Abstract
Objective:
To analyze the association of baseline biomarker data with cross sectional lung function and subsequent decline in lung function in HIV-positive persons.
Design:
Lung function was modeled in all START pulmonary substudy participants who had baseline biomarker data and good-quality spirometry. In longitudinal analyses we restricted to those participants with at least one good-quality follow up spirometry test.
Methods:
We performed linear regression of baseline FEV1, FVC, and FEV1/FVC and their longitudinal slopes on log2-transformed baseline biomarkers with adjustment for age, sex, race, region, smoking status, baseline CD4+ T-cell counts, and baseline HIV-RNA. Biomarkers included D-dimer, hsCRP, IL-6, IL-27, serum amyloid A, sICAM-1, sVCAM-1, albumin, and total bilirubin.
Results:
Among 903 included participants, baseline median age was 36 years, CD4 count was 647 cells/mm3, and 28.5% were current smokers. In adjusted analyses, elevated markers of systemic inflammation (hsCRP, IL-6, and serum amyloid A) were associated with lower baseline FEV1 and FVC. Elevated D-dimer and IL-6 were associated with worse airflow obstruction (lower FEV1/FVC). Despite these cross-sectional associations at baseline, no associations were found between baseline biomarkers and subsequent longitudinal lung function decline over a median follow-up time of 3.9 years (3,293 spirometry-years of follow up).
Conclusions:
Commonly available biomarkers, in particular markers of systemic inflammation, are associated with worse cross-sectional lung function, but do not associate with subsequent lung function decline among HIV-positive persons with early HIV infection and baseline CD4+ T-cell counts >500 cells/mm3.
Keywords: HIV, Spirometry, Biomarkers, Longitudinal studies, Pulmonary disease, chronic obstructive
INTRODUCTION
In the modern antiretroviral treatment (ART) era, the burden of HIV-associated respiratory disease has shifted away from infections and towards non-infectious pulmonary disease such as chronic obstructive pulmonary disease (COPD), pulmonary hypertension, and lung cancer.[1,2] Although tobacco smoke is clearly the most important risk factor for COPD, HIV has repeatedly been identified as an independent risk factor for the development of COPD.[3–6] However, the mechanisms remain unclear. COPD is a leading cause of death worldwide, is common among HIV positive individuals [5,7] and diminishes both quality and duration of life.[8–10]
COPD is defined by expiratory airflow limitation, when spirometry demonstrates a low ratio of forced expiratory volume in 1s to forced vital capacity (FEV1/FVC). FEV1 is the most common metric to determine severity of COPD and although FEV1 declines as part of normal aging, faster decline in FEV1 has been associated with both COPD susceptibility and increased respiratory and all-cause mortality.[11,12] Among general population COPD patients, systemic inflammation has been associated with accelerated decline in lung function, COPD hospitalization, COPD exacerbations, COPD death, and increased all-cause mortality.[13–15]
Among HIV positive individuals, systemic inflammation and persistent immune activation have been implicated in a number of non-AIDS comorbidities including chronic kidney disease, cardiovascular disease, thromboembolism, lymphoma, non-AIDS defining cancer, osteoporosis, type 2 diabetes, pneumonia, neurocognitive dysfunction, depression, and frailty.[16–19] There are few previous analyses of systemic inflammation and pulmonary function in HIV positive individuals and existing studies have been relatively small.[20–24]
We used data from the START Pulmonary Substudy to investigate the association between lung function and circulating markers of systemic inflammation (interleukin-6 [IL-6], high-sensitivity C-reative protein [hsCRP], and serum amyloid A [SAA]), coagulation (D-dimer), T-cell regulation (interleukin-27 [IL-27]), endothelial activation (soluble intercellular adhesion molecule [sICAM] and soluble vascular cell adhesion molecule [sVCAM]), and clinical lab tests previously associated with pulmonary or HIV prognosis (bilirubin and albumin). We examined both baseline lung function in a cross-sectional analysis and longitudinal lung function decline in a prospective analysis.
METHODS
Participants:
The Strategic Timing of Antiretroviral Treatment (START) trial enrolled 4685 asymptomatic HIV-positive adults, naïve to ART with baseline CD4+ T-cell counts >500 cells/mm3. Participants were randomized to initiation of ART immediately or at a CD4+ T-cell count of 350 cells/mm3 (the deferred arm). The study was unblinded early due to immediate ART reducing both AIDS and non-AIDS events.[25] The START Pulmonary Substudy co-enrolled 1,026 of these START participants, from 80 sites in 20 countries. Exclusion criteria unique to the Pulmonary Substudy included: 1) age < 25 years (the age when normal lung function begins to decline[26]); 2) respiratory illness in the previous 6 weeks; 3) asthma medication use in the previous 6 months; 4) contraindication to spirometry; and 5) allergy or contraindication to albuterol/salbutamol use. COPD was not an exclusion criteria. No significant difference was found in the primary outcome of rate of FEV1 decline between immediate and deferred ART.[27] The first participant was enrolled in March 2010, the data were unblinded in May 2015, and follow up continued through December 2016. These current analyses included all participants who had the biomarkers of interest and baseline spirometry meeting quality control review criteria as established by international spirometry standards.[28] In longitudinal analyses we further restricted our analysis to participants with at least one follow up spirometry meeting quality control review criteria.
Pulmonary function tests:
Post-bronchodilator spirometry was done in standardized fashion using the EasyOne ultrasonic flow device (ndd Medical Technologies, Andover, USA). Spirometry was done at study entry prior to randomization and annually thereafter. All spirometry results were centrally reviewed by author KMK to ensure quality control. Repeat spirometry was requested when testing did not meet quality standards. COPD was defined using a standard definition of FEV1/FVC below the 5th percentile of predicted normal, using 2012 Global Lung Function Initiative prediction equations.[29]
Biomarkers:
Blood was collected from consenting participants at baseline and stored centrally at −70 C. All assays were performed blinded to treatment group, lung function tests, or clinical outcomes. Biomarkers were not necessarily selected based on postulated associations with lung function, but rather were measured following un-blinding of the START results based on postulated associations with AIDS and non-AIDS events.[30] Rather than selectively analyzing or reporting certain biomarkers, we elected to examine all available biomarkers. IL-6 was measured using high sensitivity ELISA (R&D, Minneapolis, USA). D-dimer was measured using the VIDAS system (BioMerieux, Marcy-l’Étoile, France). IL-27 was measured using IL-27 using immunoassay (Meso Scale Diagnostics, Rockville, USA). A multiplex platform was used to measure hsCRP, SAA, sICAM, and sVCAM (Vascular Injury II Panel, Meso Scale Diagnostics, Rockville, USA). Albumin and total bilirubin were measured in the clinical laboratory facilities at each site.
