Abstract
Carbon nanotubes (CNTs) promise to advance a number of real-world technologies. Of these applications, they are a particularly attractive for uses in chemical sensors for environmental and health monitoring. However, chemical sensors based on CNTs are often lacking in selectivity and the elucidation of their sensing mechanisms remains challenging. This review is a comprehensive description of the parameters that give rise to the sensing capabilities of CNT-based sensors and the application of CNT-based devices in chemical sensing. This Review begins with the discussion of the sensing mechanisms in CNT-based devices, the chemical methods of CNT functionalization, architectures of sensors, performance parameters, and theoretical models used to describe CNT-sensors. It then discusses the expansive applications of CNT-based sensors to multiple areas including environmental monitoring, food and agriculture applications, biological sensors, and national security. The discussion of each analyte focuses on the strategies used to impart selectivity and the molecular interactions between the selector and the analyte. Finally, the Review concludes with a brief outlook over future developments in the field of chemical sensors and their prospects for commercialization.
Keywords: sensors, carbon nanotubes
Graphical Abstract
1. Introduction and Scope
Carbon nanotubes (CNTs) have now been a subject of research for more than 20 years. Mirroring this academic endeavor is the worldwide commercial interest, leading to the production capacity of several thousand tons of CNTs per year.1 These developments have paved ways to the wide array of emerging applications2,3 in microelectronics,4,5 computing,6 medicinal therapy,7 electrochemical biosensors,8 and chemical sensors.9,10 However, the field is far from mature and our understanding of the chemical and physical properties of these materials continues to grow. At the outset, it is fair to state that the chemistry of CNTs remains dubious and often imprecise.11 Although advances in the production have allowed preferential synthesis of single-walled carbon nanotube (SWCNTs) with metallic or semiconducting properties with selectivity of 90 to 95%,12,13 production of pure semiconducting tubes remains cost-prohibitive. Commercial supplies of SWCNTs, despite improvements in consistency, are still polydisperse in length, diameter, and chirality. Separations methods by density-gradient centrifugation with selective surfactants,14 conjugated polymer wrappings,15,16 or by gel chromatography17,18 are not readily scaled. Bottom-up syntheses have seen heroic efforts,19 but remain far from full realization. Similarly, multi-walled carbon nanotubes (MWCNTs) can be produced in high volume through large-scale chemical vapor deposition (CVD), however they suffer from structural deviations, contaminations, and impurities that often require costly treatment for removal.
One may ask why CNTs continue to garner such attention, given the complexity and what some chemists may even refer to as impurities. Clearly, scientific curiosity is one answer. The other motivation driving CNT research is their unusual optical, electrical, mechanical, and chemical properties. CNTs are unique organic electronic wires with shape persistence. These π-electron wires, possessing quantized electronic states with coherence lengths that are longer than what is possible for conducting polymers, making them ideal building blocks for nanoelectronic devices. Furthermore, CNTs can be organized in nanowire networks and with the addition of recognition elements are ideal for sensing applications. Indeed, it was understood from the early 1990s that molecular and nanowire architectures could produce sensors with superior sensitivity, benefitting from the restricted transport along percolative paths and the large surface area-to-volume ratio.20 Hence, these principles were translated quickly to create the first example of SWCNT chemical sensors by Dai and coworkers.21 The authors strived to realize the concept of nanowire in its purest form by connecting electrodes with an individual SWCNT to observe the change in its conductivity when exposed to oxidative p-doping (NO2) and reductive un-doping (NH3) gases. This study is certainly historic in the field of SWCNTs. However, similar to the onset of every area, much more progress was required to usher CNT platforms into versatile and useful sensors.
It is indisputable that selectivity underpins the utility of any chemical sensor. Of course, sensitivity and stability must be given the appropriate weight as discussed in the later sections of this review. The advancements in system integrations and electrical interfaces have lowered the stringent requirements of these latter parameters. For examples, electrical signals can be isolated and amplified, and trace analytes can be captured and released using pre-concentrators. However, without selectivity, the sensors are often rendered ineffective as a result of confounding effects in real-world environments such as interfering species, varying humidity, and fluctuation in temperature. Specificity, or perfect selectivity, is often not needed; and, robust sensors can be created from arrays of sensing elements, with each sensor having limited discriminating ability.22 Array-based sensors, such as a CNT-based chemical nose/tongue, are applicable to most types of chemical sensing and continue to progress toward the idea of a “universal sensor.” Inspired by the biological olfactory system, each individual channel in the sensor array needs not be perfectly orthogonal to every other channel. On the contrary, unique “fingerprint” corresponding to each analyte or group of analytes can arise when each channel of an array responds to several analytes in varying degrees. Nevertheless, it is seldom a disadvantage to incorporate sensors that are inherently selective to the target analytes. And indeed, a combinatorial approach of several highly selective and cross-selective sensing channels might lead to an optimized performance of the sensor array. SWCNTs are natural sensing materials as their transport properties are extremely responsive to their environment. They have suffered from limitation in selectivity at the inception of this field. Indeed, this limitation contributed to the relatively few commercial CNT-based sensors in spite of a massive world-wide research effort.
As a result, this review will place significant emphasis on the ways in which chemical science and engineering can be applied to create CNT-based sensors exhibiting selective responses to target analytes. Such approaches predominantly include functionalization with selectors (e.g., polymer-wrapping and sidewall attachments). Quite often, the sensing performance of CNTs depends not only on the molecular recognition, but also on the response of the collective system, which can be affected by non-specific chemical, thermal, and mechanical interactions. As will be discussed, the mechanism of chemical sensing may likely be intra-CNT and inter-CNT in nature; however, the other interfaces (CNT-electrodes and CNT-dielectric) must also be considered. In functionalization of SWCNTs, it was proposed initially that noncovalent attachments were preferred, as a result of the simplicity of the technique and the small perturbation on the base transport properties of the SWCNTs. Early applications of these methods to immobilized proteins appeared to give excellent performing biosensors.23 However, detailed follow-up studies by the same researchers later revealed that the interfaces between the metal electrodes and the CNTs were non-innocent, and the interactions at these locations constitute the major responses for these sensors.24 Hence, if the primary response occurs at locations other than sites comprising receptors/selectors, the sensor will lack predictably selective responses. In surveying the literature on chemical sensors, it is imperative to be properly skeptical regarding the advertised selectivity. Indeed, we will call out some of the results presented in this review when there is no apparent chemical rationale for the anticipated selectivity.
Although the nano-carbon area presents considerable diversity for chemical sensors, this review will strictly focus on electrical transduction using CNTs. The majority of nano-carbon sensors are based upon SWCNTs. Semiconducting CNTs are highly sensitive to carrier pinning and populations (i.e. doping levels). These are finite conductive pathways through the nanowire networks and perturbing such pathways increases the tortuosity for charge transport from one electrode to the other (i.e. resistivity). In addition to electrical transport, semiconducting SWCNTs are emissive. Similar to the concepts developed around semiconducting molecular wires,25 transport of excitons in SWCNTs can provide signal gain and emissions at long wavelengths for in vivo applications.26 This area is promising, and we direct interested readers to a review by Strano and coworkers.27 Although, there is ongoing interest in graphene-based sensors,28,29 the metallic state of these 2D materials is harder to quench or enhance, allowing carriers migration around perturbed regions. MWCNTs provide the wire architecture, however the inner tubes in these structures are prevented from interacting with the surrounding chemical environment. As a result, the intra-CNT mechanisms are not operative because the carriers can migrate through the unperturbed pathways of the inner core. Nevertheless, with suitable modulation of the inter-MWCNT transport, these materials can constitute effective sensors.
It is our intent to provide the reader a comprehensive perspective on the field of electrically-read CNT-based sensors. However, there have been previous reviews that have covered aspects of this field that may complement some of our descriptions.2,9,10,30–35 This article is conceptually self-contained and intended to serve as an informational resource to both newcomer and experienced researchers in the area of CNT-based sensors. As a result, we will cover some contributions that were highlighted in previous reviews. For researchers working in the sensor area, it is natural to think about real-world applications. It is also our perspective that these materials will become a significant commercial sensor platform in the near future. As a result, after introducing the concepts, we have organized the coverage of the literature by the respective application areas as shown in Figure 1. This approach is also intended to assist researchers with interests in the use of the sensors who are not sensor developers themselves.
Figure 1.
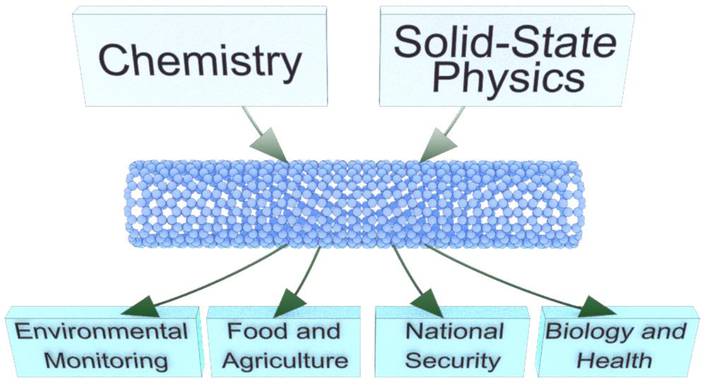
Schematic highlighting the application fields of the CNT-based chemical sensors covered in this review.
1.1. Chemical Sensing Mechanisms
The discussion on the exact mechanisms that cause the response of carbon nanotube-based sensors is very much alive. In contrast to conducting polymers, whose behavior can be described via molecular mechanisms, the properties of CNTs need to be described beyond local molecular structures, as is done in solid state physics. As a result of their extended π-system, the frontier orbitals of CNTs are best described through band structures rather than discrete molecular orbitals. Accordingly, chemical intuition is often not sufficient when trying to predict or describe CNT-based sensing mechanisms. In this section, we will discuss several mechanisms that give rise to signals in CNT-based chemical sensors of different architectures and functionalization techniques. Responses of CNT-based sensors are attributed to effects arising within the tubes (intra-CNT), effects arising at contact points between tubes (inter-CNT), or effects due to the contact between the tubes and the electrodes (Schottky barrier modulations) (Figure 2). The strength of these different mechanisms can depend strongly on the analyte, the defect concentrations in the CNTs, and the device architecture. For a historical discussion of the first investigation of CNT-sensor behavior and mechanistic investigations, we refer the interested reader to the reviews on CNT gas sensing mechanisms.36,37
Figure 2.
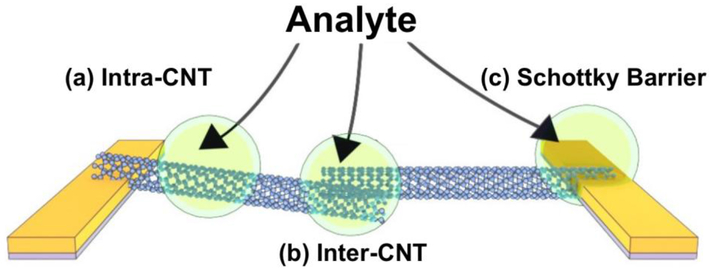
Schematic of sensing mechanisms in CNT-based sensors: (a) at the sidewall or the length of the CNT (intra-CNT), (b) at the CNT-CNT interface (inter-CNT), and (c) at the interface between the metallic electrode and the CNT (Schottky barrier).
1.1.1. Intra-CNT
Intra-CNT sensing mechanisms are modes of interaction between analyte and individual nanotubes or nanotube bundles. They include changes in the number or mobility of charge carriers and generation of defects on the walls of the tubes.
Charge transfer induced directly or indirectly by analyte interactions will modulate the conductance of the CNT by changing (decreasing or increasing) the concentration of the majority charge carriers. Under ambient conditions, CNTs are p-doped as a result of physisorption of oxygen molecules on their surface. Thus, exposure to further p-dopants will increase the hole conduction and cause a decrease in the resistance, while n-type dopants will induce the reverse effect.21,38–41 Direct charge transfer between the analyte and CNTs has been identified as a major sensing mechanism for polar analytes.21,41–43 In some cases this mechanism has a more localized nature. For example, interactions of a Lewis basic localized pair of electrons can create a local pinning force for cationic carriers as opposed to the fractional transfer of electron density to delocalized CNT states. For individual SWCNTs,21,39,44 charge transfer between analyte and tube can be observed experimentally through the current-voltage (I-V) characteristics, photoemission spectroscopy (PES), and Raman spectroscopy.
Investigation of I-V characteristics through field-effect transistor (FET) experiments is a powerful tool for probing the sensing mechanism of CNT-based devices. When plotting the current through the CNT material as a function of the applied gate voltage (transfer curve), different sensing mechanisms induce characteristic changes. Adsorption of electron donating species (charge transfer to the tube from the analyte) induces negative charge in the CNT, thus n-doping the CNT and shift the threshold voltage towards a more negative gate voltages and vice versa (Figure 3a).21 Modulation of the metal/CNT junction induces asymmetric conductance change, as electron- and hole-conductions are affected differently (Figure 3b). Lastly, a reduction of the charge carrier mobility through charge carrier trapping or scattering sites induces a reduction in conductance (Figure 3c).45,46 Any perturbation of the ideal SWCNT structure introduces charge scattering sites which reduce the mobility of the charge carriers and thus the conductance. Using I-V curves, changes in charge carrier mobility have been observed for scattering through adsorption of charged or polar species47–51 or via deformation of the tube.52
Figure 3.

Intra-CNT (semiconducting) sensing mechanism through changes in charge carrier concentration or mobility. Hypothetical transfer (I-Vg) curves and band diagrams before (black) and after (red) the exposure to the analyte for three different sensing mechanisms. The dotted line in the band diagram corresponds to the metal work function of the electrode and diagrams are given for both p- and n-type semiconductors interfaces with a metal. (a) n-Doping of the CNT induces a shift of the I-V curve to more negative voltages. The band diagram shows a hole doped CNT. (b) Schottky barrier modulation corresponds to a change of the barrier height between the work function of the metal electrode and CNT and asymmetric change in conductance for electron and hole transport. The band diagram shows change in barrier height for hole transport. (c) Change in mobility can be induced by the addition of resistive elements or carrier scattering which reduces the conductivity in both p- and n-type materials. Inspired by Ref. 53.
The effect of charge transfer on the doping levels of CNTs can also be estimated from shifts in the Raman spectrum.54–58 Wherein a shift of the G-band—stretching of the sp2 C-C bond in graphitic materials—towards higher wavenumbers is indicative of an electron-accepting analyte and a shift towards lower frequencies is indicative of electron-donating analyte (Figure 4). This shift can have a magnitude of ±30 cm−1 for strong dopants and has been observed for inorganic54 and organic dopants.59,60
Figure 4.
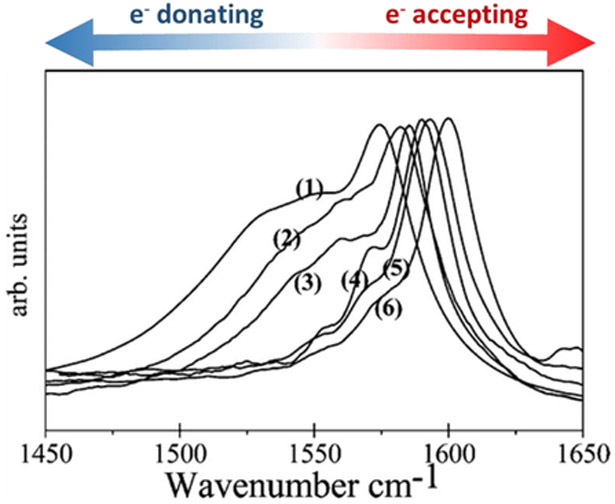
G-bands in the of Raman spectra of SWCNTs when interacting with electron donating and accepting molecules: (1) tetrathiafulvalene, (2) aniline, (3) pristine SWCNT, (4) nitrobenzene, (5) tetracyanoquinodimethane, and (6) tetracyanoethylene. Reproduced with permission from Ref. 59. Copyright 2008, American Chemical Society.
In addition to charge transfer effects, analytes can also promote the degradation of the CNT sidewalls. Particularly, the chemisorption of NO2 via formation of nitro- and nitrite-groups has been identified as a plausible sensing mechanisms.61–63 Soylemez et al.64 reported a chemiresisitive glucose sensor based on poly(4-vinylpyridine) (P4VP) wrapped SWCNTs functionalized with glucose oxidase. Upon exposure to glucose, hydrogen peroxide is formed which oxidizes the SWCNT sidewall. The degradation of the conjugated sp2 network of pristine CNTs increases the number of defect sites of the SWCNT, also observable as an increased D/G peak intensity ratio of the Raman spectrum. Strong localized interactions associated with carrier pinning can manifest increases in the D/G peak ratios.
1.1.2. Inter-CNT
For devices consisting of a network of CNTs, mechanisms at the interface between tubes can have significant influence on the electronic properties of the overall network. Small changes in distance between two CNTs dramatically influence the contact resistance as the probability of charge tunneling decreases exponentially with distance.65,66 The inter-tube conduction pathways can be modulated either by partitioning of analytes into interstitial spaces between tubes or by swelling of the supporting matrix/wrapper. Alternatively, an analyte can trigger the disassembly of a molecular/polymer wrapping of the CNTs, Figure 5.
Figure 5.
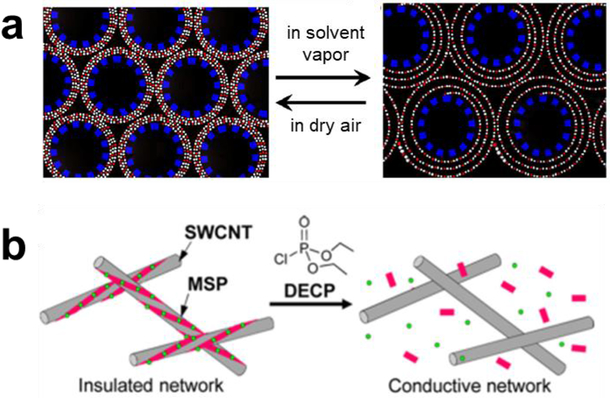
CNT-based chemical sensor designed based on inter-CNT mechanism. (a) Illustration of polymer swelling upon exposure of a CNT/polymer composite to solvent vapors. Reproduced with permission from Ref. 70. Copyright 2007, Elsevier. (b) Schematic illustration of a chemiresistive sensor comprising SWCNTs and metallosupramolecular polymer (MSP) showing the polymer degradation upon exposure to chemical warfare agent mimic diethyl chlorophosphate (DECP). Reproduced with permission from Ref. 73. Copyright 2017, American Chemical Society.
Swelling of a matrix material causes a decrease in the bulk conductance of CNT networks by increasing the width of tunneling gaps (Figure 5a). For example, Ponnamma et al.67 reported the influence of swelling on the electronic properties of a MWCNT-natural rubber composite. The swelling index was determined by quantifying the equilibrium uptake of a given solvent for all tested composites. They reported that the swelling index correlates with the magnitude of the decrease in conductance for all tested samples. Similar sensing behavior has been observed for porphyrins towards different VOCs,68 covalently69,70 and noncovalently71,72 attached polymers towards VOCs.
Alternatively, sensing systems that detect the increase in conductivity from new conducting pathways are similarly promising. Ishihara et al. reported the design of a sensor based on the de-wrapping of SWCNTs, which provided an increase of conductance by five orders of magnitude.73 In this case the SWCNTs were wrapped with a metallosupramolecular polymer designed to depolymerize upon contact with an electrophilic analyte (chemical warfare agent mimic, diethyl chlorophosphate), the depolymerization caused the formerly isolated SWCNTs to come into electronic contact (Figure 5b). Similarly, Lobez et al.74 and Zeininger et al.75 used CNTs wrapped by poly(olefin sulfone) (POS) polymers to detect ionizing radiation. Upon exposure to radiation, the meta-stable POS spontaneously depolymerizes with fragmentation resulting in an increase in the interconnections between CNTs and overall CNT network conductivity.
1.1.3. Schottky Barrier (SB) Modulation
In certain cases, the device performance is not only influenced by intra- and inter-CNT effects, but also by modulation of the junction of metal electrode and CNT (Schottky barrier). To differentiate between the previous mechanisms and effects at the electrode/CNT interface, several groups have observed the sensing behavior with and without passivation of the CNT/electrode contacts. In these experiments, passivating layers are deposited selectively over the whole device, over the areas where CNTs are in contact with the electrode, or over the length of CNTs that is not in contact with the electrodes (Figure 6a).
Figure 6.
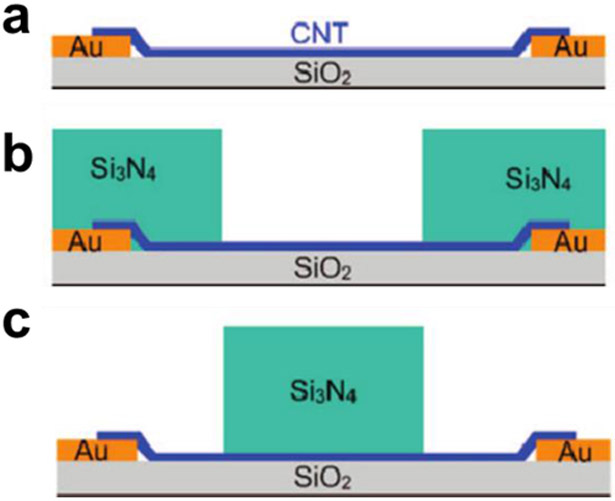
Three sensor architectures to probe Schottky vs intra-tube sensing mechanisms. Schematic for (a) device with bare CNTs, (b) device with passivated CNT-electrode contacts, and (c) device with passivated length of CNTs that are not in contact with the metal electrode. Reproduced with permission from Ref. 76. Copyright 2009, American Chemical Society.
Bradley et al. contact-passivated different areas of a pristine CNT-device with SiO2 and tested the response of the resulting sensors towards NH3.77 They found that complete coverage with SiO2 drastically attenuated the response to NH3 exposure, proving SiO2 a suitable passivating material. Coverage of the electrode/CNT contact areas resulted in a sensor with comparable responsiveness and faster reversibility than the non-passivated sensor. From this result, they concluded that the NH3 sensing mechanism is the result of processes occurring over the length of the CNT, and not at the CNT/electrode interfaces. Liu et al. used poly(methyl methacrylate) (PMMA) to passivate the channel of the electrode contact areas of a CNT-FET and detect NO2 and NH3.78 In contrast to Bradley et al., they observed changes in the transfer characteristics for both channel- and electrode-passivated devices suggesting that effect on both the metal/CNT junction and the length of the CNT have significant contributions to the sensor signal. Zhang et al. also employed PMMA to passivate the contact of a CNT device used to detect NO2, however they found that the sensing response is mainly due to the interface between electrode and CNT.79 Similarly, Peng et al. used devices partially passivated by Si3N4 and found that the sensing response towards NH3 mainly results from the metal-CNT junction.76 Considering the inconsistencies between these reports, it is understandable that the debate on the sensing mechanism persists.
To clarify the contradictory findings just discussed, Salehi-Khojin et al. investigated the sensing behaviors of CNTs with different defect levels.80 They reported that the dominating sensing mechanism is strongly dependent on the bottlenecks in the conduction pathways. For highly conductive CNTs, the sensing behavior is dominated by mechanisms influencing the electrode-tube junctions while the response of devices with less conductive, defect-rich CNTs is dominated by intra-tube effects. Generalization remains difficult as a result of the fact that there can be different types of defects in CNTs and other components in the sensor material can influence intra-tube and metal-tube electron transport.
Apart from the nature of the CNTs, the choice of metal for the electrode also influences the behavior of the CNT/electrode junction. Kim et al. reported increased sensing responses from CNT devices containing Pd instead of Au electrodes. 81 This result was attributed to the stronger interactions between the Pd surface and CNTs and the resulting barrier-free electronic transport across the junction, which agreed well with several theoretical studies.82,83 Zhang et al. demonstrated that targeted disruption of this Pd/CNT junction is a useful approach to fabricate H2 sensors using CNTs without any further functionalization. 84 A further discussion on the H2 sensing capability of Pd/CNT junctions can be found in section 2.1.2, Hydrogen (H2) and Methane (CH4).
1.2. Functionalization of CNTs
As mentioned previously, pristine CNTs have very limited selectivity when interacting with analytes. In this context, CNT functionalization is needed to tailor sensitivity and selectivity toward target analytes. Functionalization is also critical to improving the solution processability, enabling processing of these otherwise insoluble nanomaterials. Various approaches exist for the functionalization of CNTs, and they can be grouped as noncovalent and covalent modifications. Anchored chemical groups, macromolecules, or biomolecules that serve a function to recognize, interact, or react with a target analyte selectively are referred to as selectors. Noncovalent functionalization involves the adsorption of small molecules (often surfactants) to the surface of CNTs or wrapping of polymers and biomolecules around the tubes. Covalent functionalizations utilize reactions to attach chemical groups covalently to the conjugated surfaces or termini of CNTs. Covalent functionalization has the advantage that it can produce strong and stable anchors of functional groups to CNT, however the rehybridization of the carbon atoms from sp2 carbons to more sp3 character on the surface of CNT at the attachment sites lowers the electronic delocalization thereby perturbing their intrinsic optical and electronic properties. Therefore, careful control of the degree of functionalization is important to achieve an optimal balance between covalent anchoring of selectors and perturbation of the π–surface. Noncovalent approaches are generally less perturbative to the intrinsic properties of CNTs. However, physisorbed selector molecules or coatings have limited stability and can display changes in their configuration around the CNT, undergo phase segregation, and can even desorb in solution-based applications. Covalently functionalized carbon nanotubes are in general more robust for applications in environmentally challenging conditions. Several approaches to the functionalization of CNTs to impart sensor selectivity are described here. Comprehensive reviews of other functionalization methods that have not been applied in sensors are available elsewhere.11,85
1.2.1. Noncovalent Functionalization of CNTs with Small Molecule Units
CNTs can be functionalized noncovalently by physisorption of small aromatic molecules and surfactants through π-π and hydrophobic interactions. Dai and co-workers reported a simple and general method of immobilizing proteins onto CNTs using a bifunctional molecule containing a pyrene moiety for CNT adsorption and a succinimidyl ester for attachment of proteins by nucleophilic substitution reaction with the proteins’ surface amine functional groups. The immobilized proteins can be observed with atomic force microscopy (AFM) and transmission electron microscopy (TEM). This method is highly specific and efficient for immobilization of biomolecules, and the authors demonstrated the attachment of biotinyl-3,6-dioxaoctanediamine and two proteins, ferritin and streptavidin.23 Other functionalities anchored by pyrene units include amino-groups,86 cyclodextrin (Figure 7a),87 and boronic acid88 were used for the detection of trinitrotoluene (TNT), organic guest molecules and glucose respectively. Bis(trifluoromethyl) aryl groups are also suitable for noncovalent functionalization.89
Figure 7:
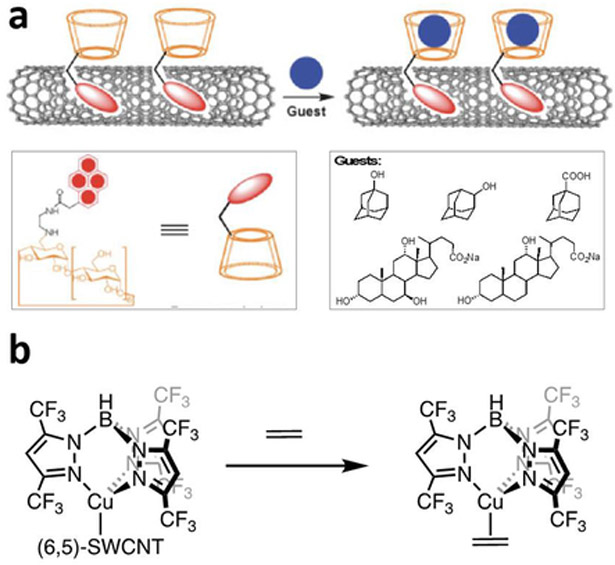
Noncovalent functionalization of CNTs with small molecules. (a) SWCNTs noncovalently functionalized with pyrenylcyclodextrins, which in a chemitransistor can detect closely related analogues of adamantane and sodium cholate. Reproduced with permission from Ref.87. Copyright 2008, Wiley-VCH Verlag GmbH & Co. (b) Chemiresistive sensor using a composite of SWCNTs functionalized by coordination of a fluorinated tris(pyrazolyl)borate copper (I) complex to the π-sidewalls to provide selectivity to ethylene gas.
