Abstract
Background:
Decades of research point to cortisol insensitivity as a biomarker of depression. Despite a vast literature on cortisol’s effects on memory, the role of cortisol insensitivity in core psychological features of depression, such as emotional memory biases, is unknown.
Methods:
Sixty-five pre-menopausal women with varying levels of depression completed this study involving an at-home low-dose dexamethasone suppression test (DST) and four experimental sessions (i.e., two visits for memory encoding of emotional pictures, each of which was followed 48 hours later by a recall test). Participants received 20 mg oral cortisol (CORT) or placebo prior to encoding. We tested whether systemic cortisol insensitivity measured with the DST predicted cognitive sensitivity to CORT, which was operationalized as change in negatively biased memory formation for pictures encoded during CORT vs. placebo.
Results:
Cortisol insensitivity was associated with more severe depression and flatter diurnal cortisol. Cortisol insensitivity predicted negative memory bias for pictures encoded during placebo and reduction in negative memory bias for pictures encoded during CORT (vs. placebo), even after accounting for psychiatric symptomatology.
Conclusions:
Our findings replicate research showing that cortisol insensitivity predicts depression severity and flatter diurnal cortisol. The results further suggest that systemic cortisol insensitivity is related to negative memory bias and its alleviation by acute cortisol administration. These novel cognitive findings tie together knowledge regarding endocrine and psychological dysfunction in depression, and suggest that boosting cortisol signal may be cognitively beneficial in individuals with cortisol insensitivity.
Trial Registration:
ClinicalTrials.gov: Depression, Adversity, and Stress Hormones (DASH) Study; https://clinicaltrials.gov/ct2/show/NCT03195933; NCT03195933.
Keywords: Depression, negative memory bias, cortisol, glucocorticoid insensitivity, glucocorticoid resistance, diurnal cortisol slope
INTRODUCTION
Glucocorticoid (GC) insensitivity is a reproducible physiological alteration observed at higher rates in depressed patients than healthy individuals (1,2). Alterations in neural sensitivity to GCs are observed in depression (3,4), and animal models suggest that altered GC effects on neuroplasticity are of utmost importance to depression (5). Despite vast literatures on GCs’ effects on neuroplasticity and emotional memory (6-8), little is known about the relevance of GC insensitivity for alterations in emotional memory in depression (9,10).
GCs are released from the adrenal gland and bind to receptors expressed in the periphery and brain: mineralocorticoid receptors (MR) and glucocorticoid receptors (GR) (11). GCs (primarily cortisol in primates and corticosterone in rodents) regulate physiologic and psychological processes, and have equally important functions during stress and in the absence of stress (9,11,12). GC insensitivity, also referred to as GC resistance, refers to decreases in sensitivity to GC signaling mechanisms across a variety of tissue types, and is associated with a hypothalamic pituitary adrenal (HPA) negative feedback deficit, in which cortisol or exogenous GCs are relatively ineffective in suppressing further HPA activation (1,2,9,13).
GC insensitivity is conceptualized as an endocrine biomarker of depression and is reflected at multiple levels of analysis, from systemic to genetic (1,9,14-16). Systemic GC sensitivity can be indexed using in vivo approaches including the dexamethasone suppression test (DST) (17-19). The DST uses the synthetic GC dexamethasone, typically administered near bedtime (e.g., 10pm or 11pm), to suppress endogenous cortisol release (20-22). To determine amount of suppression, the endogenous cortisol level from the morning(s) preceding dexamethasone administration is compared to the level from the morning following administration (2,21,23). This comparison of pre- to post-dexamethasone cortisol levels indexes HPA negative feedback viability (17,24). In the 1970s and 1980s the DST was heavily investigated as a psychiatric diagnostic indicator (23,24). Subsequent research demonstrated low diagnostic specificity and sensitivity, and the use of a categorical cut point (suppression vs. non-suppression) limits the utility of the DST (2,23). Nonetheless, research continued to indicate that a large percentage (30-70%) of patients with moderate-to-severe depression show GC insensitivity on the DST or other measures (1,2,9,25). More recent research with lower doses (0.25-0.50 mg) demonstrates that the DST can be analyzed continuously (vs. categorically) (26,27) with individual differences in sensitivity related to depression severity (21). It should be noted that bioavailability and rate of metabolism of dexamethasone are factors in determining DST results (28-30), and lower plasma concentrations of dexamethasone have been observed in depressed subjects (30,31). Thus, there may be a number of biologic alterations contributing to feedback insensitivity indexed with the DST (22,26,31).
The relevance of GC insensitivity for emotional memory biases in depression has received little empirical investigation (1,9,10,32,33). This represents a huge gap in our knowledge because GCs have potent yet variable effects on memory (6,7,34-38). GCs at times enhance memory formation and impair working memory and retrieval of already stored memories (8,39). Recent findings also show that stress – in part due to GC elevation – can enhance memory of experiences that occur at the same time or within the same context as the stressor, but can suppress memory for unrelated information (37,40). Relatedly, GC effects on memory are often most prominent when associated with emotional arousal (41-45).
Effects of acute GCs on memory are typically studied in nonclinical populations, but there is a growing body of evidence implicating altered effects of GCs in stress-related disorders (PTSD and depression), sometimes with normalization of function with acute GC administration (3,4,8,39,46,47). In young adult patients acute administration of fludrocortisone (an MR agonist) (48) or two-day treatment with dexamethasone (a GR agonist) (49) normalizes altered memory function in depression, which may be due to direct corticosteroid receptor stimulation or to reduction of circulating cortisol, as both dexamethasone and fludrocortisone suppress cortisol (48,49). Studies that administer cortisol (i.e., hydrocortisone) can help disentangle these alternative interpretations given the elevated circulating cortisol, and suggest that acutely heightened cortisol may normalize emotional memory (3,4,8,39,50).
In addition, research in rodents has shown that prior history of the organism is a potent factor in determining effects of stress and GCs on learning and neuroplasticity (51,52). For instance, adult rats with history of low levels of maternal care have a bias toward learning in contexts with GC elevation, whereas adult rats with history of high levels of maternal care have a bias toward learning when GCs are not elevated (53). Moreover, history of lower levels of maternal care is associated with impaired longterm potentiation (LTP) in hippocampal slices from adult rodents, which is normalized by corticosterone (53,54). Conversely, corticosterone reduces LTP in hippocampal slices from rodents with higher levels of maternal care (53,54). These rodent data suggest that early adverse caregiving is associated with a shifted dose-response curve, in which synaptic neuroplasticity is deficient at baseline and normalized by GCs (52-54).
In addition to effects on plasticity, early aversive caregiving in rodents alters life-long HPA axis functioning (55,56). Heim and colleagues found that depressed patients with history of adversity are more likely to show HPA dysregulation than depressed patients without adversity (57,58). In addition, childhood abuse (particularly emotional abuse) predicts incidence of depression and negative cognitive bias (59-62), but the role of GC insensitivity in these relationships is not established.
