Abstract
Objectives:
The impact of tidal volume (VT) on outcomes in mechanically ventilated children with pediatric acute respiratory distress syndrome (PARDS) remains unclear. To date, observational investigations have failed to calculate VT based on standardized corrections of weight. We investigated the impact of VT on mortality and probability of extubation in PARDS using ideal body weight (IBW)-adjusted VT.
Design:
Retrospective analysis of an ongoing prospective cohort of PARDS patients. VT was calculated based on actual body weight (ABW) and two different formulations of IBW.
Setting:
Pediatric intensive care unit at a large, tertiary care children’s hospital.
Patients:
PARDS patients on conventional ventilation with a documented height or length.
Interventions:
None.
Measurements and Main Results:
There were 483 patients with a measured height or length at PARDS onset included in the final analysis, with 73 (15%) non-survivors. At 24 hours, there remained 400 patients on conventional ventilation. When calculating VT based on IBW by either method, volumes were larger both at onset and at 24 hours compared to VT based on ABW (all p < 0.001) and the proportion of patients being ventilated with tidal volumes > 10mL/kg based on IBW was larger both at onset (12.4% and 15.5%) and 24 hours (10.3% and 11.5%) compared to ABW at onset (3.5%) and 24 hours (4.0%) (all p < 0.001). VT, based on both ABW and IBW, was not associated with either increased mortality or decreased probability of extubation after adjusting for oxygenation index in the whole cohort, while associations between higher VT and poor outcomes were seen in subgroup analyses in overweight children and in severe PARDS.
Conclusions:
Our retrospective analysis of a cohort of PARDS patients did not find a consistent association between VT adjusted for IBW and outcomes, although an association may exist in certain subgroups. Although it remains to be shown in a prospective trial whether high volumes or pressures are injurious in PARDS, VT is likely an imprecise parameter for titrating lung protective ventilation.
Keywords: ARDS, PARDS, tidal volume, ideal body weight, VILI
INTRODUCTION
The impact of tidal volume (VT)on mortality in mechanically ventilated children with acute respiratory distress syndrome (ARDS) remains unclear and understudied. Although there is evidence that ventilating adult ARDS patients with VT on the lower end of physiologic (6 mL/kg predicted body-weight with plateau pressures ≤ 30 cm H2O) lowers mortality (1), there does not exist strong data to support this practice in children (2). The Pediatric Acute Lung Injury Consensus Conference Group (PALICC) recommended VT “in or below the range of physiologic” (5-8 mL/kg predicted body-weight), with the caveat that sub-physiologic VT (3-6 mL/kg predicted body-weight) can be used for patients with poor compliance (3, 4). However, these recommendations were among the most controversial by PALICC, leading to a “weak agreement.” The Pediatric Mechanical Ventilation Consensus Conference recommended avoiding VT > 10 mL/kg ideal body weight, and considering sub-physiologic volumes for children with lung hypoplasia (5). Likely secondary to the lack of evidence to support it, pediatric intensivists in general have been slow to adopt an aggressively low VT strategy for pediatric ARDS (PARDS) patients (6), and it is unclear whether the concerns over ventilator-induced lung injury (VILI) that apply to adults are relevant in children (7, 8).
Although clinician preference has certainly contributed to the slow adoption of low VT in PARDS, inconsistent methods for calculating predicted body-weight in children has had a significant impact on this phenomenon as well. In one meta-analysis showing no association between VT and mortality in children, authors were unable to determine if actual versus ideal body-weight measurements (IBW) were used in the analyzed studies (2). Heterogeneity of weight calculations not only poses a problem for analyses of outcomes retrospectively, but also leads in practice to obese children being less likely to receive low VT (6).
Currently, the literature lacks an analysis of the association between VT and mortality in children with PARDS that has a rigorous standardization of VT calculations (9). We thus conducted an analysis of a prospective cohort of PARDS patients to test the hypothesis that VT, when calculated based on standardized weight corrections, is associated with higher mortality and decreased probability of extubation.
METHODS
PARDS Cohort
This was a retrospective analysis of an ongoing prospective cohort, approved by the Children’s Hospital of Philadelphia’s (CHOP) Institutional Review Board (IRB), with requirement for informed consent waived. The cohort has previously been described in detail (10). Briefly, intubated children meeting American-European Consensus Conference criteria for acute lung injury (two consecutive Pao2/Fio2 ≤ 300 separated by ≥ 1 hour with bilateral infiltrates) admitted to the CHOP pediatric intensive care unit (PICU) between July 1, 2011 and June 30, 2017 were enrolled. As the study was initiated prior to the Berlin definition (11), minimum positive end-expiratory pressure (PEEP) was not specified; however, CHOP PICU does not utilize PEEP < 5 cmh2o. Thus, all patients met Berlin criteria. Similarly, as the study was initiated prior to the Pediatric Acute Lung Injury Consensus Conference definition of PARDS (12), we did not screen using OI; however, all but one patient met PARDS criteria by OI. For this study, only subjects on conventional ventilation at onset of ARDS with a documented height or length for determination of IBW were eligible.