Simple physical mixtures of CNTs and a small molecule or macromolecule selectors can impart selectivity in gas sensing. Composites made of CNTs dispersed with small aromatic units can however produce inhomogeneous compositions with variable performance. Successful gas detection has been realized with physical mixtures of CNTs with selectors that can interact with desired analytes through various supramolecular interactions such as hydrogen bonding,90 halogen bonding,91 π-π,92 metal-ligand (Figure 7b),93–96 and host-guest interactions.97
1.2.2. Wrapping of CNTs with Polymers
Polymer wrapping represents a noncovalent approach of solubilizing and functionalizing CNTs, wherein the collective contacts can provide for a stable composition. CNTs can be efficiently dispersed in water with sonication in the presence of single-stranded DNA (ssDNA) as a result of multiple favorable π-π interactions.98 Hydrophobic interactions with polymers having surfactant characteristics can drive solubility in water and saccharides and polysaccharides are capable of solubilizing and functionalizing CNTs. Although CNTs are not soluble in an aqueous starch solution, they are soluble in starch-iodine complex. Stoddart and co-workers attributed these observations as a result of preorganization of amylose in starch into a helical conformation by iodine. Displacement of iodine inside the helix by CNTs by a “pea-shooting” mechanism leads to amylose wrapped CNTs. The water-soluble starch-wrapped CNTs dispersions can undergo triggered disassembly by enzymatic hydrolysis with amyloglucosidase.99 Addition of this enzyme to starch-wrapped CNTs results in quantitative precipitation within 10 minutes.100 A variety of saccharides and polysaccharides101,102 have been used for the noncovalent functionalization of CNTs.103 Conjugated polymers are a natural class of CNT-wrappers and drawn much attention. Conjugated polymer-wrapped CNTs can be obtained by polymerizing an appropriate monomer in the presence of CNTs104 or by two component mixing.105 Swager and co-workers showed that conjugated polymers attached to selector side chains can provide selectively for the detection specific analytes and even resolve very similar structural isomers. 47,73,97,106,107 CNTs can also be functionalized with polymeric surfactants containing long alkyl chains that interact via hydrophobic interactions.108,109 Dai and co-workers demonstrated the adsorption of Tween 20 conjugates containing biotin, staphylococcal protein A (SpA), or human autoantigen U1A to CNTs. Polyethylene oxide chains of Tween 20 block the surface of CNTs and are crucial to suppress nonspecific binding of proteins.108
1.2.3. CNTs Decorated with Metal Nanoparticles
Although many examples of sensors based on unfunctionalized “pristine” CNTs have been reported, it is important to note that residual metal catalysts or particles left from the CNT production process have been accredited for the sensitivity of unpurified CNTs to certain analytes.2 As a result, intentional incorporation of metal nanoparticles represents a productive approach for producing specific or stronger responses. Unfunctionalized CNTs are insensitive to H2 gas, but electron beam evaporation of 5 Å of palladium (Pd) onto CVD-grown single CNT or CNT networks provides sensitivity to H2 gas at 4 – 400 ppm level in air at room temperature. The high sensitivity is understood to stem from the dissociation of H2 to atomic hydrogen on Pd. This process lowers the work function of Pd and subsequently causes electron transfer from Pd to CNT, decreasing the carrier density and conductivity of p-type CNTs.110 Using a “dry transfer printing” process, Sun et al. showed that CNT chemiresistive devices functionalized with Pd nanoparticles could be fabricated on flexible poly(ethylene terephthalate) (PET) substrate that can withstand 1000 cycles of bending and relaxing without significant sensing performance degradation.111 Deshusses and co-workers electrochemically deposited gold nanoparticles from commercially available ready-to-use gold electroplating solutions onto spray printed carboxylated-SWCNT films on gold electrodes. The resulting chemiresistive sensors can detect H2S in air at room temperature with a limit of detection of 3 ppb.112 Similar to H2, unfunctionalized CNTs are also insensitive to carbon monoxide. The functionalization of CNTs with SnO2 nanocrystals can overcome the inherent insensitivity of MWCNTs to H2 and CO gases as well as enhancing sensitivity to NO2. Chen and co-workers showed that MWCNTs decorated with SnO2 nanocrystals can detect ppm levels of NO2, H2, and CO gases at room temperature, an improvement over existing SnO2 sensors which operate typically at temperatures over 200 °C. Uniform decoration of ~2 to 3 nm sized SnO2 nanocrystals was achieved by deposition of aerosol SnO2 onto MWCNTs on gold interdigitated electrodes by electrostatic-force-directed assembly (ESFDA).113
1.2.4. Covalent Functionalization
Although noncovalent functionalization appears attractive towards tailoring the selectivity of CNT sensors, long-term stability, robustness, and leaching of noncovalent coatings remain a concern.114,115 Covalent functionalization offers strong and stable anchoring of functional groups which allows the robust applications of functionalized CNTs in harsh environmentally challenging conditions and for in vivo studies.116,117 Using covalent modification, selectors can be precisely attached to CNTs via their termini or sidewalls for long-term stability and reproducibility with well-defined chemical composition.
The capability of the CNT graphene sidewalls to undergo chemical reactions has led to the development of an extensive collection of covalent functionalization methods.11,85 Zhang et al. developed a highly efficient modular functionalization method capable of attaching a variety of chemical groups to the sidewall of CNTs. The reaction involves the addition of zwitterionic intermediates formed in situ from 4-dimethylaminopyridine (DMAP) and disubstituted acetylenedicarboxylates to the surface of CNT.118,119 Versatile, functional handles including terminal alkynes and allyl groups can be grafted, which can allow post-functionalization procedures such as 1,3-dipolar cycloaddition, thiol-ene addition, and olefin cross-metathesis reactions for further diversification. Using this method, sidewall-functionalized MWCNTs consisting of allyl, propargyl, alkyl triazole, thioalkyl chain, carboxylic, HFIP, calix[4]arene, and crown ether groups were synthesized and fabricated into chemiresistive sensors that were able to selectively identify a diverse array of volatile organic compounds, Figure 8.
Figure 8:
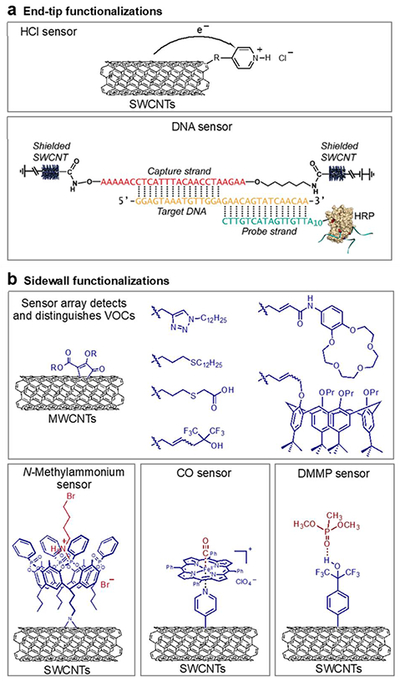
Examples of covalent functionalization of CNTs: (a) End-tip and (b) Sidewall functionalization. The DNA sensor scheme is adapted with permission from Ref. 120. Copyright 2011, American Chemical Society.
Thermal aziridination using organic azides provides access to covalently functionalized CNTs that has been theoretically suggested to provide minimal perturbation to the transport properties.121–123 Early recognition of this advantage by Schnorr et al. resulted in focused study on a variety of SWCNTs containing hydrogen bonding groups such as thiourea, urea, and squaramide via thermal aziridination of 3-azidopropan-1-amine followed by another step to attach the selectors. The resulting SWCNT chemiresistive sensor array consisting of different hydrogen bonding groups was able to detect ppm levels of cyclohexanone and nitromethane, vapor signatures of explosives. These sensors are highly reproducible between measurements and exhibit long-term stability, critical parameters to consider regarding practical applications.122 Thermal aziridination was also employed in the covalent functionalization of SWCNTs with a tetraphosphonate cavitand for N-methylammonium detection in aqueous conditions. Sarcosine, a potential biomarker for prostate cancer, and its ethyl ester hydrochloride derivative can be selectively detected in water at concentrations of 0.02 mM (Figure 8b).124 More recently, SWCNTs covalently functionalized with methyl pentafluorobenzoate and pentafluorobenzoic acid via aziridination enabled the detection of low ppm levels of ammonia and trimethylamines at room temperature. The sensors show no interference from volatile organic compounds and are operational in air and under high humidity.125
He and Swager used a reductive approach and functionalized CNTs with different aryl groups and N-heterocycles. CNTs were first reduced with sodium naphthalide in THF in situ followed by addition of aryl iodonium salts.126 CNT attachment of pyridyl groups at the 4-position is particularly useful for the anchoring of transition metal complexes. The potential of this pyridyl anchor was exemplified by the localization and electronic coupling of iron porphyrins (Fe(tpp)ClO4) to CNTs functionalized with pyridyl groups in the development of heme-inspired carbon monoxide sensors (Figure 8b).127 Although covalent functionalization is critical to achieving higher sensor sensitivity, excessive functionalization can disrupt the π-surface of CNTs increasing the base resistivity and lower sensitivity. Therefore, optimal sensor response to a targeted analyte requires a well-controlled degree of covalent functionalization.
Covalent functionalization by reactions with diazonium ions is widely used on carbon nanomaterials. Liu and co-workers functionalized SWCNTs with pendant hexafluoroisopropanol (HFIP) groups with a degree of functionalization of 1 HFIP per 75 carbons for DMMP detection. The HIFP attachment was conducted by in situ generation of an aryl diazonium ion through reaction of 2-(4-aminophenyl)-1,1,1,3,3,3-hexafluoropropan-2-ol with isoamyl nitrite at 70 °C. Johnson and co-workers covalently attached antibodies to SWCNTs functionalized with aryl groups containing sulfo-N-hydroxysuccinamide (NHS) esters.128,129 Considering the invasive characteristics of covalent functionalization, Wang and co-workers heavily functionalize the outer wall of double-walled CNTs (DWCNTs) with 4-benzoic acid moieties using diazonium chemistry. Since the inner tube is sealed and protected by the functional outer tube, the interaction between functional groups on the outer wall can induce an electrical change to the inner wall.116
Various metal oxides can be covalently attached to CNTs using sol-gel processes .130,131 The synthesis of CNT-metal oxide using a sol-gel process involves the oxidation of CNTs to generate carboxyl and hydroxyl groups on the graphitic surface. These groups can react with a colloidal solution (sol) of metal alkoxide or metal halide precursors. Subsequent hydrolysis and condensation reactions provide an integrated network (gel) of metal oxide on CNTs. Resulting CNT-metal oxides commonly give one-dimensional core-shell structures. Liang et al. provided an early example of MWCNTs coated with tin oxide nanocrystal via sol-gel method from tin(II) chloride that can detect ppm levels of NO, NO2, ethanol, and acetylene gas at 300 ºC.132 Additionally, the thickness of the tin oxide shell can influence the sensing properties.133 Other examples include MWCNTs functionalized with silica network and gold nanoparticles for the electrochemical detection of dopamine and ascorbic acid,134,135 and SWCNT-TiO2 core-shell hybrids synthesized using titanium isopropoxide as a precursor that possess a unique photoinduced acetone sensitivity.136 Moreover, SWCNT-indium oxide hybrids can be prepared from oxidized SWCNTs and indium chloride in ammonium hydroxide. The crystallinity of indium oxide, as controlled by the temperature of calcination, was reported to be important to the sensor’s response to acetone and ethanol.137 Similar to the synthesis of other metal oxides, SWCNT-CuO hybrids were prepared from copper(II) chloride in ammonium hydroxide and found sensitive to ethanol vapors and humidity at room temperature.138
Attachment of chemical groups or macromolecules to the ends of CNTs is generally achieved via amidation or esterification reactions with the carboxylic groups on oxidized CNTs that have been shortened by controlled acidic oxidation.139–141 Although these strong acid oxidative treatments can damage and shorten CNTs, subsequent end-tip functionalization doesn’t perturb the π–surface of CNTs. Haddon detected hydrogen chloride by introducing basic sites at the SWCNT ends through covalent attachment of pyridines (Figure 8a). The protonation of pyridine groups by hydrogen chloride induces the electron transfer from semiconducting SWCNTs. The sensor responded with decreasing resistivity because of holes are introduced to the valence band of semiconducting SWCNTs.142
Biomolecules can be precisely positioned at the ends of single CNT and CNT networks for sensing applications. Nuckolls and co-workers elegantly cut individual SWCNT positioned between two gold electrodes using electron-beam lithography and oxygen plasma ion etching to open a nanometer-scale gap.143 The gap was covalently bridged via amide coupling with amine-functionalized single-stranded DNA. Well-matched duplex DNA was shown to mediate charge transfer between the two SWCNT ends. The single CNT-DNA device was able to detect a single base pair mismatch in a 15-mer DNA with an increase in resistance relative to a well-matched oligomer.144 Schemes for the regiospecific covalent functionalization of CNT ends with biomolecules suffer several limitations, including nonspecific surface adsorption, the introduction of defect sites on the sidewall during oxidation process, and aggregation of tubes. Weizmann et al. exquisitely utilized a surface protection strategy to achieve regiospecific terminally linked DNA-CNT nanowires that addresses the limitations in bulk. In this strategy, strong-acid-oxidized SWCNTs were surface protected through surfactant and polymer wrapping with Triton X-100/PEG (Mn = 10,000), followed by amide coupling with amine functionalized DNA at the termini to form DNA-CNT nanowires.145 The specificity of this process was also confirmed by the selective attachment of gold nanoparticles to the CNT termini.146
1.3. Device Architecture and Fabrication
CNTs can be integrated in a straightforward manner into a variety of electronic device architectures with a variety of fabrication approaches. The design choices for devices utilizing CNTs as an electrical component of a sensor will be discussed in this section. Fluorescent sensors or other sensors that take advantage of the optical properties of CNTs have been reviewed elsewhere.26,27 This review will also not discuss the use of CNTs as tip augmentations of scanning probe microscopies.147,148
1.3.1. Single CNTs vs CNT Networks
While the chemistry of pristine and functionalized CNTs is the primary parameter to tune sensor selectivity and sensitivity, the choice of CNT quantity and morphology also plays a large role in determining sensor characteristics.5,149 Devices using individual CNTs (Figure 9a), wherein a single chemical event can interrupt all of the transport,20 can have higher sensitivity and a lower limit-of-detection than devices using multiple CNTs. Single-molecule sensors have been claimed using single-CNT architectures.144,150–157 From a purely scientific standpoint, single-CNT devices offer the ability to eliminate complexity from inter-CNT interactions, thereby simplifying the chemical sensing mechanisms (see Section 2.1) and precluding sensor drift/degradation that results from long-term movement/settling of CNT networks (see Section 2.4). However single-CNT devices are more time-consuming to fabricate and characterize than CNT network devices, and their lower signaling electrical currents also require more expensive measurement equipment. Furthermore, CNT-to-CNT variation and device-to-device reproducibility remains a challenge.
Figure 9.
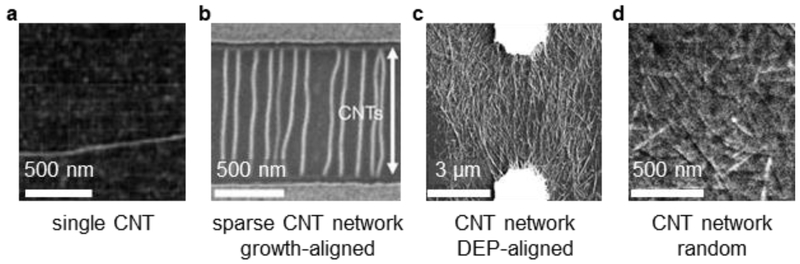
AFM/SEM images of (a) single CNT21 (b) sparse, growth-aligned CNT network162 (c) dielectrophoresis (DEP)-aligned CNT network168 and (d) random CNT network.169 Images adapted with permission from Ref. 162 Copyright 2017, Nature Publishing Group; Ref. 21, Copyright 2000, American Association for the Advancement of Science; Ref. 168, Copyright 2010, Springer; Ref. 169, Copyright 2011, American Chemical Society.
Sensors using CNT networks (Figure 9d) can be quickly and inexpensively fabricated with a variety of methods (vide infra). The response of a CNT network offers higher device-to-device reproducibility than single-CNT devices, but metallic CNTs in the network are less sensitive to analytes than semi-conducting CNTs, and overall sensor response can be attenuated.51 For optimal intra-CNT sensing responses, the CNTs should be debundled, so that charge carriers remain accessible to analyte interactions and/or coupled to selector/receptor groups.51,158 For sensors in which inter-CNT effects are proposed to determine electrical response, analogous single-CNT devices would exhibit no response. The nanotube density is also an important consideration, as lower-density films with higher surface-to-volume ratios have been shown to offer higher sensitivity and lower detection limit.159
As an intermediate between single CNTs and random CNT networks, aligned CNTs can be used (Figure 9b,c). When compared to single-CNT devices, the baseline noise of aligned CNT sensors can be much lower,160 and device yield/reproducibility is higher. Relative to random CNT networks, aligned CNTs, if sparse enough, can limit inter-CNT effects and exhibit higher sensitivity.51 Highly aligned, sparse CNT networks have been made with a variety of techniques including growth along a surface template,161,162 spin-coating,51 deposition in lithographically patterned trenches,163 and alignment along solution interfaces.164,165 Aligned CNT networks in which neighboring CNTs are intertwined and in electrical contact with each other (Figure 1c) exhibit anisotropic electrical properties that can be measured either along or perpendicular to their alignment axis.166 For a detailed review on the comparison of random CNT networks and aligned CNTs in different electronic devices we refer the reader to review articles out of John Rogers’ group.4,167
1.3.2. Device Architectures
1.3.2.1. Transistors
The field-effect transistor architecture utilizing CNT(s) as the active channel (CNT-FET) is a versatile sensor platform (Figure 10a). Early CNT-based chemical sensors, reported by Kong and Dai, were chemically responsive transistors (chemitransistors), obtained by chemical vapor deposition growth of an individual SWCNT across patterned Pd catalyst islands on SiO2/Si layered substrate.21,170 In chemitransistors, the channel current (ISD) across a range of gate voltages (VG) is compared before and after analyte exposure. Thus, one ISD-VG curve offers many features, and seemingly subtle changes can be accurately correlated with analyte exposure using linear discriminate analysis.171 To optimize sensitivity, the CNT active channel should be semiconducting and not metallic, which is an issue considering currently as-produced SWCNTs are mixtures of chiralities. In addition to the back-gate architecture, chemitransistors can utilize a top-gate architecture, in which a dielectric and metal gate electrode are deposited on top of the CNT(s). The top dielectric layer partially passivates the CNT against degradation, but also limits direct CNT–analyte interactions, which can lower sensor response.161,172
Figure 10.
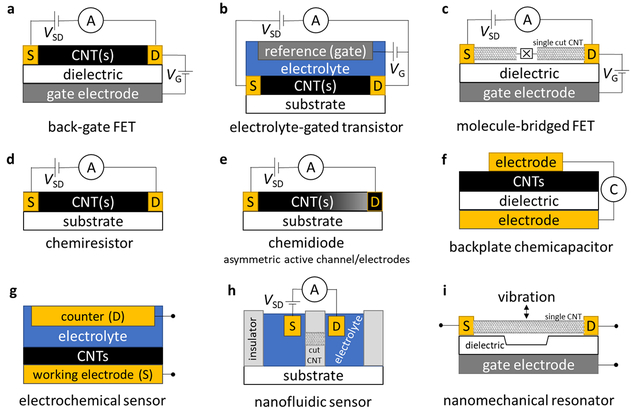
Illustrative CNT sensor architectures: (a) back-gate FET, (b) electrolyte-gated transistor, (c) molecule-bridged FET, (d) chemiresistor, (e) chemidiode, (f) back-plate chemicapacitor, (g) electrochemical sensor, (h) nanofluidic sensor, and (i) nanomechanical resonator. S = source, D = drain, Ⓐ = ammeter, V = voltage, Ⓒ = capacitance meter, ⌧ = molecular bridge.
The dielectric and gate electrode layers of a conventional solid-state FET can be replaced with liquid electrolyte and an electrochemical gate electrode. This electrolyte-gated transistor (EGT) architecture (Figure 10b), originally developed for the study of conjugated organic polymers and referred to as electrochemical transistors,173 is also referred to in the CNT literature as an electric double-layer transistor.174–176 The CNT-EGT architecture targets solution-phase analytes and can be incorporated into microfluidic sensors.177 CNT-EGTs exhibit higher on-off ratios with lower switching potentials than CNT-FETs operated via a conventional back-gate.178,179 These advantages translate to CNT-EGT sensors, as small changes (< 500 mV) in VG can result in markedly improved analyte sensitivity.179
In addition to serving as the active channel, CNTs can serve as the electrodes for molecule-bridged transistors (Figure 10c). Single CNTs are precisely cut by electrical breakdown or lithographically patterned etching, and the resulting nm-scale gap can be bridged by a variety of conductive (redox-active) molecules.143,157,180 The chemical responsiveness of the bridging unit has been leveraged to detect pH, metal ions, proteins, and DNA.143,144,157 When the CNT gap is bridged by single-stranded DNA, conductivity increases upon formation of a DNA duplex with the complementary strand. However, if the complementary strand contains one base-pair mismatch, conductivity decreases nearly 300-fold.144 Fabrication of these CNT-molecule-CNT junction chemitransistors is not trivial, but the sensing response of proposed molecular linkers can be evaluated in silico prior to fabrication.181
1.3.2.2. Two-Electrode Solid-State Sensors
While the three-electrode transistor architecture offers much more data for sensing analysis, architectures using only two electrodes offer operational simplicity that would be suited for distributed, low-cost sensors. In the absence of applied gate voltage, CNTs can exhibit changes in conductivity on exposure to analytes. These chemically responsive resistors (chemiresistors, Figure 10d) constitute a very simple sensor design with minimal power and instrumentation requirements. In liquid-phase electrochemical systems, these sensors have been referred to as chemiresistive,182,183 conductometric,184 or amperometric.185 In this review, we reserve the term amperometric sensing for electrochemical sensors in which electrolyte solution plays an integral role in the conduction pathway. Chemiresistors benefit from low cost and ease of fabrication, which allows for rapid screening of a diverse array of selectors.68,93,117,186,187 Their low operational power requirements can enable wireless sensor networks, where the CNT chemiresistor is incorporated into passively powered RFID tags (Figure 11).90,107,188,189
Figure 11.
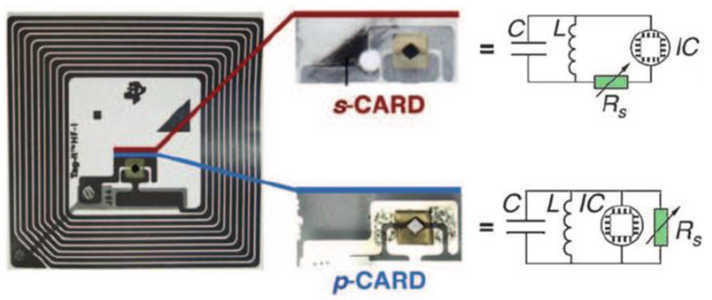
Two methods to integrate CNT chemiresistors into commercial RFID tags (left) and their equivalent circuit diagrams (right). Reproduced with permission from Ref. 90. Copyright 2016. Wiley-VCH Verlag GmbH &Co. KGaA.
Most chemiresistors are characterized using a single small bias voltage (VSD). At higher bias voltages, the ISD–VSD curve for CNTs deviates from Ohm’s law linearity for an ideal resistor.190 Analysis of the non-linear voltage regime could allow extraction of more data from chemiresistive sensors. This approach has been used for Pd-CNT-Pd devices to sense H2 via Schottky barrier modification.84
A non-linear, rectifying ISD–VSD curve is expected if the chemiresistor architecture is sufficiently electronically desymmetrized. Such a chemidiode (Figure 10e) changes its ISD–VSD curve upon exposure to analyte. If the source and drain electrodes are materials with different work functions, then a Schottky diode is formed. A Pd-CNT-Si Schottky diode has been used to sense H2.191 Symmetric metal-CNT-metal devices can detect asymmetric deposition of a second metal at one of the electrodes via the resulting rectified ISD–VSD curve.192,193 Alternatively, the p-type CNT active channel can be asymmetrically functionalized with molecular,169 polymeric,194 or metallic n-dopants to form p–n rectifying diodes.195 Current reports of chemidiodes have focused primarily on the observation of increased rectifying behavior upon exposure to a single high concentration of analyte. Calibration of chemidiodes against a range of analyte concentrations is a promising approach for future research to consider.
CNTs can also be used as the active element in a conductor/dielectric/conductor capacitor architecture. Although the capacitance between an individual CNT and a gate electrode has been studied,196 capacitive sensing studies have primarily utilized CNT networks/films (chemicapacitors, Figure 10f), often in a FET-type architecture with separate source and drain top electrodes. Using a parallel-plate capacitor model or a RC transmission line model, gate capacitive measurements of CNT films on back-gate FET devices have been used to estimate CNT coverage density.197,198 The capacitance of the film changes upon exposure to analyte, as a result of the polarization of analyte molecules near the CNT surfaces. Snow and coworkers reported that CNT-based sensors exhibit larger responses with faster recovery kinetics in capacitance rather than in resistance mode.199,200 However, a study by Esen and coworkers using an RC transmission line model attributed the bulk of the chemicapacitive response of a CNT film to changes in its resistance.201 An alternative measure of chemicapacitance is to connect a two-terminal chemiresistor-like device to an AC voltage source and impedance analyzer, and to model the source–drain capacitance and resistance of the equivalent circuit. Using this method, ammonia vapors exhibit a faster and larger capacitance than conductivity response,202 and CO2 was found to exhibit a capacitive response but not a resistive response. 203
1.3.2.3. Electrochemical Sensors
CNT films can function as electrodes for solution-phase electrochemical sensors (Figure 10g).8,204 When used with biological selectors/analytes, these are also referred to as biosensors. Their high specific surface area allows wide dynamic sensor range, resistance to fouling, and high loadings of electrocatalysts or selectors. CNT films have been functionalized with a variety of enzymes to amperometrically detect biological analytes. Potentiometry has been used for CNT-based pH sensors.185,205–207 Potential differences generated in solution can be amplified by using the CNT electrode as an extended gate electrode for a metal-oxide-semiconductor FET (MOSFET) chip.208 Voltammetry measurements, requiring a potentiostat, assist in analyzing complex mixtures by separating electroactive species by their redox potentials.209,210 This is particularly useful for distinguishing and quantifying different dissolved metal ions.211,212
A single CNT with open ends from oxidative cutting can function as a well-defined, size-selective pore to separate the cathode and anode, forming a nanofluidic sensor (Figure 10h). Amperometric measurements show a change in electroosmotic current as analytes pass through CNT pores. Based on the magnitude and duration of the current change, similar analytes can be distinguished from one another; this has been applied to discrimination of different alkali metal ions,155 oligonucleotides of varying length,156 or differently charged dye molecules.213 In a more advanced architecture, a top-gate electrode can be fabricated for the CNT pores, resulting in a nanofluidic FET which provides more data for distinguishing various analytes.214
1.3.2.4. Miscellaneous
Electronic analytical methods based on resonant frequencies have been augmented by incorporation of CNT(s), such as circular disk resonators215,216 and quartz crystal microbalance217 (QCM). The details of these analytical methods are beyond the scope of this reference. However, it should be noted that a suspended individual CNT in a modified FET-type device can be driven into resonant vibration modes by applying offset sinusoidal potentials from the gate electrode (Figure 10i). Such nanomechanical resonators have been used to detect minute mass changes on the CNT.218 A side-gate device has been used to detect adsorption of naphthalene molecules or xenon atoms to the CNT with impressive yuctogram-scale resolution (1 yg = 10 −24 g).154 Thus far, these studies have only been conducted on unfunctionalized CNTs.
