Abstract
Binge methamphetamine users have higher methamphetamine consumption, relapse rates, and depression-like symptoms during early periods of withdrawal, compared to non-binge users. The impact of varying durations of methamphetamine abstinence on depression-like symptoms and on subsequent methamphetamine intake was examined in mice genetically prone to binge-level methamphetamine consumption. Binge-level methamphetamine intake was induced using a multiple-bottle choice procedure in which mice were offered 1 water drinking tube and 3 tubes containing increasing concentrations of methamphetamine in water, or 4 water tubes (control group). In two studies, depression-like symptoms were measured using a tail-suspension test and a subsequent forced-swim test, after forced abstinence of 6 and 30 hours from a 28-day course of chronic methamphetamine intake. An additional study measured the same depression-like symptoms, as well as MA intake, after prolonged abstinence of 1 and 2 weeks. Methamphetamine high drinking mice and one of their progenitor strains, DBA/2J, escalated their methamphetamine intake with increasing methamphetamine concentration; however, methamphetamine high drinking mice consumed almost twice as much methamphetamine as DBA/2J mice. Depression-like symptoms were significantly higher early after methamphetamine access was withdrawn, compared to levels in drug-naïve controls, with more robust effects of methamphetamine withdrawal observed in methamphetamine high drinking than DBA/2J mice. When depression-like symptoms were examined after 1 or 2 weeks of forced abstinence in methamphetamine high drinking mice, depression-like symptoms dissipated, and subsequent MA intake was high. The methamphetamine high drinking genetic mouse model has strong face validity for human binge MA use and behavioral sequelae associated with abstinence.
Keywords: addiction, selected-lines, forced abstinence, 4-bottle choice, tail-suspension test, forced-swim test
1. Introduction
Abstinence from the use of psychostimulants, like methamphetamine (MA), is most difficult for a subpopulation with intermittent high-dose, binge-like patterns of use (Cheng et al., 2010; Rawson, 2013; Simon et al., 2002). A binge period typically lasts for 1–3 days, during which an individual escalates MA use until physically unable to continue or takes a break for sleep (a “crash” period); the rough amounts taken during a binge period appear to be 1–4 grams (Cho et al., 2001; Simon et al., 2002). According to Cho et al. (2001), blood MA levels in a binge cycle never drop to zero and a steady state of significant MA levels is reached after about 8–10 doses. These use patterns contribute to escalation and compulsive drug intake (Roberts et al., 2013), and are characterized by high frequency of use, depression, and psychotic symptoms occurring within the early abstinence period, namely, the first 24 hours to a few days of abstinence (McGregor et al, 2005; Newton et al., 2004). According to the American Psychiatric Association (2013), dysphoria, a symptom of depression, manifests after cessation or reduction of prolonged amphetamine-like substance use, and is a key diagnostic criterion for withdrawal. Although duration of MA use, calculated in years, does not predict binge use (Cheng et al., 2010), binge users have higher frequency of MA use and consume higher mean amounts of MA, compared to non-binge users, which may explain the greater severity of withdrawal symptoms and difficulty in maintaining abstinence (Cheng et al., 2010; Cook et al., 2017; Semple et al., 2007). The first 24 hours and up to a week, seem to be critical to whether an individual maintains abstinence for a prolonged time (Cook et al., 2017). After one week, depression symptoms are weaker, though craving for MA, sleep disturbances, and overall impulsivity may persist for much longer (Jones et al., 2016; Zorick et al., 2010; McGregor et al., 2005).
Thus, MA has dose-dependent negative subjective effects such as anxiety and dysphoria, particularly after 2–3 days of abstinence (Cruickshank & Dyer 2009). Sensitivity to initial positive psychostimulant drug effects has some predictive value for future intake in human and nonhuman subjects (de Wit et al., 1986; de Wit & Phillips, 2012; Phillips et al., 2008). Unknown is the relationship between the magnitude of symptoms occurring after MA is withdrawn and genetic risk for MA consumption. However, greater sensitivity to the initial negative subjective effects of MA may reduce subsequent MA use in humans (de Wit & Phillips, 2012), and greater sensitivity to conditioned aversive effects of MA is genetically associated with reduced MA intake in mice (Shabani et al., 2012b; Wheeler et al., 2009). Such genetically-determined traits may contribute to risk for heavy MA use and the probability of developing a MA use disorder (Phillips & Shabani, 2015). In human subject research, the inability to control MA use history, co-abuse or use of other drugs, and countless other variables, complicate studying genetic factors that may influence sensitivity to initial MA effects and magnitude or patterns of subsequent MA use. However, we have recently developed a mouse model of voluntary binge-level MA use, beginning with mice that were selectively bred for high MA intake (the MA high drinking or MAHDR mice), and found individual mice to display binge/crash-like patterns of intake across days of access (Shabani et al., 2016). Furthermore, MA intake was sustained in MAHDR mice after each of several forced abstinence periods of 30h in duration (Shabani et al., 2016). One of the 2 progenitors of the selected lines, the DBA/2J (D2) strain, but not the other progenitor, the C57BL/6J (B6) strain, voluntarily consumes MA (Eastwood & Phillips, 2014). Unlike the MAHDR mice, D2 mice progressively reduced their MA consumption after each 30h withdrawal period between MA consumption sessions (Shabani et al., 2016). However, MA withdrawal-associated behavioral symptoms were not examined in our previous study, and are the subject of the current paper.
Our genetic mouse model of binge-like MA use was developed using mice from a sequentially replicated bidirectional short-term selective breeding program that was based on voluntary consumption of MA in a two-bottle choice drinking procedure (Phillips & Shabani, 2015; Shabani et al., 2011; Wheeler et al., 2009). To create the MA drinking (MADR) lines, MA and water intake were measured for 18 h per day across an 8-day period for concentrations of 20 and then 40 mg MA/l of water (each concentration was offered for 4 consecutive days). In each replicate, mice chosen to produce the MAHDR line were those that consumed the most MA from the 40 mg MA/l concentration, and MA low drinking (MALDR) mice were produced by breeding mice that consumed the least MA. MAHDR mice consumed about 6 mg MA/kg/18h on average from the 40 mg MA/l concentration, and MALDR mice consumed less than 0.5 mg/kg/18h. These differences in MA intake cannot be explained by differences in taste sensitivity (Shabani et al., 2011; Wheeler et al., 2009). When the two-bottle choice procedure was altered to include additional gradually increasing MA concentrations, MA consumption increased to binge-like levels in MAHDR mice, with negligible impact on MA intake in MALDR mice (Shabani et al., 2016). Further increases in MA intake in MAHDR, but not MALDR, mice were obtained when 3 drinking tubes containing MA were offered vs. 1 tube of water and MA concentration was gradually increased (Shabani et al., 2016). This multiple-bottle procedure was examined because it had been found to increase alcohol intake (Tordoff & Bachmanov, 2003). Escalation of MA intake was also seen in D2 mice under these conditions (Shabani et al., 2016). In MAHDR mice, average MA consumption more than doubled and about 90% had MA consumption peaks of >10 mg/kg/18h and approximately 80% had peaks >15 mg/kg/18h, with some individuals achieving peaks as high as 40 mg/kg/18h. This mouse model has high face validity, when considering that heavy MA consumption in humans is roughly 800–1000 mg/day, or roughly 10–13 mg/kg/day (Rawson et al., 2002; Simon et al., 2002), with binge-like levels reaching 2 to 3 times those amounts (Cho et al., 2001). The primary goals of the current studies were to examine whether voluntary, binge-level MA intake (intake of ≥ 13 mg/kg/day) would induce time-dependent depression-like symptoms when MA is withdrawn, whether those symptoms would be of different magnitude in MAHDR and D2 mice, and whether amount of MA intake would be predictive of magnitude of depression-like symptoms.
MAHDR and D2 mice were tested for depression-like symptoms after either 6h or 30h periods of forced abstinence from voluntary chronic binge-level MA consumption. Next, because the MAHDR mice consumed twice as much MA as D2 mice, and exhibited greater depression-like symptoms after forced abstinence from MA, withdrawal effects were examined in MAHDR mice after longer durations of forced abstinence (1 and 2 weeks). MA consumption was then measured for 4 days, beginning the day after the 1- or 2-week time period ended. The most widely used procedures for measuring depression-like or anti-depressant-related effects in rodents have been the tail-suspension and forced-swim tests (for review see Cryan et al., 2005). Although these two procedures can offer converging outcomes, some of the biological substrates driving these behaviors are likely different (Cryan et al., 2003); therefore, both tests were used in the present studies. Both procedures are short (6 min) and measure immobility in response to an inescapable stressful situation; thus, in the tail-suspension test, mice are inescapably suspended by their tails, whereas in the forced-swim test, mice are inescapably placed in a cylinder filled with cool water. These measures were also chosen because they have been used to demonstrate that depression-like symptoms occur in mice after withdrawal from forced amphetamine or methamphetamine administration (Cryan et al., 2003; Georgiou et al., 2016).
We predicted that both MAHDR and D2 mice would voluntarily consume amounts of MA characteristic of binge-level consumption, using the multiple-bottle procedure (Shabani et al., 2016). We also predicted that forced abstinence would be associated with prolonged periods of immobility in the tail-suspension and forced-swim tests, compared to a water only control group, in both genotypes. Finally, because frequent MA users are more likely to relapse to heavy MA consumption after a prolonged period of forced abstinence, we predicted that MAHDR mice would continue to exhibit high levels of MA consumption even after 1 or 2 weeks of withdrawal. However, based on previous human and rodent studies (Cryan et al., 2003; Georgiou et al., 2016; Zorick et al., 2010), we predicted that the depression-like symptom of increased immobility would be weak or absent after these longer periods of abstinence.