Demographics, ventilator settings, and oxygenation at PARDS onset and 24 hours, and treatments for the first 3 days were recorded prospectively. Absent a standardized ventilator protocol, institutional practice is to initiate conventional ventilation with PEEP ≥ 5 cmh2o, and attempt to wean Fio2 to ≤ 0.60, keeping PaO2 ≥ 60 mmHg. Inability to wean Fio2 prompts PEEP escalation and subsequent efforts to wean Fio2. We exclusively utilize decelerating flow during conventional ventilation (either pressure control or pressure-regulated volume control [PRVC]). Persistently elevated peak pressures (≥ 35 cmh2o), hypercarbia (Paco2 ≥ 80), or oxygenation difficulties (inability to wean Fio2 ≤ 0.60 despite increasing PEEP) prompted consideration for changing mode of ventilation or escalating to extracorporeal membrane oxygenation. Actual transition was left to the discretion of the attending physician. There was no standardization of ancillary therapies, which was also left to the preference of the attending physician.
Determination of the Exposure
The primary exposure was exhaled VT, collected at the ventilator for most patients (those with VT ≥ 100 mL), using integrated software provided by the manufacturer (Dräger, Inc., Lübeck, Germany), adjusting for patient size. For VT < 100 mL, we utilized a sensor proximate to the patient at the endotracheal tube. To avoid confounding by patient effort, ventilator volumes and pressures were recorded during periods of passive breathing under deep sedation or neuromuscular blockade by data collectors (respiratory therapists) trained to identify spontaneous effort, most commonly by ensuring that the set respiratory rate matched the observed rate. As endotracheal tube leak can confound measurements of exhaled VT, we have previously demonstrated that patients in this cohort had an endotracheal tube leak < 15% (median 0 [0, 1] %), with 95% of patients having a leak ≤ 5% (13), consistent with our near-universal use of cuffed endotracheal tubes. VT was normalized to three separate calculations of body weights and expressed as mL/kg:
-
1)
measured actual body weight (ABW) at PICU admission
-
2)
IBW as determined by the Traub-Johnson (TJ) equation (14), and
-
3)
IBW determined by calculating World Health Organization (WHO) weight-for-length (for age < 2 years) or Centers for Disease Control (CDC) body mass index (BMI; for age ≥ 2 years), determining the z-score (1 z-score is 1 standard deviation away from the population mean), and assigning the 50th percentile (median) weight, as previously described (15, 16). This is effectively given by the equation IBW = BMI50 * height2. This method has been demonstrated to be superior across a wider range of ages and heights than other methods of IBW determination.
Both methods of calculating IBW incorporate length or height and gender. CDC recommends use of WHO curves for children < 2 years, and CDC curves for ≥ 2 years (15). To facilitate comparisons, we provide z-scores, rather than raw weight-for-length or BMI.
Equations and Definitions
Oxygenation was measured using Pao2/Fio2 or OI (mean airway pressure [mPaw] x Fio2 × 100)/Pao2). ΔP was defined as peak inflating pressure (PIP) minus PEEP, as we did not routinely collect plateau pressure. ΔΔP was the change in ΔP over the first 24 hours. Non-pulmonary organ failures were identified using accepted definitions in children (17). The designation “immunocompromised” required presence of an immunocompromising diagnosis (oncologic, immunologic, rheumatologic, or transplant) and active immunosuppressive chemotherapy, or a congenital immunodeficiency (10, 18). Severity of illness score used was the Pediatric Risk of Mortality (PRISM) III at 12 hours.
Outcomes
Primary outcome was PICU mortality. A secondary outcome was duration of ventilation, reported as probability of extubation given the competing risk of death. All mention of “ventilation” implies invasive ventilation; non-invasive support was not counted. “Day 1” was initiation of invasive ventilation. Liberation from invasive ventilation ≥ 24 hours defined duration of ventilation. Patients requiring re-initiation of invasive ventilation had the extra days counted towards total ventilator days.
Statistical Analysis
Analyses were performed using Stata 14.2 SE (StataCorp LLC, College Station, Texas). Data are expressed as medians [interquartile range] or percentages and analyzed using Wilcoxon rank-sum or Fisher exact tests, or their matched equivalents when appropriate. Logistic regression testing univariate associations with mortality were performed, treating VT as both a continuous variable and dichotomized at > 10 mL/kg or ≤ 10 mL/kg. Univariate association with probability of extubation given the competing risk of death was performed in the method of Fine and Gray (19). Competing risk regression is a time to event analysis that calculates a subdistribution hazard ratio (SHR) for probability of extubation (primary event), treating death as a competing event. Observations were censored at 28 days, making this analysis comparable to ventilator-free days at 28 days. We performed an additional bivariate regression including OI in the model for both logistic regression for mortality and competing risk regression for probability of extubation. We also performed a parallel analysis on ΔP and ΔΔP as a comparison with VT. Finally, we performed subgroup analyses restricting to overweight/obese subjects, in severe PARDS, and excluding patients < 2 years of age given their more compliant chest walls.
RESULTS
Description of the Cohort
During the study period, 544 patients had PARDS, of whom 521 had a height recorded. Of these, 483 were on conventional ventilation at PARDS onset (379 on PRVC [78%] and 104 on pressure control [22%]). Of this final cohort of 483 subjects, 73 (15%) died in the PICU. At 24 hours, 400 patients remained on conventional ventilation (329 on PRVC [82%] and 71 on pressure control [18%]). Demographics are presented in Table 1. Non-survivors were older (p = 0.003) and had higher PRISM III score, more organ failures, were more likely to be immunocompromised, and were more likely to have a stem cell transplant (all p < 0.001). Non-survivors had worse OI, peak pressures, and PEEP than survivors at PARDS onset. Non-survivors had higher ventilator pressures and worse oxygenation at 24 hours after PARDS onset.