1.3.2.5. Sensor Arrays
To better identify or quantify analytes from complex sample mixtures, multiple sensing elements can be integrated onto the same device, often using shared counter/drain/gate electrodes. Ishihara and coworkers elegantly used a minimal array consisting of two chemiresistors to make a formaldehyde sensor.219 One element, functionalized with hydroxylamine hydrochloride, becomes more conductive upon exposure to formaldehyde vapors, while the other element is unresponsive to formaldehyde but serves as a reference to account for conductivity changes arising from humidity and temperature variations. More elaborate multichannel arrays can be formed from a variety of cross-reactive sensing elements, in which a variety of analytes can be determined simultaneously, in a process reminiscent of olfaction/taste.22 Electronic noses/tongues comprised of CNT electrical elements have been used to discriminate between a variety of analytes such as volatile organic compounds68,117,122 or malignant vs. healthy cells.171 By giving a sensor array a training set of samples, fingerprint responses can be determined with mathematical techniques such as principal component analysis (PCA) and linear discriminant analysis (LDA).220
1.3.3. Fabrication
To incorporate CNTs into electric devices, there are two general fabrication strategies: fabrication using as-grown CNT films or the deposition of purified CNTs. The former approach, usually performed via high-temperature chemical vapor deposition onto pre-patterned electrodes, produces robust electrode/CNT contacts, and with sparse CNT deposition, bundling and inter-CNT effects can be avoided. However, for as-grown single-CNT devices, it is difficult to control the angle of CNT growth between electrodes, and device yield is low, especially for larger channel lengths.21,170 Additionally, for as-grown CNT devices, a distribution of CNT diameters and chiralities are formed, and limited on-device purification steps are available. Metallic CNTs can be preferentially disrupted using electrical breakdown162,221,222 plasma etching,223 thermal oxidation,224 or irradiation,225 resulting in higher on-off ratios for CNT transistors. CNTs aligned during growth can be alternatively transferred to different substrates with lift-off techniques.6,226
In contrast, device fabrication strategies that rely on deposition of pre-formed CNTs can take advantage of solution-based functionalization schemes (Section 2.2) and purification strategies, which can enrich specific diameters or chiralities.227,228 The purified CNTs can be further purified/treated after deposition to enhance transistor on/off ratio, chemitransistor sensitivity, and response time.229 CNTs can be deposited onto pre-patterned electrodes, or vice-versa, electrodes can be deposited onto CNTs. The former method is convenient for laboratory research, as pre-patterned electrodes can be produced in bulk. However, in these cases the CNTs are only held onto the electrode weakly, and device performance may drift as a result of a high and variable contact resistance at the CNT-electrode interface.230 In contrast, post deposition of metal electrodes onto CNTs fixes them in place with greater contact areas and lower contact resistances.231
Deposition of CNTs from a suspension by inkjet printing, spray-coating, spin-coating, dropcasting, or doctor blading can fix CNTs in place by rapid removal of solvent. Alternatively, CNTs can be deposited using external forces to make more robust CNT materials. Such techniques include layer-by-layer assembly210 and alternating current dielectrophoresis (DEP).202 Variation of AC waveform, electrode geometry, and substrate all play a role in DEP, and individual CNTs can be repeatably deposited. The resulting aligned CNT devices exhibit higher on/off ratios than random network devices.4
Solvent-free deposition methods for purified CNTs have also been explored. Mirica and coworkers pioneered abrasive deposition using compressed pellets of CNTs ball-milled with selectors.91,187,232 Abrasive deposition is a simple, low-cost, and rapid fabrication method suitable for teaching laboratories and rapid prototyping.233 Limitations of this method include the need for a rough substrate, the use of relatively large amounts of CNTs (~25–150 mg) to fabricate one compressed pellet, and the limited mechanical integrity of pellets, which is particularly acute with poorly adhering selectors (e.g. TiO2 nanoparticles). Adhesive deposition of “bucky gels”—viscous, non-volatile, CNT-containing mixtures—has been explored with ionic liquids and deep eutectic liquids,234,235 and related methods are used in the production of some commercial chemiresistive sensors.236
1.4. Performance Parameters
The promise of practical chemical sensors has motivated the constantly expanding research on CNTs. All analytical methods must possess the capability to provide quantitative, or in some cases, qualitative measurements. Chemical sensors, by definition, are devices with the capability to recognize and transduce the chemical information of the samples. In the simplest cases, the chemical information is the analyte concentration, and the read-out is a change in a readily measured signal. Ideally, a sensor is sensitive, selective, and stable. Achieving these figures of merit continues to be a challenge for the development of all sensors. Furthermore, chemical sensors are an alternative to large equipment in analytical lab and it is implicit that they are inexpensive, physically small (ideally portable), and robust under field conditions. CNT-based sensors are viewed as being well suited for these requirements. Additionally, qualities such as reproducibility, rapid response times, and low drift are demanded for practical sensors. We detail the requirements for practical sensors and its challenges from the chemical perspective. Central to our discussion are the interactions between functionalized (selector/receptor modified) CNTs and the analyte. This section will introduce and provide brief descriptions of the relevant figures of merit and how they are measured. This section will conclude with a discussion of challenges arising from the properties of CNTs.
1.4.1. Parameters of Performance and Figures of Merit: What they are and how to measure them?
The sensing response curve describes how the devices respond to the exposure of the analyte as a function of time. Figure 12a illustrates a hypothetical curve when the device is exposed to increasing concentration of the analyte. The choice of the architecture of the sensing device will govern the type of signal measured by the sensor. The sensing traces are often reported as the relative changes in measured resistance (R), current (I), conductance (G = I / V; the symbol is not to be confused with Gibb’s free energy), capacitance (C), power gain (RFID), and resonant frequency (f0) of the device vs. time. Different conventions have been adopted for plotting these measurements—most popularly normalized differences ΔX/X0, X/X0, or simply ΔX (where X = R, I, G, C, or gain). For example, the change in conductivity (–ΔG/G0) is calculated by observing the normalized difference in the current before (I0) and after (I) the exposure to the analyte using Equation 1:
| Equation 1 |
Figure 12.
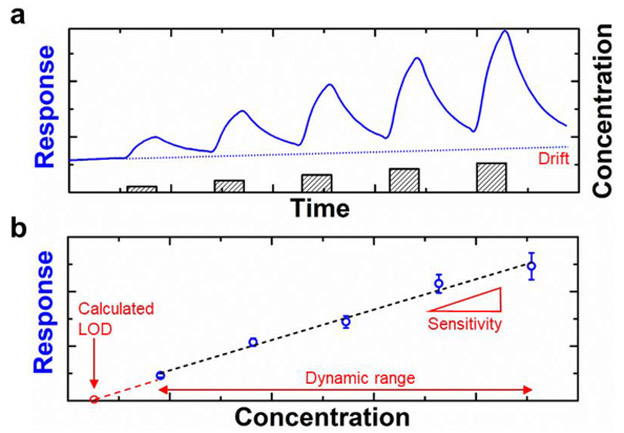
Hypothetical sensing response curve (a) and calibration curve (b) of a sensor exposed to increasing concentration of the analyte. Adapted with permission from Ref. 35. Copyright 2016, Wiley-VCH Verlag GmbH & Co. KGaA.
The calibration curve (Figure 12b) shows the relationship between the response of the sensor and the concentration of the standard solution.237 The standard solutions for the analyte should be carefully chosen to cover the relevant concentration range. Sensitivity is defined as the capability to discriminate small differences in concentration or mass of the analyte. In other words, sensitivity of the sensor is the slope of the calibration curve. The range of the concentration of the standard solution measured for the calibration curve constitutes the dynamic range; the range of which the signal is linearly proportional to the concentration is the linear range.238
Limit of detection (LOD) is the lowest amount of an analyte in a sample which can be detected with reasonable certainty. The theoretical or calculated LOD, as established in literature,239–241 is determined as the concentration that corresponds to the signal at three standard deviations of noise above the baseline, using the calibration curve. Briefly, the root-mean-square noise (rmsnoise) of the sensors is first calculated as the deviation of the sensing response curve from the appropriate polynomial fit. The LOD is then calculated using Equation 2:
| Equation 2 |
The slope in Equation 2 is the linear regression fit of the sensor response vs. concentration curve (slope of the calibration curve). The drive for achieving lower LOD is often governed by application-dependent requirements. These values for environmental safety are published by regulatory agencies. The ability to detect low concentration of the analyte is often coupled dynamic range of the sensors which describes the range of concentration the sensor can be calibrated. Sensors with low LOD often deviate from linearity at high concentrations as a result of saturation.
Selectivity of a single sensor is defined as the ability to discriminate the analyte of interest from other species within the sample.242 Specificity is also used to express selectivity: in a literal interpretation a specific sensor recognizes only the target analyte and no other compounds. Such ideal sensors are rare as a result of similarity between analytes and lack of perfect molecular recognition. Cross signaling, also known as cross reactivity, between sensors and analytes leads to a compounding of the signals and loss of selectivity. Selectivity is measured from the signals arising from the analyte and the possible interfering compounds separately with the assumption that there are no synergistic cooperative effects of multiple analytes and the sensor. The calibration curves (signal vs. concentration) of each is then compared. The ratio of the signals of the analyte to those of the interfering compounds defines the selectivity of the sensor. This method is operationally simple and generally adequately quantifies the desirable signal relative to possible cross signaling. However, it may overlook the competing effects between different compounds and the analyte. The second method comprises replicating the matrix (simple or complex) of the real-world samples in which the analyte is mixed with expected interfering compounds. In such case, the selectivity of the sensor can then be determined by the differences in signal of the analyte with and without the interfering agents.
Stability is defined as the capability of the sensors to produce repeatable outputs for an identical environment over time. To measure the stability of the sensors, repeated measurements should be taken over time or over many cycles of exposures to the analyte. In a more methodical method, full calibration curves are obtained for multiple devices over time. During the operational timeline of a sensor, issues regarding stability may arise from several sources, such as drift, hysteresis, and irreversibility. These issues are particularly prevalent in the development of sensors for highly reactive analytes. Drift is defined as the change in signal over time independent of stimuli. It remains one of the challenges to be solved for CNTs-based sensors. The common sources of drift can be identified as either the physical changes occurring to the selectors/receptors and the context of their interactions with the CNTs, very small changes in the positions of nanotubes that effect CNT-CNT junctions, or parasitic electrical effects including the electromotility of ions under small applied potentials. The perturbation of the sensing layer caused by gas flow, pressure differentials, and thermal rearrangements can lead to signal drift.33 Hysteresis is the difference between the output of the sensors when an analyte concentration is approached from an increasing and decreasing range. It is also influenced by the degree of irreversibility of the sensor, which is the background signal of the sensor before and after exposure to the analyte.
Lastly, the response and recovery times are important key factors when evaluating the performance of chemical sensors. The response time is defined as the time for the sensor to reach 90% of its steady state or maximum value upon an exposure to a given concentration of the analyte; while, the recovery time is measured as the time required for the sensor to recover to 10% of its peak value. Reversibility is coupled with recovery time as the extent to which the output can be restored after an exposure.
1.4.2. Specific Challenges on the Performance of CNT-Based Chemical Sensors
The last subsection provided definitions for evaluating the chemical sensor performance; we now introduce specific performance challenges for CNT-based chemical sensors. Significant effort has been applied to the development of practical CNT-based chemical sensors. However, many challenges still need to be addressed to realize many sensing applications.
Quality controls and reproducibility.
The quality and the purity of the CNTs often led to the differences in the sensing performance as reported by different research groups. For example, the differences in the performance can stem from the source/growth of CNTs,243,244 defect levels in the CNTs,245–249 or sensor fabrication.250,251 Obtaining high purity and specific size/helicity of the CNTs is a formidable task. Separation methods that produce high purity CNTs are inefficient and not readily scaled and thereby increase sensor costs. Establishing standardized baselines requires extensive characterization of the CNTs.
Operating temperature.
Room temperature operation is one clear advantage of CNT-based sensors over conventional chemiresistive sensors based on metal oxide semiconductors. Although operating at room temperature enables lower power consumption, it can lead to competing non-specific interactions. In this context, water sensitivity is an important parameter to be tested for practical CNT-based sensors. In addition, thermal stability of the selector/receptor-CNT composite should be tested for applications that would expose the sensors to elevated or fluctuating temperature.
Reversibility.
Strong interactions designed for ultra-trace analyte LOD can lead to slow off rates and prevent the sensor recovery in an analyte-free environment. Slow desorption of gas molecules can often be alleviated using thermal treatments or photoirradiation. These treatments, however, increase complexity (in both design and operational) and cost of the overall systems. Thus, intrinsic reversibility endowed from the interaction between the well-designed selectors and the analyte is generally preferred.
1.5. Theoretical Models
Theoretical models have been used extensively to understand and predict carbon nanotube behavior and properties. Specifically, computation on CNTs have been used to probe their electronic properties in pristine252 and deformed forms,253 thermal conductivity,254 elasticity,255–257 and flexibility.258–260 We direct interested readers to in-depth reviews on the thermal and mechanical properties of carbon nanotubes261–264 and details of the computational methods.265–268 This section highlights theoretical models for analyte-carbon nanotube interactions. These studies are used to understand the experimental data generated in sensing experiments or to computationally design novel sensors.
The possibility of probing the sensing mechanism of carbon nanotube-based sensors with computational models has been demonstrated by Cho and coworkers.269 In this early work, the interactions between CNT and adsorbed gas molecules was investigated using density functional theory (DFT) calculations. The carbon nanotube was represented as a carbon nanohoop with one-dimensional periodic boundary conditions along the tube axis. To investigate the adsorption-induced nanotube doping, the electronic and energy states of a tube with and without adsorbed molecules of NO2, NH3, CO, O2, and H2O were compared. Electron donation from the tube to the analyte was observed for NO2 and O2, while electron donation from analyte to the tube was observed for NH3. These findings were in agreement with published experimental results.21,270 Since this study, a large number of studies on adsorption of small gaseous molecules on CNT-systems have been reported. The binding energies determined through different computational methods range widely (e.g. for adsorption of NO2 on (10,0)-SWCNT binding energies from 0.92–18.6 kcal/mol), however the proposed sensing mechanisms are qualitatively consistent with the major sensing mechanism (e.g. adsorption of NO2 induces p-doping on the CNTs).21,38,63,269,271,272 Other sensing mechanisms that have been proposed for NO2 include chemisorption via formation of nitro- and nitrite-groups,61–63 changes in the density of states (DOS) of the CNT,270 electron localization effects,46 or changes in the dipole moment of NO2.273
The wide variety of results for the seemingly simple system of NO2 adsorbed on CNTs reveals the complexity of treating CNTs computationally. Challenges associated with their modelling include: (a) their large and conjugated π-system contains highly strained bonds which are inherently difficult to represent computationally,62 (b) the individual interaction energies between analyte and carbon nanotube are often very small and the resulting physisorbed states cannot be determined with great accuracy by DFT, and (c) the chirality of the CNT has a strong influence on its electronic properties which often complicates comparisons between computational studies or computational and experimental findings.274,275 Consequently, the choice of the computational method276 and length of the investigated CNT model277 can have large influences on the outcome of the theoretical study.
To create computationally tractable systems, studies have used periodic boundary conditions to model the quasi one-dimensional CNTs—a typical ratio of diameter to length is about 1:1000—which limits the selection of approximate exchange-correlation functionals and analysis methods. When truncated CNTs—with end states saturated with hydrogen atoms—are used, one runs into the risk of using a too small CNT size which cannot reflect the delocalized nature of the CNT electronic states accurately.277,278 It has become apparent that not only CNT length but also the shape of the “cut” determines computational outcomes of truncated CNT studies. Specifically, the theoretical description of truncated CNTs can be improved by taking the Clar sextet rule into account. The Clar valence bond theory, as postulated in 1964, describes polyaromatic hydrocarbons with both conventional two-electron π bonds and aromatic sextets (benzene). Clar demonstrated that the valence bond structure with the most aromatic sextets best models the reactivity of polyaromatic hydrocarbons and that structures possessing only aromatic sextets are especially stable (Figure 13).279 In CNTs, it has been shown that structures with the maximum amount of aromatic sextets for a given chirality and number of carbon atoms best describe the reactivity and geometry of CNTs and CNT-reactant studies.280–284 Accordingly, computational investigations employing “straight cut” CNT samples might misrepresent the reactivity of the studied model which might be the source of some inconsistencies in published results on CNT-analyte interactions. For detailed discussions on the Clar sextet rule in CNTs we refer the interested reader to key studies by Matsuo et al.,281 Baldoni et al.,278,283 and Ormsby et al.282,284
Figure 13.
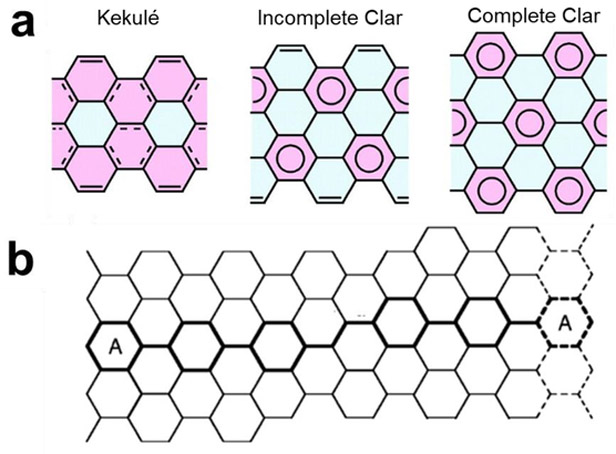
(a) Schematic representations of Kekulé, incomplete Clar, and complete Clar networks. Kekulé structure infers a 1,3,5-cyclohexatriene-type cyclic conjugate system, and the Clar structure represents a benzene structure with equivalent C-C bond lengths. Nucleus-independent chemical shift coding: red, aromatic <−4.5; blue, nonaromatic >−4.5. Reproduced with permission from Ref. 281. Copyright 2003, American Chemical Society. (b) Planar representation of a chiral (6,5) CNT. Dashed lines represent replication of hexagons when the structure is rolled up to give the nanotube. The Clar unit cell is highlighted with thick lines. For (6,5) CNTs the Clar unit cell contains five aromatic sextets and one double bond. Reproduced with permission from Ref. 285. Copyright 2009, The Royal Society of Chemistry.
Keeping the stated challenges in mind, we highlight several applications where computational studies have successfully predicted or explained experimental behavior of CNT-based chemical sensors. Intrinsic doping of nanotubes with heteroatoms can be used to increase the sensitivity and selectivity of the CNTs towards electron accepting or donating analytes. Accordingly, n-type dopants like nitrogen,286–289 phosphorous290, or sulfur291 deliver higher binding energies and stronger charge transfer between electron-accepting analytes like NO2 and the CNT. Similarly, p-type dopants like boron,286,288,292–296 and aluminum287,289,290,297,298 show strong binding with electron donating analytes like NH3, formaldehyde, cyanides or hydrogen halides.
Tubes decorated with a single metal atom or a metal cluster have been studied extensively using computational methods. A number of groups reported computational studies that probe the adsorption mechanism and reactive behavior of gaseous analytes on metal-decorated CNTs298–304 or CNTs decorated with metal nanoparticles (NPs).45,46,137,305–309 Metal-analyte combinations with higher binding energies, stronger charge transfer or stronger perturbations in the density-of-states are believed to lead to stronger signals in sensing studies. Based on these computational findings, well-informed matching between selectors and CNTs are possible.
Several studies have been conducted in which experimental sensing data is supplemented by computational studies.45,127,137,286,305,310,311 Ellis et al. fabricated CNT sensors decorated with indium oxide with sensitivity for ethanol and acetone.137 The authors noted that the sensitivity towards ethanol and acetone for materials prepared by low temperature sintering was comparable, however the sensitivity of ethanol could be improved significantly by sintering the indium oxide at 400 °C under formation of a crystalline In2O3 phase. The authors performed DFT calculations to elucidate the trends in the sensing behavior. Adsorption for both ethanol and acetone on the surface of indium oxide (In2O3)8-decorated-SWCNTs leads to comparable charge transfer to the CNT and changes in the DOS around the Fermi level. However, computational adsorption studies on the surface of crystalline In2O3 showed a different picture. Acetone is adsorbed physically on the surface; ethanol, however, undergoes dissociative adsorption via transfer of H atom to the surface with formation of an ethoxy species. The observed vibrational frequencies, from infrared spectroscopy, corresponded well with the calculated frequencies for the adsorbed ethoxy species supporting this model.
In short, computational models have proven useful to suggest or supplement sensing mechanisms and help in the design of novel CNT-based sensors. These theoretical models, however, must be chosen carefully. The CNTs can be represented using periodic boundary conditions or truncated CNTs long enough to capture the extended and delocalized nature of their electronic states. The insights gained from computational studies are certain to help elucidate sensing behavior and guide future designs of CNT sensors.
2. CNT-based Sensors for Environmental Monitoring
The promise of cost-effective, low-powered sensors has propelled the research of CNT-based sensors toward many applications, including passive environmental sensing. Detection of chemicals that are potentially harmful to human and environmental health in both gas and liquid media pose continued challenges in terms of sensor selectivity and sensitivity. Pristine CNTs have been shown to interact with many gaseous analytes and there are continuing efforts in the functionalization of CNTs to expand the analyte scope and performance. Liquid phase sensing places additional shear forces on a stationary CNT film and thereby highlights additional stability challenges relative to gas sensors. This section details sensors for environmentally relevant analytes in both gas and liquid media. The nature of the interactions between the analytes and the CNTs is particularly important and new functional synthetic selector materials integrated with CNTs are highlighted.
2.1. Gas/Vapor Sensors for Environmental Applications
Since the pioneering work by Dai and coworkers,21 many studies on CNT-based sensors for gases and vapors have been reported. The focus studies have ranged from the various detectable gases, the uses of different types of CNTs (single-walled, multi-walled, semiconducting, or metallic), functionalization methods, transduction mechanisms, and different measurable quantities. Gas sensing has been thoughtfully covered by Kauffmann and Star10 in 2008 and multiple other recent reviews.9,32,312 The purpose of this section is to focus on important analytes and how their interactions with the CNT systems can be used in sensing applications. We provide overviews of each analyte including earlier and the most recent developments. In particular, CNT sensors of interest are designed to detect ammonia (NH3), nitrogen dioxide (NO2), hydrogen (H2), methane (CH4), carbon monoxide (CO), hydrogen sulfide (H2S), and sulfur dioxide (SO2). The detection of oxygen (O2) is covered in Section 3.2 on food safety, and carbon dioxide (CO2) is discussed in Section 4.1 on breath analysis.
2.1.1. Ammonia (NH3) and Nitrogen Dioxide (NO2)
High concentrations of ammonia (NH3) threaten human health as immediate severe irritation of the nose and throat can occur with exposures of 500 ppm, and 1000 ppm vapors can cause pulmonary oedema.313 However, even at lower concentrations, ammonia can lead to irritation to the eyes, skin, and respiratory system.314 The main source of NH3 emissions comes from agricultural processes, including animal husbandry and fertilizer applications.313,315 The Occupational Safety and Health Administration (OSHA) has designated the total weight average (TWA) permissible NH3 exposure limit (PEL) over eight hours to be between 25 and 50 ppm.316 Furthermore, the Environmental Protection Agency (EPA) has warned on the toxicity of aqueous ammonia (both NH4+ and NH3) to aquatic animals and ecosystems.317 Similarly, nitrogen dioxide (NO2) has detrimental impacts on human health and the environment. NO2 in the atmosphere acts as a pollutant that causes acid rain and photochemical smog. The combustion processes from chemical plants and motor vehicles contribute significantly to the atmospheric level of both NH3 and NO2.313,315 At high concentrations, NO2 can cause irritation in the human respiratory system. Long exposures also contribute to the development of asthma and respiratory symptoms. Accordingly, the NO2 OSHA exposure limit is 5 ppm TWA over eight hours.316
The approaches in the development of CNT-based sensors for NH3 and NO2 bear heavy resemblances to each other. This section will first discuss the responsiveness of pristine CNTs and progresses to CNTs functionalized with metal and metal oxide nanoparticles (NPs), polymers, and small molecules. The adsorption of NH3 and NO2 gas onto pristine CNTs without any chemical functionalization has been shown to produce sensing responses by many groups.77,241,243–246,250,251,318–323 In accord with our earlier discussions, the performances of the sensors vary depending on the technique of fabrication and the quality of the CNTs. To avoid repetition, interested readers are referred to the discussions of chemical sensing mechanisms (Section 1.1), functionalization of CNTs (Section 1.2), and theoretical models of chemical sensing (Section 1.5).
Rigoni et al. recently demonstrated chemiresistive sensors comprising pristine SWCNTs with 20 ppb sensitivity to NH3 and a LOD of 3 ppb, Figure 14.250 Their impressive performance was attributed to the careful preparation of SWCNT layers through sonication and dielectrophoresis to minimize film thickness and to remove loosely bound agglomerates.250 Multiple groups have explored fabrication methods focusing on scalability and/or operational simplicity. For example, Huh and coworkers screen-printed SWCNTs that can detect 5 ppm of NH3.251 Mirica et al. demonstrated a solvent-free method to deposit pristine SWCNTs by mechanical abrasion of compressed powders onto the surface of various papers.241 The devices fabricated on weighing papers with low surface roughness detected NH3 over a large dynamic range (0.5–5000 ppm).241 Moreover, several studies concluded that defects along the wall of the SWCNTs enhance the response to NH3 gas.245,246 These earlier results agreed with a recent mechanistic study by Salehi-Khojin et al. that revealed a dependency of sensing response on the defect levels.324 Interestingly, Penza et al. concluded that the sensitivity of the MWCNT films to NH3 (and NO2) also depends on the catalyst used for their growth, with the Co-grown outperforming the Fe-grown MWCNTs.243 In addition, Wang et al. prepared MWCNTs using microwave plasma-enhanced chemical vapor deposition (CVD) with Ni catalyst, which detect NH3 with a linear dynamic range from 5 to 200 ppm.244 The enhanced sensing capabilities in these two cases were attributed to structural defects on the MWCNTs.
Figure 14.
Responses to NH3 (a) and calibration curve (b) of pristine SWCNT-based sensor using high-quality SWCNTs prepared through careful sonication and electrophoresis. The sensors exhibit good sensitivity to 20 ppb with a limit of detection of 3 ppb concentration of NH3. Reproduced with permission from Ref. 250. Copyright 2013, the Royal Society of Chemistry.
Analogously, multiple research groups have shown pristine individual CNTs and networks to be responsive to low NO2 concentrations. Li et al. fabricated sensors by simple casting of SWCNT dispersions onto interdigitated electrodes to produce a linear response between 6–100 ppm and a calculated LOD of 44 ppb.240 The authors noted that the recovery after each exposure could be accelerated from 10 h to 10 min by illumination of UV light.240 Cantalini et al. deposited thin films of MWCNTs by plasma enhanced CVD onto Pt electrodes that were sensitive to 10–100 ppb of NO2 in dry air.325 The sensors were operational at room temperature, but the optimal performance in terms of sensitivity and recovery time was achieved at 165°C.325 Although thermal treatment or photoirradiation is often required to recover to the original baseline after the exposure of NO2, Ammu et al. reported that inkjet-printed films of CNTs on acid-free paper display full spontaneous reversibility, Figure 15.239 These sensors were effective for NO2 detection at concentrations as low as 125 ppb in ambient air.239 The authors reasoned that full recovery upon the removal of NO2 is consistent with the initial formation of a weak charge transfer complex between NO2 and the CNTs rather than the formation of covalent bonds.239
Figure 15.
Flexible NO2 sensors fabricated from inkjet-printed films of CNTs on 100% acid-free papers. (a) Plot of relative change in resistance vs the concentration of NO2 of CNT/paper films. Inset shows plots of resistance vs. time at low concentration in ppm. (b) Plot of selectivity for a CNT/paper film showing increases in resistance for common organic vapors and decreases in resistance to NO2 and Cl2. Inset shows an optical image of the sensor printed on paper. Reproduced with permission from Ref. 239. Copyright 2012, American Chemical Society.