2. Methods
2.1. Animals
2.1.1. MAHDR mice
Male and female MAHDR mice used for experiments 1 and 3 were second or later litter offspring of fourth or fifth selection generation replicate 4 mice that were selectively bred within the Veterans Affairs Portland Health Care System (VAPORHCS) animal facility, Portland, OR. The MAHDR line was derived from an F2 cross of the B6 and D2 inbred strains, according to established procedures (Shabani et al., 2011; Wheeler et al., 2009) that were briefly described above, and were shipped from the VA Portland Health Care System in Portland, OR to Minot State University, Minot, ND. Several baseline and MA-related behavioral, physiological and neurochemical characteristics of the MAHDR mice have been published and summarized (Phillips & Shabani, 2015; Phillips et al., 2016; Shabani et al., 2016; Szumlinski et al., 2017). All mice were MA- and experimentally-naïve at the beginning of a study, and independent sets of mice were used in each experiment. MAHDR mice used in experiments 1 and 3 were 84–98 and 107–110 days old, respectively.
2.1.2. D2 mice
Male and female D2 inbred strain mice used in experiment 2 were shipped to Minot State University, Minot, ND from The Jackson Laboratory (Bar Harbor, Maine, USA) at 56 days of age and were 74 days old at the start of the experiment.
2.1.3. Housing and care
Mice were initially housed in same sex groups, 2–4 per cage, in shoe-box cages (31cm × 20cm × 15cm; l × w × h) that were fitted with wire tops and lined with Bed-O-Cobs™ bedding (The Andersons Inc., Maumee, OH, USA). They were acclimated to their new housing environment for at least 2 weeks prior to experimentation. Rodent chow (Purina 5001, 4.5% fat content; Animal Specialties Inc., Hubbard, OR or PicoLab® Laboratory Rodent Diet 5LOD, 4.5%fat content; Land O’Lakes Inc., St. Louis, MO, USA) and tap water were provided ad libitum. Room temperature was maintained at 20–22°C, and the L:D schedule was 12:12 h, with room lights turned on at 0600 h. Animal care and use were approved by the Institutional Animal Care and Use Committee of Minot State University and all procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Drinking solutions
(+) Methamphetamine hydrochloride (MA) was obtained from Sigma (St. Louis, MO, USA) and used to make fresh drinking solutions every 4 days by dissolving milligrams (mg) of MA in tap water (l) to create the appropriate mg/l solutions.
2.3. Experimental Procedures
2.3.1. Experiments 1 and 2. Depression-like symptoms after 6h and 30h forced abstinence from chronic MA intake in MAHDR and D2 mice
2.3.1.1. MA drinking
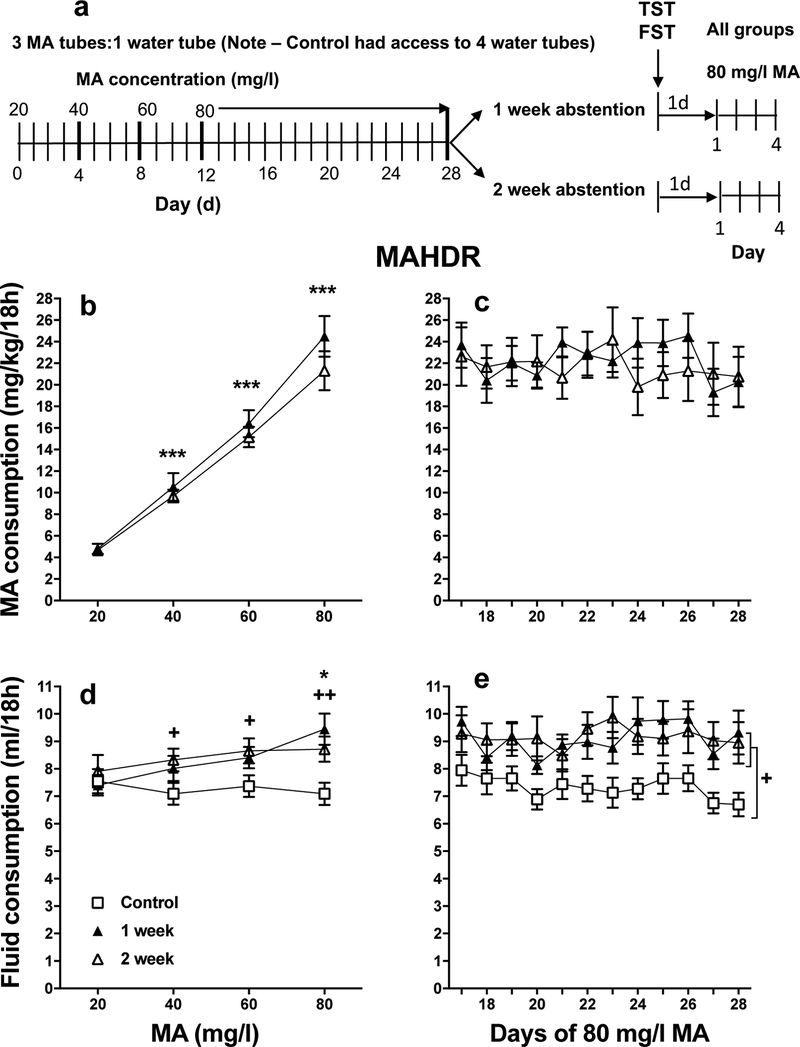
Figure 1a outlines the general design across days for experiments 1 (MAHDR mice) and 2 (D2 mice) for the mice with MA access. A 4-bottle choice procedure was used (1 water and 3 MA tubes) that was previously shown to result in binge-level MA intake (Shabani et al., 2016); a control group had access to 4 tubes of water. In experiment 1, 88 MAHDR mice (14–16/sex/group) were tested in 2 cohorts (40–48/cohort). In experiment 2, 48 D2 mice (8/sex/group) were tested in a single cohort. After a two-week acclimation period, animals were weighed and individually housed for the remainder of the experiment, so that individual drinking values could be obtained. Drinking tubes were 25-ml graduated cylinders fitted with stoppers and stainless steel sipper that were inserted between bars of the cagetops (N10SS model; Ancare, Bellmore, NY, USA). Food was evenly distributed around the tubes to avoid food association with any particular drinking solution, and a water drinking tube was provided at all times in a constant location. A control group (not shown) had access to 4 water tubes on the days shown in Figure 1. In the first 48h period of single housing, all animals were acclimated to consuming fluid from the novel drinking tubes (one water tube was offered to each mouse at this time); water consumption was measured at 24h time points during this period.
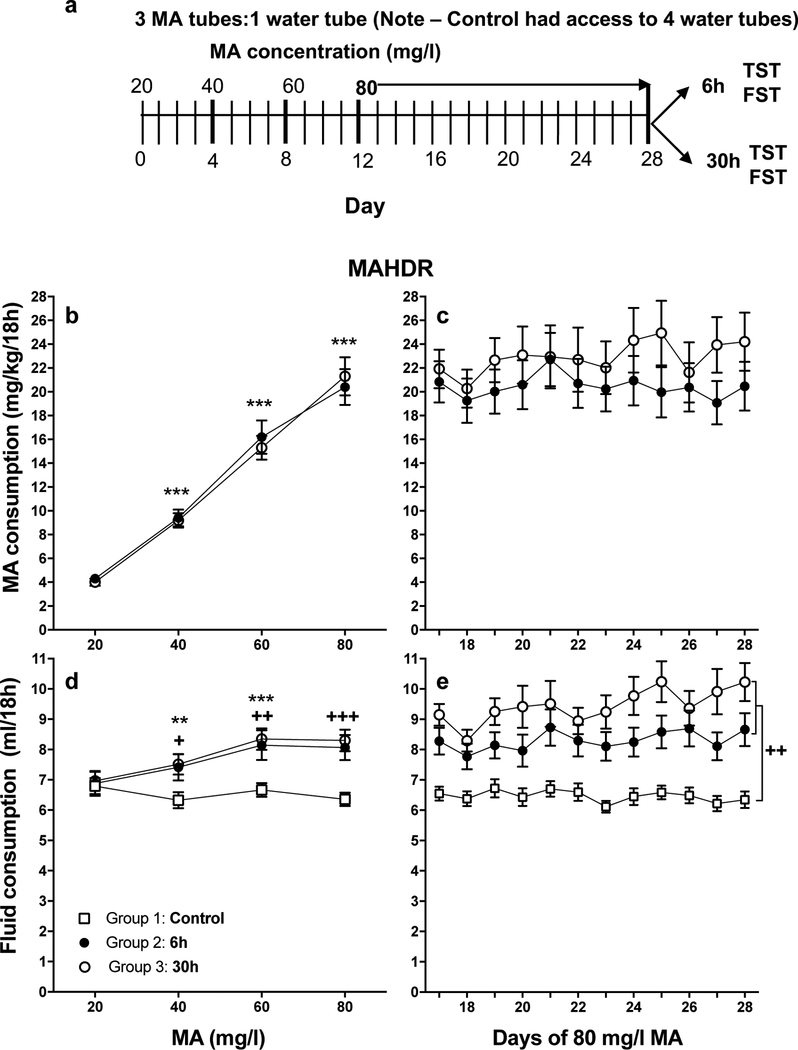
Figure 1.