Table 1:
Demographics of the cohort
| Variables | All patients (n = 483) | Survivors (n = 410) | Non-survivors (n = 73) | p value |
|---|---|---|---|---|
| Age (years) | 4.6 [1.5, 12.1] | 4.1 [1.4, 10.6] | 7.7 [3.3, 14.5] | 0.003 |
| Female (%) | 209 (43) | 185 (45) | 24 (33) | 0.055 |
| Z-score categories | ||||
| Underweight (< 3rd) | 41 (8) | 33 (8) | 7 (10) | |
| Normal (3rd to 85th) | 280 (58) | 239 (57) | 42 (58) | 0.989 |
| At-risk (85th to 97th) | 69 (14) | 59 (14) | 10 (14) | |
| Overweight (> 97th) | 93 (19) | 79 (19) | 14 (19) | |
| Severity of illness | ||||
| PRISM III at 12 hours | 10 [5, 17] | 9 [4, 15] | 16 [11, 27] | < 0.001 |
| Non-pulmonary organ failures | 2 [1, 3] | 1 [1, 2] | 3 [2, 4] | < 0.001 |
| Immunocompromised | 106 (20) | 62 (15) | 41 (56) | < 0.001 |
| Stem cell transplant | 42 (8) | 16 (4) | 24 (33) | < 0.001 |
| Cause of PARDS (%) | ||||
| Infectious pneumonia | 255 (53) | 226 (55) | 29 (40) | |
| Aspiration | 52 (11) | 44 (11) | 8 (11) | 0.038 |
| Non-pulmonary sepsis | 117 (24) | 92 (22) | 25 (34) | |
| Other | 59 (12) | 48 (12) | 11 (15) | |
| PARDS onset | ||||
| PaO2/FIO2 | 164 [113, 225] | 166 [118, 225] | 150 [89, 228] | 0.174 |
| OI | 9.9 [76.7, 15.9] | 9.5 [6.7, 14.4] | 11.1 [7.1, 23.7] | 0.034 |
| PIP (cmH2O) | 31 [26, 35] | 30 [26, 35] | 31 [28, 37] | 0.030 |
| PEEP (cmH2O) | 10 [8, 12] | 10 [8, 12] | 10 [8, 12] | 0.040 |
| ΔP (cmH2O) | 21 [17, 25] | 20 [16, 24] | 21 [18, 26] | 0.100 |
| 24 hours after onset (n = 400; 57 non-survivors) | ||||
| PaO2/FIO2 | 237 [172, 296] | 243 [180, 304] | 182 [120, 248] | < 0.001 |
| OI | 6.2 [4.4, 9.1] | 6.1 [4.3, 8.5] | 8.9 [5.7, 14.1] | < 0.001 |
| PIP (cmH2O) | 27 [24, 32] | 27 [23, 31] | 30 [25, 34] | 0.001 |
| PEEP (cmH2O) | 10 [8, 10] | 10 [8, 10] | 10 [8, 12] | < 0.001 |
| ΔP (cmH2O) | 17 [14, 22] | 17 [14, 22] | 20 [15, 23] | 0.014 |
OI: oxygenation index; PARDS: pediatric acute respiratory distress syndrome; PIP: peak inspiratory pressure; PEEP: positive end-expiratory pressure; PRISM; Pediatric Risk of Mortality; ΔP: PIP minus PEEP
Tidal Volume
VT based on ABW, IBW-TJ, and IBW-BMI at PARDS onset and 24 hours is described in Figures 1 and 2. VT based on corrected IBW were larger (all p < 0.001) both at onset and at 24 hours (IBW-TJ onset: 7.8 [6.7, 9.0] mL/kg, 24 hours: 7.7 [6.8, 8.8] mL/kg; IBW-BMI onset: 8.1 [7.0, 9.3] mL/kg, 24 hours: 8.0 [7.0, 9.0] mL/kg) compared to VT based on ABW (onset 7.5 [6.6, 8.4] mL/kg, 24 hours 7.3 [6.5, 8.1] mL/kg). When stratifying the cohort based on VT below or above 10 mL/kg, the fraction of patients with VT >10 mL/kg based on ABW at onset and 24 hours was 3.5% and 4.0%, respectively. Based on IBW-TJ, this fraction was larger both at onset (12.4%) and 24 hours (10.3%) (both McNemar’s p < 0.001). Based on IBW-BMI this fraction was also larger both at onset (15.5%) and 24 hours (11.5%; both McNemar’s p < 0.001).
Figure 1.
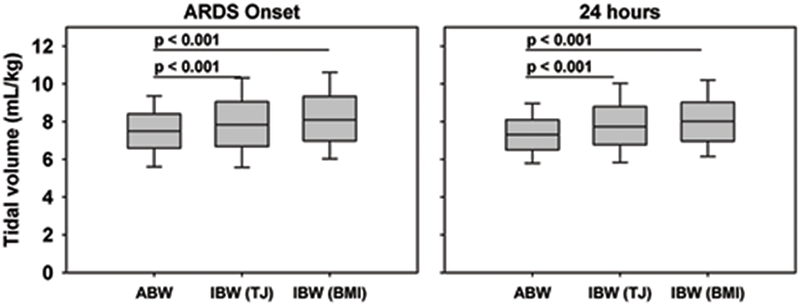
VT at PARDS onset and 24 hours using ABW and two measurements of IBW, the Traub-Johnson (TJ) equation and the WHO weight-for length and CDC body mass index (BMI). P values represent results of the signed-rank test.