Statistical Analysis:
We performed linear regression of baseline FEV1, FVC, and FEV1/FVC ratio on log2-transformed baseline biomarker concentrations. We also included the IL-6/D-dimer score [0.33 × log2 IL-6 + 0.16 × log2 D-dimer] which has been independently associated with serious non-AIDS conditions and mortality.[17] We then adjusted models for age, sex, race, region, smoking status, baseline CD4+ T-cell counts, and baseline HIV-RNA.
We also performed linear regression of longitudinal slopes of FEV1 (mL/yr), FVC (mL/yr), and FEV1/FVC (%/year) on baseline log2-transformed baseline biomarker concentrations, again including models adjusted for age, sex, race, region, smoking status, baseline CD4+ T-cell counts, and baseline HIV-RNA. We also included treatment assignment as a covariate (immediate vs. deferred ART strategy), even though treatment assignment did not affect lung function decline in our primary results.[27]
In order to determine if there was an association between baseline biomarkers and longitudinal lung function in untreated HIV-positive individuals, free of any ART exposure, we performed a secondary analysis limited to the participants randomized to the deferred arm and censoring lung function data after ART initiation. We again performed linear regression of longitudinal slopes of FEV1 (mL/yr), FVC (mL/yr), and FEV1/FVC (%/year) on baseline log2-transformed baseline biomarker concentrations adjusted for age, sex, race, region, smoking status, baseline CD4+ T-cell counts, and baseline HIV-RNA.
No adjustments were made for multiple testing. All statistical analyses were conducted using SAS 9.4 (Cary, NC, USA).
RESULTS:
Baseline characteristics
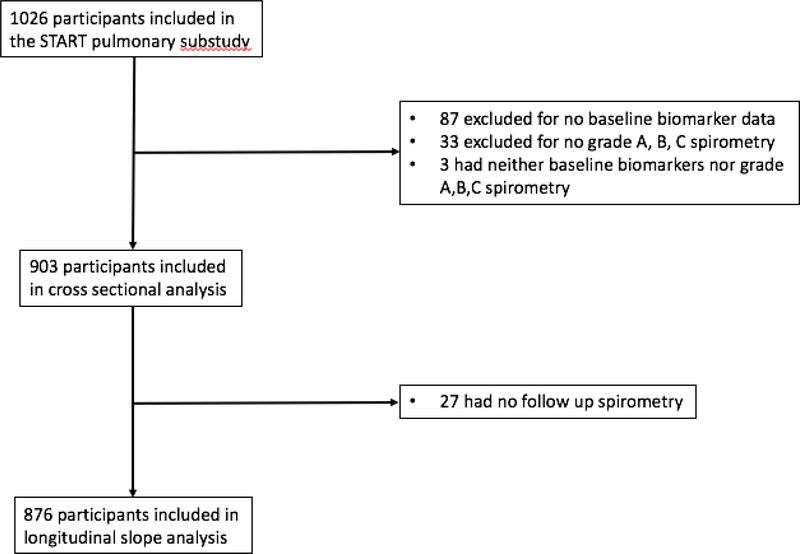
Of 1026 participants in the START pulmonary substudy, 903 (91.2%) had both baseline biomarker data and spirometry that met quality control review criteria (Figure 1). Baseline characteristics of those included and excluded are shown in Supplementary Table 1. Excluded participants were more likely to be female (43.7% vs 28.1%), Asian (19.5% vs 9.6%), and less likely to be white (33.7% vs. 23.0%). Excluded participants were less likely to be current smokers (28.5% vs 19.5%) and more likely to be never smokers (71.3% vs 60.4%). Those excluded had slightly higher CD4+ T-cell counts (both nadir and at study entry) and slightly lower CD8+ T-cell counts. Spirometry quality was similar and both groups had normal median baseline lung function.
Figure 1.
Study participant flow diagram.
Baseline characteristics of the 903 participants with baseline biomarker data who are included in our analysis are shown in Table 1. Participants had a median age of 36 (IQR 30, 44). They were most likely to be from Africa (31.8%) and Europe/Israel/Australia (31.2%). The majority likely contracted HIV via sexual contact (50.3% male with person of same sex and 43.5% with person of opposite sex). Participants had a median duration from HIV diagnosis of 1.2 years (IQR 0.4, 3.5). HIV laboratory parameters were notable for median CD4+ count of 647 (IQR 584, 766), nadir CD4+ of 550 (IQR 482, 659), log HIV-RNA 4.2 (IQR 3.5, 4.7), and 9.6% had HIV-RNA < 400 copies/mL. 28.5% were current smokers and 11.2% were previous smokers. Hypertension was present in 18.7%, but other comorbidities were infrequent. Baseline lung function was generally good, with a median FEV1 percent predicted of 96.6% (IQR 86.0, 104.1) and only 6.6% having COPD at study entry.