One common method to improve the selectivity and sensitivity of the NH3 detector is to create composites of metallic NPs and CNTs. One of the first examples were reported by Star et al., in which isolated networks of SWCNTs were decorated with different metallic NPs to create a sensor array for the detection of NH3 as well as other gases.49 Penza et al. improved the sensor performance of Fe-grown MWCNTs by sputter deposition of Au and Pt NPs.326 The authors ascribed the increase in sensitivity to the catalytic spillover effect at the surface of the NPs.326,327 Targeting wearable sensors, Lee et al. reported a flexible and transparent sensor that can detect 255 ppb NH3 using spray-deposited SWCNTs with AuNPs.328 Similarly, Ag and Co NPs were added to MWCNTs by Cui et al.329 and Nguyen et al.,330 respectively, to increase the sensor sensitivity and recovery rates. Ag NPs were added to a composite of poly(4-vinylpyridine) (P4VP) wrapped SWCNTs for enhanced NH3 detection at 20 ppm.331 Under aerobic conditions, metallic Ag NPs are oxidized to silver oxide in the SWCNT composite. These oxygen ions lead to electron depletion regions that promote the adsorption of electron-rich ammonia, leading to the additional improvement in sensitivity of the sensors.329,331
Although metal oxide gas sensors are commercially available, many require high operating temperature and thus consume large amount of power and have very limited selectivity.10 Composites of CNTs with various metal oxides have led to sensors that are operational at room temperature. For example, Hoa et al. fabricated SWCNT-tin oxide (SnO2) hybrid sensory materials by simple heat treatment and oxidation of a Sn thin film with SWCNTs produced by arc-discharge methods and created sensors with a 10 ppm LOD for NH3.332 A hybrid sensor of SnO2 prepared by sol-gel synthesis and SWCNTs was shown by Ghaddab et al. to detect 1 ppm of NH3 (and 20 ppb of O3).333 However, the authors noted that the sensitivity of the sensor depended heavily on the source of SWCNTs.333 SnO2-coated CNTs have also proved successful as NO2 sensors.113,334,335 In another example by Rigoni et al., physical mixing of SWCNTs with indium-tin oxide (ITO) NPs resulted in a threefold increase in sensitivity (compared to pristine SWCNTs) to NH3 with a limit of detection of 13 ppb.336 Interestingly, the response of the composite was p-type at low concentrations of NH3 with low humidity and switched to n-type at higher humidity levels, which was attributed to hole compensation by water.336 We speculate that hydroxide ion from NH4OH formation may be involved. Finally, CNTs have also been used as a template for layer-by-layer assembly of porous indium oxide (In2O3) nanotubes that also demonstrated room-temperature sensing capability.337 Despite the wide range of the choice of metal /metal oxide NPs, the techniques of fabrication, and architectures of the sensors, the enhancement in the sensitivity towards NH3 and NO2 was attributed to the catalytic function of the NPs and good electronic communication between CNTs and NPs.49,326,338 Although the sensitivity to reducing (NH3) and oxidizing (NO2) are enhanced by metal oxides, the analyte interactions can be largely characterized as non-specific acid-base interactions, hence other elements are likely needed to produce sensors with high selectivity.
Composites of polymers and CNTs have also been demonstrated as effective sensors as a result of strong interactions between NH3 and the selected polymers. Bekyarova et al. covalently grafted poly(m-aminobenzenesulfonic acid) (PABS), a sulfonated poly(aniline), onto SWCNTs to create chemiresistors capable of detecting 20 ppm of NH3, Figure 16.339 The authors proposed that the increase in the measured electrical resistance is the result of deprotonation of the PABS side chains, which in addition to lowering the electronic transport within the PABS, enhances the electron transfer from the PABS oligomers to the SWCNTs (Figure 16a). Specifically, the NH3-induced changes in the near-infrared (NIR) spectrum of the first semiconducting interband transition of the SWCNTs (S11) correlated well with the change in the resistance of the sensors, (Figure 16b-d).339 The sensitivity of this system of SWCNT-PABS can achieve a NH3 LOD of 100 ppb (20 ppb for NO2) by adjusting the initial resistance of the sensor.141 In addition, composites of poly(aniline) (PANI) and CNTs, prepared by electropolymerization on SWCNT electrodes340 or by in situ polymerization of aniline in SWCNT suspension,341,342 showed low limits of detection to NH3 (50 ppb Zhang et al.340) and fast recovery times. Interestingly, Li et al. reported that poly(methyl methacrylate) (PMMA) when combined with MWCNTs produced n-type gas sensing characteristics with a decrease in resistivity when exposed to NH3.343 The authors proposed that the uncommon n-type behaviors may have been related to the modification of the semiconducting properties of MWCNTs upon the pre-treatment with hydroxylamine hydrochloride.343 Lastly, Datta et al. noncovalently functionalized SWCNTs with poly(N-methyl pyrrole) (P[NMP]) that exhibited excellent linear response from 10 ppb to 1 ppm of NH3.344 When evaluating sensors that have no clear transduction designs, it likely that a major component of sensitivity enhancements are coupled to organization effects. The selectivity in many of the experimental compositions discussed here reflect the relatively high intrinsic sensitivity of the CNTs to NH3 and NO2.
Figure 16.
NH3 sensors based on films of poly(m-aminobenzenesulfonic acid)-functionalized SWCNTs (SWCNT-PABS). (a) Mechanism of the interaction between SWCNT-PABS with NH3 with the arrows indicating the charge transfer direction between SWCNTs and PABS. (b) NIR spectra after baseline correction of SWCNT-PABS recorded before (black, 1), during (blue, 2), and after (red, 3) the exposure to 20 ppm of NH3. (c) Change in the absorbance at 5320 cm–1 (S11 transition) correlates well with the change in resistance (d). Reproduced with permission from Ref. 339. Copyright 2007, American Chemical Society.
Noncovalent functionalization of SWCNTs with polymers also proved effective in detecting NO2. Qi et al. showed that a coating of polyethyleneimine (PEI) or Nafion onto SWCNTs resulted in gas sensors with selectivity for NO2 or NH3, respectively, Figure 17.345 The presence of PEI transformed the SWCNTs from p-type to n-type semiconductors; and the sensors were sensitive to 100 ppt of NO2 while being insensitive to NH3. This selectivity arises because the effect of NH3 adsorption is outcompeted by the polymeric amine. Remarkably, the authors reported that coating with Nafion, instead of PEI, allowed the sensors to detect NH3 without interference from NO2. Polymer-CNTs composites prepared by in situ polymerization also offers promising sensing results and simple fabrication. For example, An et al. polymerized uniform coatings of poly(pyrrole) (PPy) on SWCNTs.346 Sensors derived from this material, behaved as an n-type semiconductor and displayed improved sensitivity over pure SWCNTs and PPy toward NO2.346
Figure 17.

Detection of NO2 using polymer-coated SWCNTs. (a) Optical image of devices after coatings of polymer solutions. (b) SEM micrograph of SWCNTs bridging the electrodes. (c) Relative change in conductance of a polyethyleneimine-(PEI) coated SWCNT sensor when exposed to various concentration of NO2. The recovery of the sensors was facilitated by UV illumination. (d) Comparison between Nafion- and PEI-coated SWCNTs. Nafion-coated devices (top, red) responded to NH3, but not to NO2; while, PEI-coated devices only responded to NO2. Reproduced with permission from Ref. 345. Copyright 2003, American Chemical Society.
Another method of imparting selectivity toward ammonia and other amines is through functionalization with organometallics, in particular those with extended π-systems capable of strong π-stacking interactions (as discussed in Section 3.2.1). Liang et al. reported metal-phthalocyanine/MWCNT containing chemiresistive devices with high sensitivity towards NH3 for Co- and Zn-containing metal complexes and smaller interactions with other metal centers (Cu, Pb, Pd, and Ni).347 The trend in the calculated values of the binding energy between the metal centers and NH3 from DFT agreed qualitatively with the enhancement in sensitivity.347 Kaya et al. developed Cu and Co metallophthalocyanines for enhanced π-interactions with one pyrene and six polyoxyethylene substituents.348 The authors observed higher responses to NH3 for samples containing Cu than Co. The discrepancies with earlier reports regarding the sensitivity with Cu metal centers were attributed to the substituents in the macrocycle, the number of active sites, and the thickness and morphology of the thin films.348 In a study that involved all of the first row metals, Liu et al. developed chemiresistive sensors containing porphyrin complexes for the detection of food spoilage (Section 3.2 on food safety).93 In these devices, the interaction between the metal centers and the analyte as well as the redox potential of the complex were highlighted as essential to determine the selectivity and sensitivity.93,347,348
2.1.2. Hydrogen (H2) and Methane (CH4)
Hydrogen (H2) and methane (CH4) pose a threat as they can explode even at low concentrations in air.349 Additionally, methane is a potent greenhouse gas and it is important to detect sources of its release into the atmosphere. Thus, the detection of H2 and CH4 becomes paramount to prevent explosions for distribution systems/centers, mines, and petroleum fractional distillation plants. Similarly, an expanded natural gas production will require the detection of methane leakage to prevent environmental pollution and loss of revenue. The promising potential of H2 as a clean energy carrier has propelled research efforts in areas such as fuel cells, automobile engines, and various chemical and industrial processing.34,349 Such developments also place the need for hydrogen sensors in household environments and mobile transportation systems.349 Hydrogen gas and methane have no appreciable interactions with pristine CNTs and research efforts primarily focus on exploiting the composite of CNTs and metallic NPs. While theoretical350 and experimental49,351 reports have shown Pt-decorated CNTs to be sensitive to H2, the majority of CNT-based H2 sensors in literature reported focused on the use of Pd metal.352 These sensors can be grouped into two main categories depending on whether Pd metal is deposited on the CNTs (Pd-decorated) or used as the electrodes (Schottky contact-based).
Kong et al. reported one of the first examples of Pd-decorated SWCNTs that functioned as H2 sensor.110 In their report, a thin coating of Pd was deposited by electron beam evaporation on an individual SWCNT; and upon the exposure to ppm levels of H2, the measured conductance decreased.110 Pd undergoes a reversible reaction with hydrogen to produce a low electron affinity palladium hydride, which promotes electron donation to compensate hole carriers in SWCNTs and leads to higher resistance. A number of subsequent publications endeavored to improve the ease of fabrication and the performance of this type of sensor.49,111,353–361 For examples, Sayago et al. fabricated sensors by spray-coating of SWCNTs and then functionalizing with Pd NPs,354,355 and Sun and Wang created Pd-SWCNTs sensors on flexible substrates.111,358 In addition, Sippel-Oakley et al. observed that thermally evaporated Pd exhibited faster responses and recoveries than sensors prepared by sputter-coating Pd on SWCNT thin films, suggesting that sputtering damages the π-sidewalls.356 Similar to the detection of NH3 and NO2, differences in performance of H2 sensors may depend directly or indirectly on CNT sidewall defects. Khalap et al. compared the sensing responses between sensors comprising individual Pd-decorated SWCNTs with and without sidewall defects.359 Their results demonstrated that a positive relationship between the defect sites and Pd NPs led to the thousand-fold increases in hydrogen response (resistance), and only two-fold increases were observed in defect-free samples. More recently, Penner and coworkers reported the effects of the size of Pd NPs electrodeposited on CNT ropes, Figure 18.362 From their results, modest changes in the diameter of the Pd NPs greatly affected the amplitude and the time of the responses.
Figure 18.
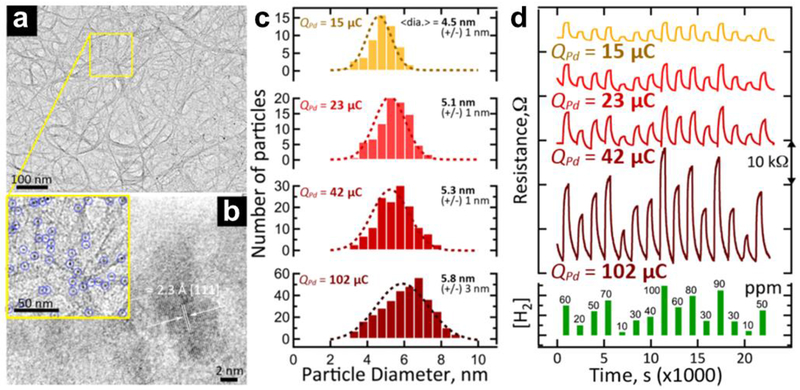
Palladium NP-decorated CNT ropes as H2 chemiresistors. (a) TEM image of a CNT rope after deposition of Pd NPs. (b) (Inset) Pd NPs are highlighted by blue circles and a high resolution TEM image of a NP is shown in the main picture. (c) Histogram of the size of the Pd NPs for electrodepositions of various quantities of Pd in terms of the Coulombic loading (QPd). (d) Sensing responses to ppm levels of H2 for the different Coulombic loading of Pd nanoparticles. Reproduced with permission from Ref. 362. Copyright 2017, American Chemical Society.
Schottky contact-based H2 sensors rely on the modulation of the contact electronic barrier height between the electrons and CNTs upon the exposure to hydrogen gas. The work function of Pd is sensitive to H2 and hydride formation reduces the work function. Javey et al. demonstrated this mechanism in a Pd-contacted SWCNTs field-effect transistor.363 The contacts between metallic Pd and semiconducting SWCNTs exhibited ohmic behavior before H2 and Schottky diode behavior after H2.363 Their results suggested that the lower work function of Pd led to the formation of higher Schottky barriers, and electron tunneling through these structures decreases exponentially with length for dramatic reductions in current. To exploit this phenomenon for H2 sensing, Wong et al. fabricated a microelectronic diode with the structure of the thin layer of Pd deposited on top of MWCNTs on doped silicon and detailed response with 100% H2.191 Myung and coworkers later improved on this configuration to achieve sensors with high sensitivity, large dynamic range (25 to 2000 ppm), and fast response/recovery times, Figure 19.84 The authors aligned SWCNTs across prefabricated Pd microelectrodes by AC dielectrophoresis and stressed the importance of using semiconducting SWCNTs.84 Carriers are more likely to flow through metallic SWCNTs, which do not produce Schottky barriers at the electrode interfaces, leading to the attenuation of the signal and sensitivity. Choi et al. demonstrated an alternative solution by using pre-separated semiconducting SWCNTs to fabricate networks between Pd electrodes.364 The results highlighted the importance of minimizing the amount of metallic CNTs and optimizing the density of the SWCNT networks for the performance of the sensors.
Figure 19.
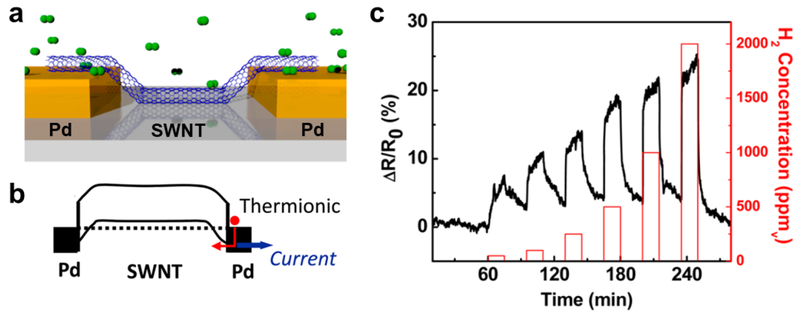
Schottky contact-based H2 gas sensor. (a) Schmatic representation of the sensor comprising aligned SWCNTs bridged across the Pd microelectrode. (b) Band diagram of the sensor when exposed to H2, which decreased the work function of Pd and created a Schottky barrier. (c) Sensing response to various concentrations of H2. Reproduced with permission from Ref. 84. Copyright 2014, American Chemical Society.
CNT-based sensors for methane are not as common as H2 sensors, as a result of the difficulty of obtaining selectivity towards CH4. For metal-decorated SWCNTs, Star et al. reported that CH4 showed no significant response at room temperature.49 In an earlier example, Lu et al. reported the fabrication of CH4 sensors comprising Pd-decorated SWCNTs that showed increases in conductance when exposed to 6–100 ppm of methane.365 Subsequent exposure to UV light facilitated the desorption of the gas molecules and recovered the sensing baseline.365 More recently, Paprotny and coworkers demonstrated CH4 sensors using MWCNTs functionalized with metal oxide NPs (either SnO2 or ZnO) through atomic layer deposition (ALD).366,367 Both chemiresistors functioned at room temperature and were sensitive to ppm levels of methane in dry air.366,367 The proposed mechanism involved the adsorption of CH4 molecules on the metal oxide NPs, leading to the increase in the sensor’s relative resistance. However, cross reactivity to other gases will remain a challenge for CH4 sensors based on metal-decorated CNTs. In cases where reactivity of the constituent metal oxides or NPs have not been established under the reported conditions in the absence of the CNTs, it is proper to be suspect of results, which could be coupled to changes in the gas (oxygen levels) or humidity rather than methane. Hence, we consider the development of chemiresistive CH4 sensors to be at an early stage and encourage the readers to consider new approaches to detect this challenging analyte.
2.1.3. Carbon Monoxide (CO)
Carbon monoxide (CO) is the colorless, tasteless, and odorless toxic gas that is responsible for the majority of fatal poisoning worldwide.368 CO is produced from the incomplete combustion of carbon fuels, and emissions from vehicles and industrial processes are major sources. OSHA has designated the PEL of 35 ppm time weighted average (TWA over 8 hours) and 200 ppm ceiling (5 min exposure).369 The higher affinity of CO over O2 towards the iron porphyrin complexes found in hemoglobin and myoglobin leads to CO poisoning.370,371 Although detectors for CO are available with metal oxide semiconductors (MOS) and solid electrolyte (SE),10 CNT-based sensors could fill the need for massively distributed or even wearable sensors to prevent poisoning in domestic and industrial environments.372
Although CO does not engage in strong binding or charge transfer interactions with pristine CNTs,127,248,270,311 successful examples of sensors comprising pristine CNTs exist in the literature. Varghese et al. demonstrated the capacitive sensing of CO with pristine CNTs, albeit only at concentrations of 100%.203 Pristine CNTs have also been used in the resonance frequency-based detection of CO; in these sensors the adsorption of CO was detected as a change in the resonance frequency of the active layer.215,216 Increased interaction between the CNTs and CO have been observed theoretically for deformed,373 boron-doped, nitrogen-doped CNTs,374 and aluminum-doped CNTs.375 Sensors capable of ~1 ppm detection have been demonstrated using CNTs with carboxylic acid (C(CNT)-COOH) sidewall defects248,376 and sulfonated SWCNTs,377 which are assumed to have covalent C(CNT)-SO3H or C(CNT)-OSO3H groups. In an alternative chemical modification, Zhao et al. used oxygen plasma to create disordered structures and eliminate metallic CNTs, to produce a CO sensor capable of detecting 5 ppm of CO.249 The authors, however, noted that the presence of water molecules led to complications suggesting that the oxygenated defects can give rise to unwanted side reactivity.249
CNTs functionalized with NPs of metals and metal-oxides have been reported as effective CO sensors. Changes in the transfer of electron density from the metal NPs to the CNTs upon the adsorption of CO molecules is reported to give rise to the sensing response.378 Star et al. reported the sensing properties of 18 chemiresistive SWCNT-channels functionalized with different metals.49 Particularly, the Pt-decorated SWCNTs showed sensitivity towards 2500 ppm of CO.49 SWCNTs modified with Au,378,379 Pd380 (ab initio study), and tin oxide (SnO2)381,382 were also reported as room temperature CO detectors. In addition, composites of polymers and CNTs have been shown by several groups to detect CO molecules. For examples, Wanna et al.383 showed reproducible responses to CO exposure from 100–500 ppm with a composite of CNTs and maleic acid-doped PANI, while Lin et al.384 fabricated a CO (and NH3) sensor using a composite of Co3O4/PEI-CNTs. In the metal free PANI modified material, the basis of sensitivity to CO is not clear.
The translation of known reactivity or bonding of analytes to sensors optimally leverages chemical knowledge. In this context, recently efforts towards noncovalent modification of CNTs with organometallic complexes have proved promising. The composites of these two materials improve the selectivity of the CNTs and overcome the inherently low conductivity of the organometallic compounds. Liu et al. employed a metal complex with a well-defined, experimentally supported molecular sensing mechanism.311 The organocobalt complex contains a pendant amino ligand internally bound to the central metal atom, which can be trapped in the open form with a free amine upon binding of CO. The large geometricaldifferences between the cobalt complexes requires a solution phase to display an efficient equilibrium. Hence in this case a network of unfunctionalized SWCNTs is coated with an inert fluorocarbon oil containing the selector complex. The CO favors the free amine form of the complex, which subsequently engages in charge transfer (electron donation) to p-doped SWCNTs and produces decrease in conductance, Figure 20a-b.311 The sensor was shown to be stable in air and with near perfect selectivity to CO over CO2, C2H2, C2H4, and H2. Biological based recognition mechanisms also provide important opportunities and heme-inspired materials have been reported both experimentally127,385 and computationally181,386 to detect CO. He et al. computationally investigated the electronic transport properties of a system comprising two SWCNTs covalently linked via an iron(II) porphyrin when exposed to CO.181,386 They found that the binding of the CO molecule to the Fe center of the porphyrin hinders the electron transport between the two SWCNTs.181,386 Savagatrup et al.127 demonstrated the use of the inactive iron(III) porphyrin anchored to aniline-functionalized SWCNTs that was reduced in situ to active iron(II) structure via the application of a moderate gate voltage. Upon the fractional in situ reduction to the nominally air sensitive iron(II) state, sensing responses to CO increased significantly, Figure 20c-d.127 This method is consistent with solution studies, wherein iron(III) porphyrins do not interact with CO, but the iron(II) porphyrins bind CO strongly.
Figure 20.
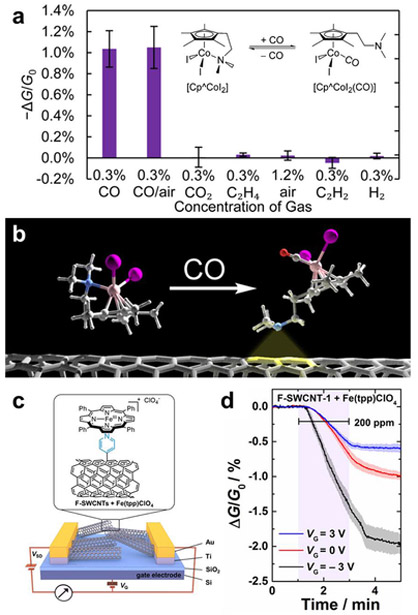
SWCNT-based CO sensors. (a, b) Sensing scheme of binding event of CO by cobalt complex showing the freeing of amine ligand upon binding of CO. The averaged change in conductance of cobalt complex/SWCNT chemiresistor in response to several gases and gas mixtures. Reproduced with permission from Ref. 311. Copyright 2016, American Chemical Society. (c) Schematic depiction of CO sensor comprising aniline-functionalized SWCNTs and iron porphyrin (Fe(tpp)ClO4). The covalent pyridyl ligands facilitate the in situ reduction of iron porphyrin to modulate the sensitivity of the sensor. (d) Sensing responses of iron porphyrin/aniline-functionalized SWCNTs towards 200 ppm CO with applied gat voltage of −3, 0, and +3V. Reproduced with permission from Ref. 127. Copyright 2017, Wiley-VCH Verlag GmbH & Co. KGaA.
2.1.4. Hydrogen Sulfide (H2S) and Sulfur Dioxide (SO2)
Both hydrogen sulfide (H2S) and sulfur dioxide (SO2) are common waste gases and atmospheric pollutants. H2S is a colorless, foul smelling, and poisonous gas that is generated/released in large amounts from coal, natural gas, and petroleum refineries as well as bacterial decomposition of animal and human waste.387 H2S acts as neurotoxin at low concentration and causes severe eye, nose, and throat irritation at moderate concentration.388 Prolonged exposure to levels above 100 ppm can cause death and individuals can become desensitized to the smell of H2S, thereby compromising their ability to detect this danger.388 SO2 is highly corrosive and readily oxidized in air to create sulfuric acid. Both gases pose significant threats to the environment and human health.389 The 8-hour TWA PELs are set at 10 ppm for H2S and 5 ppm for SO2.316 Thus, accurate measurement of the two sulfur-containing vapors are of crucial interest.
Although most reports of CNT-based detection of H2S rely on NPs-decorated CNTs, there are examples suggesting that pristine and defective CNTs can provide responses. For example, ink-jet printed SWCNTs have been used to create both chemiresistive390 and chemFET sensors.391 In addition, plasma treatment of MWCNTs to introduce carboxyl and nitrogen-containing groups has been shown to increase the selectivity towards H2S over SO2.392 Such sensors are likely to be less selective, but could find utility in applications wherein other interferants are not present.
CNTs decorated with metal or metal oxide NPs have proved to be most promising for the detection of H2S. Guided by DFT calculations, Zhang et al. suggested the potential of Au- and Pt-decorated CNTs for sensing of both H2S and SO2.299,393 In these system, SO2 accepts electrons from the metal-SWCNT networks while the opposite occurs with H2S.299,393 Experimentally, several examples of composites of Au nanostructures and SWCNTs have been demonstrated.112,327,394,395 Deshusses and coworkers decorated SWCNTs with Au NPs using a electrodeposition method and reported a 3 ppb H2S LOD at room temperature.112 The sensing mechanism was reported as H2S modulation of the electron exchange between the Au NPs and the defects on the SWCNTs. Similarly, Ding et al. reported a synthesis of gold nanowires (Au NWs) using SWCNTs as templates, Figure 21.394 Citrate-stabilized Au NPs were self-assembled on 1-pyrenesulfonic acid (PSA)-decorated SWCNTs template and fused to create Au NWs after heating. The hybrid network of Au NW-SWCNTs showed improved sensitivity to H2S when compared to pristine SWCNTs and Au NP-SWCNTs and can detect ppb concentrations of H2S without cross-sensitivity to CH4, CO2 and O2.394
Figure 21.

Hydrogen sulfide (H2S) detection using gold nanowires-SWCNTs hybrid network (Au NW-SWCNTs). (a) 1-pyrenesulfonic acid (PSA)-functionalized SWCNTs suspended in aqueous solution were used as the template for citrate reduction of HAuCl4 to generate AuNPs; subsequent heating produced Au NWs. (b) TEM images of Au NPs and (c) Au NWs on SWCNTs. (d) The relative change in conductivity of Au NW-SWCNTs network to 5 min exposures of various concentration of H2S gas. (e) Detection of H2S in methane (CH4); inset shows the sensor response to 100% CH4 and 10 ppm H2S in methane. Reproduced with permission from Ref. 394. Copyright 2017, American Chemical Society.
A major challenge of sensors composed of Au-SWCNTs (and Ag-SWCNTs396) is the strong affinity of metallic NPs to sulfur containing molecules, thereby leading to irreversible responses. Thus, most H2S sensors rely on mild heating in ambient condition for recovery.112,394 Similarly, UV irradiation can accelerate desorption of H2S in sensors containing organometallics.397 Several groups have made efforts towards the development of H2S sensors with fast self-recovery by replacing gold with other metal or metal oxide NPs. For example, SnO2 has proved to be an attractive alternative.398–400 The combination of SnO2 and CNTs allows for room temperature operations, which is unobtainable for system with SnO2 alone.113 Mendoza et al. reported a sensor based on SnO2-CNT composite films fabricated by CVD with recovery times of one minute at room temperature.398 In addition, Asad et al. demonstrated that copper and copper oxide NPs enhance the sensitivity and selectivity of SWCNTs for H2S, Figure 22.401,402 The authors fabricated flexible chemiresistive sensors and RFID-based wireless sensors that detect ppm levels of H2S at room temperature with rapid self-recovery.401,402
Figure 22.