It is unknown whether GC insensitivity is associated with effects of acute manipulation of GCs on negatively biased memory formation in depression (8,9). In the current study, we administered exogenous cortisol and placebo during memory formation for emotional pictures, and operationalized “cognitive GC sensitivity” as magnitude of change in negatively biased memory formation for pictures encoded during cortisol administration (CORT) vs. placebo. Our goal was to determine whether individuals with systemic GC insensitivity measured with the DST benefitted from the acute pharmacological boost in cortisol as evidenced by reduction in negatively biased memory formation. We hypothesize that exogenous cortisol administration overrides an endogenous GC insensitivity in neural tissues, and thus transiently normalizes neurocognitive function. A secondary goal was to investigate whether prior experience of adverse caregiving was associated with systemic and cognitive GC sensitivity. Finally, the current study only recruited women. As cortisol’s effects on cognition differ by sex (63,64), and women are twice as likely as men to suffer from depression (65,66), it is essential to adequately power studies to investigate etiological mechanisms of depression in women.
METHODS AND MATERIALS
Participants
Participants were a community-based sample of unmedicated premenopausal women aged 18-45 years old, who took part in an NIMH-funded study of cortisol-related neurocognition (3). The current report includes 65 participants who adhered to the at-home cortisol protocol, completed a DST, and were not taking psychotropic medication. See Supplemental Methods for eligibility criteria. Participants provided written informed consent and were paid for participation. The University of Wisconsin Health Sciences IRB approved study procedures.
Consistent with the NIH Research Domain Criteria initiative, depression was investigated along a continuum of severity (67). Depression severity was indexed by averaging Beck Depression Inventory II (BDI-II) scores taken prior to drug administration during encoding sessions (68). Psychiatric diagnoses were determined using the SCID-I/P for DSM-IV-TR with additional questions for DSM-5 to identify women meeting criteria for depressive disorders (n=39) and never-depressed controls (n=26) (69). Depression diagnoses were further categorized as current major depressive disorder (MDD; n=15) or other depression (n=24, i.e., depressive disorders other than MDD and/or past MDD). See Supplemental Table S1 for a complete list of diagnoses.
Childhood emotional abuse
We assessed severity of childhood emotional abuse (EA) with the Emotional Abuse subscale of the Childhood Trauma Questionnaire (CTQ) (70). Of the final sample, 13 women experienced moderate-to-extreme (“severe”), 9 experienced low-to-moderate (“moderate”), and 43 experienced none-to-minimal (“minimal”) EA. Consistent with the close association between EA and adult depression (59-62), our sample does not fully disentangle variation in EA and depression, although we intentionally recruited a sample in which they were not entirely overlapping (Table 1); correlation between EA and depression severity is r(64)=0.36, p<.01.
Table 1.
Demographic Characteristics
| Characteristics | Depression Groups | ||
|---|---|---|---|
| Never Depressed (n = 26) |
Other Depression (n = 24) |
Current MDD (n = 15) |
|
| Age, Years | 27.2 ±7.9 | 29.4 ±7.7 | 26.1 ±5.7 |
| Education level | 4.5 ±1.4 | 4.7 ±1.3 | 4.5 ±1.2 |
| Overall CTQ Score | 30.6 ±7.8 | 39.9 ±16.5 | 47.5 ±15.3 |
| CTQ Emotional Abuse Score | 6.9 ±2.6 | 8.7 ±5.0 | 12.1 ±4.8 |
| Number of participants with moderate-to-severe Emotional Abusea | 5 (19) | 8 (33) | 9 (60) |
| Racea | |||
| White | 18 (69) | 23 (96) | 9 (60) |
| Asian | 5 (19) | 0 | 5 (33) |
| African American | 3 (12) | 0 | 0 |
| Unknown | 0 | 1 (4) | 1 (7) |
| Ethnicity | |||
| Hispanic/Latina | 0 | 3 (13) | 1 (7) |
| Not Hispanic/Latina | 26 (100) | 20 (83) | 14 (93) |
| Unknown | 0 | 1 (4) | 0 |
Values are mean ±SD or n (%).
CTQ, Childhood Trauma Questionnaire; MDD, major depressive disorder.
Education categories: 1 = Less than high school; 2 = High school diploma or equivalent (i.e., GED); 3 = Some college, no degree; 4 = Associate’s degree; 5 = Bachelor’s degree; 6 = Master’s degree; 7 = Doctoral degree.
Groups differed on childhood emotional abuse (Chi-Square=15.0, p<.01) and race (Chi-Square=13.5, p<.01, apparent in the lack of racial diversity in the “Other Depression” group). Groups did not differ on demographic characteristics of age, education level, or ethnicity, p’s>.17.
Procedure
At-home saliva collection and DST
Participants collected saliva at home on 4 days (Monday through Thursday) during a typical week to assess cortisol concentrations (71). Participants were instructed to abstain from the following activities for 60 minutes prior to sample collection: oral hygiene; strenuous activities/exercise; nicotine use; eating or drinking (anything other than water, which could not be consumed within 20 minutes of sample collection). Participants used Salivettes (Sarstedt, Nümbrecht, Germany) to provide one sample upon awakening, 40 minutes post-awakening (“morning peak”), and in the evening at 10pm. See Supplemental Methods for additional procedures.
Participants took a pill containing a low dose of dexamethasone (0.25 mg) immediately following their 10pm sample on Day 3, and the cortisol response to dexamethasone was assessed on Day 4. This dexamethasone dose is lower than the common dose range of 0.5-1 mg (72-74), although 0.25 mg has revealed heightened GC sensitivity in studies of PTSD (75). We used a low dose with the goal of obtaining a broad range of responses to dexamethasone.
Experimental manipulation of cortisol during memory formation
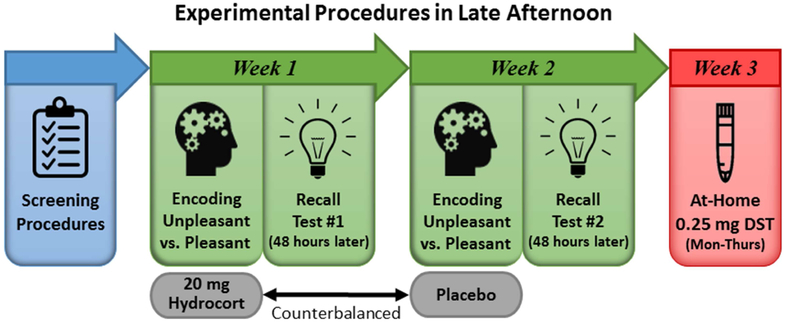
Cortisol was pharmacologically manipulated during memory formation with oral administration of 20 mg encapsulated cortisol (i.e., hydrocortisone; CORT) vs. an identically appearing placebo capsule. Twenty mg oral cortisol causes significant cortisol elevations (i.e., commensurate with vigorous exercise or moderate-to-extreme stress). Participation included two memory encoding visits, which also included MRI (data published elsewhere (3,76)), and two recall test sessions (see Figure 1 for study timeline). During encoding sessions, study drug (i.e., CORT or placebo) was administered 90 minutes before the encoding task. Drug order was randomized and double-blinded. The University of Wisconsin Pharmaceutical Research Center prepared and randomized study drugs. Both encoding sessions began at ~4:15pm (earliest start time was 4:03pm and latest was 4:43pm) and were typically separated by 1 week, with a minimum separation of 5 days. During memory encoding, 84 pictures from the International Affective Picture System (IAPS) (77) were presented for 5 seconds each. Two sets of pictures that were unique yet psychometrically-matched on normative ratings of affective valence (i.e., pleasantness) and arousal were presented during CORT and placebo (see Supplemental Table S2 for IAPS picture numbers). Free recall tests were conducted 48 hours after each encoding session. Participants had 10 minutes to provide written descriptions of as many pictures as they could recall. If participants had not exhausted recall by 10 minutes, they were given additional time. Scoring was conducted blind to drug condition, depression, and EA. Two scorers coded the recall descriptions. Discrepancies between scorers were rectified by a third individual (RMH).