Table 1:
Baseline characteristics of START pulmonary substudy participants with baselinebiomarkers and spirometry meeting standardized quality control criteria.
| N (%) or Median (IQR) | |
|---|---|
| No. of participants | 903 (91.2%) |
| Demographics | |
| Age (years) | 36 (30, 44) |
| Female | 254 (28.1%) |
| Race | |
| Black | 345 (38.2%) |
| Latino/Hispanic | 158 (17.5%) |
| Asian | 87 (9.6%) |
| White | 304 (33.7%) |
| Other | 9 (1.0%) |
| Region | |
| Africa | 287 (31.8%) |
| Asia | 85 (9.4%) |
| Europe/Israel/Australia | 282 (31.2%) |
| Mexico/South America | 169 (18.7%) |
| United States | 80 (8.9%) |
| HIV history | |
| Likely mode of HIV infection | |
| Injection drug use | 13 (1.4%) |
| Male sexual contact with person of same sex | 454 (50.3%) |
| Sexual contact with person of opposite sex | 393 (43.5%) |
| Other/unknown | 43 (4.8%) |
| Years HIV positive | 1.2 (0.4, 3.5) |
| Lab results | |
| CD4 count (cells/mm3) | 647 (584, 766) |
| CD8 count (cells/mm3) | 1026 (760, 1411) |
| CD4/CD8 ratio | 0.66 (0.47, 0.93) |
| Nadir CD4 (cells/mm3) | 550 (482, 659) |
| Log HIV-RNA (copies/ml) | 4.2 (3.5, 4.7) |
| HIV-RNA ≤ 400 copies/mL | 87 (9.6%) |
| Smoking History | |
| Current smoker | 257 (28.5%) |
| Previous smoker | 101 (11.2%) |
| Never smoked | 545 (60.4%) |
| Pack years smoking (current and previous smokers) | 6.5 (2.0, 15.0) |
| Medical History | |
| Prior CVD | 7 (0.8%) |
| Hypertension | 169 (18.7%) |
| Diabetes | 32 (3.5%) |
| Hepatitis B | 39 (4.4%) |
| Hepatitis C | 28 (3.2%) |
| Body mass index (kg/m2) | 24.8 (22.2, 28.4) |
| Respiratory History | |
| FEV1 (L) | 3.4 (2.7, 4.0) |
| FVC (L) | 4.1 (3.3, 4.9) |
| FEV1/FVC ratio | 0.83 (0.78, 0.86) |
| < 0.7 | 50 (5.5%) |
| < lower limit or normal | 60 (6.6%) |
| FEV1 as % of predicted | 96.6 (86.0, 104.0) |
| ≥ 80% | 778 (86.2%) |
| 50% – 79% | 119 (13.2%) |
| 30% – 49% | 6 (0.7%) |
| < 30% | 0 (0.0%) |
876 (97%) of the participants included in the cross-sectional analysis had at least one good-quality follow up spirometry test for a total of 3293 spirometry-years of follow up. Median follow up for the longitudinal slope analyses was 3.9 years.
Baseline biomarker levels in smokers, non-smokers, and in all participants are shown in Table 2. Smokers and non-smokers had similar concentrations of D-dimer, IL-6, IL-27, SAA, albumin and bilirubin, but smokers had higher concentrations of hsCRP, sICAM, and sVCAM.
Table 2:
Baseline biomarkers among smokers and non-smokers.
| Smokers | Non-smokers | Total | ||||
|---|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | |
| D-dimer (mcg/ml) | 257 | 0.32 (0.24, 0.45) | 644 | 0.35 (0.22, 0.54) | 901 | 0.33 (0.23, 0.51) |
| hsCRP (mcg/ml) | 257 | 1.83 (0.76, 3.34) | 646 | 1.53 (0.66, 3.74) | 903 | 1.62 (0.69, 3.69) |
| IL-6 (pg/ml) | 257 | 1.43 (0.99, 2.13) | 646 | 1.27 (0.92, 1.94) | 903 | 1.32 (0.93, 2.01) |
| IL-27 (pg/ml) | 257 | 259.20 (145.50, 514.90) | 646 | 255.25 (123.20, 522.60) | 903 | 255.70 (128.60, 522.60) |
| Serum amyloid A (mg/L) | 257 | 4.60 (2.76, 8.48) | 646 | 4.39 (2.47, 8.85) | 903 | 4.42 (2.56, 8.65) |
| sICAM-1 (mcg/mL) | 257 | 599.56 (446.64, 740.00) | 646 | 524.93 (398.31, 665.61) | 903 | 540.74 (408.55, 692.54) |
| sVCAM-1 (mcg/mL) | 257 | 760.76 (609.82, 986.58) | 646 | 703.51 (537.21, 906.07) | 903 | 722.46 (552.26, 937.23) |
| IL-6/D-dimer score1 | 257 | −0.05 ± 0.34 | 644 | −0.09 ± 0.38 | 901 | −0.08 ± 0.37 |
| Albumin (g/dL)2 | 256 | 4.40 (4.20, 4.60) | 628 | 4.40 (4.10, 4.64) | 884 | 4.40 (4.19, 4.60) |
| Total bilirubin (mg/dL)2 | 256 | 0.50 (0.40, 0.70) | 642 | 0.50 (0.40, 0.70) | 898 | 0.50 (0.40, 0.70) |
The IL-6/D-dimer score [0.33 × log2 IL-6 + 0.16 × log2 D-dimer] has been independently associated with serious non-AIDS conditions and mortality. [17]
Albumin and bilirubin were measured in clinical laboratory facilities at each site and not available in every participant.
Baseline lung function and biomarkers
Unadjusted and adjusted regressions of baseline FEV1, FVC, and FEV1/FVC on log2-transformed baseline biomarkers are shown in Table 3. In log2-transformed linear regression, the β coefficients represent the difference in lung function associated with a doubling of the biomarker of interest. In unadjusted analyses, all included biomarkers, with the exception of sICAM, were associated with FEV1 and FVC at baseline. Higher concentrations of IL-27, sVCAM, albumin, and total bilirubin were associated with higher FEV1 and FVC, while higher concentrations of D-Dimer, hsCRP, IL-6, SAA, and the IL-6/D-dimer score were associated with lower FEV1 and FVC. When analyzing FEV1/FVC ratio, higher D-dimer, hsCRP, IL-6, and the IL-6/D-dimer score were associated with more airflow obstruction (i.e. lower FEV1/FVC), while higher albumin was associated with less airflow obstruction (i.e higher FEV1/FVC ratio). The other biomarkers had no significant associations with FEV1/FVC.