Design, MA consumption, and fluid consumption data for experiment 1. With increasing MA concentration, the selectively bred MAHDR mice increase their MA consumption to binge-like levels and sustain that level of consumption for another 12 days. a: Experimental design. The MA group had 18h daily access to 1 water tube and 3 MA tubes, all of which contained the same concentration of MA in water. MA concentration was increased as shown in the diagram and then there was a protracted period of 80 mg/l MA access. The controls had daily access to 4 water tubes, during the same 18h period (control group details not shown). During the remaining 6h of each day, both groups had access to 1 water tube (not shown). After the 28-day MA drinking period, the MA group was divided into two groups and one was tested for immobility using the tail-suspension test (TST) and then the forced swim test (FST) 6h after MA access was withdrawn (Group 2); the other was tested 30h after withdrawal of MA access (Group 3). Control group mice (Group 1) were also divided in half and tested at these times. b: mean (± SEM) mg/kg/18h MA consumed for MA concentrations offered for 4 days each; c: mean (± SEM) mg/kg/18h MA consumed when access to 80 mg/l MA was extended for an additional 12 days. d and e: mean (± SEM) total fluid consumed during the periods in B and C. n = 14–16/group/sex. **p<.01, ***p<.001, compared to the next lower MA concentration for Group 2 and 3 data in b and d; +p<.05, ++p<.01, +++p<.001 for the comparison of each MA group (Groups 2 and 3) to the control (Group 1) at the indicated MA concentration (d) or for the main effect of group (e).
For MA drinking acquisition, mice were weighed and 3 tubes containing 20 mg MA/l of water or 3 tubes containing water (control group mice) were added to the existing water tube. These 3 additional tubes remained in place during the 18h period, which began 3h before onset of the dark phase of the L:D cycle and ended 3 h after onset of the light phase. The 18h MA access period is consistent with the MA drinking procedures used for selective breeding and our prior multiple-bottle choice study (Shabani et al., 2011; 2016; Wheeler et al., 2009). Fluid consumption was measured for this 18h period from all 4 tubes. For the remaining 6h of each day, a single water tube was available. These procedures continued as the MA concentration was increased in 20 mg/l intervals from 20 to 80 mg/l, every 4 days. Mice were then given access to the 80 mg/l MA concentration (3 tubes) vs. water (1 tube) for an additional 12 days; the control group had access to 4 tubes of water. Mice were weighed every 4 days, always at the end of an 18h drinking period.
2.3.1.2. Tail-suspension and forced-swim tests
After the final 80 mg MA/l drinking session, control and MA drinking animals were tested in tail-suspension and forced-swim procedures. Water drinking controls were designated as Group 1, and tested at the same time as the MA-drinking mice. MA-drinking mice were divided into 2 groups, matched for MA intake during the chronic MA drinking phase, and designated as either Group 2 or Group 3, based on the duration of forced abstinence (6h or 30h, respectively) after which they were tested. The protocol for the tail-suspension test was conducted according to Castagne et al. (2011). Animals were weighed and then a small plastic cylinder was fitted around the tail at roughly three-quarters of the distance from the base to the tip of the tail to prevent climbing up the tail during the test. Adhesive tape was then wrapped around the tail and the tail was firmly attached via the tape to the apparatus hook (TST System; Bioseb, Pinellas Park, FL), thus suspending the animal in an upside down orientation for 6 minutes. Animals were videotaped and scored for time spent immobile in seconds (s). Videoclips were scored without knowledge of experimental group.
At the conclusion of the tail-suspension test, animals were returned to their homecages within the test room for ~80 min, and then tested in the forced-swim test in the same order that they were tested in the tail-suspension test. The forced-swim test procedure was conducted according to Can et al. (2012). Forced-swim test enclosures (20 cm diameter and 40 cm height; San Diego Instruments, San Diego, CA) were filled to a level of 18 cm with water at a temperature of 22–25°C and then the animal was placed gently into the water for 6 minutes. Behavior was videotaped for the entire 6-minute session; however, only the last 4 minutes of the session were scored for total immobility time in s, consistent with Can et al. (2012). Videoclips were scored blind to group.
2.3.2. Experiment 3. Depression-like symptoms after prolonged forced abstinence from chronic MA intake in MAHDR mice
The procedure was the same as for experiment 1, except that mice were tested either 1 week (Group 2) or 2 weeks (Group 3) after forced abstinence from MA for the same depression-like symptoms; Group 1 was again a water only control. In experiment 3, 55 MAHDR mice (7–12/sex/group) were tested. In addition, beginning one day after measurement of depression-like symptoms, all mice were given access to a 80 mg MA/l solution for 4 consecutive days. This allowed for a between-groups comparison of MA intake between MA-naïve animals and those with a history of chronic MA intake, as well as a within-group comparison of MA intake before and after prolonged forced abstinence.
2.4. Data Analysis
The dependent variables for drinking were MA consumption (mg/kg) and total fluid consumption (ml) during the 18h periods. Consistent with our established methods, data from days 2 and 4 for each MA concentration were averaged to represent drinking for a particular MA concentration. The total fluid consumption of mice with MA access was compared to that of water-only controls to determine if MA intake impacted overall fluid intake. For the tail-suspension and forced-swim tests, immobility time (s) was the dependent measure. Possible independent variables were MA concentration, group, and sex. All data analysis was performed using SPSS software (IBM® SPSS® Statistics software). First, data were analyzed by repeated measures (drinking data) or univariate (immobility data) ANOVA. If the assumption of sphericity was not met, according to Mauchly’s test (p<.05), a conservative correction of degrees of freedom, known as the Greenhouse-Geisser estimate, was used to assess significance of the F ratio. Significant 3-way interactions were considered by 2-way ANOVAs at each level of a third variable (e.g., group). If there were no significant interactions involving sex, data for the sexes were combined in further analyses. Two-way interactions were analyzed for simple effects, and the Tukey HSD test was used for post-hoc mean comparisons, when appropriate. Simple linear regression (Pearson’s r) was used to calculate correlations between MA intake and depression-like symptom variables. Data were first examined for outliers using Cook’s D test. Significance value (α) was set at .05. P values for group and mean comparisons are indicated by symbols in the figures.
3. Results
3.1. Experiment 1. Depression-like symptoms after 6h and 30h forced abstinence from chronic MA intake in MAHDR mice
During the acquisition period, MAHDR mice increased their MA consumption with each incremental increase in MA concentration (Figure 1b), and consumed a stable amount of MA during the chronic 80 mg/l MA period (Figure 1c). In the initial repeated measures analysis of MA intake data for Groups 1 and 2, there were no significant effects of sex or group. There was a significant main effect of MA concentration (F(1.5, 86.6) = 236.4, p<.001), and within-subjects mean contrasts performed between each lower and the next higher concentration of MA detected significantly increasing intake as concentration increased (Figure 1b). There were no significant results from the repeated measures ANOVA for MA consumption during the chronic MA intake period (days 17–28; Figure 1c).
For total fluid consumption during the water drinking (Group 1) and MA acquisition (Groups 2 and 3) periods, there were no significant effects involving sex. However, there were group differences that were dependent on MA concentration (F(4.9, 206.9) = 10.2, p<.001 for the interaction; Figure 1d). Simple effects analysis at each concentration revealed significant group differences in total fluid consumption at all MA concentrations except 20 mg/l. Tukey post-hoc tests determined that the MA groups did not differ from each other, but each consumed significantly more fluid than the water control group. The fluid (water) consumption in the control group varied little across time, but it was significantly lower (by ~0.5 ml) after the initial 4-day period. In contrast, fluid consumption significantly increased with increasing MA concentration in both MA groups. Within-subjects mean contrasts for each group indicated significant effects for the 20 to 40 mg/l and 40 to 60 mg/l transitions, but not when concentration was increased from 60 to 80 mg/l.
For total fluid consumption during the chronic MA drinking period (Figure 1e), there were no significant effects of sex. There was a main effect of group (F(2, 82) = 14.1, p<.001) that was not dependent on day. Post-hoc analyses indicated that the 2 MA groups did not significantly differ in fluid consumption, but each consumed significantly more total fluid than the control group.
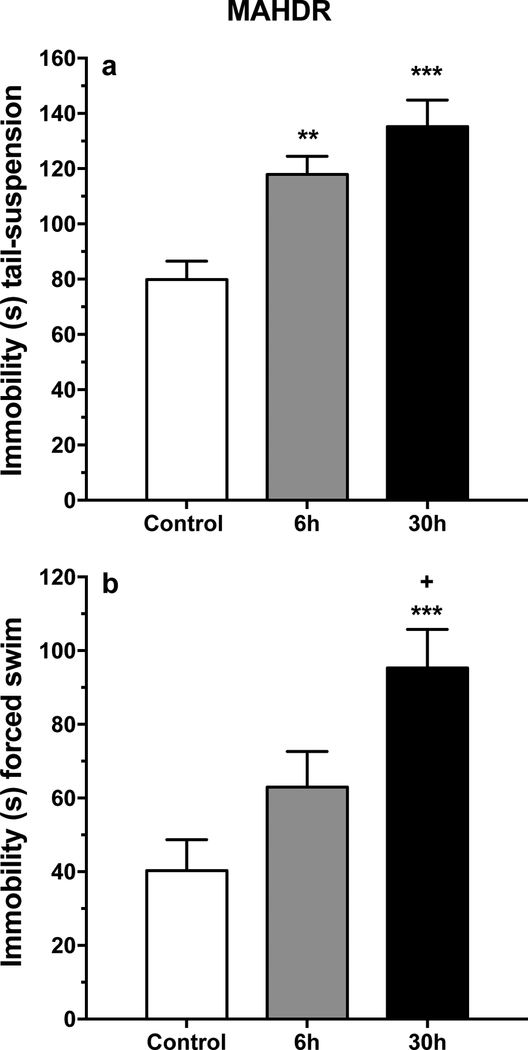
For duration of immobility during the tail-suspension test (Figure 2a), there were no significant effects involving sex; however, there was a significant main effect of group (F(2, 82) = 13.0, p<.001). Post-hoc mean comparisons revealed significantly longer duration of immobility for the 6h and 30h MA abstinence groups, compared to the water control group. For the forced-swim test (Figure 2b), there was again only a significant main effect of group (F(2, 82) = 8.1, p<.005). Post-hoc comparisons identified significant differences in duration of immobility between the 30h MA abstinence group and both the water control and 6h MA abstinence groups, with a strong statistical trend for a difference between the 6h and 30h MA abstinence groups (p=.052).