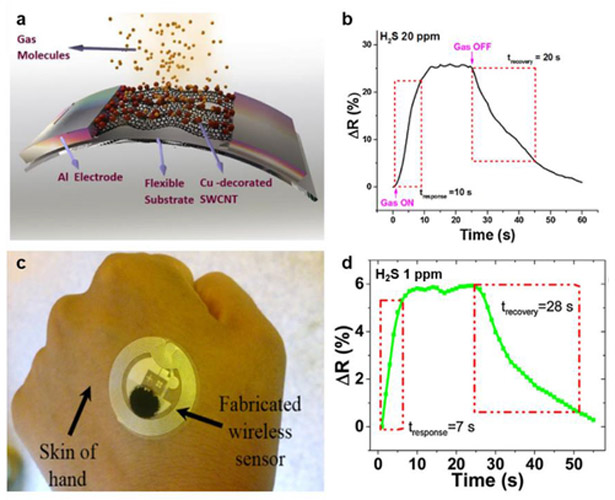
H2S sensors based on SWCNTs decorated with Cu or CuO NPs. (a) Schematic of flexible sensors comprising Cu-decorated SWCNTs as the conduction channel between Al electrodes on polyethylene terephthalate (PET) substrates. (b) Response to the exposure of 20 ppm of H2S. Reproduced with permission from Ref. 401. Copyright 2015, Elsevier. (c) Wireless sensor fabricated using a commercial 13.56 MHz RFID tag with CuO-SWCNT as the sensing material. (d) Response and recovery times of the sensor when exposed to 1 ppm of H2S. Reproduced with permission from Ref 402. Copyright 2016, Elsevier.
Examples of CNT-based SO2 sensors are limited, however non-functionalized SWCNTs and MWCNTs have been shown to respond to SO2 experimentally.403,404 In addition, Li et al. predicted computationally that Ni-doped CNTs should be more sensitive to SO2 than pristine CNTs.405 Goldoni et al. used spectroscopic analysis to reveal that interactions between SO2 and SWCNTs are similar to those between NO2 and SWCNTs.323 Correspondingly, poor selectivity between SO2 and NO2 limits further development of commercialized sensors. Interestingly, Yao et al. achieved selective discrimination between the two gases using chemiresistive random networks of SWCNTs.389 The authors reported a differentiation of SO2 and NO2 at various humidity levels. At high humidity level (92%), the resistance of the device decreased in response to NO2, but the resistance increased with SO2.389 Their experimental results were consistent with DFT calculations that indicated electron donation should occur between the SO2/H2O complex and the p-type CNTs.389 Complicating the development of sensors for SO2 and NO2 is the fact that both species are sufficiently reactive that they chemically evolve in ambient atmospheres. Hence, the actual responses from measurements under realistic conditions with moisture and humidity may arise from a daughter product of these gases.
2.1.5. Benzene, Toluene, and Xylene (BTX)
Non-polar compounds and aromatic hydrocarbons—namely benzene, toluene, (ethylbenzene), and xylene (BTX or BTEX)—are considered highly hazardous volatile pollutants. Benzene, in particular, is classified as both an acute and chronic health hazard.406–408 The PEL concentrations given by OSHA are 1 ppm, 200 ppm, and 100 ppm for benzene, toluene, and xylene, respectively.409 These compounds are present in the vicinity of natural gas and petroleum deposits and are produced from incomplete combustions of fossil fuels and automobile exhausts. In addition, BTX are used widely as components for petrochemical products, gasoline thinner, and solvents for chemical reactions. BTX in consumer products have also been reported.408 In addition to gas chromatography (GC) coupled with mass spectrometer (MS) or flame ionization detection (FID), several methods for the detection of BTX have been reported including the use of quartz crystal microbalance (QCM)-based sensors410,411 and spectroscopy.412 CNT-based devices have also been used as selective sensors92,97,413 or as part of an array.117,414
For examples, Rushi et al. reported ChemFET devices comprising SWCNTs functionalized noncovalently with iron tetraphenyl porphyrin (FeTPP) or cobalt tetraphenyl porphyrin (CoTPP) that responded to BTX at concentrations well below those given by the PEL.413 The authors found that FeTPP functionalized devices performed significantly better than the CoTPP counterparts, suggesting that the choice of the central metal ion is important for device optimization. An alternative method using chemiresistors integrated with polymer concentrator was reported by Im et al., Figure 23.92 The authors synthesized cellulose acetates functionalized with 2,3,4,5,6-pentafluorophenylacetyl (F5Ph) and deposited the concentrating layer on top of SWCNTs. The integrated sensors performed significantly better than devices with pristine SWCNTs at detecting BTX; however, interference from ethanol, commonly found in petrochemicals, was reported. While the sensitivity and recovery are promising in both examples, selectivity among BTX or from interfering compounds remains challenging. Distinguishing among BTX or among the different isomers of xylene often requires sensing arrays.97,117,414 Further discussions on CNT-based sensing arrays in the context of sensors for BTX and other volatile organic compounds (VOCs) can be found in section 4.1.1.
Figure 23:
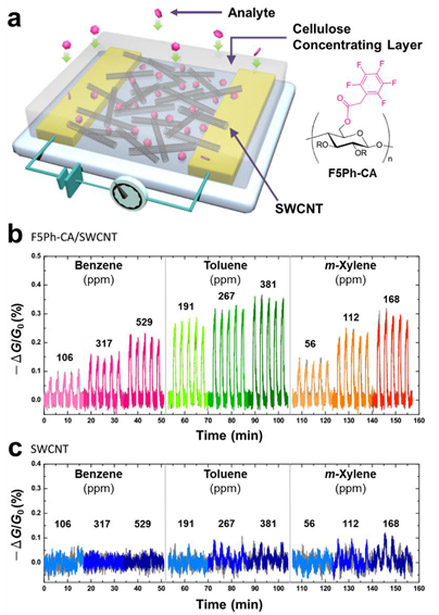
BTX gas sensing system of SWCNTs and cellulose polymer concentrator. (a) Schematic diagram of chemiresistor with SWCNTs and cellulose concentrating layer. (b) Change in conductance of the sensors in response to BTX gases at various concentration. (c) Responses of controlled devices without the cellulose concentrating layer. Reproduced with permission from Ref.92. Copyright 2016, MDPI.
2.2. Aqueous Environmental Sensing
Detecting trace analytes in the liquid phase is important for environmental monitoring, assessment of water quality, and health diagnostics.51,415 The large surface area and excellent chemical stability of CNTs make them promising aqueous chemical sensors.51 In this section, we will detail CNT-based sensors developed for aqueous environmental monitoring. Discussions on the following specialized aqueous sensors will be addressed in the later sections: detection of pesticides (Section 3.2.3), health diagnostics and bio-related applications (Section 4.2), and detection of warfare agents (Section 5).
2.2.1. CNT-based pH Sensors
Fluctuations in pH can have significant effects on chemical processes, thus measuring and controlling pH are important in environmental, industrial, and biological applications. Monitoring the pH values in real-time can provide high temporal resolution and requires sensitive and stable sensors. CNTs-based pH sensors provide a simple alternative to conventional sensors that could be costly, large, and incompatible with integrated circuits.416 Specifically, CNT-based chemiresistors relinquish the need for a reference electrode, glass membrane, and a significant power supply.
Several groups have reported that non-functionalized CNTs respond to changes in the pH of aqueous solutions. Takeda et al. fabricated FET devices using acid-treated SWCNTs that showed a reliable response and stability in liquid environments.417 Similarly, Li et al. introduced carboxyl groups on the surface and termini of the SWCNTs before alignment on microelectrodes using dielectrophoresis.416 The resulting sensors demonstrated changes in the resistance over the pH range of 5–9.416 The sensors were stable in buffer solution over the 10-day experimental period.416 Despite these successful examples, concerns regarding the selectivity persist. Without functionalization, it is likely CNTs will have limited selectivity between hydronium ions and metal cations.
Similar to the gas phase sensors, composites of conductive polymers and CNTs proved to be an attractive platform for pH sensing. The electrical properties of poly(aniline) (PANI) depend significantly on the degree of protonation, leading to rapid response to changes in solution pH. Liao et al. demonstrated that PANI/SWCNTs composite possessed tunable conductivity overa wide pH range in water.418 In addition, Roth and coworkers reported a simple process for the fabrication of PANI/CNTs and PPy/CNTs on transparent and flexible electrodes.207,419 Although conductive polymers provide the necessary selectivity, long-term stability is potentially problematic. To improve the stability of the sensors, Gou et al. functionalized oxidized SWCNTs by electropolymerization of poly(1-amino anthracene) (PAA), Figure 24, which was designed to have stronger interactions as a result of the polymer’s extended π-system.206 PAA/SWCNT displayed high electro-conductivity and thermal stability compared to the other systems (PPy/SWCNT and PANI/SWCNTs) and retained sensitivity to pH 2–12 over 120 days.206 The authors also demonstrated selectivity to hydronium ion over potential interferants (Ca2+ and Na+).206
Figure 24.

Chemiresistive pH sensor based on oxidized SWCNTs functionalized with conductive polymer poly(1-amino anthracene) (PAA). (a) Electropolymerization of PAA from 1-amino anthracene onto oxidized SWCNTs. (b) Sensing responses of the pH sensors to various pH solutions. The vertical bars represent the pH values. (c) Calibration curve of conductivity vs. pH values. Reproduced with permission from Ref 206. Copyright 2015, Nature Publishing Group.
2.2.2. CNT-based Metal Ion Sensing
Toxic metal ions in water represent serious personal and environmental health concerns. Some species—such as iron, cobalt, zinc, copper, and manganese—are required for metabolic and signaling processes in living organisms at low concentrations but can be toxic at higher concentrations.420 Other metals/elements (cadmium, lead, arsenic, chromium, and mercury) are highly toxic even at trace-levels.420 The World Health Organization (WHO) and the US Environmental Protection Agency (EPA) issued the permissible exposure limit of these toxic metal ions in water. For example, the limits for iron, zinc, and copper are on the order of 1–10 ppm; while the limits are more stringent for cadmium, lead, arsenic, chromium, and mercury at the range of 1–10 ppb.420,421 The mechanisms of the toxicity of each species on the living cells are beyond the scope of this review; interested parties will benefit from reading recent reviews on the topic.420 For the detection of toxic metal ions, CNTs are most often used as an electrode material because of their large surface area, small size, ease of functionalization, and excellent electrical conductivity. In addition, CNTs have been shown to be an excellent sorbent for metal ions.422 Considering the large amount of work reported in this field, we can only present representative examples. We direct interested readers to specialized reviews.211,420,422,423
Electrochemical sensors using CNT-covered electrodes proved promising for the detection of multiple toxic metal ions. The main technique is anodic stripping voltammetry (ASV), which involves the electrochemical deposition of metals (analyte) at a constant potential and subsequent stripping of the deposited analyte from the electrodes. While the inherent selectivity towards toxic metals arises from a specific redox potential, the addition of molecular recognition by functionalization is often needed to selectively accumulate the analyte at the electrode interface. For example, Wanekaya and coworkers demonstrated the trace detection of Pb2+ and Cu2+ using cysteine-modified MWCNTs with a LOD of 1 and 15 ppb, respectively.424 Cysteine, an amino acid with high affinity towards the target metals, was covalently functionalized onto MWCNTs which were casted onto the electrode surface for ASV analysis.424 The authors reported no interference for the Cu2+ peak from common anions (Cl–, SO42−, or CO32−) and cations (Cd2+ and Ni2+); however, excessive Cu2+, Cd2+, and Ni2+ reduced the peak current associated with Pb2+.424 Calixarene-functionalized CNTs have proved promising for molecular recognition.425 To selectively detect Pb2+, Wang et al. covalently attached thiacalixarene onto MWCNTs, Figure 25.212 Due to the highly selective recognition of thiacalixarene to Pb2+, the authors reported that the sensing of Pb2+ was not affected by 100-fold excess amounts of Zn2+, Ni2+, or Cd2+.212
Figure 25.
Determination of Pb2+ using thiacalixarene-functionalized MWCNTs for anodic stripping voltammetry (ASV). (a) Schematic representation of the fabrication thiacalixarene/MWCNTs functionalized glassy carbon electrode (GCE) and the detection of Pb2+. (b) Response signals after 15 min accumulation of 10–7 M Pb2+ with 100-fold excess amounts of Zn2+, Cd2+, and Ni2+. Reproduced with permission from Ref. 212,425. Copyright 2016, the Royal Society of Chemistry. Copyright 2012, American Chemical Society.
In addition to electrochemical analysis, chemiresistive and FET-based sensors have been used for the detection of toxic metals. FET sensors provide the advantage of real-time detection without the accumulation step necessary for ASV detection. Kim et al. reported the detection of mercury ions using pristine SWCNTs, Figure 26.426 The selectivity towards Hg2+ over other metal cations was attributed to the favorable and irreversible reduction from Hg2+ to Hg0 by SWCNTs at less negative reduction potentials than needed for other ions.426 Star and coworkers have also reported the detection of Co2+ using a combined optical spectroscopic and chemiresistive measurement on SWCNTs noncovalently functionalized with polyazomethine (PAM).427 The large conformational change of PAM upon complexation with Co2+ gave rise to the selectivity of the sensors.427 In another example, Forzani et al. introduced coatings of polymers functionalized with different peptide sequences and lengths in SWCNT-based FET sensors.428 The authors demonstrated a selective platform by leveraging the well-known selectivity of His6 for Ni2+ and Gly-Gly-His for Cu2+ ions.426
Figure 26.
SWCNT-FET sensor for the detection of Hg2+. (a) Schematic of the proposed mechanism for the selectivity toward Hg2+. (b) Real-time measurement of the change in current after exposures to various concentration of Hg2+. (c) Sensitivity vs. concentration of various metal cations. Reproduced with permission from Ref. 426. Copyright 2009, American Chemical Society.
3. CNT-based Sensors for Food and Agriculture Applications
Food (along with water) is the most important consumable. Several processes in food management and agriculture production can be monitored and improved through chemical sensing. Specifically, these processes include monitoring of food ripeness, prevention of food spoilage, ensuring the quality of food storage, and detection of pesticides. Worldwide, a third of the food produced for human consumption—approximately 1.3 billion tons—are wasted or lost, due partially to the lack of accurate and real-time measurements of the condition of perishable goods along the supply chain, Figure 27.429,430 Economically viable sensing systems can inform both the suppliers and consumers, improve coordination of harvest and transportation, monitor storage in both industrial and consumer settings, and identify pesticide-contaminated food.
Figure 27.

Distribution of food losses and waste along the supply chain by region and by stage (consumption, distribution, processing/packaging, post-harvest, and harvest). Reproduced with permission from Ref. 429. Copyright 2017, Food and Agriculture Organization of the United Nations.
CNT-based sensors offer several advantages that are required in food applications. Their small size, low power-consumption, and simplicity combined with their capability to detect complex analytes allows the production of economically viable sensors that are ideal for product tracking and supply chain management. For example, CNT-based sensors have been incorporated into circuitry that allows for real-time information of the state of food using smartphones or other connected devices for use in smart packaging,189 detection of fruit ripening,96 food spoilage,93 and pesticide detection.431 Apart from improving food safety and minimizing food loss, CNT-based gas sensors can be used for characterizing the flavor (taste) and odor (smell) of products to authenticate and control the quality of food products.
3.1. Food Quality
3.1.1. Fruit Ripeness
Climacteric fruits (e.g., apples and bananas) produce the fruit-ripening hormone ethylene at the onset of ripening. Ethylene is one of the smallest molecules with biological activity and this volatile, signaling molecule controls changes in color, aroma, texture, and flavor of fruit, regardless of whether it is produced naturally in the fruit or applied artificially to induce ripening.432,433 Thus, the processes of ripening and senescing of these fruits can be controlled through the concentration of ethylene in the atmosphere.434 Monitoring/managing ethylene concentration is useful for both the determination of the optimal harvesting time and preservation of freshness in storage and transportation.
The detection of ethylene using CNT-based sensors was investigated both experimentally96,435,436 and computationally.437 Pristine CNTs show no sensitivity towards ethylene gas at room temperature96 and the addition of selector moieties is required. To this end, Leghrib et al. reported the detection of ethylene at room temperature using MWCNTs decorated with tin oxide (SnO2) nanoparticles.435 Although the chemiresistors responded to as low as 3 ppm of ethylene, interference from NO2 was reported. Esser et al.96 developed an ethylene selective sensor by coordinating a fluorinated tris(pyrazolyl)borate copper (I) complex to SWCNTs that was capable of forming air stable complexes with ethylene, Figure 28a. The authors demonstrated the sensor’s selectivity and utility by conducting studies on a range of fruit samples. The climacteric fruits (banana, avocado, apple, and pear) showed a decrease of ethylene production over time after they reached peak ripeness, while the non-climacteric fruit (orange) showed an overall low production of ethylene, Figure 28b. Interestingly, the detector could differentiate between an apple stored at room temperature (“Apple 1”) and an apple stored in the refrigerator (“Apple 2”), demonstrating the ability to monitor the state of the fruit simply by sampling the headspace. Additionally, Zhang et al. used the composite of MOF-199 containing copper metal centers and MWCNT to adsorb ethylene from the headspace of durian husk, wampee, blueberry, and grapes for gas chromatography. This method showed an exceptionally low LOD of 13 ppb .436
Figure 28.
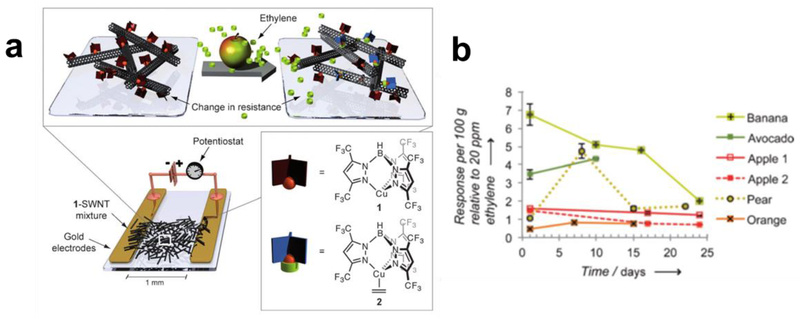
Detection of ethylene in real fruit samples. (a) Chemiresistive sensor using SWCNTs and a fluorinated tris(pyrazolyl)borate copper (I) complex, 1, that can be bound directly to SWCNTs to detect ethylene gas. This complex is known to bind ethylene as shown, 2. (b) Ethylene response of SWCNT-based sensor to banana, avocado, apple, pear, and orange samples over 25 days. Reproduced with permission from Ref. 96. Copyright 2014, Wiley-VCH Verlag GmbH & Co. KGaA.
3.1.2. Taste and Smell
In addition to monitoring fruit ripeness, CNT-sensors are also used to effectively determine the taste and smell of various food. Sensors for this application are often referred to as electronic tongues and noses (e-tongues and e-noses). We refer interested readers to reviews of metal oxide- and polymer-based electronic noses,438 bioelectronic noses,439–442 electronic noses for food443,444 and medical applications,445 and the computational analysis of electronic noses.446 The goal is to imitate the senses of taste and smell through a sensor readout. Humans have around 400 different olfactory receptors that collaboratively give rise to the sense of smell.447 Once in your olefactory system, the binding of an odorant (a molecule with a smell) to a receptor triggers a neural signal.448 To imitate such processes in sensors, researchers have incorporated olfactory receptors in CNT-based sensors to discriminate between different odorants differing by a single carbon center.
CNT-sensors have been developed using olfactory receptors from humans,415,449–458 rodents,459–462 insects,463 and canines464. Park and coworkers first demonstrated the use of human olfactory proteins for the detection of odorants in 2009.451,458 In early generations of these devices, lipid membranes containing human olfactory receptors were coated on SWCNT networks.451 Upon binding of the odorant, the olfactory receptor shifts from the inactive, neutral state to the active, negatively charged state, which reduces the mobile charge-carrier density in the SWCNT-network. This sensor was able to differentiate between butyrate molecules that only differ by a few carbon atoms (amyl butyrate, butyl butyrate, propyl butyrate, and pentyl valerate, Figure 29b) and to detect the amyl butyrate at pM concentrations.451 A follow-up study by the same group further improved on the sensitivity of the sensors by mimicking cell signaling pathways in natural olfactory systems more closely.449 Here nanovesicles decorated with olfactory receptors trigger an influx of Ca2+ ions into the nanovesicles upon the exposure to odorants, Figure 29. The accumulation of positive charges in the nanovesicles induce a field effect on the SWCNT, reducing their conductance. Devices of this architecture are able to detect amyl butyrate at concentrations as low as 100pM, Figure 29c. Additionally, CNT-based sensors employing olfactory receptors have been used to quantify sour,462 sweet,452 salty,460 bitter,453,455,461 and umami tastes.463
Figure 29.
Detection of taste and smell. (a) Schematic of the response of nanovesicle towards exposure to odorant molecule. The odorant molecule activates the olfactory receptor (blue) which triggers the uptake of Ca2+ ions through the calcium ion channels (purple), inducing a field-effect gating on the poly-d-lysine (PDL) and CNT active channel. (b) Structure of different odorant molecules that can be differentiated using human olfactory receptor hOR2AG1. (c) Change in conductance upon exposure of sensing device to different odorant molecules. Reproduced with permission from Ref. 449 Copyright 2012, Elsevier.
Beyond quantifying human taste or smell using receptor proteins, several research groups have developed CNT-based sensors for the differentiation between high-quality products. These sensors can authenticate the product by quantifying the differences between similar products based on taste or smell. Wei et al. developed CNT- and graphene-based electrochemical sensors using Ni and Cu foams as the selectors to differentiate Chinese rice wines by age and manufacturer.465 Similarly, Tang and coworkers developed a MWCNT/polymer-composite sensor-array comprising eight different sensing channels to differentiate between several complex alcohol vapors (sake, sorghum liquor, medical liquor, and whiskey), Figure 30.466,467 The signals here were attributed to differential swelling behaviors of the composites. Interestingly, Kachoosangi et al. reported electrochemical CNT sensors that quantified the “heat” in several hot sauces by measuring the concentration of capsaicin.468
Figure 30.
Detection of taste and smell. (Differentiation between four different complex alcohol vapors using MWCNT/polymer composites: styrene/allyl alcohol copolymer (SAA), poly(vinylidene chloride-co-acrylonitrile) (P(VDC-AN)), polyvinylpyrrolidone (PVP), poly(methyl vinyl ether-alt-maleic acid) (PMVEMA), poly(alpha-methylstyrene) (PMS), hydroxypropyl methyl cellulose (HPMC), poly(ethylene adipate) (PEA), and poly(vinyl benzyl chloride) (PVBC).466 Reproduced with permission from Ref. 466. Copyright 2011, Elsevier.
3.2. Food Safety
Ensuring food safety is of great societal concerns. Food related illness was estimated to have large economic cost due to premature death, medical care, loss of productivity, and litigation.469,470 To address the increasing need for quality control, comprehensive sensing technologies for monitoring the quality of food along the entire supply line are needed. CNT-based sensors have been developed to detect food spoilage through biomarkers associated with food spoilage directly or by confirming the integrity of the packaging. In addition, we will discuss the detection of residual pesticides and foodborne pathogens.
3.2.1. Food Spoilage
Monitoring food spoilage in real-time can help prevent the consummation of potentially harmful products as well as the unnecessary disposal of food that is still fit for human consumption. To inform a consumer about the state of the packaged consumable, inexpensive and disposable sensors can be incorporated on the inside of the packaging.471 Several CNT-based sensors have been developed to fill this specific need. These sensors detect volatile biomarkers indicative of food spoilage. For examples, biomarkers include biogenic amines and ammonia for spoilage in meat and fish products,472,473 hexanal for spoiled milk,474 and 1-octen-3-ol for fungal infections of grain.475 A large number of sensors for ammonia have been developed, which we detailed in Section 2.1.1. In this section, we will strictly discuss devices that have been tested on food samples.
Liu et al. developed a chemiresistive sensor containing a series of cobalt meso-aryl-porphyrin complexes for the selective detection of ammonia and biogenic amines.93 By investigating porphyrins with different substitution patterns and counter ions, the authors found that the sensor response correlates with the electron deficiency of the metal center and that the highest response to biogenic amines was achieved with the electron-poor [Co(tetrakis(pentafluorophenyl)porphyrinato)][ClO4]. This sensor was used to monitor the spoilage of meat and seafood samples over several days, showing clear differentiation between samples stored at room temperature and samples stored at 4 °C in the refrigerator (Figure 31a). Similar results were obtained from chemiresistive CNT-based sensors containing human olfactory receptors to detect spoilage of meat,454 oysters,476,477 and a wide variety of seafood.454,478 The detection of more complex biomarkers of spoilage is less explored, however great progress has been made by using olfactory receptors to detect specific small organic molecules. For example, increases in geosmin and 2-methylisoborneol, metabolites of bacteria, are indicative of bacterial contamination of water.415 Using SWCNTs functionalized with human olfactory proteins, both molecules can be detected chemiresistively at nM concentrations.415 Human olfactory protein/CNT devices have also been developed for the detection of spoiled milk. The spoiling of milk correlates with the amount of hexanal in the headspace over the liquid.474 This analyte can be detected using olfactory receptors that bind hexanal at concentrations of 1 µM as demonstrated by Son et al.474 The authors used their olfactory receptor/CNT sensor to monitor the spoiling of milk over a week and recorded a steady increase in hexanal concentration (Figure 31b).454
Figure 31:
Detection of food spoilage. (a) Response of chemiresistive sensor using cobalt meso-aryl-porphyrins as the selector toward meat and seafood samples stored at RT and in the refrigerator over four days. Reproduced with permissions from Ref. 93. Copyright 2015, Wiley-VCH Verlag GmbH & Co. KGaA. (b) Response of CNT-based sensor functionalized with four different human olfactory receptors (OR2J2 for octanol, OR2W1 for hexanal, TAAR5 for trimethylamine, TASR38 for goitrin) while monitoring spoiling of milk over one week. Reproduced with permissions from Ref. 454. Copyright 2017, Elsevier.
3.2.2. Integrity of Packaging and Oxygen Sensors (O2)
While sensing biogenic decomposition products is a viable method to detect food spoilage, monitoring the integrity of food packaging can provide a general indicator of the quality of the content. Food and pharmaceuticals can be protected from oxidative degeneration via packaging in inert atmosphere,479,480 and the detection of oxygen inside of the packaging is a vital method to monitor the intactness of this barrier.481 Apart from monitoring food packaging, detection of oxygen has many important applications including environmental monitoring, and biological and medical applications. For example, the control of automobile exhaust emissions and optimization of industrial processes are made possible by oxygen sensors.482,483 Furthermore, the measurement of biochemical oxygen demand (BOD) can be used to characterize the amount of organic waste contained in water.484 Measuring the dissolved oxygen partial pressure in arterial blood provides an evaluation of a patient’s condition.485 Several review articles cover the luminescence-based and colorimetric detection of oxygen using optically active organic or inorganic materials sensors;486,487 herein we discuss the CNT-based sensors. Both pristine and functionalized CNTs show responsiveness towards O2 adsorption and are promising materials for the development of oxygen sensing devices.
The consensus on the exact description of the interaction between O2 molecules and CNTs has not been reached, however physisorbed O2 seems to induce p-doping of CNTs. Zettl and coworkers originally reported that the physical properties of CNTs, including local densities of electrical states and electrical resistance, are extremely sensitive to the presence of oxygen.39,488 The doping effect of O2 on the electronic structure of CNTs has been reported for both experimental investigations and computational results.40,489–493 However, Derycke et al. concluded that the adsorption of O2 mainly modifies the Schottky barriers of the CNT-metal contacts rather than doping of the CNTs themselves.494 These conflicting experimental reports may originate from the differences in the quality of the CNTs. For example, Goldoni et al. demonstrated that the removal of contaminants (Na, and Ni) and defect sites renders the electronic spectra of CNTs insensitive to O2, CO, H2O, and N2.323
An additional limitation towards the development of CNT-based oxygen detectors arises from the slow desorption rate of O2 from the nanotubes at room temperature. In earlier reports, reversible sensitivity of oxygen relied on the application of high vacuum.39,488,494 To develop a lower-power and portable platform, Kauffman et al. reported a chemiresistive device that can be refreshed to the original conductance using ultraviolet illumination (UV, 365 nm) after the exposure to O2, Figure 32.495 The device comprised SWCNTs decorated with an oxygen-sensitive Eu3+ dendrimer complex (Eu8). The authors found that the initial rate of change in the conductance scaled linearly with the concentration of oxygen in the range of 5–27%.495 Furthermore, the sensors showed good selectivity to O2 compared to CO2, NH3, and NO2.495
Figure 32.