Table 3:
Cross-sectional analyses. Univariable (unadjusted) and multivariable1 (adjusted) linear regressions of baseline FEV1 (mL), FVC (mL), and FEV1/FVC on log2-transformed baseline biomarkers
|
Univariable |
Multivariable1 |
||||||
|---|---|---|---|---|---|---|---|
| β3 | 95% C.I. | P-value | β3 | 95% C.I. | P-value | ||
| FEV1 | |||||||
| D-dimer (mcg/ml) | −347.6 | −403.6, −291.7 | < 0.001 | 4.7 | −39.2, 48.6 | 0.83 | |
| hsCRP (mcg/ml) | −98.9 | −130.3, −67.4 | < 0.001 | −36.2 | −56.5, −16.0 | <0.001 | |
| IL-6 (pg/ml) | −109.7 | −173.4, −46.0 | < 0.001 | −73.8 | −114.8, −32.7 | <0.001 | |
| IL-27 (pg/ml) | 60.2 | 27.1, 93.3 | < 0.001 | 3.8 | −17.6, 25.1 | 0.73 | |
| Serum amyloid A (mg/L) | −67.4 | −103.2, −31.6 | < 0.001 | −23.1 | −45.7, −0.5 | 0.05 | |
| sICAM-1 (mcg/mL) | −33.7 | −123.2, 55.8 | 0.46 | −26.7 | −83.9, 30.5 | 0.36 | |
| sVCAM-1 (mcg/mL) | 215.1 | 126.5, 303.8 | < 0.001 | 24.0 | −36.1, 84.1 | 0.43 | |
| IL-6/D-dimer score2 | −580.5 | −731.4, −429.6 | < 0.001 | −146.7 | −249.9, −43.5 | 0.005 | |
| Albumin (g/dL) | 1856.8 | 1453.1, 2260.6 | < 0.001 | −17.0 | −304.7, 270.8 | 0.91 | |
| Total bilirubin (mg/dL) | 237.7 | 166.0, 309.4 | < 0.001 | 12.2 | −37.4, 61.7 | 0.63 | |
| FVC | |||||||
| D-dimer (mcg/ml) | −382.2 | −452.6, −311.8 | < 0.001 | 33.1 | −20.1, 86.3 | 0.22 | |
| hsCRP (mcg/ml) | −109.5 | −148.6, −70.5 | < 0.001 | −41.4 | −65.9, −16.8 | <0.001 | |
| IL-6 (pg/ml) | −88.3 | −167.4, −9.3 | 0.03 | −64.3 | −114.3, −14.4 | 0.01 | |
| IL-27 (pg/ml) | 71.0 | 30.0, 112.0 | < 0.001 | −4.0 | −29.9, 21.9 | 0.76 | |
| Serum amyloid A (mg/L) | −82.5 | −126.8, −38.2 | < 0.001 | −29.2 | −56.6, −1.8 | 0.04 | |
| sICAM-1 (mcg/mL) | −18.2 | −128.9, 92.6 | 0.75 | −36.7 | −106.1, 32.6 | 0.30 | |
| sVCAM-1 (mcg/mL) | 287.4 | 177.9, 396.8 | < 0.001 | 14.9 | −57.9, 87.8 | 0.69 | |
| IL-6/D-dimer score2 | −575.3 | −764.1, −386.5 | < 0.001 | −100.6 | −226.1, 24.9 | 0.12 | |
| Albumin (g/dL) | 2079.3 | 1573.6, 2584.9 | < 0.001 | −142.0 | −493.1, 209.1 | 0.43 | |
| Total bilirubin (mg/dL) | 271.4 | 182.4, 360.4 | < 0.001 | 19.7 | −40.4, 79.7 | 0.52 | |
| FEV1/FVC | |||||||
| D-dimer (mcg/ml) | −0.89 | −1.3, −0.5 | < 0.001 | −0.57 | −1.0, −0.1 | 0.02 | |
| hsCRP (mcg/ml) | −0.26 | −0.5, −0.0 | 0.03 | −0.10 | −0.3, 0.1 | 0.38 | |
| IL-6 (pg/ml) | −0.92 | −1.4, −0.5 | < 0.001 | −0.54 | −1.0, −0.1 | 0.02 | |
| IL-27 (pg/ml) | 0.03 | −0.2, 0.3 | 0.84 | 0.13 | −0.1, 0.4 | 0.27 | |
| Serum amyloid A (mg/L) | 0.01 | −0.2, 0.3 | 0.92 | 0.00 | −0.2, 0.2 | 0.98 | |
| sICAM-1 (mcg/mL) | −0.46 | −1.1, 0.2 | 0.17 | 0.08 | −0.5, 0.7 | 0.81 | |
| sVCAM-1 (mcg/mL) | −0.43 | −1.1, 0.2 | 0.20 | 0.30 | −0.3, 0.9 | 0.36 | |
| IL-6/D-dimer score2 | −2.74 | −3.9, −1.6 | < 0.001 | −1.65 | −2.7, −0.6 | 0.003 | |
| Albumin (g/dL) | 3.74 | 0.7, 6.8 | 0.02 | 2.35 | −0.7, 5.4 | 0.13 | |
| Total bilirubin (mg/dL) | 0.30 | −0.2, 0.8 | 0.27 | −0.18 | −0.7, 0.3 | 0.51 | |
adjusted for age, sex, race, region, smoking status, CD4+ T-cell count, and HIV-RNA.
[0.33 × log2 IL-6 + 0.16 × log2 D-dimer] [17]
β represent tde difference in lung function (FEV1, FVC, or FEV1/FVC) associated witd a doubling of tde corresponding biomarker
After adjusting for age, sex, race, region, smoking status, CD4+ T-cell counts, and HIV-RNA, higher hsCRP and IL-6 were associated with lower baseline FEV1 and FVC. Higher SAA had a borderline statistically significant association with lower baseline FEV1 and FVC (p=0.05 and 0.04, respectively). The IL-6/D-dimer score was associated with lower FEV1 but not FVC. Higher D-dimer, IL-6, and the IL-6/D-dimer score were associated with more airflow obstruction.