Figure 2.
Depression-like symptoms are elevated in MAHDR mice after shorter periods of forced abstinence from chronic MA intake in both the tail-suspension and forced-swim tests in experiment 1. a: mean (± SEM) immobility time in seconds (s) in the tail-suspension test. b: mean (± SEM) immobility time (s) in the forced-swim test. **p<.01, ***p<.001, compared to control; +p<.05, compared to 6h group.
3.2. Experiment 2. Depression-like symptoms after 6h and 30h forced abstinence from chronic MA intake in D2 mice
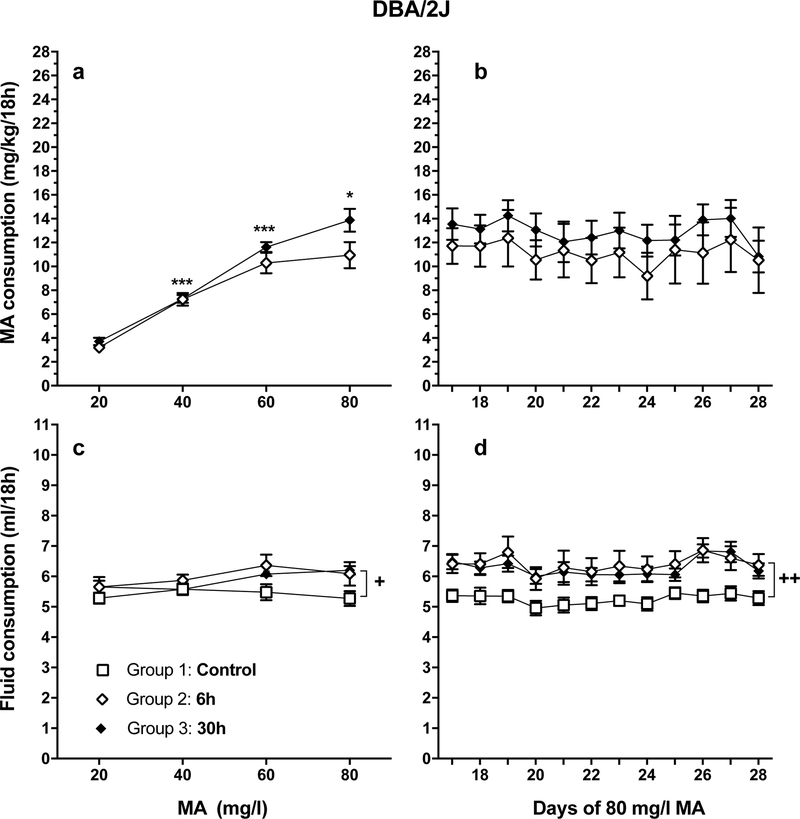
Similar to MAHDR mice, D2 mice increased their MA intake with each increase in MA concentration (Figure 3a) and their MA intake remained stable during the chronic intake phase (Figure 3b). Statistical analyses identified no significant effects involving sex or group (Groups 2 and 3) during either phase. For acquisition, there was a significant main effect of concentration (F(1.8, 73.1) = 149.6, p<.001); within-subjects mean contrasts between each lower concentration and the next higher concentration revealed significant increases in MA intake for all concentration comparisons (Figure 3a). There were no significant results from the repeated measures ANOVA for MA consumption during the chronic MA intake period (days 17–28; Figure 3b).
Figure 3.
MA and fluid intake data for experiment 2. See Figure 1A for the experimental design. With increasing MA concentration, DBA/2J (D2) mice increase their MA consumption and sustain high levels of intake for another 12 days, though at a level about half that of MAHDR mice (see Figure 1). a: mean (± SEM) mg/kg/18h MA consumed for MA concentrations offered for 4 days each; b: mean (± SEM) mg/kg/18h MA consumed when access to 80 mg/l MA was extended for an additional 12 days. cand d: mean (± SEM) total fluid consumed during the periods in a and b. n = 8/group/sex. *p<.05, ***p<.001, compared to the next lower MA concentration for Group 2 and 3 data; +p<.05, ++p<.01 for the comparison of each MA group (Groups 2 and 3) to the control group (Group 1).
Analysis of total fluid consumption data for D2 mice during the water (Group 1) and MA acquisition (Groups 2 and 3) period (Figure 3c) identified significant main effects of concentration (F(2.5, 103.7) = 3.7, p < .05), group (F(2, 42) = 4.5, p < .05), and sex (F(1, 42) = 7.0, p < .05). The effect of concentration was due to greater overal fluid intake during the time that the 60 mg/l MA concentration was offered, compared to the next lower MA concentration. Post-hoc mean comparisons for the effect of group revealed significant differences between the control and each MA group, due to greater fluid intake in the MA groups. Fluid consumption was higher in males than females, regardless of group, by about 0.5 ml (mean ± SEM = 6.02 ± 0.12 and 5.56 ± 0.12 ml for males and females, respectively). Analysis of fluid consumption data during the chronic period (Figure 3d) identified significant group (F(2, 42) = 8.2, p < .001) and sex (F(1, 42) = 22.9, p < .001) effects. Post-hoc mean comparisons revealed significant differences between the control group and each MA group. Fluid consumption during the chronic period was higher in males than females, regardless of group, by about 1.2 ml (mean ± SEM = 6.53 ± 0.17 and 5.37 ± 0.18 ml for males and females, respectively).
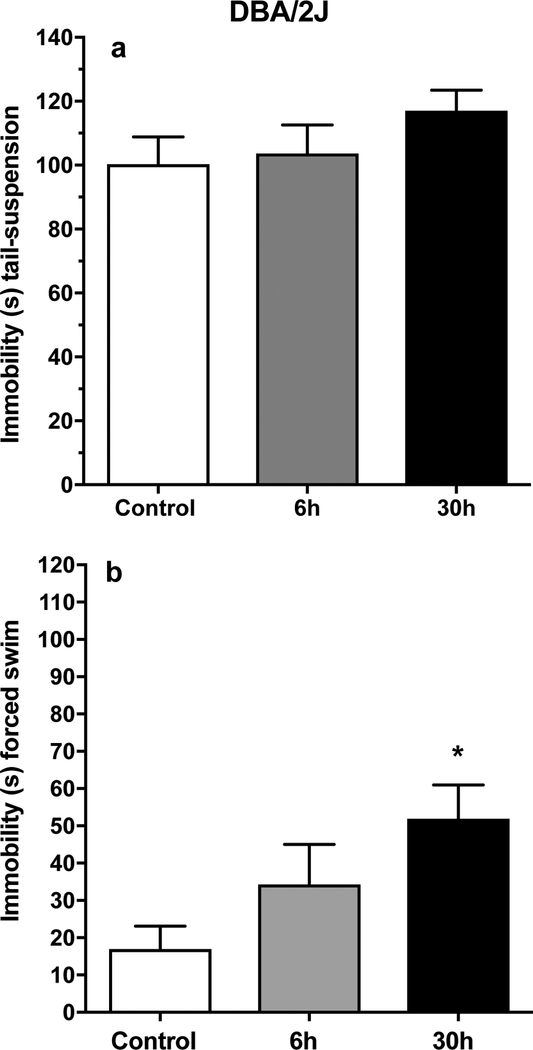
For the tail-suspension test (Figure 4a), there were no differences between the sexes or among the groups for duration of immobility in D2 mice. For duration of immobility in the forced-swim test (Figure 4b), there were significant main effects of group (F(2, 42) = 3.8, p<.05) and sex (F(2, 42) = 10.5, p<.01), but no significant interaction effects. Post-hoc comparisons identified a significant difference in duration of immobility between the 30h MA abstinence group and the water control group. Duration of immobility was lower in females than males, regardless of group (mean ± SEM = 19. 7 ± 6.2 vs. 54.2 ± 9.6 s for females and males, respectively).
Figure 4.
Depression-like symtoms are elevated in DBA/2J (D2) mice after shorter periods of forced abstinence from chronic MA intake only in the forced-swim test in experiment 2. a: mean (± SEM) immobility time in seconds (s) in the tail-suspension test. b: mean (± SEM) immobility time (s) in the forced-swim test. *p<.05, compared to control.