Figure 2.

Distribution of VT at ARDS onset and 24 hours using ABW and two measurements of IBW, the Traub-Johnson (TJ) equation and the WHO weight-for length and CDC body mass index (BMI).
Tidal Volume and Outcomes
Between survivors and non-survivors, there were no differences in VT recorded at onset and 24 hours when calculated by any of the different weight categorizations (Figure 3). VT was not associated with an increased mortality either before or after adjusting for OI (Table 2). In the case of VT based on IBW-BMI at 24 hours when adjusting for OI, higher VT was associated with lower mortality (OR 0.80, 95% CI 0.66 to 0.98, p = 0.029). Among patients with VT > 10 mL/kg based on IBW-BMI at onset of PARDS, there was an unadjusted association with increased mortality (OR 1.84, 95% CI 1.00 to 3.39, p = 0.049); however, after adjusting for OI, there was no longer an association with mortality.
Figure 3.
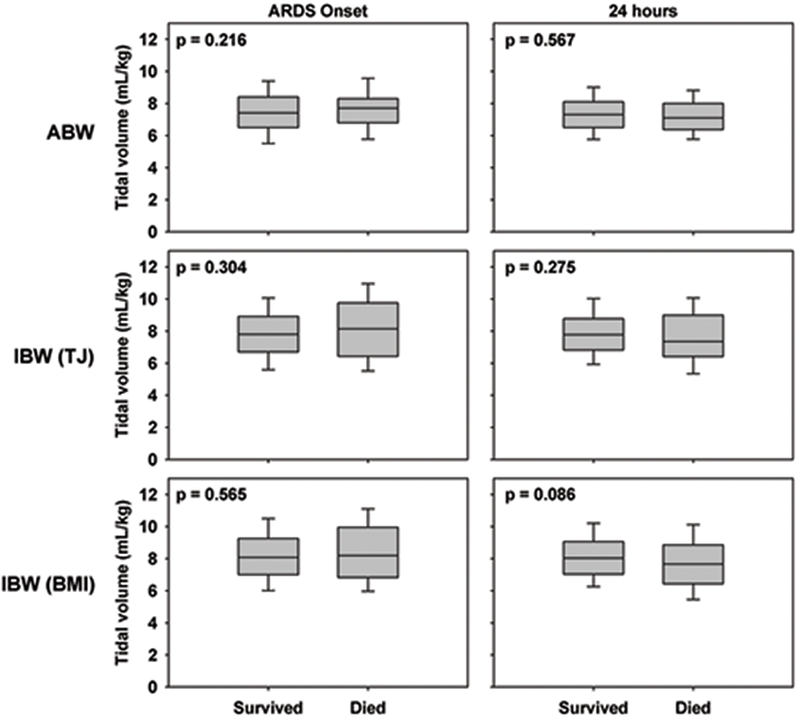
Comparison of VT in survivors and non-survivors at ARDS onset and 24 hours using ABW and two measurements of IBW, the Traub-Johnson (TJ) equation and the WHO weight-for length and CDC body mass index (BMI). P values represent results of the Wilcoxon rank-sum test.
Table 2:
Association between tidal volumes and mortality
| Unadjusted | Adjusted for OI | |||
|---|---|---|---|---|
| Variable | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value |
| Tidal volume (mL/kg) onset | ||||
| ABW | 1.13 (0.96 to 1.33) | 0.134 | 1.12 (0.95 to 1.32) | 0.162 |
| IBW (TJ) | 1.09 (0.96 to 1.24) | 0.189 | 1.08 (0.95 to 1.23) | 0.241 |
| IBW (BMI) | 1.06 (0.93 to 1.21) | 0.379 | 1.04 (0.90 to 1.20) | 0.596 |
| Tidal volume (mL/kg) 24h | ||||
| ABW | 0.94 (0.76 to 1.15) | 0.533 | 0.94 (0.76 to 1.16) | 0.561 |
| IBW (TJ) | 0.91 (0.76 to 1.08) | 0.265 | 0.89 (0.74 to 1.07) | 0.209 |
| IBW (BMI) | 0.83 (0.69 to 1.01) | 0.057 | 0.80 (0.66 to 0.98) | 0.029 |
| Tidal volume > 10 mL/kg onset | ||||
| ABW | 2.44 (0.83 to 7.14) | 0.104 | 2.51 (0.84 to 7.53) | 0.100 |
| IBW (TJ) | 1.88 (0.97 to 3.63) | 0.061 | 1.71 (0.87 to 3.36) | 0.117 |
| IBW (BMI) | 1.84 (1.00 to 3.39) | 0.049 | 1.67 (0.89 to 3.12) | 0.107 |
| Tidal volume > 10 mL/kg 24h | ||||
| ABW | 0.39 (0.05 to 2.98) | 0.361 | 0.34 (0.04 to 2.96) | 0.329 |
| IBW (TJ) | 1.02 (0.41 to 2.55) | 0.963 | 1.10 (0.43 to 2.82) | 0.839 |
| IBW (BMI) | 1.05 (0.44 to 2.46) | 0.917 | 0.87 (0.34 to 2.19) | 0.767 |
ABW: actual body weight; CI: confidence intervals; IBW (BMI): ideal body weight (using combined body mass index and weight-for-length curves); IBW (TJ): ideal body weight (Traub-Johnson method); OI: oxygenation index
In addition to investigating the association between VT and mortality, we looked at whether increasing VT was associated with an increased or decreased probability of extubation (Table 3). For patients ventilated with VT > 10 mL/kg based on IBW-BMI as measured at onset of PARDS, there was an unadjusted lower probability of extubation (SHR 0.74, 95% CI 0.58 to 0.96, p = 0.021). However, after adjusting for OI, this association as no longer significant. Both conclusions regarding mortality and probability of extubation were confirmed when restricting analysis to the subgroup of subjects on PRVC with preset VT (Supplementary Tables 1 and 2).