Figure 1. Study Timeline.
Participant eligibility was determined by conducting screening interviews over the phone and in person. Study participation consisted of two memory encoding sessions and two recall test sessions in the lab, in addition to a dexamethasone suppression test (DST) at home. During encoding sessions, which typically occurred 1 week apart, participants completed an emotional memory encoding task approximately 90 minutes after taking a pill containing either 20 mg cortisol (CORT) or placebo. Drug order was randomized across the two sessions and double-blinded. Memory recall for the pictures was tested 48 hours later. All experimental sessions were conducted late in the day when endogenous cortisol levels are relatively low. Participants also completed a DST, which included saliva sampling at home for 4 days (Monday through Thursday). Immediately after collecting the 10pm sample on Day 3, participants took a pill containing a low dose of dexamethasone (0.25 mg). Cortisol response to dexamethasone was measured on Day 4. The majority of DSTs were completed within 10 days of memory testing.
Quantification of DST feedback sensitivity and diurnal cortisol slope
Saliva samples were stored in participants’ refrigerators and at −80°C when returned to the lab until they were shipped to Technische Universität Dresden for analysis. Cortisol concentrations were measured with a high sensitivity chemiluminescence immunoassay (IBL International, Hamburg, Germany). Intra- and inter-assay CVs were below 8%. Salivary data were cleaned by inspecting collection times for sampling accuracy. Samples collected 30-60 minutes after awakening were used as the morning peak sample. Samples collected outside of this timeframe were excluded. DST feedback sensitivity was indexed as the difference between pre-dexamethasone cortisol levels for the morning peak sample (averaged across Days 1-3) and the post-dexamethasone morning peak sample from Day 4 (see Supplemental Table S3 for raw cortisol levels). Suppression was scored continuously, and higher numbers refer to greater DST feedback sensitivity (i.e., greater suppression of cortisol on Day 4 compared to the average of Days 1-3). Computation of diurnal cortisol slope was modeled after Jarcho et al. (21) by indexing diurnal cortisol slope as the absolute value of the change in cortisol levels from the morning peak sample to the 10pm sample divided by the time between the two samples, averaged across Days 1-3. Higher numbers indicate a steeper diurnal cortisol slope. As described by Tukey (78), and used in previous research examining cortisol (79,80), we winsorized one value >2 SD above the mean to the 2 SD value.
Computation of memory bias and data analysis
Memory bias is expressed as a ratio (i.e., the difference between pleasant and unpleasant pictures recalled divided by the total number of pleasant and unpleasant pictures recalled), which adjusts for variation in overall recall performance. Memory bias was calculated separately for CORT and placebo sessions, and higher numbers reflect more negatively biased memory formation (see Table 2 and Figures 3A & 3B for values). Change in memory bias values (i.e., cognitive GC sensitivity) reflects the difference for CORT minus placebo, i.e., bias for pictures encoded during CORT minus bias for pictures encoded during placebo; lower numbers reflect a greater reduction in negative memory bias for pictures encoded during CORT compared to placebo (see Figure 3C).
Table 2.
Cortisol Levels, Dexamethasone Suppression Test, and Memory for Pictures Encoded during Placebo and Cortisol Administration
| Depression Groups | Depression Group Comparisons |
|||
|---|---|---|---|---|
| Never Depressed (n = 26) |
Other Depression (n = 24) |
Current MDD (n = 15) |
||
| Cortisol levels, μg/dL | ||||
| Morning peak cortisol | 0.60 ±0.24 | 0.58 ±0.27 | 0.57 ±0.26 | F(2,62)=0.09, n.s. |
| Post-dexamethasone morning cortisol | 0.30 ±0.25 | 0.29 ±0.24 | 0.42 ±0.33 | F(2,62)=1.23, n.s. |
| DST feedback sensitivitya | 0.30 ±0.19 | 0.28 ±0.17 | 0.15 ±0.24 | F(2,62)=3.10, p=.05 |
| Memory biasb | ||||
| Placebo | 0.03 ±0.19 | 0.11 ±0.18 | 0.26 ±0.21 | F(2,62)=6.91, p=.002 |
| CORT | 0.07 ±0.19 | 0.10 ±0.17 | 0.11 ±0.22 | F(2,62)=0.23, n.s. |
| Total memoryc | ||||
| Placebo | 24.0 ±9.6 | 22.7 ±6.9 | 20.0 ±5.6 | F(2,62)=1.22, n.s. |
| CORT | 25.0 ±9.0 | 24.3 ±7.6 | 21.5 ±5.1 | F(2,62)=1.05, n.s. |
Values are mean ±SD.
CORT, cortisol administration; DST, dexamethasone suppression test; MDD, major depressive disorder. International System of Units (SI) conversion factors: To convert cortisol to nmol/L, multiply values by 27.588.
DST feedback sensitivity is the difference between morning peak cortisol and post-dexamethasone morning cortisol, with higher numbers reflecting greater DST feedback sensitivity (i.e., greater dexamethasone suppression of cortisol). Groups differed on DST feedback sensitivity, F(2,62)=3.10, p=.05, reflected in the lower feedback sensitivity in the MDD group. However, groups did not differ significantly on absolute morning peak cortisol or post-dexamethasone morning cortisol, p’s>.29.
Memory bias is expressed as a ratio, in which higher numbers reflect more negatively biased memory (see method section for detail). When DST feedback sensitivity is not included in the model, there is a main effect of Group for memory bias, F(2,62)=3.58, p=.03, such that depressed subjects show greater negative memory bias, particularly in the MDD group for pictures encoded during placebo.
There is also a Group X Drug interaction for memory bias, F(2,62)=3.61, p=.03, reflected in the normalization of memory bias in MDD participants for pictures encoded during CORT. See text and Figure 3 for findings when DST Feedback Sensitivity is included in the model. Also see text for analyses with depression treated as a continuous rather than categorical variable.
Total memory refers to values for unpleasant plus pleasant pictures recalled. Effects of CORT (vs. placebo) trended toward facilitation of total free recall, F(1,62)=3.20, p=.078, across all levels of depression severity.
