–59. [DOI] [PubMed] [Google Scholar]
- 6.Li TK, Lumeng L (1984): Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res 8:485–486. [DOI] [PubMed] [Google Scholar]
- 7.Lancaster FE, Spiegel KS (1992): Sex differences in pattern of drinking. Alcohol. 9:415–420. [DOI] [PubMed] [Google Scholar]
- 8.Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK (1998): Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest 101:2677–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blanchard BA, Steindorf S, Wang S, Glick SD (1993): Sex differences in ethanol-induced dopamine release in nucleus accumbens and in ethanol consumption in rats. Alcohol Clin Exp Res 17:968–973. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL (2010): Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav 96:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meliska CJ, Bartke A, McGlacken G, Jensen RA (1995): Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav 50:619–626. [DOI] [PubMed] [Google Scholar]
- 12.Patchev VK, Hayashi S, Orikasa C, Almeida OF (1995): Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J 9:419–423. [DOI] [PubMed] [Google Scholar]
- 13.Klein SL, Flanagan KL (2016): Sex differences in immune responses. Nat Rev Immunol 16:626–638. [DOI] [PubMed] [Google Scholar]
- 14.Kawai T, Akira S (2011): Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 34:637–650. [DOI] [PubMed] [Google Scholar]
- 15.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW (2011): Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 118:5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S (2006): Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 312:1669–1672. [DOI] [PubMed] [Google Scholar]
- 17.Marriott I, Bost KL, Huet-Hudson YM (2006): Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: a possible mechanism for gender-based differences in endotoxic shock susceptibility. J Reprod Immunol 71:12–27. [DOI] [PubMed] [Google Scholar]
- 18.Hannah MF, Bajic VB, Klein SL (2008): Sex differences in the recognition of and innate antiviral responses to Seoul virus in Norway rats. Brain Behav Immun 22:503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klein SL, Jedlicka A, Pekosz A (2010): The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis 10:338–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grossman CJ, Nienaber M, Mendenhall CL, Hurtubise P, Roselle GA, Rouster S, et al. (1993): Sex differences and the effects of alcohol on immune response in male and female rats. Alcohol Clin Exp Res 17:832–840. [DOI] [PubMed] [Google Scholar]
- 21.Spitzer JA, Zhang P (1996): Gender differences in phagocytic responses in the blood and liver, and the generation of cytokine-induced neutrophil chemoattractant in the liver of acutely ethanol-intoxicated rats. Alcohol Clin Exp Res 20:914–920. [DOI] [PubMed] [Google Scholar]
- 22.Spitzer JA, Zhang P (1996): Gender differences in neutrophil function and cytokine-induced neutrophil chemoattractant generation in endotoxic rats. Inflammation. 20:485–498. [DOI] [PubMed] [Google Scholar]
- 23.Alfonso-Loeches S, Pascual M, Guerri C (2013): Gender differences in alcohol-induced neurotoxicity and brain damage. Toxicology. 311:27–34. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs EJ, Messingham KA (2002): Influence of alcohol and gender on immune response. Alcohol Res Health. 26:257–263. [PMC free article] [PubMed] [Google Scholar]
- 25.Pascual M, Montesinos J, Marcos M, Torres JL, Costa-Alba P, García-García F, et al. (2017): Gender differences in the inflammatory cytokine and chemokine profiles induced by binge ethanol drinking in adolescence. Addict Biol 22:1829–1841. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer JA (1999): Gender differences in some host defense mechanisms. Lupus. 8:380–383. [DOI] [PubMed] [Google Scholar]
- 27.Blednov YA, Bergeson SE, Walker D, Ferreira VM, Kuziel WA, Harris RA (2005): Perturbation of chemokine networks by gene deletion alters the reinforcing actions of ethanol. Behav Brain Res 165:110–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blednov YA, Black M, Benavidez JM, Da Costa A, Mayfield J, Harris RA (2017): Sedative and Motor Incoordination Effects of Ethanol in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res 41:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blednov YA, Black M, Chernis J, Da Costa A, Mayfield J, Harris RA (2017): Ethanol Consumption in Mice Lacking CD14, TLR2, TLR4, or MyD88. Alcohol Clin Exp Res 41:516–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayfield J, Arends MA, Harris RA, Blednov YA (2016): Genes and Alcohol Consumption: Studies with Mutant Mice. Int Rev Neurobiol 126:293–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blednov YA, Benavidez JM, Geil C, Perra S, Morikawa H, Harris RA (2011): Activation of inflammatory signaling by lipopolysaccharide produces a prolonged increase of voluntary alcohol intake in mice. Brain Behav Immun 25 Suppl 1 :S92–S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.June HL, Liu J, Warnock KT, Bell KA, Balan I, Bollino D, et al. (2015): CRF-amplified neuronal TLR4/MCP-1 signaling regulates alcohol self-administration. Neuropsychopharmacology. 