3.3. Experiments 1 and 2. Correlational analysis of MA intake and depression-like symptoms in MAHDR and D2 mice
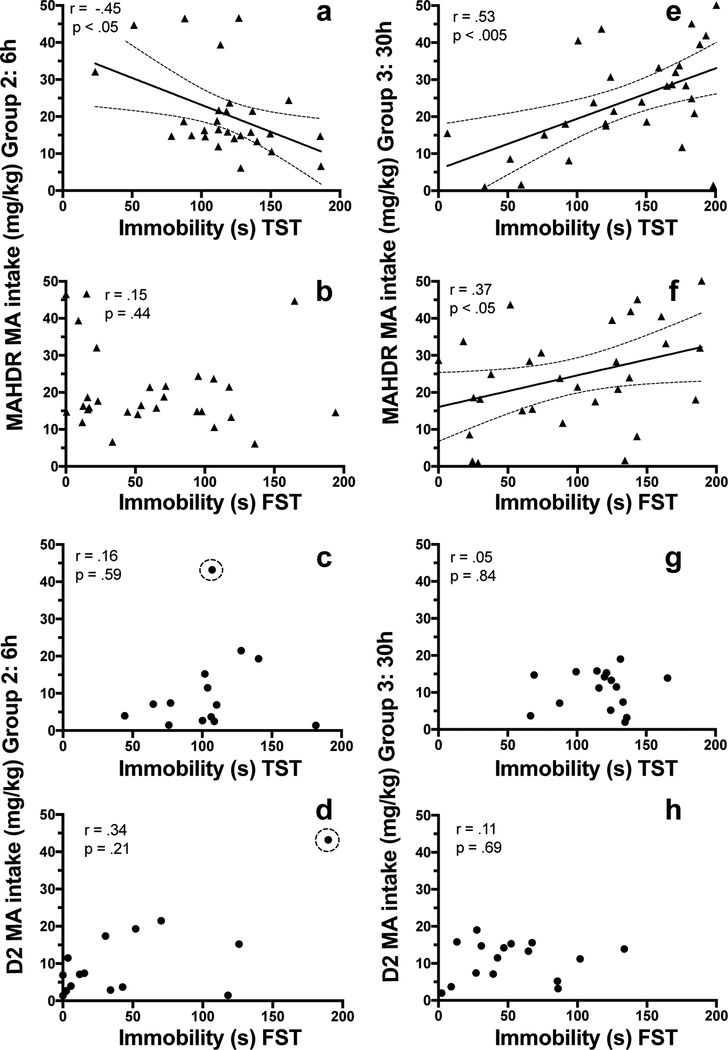
Correlations were calculated separately for data from MAHDR and D2 mice, between MA intake amounts on the last day of MA access (Day 28) and immobility time (s) for the tail- suspension and forced-swim tests (Figure 5). Data were first examined for outliers with undue influence on the correlation, using Cook’s D test. Distance values above 1 indicate that a data point should be excluded from the analysis. Data points for one D2 mouse from Group 2 met this criterion (Cook’s D = 1.3). These data points are shown in Figure 5c and d, but were not included in the correlation analyses illustrated. For the immobility time data collected 6h after the final MA intake session, the only significant correlation was a negative correlation between MA intake and immobiiity time in the tail-suspension test for MAHDR mice (Figure 5a–d). Significant positive associations between amount of MA consumed and immobility time after 30h of abstinence were identified for MAHDR, but not D2, mice for both tests (Figure 5e–h).
Figure 5.
Depression-like symptoms after forced abstinence from MA are associated with amount of MA consumed on the final day of MA access (Day 28) in MAHDR, but not D2, mice. Presented are simple linear regression plots and Pearson’s r values for MA intake (mg/kg/18h) on Day 28 vs. immobility time (s) in the tail-suspension test (TST) and forced-swim test (FST). a-b: Correlation between MA intake and TST (a) or FST (b) immobility time for MAHDR mice for the 6h (Group 2) forced abstinence time point; c-d: Correlation between MA intake and TST (c) or FST (d) immobility time for D2 mice for the 6h forced abstinence time point; an outlier is denoted by the dotted circle (◌), and the data point was not included in the analysis; e-f: Correlation between MA intake and TST (e) or FST (f) immobility time for MAHDR mice for the 30h (Group 3) forced abstinence time point; g-h: Correlation between MA intake and TST (c) or FST (d) immobility time for D2 mice for the 30h forced abstinence time point. Dotted lines on either side of the regression lines indicate 95% confidence intervals.
Associations were also examined between mean MA intake across all days of 80 mg/l MA access (Days 13–28) and immobility times to determine if mean intake over a more protracted period had a similar relationship with severity of the depression-like symptoms. There was a significant correlation for MAHDR mice from the 30h MA abstinence group for the tail-suspension test (r = .57, p < .001), and a strong trend for the forced-swim test (r = .35, p = .06). For D2 mice (excluding the outlier), no significant relationships between protracted MA intake and immobility time in either test were found.
3.4. Experiment 3. Depression-like symptoms after prolonged forced abstinence from chronic MA intake in MAHDR mice
Figure 6a outlines the study design. Drinking results largely mimicked those for experiment 1. During the acquisition period, MAHDR mice increased their MA consumption with each incremental increase in MA concentration (Figure 6b), and exhibited stable intake during the chronic intake period (Figure 6c). For acquisition period data, there were no significant effects of sex or group, and examination of the significant concentration effect (F(1.4, 39.9) = 178.9, p < .001) by within-subjects mean contrasts detected significantly increasing intake as concentration increased (Figure 6b). There were no significant results for MA consumption during the chronic MA intake period (days 17–28; Figure 6c).
Figure 6.
Design, MA consumption, and fluid consumption data for experiment 3. MAHDR mice increase their MA consumption with increasing MA concentration to a binge-like level and sustain that level of intake. a: Experimental design. The design was identical to that for experiments 1 and 2, except that testing for depression-like behavior (FST and TST) occurred after a more protracted period of forced abstinence; 1 week (Group 2 and half of Group 1) or 2 weeks (Group 3 and half of Group 1). In addition, one day after the FST and TST, all animals were given access to 1 tube of water and 3 tubes of 80 mg/l MA during 4 daily 18h sessions. b: mean (± SEM) mg/kg/18h MA consumed for MA concentrations offered for 4 days each; c: mean (± SEM) mg/kg/18h MA consumed when access to 80 mg/l MA was extended for an additional 12 days. d and e: mean (± SEM) total fluid consumed during the periods in b and c. n = 7–12/group/sex. *p<.05, ***p<.001, compared to the next lower MA concentration for Group 2 and 3 data in b and d; +p<.05 for the comparison of each MA group (Groups 2 and 3) to the control (Group 1) at the indicated MA concentration (d) or for the main effect of group (e).
For total fluid consumption during the MA acquisition period (Figure 6d), the only significant result was a treatment group by MA concentration interaction (F(4.0, 97.7) = 6.1, p < .001). Simple effects analysis at each concentration revealed significant group differences in total fluid consumed at all MA concentrations except 20 mg/l, with both MA groups consuming significantly more fluid than the water control group. Simple effects analysis within each group indicated a significant increase in fluid intake across MA concentrations only in the 1 week MA abstinence group, that was identified by post-hoc analysis as an increase when the MA concentration changed from 60 to 80 mg/l. For fluid consumption during the chronic period (Figure 6e), there were significant effects of treatment group (F(2, 49) = 6.1, p < .01) and day (F(8.1, 399.0) = 2.2, p < .05), but no significant sex or interaction effects. Mean comparisons for the main effect of group revealed significant differences between each MA group and the control group.
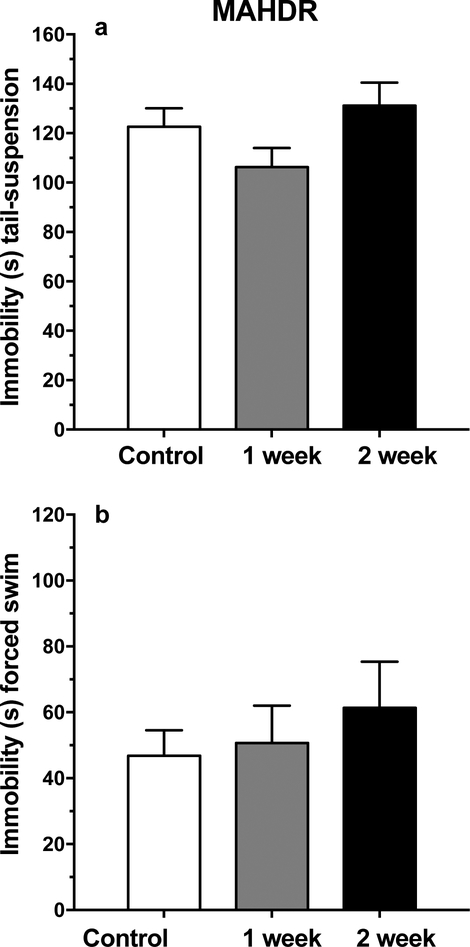
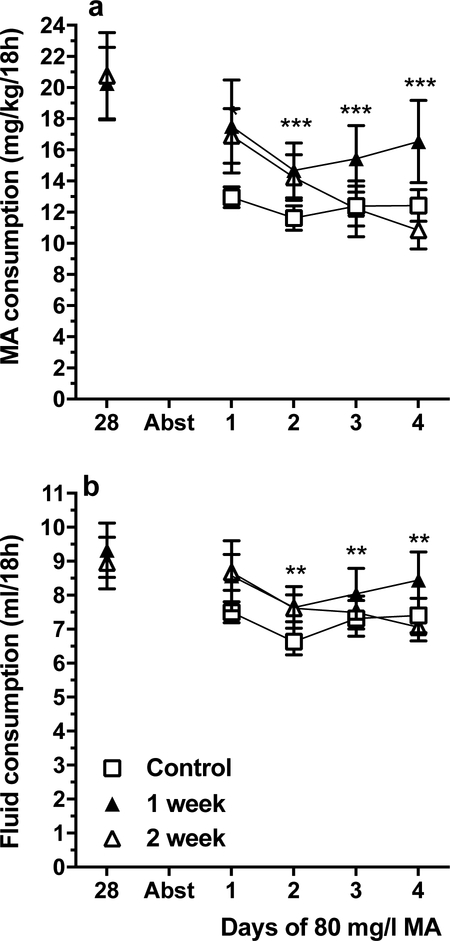
There were no statistically significant findings for duration of immobility in either the tail-suspension or forced-swim tests that were conducted 1 or 2 weeks after forced abstinence from MA (Figure 7). To examine the impact of prior MA consumption and of abstinence on MA intake, MA consumption data were collected beginning 1 day after immobility testing (Figure 8a). Two analyses were conducted. First, data for the 1 and 2 week MA abstinence groups were analyzed by repeated measures ANOVA, including data for the last day of chronic intake prior to MA withdrawal and for the 4 days after withdrawal. There were no significant sex, group or interaction effects, but there was a significant effect of day (F(2.7, 83.5) = 10.4, p < .001). Follow-up mean comparisons identified significantly lower MA consumption on all post-abstinence days, compared to the pre-abstinence day. Next, all 3 groups were included in an analysis of MA intake for the 4 post-abstinence days. No significant differences were found, indicating that there was no impact of prior MA consumption on post-abstinence MA intake.