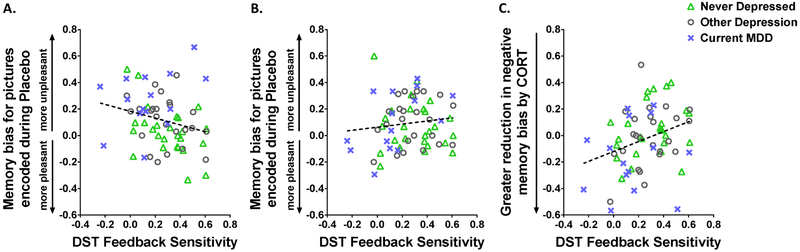
Figure 3. Dexamethasone Suppression Test (DST) Feedback Sensitivity and Memory Bias.
Scatter plots show how variation in DST feedback sensitivity predicts memory bias. For DST feedback sensitivity, higher values reflect greater feedback sensitivity (i.e., greater post-dexamethasone cortisol suppression). For memory bias in panels A & B, higher values reflect more negatively biased memory formation. A) Lower DST feedback sensitivity was associated with more negatively biased memory for pictures encoded during placebo, r(64)=−0.25, p=.05. B) Feedback sensitivity and memory bias were unrelated for pictures encoded during CORT, r(64)=0.12, n.s. C) Panel C illustrates change in memory bias (CORT minus placebo), with lower numbers representing a greater reduction in negative bias for pictures encoded during CORT compared to placebo (reflected on Y-axis). Lower feedback sensitivity was associated with greater reduction in negative memory bias for pictures encoded during CORT compared to placebo, r(64)=0.32, p=.009. Depression groups are differentiated with symbols in the plots (see legends), which show the wide variability in DST feedback sensitivity within and across groups. The symbols are provided merely for illustration purposes, as DST feedback sensitivity interacted with effects of CORT on memory bias even after controlling for depression severity (see text for detail).
Analyses were conducted in SAS Enterprise Guide 7.1. ANCOVA and zero-order correlations were used to test relations among continuous measures of depression and/or EA severity and measures of GC sensitivity, including DST feedback sensitivity and cognitive sensitivity to CORT (i.e., memory bias for pictures encoded during CORT vs. placebo). We used ANOVA to evaluate how GC sensitivity varied by depression group. To test the relation between DST feedback sensitivity and cognitive sensitivity to CORT, we used repeated measures ANCOVA with negative memory bias as the dependent variable and the following predictors: Drug (CORT vs. placebo), DST feedback sensitivity, depression severity, and EA severity. We also estimated menstrual phase for each segment of the study using dates of first day of last period for 2-to-3 cycles, and tested whether it moderated effects (see Supplement for null results for menstrual phase).
RESULTS
DST feedback sensitivity and diurnal cortisol slope
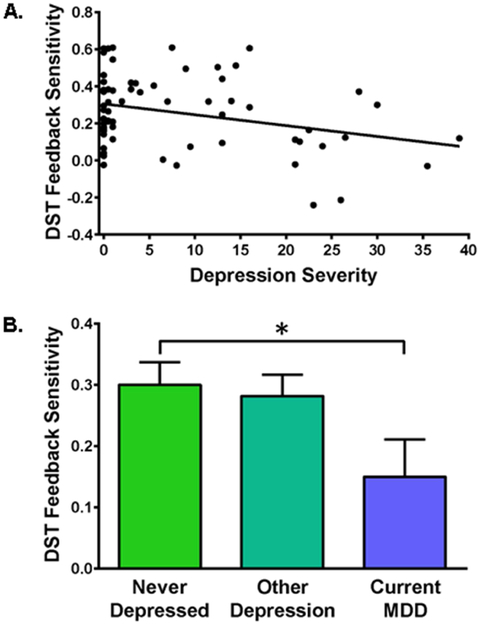
DST feedback sensitivity was inversely correlated with depression severity, r(64)=−0.27, p=.03 (Figure 2A), such that lower DST feedback sensitivity (i.e., less suppression of morning peak cortisol reflecting GC insensitivity) was associated with greater depression severity. The association between depression severity and DST feedback sensitivity remained when EA was added to the model, F(1,59)=6.62, p=.01. There was also a marginal association between EA and DST feedback sensitivity, F(2,59)=2.84, p=.07, but no interaction between EA and depression severity, F(2,59)=0.63, n.s. (see Supplemental Figure S1).
Figure 2. Associations between Dexamethasone Suppression Test (DST) Feedback Sensitivity and Depression.
DST feedback sensitivity values reflect the difference between pre- and post-dexamethasone morning cortisol levels in μg/dL, with higher values representing greater DST feedback sensitivity (i.e., greater post-dexamethasone cortisol suppression). A) DST feedback sensitivity was inversely correlated with depression severity indexed with the Beck Depression Inventory II (BDI-II), r(64)=−0.27, p=.03, reflecting lower (impaired) DST feedback sensitivity associated with greater depression. B) Depressive disorder diagnosis (“Group”) was related to feedback sensitivity, F(2,62)=3.10, p=.05, reflecting lower (impaired) DST feedback sensitivity in women with current Major Depressive Disorder (MDD) compared to never-depressed controls, F(1,40)=3.96, p=.05. Feedback sensitivity was not significantly impaired for women with mild depressive disorders other than current MDD, F(1,47)=0.18, n.s.
Consistent with findings using a continuous measure of depression, depressive disorder diagnosis (“Group”) was also related to feedback sensitivity, F(2,62)=3.10, p=.05. Women with MDD, compared to never-depressed controls, showed impairment in DST feedback sensitivity, F(1,40)=3.96, p=.05 (Table 2 & Figure 2B). DST feedback sensitivity was not impaired for women with depressive disorders other than current MDD, F(1,47)=0.18, n.s. (Table 2 & Figure 2B). Depression groups did not differ on absolute cortisol levels assessed on days before or after dexamethasone administration, p’s>.29 (Table 2).
Diurnal cortisol slope and DST feedback sensitivity were positively correlated, r(64)=0.32, p=.01 (see Supplemental Figure S2), such that steeper decline of cortisol throughout the day was related to greater feedback sensitivity (i.e., more DST suppression). When including EA and depression severity in the model, diurnal cortisol slope remained a significant predictor of DST feedback sensitivity, F(1,53)=7.87, p=.007 and did not interact with either EA or depression severity, p’s>.12. Diurnal cortisol slope was unrelated to EA or depression severity, p’s>.54.
Cognitive sensitivity to CORT
There was a main effect of depression severity for memory bias, F(1,63)=7.84, p=.007, such that greater depression severity was associated with greater negative memory bias. There was also a Drug (CORT vs. placebo) X Depression Severity interaction for memory bias, F(1,63)=5.93, p=.02, reflecting normalization of depression-related memory bias for pictures encoded during CORT compared to placebo. See Table 2 for means by depression group.