Chemiresistors based on SWCNT networks decorated with an oxygen-sensitive Eu3+-containing dendrimer complex. (a) Chemical structure of the europium containing dendrimer complex (Eu8) containing eight Eu3+ cations coordinated within a 1,8-naphthalimide-terminated, G3-PAMAM dendrimer core. (b) Electrical response rate of the Eu8-SWCNT chemiresistor to increasing oxygen concentration. (c) Comparison of the normalized conductance between Eu8-SWCNTs (black curve) and pristine SWCNTs (green curve) when exposed to illumination with 365 nm ultraviolet light in flowing N2, pure O2, 10.5% CO2, 100 ppm NH3, and 10 ppm NO2. Reproduced with permission from Ref. 495. Copyright 2009, Nature Publishing Group.
For applications wherein the presence of oxygen leads to detrimental effects, such as in modified atmosphere packaging (MAP) for oxygen-sensitive food and drugs, irreversible responses to oxygen could prove useful as they will report on the cumulative level of oxygen.189 To this end, Zhu et al. described the development of a wireless oxygen dosimeter using FeII-poly(4-vinylpyridine)-SWCNTs composites with passive radio frequency identification (RFID) tags, Figure 33.189 Poly(4-vinylpyridine) (P4VP) was used to disperse the SWCNTs331 and to bind FeII species through the pyridyl ligands. FeII is reducing and transfers electron density to the carbon nanotubes thereby eliminating the hole carriers and increasing the resistivity. Oxygen exposure of varying concentrations (2–21%) led to irreversible responses that can be detected by a smartphone through passive RFID tags, which allowed the wireless detection of oxygen inside of food packaging.189 To limit the effects of humidity and prevent contact with food, the device was covered with a thin layer of polydimethylsiloxane (PDMS), which functioned as an oxygen-permeable moisture barrier. Exposure to oxygen irreversibly oxidizes the selector (FeII to FeIII) and induces oxidation of the SWCNTs, increasing the number of hole charge carriers and the conductance. For the in vivo detection of oxygen in water, Xiang et al. developed an electrochemical sensor comprising microsized Pt NP-covered CNT-based electrodes. Although the authors demonstrated the detection of oxygen in the brains of anesthetized rats, a similar device could be used to detect oxygen on the inside of air-sensitive liquid food packaging.
Figure 33.
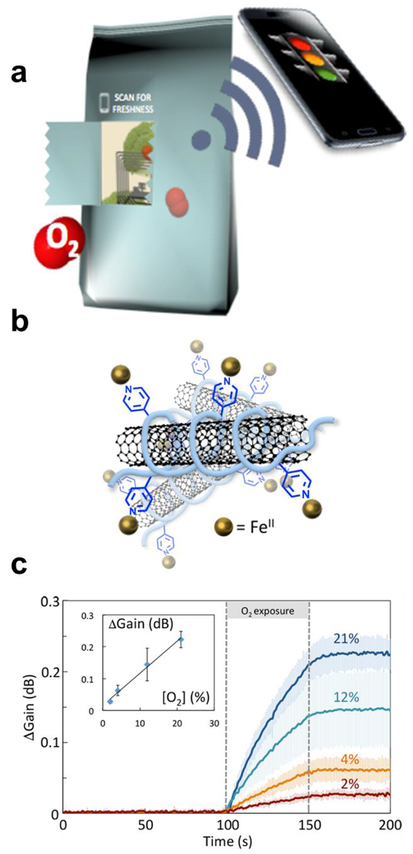
Wireless oxygen sensors enabled by near-field communication (NFC) tag. (a) Schematic drawing of a smart packaging that measures oxygen exposure from the inside. A radio frequency reader, in this case, a smartphone, can remotely read the sensor’s state, and access the quality of the package contents without a line of sight. (b) Schematic of poly(4-vinylpyridine)-dispersed SWCNTs and coordinating FeII ions as the sensing element. (c) Sensing responses to various O2 concentrations (diluted in N2). The inset shows the calibration curve of the sensor. Reproduced with permission from Ref. 189. Copyright 2017, American Chemical Society.
3.2.3. Pesticide Contamination
Overuse of pesticides and contamination of food poses a risk for human health and biodiversity.496 Millions of tons per year of pesticides are used to protect crops from insects to improve agricultural production.496 Nevertheless, the use of pesticide has been linked to the collapse of honeybee colonies,497 increase in amphibian mortality,498 and decrease of biodiversity in aquatic ecosystems.496 Additionally, residual pesticides in agricultural products can threaten human health. These exposures have been linked to the development of several cancers (breast, pancreatic, non-Hodgkin lymphoma, leukemia, brain, prostate, and kidney),499 neurotoxicity,500 genotoxicity,500 birth defects,500 fetal death,500 and decreased neurodevelopment.501 Additionally, pesticides have been used as chemical nerve agents in chemical attacks as will be described in Section 5.1 on Chemical Warfare Agents.502 Overall, the use of pesticides causes an estimated $10 billion in environmental and societal damages annually in the US alone.503 Monitoring the concentration of pesticides can prevent overuse and help protect the environment and human health.
Several CNT-based sensors have been developed that can quantify pesticide concentration in complex matrixes of food samples. The examples include soybean sprout, potato, tomato, pear, apple, cabbage, onion, carrot, and celery samples, orange juice, strawberry juice, beer, and milk. 431,504–507 A large number of these sensors rely on the selective oxidation or reduction of the pesticide on a functionalized CNT-based electrode.431,504–510 Possible selectors for the detection of organophosphorous pesticides (OPs) are the enzymes that are effected by the OPs. The bio-catalytic activity of the enzyme acetylcholinesterase (AChE) is the removal of the neurotransmitter acetylcholine (ACh) through hydrolysis. Poisoning with OPs inhibits AChE, which leads to a buildup of ACh at the nerve synapses, causing rapid twitching of voluntary muscles and paralysis. This same reaction cascade was used in CNT-based sensors to detect OPs in many reports.431,505,507,509–511 For example, Yu et al. observed that with addition of ACh to AChE functionalized CNT-electrodes generated a concentration-dependent amperometric current increase (Figure 34).505 Addition of the OP paraxon reduced the current increase as a result of the inhibition of the enzymatic hydrolysis of ACh. Alternatively, to these enzymatic transduction schemes, sensors with non-enzymatic selector moieties have been identified for the electrochemical sensing of pesticides on CNT-enhanced electrodes. These systems include iron porphyrin508,512, ruthenium phthalocyanine,506 patterned polymers504,513,514, and metal nanoparticles.515
Figure 34.
Detection of pesticides. (a) Schematic representation of amination process of CNT (3-APTES, 3–aminopropyltriethoxysilane). (b) Schematic of acetylcholinesterase (AChE) the immobilization of onto the NH2-functionalized CNTs (acetylthiocholine chloride ATCh). Reproduced with permission from Ref. 505. Copyright 2015, Elsevier.
3.2.4. Foodborne Pathogens
Apart from detection of biomarkers for the spoilage of food or the monitoring of food packaging, foodborne pathogens can be detected directly. Infections with pathogenic microorganisms—such as Campylobacter, Salmonella, Listeria monocytogenes, and Escherichia coli (E. coli)516—can cause diarrheal diseases, which remain a leading cause of preventable death of children in developing countries.517,518 According to the World Health Organization (WHO), even in the United States, 76 million cases of foodborne diseases occur annually, out of which 325,000 require hospitalization and 5,000 lead to death.518 Infection can occur though ingestion of contaminated foods or water, person-to person transmission, or contact with infected animals.519 To minimize the risk of infection, rapid and real-time identification of infected food items poses a pressing need.519 Current methods for the detection of foodborne pathogens include optical-based biosensors, mass-sensitive biosensors, electrochemical biosensors, culture and colony counting, immunology-based methods, and polymerase chain reaction. These methods are detailed in several excellent review articles focused on the detection of foodborne pathogens.516,520 A review of nanomaterial-based pathogen detection was also presented by Inbaraj et al.521 In this section we discuss the CNT-based detection of pathogens in water or biological media.
To detect pathogenic microorganisms in complex media, several groups have functionalized CNTs with antibodies with specific interactions with the target bacteria or toxin. Bhardwaj et al. developed a paper-based electrochemical sensor using antibodies (Ab) as the selector unit to detect Staphylococcus aureus (S. aureus).522 The antibodies were covalently attached to the sidewalls of the CNTs using 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (EDC/NHS) coupling. After functionalization, a dispersion of the Ab-CNTs was deposited on the working electrode of the electrochemical device where the biding of S. aureus can be observed as an increase of the peak current. The response time of sensors was shown to be 30 min, and the sensor was operational in milk. Similarly, Zhao et al. investigated an electrochemical sensor for Shigella flexneri based on an Ab functionalized MWCNT/sodium alginate composite (MWCNT/SA).523 Sodium alginate acts as a dispersing agent for the MWCNTs and as a biocompatible scaffold to facilitate binding of horseradish peroxidase labeled antibodies to Shigella flexneri. Binding of S. flexneri inhibits the activity of the horseradish peroxidase which can be seen as a decrease in the peak current in the electrochemical signature of the device. Viswanathan et al. investigated the electrochemical detection of cholera toxin (CT), a bacterial protein toxin secreted by the bacterium Vibrio cholerae.524 The device is operated in a two-step process: the first step consists of the binding of CT through a poly(3,4-ethylenedioxythiophene)/antibody (PEDOT/Ab) composite on a MWCNT electrode, and the second step consists of the binding of a K4[Fe(CN)6]-containing liposome, Figure 35a. The binding of the potassium ferritin-containing liposome is proportional to the CT concentration and enhances the peak current of the electrochemical feature of the sensing device. Using this two-step process, the authors were able to determine the concentration of CT in tap water and in kitchen wastewater with a detection time of 60 min.
Figure 35.

CNT-based pathogen detection. (a) Schematic representation of electrochemical sensor for cholera toxin (CT). In the first step, CT is bound by an antibody containing MWCNT-electrode. In the second step a potassium ferrocyanide-containing liposome is bound to amplify the binding event of CT.524 (b) Chemiresistive detection of E. coli using a CNT-coated nano-junction functionalized with antibodies.525 Reproduced with permission from Ref. 524. Copyright 2006, American Chemical Society, and Ref.525, Copyright 2014, Public Library of Science.
The rapid detection of foodborne pathogens using functionalized nano-junctions has been reported by the group of Jun and coworkers.525,526 PEI/SWCNT-coated microwires are assembled into crossbar junctions (Figure 35b) with a 10 µm gap between the wires. The wire junction is functionalized by self-assembly of streptavidin and biotinylated antibodies to E. coli, which binds strongly to the streptavidin binding pocket, Figure 35b. The current through the nano-junction decreases upon each subsequent addition of biomolecule (streptavidin, biotinylated antibodies, E. coli), possibly via a deformation of the CNT-sidewall or electrostatic disturbance of the CNT-network by the biomolecules. Using a single junction, the authors were able to detect E. coli in water with a detection time of 5 min.525 Using a 4-wire design, arranged in a 2×2 grid, the authors were able to detect E. coli and S. aureus simultaneously.526 Two adjacent junctions were functionalized using antibodies to E. coli while the remaining two junctions were functionalized using antibodies to S. aureus. When exposed to a mixture of bacteria, the multi-junction device can determine the concentration of both strains of bacteria independently. Expanding on this 2×2 array might allow the detection of all major foodborne pathogenic bacteria in a rapid fashion (detection time 5 min).
4. CNT-based Biological Sensors
Health monitoring and the detection of biomolecules are crucial for many areas of healthcare ranging from the diagnosis of diseases and real-time monitoring the conditions of patients. These applications can assist in a global reduction of mortality and medical costs. For example, early discrimination of different infections can facilitate appropriate treatment and preventative measures. Present medical testing protocols require expensive equipment and/or dedicated laboratories with long turnaround time. Hence, CNT-based sensors offer the attractive features of portability and cost-effectiveness for point-of-care diagnostics. This section describes CNT-based sensors addressing biological applications. We aim to highlight examples of sensors that derive selectivity from the choice of functionalization and/or the use of sensor arrays for two main topics: breath analysis and the detection of biomolecules. Interested readers may benefit from full reviews on this topic.7,31,33,527
4.1. Breath Analysis
The analysis of exhaled breath provides a noninvasive and potentially portable method for the detection of diseases and monitoring a person’s physical condition.528–531 Volatile molecules from human breath have been linked to various diseases including breast, lung, gastric, colon, and prostate cancers,532–536 Alzheimer’s and Parkinson’s diseases,537 multiple sclerosis,538 diabetes,539 and hyperglycemia.539 Extensive clinical trials are often required to establish the link between the presence of biomarkers in human breath with the indication and state of a disease. These efforts are best initially quantified by precision analytical assessments using standardized chromatographic techniques, such as gas-chromatography mass-spectrometry (GC-MS).540 We highlight that a large volume of work on breath analysis has been accomplished by the group of Haick42,541,542 and Owlstone Medical.543 Nanomaterials, including CNTs, are ideal for breath analysis due to their chemical versatility and ease of incorporation into sensing platforms.530 We will focus on two main categories of analytes: volatile organic compounds (VOCs) and inorganic gases.
4.1.1. Volatile Organic Compounds (VOCs)
VOCs in a biological context are the products or byproducts of cellular metabolism or oxidative stress caused by reactive oxidative species (ROS).536,544,545 These compounds are often separated into different categories based on their functional groups: hydrocarbons, alcohols, aldehydes, ketones, esters, nitriles, aromatic compounds.536 The concentration of these compounds in bodily excreted fluids and breath is affected by changes in diet, environmental exposures, and disease states of the patient.546–548 The typical VOCs originated from cellular activity in humans include hydrocarbons, aldehydes, and ketones.33 For example, peroxidation of lipids by ROSs can produce ethane, pentane, and aldehydes; high level fat metabolism produces detectable levels of acetone; and halitosis can lead to high concentration of hydrogen sulfide, methyl mercaptan, and dimethyl sulfide.33
As a result of the large number of VOCs present in typical breath samples, it is natural to construct arrays of sensors to discriminate the different chemical species. As described in the Section 1.3 on device architectures, sensing arrays provide “finger prints” for a given compound or class of compounds, which can then be analyzed by computational methods (linear discriminant analysis, LDA and principal component analysis, PCA). Some of the first examples of CNTs-based detection of VOCs via a sensing array was demonstrated by Haick and co-workers with the goal to discriminate healthy subjects from those suffering from lung cancer and chronic renal failure.42,541 The authors constructed arrays from semiconducting SWCNTs coated with ten different non-polymeric organic materials and tested their performance in the differentiation between simulated breath of healthy and diseased subjects, Figure 36.541 PCA of the obtained signal revealed that the discrimination between cancerous and healthy breath were confounded by the effects from humidity and reduction of the relative humidity from 80% to below 10% led to excellent discrimination.541 In their follow-up study, FET devices were used instead of chemiresistors with less debilitating effects from humidity.42 Recently, the same group has reported the use of functionalized SWCNTs arrays in combination with molecularly modified gold nanoparticles to diagnose 17 different disease conditions from 1404 subjects with 86% accuracy.542
Figure 36.
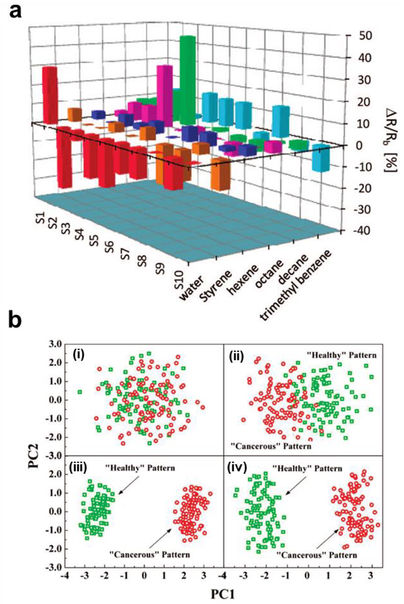
Detection of simulated patters of lung cancer biomarkers using arrays of SWCNTs coated noncovalently with thin films of organic materials. (a) Patterns of responses of chemiresistive networks of SWCNTs coated with ten different organic materials (S1 to S10) when exposed to the representative VOCs for 10 min. (b) PCA score plots of the ten sensing arrays upon the exposure to “healthy” and “cancerous” mixtures of VOCs at (i) 80% RH, (ii) 10% RH, (iii) 1% RH, and (iv) 80% RH with preconcentration of VOCs of 50 times. Reproduced with permission from Ref. 541. Copyright 2008, American Chemical Society.
Several groups have developed CNT-based sensor arrays that can differentiate between different VOCs. For example, Liu et al. used noncovalently functionalized SWCNTs with a series of metalloporphyrin complexes with a diversity of different metal centers.68 The authors constructed PCA plots from signals obtained from chemiresistors comprising SWCNTs and eight metalloporphyrins to distinguish five classes of VOCs (amines, hydrocarbons, aromatic, ketones, and alcohols).68 They reported a large separation of the amines from other VOCs as a result their charge transfer capabilities and sufficient discrimination between the remaining four classes based on their intermolecular interaction or swelling effects.68 Hybrids of SWCNTs and metalloporphyrins have also been reported by Shirsat et al. to distinguish acetone, ethanol, methanol, and methyl ethyl ketone.549 Common surfactants have also been incorporated with CNTs to create VOC sensing arrays. Chatterjee et al. prepared aqueous solutions of MWCNTs dispersed in common surfactants—sodium deoxycholate (DOC), sodium dodecylbenzenesulfonate (SDBS), 1-hexadecyl trimethyl ammonium bromide (CTAB), benzalkonium chloride (BnzlkCl), and triton x-405 (TX405)—and spray-coated CNT-films onto interdigitated electrodes using layer-by-layer technique.550 The authors reported that the sensing responses of the array depended on the interactions between the surfactants and the analytes, as well as their supramolecular assembly with MWCNTs.550 Individually, each sensor provided limited discrimination; however, PCA demonstrated separation between methanol, ethanol, water, acetone, chloroform, and toluene.550
Although noncovalent functionalization has been successful at producing selective sensing arrays, they often do not produce sensors with sufficient robustness toward the harsh conditions. With the goal of creating robust CNT-based chemiresistive arrays, Sarkar et al. covalently anchored poly(tetraphenylporphyrin) on SWCNTs to detect acetone, and the resulting sensors showed good stability over a period of 180 days.551 Wang and Swager synthesized a series of covalently functionalized MWCNTs with cross-sensitive recognition groups via a two-step synthetic protocol, Figure 37.117 The authors chose each selector to maximize the differential interactions with the range of targeted analytes. Propargyl- and allyl-MWCNTs (1 and 2) are both polar and hydrogen bond accepting and were expected to interact strongly with vapors with large dipoles. Selectors with long alkyl chains (3 and 4) favor dispersion interactions that were aimed to detect aliphatic hydrocarbons. Hydrogen bond accepting vapors (e.g., ethers and ketones) were targeted by carboxylic acid group and hexafluoroisopropanol (5 and 6). Calix[4]arenes (7) favor the adsorption of aromatic and chlorinated hydrocarbons due to their highly polarizable pocket; and lastly, crown-ether (8) provided hydrogen bonding basicity to interact with acids and alcohols. As a result, the sensor array successfully discriminated the 20 tested VOCs into five difference classes, without interfering effects from humidity. Moreover, the distinct pattern of responses when subjected to the linear discriminant analysis (LDA) accurately identified all 20 VOCs. The carefully selected selectors in this array further discriminated chemical space in an intuitively meaningful way. For example, in the PCA plot, Figure 37, the analytes at the boundaries between functional classes have characteristics of both classes. Within the ethers and ketones, the analytes proximate to the hydrocarbons are dihexylether and 2-decanone, which have hydrocarbon character. Similarly, for alcohols 1-octanol is closest to the hydrocarbons. And for the aromatics, 1,3,5-trimethylbenzene wherein the aromatic structure is masked by the methyl groups is also closest to the hydrocarbons. Hence, this study clearly illustrates that careful chemical designs, rather than random collections of selectors, are best at selectively characterizing complex VOCs.
Figure 37.

Sensing arrays of pristine and covalently functionalized MWCNTs for discrimination of 20 representative VOCs. (a) Chemical structures and synthetic routes for the covalently functionalized MWCNTs. (b) Patterns of changes in the conductance of pristine MWCNT and eight functionalized MWCNTs to VOCs at 1% of their saturated vapor pressure. (c) Principal component score plots of an array of pristine MWCNTs and eight functionalized MWCNTs to the 20 VOCs. Reproduced with permission from Ref. 117. Copyright 2011, American Chemical Society.
An advantage of array-based sensors comprising multiple sensors with limited selectivity is that the library of the “finger prints” can be easily updated to detect a new class of analyte. In applications where one specific biomarker has been identified as sufficient proof that a disease is present, the detection of a single analyte can be a powerful diagnostic tool.22 Thus, array-based sensors will benefit when used in parallel with sensors capable of detecting a single analyte. For example, Wang et al. fabricated vertically aligned-CNTs with conductive polymer coating to detect n-pentane with a projected LOD of 50 ppm with good selectivity over methanol and toluene.552 Vertically aligned CNTs were coated with poly(3,4-ethylenedioxythiophene) (PEDOT) via oxidative chemical vapor deposition, followed by a coating of non-conducting polystyrene (PS). Adsorption of pentane on the surface of PEDOT disrupts the conductive pathway, and added selectivity is achieved by the layer of PS that excludes polar VOCs.552 As discussed above, calix[4]arenes have the tendency to interact with aromatic hydrocarbons. Wang et al. demonstrated that chemiresistors of SWCNTs wrapped with calix[4]arenes-substituted polythiophene could distinguish between different isomers of xylene, Figure 38.97 Using quartz crystal microbalance (QCM) studies and NMR binding studies, the authors verified that the selectivity arises from the preferential binding of p-xylene within the calixarene cavity over the other two isomers.97 Selectivity based on structural isomers that do not have large differences in dipoles is rare for chemical sensors, and this study is another demonstration of merits of integrating selective recognition elements into CNT sensors.
Figure 38.
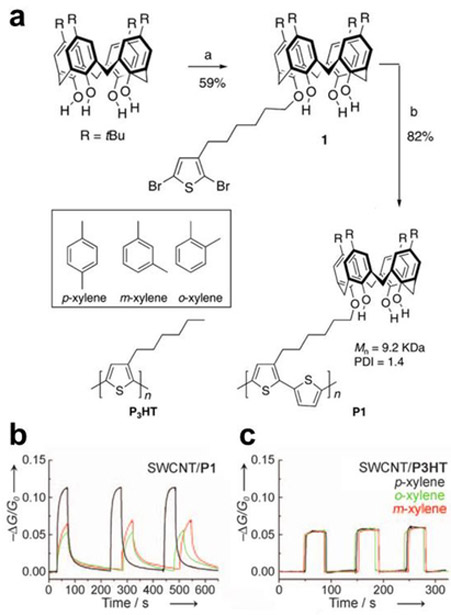
Molecular recognition of SWCNTs/polythiophene chemiresistor toward xylene isomers. (a) Chemical structures of xylene isomers, poly(3-hexylthiophene) (P3HT), and synthesis scheme of p-tert-butylcalix[4]arene-substituted polythiophene (P1). Conductance change of the chemiresistor of (b) SWCNTs and P1 and (c) SWCNT and P3HT to p-xylene, o-xylene, and m-xylene at 400 ppm. Reproduced with permission from Ref. 97. Copyright 2008, Wiley-VCH Verlag GmbH & Co.
In addition to composites of polymers and CNTs, metal and metal oxide nanoparticles have also been demonstrated as selectors for CNT-sensor VOC arrays. Ding et al. fabricated chemFET sensors with the composite of SWCNTs and TiO2 that exhibited responses to acetone vapor at 400 ppb.136 In this study, oxidized SWCNTs with defect sites on the sidewall directed covalent nucleation and growth of titanium oxide layers. The proposed sensing mechanism relies on the UV photoinduced generation of electron/hole pairs in the TiO2 layer and the adsorption of acetone that prevents recombination to cause a detectable drop in conductance.136 This sensor showed high selectivity toward acetone over NH3, H2, CO, and NO, and was able to detect 20 ppm of acetone in both air and high humidity despite interferences from O2 and water vapor.136 Yoon et al. has also shown the incorporation of soft Lewis acid Pd2+ cations in a polymer corona round SWCNTs. In this case PdCl2 is coordinated to P4VP-wrapped SWCNTs (described previously in the section on functionalization) to produce a highly selective sensor toward vapors of thioethers.331 Specifically, the PdCl2 salt imparted large dosimetric response to methyl n-propyl sulfide diluted in air, breath biomarker for diseases such as malaria.331
4.1.2. Inorganic Gases
In addition to VOCs, inorganic gases are important biomarkers for medical monitoring. The primary gases of interest are carbon dioxide (CO2), nitric oxide (NO), ammonia (NH3), and hydrogen sulfide (H2S). Monitoring the concentration or the partial pressure of CO2 in the respiratory gases, called capnography, is commonly used during medical procedures such as during anesthesia and intensive care. Using capnography, it is possible to monitor the patients’ metabolic status and infer any alterations to obstructive conditions such as cardiac arrest, bronchitis, asthma, and anesthesia.553,554 NO is involved in many physiological processes including immune response, regulation of blood pressure, and neural communication.555 However, direct measurement of NO is difficult due to its extremely short physiological half-life in aerobic environments. Increased level of NH3 in exhaled breath can also be used as the biomarker of patients with renal failure or disease (ppm levels compared to ppb range for healthy individuals).33,556,557 Various types of NH3 sensors are discussed in Section 2.1.1 on environmental application). In addition, H2S (discussed previously in Section 2.1.4 on environmental monitoring) can serve as a breath marker for some diseases such as diabetes and halitosis.
Although earlier reports of CNT-based sensors exhibiting sensitivity to CO2 exist, selective sensors have predominantly relied on polymer-wrapped CNTs as the sensing materials. Varghese et al.203 and Ong et al.492 demonstrated the sensing capability of MWCNT-SiO2 composite toward CO2; however, cross sensitivity with other gases and humidity were also reported. Star et al. incorporated CNTs functionalized with poly(ethyleneimine) (PEI) and starch polymers in FET devices to show excellent sensitivity to CO2 dynamic operating range was shown to be from 500 ppm to 10% of CO2 in air.48 The authors attributed the sensing behaviors to the reduction of electron-donating character of PEI and structural rearrangement of the starch polymer upon the exposure to CO2. Li et al. reported chemiresistive sensors comprising poly(ionic liquid)-wrapped SWCNTs, Figure 39.558 Dispersions of poly[1-(4-vinylbenzyl)-3-methylimidazolium tetrafluoroborate (PIL) and SWCNTs were deposited onto interdigitated microelectrodes and displayed a dynamic range of CO2 responses between 500 ppt to 10 ppm. The interactions between [BF4–] anion and CO2 were attributed as the sensing mechanism that gave rise to the selectivity to CO2 over CO, H2, CH4, ethanol, O2, and water vapor. Lastly, Olney et al. prepared a composite of PEDOT:PSS and SWCNTs that showed sensitivity to CO2 down to 10 ppm.559 The observed increase in conductivity upon the adsorption of CO2 was suggested to be caused by conformational change and phase separation of the PEDOT:PSS. Although cross-sensitivity with methane was noted, in this case the conductivity decreases, which provides a means to distinguish between the two gases. The robustness of this rather indirect and seemingly chemically nonspecific mechanism remains to be determined.
Figure 39.

Poly(ionic liquid)-wrapped SWCNTs for detection of CO2. (a) Schematic of the sensors comprising poly[1-(4-vinylbenzyl)-3-methylimidazolium tetrafluoroborate (PIL) and SWCNTs. (b) TEM image of an individual SWCNTs partially wrapped by PIL. (c) Change in the resistance of the PIL/SWCNTs sensors when exposed to increasing concentration of CO2. Exposure to UV light in N2 was used to recover the sensors to the baseline. (d) Sensitivity toward interfering gases and the effects of humidity. Reproduced with permission from Ref. 558. Copyright 2012, The Royal Society of Chemistry.