Figure 7.
There is no elevation in depression-like symptoms in MAHDR mice after prolonged abstinence from chronic MA intake in experiment 3. b: mean (± SEM) immobility time (s) in the tail-suspension test. b: mean (± SEM) immobility time (s) in the forced-swim test.
Figure 8.
MA consumption is reduced in MAHDR mice after prolonged periods of forced MA abstinence (Abst) to levels not significantly different from those of controls given access to MA for the first time. a: mean (± SEM) mg/kg/18h MA consumed across 4 days in MA-naïve controls (Group 1), and in MA-consuming mice before (day 28), and 1 week (Group 2) or 2 weeks (Group 3) after MA access is withdrawn. No MA consumption data are shown on Day 28 for Group 1, because they did not have access to MA until day 1 post-Abst. During the 4-day post-Abst drinking period, all animals had access to 1 water tube and 3, 80 mg/l MA tubes. b: mean (± SEM) total fluid consumed during the periods in A. **p<.01, ***p<.001, compared to the mean on day 28 for the main effect of day for the MA groups combined.
Data for the same days are shown for total fluid consumption in Figure 8b, and were analyzed in the same way as for the MA consumption data. For the analysis including data for only the MA groups, there was a significant effect of day (F(2.5, 76.4) = 6.4, p < .001), but no other significant effects. Total fluid consumption was lower on post-abstinence days 2–4, compared to the pre-abstinence day. For the second analysis, comparing total fluid consumption for all groups only on the post-abstinence days, there were no significant differences.
4. Discussion
The present research utilized mice that are genetically susceptible to voluntary, binge-level MA intake to examine depression-like symptoms after various periods of forced abstinence. Selectively bred MAHDR mice displayed elevated immobility times in both the tail-suspension and forced-swim tests after shorter periods of abstinence from MA, but not after prolonged abstinence. Mice of the D2 progenitor strain exhibited elevated mean immobility time only at the 30 h MA withdrawal test time, and only in the forced-swim test. Data for MAHDR and D2 mice were not statistically compared, because they were collected in separate studies; however, mean MA intake of the D2 mice was about half that of MAHDR mice and this could have contributed to their apparent reduced sensitivity to the effects of withdrawal of MA. In addition, the tail-suspension test has been reported to be problematic in specific strains, and that might be the case for D2 mice (Cryan et al., 2005; Jacobson & Cryan, 2007; Ripoll et al., 2003; Trullas et al., 1989); D2 mice had a high immobility score in the tail-suspension test, which could have impacted the ability to detect an increase.
A strong positive relationship was found, only in MAHDR mice, between level of MA intake (Day 28 and mean Days 13–28) and severity of depression-like symptoms at the 30h abstinence time point. However, when MA intake data were examined for associations with depression-like symptoms at the earlier 6h withdrawal point, there was a negative relationship for MAHDR mice, only between Day 28 MA intake and immobility time in the tail-suspension test. As shown in Figure 5a, 5 MAHDR mice consumed > 30 mg MA/kg on this day and several others consumed ≥ 20 mg MA/kg. Only 2 D2 mice consumed this much MA, and one was determined to be an outlier. One interpretation of the negative correlation of MA intake with immobility time at the 6h time point for the forced-swim test for MAHDR mice is that MA had not been completely cleared and had a direct impact on behavior during the test. Thus, animals with higher intake may not have been in complete withdrawal, and may have had reduced immobility because of direct stimulant effects of MA. An acute injection of 2 mg/kg MA is cleared from blood in MAHDR mice in about 4h (Shabani et al., 2012). Animals in the current studies likely had variable intake patterns across the 18h access periods, and blood samples were not taken at the end of the Day 28 drinking period to avoid disrupting the behavioral analysis. However, this is something we will consider exploring in future studies. Overall, the results, most markedly for the 30h time point, indicate that binge-level MA intake can lead to abstinence-associated depression-like symptoms that, in turn, sustain high levels of MA intake.
Voluntary MA intake in MAHDR mice was significantly attenuated after prolonged forced abstinence of one or two weeks, compared to intake on the day prior to the prolonged abstinence periods. However, their MA intake remained at binge-like levels. This outcome is consistent with higher vulnerability to relapse (Cheng et al., 2010; Cook et al., 2017; Semple et al., 2007). MA-dependent individuals that use MA daily are more likely to experience more severe negative affective and psychotic symptoms and are more likely to relapse (McKetin et al., 2013; Newton et al., 2004; Zorick et al., 2010). Furthermore, binge users report higher quantity and frequency of MA use than non-binge users, and exhibit higher Beck Depression Inventory scores (Cheng et al., 2010). Although MA intake amounts for individual MAHDR mice overlap with those for D2 mice (for example, see Figure 5), the distribution of individual MA intake amounts in the 4-bottle choice test for MAHDR mice is markedly skewed to the right of D2 mice (Shabani et al., 2016). That the magnitude of depression-like symptoms was associated with MA intake in MAHDR, but not D2, mice suggests that the development of depression-like symptoms is dependent upon a certain level of MA intake. It is also possilble that MAHDR mice experience negative withdrawal symptoms during the 6h forced abstinence periods as they are establishing MA consumption, and that this contributes to higher subsequent MA intake. Higher MA reinforcement may also lead to higher MA intake and stronger withdrawal-related symptoms. The success rate of achieving continued abstinence and remaining in a clinical treatment trial is attenuated when MA use has been heavy (Cook et al., 2017). Thus, individuals who reported higher MA use were more likely to drop out of the study before early abstinence was achieved, and among those of this group who were able to achieve early abstinence, more were likely to drop out of the study at some time. In contrast, individuals with less frequent use of MA were more likely to achieve early abstinence and were more receptive to treatment and more likely to abstain from MA use for prolonged periods of time.
Genetic factors may impact MA intake levels following periods of forced abstinence. This view is supported by previous results for which introduction of forced abstinence periods longer than 6 h resulted in reduced MA intake in progenitor D2, but not MAHDR, mice (Shabani et al., 2016). MAHDR mice possess alleles from the B6 progenitor strain that are not present in D2 mice, which may act additively or interactively to further increase MA intake (Belknap et al., 2013). Whether genetic factors play a significant role in the ability to remain abstinent in humans is not known. Furthermore, whether genetic susceptibility to negative symptoms following the withdrawal of MA plays a role in risk for voluntary MA intake has not been studied, to the best of our knowledge. This could be studied in the MA drinking lines using forced MA exposure (such as by minipump), so that symptoms after cessation could be measured after equal exposure. A similar approach has been taken in lines bred for high and low severity of ethanol withdrawal symptoms or bred for high and low ethanol intake to examine the relationship between these traits. In populations of mice derived from the D2 and B6 progenitors, high susceptibility to ethanol-withdrawal symptoms was associated with reduced voluntary ethanol intake (Metten et al., 1998), suggesting that adverse symptoms of withdrawal can play a role in risk for continued use. Of course, it is important to recognize that forced drug exposure effects may differ from the effects of voluntary self-administration.
Depression-like symptoms were apparent in MAHDR mice early after the withdrawal of MA (6 and 30h), but not after prolonged abstinence (1 and 2 weeks). Disappearance of depression-like symptoms suggests that prolonged abstinence allows for depression-related neuroadaptations to weaken or reverse over time. DBA/2HA mice displayed depression-like symptoms in the tail-suspension test 24h after 7 days of 5 or 10 mg/kg/day amphetamine administration via minipumps (Cryan et al., 2003). B6 mice did not exhibit depression-like symptoms in a forced-swim test 1 week after 10 daily bolus 2 mg/kg MA administrations (Georgiou et al., 2016). An earlier time period after cessation of MA treatment was not examined in that study, and it is possible that this lower dose of MA was not enough to produce changes in the central nervous system that would induce such symptoms. In Wistar rats with access to MA for two weeks or longer, a 24h withdrawal period was associated with significant depression-like symptoms in a forced swim test and in other tests, such as open field, and novelty-suppressed feeding (Jang et al., 2013); rats took about 4 mg/kg MA per day in that study. An increase in brain reward threshold, assessed using intracranial self-stimulation (ICSS), may also provide evidence of a depressive-like or dysphoric state. During minipump administration of amphetamine (10 mg/kg/day) to Wistar rats, ICSS threshold decreased, compared to that of vehicle controls; thus, less stimulation was needed to reach the reward threshold. However, ICSS threshold increased significantly above the threshold for control rats during the initial 24h of amphetamine withdrawal, indicating that more stimulation was needed to reach the brain reward threshold. This elevation was sustained for 48h and declined over the next 3 days. ICSS thresholds for amphetamine and control rats were comparable thereafter (Cryan et al., 2003). A similar result was found for Wistar rats that self-administered ~4 mg/kg MA per day, although ICSS thresholds remained elevated for up to 7 days (Jang et al., 2013). Immobility time for the control group in the tail-suspension test was greater in the latter study of MAHDR mice, which could have impaired the ability to observe an increase; however, immobility time for the forced-swim test was comparable in the 2 studies, yet a time-dependent increase was observed only for the early withdrawal times. One potential limitation is that the immobility data for the shorter and longer withdrawal time studies of MAHDR mice were collected in separate experiments; however, MA consumption results were quite comparable in the 2 studies.