Table 3:
Association between tidal volumes and probability of extubation by day 28, given the competing risk of death
| Unadjusted | Adjusted for OI | |||
|---|---|---|---|---|
| Variable | SHR (95% CI) | P value | SHR (95% CI) | P value |
| Tidal volume (mL/kg) onset | ||||
| ABW | 0.99 (0.94 to 1.05) | 0.784 | 1.01 (0.95 to 1.07) | 0.846 |
| IBW (TJ) | 0.98 (0.94 to 1.03) | 0.435 | 0.99 (0.95 to 1.04) | 0.696 |
| IBW (BMI) | 0.98 (0.94 to 1.03) | 0.452 | 0.99 (0.94 to 1.04) | 0.701 |
| Tidal volume (mL/kg) 24h | ||||
| ABW | 1.03 (0.95 to 1.11) | 0.427 | 1.02 (0.94 to 1.11) | 0.587 |
| IBW (TJ) | 1.01 (0.95 to 1.07) | 0.716 | 1.01 (0.95 to 1.07) | 0.815 |
| IBW (BMI) | 1.01 (0.95 to 1.08) | 0.672 | 1.03 (0.96 to 1.10) | 0.437 |
| Tidal volume > 10 mL/kg onset | ||||
| ABW | 0.79 (0.47 to 1.32) | 0.370 | 0.79 (0.45 to 1.36) | 0.387 |
| IBW (TJ) | 0.76 (0.58 to 0.99) | 0.050 | 0.79 (0.60 to 1.05) | 0.100 |
| IBW (BMI) | 0.74 (0.58 to 0.96) | 0.021 | 0.78 (0.60 to 1.01) | 0.062 |
| Tidal volume > 10 mL/kg 24h | ||||
| ABW | 0.93 (0.53 to 1.64) | 0.808 | 0.85 (0.45 to 1.62) | 0.625 |
| IBW (TJ) | 1.02 (0.74 to 1.42) | 0.891 | 1.03 (0.71 to 1.49) | 0.897 |
| IBW (BMI) | 0.72 (0.50 to 1.04) | 0.078 | 0.81 (0.59 to 1.18) | 0.268 |
ABW: actual body weight; CI: confidence intervals; IBW (BMI): ideal body weight (using combined body mass index and weight-for-length curves); IBW (TJ): ideal body weight (Traub-Johnson method); OI: oxygenation index; SHR: subdistribution hazard ratio
Comparison with ΔP
In contrast to measures of VT, ΔP at both PARDS onset, at 24 hours, and ΔΔP were associated with decreased probability of extubation (Supplementary Table 3). ΔΔP retained significant association with decreased probability of extubation when adjusting for ΔOI.
Subgroup Analyses
When restricting analyses to overweight and obese subjects (> 97th %ile in Table 1), VT based on IBW were substantially larger (all p < 0.001) both at onset and at 24 hours (IBW-TJ onset: 9.1 [7.5, 10.0] mL/kg, 24 hours: 8.8 [7.8, 9.9] mL/kg; IBW-BMI onset: 9.6 [8.1, 10.5] mL/kg, 24 hours: 9.1 [8.2, 10.1] mL/kg) compared to VT based on ABW (onset 6.8 [5.6, 7.6] mL/kg, 24 hours 6.5 [5.6, 7.4] mL/kg). When assessing for the association between VT and outcomes in overweight/obese subjects (Supplementary Table 4), results largely conformed to the main analysis, with two exception. First, in overweight/obese subjects, VT > 10 mL/kg at 24 hours based on IBW-TJ was associated with increased mortality (OR 7.83, 95% CI 1.52 to 40.26, p = 0.014). Second, higher VT at 24 hours (analyzed as a continuous variable) using IBW-TJ was associated with decreased probability of extubation (SHR 0.88, 95% CI 0.79 to 0.98, p = 0.024). Otherwise, there was no association between any measure of VT and outcome.
Because severe PARDS plausibly has a smaller amount of functional lung and is thus more susceptible to the damaging effects of high VT, we repeated the above analyses restricted to children with severe PARDS (OI ≥ 16). In this subgroup, there were no significant associations between VT and mortality (Supplementary Table 5). However, higher VT at PARDS onset using ABW (SHR 0.84, 95% CI 0.73 to 0.95, p = 0.007) and IBW-BMI (SHR 0.89, 95% CI 0.79 to 0.99, p = 0.044) was associated with decreased probability of extubation in severe PARDS.