Longitudinal change in lung function relative to baseline biomarkers
Unadjusted and adjusted regressions of longitudinal changes in FEV1, FVC, and FEV1/FVC on baseline biomarkers are shown in Table 4. None of the baseline biomarkers had any association with subsequent slopes of FEV1, FVC, or FEV1/FVC in unadjusted or adjusted analyses.
Table 4:
Longitudinal analyses. Univariable (unadjusted) and multivariable1 (adjusted) linear regression of longitudinal changes in FEV1 (mL/yr), FVC (mL/yr), and FEV1/FVC (x1000/yr) on log2-transformed baseline biomarkers.
|
Univariable |
Multivariable1 |
||||||
|---|---|---|---|---|---|---|---|
| Lab value (log 2) | β3 | 95% C.I. | P-value | β3 | 95% C.I. | P-value | |
| FEV1 Slope | |||||||
| D-dimer (mcg/ml) | −0.4 | −5.7, 4.9 | 0.88 | −0.5 | −5.8, 4.8 | 0.86 | |
| hsCRP (mcg/ml) | 2.6 | −0.1, 5.3 | 0.06 | 2.5 | −0.2, 5.2 | 0.07 | |
| IL-6 (pg/ml) | 0.9 | −4.5, 6.4 | 0.73 | 0.9 | −4.6, 6.3 | 0.75 | |
| IL-27 (pg/ml) | −1.0 | −3.8, 1.9 | 0.52 | −0.9 | −3.8, 2.0 | 0.54 | |
| Serum amyloid A (mg/L) | 2.3 | −0.8, 5.5 | 0.15 | 2.3 | −0.8, 5.5 | 0.14 | |
| sICAM-1 (mcg/mL) | 1.4 | −6.0, 8.7 | 0.72 | 1.3 | −6.1, 8.6 | 0.73 | |
| sVCAM-1 (mcg/mL) | −4.2 | −11.6, 3.1 | 0.26 | −4.2 | −11.5, 3.2 | 0.27 | |
| IL-6/D-dimer score2 | 2.0 | −11.3, 15.3 | 0.77 | 1.7 | −11.7, 15.0 | 0.81 | |
| Albumin (g/dL) | −10.7 | −47.5, 26.2 | 0.57 | −9.1 | −45.9, 27.8 | 0.63 | |
| Total bilirubin (mg/dL) | −2.2 | −8.7, 4.3 | 0.50 | −2.1 | −8.6, 4.4 | 0.53 | |
| FVC Slope | |||||||
| D-dimer (mcg/ml) | −1.4 | −7.5, 4.7 | 0.66 | −1.6 | −7.7, 4.6 | 0.62 | |
| hsCRP (mcg/ml) | 1.4 | −1.7, 4.6 | 0.38 | 1.4 | −1.8, 4.5 | 0.40 | |
| IL-6 (pg/ml) | −0.6 | −6.9, 5.7 | 0.86 | −0.7 | −7.0, 5.6 | 0.83 | |
| IL-27 (pg/ml) | −0.8 | −4.1, 2.5 | 0.64 | −0.7 | −4.0, 2.6 | 0.69 | |
| Serum amyloid A (mg/L) | 1.8 | −1.9, 5.4 | 0.34 | 1.8 | −1.9, 5.4 | 0.34 | |
| sICAM-1 (mcg/mL) | 4.3 | −4.1, 12.8 | 0.31 | 4.4 | −4.1, 12.8 | 0.31 | |
| sVCAM-1 (mcg/mL) | −1.6 | −10.1, 6.9 | 0.71 | −1.3 | −9.8, 7.3 | 0.77 | |
| IL-6/D-dimer score2 | −2.1 | −17.5, 13.4 | 0.79 | −2.7 | −18.2, 12.8 | 0.73 | |
| Albumin (g/dL) | −6.7 | −49.6, 36.1 | 0.76 | −2.9 | −45.9, 40.1 | 0.89 | |
| Total bilirubin (mg/dL) | 1.6 | −5.9, 9.2 | 0.67 | 1.8 | −5.7, 9.4 | 0.63 | |
| FEV1/FVC Slope | |||||||
| D-dimer (mcg/ml) | −0.1 | −0.8, 0.7 | 0.84 | −0.1 | −0.8, 0.7 | 0.89 | |
| hsCRP (mcg/ml) | 0.3 | −0.1, 0.7 | 0.11 | 0.3 | −0.1, 0.7 | 0.10 | |
| IL-6 (pg/ml) | 0.3 | −0.4, 1.1 | 0.38 | 0.4 | −0.4, 1.1 | 0.36 | |
| IL-27 (pg/ml) | 0.0 | −0.4, 0.4 | 0.84 | 0.0 | −0.4, 0.4 | 0.87 | |
| Serum amyloid A (mg/L) | 0.2 | −0.2, 0.7 | 0.29 | 0.2 | −0.2, 0.7 | 0.29 | |
| sICAM-1 (mcg/mL) | −0.4 | −1.4, 0.6 | 0.40 | −0.4 | −1.4, 0.6 | 0.40 | |
| sVCAM-1 (mcg/mL) | −0.7 | −1.7, 0.3 | 0.20 | −0.7 | −1.7, 0.3 | 0.18 | |
| IL-6/D-dimer score2 | 0.6 | −1.2, 2.5 | 0.50 | 0.7 | −1.1, 2.5 | 0.47 | |
| Albumin (g/dL) | 0.2 | −4.8, 5.2 | 0.94 | 0.0 | −5.1, 5.0 | 0.99 | |
| Total bilirubin (mg/dL) | −0.7 | −1.6, 0.2 | 0.12 | −0.7 | −1.6, 0.2 | 0.12 | |
adjusted for age, sex, race, region, smoking status, treatment group (immediate vs. deferred antiretroviral treatment initiation), CD4+ T-cell count, and HIV-RNA.