The similarity of rodent and human MA/amphetamine withdrawal effect timelines encourages further study of short-term and protracted effects in rodent models of MA use. Symptoms of depression in humans with MA use disorders are most profound in the first 24h after MA use is terminated, but remain high for about a week. Milder symptoms of depression or complete abatement may be seen over subsequent days or weeks (Mancino et al., 2011; McGregor et al., 2005; Zorick et al., 2010). However, the timecourse of MA withdrawal-associated depression is highly variable among MA users and does not seem to be associated with absolute values of MA intake (Newton et al., 2004).
A major genetic contributor to relative risk for MA intake that was identified using our bidirectionally selected MA drinking lines is the trace amine-associated receptor 1 (Taar1) gene (Harkness et al., 2015; Reed et al., 2018; Shi et al., 2016). The allele derived from the B6 progenitor strain is absent in the MAHDR line, and codes for a functional stimulatory G protein-coupled receptor, TAAR1, that confers avoidance of MA intake and high sensitivity to aversive effects of MA (Harkness et al., 2015). The alternative allele derived from the D2 progenitor, codes for a non-functional receptor. Avoidance of MA intake in mice with functional TAAR1 (i.e., MALDR) occurs with a short incubation period. Thus, MAHDR and MALDR mice consume the same average amount of MA during the first session that it is offered, in both a two-bottle choice and an operant oral self-administration procedure, and then MALDR mice reduce their MA intake to negligible amounts during the next and all following sessions, whereas MAHDR mice increase their MA intake (Eastwood et al., 2014; Shabani et al., 2012a). MA is a direct agonist at TAAR1 and the negative regulation of monoamines in brain reward pathways by TAAR1 has been suggested to be a mechanism for its impact on MA-related and other addiction-related and psychiatric traits (Berry et al., 2017). Whether TAAR1 function has a role in MA withdrawal-associated symptoms of depression or in susceptibility to the effects of MA withdrawal remains to be studied. However, because mice with functional TAAR1 will not voluntarily consume MA (Reed et al., 2018), a forced exposure model would be required to study this, as has been used to examine the role of TAAR1 in sensitivity to MA-induced neurotoxicity (Miner et al., 2017).
Some studies have suggested that the maintenance of drug intake and relapse to drug intake after forced abstinence, are associated with functional switches of monoaminergic and glutamatergic systems (Krawczyk et al., 2011; Neisewander et al., 2014; Pentkowski et al., 2012; Szumlinski et al., 2017). Microdialysis studies reported higher basal levels of serotonin in the nucleus accumbens (NAc) of MAHDR mice, compared to MALDR mice, as well as resistance in MAHDR mice to MA-induced elevations of serotonin in the medial prefrontal cortex that occurred in MALDR mice (Lominac et al., 2014). In addition, basal extracellular glutamate levels in the NAc were twice as high in MAHDR, compared to MALDR, mice and NAc glutamate levels increased after an acute 2 mg/kg MA injection in MAHDR, but not MALDR, mice (Szumlinski et al., 2017). Mice of the B6 progenitor strain, which resemble MALDR mice for MA intake and Taar1 genotype, displayed a hyperglutamatergic state after 21 days, but not 1 day, of withdrawal from 10 daily IP injections of 2 mg/kg MA. Furthermore, a MA challenge injection after 21 days, but not 1 day, of withdrawal induced a robust glutamate response in these mice. It would be of interest to examine such effects in D2 progenitor strain mice, which resemble MAHDR mice for MA intake and Taar1 genotype, and in the MADR lines. Overall, additional studies are needed to determine how the genetically correlated differences in serotonergic and glutamatergic systems in the MADR lines play roles in MA intake and what their roles might be in depression-like symptoms after cessation of MA access or exposure.
Total fluid consumption was greater in mice consuming both water and MA, compared to mice consuming water only. One speculation is that the increased fluid consumption is associated with stimulated behavior induced by high MA consumption, leading to greater thirst. A more parsimonious interpretation is that the reinforcing effects of MA lead to its consumption and contriubte to the increase in total fluid consumption. In fact, during the creation of the MADR lines, MAHDR mice consumed about 0.5 ml more total fluid than MALDR mice, commensurate with their higher MA consumption (Shabani et al., 2011; Wheeler et al., 2009). Sex had no impact on the results for MAHDR mice. Although females have sometimes been found to consume more MA than males, particularly when the group size was very large, as for our selective breeding experiments (Shabani et al., 2011; Wheeler et al., 2009), we have commonly found no sex differences (Harkness et al., 2015; Shabani et al., 2016). In D2 mice, main effects of sex were found only for total amount of fluid consumed.
Recent reports indicate that MA is one of the top 10 drugs associated with death from overdose in the United States, and between 2010 and 2014, the number of MA overdose deaths more than doubled (Warner et al., 2016). Taar1 has been identified as a gene that impacts risk for MA consumption (Harkness et al., 2015; Reed et al., 2018). A quantitative trait locus on mouse chromosome 10 that includes Taar1 within its confidence interval accounts for ~50% of the genetic variance in MA intake in our selected lines (Belknap et al., 2013), so there are other genetic influences yet to be identified. The mu opioid receptor gene, Oprm1, at the proximal end of mouse chromosome 10, has been ruled out as a quantitative trait gene for MA intake, although it is regulated by the top-ranked transcription factor network of genes that are differentially expressed in MA-naïve MALDR and MAHDR mice (Belknap et al., 2013; Eastwood et al., 2018). The current genetic mouse model is being used to identify other genetic influences, but also provides us with a unique opportunity to examine the consequences of high levels of MA intake, and to explore drugable targets that curb MA intake.
Acknowledgments
We thank Chelsey Casiquin for her instrumental help in experiment 3. Support was provided by the Department of Veterans Affairs Senior Research Career Scientist Program and grant I01BX002106 (TP), NIH NIDA P50 DA018165 (TP), NIH NIDA U01 DA041579 (TP), NIH NIAAA R24 AA020245 (TP), and an Institutional Development Award from NIH NIGMS P20GM103442 (SS).
Footnotes
Conflict of interest
The authors declare no conflict of interest. They or their institution did not receive services from a third party for any aspect of the submitted work. They have no financial relationships with entities that could be perceived to influence, or give the appearance of potentially influencing, statements in this paper. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- APA, 2013. American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 5th ed; DSM-5; Arlington VA. [Google Scholar]
- BELKNAP JK, MCWEENEY S, REED C, BURKHART-KASCH S, MCKINNON CS, LI N, BABA H, SCIBELLI AC, HITZEMANN R & PHILLIPS TJ 2013. Genetic factors involved in risk for methamphetamine intake and sensitization. Mamm Genome, 24, 446–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRY MD, GAINETDINOV RR, HOENER MC & SHAHID M 2017. Pharmacology of human trace amine-associated receptors: Therapeutic opportunities and challenges. Pharmacol Ther, 180, 161–180. [DOI] [PubMed] [Google Scholar]
- CAN A, DAO DT, ARAD M, TERRILLION CE, PIANTADOSI SC & GOULD TD 2012. The mouse forced swim test. J Vis Exp, e3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTAGNE V, MOSER P, ROUX S & PORSOLT RD 2011. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci, Chapter 8, Unit 810A. [DOI] [PubMed] [Google Scholar]
- CHENG WS, GARFEIN RS, SEMPLE SJ, STRATHDEE SA, ZIANS JK & PATTERSON TL 2010. Binge use and sex and drug use behaviors among HIV(−), heterosexual methamphetamine users in San Diego. Subst Use Misuse, 45, 116–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO AK, MELEGA WP, KUCZENSKI R & SEGAL DS 2001. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse, 39, 161–6. [DOI] [PubMed] [Google Scholar]
- COOK R, QUINN B, HEINZERLING K & SHOPTAW S 2017. Dropout in clinical trials of pharmacological treatment for methamphetamine dependence: the role of initial abstinence. Addiction, 112, 1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUICKSHANK CC & DYER KR 2009. A review of the clinical pharmacology of methamphetamine. Addiction, 104, 1085–99. [DOI] [PubMed] [Google Scholar]
- CRYAN JF, HOYER D & MARKOU A 2003. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry, 54, 49–58. [DOI] [PubMed] [Google Scholar]
- CRYAN JF, MOMBEREAU C & VASSOUT A 2005. The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev, 29, 571–625. [DOI] [PubMed] [Google Scholar]