40:1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Yang AR, Kelly T, Puche A, Esoga C, June HL, et al. (2011): Binge alcohol drinking is associated with GABAA alpha2-regulated Toll-like receptor 4 (TLR4) expression in the central amygdala. Proc Natl Acad Sci U S A. 108:4465–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Truitt JM, Blednov YA, Benavidez JM, Black M, Ponomareva O, Law J, et al. (2016): Inhibition of IKβ Reduces Ethanol Consumption in C57BL/6J Mice. eneuro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C (2010): Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J Neurosci 30:8285–8295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt JM, et al. (2017): Genetic and Pharmacologic Manipulation of TLR4 Has Minimal Impact on Ethanol Consumption in Rodents. J Neurosci 37:1139–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCarthy GR, Warden A, Bridges C, Blednov YA, Mayfield RD, Harris RA. (2018): Chronic ethanol consumption: Role of TLR3/TRIF-dependent signaling. Addiction Biology. 23(3):889–903. doi: 10.1111/adb.12539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J (2013): High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 73:602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD (2012): Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci 32:1884–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Warden AS, Azzam M, DaCosta A, Mason S, Blednov YA, Messing RO, Mayfield RD, Harris RA (2019): Toll-like receptor 3 activation increases voluntary alcohol intake in C57BL/6J male mice. Brain, Behavior & Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou B, Reizis B, DeFranco AL (2008): Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity. 29:272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. (2008): Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res 32:1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise RA (1973): Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 29:203–210. [DOI] [PubMed] [Google Scholar]
- 44.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011): Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melendez RI (2011): Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol Clin Exp Res 35:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenwasser AM, Fixaris MC, Crabbe JC, Brooks PC, Ascheid S (2013): Escalation of intake under intermittent ethanol access in diverse mouse genotypes. Addict Biol 18:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (2009): The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622. [DOI] [PubMed] [Google Scholar]
- 48.Osterndorff-Kahanek E, Ponomarev I, Blednov YA, Harris RA (2013): Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PLoS One. 8:e59870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osterndorff-Kahanek EA, Becker HC, Lopez MF, Farris SP, Tiwari GR, Nunez YO, et al. (2015): Chronic ethanol exposure produces time- and brain region-dependent changes in gene coexpression networks. PLoS One. 10:e0121522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warden A, Truitt J, Merriman M, Ponomareva O, Jameson K, Ferguson LB, et al. (2016): Localization of PPAR isotypes in the adult mouse and human brain. Sci Rep 6:27618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Torcia MG, Nencioni L, Clemente AM, Civitelli L, Celestino I, Limongi D, et al. (2012): Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS One. 7:e39853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aomatsu M, Kato T, Kasahara E, Kitagawa S (2013): Gender difference in tumor necrosis factor-α production in human neutrophils stimulated by lipopolysaccharide and interferon-γ. Biochem Biophys Res Commun 441:220–225. [DOI] [PubMed] [Google Scholar]