Few examples of NO sensors based on CNTs are reported. Star and coworkers extended the use of their FET devices with PEI coated CNTs to detect NO in simulated breath.560 The composite of PEI and CNTs is moderately sensitive to NO, thus the authors incorporated a CrO3 converter to oxidize NO to NO2 prior to the sensors. They noted that with the addition of an ascarite scrubber was required to remove the interfering signal from CO2.
4.2. Health Monitoring and Detection of Biomolecules
This section focuses on designs of CNT-based biosensors and their uses for the detection of biomolecules. CNTs can serve as both the electrodes for electrochemical methods and as the transducer in a chemiresistor or chemFET.527 For practical sensors, the dynamic range of the sensors must correspond with the physiological concentrations of the targeted biomarker. For example, the difference in the concentration of glucose in blood of a person with diabetes can be as low as 1 mM, with the average values range between 4 and 9 mM.527 Significant work has been done over the past two decades to incorporate CNTs as biosensors. This section highlights selected reports, mainly focusing on the detections of glucose and DNA. Not surprisingly, CNT-based sensors have been developed for the detection of many other biomolecules. Enzymatic biosensors are relatively simple to incorporate into CNT electrodes for electrochemical analysis, leading to analyte-specific recognition. The common analytes found in literature includes proteins,52 cholesterol,561–563 dopamine,564–568 serotonin,567–569 nicotinamide adenine dinucleotide (NAD),570–572 and cytochrome C.573,574 We direct interested readers to recent full reviews that cover these other analytes.8,31,527,575–577
4.2.1. Glucose Detection
One of the most frequently performed medical tests is the detection of blood glucose level. Monitoring this concentration is used to diagnose and manage diabetes, resulting in a high demand for glucose sensors. Both electrochemical and chemFET sensors have been reported with glucose oxidase (GOx) as the selector. The dramatic reduction in the overpotential for the generation of hydrogen peroxide (H2O2) and the direct electron transfer from GOx to the CNT electrodes proved extremely useful for electrochemical sensors using GOx/CNT compsoites.8 However, the immobilization of the enzymes onto CNT electrodes via simple adsorption faces several problems, namely the lowering of the enzyme activity (denaturation) and leaching of adsorbed enzymes.527 One solution to overcome these problems was the use of metal and metal oxide NPs to anchor GOx on CNTs. To this end, Tang et al. reported an electrochemical sensor based on the adsorption of GOx onto Pt nanoparticles-decorated CNT electrodes. The authors attributed the excellent electrocatalytic activity to the large surface area obtained by the addition of both CNTs and Pt nanoparticles, resulting in the large linear range (0.1 – 13.5 mM) and high sensitivity of 14 µA / mM.578 The sensor showed moderate stability, retaining 73.5% of the initial activity after 22 days of testing.578 Successful examples were also reported for MWCNTs decorated with ZnO nanoparticles by Wang et al.579 and with Pt-Pd bimetallic nanoparticles by Chen et al.580 In these examples, a layer of polymeric film was used to eliminate common interferents—such as uric acid, ascorbic acid, and fructose—and improve stability. Wang et al. showed only 10% decrease in detection current after 160 days, and Chen et al. reported 15% loss of sensitivity after 28 days.
In addition to the adsorption of GOx onto CNTs electrodes decorated with NPs, multiple groups have reported immobilization of GOx using electropolymerization of conductive polymers on CNTs. In this method, GOx is mixed with the monomer which is then electropolymerized at a CNT electrode. Gao et al. electropolymerized polypyrrole (PPy) on aligned MWCNTs decorated with Fe particles in the presence of GOx.581 They observed a large linear range (2.5 – 20 mM), and concluded that both the alignment of the MWCNTs and the Fe particles were critical to lower the oxidation potential of H2O2, thereby preventing the overoxidation of PPy.581 Similarly, Wang and Musameh prepared sensors using a PPy/GOx composite that demonstrated a LOD of 0.2 nM and a linear range from 0–50 mM.582 Pilan and Raicopol reported a composite of PANI/functionalized SWCNT/Prussian Blue for the electrochemical detection of glucose.583 They demonstrated selectivity to glucose over the common interfering species of acetaminophen, uric acid, lactate, and ascorbic acid.583 Alternatively, Patolsky et al. demonstrated a covalent attachment of a reconstituted GOx onto the edge of SWCNTs that are linked to an electrode surface, Figure 40.584 SWCNTs was first coupled to the surface of Au electrode with a mixed monolayer of thioethanol and cystamine in the presence of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC). The amino derivative of the flavin adenine dinucleotide (FAD) cofactor was then coupled to the carboxyl group at the end of the SWCNTs; subsequently, apo-glucose oxidase (apo-GOx) was reconstituted on the FAD units. Cyclic voltammograms of the bioelectrocatalytic oxidation of glucose provided the calibration curve of amperometric current as a function of the glucose concentration and revealed that the SWCNTs behaved as electrical contacts between the active site of the GOx and the electrode.
Figure 40:
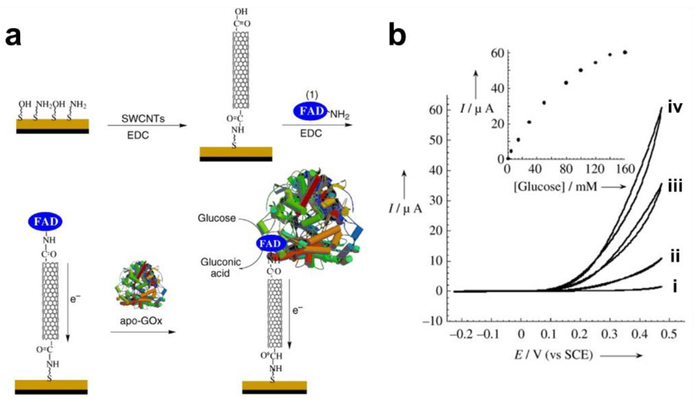
Covalent immobilization of GOx onto the edge of SWCNTs for amperometric sensing of glucose. (a) Assembly of a SWCNT-based GOx electrode. (b) Cyclic voltammograms of the electrocatalyzed oxidation of different concentrations of glucose by the GOx at (i) 0 mM, (ii) 20 mM, (iii) 60 mM, and (iv) 160 mM glucose. The inset shows the calibration curve of the amperometric responses as a function of the concentration of glucose. Reproduced with permission from Ref.584. Copyright 2004, Wiley-VCH Verlag GmbH & Co.
Alternatively, non-enzymatic CNT-based sensors for glucose have been reported.585 These sensors rely on the catalytic properties of metallic nanoparticles decorated on the CNTs to generate an electrochemical response of glucose. Lin et al. used nickel and copper NPs decorated MWCNTs as the electrodes that showed activity toward glucose oxidation,586 while Gougis et al. used pulsed laser deposition of gold NPs onto CNT electrodes.587 Recently, Baghayeri et al. demonstrated selective glucose electrochemical sensors using Ag NPs electrodeposited onto MWCNTs functionalized with metformin, Figure 41.588 The reported sensor showed a low LOD at 0.3 nM with the linear range from 1 nM to 350 µM without interference from common molecules found in human blood serum and urine samples.
Figure 41.
Non-enzymatic glucose sensor based on Ag nanoparticles (NPs) on functionalized CNTs. (a) Scheme for the preparation of Ag NPs on metformin functionalized MWCNTs. (b) Amperometric response of the sensor to successive addition of different concentration of glucose in 0.1 M NaOH at a working potential of 0.70 V. Inset shows the plot of electrocatalytic peak current vs. concentration of glucose from 1.0 nM to 500 nM. (c) Amperometric response to a) 100 µM glucose and 400 µM of b) acetic acid, c) ethanol, d) ascorbic acid, e) uric acid, f) dopamine, g) L-Dopa, h) epinephrine, and i) L-tyrosine. Reproduced with permission from Ref. 588. Copyright 2016, Elsevier.
CNTs also enable chemiresistor and chemFET approaches to glucose sensors. Besteman et al. reported one of the earliest examples of SWCNT-based glucose sensors.589 GOx was attached to the sidewall of individual SWCNTs; upon the addition of glucose, modest increase in conductance was observed.589 Soylemez et al. reported the functionalization of a poly(4-vinylpyridine) (P4VP) wrapped surface anchored array of SWCNTs with alkylation of the pyridyl groups electrostatically assembled GOx.64 The P4VP-SWCNT scaffold provided prolonged stability, retaining 83.3% of its initial response, and excellent electrical communication between the GOx and the SWCNTs, enabling real-time chemiresistive sensing of glucose. The authors reported the dosimetric responses with the linear range from 0.08 to 2.2 mM and validated their method using commercial beverages. In another example, Lee and Cui developed flexible sensors via layer-by-layer self-assembly of SWCNTs, poly(diallyldimthyammonium chloride) (PDDA), polystyrene sulfonate (PSS), and GOx on polyethylene terephthalate (PET).590 The sensors were capable of detecting glucose down to 0.5 mM with a linear range of 0.5 to 25 mM. Using molecular based recognition principles, Lerner et al. reported the detection of glucose using the complexation by boronic acid moieties, Figure 42.88 The reduction in source-drain current occurred due to the increased carrier scattering upon the formation of boronate anion complex. As a control, exposure to lactose showed negligible response as a result of its lower binding affinity to the pyrene boronic acid moieties. Lastly, Cella et al. employed a displacement sensing scheme on a SWCNT-based chemiresistive platform.101 In this study, SWCNTs were initially functionalized with hydrophobic dextran derivative (DexP) that subsequently formed complexes with concanavalin A (ConA). The lower affinity of ConA to DexP when compared to glucose, resulted in the release of ConA from the SWCNTs upon the addition of glucose, leading to detectable changes in the resistance.
Figure 42.
Boronic acid functionalized CNT-based FET sensor for the detection of glucose. (a) Schematic of the FET sensors and the illustration of binding of glucose to a CNT functionalized with pyrene-1-boronic acid. Bound glucose forms a boronate anion complex. (b) Sensing response as a function of the concentration of glucose (blue circles). Control experiments included responses from unfunctionalized devices to glucose (red squares), responses from functionalized devices to lactose (black triangles), and null response of functionalized devices to DI water. Reproduced with permission from Ref. 88. Copyright 2013, AIP Publishing LLC.
4.2.2. DNA Sensors
Detection of DNA is particularly important for the diagnosis and treatment of genetic disorders, prevention against bio-warfare agents, detection of infectious agents and pathogens, and drug discovery.591 Selective predictable base pairing interactions between and within DNA strands have led the development of sensors employing optical, piezoelectric, and electrochemical transductions.592 CNT-based electrochemical DNA sensors were originally pursued as a result of their high sensitivity, selectivity, and reproducibility. These early sensors relied on the immobilization of single-stranded DNA (ssDNA) on the electrode and changes in electrical current triggered by hybridization of the complementary sequence.527 For example, Wang et al. used CNTs for amplification of enzyme-based electrical sensing of proteins and DNA.591 In this study, CNTs were loaded with alkaline phosphatase (ALP) enzyme tracers, which carried the enzyme tags and preconcentrated the analyte to yield enhanced sensitivity. This scheme generated a low LOD of the target DNA of 1 fg mL−1 (54 aM, 820 copies or 1.3 zmol in the 25 µL sample).591 Similarly, He and Dai reported the covalently functionalization of ssDNA chains onto aligned CNT electrodes to detect complementary DNA and target sequences of DNA.593 The authors used an acetic acid-plasma treatment on aligned CNTs to generate carboxyl groups on the tips and walls of CNTs. They then grafted ssDNA chains through the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) based amide coupling and demonstrated reversible electrochemical responses.
Multiple groups have reported the use of chemFETs to detect the change in resistance of an individual CNT or networks of CNTs in the presence of DNA. Star et al. reported SWCNT-based FET devices that selectively detect the immobilization and hybridization of DNA.594 By monitoring the FET transfer characteristics of the networks of pristine SWCNTs during the addition of ssDNA oligonucleotides, the authors observed a shift of the threshold voltage toward more negative gate voltages upon the initial noncovalent adsorption of ssDNA molecules. Subsequent DNA hybridization with complementary target DNA resulted in a reduction of the measured conductance at a given gate voltage. This detection scheme proved successful in differentiation between mutant and wild-type alleles of the HFE gene that is responsible for hereditary hemochromatosis. To improve the sensitivity of FET-based devices in detecting complementary strands of DNA, Gui et al. introduced a naphthalene-based DNA intercalator that binds selectively to double-stranded DNA.595 After the addition of the intercalator, the sensors hybridized with complementary DNA showed significant reduction in conductivity compared to samples with mismatched DNA. Building on the previous study, Dong et al. also reported the use of DNA-functionalized Au NPs to enhance SWCNT-based FET sensors with a 100 fM LOD, Figure 43.596 In this study, each target DNA binds to the DNA-functionalized Au NPs and the probe DNA immobilized on the SWCNT. The close proximity of the Au NPs to the electrode-SWCNT contacts significantly enhance the change in conductivity upon the binding of the target DNA as measured by transfer characteristics. Interestingly, the authors reported that devices with Ta electrodes exhibited larger enhancement than devices with Au electrodes.596 In the detailed mechanistic study by the same group, they reported that the change in metal-SWCNT junctions, rather than the channel conductance, dominates the sensing of DNA.597 This feature highlights the different electrical features of CNT devices described in the introduction and that there are multiple features that require consideration in developing CNT sensors.
Figure 43.
Detection of femtomolar DNA using Au NPs to enhance SWCNT-based FET sensors. (a) Schematic illustration of the enhancement of DNA detection using reporter DNA-functionalized Au NPs. The right panel illustrates the proposed molecular binding on the FET sensors. (b) Relative decrease in source-drain current for four sensing experiments. The reporter DNA used in this graph is 6A DNA. (c) Comparison of the relative decrease in current versus the concentration of the target DNA enhanced by 6A and 11A reporter DNA-AuNPs. Reproduced with permission from Ref. 596. Copyright 2008, Wiley-VCH Verlag GmbH & Co.
The selectivity endowed by the composites of DNA-CNT sensors is highlighted by the ability to detect of base pair mismatches. Because charge transport can occur over significant distances through the stacked aromatic base pairs of DNA, it is found to be extremely sensitive to the integrity of base pairing. Single base mismatches can attenuate the charge transport through the DNA strand.598 Nuckolls and coworkers described the first measurements of the conductivity of a single DNA duplex wired between an individual SWCNT through covalent bonds, Figure 44a.144 The authors fabricated the devices by cutting individual SWCNTs with an electron beam and then used oxygen plasma to ensure the presence of the carboxylic acid functionalities on both sides of the gap. The gaps were then bridged by ssDNA anchored at both sides by robust amide linkages. Through this device, the difference in conductivity between well-matched and mismatched duplex was clearly observed. Figure 44b shows that a single mismatch (both CA and GT) attenuated the current through the DNA strand. In addition to the use of individual SWCNTs, Weizmann et al. reported networks of ssDNA-bridged CNTs for the chemiresistive detection of complementary DNA, Figure 45.120 This approach made use of formation of oligomeric (SWCNT-ssDNA)n sequences between electrodes. The ssDNA gaps rendered the materials insulating. Selective binding of the ssDNA analyte resulted in the formation of double-stranded DNA assemblies, however the transport through extended sequences of DNA did not introduce sufficient conductivity for detection. To produce robust detection events, horse radish peroxidase (HRP) enzyme functionalized to complement the target DNA was used. The target DNA with HRP would recognize and localize at the gaps between the SWCNTs, and HRP could then be used to deposit silver metal. The net result, shown schematically in Figure 45, was a conductive network wherein the gaps between SWCNTs were connected by conductive silver. Simple measurements of conductivity demonstrated a LOD of 10 fM with ability to discriminate single, double, and triple base pair mismatches.120
Figure 44.
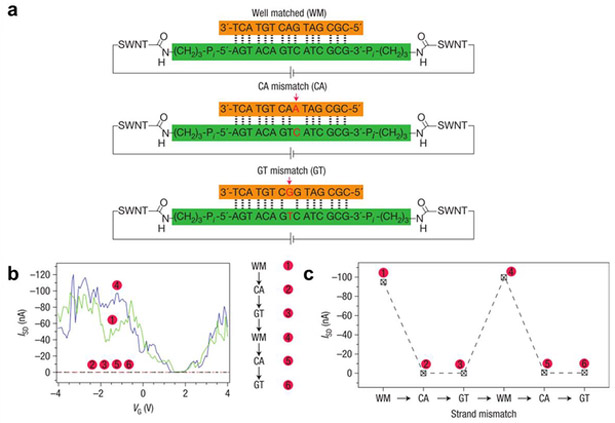
Conductivity of a single DNA duplex bridged between SWCNT. (a) Schematic of the covalent functionalization of SWNCT and DNA strands. Replacing the well-matched (WM) duplexes with DNA CA and GT mismatches resulted in a large effect on the conductance. (b) Transfer characteristics of a SWCNT device taken through the sequence 1 through 6 at source-drain voltage of 50 mV. The current levels for mismatch points (2, 3, 5, and 6) are ~300 times lower than the well-matched points (1 and 4). (c) Current at constant gate voltage (VG) of –3V and source-drain voltage of 50 mV through sequence 1 through 6. Reproduced with permission from Ref. 144. Copyright 2008, Nature Publishing Group.
Figure 45.
CNT-network based DNA detection scheme. (a) Schematic representation of a single junction of a ssDNA/SWCNT construction. (b) Schematic of device architecture and sensing scheme. Hybridization with the target DNA strand results in the spatial localization of horse radish peroxidase (HRP) that can be used to deposit Ag metal to create a conductive bridge. Reproduced with permission from Ref. 120. Copyright 2011, American Chemical Society.
5. CNT-based Sensors for National Security
The use of chemical warfare agents and explosives is unfortunately an ongoing menace to society that is unlikely to cease in the near future. Considering the continued threat of domestic and international terrorism, early detection of these agents can minimize casualties and injuries to military personnel and civilians alike.599 In this part of the review, we will cover the detection of chemical weapons and explosives.
5.1. Chemical Warfare Agents
Chemical Warfare Agents (CWAs) have been used as weapons of mass destruction in military conflicts since World War I, and the use of mustard gas, phosgene, and chlorine caused 1.3 million casualties.600 In recent years, chemical weapons in the hands of terrorists and rogue nations also pose a threat even in peaceful times.601 In this section, we will discuss CNT-based sensors for the detection of CWAs categorized by their biochemical interaction with the victim: nerve agents, vesicating agents, respiratory agents, and blood agents.600,602 For a more general review including the historical context, toxicology and destruction of chemical warfare agents, we refer the interested reader to a review article by Jang et al.603 For a review on the biomonitoring of exposure for diagnostics and verification of CWA usage, we refer to Noort et al.600
Current methods for the detection of CWAs include ion mobility spectroscopy,604 mass spectrometry,604 surface acoustic wave (SAW),605 electrochemical sensors,606 infrared spectroscopy,607 flourescence,608–610 and colorimetric sensors.611,612 Although all of these techniques offer excellent sensitivity and specificity, there are limitations inherent to the different approaches. Analytical techniques like mass and infrared spectroscopies require bulky, sensitive, and/or power-intensive instrumentation that is unsuitable for field work. Colorimetric detectors have excellent portability, however they cannot be used to monitor real-time data and are not readily incorporated into more sophisticated circuitry.372 CNTs offer the opportunity to produce portable sensing methods that naturally couple into electrical devices—based on chemiresisitive, electrochemical, or SAW elements—capable of continuous monitoring of the environment.
5.1.1. Nerve Agents and Vesicant Agents
Nerve agents are compounds that disrupt the nervous system by irreversibly inhibiting the activity of acetylcholinesterase (AChE)—an enzyme responsible for breaking down the neurotransmitter acetylcholine (ACh).602,613 The inhibition of AChE leads to accumulation of ACh in the nerve, which leads to over-stimulation. Early symptoms include agitation and muscle weakness, severe poisoning and respiratory failure, unconsciousness, confusion, convulsions, and death.602,613 Lethal concentrations for typical organophosphorous nerve agents are in concentrations as low as 10–100 ppb.614 As a result of the severe consequences of exposures to these nerve agents, sensors are usually developed by testing on chemically similar but less harmful nerve agent mimics.603 Figure 46 shows the chemical structures of a select number of nerve agents, nerve agent mimics, and decomposition products that were used in the studies covered in this review.
Figure 46.
Chemical structures of selected nerve agents, nerve agent mimics, and decomposition products
Vesicant agents—such as sulfur mustard and nitrogen mustard, Figure 47, cause skin blisters, eye injuries, and respiratory disorders, and were first used as in World War I. Although the cellular and biochemical consequences of exposures are not fully understood, it is known that mustard gas acts as a bifunctional alkylating agent able to react with nucleophiles under physiological conditions.600
Figure 47.
Chemical structures of selected vesicant agents and vesicant agent mimics.
As a result of the high reactivity of nerve agent mimics, even pristine SWCNTs display non-specific sensing responses. Novak et al. first reported the sensitivity of CNT-films to ppb levels of the simulant DMMP in air.41 In addition to a decrease in conductance of the p-doped CNTs, the authors observed a shift in the FET threshold voltage by −2 V, which indicates electron charge donation from the adsorbent to the CNT network. These findings are consistent with the strong electron donating properties of DMMP through the terminal phosphonate oxygen. The decrease in conductance of pristine CNT sensors was also demonstrated with single tube devices,81 and pristine-CNT sensors assembled on flexible substrates.615 In addition to gas phase sensors, Roberts et al. reported liquid phase detectors to determine the concentration of DMMP.51 The DMMP used in the water-based sensors may be partially hydrolyzed which complicates the evaluation of liquid phase sensors of DMMP. Lastly pristine CNTs were also used in chemocapacitors to detect DMMP at ppb levels.616
Several groups have explored sensors based on CNTs functionalized with polymeric materials. Lee et al. applied polypyrrole/CNT composites to increase the chemiresistive response of pristine CNT sensors threefold.617 Complementary, Cattanach et al. used a passivating layer made of polyisobutylene to selectively block interfering vapors (water, hexane, and xylene) and fabricate a chemiresisitive sensor.618 Chuang et al.619 developed a sensor array consisting of 30 channels with 15 different polymer/CNT composites to differentiate between several chemical warfare agents and organic solvents. Although none of the channels show exceptional sensitivity towards DMMP or mustard gas, the array successfully generates unique fingerprints for all tested compounds.
Alternatively, hydrogen-bonding acidic selectors have yielded highly selective and sensitive sensors. A popular molecular moiety is hexafluoroisopropanol (HFIP), first introduced by Snow et al.199 The authors observed a 100 fold increase of the LOD for devices covered in a HFIP-terminated monolayer. Our group has demonstrated the wrapping of SWCNTs with HFIP-substituted polythiophene47 and poly(3,4-ethylenedioxythiophene)106 to increase their sensitivity towards DMMP in chemiresistive sensors (threefold increase over unsubstituted polymer, Figure 48.47 Sensors containing the HFIP-substituted polymer showed high sensitivity toward DMMP over potential interfering VOC gases and water.47,106 Investigations of the sensing mechanism showed a shift of the threshold voltage in FET-measurements to a more negative voltage, suggesting charge donation or trapping of holes by the interaction of the analyte and the SWCNTs. 47 Changes in the Schottky-barrier between SWCNTs and electrodes were eliminated from consideration by comparing the response of devices with polymethylmethacrylate (PMMA) passivation of the electrodes, active SWCNT channel, or both. The completely passivated devices showed no response towards DMMP demonstrating the effectiveness of PMMA to block the diffusion of DMMP, and devices with passivated electrodes showed the same response as sensors containing no PMMA. In addition to HFIP, Kumar et al. reported that the noncovalent functionalization of CNTs with p-hexafluoroisopropanol aniline via dropcasting resulted in a 3.7 fold increase of the chemiresisitive sensing of DMMP.620 Covalent attachment of the same molecule by Kong et al. through diazonium chemistry leads to a 13 fold increase in response towards DMMP.621 Investigation of the transfer characteristics confirmed charge transfer as a significant sensing mechanism. Another hydrogen-bonding molecule used in the sensing of DMMP is tetrafluorohydroquinone (TFQ). Wei et al.622 demonstrated the chemiresistive detection of 20 ppt DMMP using SWCNTs noncovalently with TFQ and identified heavy hole doping as the cause of increased conductance upon exposure to DMMP.
Figure 48.
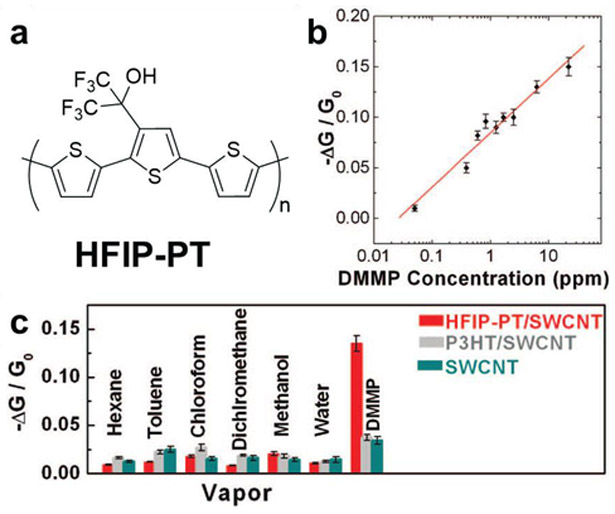
CNT-based sensing of nerve agents. (a) Chemical structure of HFIP-substituted polythiophene, HFIP-PT, (b) change in conductance of the sensor at DMMP concentration of 0.05–25 ppm, and (c) comparison of response towards DMMP and common VOCs. Reproduced with permission from Ref.47. Copyright 2008, American Chemical Society.
Kraatz and coworkers reported several electrochemical sensors for nerve agents and mustard gas mimics based on noncovalent623 and covalent modification of SWCNTs.624,625 As the nerve agents themselves are electrochemically inactive, the authors employed a redox active selector. Specifically, the authors employed ferrocene-amino acid conjugates that were either blended with MWCNTs or attached covalently. Electrochemical or impedance-based sensing allows detection of CWA mimics in water at concentrations in the pM range.
Single-strand DNA (ssDNA) can be used to create stable SWCNT dispersions to create CNT-based sensors. Practitioners of this method make use of the ability of specific DNA dispersants (a) to interact with specific analytes, and (b) to effectively isolate individual SWCNTs making use of π-π-stacking between DNA bases and nanotube sidewalls.98,626 Johnson and coworkers reported the sensing of DMMP using devices containing individual p-type semi-conducting nanotubes that were noncovalently functionalized with different DNA base sequences.627 Although the DNA-DMMP recognition mechanism was not investigated in detail, ssDNA functionalization increased the sensitivity towards DMMP by a factor of 2.5 compared to the pristine device. In a second study, the group developed a CNT-based gravimetric sensor functionalized with the same two ssDNA sequences.628 In the gravimetric device, the CNTs improve the device performance by increasing the surface area and providing the sensor with a large number of mass adsorption sites. However, both base sequences showed the same sensitivity towards DMMP, a threefold increase over pristine CNT sensors. Most recently Johnson and coworkers expanded their work on ssDNA-functionalized CNT-sensors to a wider scope of analytes (enantiomers of limonene, pinene, and homologous carboxylic acids).629,630 Using different ssDNA sequences, the authors were able to differentiate between different isomers and enantiomers. The authors hypothesized that the assembly of DNA on the surface of CNTs generates sequence-specific sets of binding pockets near the CNT sidewall and that binding of analytes in these pockets results in a sequence specific response for different ssDNA-CNT devices. The authors suggest that ssDNA functionalized sensors make promising candidates for the fabrication of electrical noses. Lee at al. used ssDNA- and SDS-wrapped aligned CNTs to detect DMMP where DNA was used as a dispersing agent without investigation of the DNA-analyte interaction.43 Liu et al. developed a chemiresistive sensor in which the CNTs were functionalized with ssDNA after deposition of the tubes on the device.631
Several groups have developed sensors comprising selectors designed to react with chemical warfare agent molecules. Liu et al. 632 used acetylcholinesterase, the enzyme interacting with nerve agents in the body, to selectively react with organophosphates in the electrochemical detection of paraoxon in water. Delalande et al.633 reported the design of a DPCP sensor based on the modulation of the Schottky Barrier between CNTs and gold electrodes. The gold electrodes were functionalized with an organophosphorous-sensitive self-assembled monolayer such that exposure to DPCP leads to intramolecular cyclization under formation of a quaternary ammonium salt, Figure 49. For exposures of just 1 ppm DCPC, this reaction induces a 100-fold increase in conductance of the Au-CNT-Au channel. Ishihara et al.73,107 reported the chemiresistive sensing of DMMP using SWCNTs wrapped with a metallosupramolecular polymers (see Figure 5b). The polymers are designed to depolymerize upon exposure to DMMP, which increases the conduction pathways through the nanotube network and the conductance by five orders of magnitude. This extraordinarily large effect is caused by a non-linear disassembly process, wherein SWCNTs are initially isolated from each other by the metallosupramolecular polymer. Disassembly caused the formation of a conductive SWCTN network.