When DST feedback sensitivity was included in the model predicting negative memory bias, there was a main effect of Drug (CORT vs. placebo), F(1,57)=4.37, p=.04, which was qualified by a Drug X DST interaction, F(1,57)=5.2, p=.03, such that individuals with lower DST feedback sensitivity showed greater reduction in negative memory bias with CORT administration, even with EA and depression severity in the model.1 Within this model, none of the interactions with EA or depression severity reached significance, p’s>.09. The Drug X DST interaction is illustrated by the zero-order correlations: For pictures encoded during placebo, feedback sensitivity was inversely correlated with memory bias, r(64)=−0.25, p=.05, as lower feedback sensitivity was associated with more negatively biased memory (Figure 3A). For pictures encoded during CORT, feedback sensitivity and memory bias were unrelated, r(64)=0.12, n.s. (Figure 3B). Most importantly, feedback sensitivity predicted change in memory bias for pictures encoded during CORT compared to placebo, r(64)=0.32, p=.009, such that women showing lower feedback sensitivity exhibited greater reduction in negative memory bias for pictures encoded during CORT compared to placebo (Figure 3C). Diurnal cortisol slope did not predict memory bias for pictures encoded in either drug condition, p’s>.44.
DISCUSSION
We investigated associations among systemic and cognitive GC sensitivity in premenopausal women. We replicated findings showing that lower DST feedback sensitivity (reflecting GC insensitivity) was associated with: 1) greater depression severity (1,2,13,21); and 2) flatter decline in diurnal cortisol, suggesting that variation in GC sensitivity is associated with systemic HPA regulation as indexed by diurnal cortisol slope (21). We extended these findings by examining relations between systemic GC sensitivity and negatively biased memory formation. Lower DST feedback sensitivity (GC insensitivity) was associated with more negatively biased memory for pictures encoded during placebo (when cortisol levels were not manipulated). This finding extends prior research suggesting that peripheral GC sensitivity is related to emotional memory in healthy adults (9,81). Furthermore, lower DST feedback sensitivity was associated with greater reductions in negative memory bias for pictures encoded during CORT (compared to placebo), even after statistically adjusting for severity of psychiatric symptomatology. That is, women with systemic GC insensitivity showed the greatest cognitive sensitivity to CORT, and appeared to benefit from acute cortisol administration as evidenced by a cortisol-related reduction in negatively biased memory formation. These findings suggest that GC insensitivity may be involved in depression-related emotional cognition. Acutely boosting the cortisol signal may ameliorate this cognitive alteration in those with systemic GC insensitivity.
As a secondary goal, we tested whether prior experience of adverse childhood caregiving was associated with feedback sensitivity (57). In our study, severity of EA was marginally related to DST feedback sensitivity and did not explain the relation between depression and DST feedback sensitivity. However, prior research has shown that HPA alterations occur more frequently in depressed individuals with (vs. without) history of early adversity. Although our current findings are marginally significant, they align with prior findings suggesting that measures of early adversity explain unique variance above depression. In our study, women with severe EA showed marginally impaired DST feedback sensitivity, but women with moderate EA showed relatively greater feedback sensitivity, which has been previously found in PTSD (27) and in non-human primates following moderate early adversity (82,83). These results warrant future investigation, as early adverse caregiving causes life-long changes in GC cellular signaling in non-human animals (53,84,85).
Clinical implications
The use of the DST in clinical psychiatry has fallen out of favor (20). Dexamethasone is primarily a GR agonist with little to no action at mineralocorticoid receptors (MRs), and the blood-brain barrier is relatively impermeable to a one-time dose of dexamethasone (though permeability increases with repeated doses) (2,13,86). Few individuals fail to suppress cortisol with typical doses (e.g., 1 mg) (2,21,87). However, DSTs using lower doses (e.g., 0.25 and 0.5 mg) suppress cortisol yet leave more room for variability in the cortisol response (21,88,89). Research into standardization of the low dose DST is warranted, which would allow use across research and clinical settings. Rather than as a proxy for clinical diagnosis, the value of the DST is likely in its ability to identify individuals in whom altered cortisol signaling plays a role in their depressive illness. The current study and prior research suggest that GC sensitivity and early adversity should be investigated as relevant indices for personalization of depression treatment (57,86,90-92).
Relatedly, the evidence reported herein supports research suggesting that therapeutics targeting cortisol signaling hold promise as antidepressant treatments (48,90,91,93,94). Unfortunately, it ineffective to boost the cortisol signal chronically by administering the steroid cortisol itself, for a variety of reasons including deleterious effects of chronically high levels of circulating cortisol (11). However, brief treatment with cortisol and corticosteroid agonists have shown beneficial effects in depression and PTSD (39,48,50,93). Especially promising may be therapeutics that target mechanisms underlying altered cellular response to cortisol, such as expression of the FKBP5 gene, which codes a protein that regulates GR function (91,94). Future research on therapeutics targeting cortisol signaling should identify individuals with GC insensitivity, who may respond differently to these therapeutics than patients without GC insensitivity (90).
Limitations and future directions
Previous research has shown that dexamethasone bioavailability is a key factor determining DST results. There is interindividual variability in rates of dexamethasone metabolism (28-30), which may present differently according to one’s history of depression (95). For example, lower plasma dexamethasone concentrations have been found in depressed patients and is shown to explain significant variance in DST results (31). Plasma dexamethasone concentrations should be measured and incorporated into analyses to improve the assessment of HPA dysfunction (26,31).
This data set includes relatively few women with EA. With greater power the study could have potentially replicated prior research suggesting that history of early adversity accounts for HPA alterations in depression. There is also potential bias inherent in retrospective reports of adverse childhood experiences and thus conclusions regarding EA can only be drawn tentatively (96). Our study was conducted only in younger pre-menopausal women and findings may differ for men or an older sample of women (64,97,98). In addition, results may differ in psychotic depression or depression associated with gross memory deficits (99), or with cortisol administration at a different time of day (36). Furthermore, cortisol dynamics might differ between non-Hispanic whites and other ethnic groups (100,101). Future research is required to assess whether racial and ethnic background moderates cognitive and systemic GC sensitivity.
Results may have differed if cortisol had been manipulated with an acute stressor rather than hydrocortisone administration. Acute effects of hydrocortisone administration in our study could be due to either MR and/or GR activation given that the experiment occurred in the evening when neither receptor type tends to be fully occupied. A benefit of using hydrocortisone is its identity with the endogenous hormone cortisol and consequent potential to reveal alterations in signaling of the endogenous hormone. Our goal was not to determine whether MR or GR are key, but to make inferences about depression-related cortisol signaling alterations. Future research is needed to determine whether alterations in cortisol signaling in depression are due to alterations of MR, GR, or activity at both receptor types.
The current report uses behavioral measures of memory function as an index of neurocognitive function. Our prior research in depressed samples has shown wide variation in the effects of cortisol on neural function (3,4). Future research should determine whether this variation is explained by differences in systemic GC sensitivity. However, to investigate this association, large samples will be required to adequately power neuroimaging investigations of depressed samples stratified by variation in GC sensitivity. Concurrent measurement of cortisol’s effects on cognition, neural function, and HPA feedback are highly needed.