- 53.Villapol S, Loane DJ, Burns MP (2017): Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia. 65:1423–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayfield J, Harris RA (2017): The Neuroimmune Basis of Excessive Alcohol Consumption. Neuropsychopharmacology. 42:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cunningham C, Campion S, Teeling J, Felton L, Perry VH (2007): The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C). Brain Behav Immun 21:490–502. [DOI] [PubMed] [Google Scholar]
- 56.Squeglia LM, Schweinsburg AD, Pulido C, Tapert SF (2011): Adolescent binge drinking linked to abnormal spatial working memory brain activation: differential gender effects. Alcohol Clin Exp Res 35:1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Squeglia LM, Sorg SF, Schweinsburg AD, Wetherill RR, Pulido C, Tapert SF (2012): Binge drinking differentially affects adolescent male and female brain morphometry. Psychopharmacology (Berl). 220:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaillon S, Berthenet K, Garlanda C (2017): Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol [DOI] [PubMed] [Google Scholar]
- 59.Schwarz JM, Sholar PW, Bilbo SD (2012): Sex differences in microglial colonization of the developing rat brain. J Neurochem 120:948–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin L, Crews FT (2012): Chronic ethanol increases systemic TLR3 agonist-induced neuroinflammation and neurodegeneration. J Neuroinflammation. 9:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhu X, Levasseur PR, Michaelis KA, Burfeind KG, Marks DL (2016): A distinct brain pathway links viral RNA exposure to sickness behavior. Sci Rep 6:29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doyle HH, Eidson LN, Sinkiewicz DM, Murphy AZ (2017): Sex Differences in Microglia Activity within the Periaqueductal Gray of the Rat: A Potential Mechanism Driving the Dimorphic Effects of Morphine. J Neurosci 37:3202–3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bollinger JL, Bergeon Burns CM, Wellman CL (2016): Differential effects of stress on microglial cell activation in male and female medial prefrontal cortex. Brain Behav Immun 52:88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tonelli LH, Holmes A, Postolache TT (2008): Intranasal immune challenge induces sex-dependent depressive-like behavior and cytokine expression in the brain. Neuropsychopharmacology. 33:1038–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF (2008): Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang PF, Fang H, Chen J, Lin S, Liu Y, Xiong XY, et al. (2014): Polyinosinic-polycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3. J Immunol 192:4783–4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pan LN, Zhu W, Li Y, Xu XL, Guo LJ, Lu Q, et al. (2014): Astrocytic Toll-like receptor 3 is associated with ischemic preconditioning-induced protection against brain ischemia in rodents. PLoS One. 9:e99526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Becker JB, Koob GF (2016): Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68:242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roberts AJ, Smith AD, Weiss F, Rivier C, Koob GF (1998): Estrous cycle effects on operant responding for ethanol in female rats. Alcohol Clin Exp Res 22:1564–1569. [PubMed] [Google Scholar]
- 70.Dazzi L, Seu E, Cherchi G, Barbieri PP, Matzeu A, Biggio G (2007): Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology. 32:892–901. [DOI] [PubMed] [Google Scholar]
- 71.Cha YM, Li Q, Wilson WA, Swartzwelder HS (2006): Sedative and GABAergic effects of ethanol on male and female rats. Alcohol Clin Exp Res 30:113–118. [DOI] [PubMed] [Google Scholar]
- 72.Ford MM, Eldridge JC, Samson HH (2002): Microanalysis of ethanol self-administration: estrous cycle phase-related changes in consumption patterns. Alcohol Clin Exp Res 26:635–643. [PubMed] [Google Scholar]
- 73.Moore CF, Lynch WJ (2015): Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav 132:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Priddy BM, Carmack SA, Thomas LC, Vendruscolo JC, Koob GF, Vendruscolo LF (2017): Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav 152:61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Straub RH (2007): The complex role of estrogens in inflammation. Endocr Rev 28:521–574. [DOI] [PubMed] [Google Scholar]
- 76.Whitacre CC, Reingold SC, O’Looney PA (1999): A gender gap in autoimmunity. Science. 283:1277–1278. [DOI] [PubMed] [Google Scholar]
- 77.Chakrabarti M, Haque A, Banik NL, Nagarkatti P, Nagarkatti M, Ray SK (2014): Estrogen receptor agonists for attenuation of neuroinflammation and neurodegeneration. Brain Res Bull 109:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ghisletti S, Meda C, Maggi A, Vegeto E (2005): 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol 25:2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Correale J, Arias M, Gilmore W (1998): Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol 161:3365–3374. [PubMed] [Google Scholar]