Finally, we repeated the above analyses restricting to subjects ≥ 2 years of age, as we reasoned that the stiffer chest walls of older children may be less forgiving of larger VT (Supplementary Table 6). The results of this subgroup analysis confirmed the results of the larger cohort, with no evidence of any association between any measure of VT and mortality or probability of extubation.
DISCUSSION
In our retrospective analysis of a prospective database of mechanically ventilated children with PARDS, there was no consistent association between any weight-normalized measure of VT and outcome. VT in mL/kg was higher when using IBW than ABW, particularly for overweight and obese children. There was a trend noted for VT > 10 mL/kg at PARDS onset and poor outcome, which was generally absent after adjusting for OI. In subgroups of overweight/obese children and in severe PARDS there were observed associations between higher VT and poor outcomes. Overall, our analysis did not provide strong evidence for VT on outcomes in PARDS.
Our hypothesis that there would be a positive association between VT and mortality was primarily based on adult data (1). The relevant retrospective data that exists in children is, on the whole, ambiguous as to whether low tidal volumes are positively (20) or negatively associated with outcome. Two studies demonstrated lower mortality with higher tidal volumes (21, 22). Khemani et al (22) used primarily pressure control (> 90%) and ABW in their analysis, which may confound the relationship between VT and outcomes, as sicker patients in pressure control ventilation will achieve lower VT. We extend the findings of these previous reports by normalizing VT to IBW, rather than ABW, and analyzing a cohort ventilated predominantly with PRVC with volume preset.
We do note that VT > 10 mL/kg at PARDS onset demonstrated a trend towards increased mortality and decreased probability of extubation (Table 2), with significant associations between VT at 24 hours and outcomes when using IBW-TJ in overweight/obese children (Supplementary Table 4). It is possible that a larger cohort of PARDS subjects would have found an association between VT > 10 mL/kg and outcomes. However, this trend was no longer evident when restricted to subjects on PRVC and preset VT (Supplementary Tables 1 and 2) or by 24 hours (Table 2). Nevertheless, we cannot entirely exclude that VT > 10 mL/kg are associated with worse outcomes in PARDS, and should probably be avoided. We also demonstrate that normalization of VT to IBW may assist in identifying unintentionally high VT, particularly in overweight and obese children. Using IBW, the actual proportion of subjects exposed to VT > 10 mL/kg can be as high as 15%, which is 3- to 4-fold higher than what is appreciated when adjusting solely on ABW. Our results highlight the potential for exposing subjects to high VT even when providers believe they are providing lung-protective ventilation.
The failure to find a consistent trend for the prognostic role of VT in PARDS could have several etiologies, including an actual lack of association. Our study sought to clarify if the failure to find an association with mortality was due to miscalculations of VT. Therefore, we calculated VT in multiple ways, including two formulations of IBW. No other prior retrospective study has examined VT in PARDS using this standardization. Nonetheless, we still did not find a consistent positive association between VT and mortality. We did, however, note associations between higher VT and poor outcomes in subgroup analyses of overweight/obese children and in severe PARDS. Findings in these subgroups should be interpreted with caution, as multiple hypothesis-generating comparisons were made, without correction for multiple testing. Nevertheless, the possibility exists that in overweight/obese children and in severe PARDS, higher VT (particularly > 10 mL/kg using IBW) are harmful. Further studies dedicated to these sub-groups are warranted.
Our methodology regarding corrected body-weight also provided a useful description of the distribution of VT used in the PICU. This information suggests two important, related concepts: obesity is prevalent in the PICU and clinicians are using ABW as opposed to predicted body-weight to calculate VT, even in the setting of PARDS, and despite recommendations (1, 3, 4). In a survey of practice patterns, no pediatric intensivists stated willingness to ventilate subjects with VT > 10 mL/kg (23); however, when using IBW, this occurs at a substantial frequency in the present cohort.
If miscalculation does not explain the lack of association between VT and mortality, then two questions remain. The first is whether mortality is the best outcome to measure in PARDS. Given the relatively lower mortality rate in PARDS compared to adult ARDS (10, 24), other parameters of disease severity, such as ventilator-free days (25), have been proposed as useful outcome measurements. We therefore also analyzed whether VT was associated with a decreased probability of extubation by day 28. After correcting for OI, however, we did not find any association between VT and probability of extubation in the whole cohort, although we did find evidence for harm in subgroup analyses.
The second question is whether VT itself is a reliable measurement of lung distension that, in excess, causes VILI. While thus far, the only clinical trial has compared VT (albeit with plateau pressure limits) (1), a more recent analysis demonstrated that driving pressure (plateau pressure minus PEEP) is the parameter that most strongly predicts survival in adults with ARDS (26). They also observed that increases in VT were not necessarily associated with mortality if the increased VT did not result in increased driving pressures. Driving pressure may be a more useful surrogate of lung distension because it implicates the compliance of the respiratory system. In the present study, we confirmed an association between ΔP and ΔΔP with probability of extubation, as we have shown before (8). Importantly, we defined ΔP as PIP minus PEEP, as this cohort was ventilated exclusively with decelerating flow. Since PIP partly reflects airway resistance, it is inherently going to provide a larger value for ΔP than plateau pressure. While ΔP as we defined it is not true driving pressure, decelerating flow is the most common method of ventilation in PARDS (22, 27, 28), and PIP is more commonly tracked and acted upon than plateau pressure, making this metric more relevant for pediatrics. If there is a future prospective randomized clinical trial comparing a protective ventilation strategy to a non-protective strategy in PARDS, our data suggest that VT would not be a useful guiding measurement. Therefore, a trial in which two levels of driving pressure or ΔP are compared should be considered, since our data confirm an association between ΔP and outcome.