Summary
Altogether, these findings suggest that GC insensitivity is not merely an endocrine biomarker of depression, but also related to a core psychological feature of depression (i.e., negative memory bias). Pharmacologically-induced cortisol elevation alleviated negative bias particularly in individuals exhibiting systemic GC insensitivity, suggesting that boosting the cortisol signal may override neurocognitive alterations related to GC insensitivity. The results suggest that GC insensitivity plays a role in negative memory bias in depression and suggest that treatments aimed at cortisol signaling may be beneficial. These findings add relevance to prior research suggesting that measures of GC sensitivity are important indices in the personalization of psychiatric treatment, and suggest that research on novel therapeutics for depression should index GC sensitivity.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded by grants to H. Abercrombie from the National Institute of Mental Health (R01MH094478), the University of Wisconsin-Madison Office of the Vice Chancellor for Research and Graduate Education with funding from the Wisconsin Alumni Research Foundation, and the University of Wisconsin-Madison Women in Science & Engineering Leadership Institute (WISELI)/The Office of the Provost; and a National Center for Advancing Translational Sciences (NCATS) grant (KL2TR002490) to E. Walsh. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The NIH had no further role in study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the article for publication.
We thank all of the volunteers who participated in this study, as well as A. Blumenfeld, C. Siwik, M. Dennison, A. Ehlers, C. Ernstoff, C. Frost, S. Goldberg, M. Kalambokidis, A. Lang, J. Nelson, E. Osterbauer, D. Plante, M. Sampe, R. Svoboda, R. Vohnoutka, and A. Winter for assistance with data collection. We thank Clemens Kirschbaum’s laboratory for conducting salivary cortisol assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We confirmed that the Drug X DST feedback sensitivity interaction was a significant predictor of negative memory bias when depression severity and EA severity were not included in the model, F(1,63)=7.24, p=.01. Removal of three women who used nicotine during the study does not change findings, e.g., for this Drug X DST interaction, F(1,54)=4.82, p=.03. When accounting for racial and ethnic background in the analyses, results are unchanged except for this major finding, which holds for non- Hispanic white participants, F(1,40)=4.56, p=.04. However, when tested separately in the subset of 17 participants from racial and ethnic minorities, variation in DST feedback sensitivity does not predict the effects of CORT on negative memory bias, F(1,9)=0.32, n.s., which may be due to low statistical power rather than a true difference related to racial and ethnic background.
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Pariante CM (2018): Too much is still not enough, when talking about cortisol. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 3: 207–208. [DOI] [PubMed] [Google Scholar]
- 2.Holsboer F (2001): Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disorders 62: 77–91. [DOI] [PubMed] [Google Scholar]
- 3.Abercrombie HC, Frost CP, Walsh EC, Hoks RM, Cornejo MD, Sampe MC, et al. (2018): Neural signaling of cortisol, childhood emotional abuse, and depression-related memory bias. Biol Psychiatry Cogn Neurosci Neuroimaging 3: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abercrombie HC, Jahn AL, Davidson RJ, Kern S, Kirschbaum C, Halverson J (2011): Cortisol's effects on hippocampal activation in depressed patients are related to alterations in memory formation. J Psychiatr Res 45: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pittenger C, Duman RS (2008): Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33: 88–109. [DOI] [PubMed] [Google Scholar]
- 6.van Ast VA, Cornelisse S, Marin MF, Ackermann S, Garfinkel SN, Abercrombie HC (2013): Modulatory mechanisms of cortisol effects on emotional learning and memory: Novel perspectives. Psychoneuroendocrinology 38: 1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL (2006): Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience 138: 901–910. [DOI] [PubMed] [Google Scholar]
- 8.Wolf OT, Atsak P, de Quervain DJ, Roozendaal B, Wingenfeld K (2016): Stress and memory: A selective review on recent developments in the understanding of stress hormone effects on memory and their clinical relevance. J Neuroendocrinol 28: 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Rohleder N, Wolf JM, Wolf OT (2010): Glucocorticoid sensitivity of cognitive and inflammatory processes in depression and posttraumatic stress disorder. Neurosci Biobehav Rev 35: 104–114. [DOI] [PubMed] [Google Scholar]
- 10.Behnken A, Bellingrath S, Symanczik JP, Rieck MJ, Zavorotnyy M, Domschke K, et al. (2013): Associations between cognitive performance and cortisol reaction to the DEX/CRH test in patients recovered from depression. Psychoneuroendocrinology 38: 447–454. [DOI] [PubMed] [Google Scholar]
- 11.McEwen BS (2008): Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583: 174–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raison CL, Miller AH (2003): When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 160: 1554–1565. [DOI] [PubMed] [Google Scholar]
- 13.Pariante CM (2009): Risk factors for development of depression and psychosis. Glucocorticoid receptors and pituitary implications for treatment with antidepressant and glucocorticoids. Ann N Y Acad Sci 1179: 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cattaneo A, Macchi F, Plazzotta G, Veronica B, Bocchio-Chiavetto L, Riva MA, et al. (2015): Inflammation and neuronal plasticity: a link between childhood trauma and depression pathogenesis. Frontiers in cellular neuroscience 9: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebner K, Singewald N (2017): Individual differences in stress susceptibility and stress inhibitory mechanisms. Current Opinion in Behavioral Sciences 14: 54–64. [Google Scholar]
- 16.Leistner C, Menke A (2018): How to measure glucocorticoid receptor's sensitivity in patients with stress-related psychiatric disorders. Psychoneuroendocrinology 91: 235–260. [DOI] [PubMed] [Google Scholar]
- 17.Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, et al. (1994): Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann N Y Acad Sci 746: 22–31; discussion 31-22, 64-27. [DOI] [PubMed] [Google Scholar]
- 18.de Kloet ER, Joels M, Holsboer F (2005): Stress and the brain: from adaptation to disease. Nat Rev Neurosci 6: 463–475. [DOI] [PubMed] [Google Scholar]
- 19.de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1997): Glucocorticoid feedback resistance. Trends Endocrinol Metab 8: 26–33. [DOI] [PubMed] [Google Scholar]
- 20.The APA Task Force on Laboratory Tests in Psychiatry (1987): The dexamethasone suppression test: An overview of its current status in psychiatry. Am J Psychiatry 144: 1253–1262. [DOI] [PubMed] [Google Scholar]
- 21.Jarcho MR, Slavich GM, Tylova-Stein H, Wolkowitz OM, Burke HM (2013): Dysregulated diurnal cortisol pattern is associated with glucocorticoid resistance in women with major depressive disorder. Biol Psychol 93: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menke A, Arloth J, Best J, Namendorf C, Gerlach T, Czamara D, et al. (2016): Time-dependent effects of dexamethasone plasma concentrations on glucocorticoid receptor challenge tests. Psychoneuroendocrinology 69: 161–171. [DOI] [PubMed] [Google Scholar]