- 80.Lesmeister MJ, Jorgenson RL, Young SL, Misfeldt ML (2005): 17Beta-estradiol suppresses TLR3-induced cytokine and chemokine production in endometrial epithelial cells. Reprod Biol Endocrinol 3:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jorgenson RL, Young SL, Lesmeister MJ, Lyddon TD, Misfeldt ML (2005): Human endometrial epithelial cells cyclically express Toll-like receptor 3 (TLR3) and exhibit TLR3-dependent responses to dsRNA. Hum Immunol 66:469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Soucy G, Boivin G, Labrie F, Rivest S (2005): Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J Immunol 174:6391–6398. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2: Poly(I:C) prevents reductions in alcohol intake when alcohol access occurs after peak innate immune activation in females on 20% ethanol. Two-bottle choice every-other-day (20%v/v) drinking in female mice on 5-day injection schedule. Ethanol (EtOH) intake (g/kg/24 h) (A), preference for EtOH (B), and total fluid intake (C) in saline-treated versus poly(I:C)-treated female mice (C57BL/6J, n=10 per group). Data were analyzed by repeated-measures 2-way ANOVA. There was no effect of poly(I:C) on ethanol consumption [(A) Ftreatment × time(8,144)=0.59, p=0.78; Ftreatment(1,18)=1.99, p=0.17; Ftime(8,144)=25.74, p<0.0001] or preference [(B), Ftreatment × time(8,144)=0.18, p=0.99; Ftreatment(1,18)=0.79, p=0.38; Ftime(8,144)=17.1, p<0.0001]. Total fluid intake was unchanged by treatment [(C), Ftreatment × time(8,144)=0.80, p=0.60; Ftreatment(1,18)=0.06, p=0.80; Ftime(8,144)=6.34, p<0.0001].
Supplemental Figure 5: Quantification of TRIF-dependent, MyD88-dependent and interferon proteins in animals on different poly(I:C) injection schedules. Data were analyzed by 2-way ANOVA followed by Tukey HSD post hoc tests (p<0.0001 (****), p<0.0002 (***), p<0.0021 (**), p<0.0332 (*) compared with saline group). Data are represented as mean + SEM, n = 2 per group.
Supplemental Figure 6: Representative images for protein confirmation of mRNA changes in poly(I:C) drinking experiments. 4-day injection schedule: MYD88 (A), IKKB (C), TRIF (E), IKKI (G), IFNB (I). 5-day injection schedule: MYD88 (B), IKKB (D), TRIF (F), IKKI (H), IFNB (J). Scale bar = 100 μm.
Supplemental Figure 1: Raw qRT-PCR data for poly(I:C) timecourse. The levels of transcripts are presented using fold-change values normalized to endogenous control. Data were analyzed by 2-way ANOVA followed by Tukey HSD post hoc tests (p<0.0001 (****), p<0.0002 (***), p<0.0021 (**), p<0.0332 (*) compared to saline group).
Supplemental Figure 3: Lack of correlation between ethanol intake and severity of sickness response after poly(I:C) injections. Body weight (g) during (A) 4-day injection schedule and (B) 5-day injection schedule drinking experiments. (C) Correlation between average alcohol intake (g/kg) for 4-day injection schedule and % body weight change. The severity of sickness was calculated % change in body weight after the first injection of poly(I:C) then averaged across the ten injections. Body weight data was analyzed by repeated-measures 2-way ANOVA followed by Bonferroni post hoc tests (p<0.0332 (*) compared with saline group). Pearson correlation (α=0.05) was used to correlate % weight change to average alcohol intake (g/kg), (Saline r=0.3717, R2=0.1382, p=0.29, poly(I:C) r= −0.1785, R2=0.03185, p=0.59).
Supplemental Figure 4: Raw qRT-PCR data for 4-day and 5-day poly(I:C) schedules after chronic ethanol exposure. The transcript levels of genes are presented as fold-change values normalized to respective endogenous control. Data were analyzed by 2-way ANOVA followed by Tukey HSD post hoc tests (p<0.0001 (****), p<0.0002 (***), p<0.0021 (**), p<0.0332 (*) compared with saline group).
Supplemental Figure 7: Replication experiment for poly(I:C)-induced decreases in alcohol intake in females. C57BL/6J female mice were injected with saline or poly(I:C) (5mg/kg) every fourth day for a total of ten injections during two-bottle choice, every-other-day drinking (EOD,15%v/v) using the same batch of poly(I:C). (A) 4-day injection schedule; (B) Ethanol (EtOH) intake (g/kg/24 h); (C) preference for EtOH; (D) total fluid intake. Arrows indicate days when animals received injection; (n=10 per group).
Supplemental Table 1: Antibody information