- DE WIT H & PHILLIPS TJ 2012. Do initial responses to drugs predict future use or abuse? Neurosci Biobehav Rev, 36, 1565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE WIT H, UHLENHUTH EH & JOHANSON CE 1986. Individual differences in the reinforcing and subjective effects of amphetamine and diazepam. Drug Alcohol Depend, 16, 341–60. [DOI] [PubMed] [Google Scholar]
- EASTWOOD EC & PHILLIPS TJ 2014. Opioid sensitivity in mice selectively bred to consume or not consume methamphetamine. Addict Biol, 19, 370–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASTWOOD EC, BARKLEY-LEVENSON AM & PHILLIPS TJ 2014. Methamphetamine drinking microstructure in mice bred to drink high or low amounts of methamphetamine. Behav Brain Res, 272, 111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASTWOOD EC, ESHLEMAN AJ, JANOWSKY A & PHILLIPS TJ 2018. Verification of a genetic locus for methamphetamine intake and the impact of morphine. Mamm Genome, 29, 260–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGIOU P, ZANOS P, GARCIA-CARMONA JA, HOURANI S, KITCHEN I, LAORDEN ML & BAILEY A 2016. Methamphetamine abstinence induces changes in mu-opioid receptor, oxytocin and CRF systems: Association with an anxiogenic phenotype. Neuropharmacology, 105, 520–532. [DOI] [PubMed] [Google Scholar]
- HARKNESS JH, SHI X, JANOWSKY A & PHILLIPS TJ 2015. Trace Amine-Associated Receptor 1 Regulation of Methamphetamine Intake and Related Traits. Neuropsychopharmacology, 40, 2175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON LH & CRYAN JF 2007. Feeling strained? Influence of genetic background on depression-related behavior in mice: a review. Behav Genet, 37, 171–213. [DOI] [PubMed] [Google Scholar]
- JANG CG, WHITFIELD T, SCHULTEIS G, KOOB GF & WEE S 2013. A dysphoric-like state during early withdrawal from extended access to methamphetamine self-administration in rats. Psychopharmacology (Berl), 225, 753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES HW, DEAN AC, PRICE KA & LONDON ED 2016. Increased self-reported impulsivity in methamphetamine users maintaining drug abstinence. Am J Drug Alcohol Abuse, 42, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAWCZYK M, SHARMA R, MASON X, DEBACKER J, JONES AA & DUMONT EC 2011. A switch in the neuromodulatory effects of dopamine in the oval bed nucleus of the stria terminalis associated with cocaine self-administration in rats. J Neurosci, 31, 8928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOMINAC KD, MCKENNA CL, SCHWARTZ LM, RUIZ PN, WROTEN MG, MILLER BW, HOLLOWAY JJ, TRAVIS KO, RAJASEKAR G, MALINIAK D, THOMPSON AB, URMAN LE, PHILLIPS TJ & SZUMLINSKI KK 2014. Mesocorticolimbic monoamine correlates of methamphetamine sensitization and motivation. Front Syst Neurosci, 8, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANCINO MJ, GENTRY BW, FELDMAN Z, MENDELSON J & OLIVETO A 2011. Characterizing methamphetamine withdrawal in recently abstinent methamphetamine users: a pilot field study. Am J Drug Alcohol Abuse, 37, 131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGREGOR C, SRISURAPANONT M, JITTIWUTIKARN J, LAOBHRIPATR S, WONGTAN T & WHITE JM 2005. The nature, time course and severity of methamphetamine withdrawal. Addiction, 100, 1320–9. [DOI] [PubMed] [Google Scholar]
- MCKETIN R, LUBMAN DI, BAKER AL, DAWE S & ALI RL 2013. Dose-related psychotic symptoms in chronic methamphetamine users: evidence from a prospective longitudinal study. JAMA Psychiatry, 70, 319–24. [DOI] [PubMed] [Google Scholar]
- METTEN P, PHILLIPS TJ, CRABBE JC, TARANTINO LM, MCCLEARN GE, PLOMIN R, ERWIN VG & BELKNAP JK 1998. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome, 9, 983–90. [DOI] [PubMed] [Google Scholar]
- MINER NB, ELMORE JS, BAUMANN MH, PHILLIPS TJ & JANOWSKY A 2017. Trace amine-associated receptor 1 regulation of methamphetamine-induced neurotoxicity. Neurotoxicology, 63, 57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEISEWANDER JL, CHEUNG TH & PENTKOWSKI NS 2014. Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: Implications for medications development. Neuropharmacology, 76 Pt B, 301–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON TF, KALECHSTEIN AD, DURAN S, VANSLUIS N & LING W 2004. Methamphetamine abstinence syndrome: preliminary findings. Am J Addict, 13, 248–55. [DOI] [PubMed] [Google Scholar]
- PENTKOWSKI NS, CHEUNG TH, TOY WA, ADAMS MD, NEUMAIER JF & NEISEWANDER JL 2012. Protracted withdrawal from cocaine self-administration flips the switch on 5-HT(1B) receptor modulation of cocaine abuse-related behaviors. Biol Psychiatry, 72, 396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS TJ, MOOTZ JR & REED C 2016. Identification of treatment targets in a genetic mouse model of voluntary methamphetamine drinking. Int Rev Neurobiol, 126, 39–85. [DOI] [PubMed] [Google Scholar]
- PHILLIPS TJ & SHABANI S 2015. An animal model of differential genetic risk for methamphetamine intake. Front Neurosci, 9, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS TJ, KAMENS HM & WHEELER JM 2008. Behavioral genetic contributions to the study of addiction-related amphetamine effects. Neurosci Biobehav Rev, 32, 707–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAWSON RA 2013. Current research on the epidemiology, medical and psychiatric effects, and treatment of methamphetamine use. J Food Drug Anal, 21, S77–s81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAWSON RA, HUBER A, BRETHEN P, OBERT J, GULATI V, SHOPTAW S & LING W 2002. Status of methamphetamine users 2–5 years after outpatient treatment. J Addict Dis, 21, 107–19. [DOI] [PubMed] [Google Scholar]
- REED C, BABA H, ZHU Z, ERK J, MOOTZ JR, VARRA NM, WILLIAMS RW & PHILLIPS TJ 2018. A Spontaneous Mutation in Taar1 Impacts Methamphetamine-Related Traits Exclusively in DBA/2 Mice from a Single Vendor. Front Pharmacol, 8, 993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIPOLL N, DAVID DJ, DAILLY E, HASCOET M & BOURIN M 2003. Antidepressant-like effects in various mice strains in the tail suspension test. Behav Brain Res, 143, 193–200. [DOI] [PubMed] [Google Scholar]
- ROBERTS DC, GABRIELE A & ZIMMER BA 2013. Conflation of cocaine seeking and cocaine taking responses in IV self-administration experiments in rats: methodological and interpretational considerations. Neurosci Biobehav Rev, 37, 2026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEMPLE SJ, ZIANS J, STRATHDEE SA & PATTERSON TL 2007. Psychosocial and behavioral correlates of depressed mood among female methamphetamine users. J Psychoactive Drugs, Suppl 4, 353–66. [DOI] [PubMed] [Google Scholar]
- SHABANI S, DOBBS LK, FORD MM, MARK GP, FINN DA & PHILLIPS TJ 2012. A genetic animal model of differential sensitivity to methamphetamine reinforcement. Neuropharmacology, 62, 2169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABANI S, HOULTON SK, HELLMUTH L, MOJICA E, MOOTZ JR, ZHU Z, REED C & PHILLIPS TJ 2016. A Mouse Model for Binge-Level Methamphetamine Use. Front Neurosci, 10, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABANI S, MCKINNON CS, CUNNINGHAM CL & PHILLIPS TJ 2012. Profound reduction in sensitivity to the aversive effects of methamphetamine in mice bred for high methamphetamine intake. Neuropharmacology, 62, 1134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABANI S, MCKINNON CS, REED C, CUNNINGHAM CL & PHILLIPS TJ 2011. Sensitivity to rewarding or aversive effects of methamphetamine determines methamphetamine intake. Genes Brain Behav, 10, 625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI X, WALTER NA, HARKNESS JH, NEVE KA, WILLIAMS RW, LU L, BELKNAP JK, ESHLEMAN AJ, PHILLIPS TJ & JANOWSKY A 2016. Genetic Polymorphisms Affect Mouse and Human Trace Amine-Associated Receptor 1 Function. PLoS One, 11, e0152581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON SL, RICHARDSON K, DACEY J, GLYNN S, DOMIER CP, RAWSON RA & LING W 2002. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis, 21, 35–44. [DOI] [PubMed] [Google Scholar]
- SZUMLINSKI KK, LOMINAC KD, CAMPBELL RR, COHEN M, FULTZ EK, BROWN CN, MILLER BW, QUADIR SG, MARTIN D, THOMPSON AB, VON JONQUIERES G, KLUGMANN M, PHILLIPS TJ & KIPPIN TE 2017. Methamphetamine Addiction Vulnerability: The Glutamate, the Bad, and the Ugly. Biol Psychiatry, 81, 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORDOFF MG & BACHMANOV AA 2003. Influence of the number of alcohol and water bottles on murine alcohol intake. Alcohol Clin Exp Res, 27, 600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRULLAS R, JACKSON B & SKOLNICK P 1989. Genetic differences in a tail suspension test for evaluating antidepressant activity. Psychopharmacology (Berl), 99, 287–8. [DOI] [PubMed] [Google Scholar]
- WARNER M, TRINIDAD JP, BASTIAN BA, MININO AM & HEDEGAARD H 2016. Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2010–2014. Natl Vital Stat Rep, 65, 1–15. [PubMed] [Google Scholar]
- WHEELER JM, REED C, BURKHART-KASCH S, LI N, CUNNINGHAM CL, JANOWSKY A, FRANKEN FH, WIREN KM, HASHIMOTO JG, SCIBELLI AC & PHILLIPS TJ 2009. Genetically correlated effects of selective breeding for high and low methamphetamine consumption. Genes Brain Behav, 8, 758–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZORICK T, NESTOR L, MIOTTO K, SUGAR C, HELLEMANN G, SCANLON G, RAWSON R & LONDON ED 2010. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction, 105, 1809–18. [DOI] [PMC free article] [PubMed] [Google Scholar]