[0.33 × log2 IL-6 + 0.16 × log2 D-dimer] [17]
β represent the difference in lung function slope (FEV1, FVC, or FEV1/FVC) associated with a doubling of the corresponding biomarker
Unadjusted and adjusted regression of longitudinal changes in FEV1, FVC, and FEV1/FVC on baseline biomarkers limited to the deferred arm and with lung function data censored after initiation of ART are shown in Supplementary Table 2. In these analyses, free of any ART exposure, only sICAM-1 was associated with FEV1 slope (p=0.02) and FVC slope (p=0.02) in unadjusted analyses. However, these associations were attenuated and no longer significant in adjusted analysis (p=0.07 and p=0.06, respectively).
DISCUSSION
We found that a number of biomarkers, specifically markers of systemic inflammation and coagulation (hsCRP, IL-6, SAA, and D-dimer), were associated with cross-sectional measures of baseline lung function, but not with subsequent decline in lung function. These findings suggest that changes in circulating biomarker concentrations may accompany better or worse lung function, but a single measurement of these biomarkers does not help identify those at higher or lower risk of more rapid lung function decline.
There are few previous data on the association of biomarkers and pulmonary function in HIV-positive individuals. In a single center (Pittsburgh, USA) cross-sectional analysis, 147 HIV-positive individuals underwent pulmonary function testing, blood flow cytometry for T-cell surface markers, blood biomarker measurement (hsCRP, IL-6, and IL-8). hsCRP > 1 mg/L was associated with lower diffusing capacity for carbon monoxide (DLCO), lower FEV1, and more airflow obstruction (FEV1/FVC < 70% post-bronchodilator). Higher IL-6 was associated with lower DLCO and FEV1, while IL-8 did not show any significant associations. T-cell activation was also associated with pulmonary function abnormalities. [20]
In a recent single center (Mbarara, Uganda) cross sectional analysis of 125 HIV-positive individuals and 109 HIV-negative controls, investigators modeled the relationship between baseline lung function and markers of systemic inflammation (hsCRP and IL-6) and macrophage activation (sCD14 and sCD163).[24] Participants were enrolled in the Uganda Non-Communicable Diseases and Aging Cohort and HIV-positive patients had been on ART for at least 3 years (median 9 years). Higher hsCRP was associated with lower FEV1 and FVC among HIV-positive and negative participants. Higher IL-6 was associated with lower FEV1 and FVC among HIV-positive, but not HIV-negative, participants. Higher sCD163 was associated with lower FVC among HIV-positive participants but had no association with FEV1 among HIV-positive individuals or either FEV1 or FVC in HIV-negative individuals. sCD14 had no significant associations and there were no associations with biomarkers and FEV1/FVC.
Similar to these previous analyses, we found that markers of systemic inflammation were associated with lower cross-sectional pulmonary function. We did not specifically test markers of T-cell or macrophage activation, but we did not find an association between IL-27, which regulates T-cell function, and lung function in either cross-sectional or longitudinal analyses.
In an additional single center (Baltimore, USA) cross-sectional analysis, among 650 participants in the ALIVE (AIDS Linked to the IntraVenous Experience) cohort, 41% of whom were HIV-positive, lower cathelicidin (an antimicrobial peptide which is regulated by vitamin D) was associated with lower FEV1 (−115 mL, [–8,–221]). Although ALIVE has collected longitudinal lung function measures, the authors did not report longitudinal analyses.[21] We did not test antimicrobial peptides or vitamin D related markers. In a small case-control study from the START pulmonary substudy that included 38 HIV-positive individuals with COPD compared to 40 HIV-positive individuals without COPD, we also identified a unique metabolite profile in HIV-associated COPD that included sphingolipids.[31] We have not measured sphingolipids more broadly in our START pulmonary substudy cohort.
There are few data regarding biomarkers and longitudinal lung function in HIV-positive individuals. Among 124 HIV-positive persons from 2 sites (Pittsburgh and Los Angeles, USA) in the Lung HIV study who completed pulmonary function testing, IL-6, sCD163 (a marker of monocyte activation), and endothelin-1 (ET-1, a vasoconstrictor and marker of endothelial dysfunction) were associated with cross-sectional lung function measures. Among these participants, 70 (56%) underwent repeat pulmonary function testing over 18–36 months and in longitudinal analyses, only higher baseline concentrations of ET-1 were associated with faster decline in FEV1. [22] We tested sICAM and sVCAM which are both markers of endothelial activation, but found no associations with baseline or longitudinal lung function. We did not measure ET-1.
Finally, in a prospective study of lung function in 730 South African HIV-positive patients, higher CRP was associated with obstructive lung disease at baseline but had no association with longitudinal lung function over a relatively short median follow-up time of 18 months. Other biomarkers were not analyzed in that study. [32]
Although markers of systemic inflammation have been associated with a multitude of COPD and HIV-related outcomes, our finding that there was not association with longitudinal lung function decline has precedent. In an analysis of 1793 participants in the ECLIPSE (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) study several biomarkers, including CRP and fibrinogen (a marker of coagulation) were associated with baseline FEV1 however only Club Cell 16 (CC16), a pulmonary specific biomarker, was associated with longitudinal FEV1 decline. Surfactant protein D (SP-D), another more pulmonary-specific biomarker, was not associated with baseline or longitudinal lung function. [33] We have previously demonstrated that ART initiation is associated with a subsequent decline in blood SP-D concentrations, but we have not tested the ability of SP-D (or CC16) to predict longitudinal lung function decline in HIV. [34]
Our study has several strengths. We enrolled a large cohort of HIV-positive participants from 80 sites in 20 countries, thus improving the generalizability of our findings to HIV-positive persons from high and low-to-middle income settings. To our knowledge, our study also represents the largest collection of longitudinal lung function measures in an HIV population, having collected post-bronchodilator spirometry, using standardized equipment across all sites, with rigorous quality control, over a median of 3.9 years. This is the largest study to date analyzing the association of biomarkers and pulmonary function in HIV.