We have thus far assumed that VILI applies to children similarly as it does to adults. Several studies have reported less severe lung injury in young mice/rats compared to older animals ventilated with high VT (29–31). Questions remain whether children have a similar inflammatory response to positive pressure ventilation as adults (7). A prospective randomized trial as mentioned above is needed to clarify the clinical relevance of this issue. It is unlikely that additional observational studies will contribute more information, as all will suffer from the same limitations present in our and related studies. Specifically, children with worse prognoses have stiffer lungs, reflected in higher pressures and de facto lower VT. This confounding by indication limits many conclusions one can draw regarding the safe range of VT (or PIP and ΔP). A trial assigning two distinct ΔP targets is the only way to overcome this issue.
Our study has limitations. It is a single-center cohort and generalizability may be limited. While severity of illness and PARDS etiologies were similar to other cohorts (21, 32, 33), specific management strategies, including use of ancillary therapies or non-conventional ventilation, may affect mortality, thus affecting both our conclusions and the generalizability to other centers. Ventilator management was not protocolized, including use of pressure control or PRVC, or decisions to switch to alternative ventilator modes, which may introduce bias regarding the association between VT and outcomes. We note that our primary findings were confirmed in the subset of children on volume-preset PRVC. There is limited information regarding the accuracy VT measurements of the Dräger ventilators used in this study. Bench studies have suggested an approximately 6% under-estimation of delivered VT during pressure control (34), although fairly accurate estimates during PRVC as long as there is not substantial obstructive physiology (35). By necessity, we could only analyze subjects with recorded VT, and some of the sickest subjects had likely been transitioned to alternative ventilator modes, thereby biasing the cohort. This could also likely affect our conclusions, as the VT of these subjects had they remained on conventional ventilation cannot be known. However, it is more likely subjects who transitioned had the decision to switch made due to peak pressures, rather than VT, as that is more typical practice at our institution and in surveyed PICUs (23). Finally, as mortality was uncommon in this cohort, we may be underpowered to detect an association between VT and mortality. However, our analysis also did not demonstrate any consistent association with probability of extubation, which is also a commonly used endpoint in PARDS. Furthermore, we were able to demonstrate an association between ΔP and ΔΔP and probability of extubation, lending plausibility to the relative importance of this variable, rather than VT, on PARDS outcomes. Given this limitation, we cannot entirely exclude that VT > 10 mL/kg are associated with poor outcomes, as VT > 10 mL/kg using IBW-BMI approached significance for adversely affecting probability of extubation, and VT > 10 mL/kg using IBW-TJ was associated with mortality in overweight/obese children.
Our study has several strengths. The data was from a large, recent, prospectively enrolling PARDS cohort from a large PICU with detailed data collection. Ventilator pressures and volumes were collected by trained data collectors minimizing the effects of spontaneous effort, increasing reliability of the measurements. Our analysis included two different corrected measurements of VT. In addition to investigating the association between VT, and mortality, we performed a similar analysis of probability of extubation. Despite the low mortality, our sample size and number of non-survivors is one of the largest reported for PARDS, making ours one of the few centers capable of performing this analysis.
CONCLUSIONS
Our retrospective analysis of a cohort of PARDS patients did not find a consistent association between VT and mortality or probability of extubation, even when using multiple different formulations for IBW. VT is likely an imprecise parameter for titrating lung protective mechanical ventilation in children. Future studies and prospective trials should consider using driving pressure or ΔP as a guiding parameter, rather than VT.
Supplementary Material
Acknowledgments
Financial Support: NIH K23-136688 (NY)
Copyright form disclosure: Dr. Yehya’s institution received funding from the National Institutes of Health (NIH), and he received support for article research from the NIH. Dr. Thomas’s institution received funding from GeneFluidics, and he received funding from Therabron and Care Fusion. Mr. Imber disclosed that he does not have any potential conflicts of interest.