- 23.Arana GW, Baldessarini RJ, Ornsteen M (1985): The dexamethasone suppression test for diagnosis and prognosis in psychiatry. Arch Gen Psychiatry 42: 1193–1204. [DOI] [PubMed] [Google Scholar]
- 24.Carroll BJ (1982): The dexamethasone suppression test for melancholia. Br J Psychiatry 140: 292–304. [DOI] [PubMed] [Google Scholar]
- 25.Pariante CM, Lightman SL (2008): The HPA axis in major depression: classical theories and new developments. Trends Neurosci 31: 464–468. [DOI] [PubMed] [Google Scholar]
- 26.Huizenga NATM, Koper JW, de Lange P, Pols HAP, Stolk RP, Grobbee DE, et al. (1998): Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitary-adrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab 83: 47–54. [DOI] [PubMed] [Google Scholar]
- 27.Yehuda R, Halligan SL, Golier JA, Grossman R, Bierer LM (2004): Effects of trauma exposure on the cortisol response to dexamethasone administration in PTSD and major depressive disorder. Psychoneuroendocrinology 29: 389–404. [DOI] [PubMed] [Google Scholar]
- 28.Johnson GF, Hunt GE, Caterson I (1988): Plasma dexamethasone and the dexamethasone suppression test. Initial and follow-up tests in depressed patients. J Affect Disord 15: 93–100. [DOI] [PubMed] [Google Scholar]
- 29.Lowy MT, Meltzer HY (1987): Dexamethasone bioavailability: Implications for DST research. Biol Psychiatry 22: 373–385. [DOI] [PubMed] [Google Scholar]
- 30.O'Sullivan BT, Cutler DJ, Hunt GE, Walters C, Johnson GF, Caterson ID (1997): Pharmacokinetics of dexamethasone and its relationship to dexamethasone suppression test outcome in depressed patients and healthy control subjects. Biol Psychiatry 41: 574–584. [DOI] [PubMed] [Google Scholar]
- 31.Menke A, Arloth J, Putz B, Weber P, Klengel T, Mehta D, et al. (2012): Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology 37: 1455–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wingenfeld K, Wolf OT (2015): Effects of cortisol on cognition in major depressive disorder, posttraumatic stress disorder and borderline personality disorder - 2014 Curt Richter Award Winner. Psychoneuroendocrinology 51: 282–295. [DOI] [PubMed] [Google Scholar]
- 33.Gotlib IH, Joormann J (2010): Cognition and depression: current status and future directions. Annu Rev Clin Psychol 6: 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lupien SJ, McEwen BS (1997): The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev 24: 1–27. [DOI] [PubMed] [Google Scholar]
- 35.Wolf OT (2009): Stress and memory in humans: twelve years of progress? Brain Res 1293: 142–154. [DOI] [PubMed] [Google Scholar]
- 36.Het S, Ramlow G, Wolf OT (2005): A meta-analytic review of the effects of acute cortisol administration on human memory. Psychoneuroendocrinology 30: 771–784. [DOI] [PubMed] [Google Scholar]
- 37.Shields GS, Sazma MA, McCullough AM, Yonelinas AP (2017): The effects of acute stress on episodic memory: A meta-analysis and integrative review. Psychol Bull 143: 636–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abercrombie HC, Kalin NH, Thurow ME, Rosenkranz MA, Davidson RJ (2003): Cortisol variation in humans affects memory for emotionally laden and neutral information. Behav Neurosci 117: 505–516. [DOI] [PubMed] [Google Scholar]
- 39.de Quervain D, Schwabe L, Roozendaal B (2017): Stress, glucocorticoids and memory: implications for treating fear-related disorders. Nat Rev Neurosci 18: 7–19. [DOI] [PubMed] [Google Scholar]
- 40.Schwabe L, Joëls M, Roozendaal B, Wolf OT, Oitzl MS (2012): Stress effects on memory: an update and integration. Neurosci Biobehav Rev 36: 1740–1749. [DOI] [PubMed] [Google Scholar]
- 41.Abercrombie HC, Speck NS, Monticelli RM (2006): Endogenous cortisol elevations are related to memory facilitation only in individuals who are emotionally aroused. Psychoneuroendocrinology 31: 187–196. [DOI] [PubMed] [Google Scholar]
- 42.Abercrombie HC, Wirth MM, Hoks RM (2012): Inter-individual differences in trait negative affect moderate cortisol's effects on memory formation: preliminary findings from two studies. Psychoneuroendocrinology 37: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okuda S, Roozendaal B, McGaugh JL (2004): Glucocorticoid effects on object recognition memory require training-associated emotional arousal. P Natl Acad Sci USA 101: 853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buchanan TW, Lovallo WR (2001): Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology 26: 307–317. [DOI] [PubMed] [Google Scholar]
- 45.Roozendaal B, McEwen BS, Chattarji S (2009): Stress, memory and the amygdala. Nat Rev Neurosci 10: 423–433. [DOI] [PubMed] [Google Scholar]
- 46.Terfehr K, Wolf OT, Schlosser N, Fernando SC, Otte C, Muhtz C, et al. (2011): Hydrocortisone impairs working memory in healthy humans, but not in patients with major depressive disorder. Psychopharmacology 215: 71–79. [DOI] [PubMed] [Google Scholar]
- 47.Terfehr K, Wolf OT, Schlosser N, Fernando SC, Otte C, Muhtz C, et al. (2011): Effects of acute hydrocortisone administration on declarative memory in patients with major depressive disorder: a placebo-controlled, double-blind crossover study. J Clin Psychiatry 72: 1644–1650. [DOI] [PubMed] [Google Scholar]
- 48.Otte C, Wingenfeld K, Kuehl LK, Kaczmarczyk M, Richter S, Quante A, et al. (2015): Mineralocorticoid Receptor Stimulation Improves Cognitive Function and Decreases Cortisol Secretion in Depressed Patients and Healthy Individuals. Neuropsychopharmacology 40: 386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bremner JD, Vythilingam M, Vermetten E, Anderson G, Newcomer JW, Charney DS (2004): Effects of glucocorticoids on declarative memory function in major depression. Biol Psychiat 55: 811–815. [DOI] [PubMed] [Google Scholar]
- 50.de Kloet ER, Otte C, Kumsta R, Kok L, Hillegers MHJ, Hasselmann H, et al. (2016): Stress and depression: a crucial role of the mineralocorticoid receptor. J Neuroendocrinol 28: 1–12. [DOI] [PubMed] [Google Scholar]
- 51.Joëls M, Krugers HJ (2007): LTP after stress: up or down? Neural Plast 2007: 93202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moriceau S, Raineki C, Holman JD, Holman JG, Sullivan RM (2009): Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front Behav Neurosci 3: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, et al. (2008): Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci 28: 6037–6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M (2009): Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem 92: 292–300. [DOI] [PubMed] [Google Scholar]
- 55.Meaney MJ, Szyf M (2005): Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 7: 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. (1997): Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659–1662. [DOI] [PubMed] [Google Scholar]
- 57.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB (2008): The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33: 693–710. [DOI] [PubMed] [Google Scholar]
- 58.Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB (2008): The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry 63: 398–405. [DOI] [PubMed] [Google Scholar]
- 59.Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T (2012): The long-term health consequences of child physical abuse, emotional abuse, and neglect: A systematic review and meta-analysis. PLoS Med 9: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gibb BE, Alloy LB, Abramson LY, Rose DT, Whitehouse WG, Donovan P, et al. (2001): History of childhood maltreatment, negative cognitive styles, and episodes of depression in adulthood. Cognitive Ther Res 25: 425–446. [Google Scholar]
- 61.Gibb BE, Abela JRZ (2008): Emotional abuse, verbal victimization, and the development of children's negative inferential styles and depressive symptoms. Cognitive Ther Res 32: 161–176. [Google Scholar]