Our study also has several limitations. We leveraged biomarker data that were generated for other primary purposes, so we were unable to test more potentially pulmonary-specific circulating biomarkers such as CC16 and SP-D, nor other biomarkers that smaller studies suggest might relate to lung function in HIV (e.g. ET-1, antimicrobial peptides, markers of activated T-cells/macrophages, sphingolipids). We did not adjust for multiple statistical testing and although we adjusted for multiple confounders, there may be unmeasured confounding. We also did not collect lung-specific specimens, such as bronchoalveolar lavage fluid, so we were unable to assess local inflammatory or immune responses in the lung. Although we have one of the largest sample sizes of spirometry measures in an HIV-positive cohort, we did not collect other non-spirometry lung assessments, such as carbon monoxide diffusing capacity (DLCO) or quantitative computed tomography CT imaging of the lung. We did not have the ability to evaluate for reverse causality whereby rather than systemic inflammation and coagulation leading to lung disease, pathologic processes in the lungs might subsequently lead to systemic inflammation. This is one possible explanation for the observed cross-sectional associations, but lack of longitudinal associations, between systemic inflammation/coagulation and lung function. We also only tested biomarkers at baseline, prior to any antiretroviral treatment. Future work in virologically suppressed persons could yield different results.
Importantly, we enrolled a relatively young cohort of HIV-positive patients with a short duration of HIV diagnosis and high CD4+ T-cell counts (>500 cells/mm3) at study entry, so we cannot extrapolate our findings to those who are diagnosed at older ages or later in the course of HIV infection. Several studies have suggested that among HIV-positive persons, those with low nadir CD4+ T-cell counts in the 200–250 cells/mm3 range are those at highest risk for COPD.[23,35–37] Given that our study participants had far higher average CD4+ T-cell counts, our study participants may have been at low risk for developing lung disease. Additionally, of the 40% of our study participants who reported current or former smoking, the median pack-years smoked was only 6.5 pack-years, so our data may not apply to HIV-positive persons with more extensive smoking histories. However, we believe that the presence of biomarker associations within our cross-sectional spirometry analysis, but absence of associations within our longitudinal data suggest reverse causality rather than unique characteristics of our cohort. Nevertheless, we can’t exclude the possibility that longitudinal associations might be present in older persons, those with more advanced HIV infection, and/or those with more extensive smoking.
Lastly, we note that although our results might be limited by the relatively young age and early HIV diagnosis of our study participants compared to many other previous HIV cohorts, these characteritsics reflect the future goals of global HIV, where people with HIV should be diagnosed and treated early in order to preserve optimal health. Therefore, our study participants should increasingly reflect a larger proportion of those living with HIV in coming years, both in high and low-to-middle income countries where we conducted this study.
CONCLUSION
A number of common and readily available biomarkers, including markers of systemic inflammation and coagulation, are associated with concurrent measures of lung function in HIV-positive persons with CD4+ T-cell counts >500 cells/mm3, but not with subsequent longitudinal changes in lung function.
Supplementary Material
ACKNOWLEDGEMENTS:
The study team expresses our gratitude to each of the START Pulmonary Substudy participants for their contributions to our scientific understanding of lung health in HIV.
Support for this work:
Funding sources: The START Pulmonary Substudy reported here was supported by the National Heart Lung and Blood Institute (R01 HL096453); the parent START trial was primarily supported by the National Institute of Allergy and Infectious Diseases Division of AIDS (UM1 AI068641 and UM AI120197) with additional support from the German Ministry of Education and Research, the European AIDS Treatment Network (NEAT), the Australian National Health and Medical Research Council, Agence Nationale de Recherches sur le SIDA et les Hipatites Virales (France), National Research Foundation (Denmark), and the UK Medical Research Council and National Institute for Health Research. The Veterans Health Administration Office of Research and Development also provided protected research time in support of this study. The University of Minnesota served as sponsor of the study. None of the funders nor sponsor had any input regarding the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Study Drugs: Antiretroviral drugs were donated to the central drug repository by AbbVie, Bristol-Myers Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, Janssen Scientific Affairs, and Merck.
St. George’s Respiratory Questionnaire for COPD: Permission for use granted to investigators by Dr. Paul Jones (St. George’s, University of London, London, UK).
Disclaimer: The views expressed in this article are those of the authors and do not reflect the views of the United States Government, the National Institutes of Health, the Department of Veterans Affairs, the funders, the sponsors, or any of the authors’ affiliated academic institutions.
Footnotes
Declaration of interests:
GC, JVB, MC, EL, VP, DEN and KMK received grant support from the National Institutes of Health (R01 HL096453, UM1 AI068641 and UM AI120197) for conduct of this study.