Footnotes
Plan for Reprints: No
REFERENCES
- 1.Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, et al. : Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 2.de Jager P, Burgerhof JG, van Heerde M, et al. : Tidal volume and mortality in mechanically ventilated children: A systematic review and meta-analysis of observational studies*. Crit Care Med 2014;42:2461–2472 [DOI] [PubMed] [Google Scholar]
- 3.Khemani RG, Smith LS, Zimmerman JJ, et al. : Pediatric acute respiratory distress syndrome: Definition, incidence, and epidemiology: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015;16:S23–40 [DOI] [PubMed] [Google Scholar]
- 4.Rimensberger PC, Cheifetz IM and Pediatric G Acute Lung Injury Consensus Conference: Ventilatory support in children with pediatric acute respiratory distress syndrome: Proceedings from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015;16:S51–60 [DOI] [PubMed] [Google Scholar]
- 5.Kneyber MCJ, de Luca D, Calderini E, et al. : Recommendations for mechanical ventilation of critically ill children from the paediatric mechanical ventilation consensus conference (pemvecc). Intensive Care Med 2017;43:1764–1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward SL, Quinn CM, Valentine SL, et al. : Poor adherence to lung-protective mechanical ventilation in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 2016;17:917–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kneyber MC, Zhang H and Slutsky AS: Ventilator-induced lung injury. Similarity and differences between children and adults. Am J Respir Crit Care Med 2014;190:258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yehya N and Thomas NJ: Disassociating lung mechanics and oxygenation in pediatric acute respiratory distress syndrome. Crit Care Med 2017;45:1232–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim GJ, Newth CJL, Khemani RG, et al. : Does size matter when calculating the “correct” tidal volume for pediatric mechanical ventilation?: A hypothesis based on fvc. Chest 2018;154:77–83 [DOI] [PubMed] [Google Scholar]
- 10.Yehya N, Servaes S and Thomas NJ: Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med 2015;43:937–946 [DOI] [PubMed] [Google Scholar]
- 11.Force ADT, Ranieri VM, Rubenfeld GD, et al. : Acute respiratory distress syndrome: The berlin definition. JAMA 2012;307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 12.Pediatric G Acute Lung Injury Consensus Conference: Pediatric acute respiratory distress syndrome: Consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med 2015;16:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yehya N, Bhalla AK, Thomas NJ, et al. : Alveolar dead space fraction discriminates mortality in pediatric acute respiratory distress syndrome. Pediatr Crit Care Med 2016;17:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traub SL and Johnson CE: Comparison of methods of estimating creatinine clearance in children. Am J Hosp Pharm 1980;37:195–201 [PubMed] [Google Scholar]
- 15.Ross PA, Newth CJ, Leung D, et al. : Obesity and mortality risk in critically ill children. Pediatrics 2016;137:e20152035. [DOI] [PubMed] [Google Scholar]
- 16.Callaghan LC and Walker JD: An aid to drug dosing safety in obese children: Development of a new nomogram and comparison with existing methods for estimation of ideal body weight and lean body mass. Anaesthesia 2015;70:176–182 [DOI] [PubMed] [Google Scholar]
- 17.Goldstein B, Giroir B, Randolph A, et al. : International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6:2–8 [DOI] [PubMed] [Google Scholar]
- 18.Yehya N, Topjian AA, Thomas NJ, et al. : Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome*. Pediatr Crit Care Med 2014;15:e147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP and Gray RJ: A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association 1999;94:496–509 [Google Scholar]
- 20.Albuali WH, Singh RN, Fraser DD, et al. : Have changes in ventilation practice improved outcome in children with acute lung injury? Pediatr Crit Care Med 2007;8:324–330 [DOI] [PubMed] [Google Scholar]
- 21.Erickson S, Schibler A, Numa A, et al. : Acute lung injury in pediatric intensive care in australia and new zealand: A prospective, multicenter, observational study. Pediatr Crit Care Med 2007;8:317–323 [DOI] [PubMed] [Google Scholar]
- 22.Khemani RG, Conti D, Alonzo TA, et al. : Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med 2009;35:1428–1437 [DOI] [PubMed] [Google Scholar]
- 23.Santschi M, Randolph AG, Rimensberger PC, et al. : Mechanical ventilation strategies in children with acute lung injury: A survey on stated practice pattern*. Pediatr Crit Care Med 2013;14:e332–337 [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman JJ, Akhtar SR, Caldwell E, et al. : Incidence and outcomes of pediatric acute lung injury. Pediatrics 2009;124:87–95 [DOI] [PubMed] [Google Scholar]
- 25.Schoenfeld DA, Bernard GR and Network A: Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med 2002;30:1772–1777 [DOI] [PubMed] [Google Scholar]
- 26.Amato MB, Meade MO, Slutsky AS, et al. : Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372:747–755 [DOI] [PubMed] [Google Scholar]
- 27.Santschi M, Jouvet P, Leclerc F, et al. : Acute lung injury in children: Therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med 2010;11:681–689 [DOI] [PubMed] [Google Scholar]
- 28.Khemani RG and Newth CJ: The design of future pediatric mechanical ventilation trials for acute lung injury. Am J Respir Crit Care Med 2010;182:1465–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Copland IB, Martinez F, Kavanagh BP, et al. : High tidal volume ventilation causes different inflammatory responses in newborn versus adult lung. Am J Respir Crit Care Med 2004;169:739–748 [DOI] [PubMed] [Google Scholar]
- 30.Kornecki A, Tsuchida S, Ondiveeran HK, et al. : Lung development and susceptibility to ventilator-induced lung injury. Am J Respir Crit Care Med 2005;171:743–752 [DOI] [PubMed] [Google Scholar]
- 31.Smith LS, Gharib SA, Frevert CW, et al. : Effects of age on the synergistic interactions between lipopolysaccharide and mechanical ventilation in mice. Am J Respir Cell Mol Biol 2010;43:475–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flori HR, Glidden DV, Rutherford GW, et al. : Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med 2005;171:995–1001 [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Fernandez Y, Azagra AM, de la Oliva P, et al. : Pediatric acute lung injury epidemiology and natural history study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med 2012;40:3238–3245 [DOI] [PubMed] [Google Scholar]
- 34.Chatburn RL, Mireles-Cabodevila E and Sasidhar M: Tidal volume measurement error in pressure control modes of mechanical ventilation: A model study. Comput Biol Med 2016;75:235–242 [DOI] [PubMed] [Google Scholar]
- 35.Medina A, Modesto-Alapont V, Lobete C, et al. : Is pressure-regulated volume control mode appropriate for severely obstructed patients? J Crit Care 2014;29:1041–1045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