- 62.Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, et al. (2014): Stressful life events and depression symptoms: The effect of childhood emotional abuse on stress reactivity. J Clin Psychol 70: 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shors TJ (2006): Stressful experience and learning across the lifespan. Annu Rev Psychol 57: 55–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf OT, Schommer NC, Hellhammer DH, McEwen BS, Kirschbaum C (2001): The relationship between stress induced cortisol levels and memory differs between men and women. Psychoneuroendocrinology 26: 711–720. [DOI] [PubMed] [Google Scholar]
- 65.Lucht M, Schaub RT, Meyer C, Hapke U, Rumpf HJ, Bartels T, et al. (2003): Gender differences in unipolar depression: a general population survey of adults between age 18 to 64 of German nationality. J Affect Disord 77: 203–211. [DOI] [PubMed] [Google Scholar]
- 66.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB (1993): Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord 29: 85–96. [DOI] [PubMed] [Google Scholar]
- 67.Cuthbert BN, Insel TR (2013): Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Medicine 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beck AT, Steer RA, Brown GK (1996): Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 69.First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured clinical interview for DSM-IV-TR axis I disorders. New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- 70.Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, et al. (2003): Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl 27: 169–190. [DOI] [PubMed] [Google Scholar]
- 71.Adam EK, Kumari M (2009): Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology 34: 1423–1436. [DOI] [PubMed] [Google Scholar]
- 72.Kaye TB, Crapo L (1990): The Cushing Syndrome - an Update on Diagnostic-Tests. Annals of Internal Medicine 112: 434–444. [DOI] [PubMed] [Google Scholar]
- 73.Savic D, Knezevic G, Damjanovic S, Spiric Z, Matic G (2012): Is there a biological difference between trauma-related depression and PTSD? DST says 'NO'. Psychoneuroendocrinology 37: 1516–1520. [DOI] [PubMed] [Google Scholar]
- 74.Klaassens ER, Giltay EJ, Cuijpers P, van Veen T, Zitman FG (2012): Adulthood trauma and HPA-axis functioning in healthy subjects and PTSD patients: A meta-analysis. Psychoneuroendocrinology 37: 317–331. [DOI] [PubMed] [Google Scholar]
- 75.Morris MC, Compas BE, Garber J (2012): Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev 32: 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frost CP, Meyerand ME, Birn RM, Hoks RM, Walsh EC, Abercrombie HC (2018): Childhood emotional abuse moderates associations among corticomotor white matter structure and stress neuromodulators in women with and without depression. Front Neurosci 12: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lang PJ, Bradley MM, Cuthbert BN (2008): International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida. [Google Scholar]
- 78.Tukey JW (1977): Exploratory Data Analysis. Reading, MA: Addison-Wesley Publishing Company. [Google Scholar]
- 79.Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, et al. (2015): Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry 20: 615–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.LeMoult J, Chen MC, Foland-Ross LC, Burley HW, Gotlib IH (2015): Concordance of mother-daughter diurnal cortisol production: Understanding the intergenerational transmission of risk for depression. Biol Psychol 108: 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohleder N, Wolf JM, Kirschbaum C, Wolf OT (2009): Effects of cortisol on emotional but not on neutral memory are correlated with peripheral glucocorticoid sensitivity of inflammatory cytokine production. Int J Psychophysiol 72: 74–80. [DOI] [PubMed] [Google Scholar]
- 82.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM (2006): Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci U S A 103: 3000–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parker KJ, Maestripieri D (2011): Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev 35: 1466–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ (2004): Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci 1024: 182–212. [DOI] [PubMed] [Google Scholar]
- 85.Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, et al. (2004): Epigenetic programming by maternal behavior. Nat Neurosci 7: 847–854. [DOI] [PubMed] [Google Scholar]
- 86.Pariante CM, Miller AH (2001): Glucocorticoid receptors in major depression: Relevance to pathophysiology and treatment. Biol Psychiat 49: 391–404. [DOI] [PubMed] [Google Scholar]
- 87.Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW (1993): Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 150: 83–86. [DOI] [PubMed] [Google Scholar]
- 88.Newport DJ, Heim C, Bonsall R, Miller AH, Nemeroff CB (2004): Pituitary-adrenal responses to standard and low-dose dexamethasone suppression tests in adult survivors of child abuse. Biol Psychiatry 55: 10–20. [DOI] [PubMed] [Google Scholar]
- 89.Stein MB, Yehuda R, Koverola C, Hanna C (1997): Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry 42: 680–686. [DOI] [PubMed] [Google Scholar]
- 90.Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, et al. (2013): Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline 'predictors' and longitudinal 'targets'. Neuropsychopharmacology 38: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Matosin N, Halldorsdottir T, Binder EB (2018): Understanding the molecular mechanisms underpinning gene by environment interactions in psychiatric disorders: The FKBP5 model. Biol Psychiatry 83: 821–830. [DOI] [PubMed] [Google Scholar]
- 92.Holsboer F (2008): How can we realize the promise of personalized antidepressant medicines? Nature Reviews Neuroscience 9: 638–646. [DOI] [PubMed] [Google Scholar]
- 93.Otte C, Hinkelmann K, Moritz S, Yassouridis A, Jahn H, Wiedemann K, et al. (2010): Modulation of the mineralocorticoid receptor as add-on treatment in depression: a randomized, double-blind, placebo-controlled proof-of-concept study. J Psychiatr Res 44: 339–346. [DOI] [PubMed] [Google Scholar]
- 94.Schmidt MV, Paez-Pereda M, Holsboer F, Hausch F (2012): The prospect of FKBP51 as a drug target. ChemMedChem 7: 1351–1359. [DOI] [PubMed] [Google Scholar]
- 95.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM (2010): Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hardt J, Rutter M (2004): Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry 45: 260–273. [DOI] [PubMed] [Google Scholar]
- 97.Otte C, Wingenfeld K, Kuehl LK, Richter S, Regen F, Piber D, et al. (2015): Cognitive function in older adults with major depression: Effects of mineralocorticoid receptor stimulation. J Psychiatr Res 69: 120–125. [DOI] [PubMed] [Google Scholar]
- 98.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE (2007): The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65: 209–237. [DOI] [PubMed] [Google Scholar]
- 99.Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr., et al. (2016): HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chong RY, Uhart M, McCaul ME, Johnson E, Wand GS (2008): Whites have a more robust hypothalamic-pituitary-adrenal axis response to a psychological stressor than blacks. Psychoneuroendocrinology 33: 246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yanovski JA, Yanovski SZ, Gold PW, Chrousos GP (1993): Differences in the hypothalamic-pituitary-adrenal axis of black and white women. J Clin Endocrinol Metab 77: 536–541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.