Other interests:
DMM: No conflicts to report
ADZ: No conflicts to report
GC: No other conflicts to report
JVB: No other conflicts to report
MC: No other conflicts to report
EL: No other conflicts to report
DEN: No other conflicts to report
VP: No other conflicts to report
CHW: No other conflicts to report
RW: No other conflicts to report
KMK: Consultancy fees from GlaxoSmithKline
References:
- 1.Triplette M, Crothers K, Attia EF. Non-infectious Pulmonary Diseases and HIV. Curr HIV/AIDS Rep 2016; 13:140–148. [DOI] [PubMed] [Google Scholar]
- 2.Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, et al. Bacterial Pneumonia in Persons Infected with the Human Immunodeficiency Virus. N Engl J Med 1995; 333:845–851. [DOI] [PubMed] [Google Scholar]
- 3.Pefura-Yone EW, Fodjeu G, Kengne AP, Roche N, Kuaban C. Prevalence and determinants of chronic obstructive pulmonary disease in HIV infected patients in an African country with low level of tobacco smoking. Respir Med 2015; 109:247–254. [DOI] [PubMed] [Google Scholar]
- 4.Crothers K, Butt AA, Gibert CL, Rodriguez-Barradas MC, Crystal S, Justice AC. Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006; 130:1326–1333. [DOI] [PubMed] [Google Scholar]
- 5.Bigna JJ, Kenne AM, Asangbeh SL, Sibetcheu AT. Prevalence of chronic obstructive pulmonary disease in the global population with HIV: A systematic review and meta-analysis. Lancet Glob Health 2017; 6:e193–e202. [DOI] [PubMed] [Google Scholar]
- 6.Makinson A, Hayot M, Eymard-Duvernay S, Ribet C, Raffi F, Pialoux G, et al. HIV is associated with airway obstruction: A matched controlled study. AIDS 2018; 32:227–232. [DOI] [PubMed] [Google Scholar]
- 7.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gingo MR, Nouraie M, Kessinger CJ, Greenblatt RM, Huang L, Kleerup EC, et al. Decreased lung function and all-cause mortality in HIV-infected individuals. Ann Am Thorac Soc 2018; 15:192–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triplette M, Justice A, Attia EF, Tate J, Brown ST, Goetz MB, et al. Markers of Chronic Obstructive Pulmonary Disease are associated with mortality in people living with HIV. AIDS 2018; 32:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gingo MR, Balasubramani GK, Rice TB, Kingsley L, Kleerup EC, Detels R, et al. Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med 2014; 14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelkonen M, Notkola I-L, Tukiainen H, Tervahauta M, Tuomilehto J, Nissinen A. Smoking cessation, decline in pulmonary function and total mortality: a 30 year follow up study among the Finnish cohorts of the Seven Countries Study. Thorax 2001; 56:703–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-Function Trajectories Leading to Chronic Obstructive Pulmonary Disease. N Engl J Med 2015; 373:111–122. [DOI] [PubMed] [Google Scholar]
- 13.Man SFP, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax 2006; 61:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahl M, Vestbo J, Lange P, Bojesen SE, Tybjrg-Hansen A, Nordestgaard BG. C-reactive protein as a predictor of prognosis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175:250–255. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y-WR, Leung JM, Sin DD. A Systematic Review of Diagnostic Biomarkers of COPD Exacerbation. PLoS One 2016; 11:e0158843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunt PW, Lee SA, Siedner MJ. Immunologic Biomarkers, Morbidity, and Mortality in Treated HIV Infection. J Infect Dis 2016; 214:S44–S50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grund B, Baker JV., Deeks SG, Wolfson J, Wentworth D, Cozzi-Lepri A, et al. Relevance of interleukin-6 and D-dimer for serious non-AIDS morbidity and death among HIV-positive adults on suppressive antiretroviral therapy. PLoS One 2016; 11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008; 5:1496–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkegaard-Klitbo DM, Mejer N, Knudsen TB, Mller HJ, Moestrup SK, Poulsen SD, et al. Soluble CD163 predicts incident chronic lung, kidney and liver disease in HIV infection. AIDS 2017; 31:981–988. [DOI] [PubMed] [Google Scholar]
- 20.Fitzpatrick ME, Singh V, Bertolet M, Lucht L, Kessinger C, Michel J, et al. Relationships of pulmonary function, inflammation, and T-cell activation and senescence in an HIV-infected cohort. AIDS 2014; 28:2505–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lambert AA, Kirk GD, Astemborski J, Neptune ER, Mehta SH, Wise RA, et al. A cross sectional analysis of the role of the antimicrobial peptide cathelicidin in lung function impairment within the ALIVE cohort. PLoS One 2014; 9:e95099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzpatrick ME, Nouraie M, Gingo MR, Camp D, Kessinger CJ, Sincebaugh JB, et al. Novel relationships of markers of monocyte activation and endothelial dysfunction with pulmonary dysfunction in HIV-infected persons. AIDS 2016; 30:1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Attia EF, Akgün KM, Wongtrakool C, Goetz MB, Rodriguez-Barradas MC, Rimland D, et al. Increased Risk of Radiographic Emphysema in HIV Is Associated With Elevated Soluble CD14 and Nadir CD4. Chest 2014; 146:1543–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.North CM, Muyanja D, Kakuhikire B, Tsai AC, Tracy RP, Hunt PW, et al. Systemic Inflammation, Immune Activation and Impaired Lung Function among People Living with HIV in Rural Uganda. J Acquir Immune Defic Syndr 2018; 78:543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The INSIGHT START Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med 2015; 373:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerstjens HAM, Rijcken B, Schouten JP, Postma DS. Decline of FEV1 by age and smoking status : facts, figures, and fallacies. Thorax 1997; 52:820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunisaki KM, Niewoehner DE, Collins G, Aagaard B, Atako NB, Bakowska E, et al. Pulmonary effects of immediate versus deferred antiretroviral therapy in HIV-positive individuals: a nested substudy within the multicentre, international, randomised, controlled Strategic Timing of Antiretroviral Treatment (START) trial. Lancet Respir Med 2016; 4:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Standardization of Spirometry. 1994 Update. Am J Respir Crit Care Med 1995; 152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 29.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker JV, Sharma S, Grund B, Rupert A, Metcalf JA, Schechter M, et al. Systemic Inflammation, Coagulation, and Clinical Risk in the START Trial. Open Forum Infect Dis 2017; 4:ofx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodgson S, Griffin TJ, Reilly C, Harvey S, Witthuhn BA, Sandri BJ, et al. Plasma sphingolipids in HIV-associated chronic obstructive pulmonary disease. BMJ Open Respir Res 2017; 4:e000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupte AN, Wong ML, Msandiwa R, Barnes GL, Golub J, Chaisson RE, et al. Factors associated with pulmonary impairment in HIV-infected South African adults. PLoS One 2017; 12:e0184530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in Forced Expiratory Volume in 1 Second over Time in COPD. N Engl J Med 2011; 365:1184–1192. [DOI] [PubMed] [Google Scholar]
- 34.Kunisaki KM, Quick H, Baker JV. HIV Antiretroviral Therapy Reduces Circulating Surfactant Protein-D Levels. HIV Med 2011; 12:580–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Risso K, Guillouet-De-salvador F, Valerio L, Puglièse P, Naqvi A, Durant J, et al. COPD in HIV-infected patients: CD4 cell count highly correlated. PLoS One 2017; 12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shirley DK, Kaner RJ, Glesby MJ. Screening for Chronic Obstructive Pulmonary Disease (COPD) in an Urban HIV Clinic: A Pilot Study. AIDS Patient Care STDS 2015; 29:232–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crothers K, McGinnis K, Kleerup E, Wongtrakool C, Hoo GS, Kim J, et al. HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr 2013; 64:271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.