Figure 49.
CNT-based sensing of nerve agents. (a) Chemical structure of molecule 1 sensitive towards DPCP under formation of a quaternary ammonium salt. (b) Schematic of CNTFET with gold electrodes functionalized with 1, and (c) response of CNTFET sensor with and without 1-covered Au-electrodes upon exposure to DCPC. Reproduced with permission from Ref.633. Copyright 2011, American Chemical Society.
5.1.2. Pulmonary Agents
Pulmonary agents, like chlorine and phosgene, cause lung damage, coughing, dyspnea, and pulmonary edema at high dosage.634,635 Chlorine acts by dissociation to give HCl and HOCl that produce other reactive intermediates capable of causing nitration, chlorination, and dimerization of aromatic amino acids.636 Phosgene acts by acylation of nucleophilic moieties in the body— (i.e. aminio, hydroxyl, and sulfhydryl groups).637 Several groups have developed CNT-based sensors to detect mimics of the pulmonary agents Cl2, phosgene, and SOCl2.
Wongwiriyapan et al. reported a chemiresistive sensor that comprised pristine CNTs grown between Pt electrodes.638 They reported sensitivity towards oxidizing gases like NO2 and Cl2 – 6% and 50% change in resistance for 50 ppb of NO2 and Cl2 respectively - but insensitive towards CO2, H2, alkanes, aromatic hydrocarbons, alcohols, ketones, and carboxylic acids. This detection limit is well within the limit of 1 ppm set by OSHA.316 Lee et al. reported the chemiresistive sensing of thionyl chloride (SOCl2) using sodium dodecyl sulfate (SDS)-wrapped CNTs.43 The sensing mechanism towards SOCl2 was investigated in detail, passivating of the electrode surface did not decrease the response towards SOCl2 excluding sensing Schottky barrier mechanisms, Figure 50a. Raman spectra before and after exposure indicated that the increase in conductance upon exposure was the result of electron transfer form metallic tubes to SOCl2. Additionally, the initial conductance of the sensor is restored by hydrolysis of SOCl2 via exposure of the device to humid air, Figure 50b.
Figure 50.

Sensing behavior of a chemiresistive device containing SDS-dispersed CNTs towards thionyl chloride (SOCl2). (a) Response towards thionyl chloride with and without passivation of the device surface. Left: fully passivated devices showed no response towards thionyl chloride. Right: contact passivated devices, matching the response of non-passivated responses towards thionyl chloride. (b) Regeneration of nanotube surface after exposure to thionyl chloride by exposure of the device. SOCl2 is hydrolyzed and desorbed from the nanotube surface. Reproduced with permission from Ref. 43. Copyright 2006, American Chemical Society.
Several groups have reported sensors for pulmonary agents containing selector units that impart selectivity. Li et al.639 developed sensors that responded selectively to HCl and Cl2 by functionalizing CNTs with chlorosulfonated polyethylene and hydroxypropyl cellulose respectively. The authors hypothesized that the selectivity of the chlorosulfonated polymer towards Cl2 can be attributed to the like-dissolves-like principle of solubility. Likewise, the increased polarity of the –OH groups in the hydroxypropyl-cellulose-coated CNTs was thought to be responsible for the increased response towards HCl. Building on these findings, they developed CNT-arrays containing 32 sensing channels – containing chlorosulfonated polyethylene and hydroxypropyl cellulose functionalized CNTs– to differentiate between Cl2, NO2, HCN, HCl, and a number of VOCs.640,641
Furthermore, CNTs doped with nitrogen, or nitrogen containing functional groups, have been used to detect Cl2. Gohier et al. used as-grown, annealed, nitrogen-doped, and polyethyleneimine (PEI) functionalized CNTs to detect Cl2.642 The authors first investigated the influence of structural defects on the CNT sensor. Nitrogen-doped CNTs where fabricated by adding 35 vol% NH3 to the argon carrier gas during CNT-synthesis. For both the as-grown and the annealed CNTs exposure to Cl2 induces an increase in the conductance of the CNT network. For nitrogen-doped or PEI coated CNTs however, exposure to Cl2 induces a decrease in the conductance of the CNT network. Overall, the annealed CNTs showed the strongest response towards Cl2. The authors hypothesized that the difference in response is due to the initial doping level of the CNT material and that the decrease in conductance is indicative of the n-type semiconducting properties of nitrogen containing sensors. Lee et al. investigated the influence of nitrogen-containing dopants on the semiconducting properties and the resulting sensitivity to SOCl2.643 In agreement with Gohier at al.,642 the authors observed sensing behavior indicative of an n-typed semiconductor for PEI-functionalized CNTs.
Apart from these organic selectors, several inorganic materials have found application in the sensing of pulmonary agents. Multiple groups reported the detection of chlorine using metal NPs/CNT composites which are based on the catalytic interaction between metal and Cl2. Popa et al. developed Cl2 sensors that comprised CNTs blended with hollow Pt-nanocubes, exhibiting a 6-fold increase in sensitivity over pristine CNT sensors.644 The increased response upon addition of nanostructured Pt was attributed to the increases surface area and the reductive dissociation of Cl2 catalyzed by Pt. Similarly, Choi et al. fabricated sensors in which the Pt-nanoparticles were grafted directly on the sidewalls of the CNTs.645 Their sensors showed excellent long-term stability with10.3% change in resistance for 1 ppm of Cl2 for newly fabricated sensors that is retained after storing under humid conditions for 6 months. Sharma et al. reported a chemiresistive sensor containing CNT/zinc phthalocyanine composites that display an increase in conductance upon exposure to Cl2.646 Raman spectroscopic investigations indicated that Cl2 is bound to the Zn center during exposure. A shift of the Zn-core levels towards higher binding energies, as observed from X-ray photoelectron spectra before and during exposure of the selector to Cl2, indicates a strong electron transfer to Cl2.
5.1.3. Blood Agents
Blood agents act by disrupting the transport of oxygen through the body. Common examples of these agents are carbon monoxide (CO) and cyanide. CO, covered previously in Section 2.1.3 Carbon Monoxide (CO), binds to the heme groups in hemoglobin, compromising the transport of oxygen from the lungs to the tissue.647,648 Cyanide binds to the heme center in the enzyme cytochrome c oxidase, and thus prevents the utilization of oxygen in the body. Poisoning with cyanide or carbon monoxide causes nausea, dizziness, and headaches, and larger doses can cause loss of consciousness and death.649
Only a few examples of CNT-based cyanide sensors have been reported. Srivastava et al. reported the theoretical investigation of HCN binding on pristine CNTs.650 In this study, HCN was found to physisorb weakly on the CNT sidewalls with negligible charge transfer. B-doped CNTs, as investigated by Zhang et al. computationally, demonstrate electron transfer from HCN to the CNTs with a much increased binding energy when compared to pristine CNTs.295 These findings indicate that pristine CNT sensors are unlikely to show significant responses to HCN, but that sensors including selectors with electron withdrawing functionality might impart sensitivity. Yari et al. reported the electrochemical detection of cyanide using MWCNTs filled with AgNO3, Figure 51.651 Cyanide is detected selectively over thiocyanate, chloride, iodide, hydroxide, and acetate, the authors attribute the selectivity to the capability of Ag(I) ions to form well-known complexes with cyanide as AgCN2−and Ag[AgCN2].
Figure 51:
Sensing of blood agent HCN. (a) Schematic representation of sensing mechanism, nitrate anion in AgNO3 filled CNTs (orange) is replaced by cyanide at electrode-solution interface. (b) Potential response of electrochemical sensor towards common anions compared to cyanide (filled circles). Reproduced with permission from Ref. 651. Copyright 2011, Springer.
5.2. Explosives
Explosive-based weapons are the most common method of carrying out terrorist attacks, they are highly destructive and relatively easy to construct.652,653 To prevent terrorist activity, fast and reliable distributed sensing of explosives is of utmost importance. As the detection of high boiling explosive nitroaromatic compounds like 2,4,6-trinitrotoluene (TNT) in air is complicated by their low vapor pressure, several groups have developed sensors for the precursors or impurities associated with TNT, including nitrotoluene or dinitrotoluene. Additionally, there are non-explosive markers such as cyclohexanone, which is used to recrystallize the highly explosive RDX as part of its production. Figure 52 shows the chemical structure of the explosive compounds and markers covered in this section.
Figure 52.
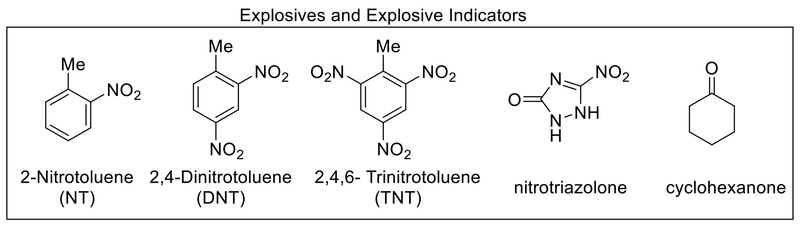
Chemical structures of explosives and secondary explosive indicators.
Pristine CNTs have been demonstrated to be sensitive towards of nitroaromatic compounds. Theoretical and experimental investigations confirm that interactions between the electron accepting nitroaromatics and CNT are dominated by π-π stacking with minor charge transfer characteristics.654,655 These weaker interactions would seem to indicate that the response of pristine CNT sensors is significantly lower than the response towards the strong electron acceptor, NO2. Li et al. compared the chemiresistive detections between nitrotoluene and NO2 in which the exposure to nitrotoluene induced a semi-reversible increase in conductance with two orders of magnitude lower than the response of the same sensor towards NO2.240 Ruan et al. coated a piezo-electrical microcantilever with pristine CNTs to detect TNT.656 During heating of the cantilever, adsorbed nitroaromatic compounds decompose exothermically and this extra heat increases the bending of the microcantilever, this bending can be read out through the piezo electrical element. Liu et al. reported the chemiresistive sensing of TNT in water in a microfluidic system, with a detection limit of 1 ppm.657 Chen et al. fabricated flexible pristine CNT sensors which can detect TNT down to 8 ppb.658 Kumar et al. used films of pristine CNTs to detect DNT at 0.2–2 ppm chemiresistively. Here the response to DNT is semi-reversible, and exposure to UV light allows the complete recovery of the sensor.659 Although sensitive, these pristine CNT explosive sensors are of limited selectivity.
Biosensors consisting of DNA or peptide functionalized CNTs show promise in the selective detection of explosives. The ssDNA sensors covered in Section 5.1.1 Nerve Agents and Vesicant Agents have also been used to detect explosives. Different base sequences lead to different response towards specific explosives allowing selective detection of specific targets.627,628,631 Kim et al. used the tripeptide receptor tryptophan-histidine-triptophan to selectively detect TNT with CNTs. In this scheme they anchored the tripeptide to a polydiacetylene polymer which was then used to form a lipid layer over a SWCNT device, Figure 53.660 The binding of TNT by the receptor induces an increase in the conductance of the active layer, which the authors attribute to the charge acceptor properties of TNT.
Figure 53.
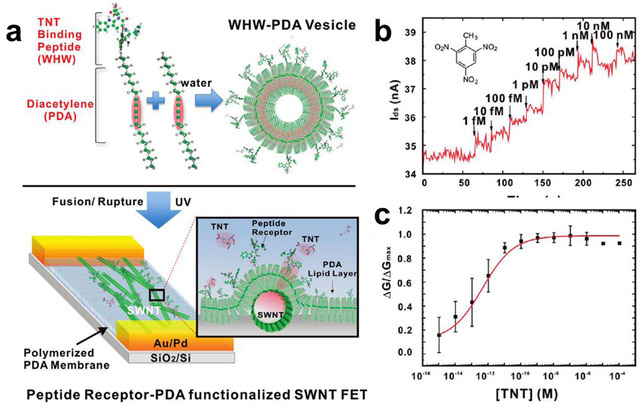
Chemiresistive sensing of 2,4,6-trinitrotoluene (TNT). (a) Schematic of peptide-based TNT sensor. TNT-binding peptide (WHW) conjugated with diacetylene (PDA), after assembly of WHW-PDA nanovesicles in water, the nanovesicle is applied to the SWCNT-device. The diacetylene is then polymerized via application of UV light. (b) Real-time change in conductance of WHW-PDA coated SWCNTs upon injection (arrow) of TNT and (c) calibration curve of response as a function of TNT concentration. Reproduced with permission from Ref. 660. Copyright 2011, American Chemical Society.
Zhang et al. used carbazolylethynylene oligomer-wrapped CNTs to selectively sense nitroaromatics in a chemiresistive devices, Figure 54.661 When comparing devices containing pristine CNTs with devices containing wrapped CNTs, the authors observed the opposite responses towards nitroaromatics. For devices containing pristine CNTs, an increase in conductance was observed, consistent with the charge accepting character of nitroaromatics. For devices containing wrapped CNTs, a decrease in conductance was observed which the authors attributed to swelling of the oligomeric material and the resulting increase in separation between CNTs, Figure 54c. By employing channels of both pristine and wrapped CNTs, the authors were able to separate vapors of TNT, DNT, and NT.
Figure 54.
Chemiresistive sensing of nitroaromatics. (a) Molecular structure of Tg-Car oligomer with strong affinity to nitroaromatic explosive compounds. (b) Sensing behavior of wrapped (red) and pristine (blue) CNTs towards 4-nitrotoluene (NT), 2,4-dinitrotoluene (DNT), and 2,4,6-trinitrotoluene (TNT). (c) Schematic of sensing mechanism demonstrating the swelling of oligomer wrapped CNTs when exposed to nitroaromatics. Reproduced with permission from Ref. 661. Copyright 2015, American Chemical Society.
In addition, Hrapovic et al. used Cu nanoparticle/MWCNT composites to detect TNT electrochemically.662 Using Cu NP electrodes, TNT exhibits three well-defined redox peaks corresponding to sequential reduction of the three nitro groups to hydroxylamines, permitting the detection of 1 ppb of TNT in Milli-Q water and 50 ppb in complex backgrounds (river water or soil samples). Li et al. used a β-cyclodextin/MWCNT/graphene oxide composites to detect DNT.663 Similarly, the nitroaromatic can be detected in complex backgrounds including groundwater and soil samples. The cylic oligosaccharide, β-cyclodextin, forms hydrophobic pockets in water that can bind DNT and facilitate the detection of TNT at much lower concentrations than is possible with plain carbon electrodes. In addition to electrochemical sensing in water, Wei et al. reported the chemiresistive sensing of nitroaromatics in water using 1-pyrenemethylamine functionalized CNTs.86 The authors report sensitivity for concentrations as low as 10 ppt and good selectivity for TNT over other nitroaromatics.
An alternative approach to sensing the explosive compound or precursors to the explosive compound, is the detection of secondary signatures such as solvents that are commonly used in the production of explosive compounds. Cyclohexanone is a recrystallizing agent for RDA and is found in the headspace of plastic explosives and thereby is a targets for explosives detection.664 Frazier and Swager demonstrated the chemiresistive sensing of cyclohexanone using thiourea and/or urea functionalized SWCNTs.89,122 The thiourea motif is known to bind ketones (e.g., cyclohexanone) through a two-point hydrogen bonding. They reported the covalent functionalization of polymer matrix materials that surround the SWCNTs with thiourea derivatives, which increased the response towards cyclohexanone by 100% when compared to pristine CNTs.122 They also demonstrated a selective and robust sensors using a trifunctional selector (Figure 55a) which can form a cross-linked polymeric network on the surface of the device to prevent the phase separation of selector and CNT.89 Figure 55b shows the response of a sensor containing this trifunctional selector towards 10–150 ppm cyclohexanone; the selector showed a two-fold increase over pristine CNTs and polymerization of the triethoxysilane substituents increases the robustness of the selector to heat treatment. The sensors had a highly reproducible response and were sufficiently robust to survive sonication in methanol solution.
Figure 55.
Chemiresistive sensing of cyclohexanone. (a) Schematic sensor containing the chemical structures of trifunctional Selector 1, SWCNT, and sensing device. (b) Sensing traces using a blend of Selector 1 and SWCNTs to detect cyclohexanone at different concentrations. Reproduced with permission from Ref. 89. Copyright 2013, American Chemical Society.
6. Summary and Concluding Remarks
CNTs are promising active elements of electronic chemical sensors. In this review, we first present the fundamentals of these sensors: sensing mechanism, chemical CNT functionalization, device architectures, fabrication methods, performance parameters, and computational models. The reader is then introduced to CNT-based sensors and target analytes for a variety of applications: environmental monitoring, food and agricultural applications, biological sensors, and national security. These discussions are intended to impart a working knowledge of the current state of the field to the reader.
The diversity of sensing schemes that have been reported with CNTs is clearly expansive. There are many examples that are not designed from an intuitive chemical basis or from known solution chemistry and reactivity. This approach is not specific to CNT sensors, and although it is tempting to be dismissive of these empirical methods, some of these schemes may be highly effective in a specific environment. Indeed, in specific applications, there may not be many interferants or a pressing need for precise selectivity. For example, inorganic gases like SO2 and NO2, generate a response from almost all CNT-based chemical sensors. In applications however, where these gasses do not occur, their interference becomes a non-issue. Similarly, sensors that respond to NH3 or biogenic amines will likely have cross reactivity with DMSO, DMF, THF, phosphines, or organic sulfides. If such sensors were designed to ensure the integrity of food packaging, these chemicals are just as problematic as the amines. Thus, preventing reactivity towards these interferants is not always required. However, in general the best and most robust sensors involve well-conceived chemical or biological principles that recognize unique molecular attributes and reactivity of the analytes.
Advances in the sophistication by which CNTs can be functionalized and organized are certain to play a role in evolving this technology. The modularity by which CNTs can be functionalized is allowing for rapid development of sensor methods and it is clear that we will see a wave of commercial electrical CNT sensors that rival and likely surpass most other chemical sensor types. It is our hope that this review will serve as a guide to developments that can contribute to the adoption of new sensory methods that can protect and improve our environment, safety and health.
Acknowledgements
The authors of this article were supported by: the KAUST sensor project CRF-2015-SENSORS2719 and the Army Research Office through the Institute for Soldier Nanotechnologies and National Science Foundation (DMR-1410718). S.S. and S.L. were supported by F32 Ruth L. Kirschstein National Research Service Awards. M.H. was supported by NIH Training Grant T32ES007020.
Author Biographies
Vera Schroeder received her B.S. degree in chemistry and her M.S. degree in materials chemistry from RWTH Aachen University, where she conducted research with Prof. Paul Kögerler in the Department of Inorganic Chemistry and Prof. Ting Xu in the Department of Materials Science & Engineering at the University of California, Berkeley. In 2014, Vera started her Ph.D. in Inorganic Chemistry at MIT under the guidance of Prof. Timothy M. Swager. Her Ph.D. research focusses on the investigation of carbon nanotube-based chemical sensors and their application.
Suchol Savagatrup earned his B.S. in chemical engineering from University of California, Berkeley in 2012, where he conducted research with Prof. Rachel Segalman in the Department of Chemical and Biomolecular Engineering and Dr. Adam Weber at Lawrence Berkeley National Laboratory. He completed his Ph.D. in 2016 at the University of California, San Diego, under the direction of Prof. Darren Lipomi where his work was funded by the NSF Graduate Research Fellowship and the Kaplan Dissertation Year Fellowship. Suchol is currently a postdoctoral fellow in the Swager lab at MIT and a recipient of the Ruth L. Kirschstein Postdoctoral Fellowship.
Maggie He completed her undergraduate studies at the City College of New York and earned her B.S. degree in chemistry in 2008, where she performed research with Prof. Barbara Zajc. She received her M.S. degree in chemistry in 2010 from University of Pennsylvania and Ph.D. degree in chemistry in 2014 from ETH Zürich under the supervision of Prof. Jeffrey Bode. Maggie is currently a postdoctoral fellow in the Swager group and a recipient of the Swiss National Science Foundation (SNF) Early Postdoc Mobility Fellowship.
Sibo Lin began his studies at Indiana University, where he conducted computational chemistry research under the guidance of Prof. My-Hyun Baik and Prof. Jennifer C. Green at the University of Oxford, UK, and earned B.S. degrees in Mathematics and Chemistry in 2008. Sibo then continued his studies at the California Institute of Technology with Prof. Theodor Agapie in synthetic inorganic chemistry with support from the NSF Graduate Research Fellowship, finishing his Ph.D. in 2014. Sibo is currently a postdoctoral researcher at MIT with the Swager group and a recipient of the Ruth L. Kirschstein Postdoctoral Fellowship.
Timothy M. Swager is the John D. MacArthur Professor of Chemistry and the Director, Deshpande Center for Technological Innovation at the Massachusetts Institute of Technology. A native of Montana, he received a BS from Montana State University in 1983 and a Ph.D. from the California Institute of Technology in 1988. After a postdoctoral appointment at MIT he was on the chemistry faculty at the University of Pennsylvania and returned to MIT in of 1996 as a Professor of Chemistry. His research has been focused at the interface of chemistry and materials science with a particular interest on chemical and biological sensing.
References
- (1).De Volder MFL; Tawfick SH; Baughman RH; Hart AJ Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [DOI] [PubMed] [Google Scholar]
- (2).Schnorr JM; Swager TM Emerging Applications of Carbon Nanotubes. Chem. Mater 2011, 23, 646–657. [Google Scholar]
- (3).Hu L; Hecht DS; Grüner G Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev 2010, 110, 5790–5844. [DOI] [PubMed] [Google Scholar]
- (4).Cao Q; Rogers J. a. Ultrathin Films of Single-Walled Carbon Nanotubes for Electronics and Sensors: A Review of Fundamental and Applied Aspects. Adv. Mater 2009, 21, 29–53. [Google Scholar]
- (5).Park S; Vosguerichian M; Bao Z A Review of Fabrication and Applications of Carbon Nanotube Film-Based Flexible Electronics. Nanoscale. 2013, pp 1727–1752. [DOI] [PubMed] [Google Scholar]
- (6).Shulaker MM; Hills G; Patil N; Wei H; Chen H-YY; Wong H-SPSP; Mitra S Carbon Nanotube Computer. Nature 2013, 501, 526–530. [DOI] [PubMed] [Google Scholar]
- (7).Hong G; Diao S; Antaris AL; Dai H Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev 2015, 115, 10816–10906. [DOI] [PubMed] [Google Scholar]
- (8).Wang J Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis 2005, 17, 7–14. [Google Scholar]
- (9).Meyyappan M Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [DOI] [PubMed] [Google Scholar]
- (10).Kauffman DR; Star A Carbon Nanotube Gas and Vapor Sensors. Angew. Chem. Int. Ed. Engl 2008, 47, 6550–6570. [DOI] [PubMed] [Google Scholar]
- (11).Tasis D; Tagmatarchis N; Bianco A; Prato M Chemistry of Carbon Nanotubes. Chem. Rev 2006, 106, 1105–1136. [DOI] [PubMed] [Google Scholar]
- (12).Ding L; Tselev A; Wang J; Yuan D; Chu H; McNicholas TP; Li Y; Liu J Selective Growth of Well-Aligned Semiconducting Single-Walled Carbon Nanotubes. Nano Lett 2009, 9, 800–805. [DOI] [PubMed] [Google Scholar]
- (13).Harutyunyan AR; Chen G; Paronyan TM; Pigos EM; Kuznetsov OA; Hewaparakrama K; Kim SM; Zakharov D; Stach EA; Sumanasekera GU Preferential Growth of Single-Walled Carbon Nanotubes with Metallic Conductivity. Science 2009, 326, 116–120. [DOI] [PubMed] [Google Scholar]
- (14).Arnold MS; Green AA; Hulvat JF; Stupp SI; Hersam MC Sorting Carbon Nanotubes by Electronic Structure Using Density Differentiation. Nat. Nanotechnol 2006, 1, 60–65. [DOI] [PubMed] [Google Scholar]
- (15).Lee HW; Yoon Y; Park S; Oh JH; Hong S; Liyanage LS; Wang H; Morishita S; Patil N; Park YJ; et al. Selective Dispersion of High Purity Semiconducting Single-Walled Carbon Nanotubes with Regioregular Poly(3-Alkylthiophene)S. Nat. Commun 2011, 2, 541. [DOI] [PubMed] [Google Scholar]
- (16).Wang H; Koleilat GI; Liu P; Jimenez-Oses G; Lai Y; Vosgueritchian M; Fang Y; Park S; Houk KN; Bao Z High-Yield Sorting of Small-Diameter Carbon Nanotubes for Solar Cells and Transistors. ACS Nano 2014, 8, 2609–2617. [DOI] [PubMed] [Google Scholar]
- (17).Tanaka T; Urabe Y; Nishide D; Kataura H Continuous Separation of Metallic and Semiconducting Carbon Nanotubes Using Agarose Gel. Appl. Phys. Express 2009, 2, 125002. [Google Scholar]
- (18).Yomogida Y; Tanaka T; Zhang M; Yudasaka M; Wei X; Kataura H Industrial-Scale Separation of High-Purity Single-Chirality Single-Wall Carbon Nanotubes for Biological Imaging. Nat. Commun 2016, 7, 12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Povie G; Segawa Y; Nishihara T; Miyauchi Y; Itami K Synthesis of a Carbon Nanobelt. Science. 2017, 356, 172–175. [DOI] [PubMed] [Google Scholar]
- (20).Swager TM The Molecular Wire Approach to Sensory Signal Amplification. Acc. Chem. Res 1998, 31, 201–207. [Google Scholar]
- (21).Kong J; Franklin NR; Zhou C; Chapline MG; Peng S; Cho K; Dai H; Franklin RN; Zhou C; Chapline MG; et al. Nanotube Molecular Wires as Chemical Sensors. Science 2000, 287, 622–625. [DOI] [PubMed] [Google Scholar]
- (22).Peveler WJ; Yazdani M; Rotello VM Selectivity and Specificity: Pros and Cons in Sensing. ACS Sensors 2016, 1, 1282–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Chen RJ; Zhang Y; Wang D; Dai H Noncovalent Sidewall Functionalization of Single-Walled Carbon Nanotubes for Protein Immobilization. J. Am. Chem. Soc 2001, 123, 3838–3839. [DOI] [PubMed] [Google Scholar]
- (24).Chen RJ; Choi HC; Bangsaruntip S; Yenilmez E; Tang X; Wang Q; Chang YL; Dai H An Investigation of the Mechanisms of Electronic Sensing of Protein Adsorption on Carbon Nanotube Devices. J. Am. Chem. Soc 2004, 126, 1563–1568. [DOI] [PubMed] [Google Scholar]
- (25).Zhou Q; Swager TM Fluorescent Chemosensors Based on Energy Migration in Conjugated Polymers: The Molecular Wire Approach to Increased Sensitivity. J. Am. Chem. Soc 1995, 117, 12593–12602. [Google Scholar]
- (26).Barone PW; Baik S; Heller DA; Strano MS Near-Infrared Optical Sensors Based on Single-Walled Carbon Nanotubes. Nat. Mater 4, 86–92. [DOI] [PubMed] [Google Scholar]
- (27).Boghossian AA; Zhang J; Barone PW; Reuel NF; Kim JH; Heller DA; Ahn JH; Hilmer AJ; Rwei A; Arkalgud JR; et al. Near-Infrared Fluorescent Sensors Based on Single-Walled Carbon Nanotubes for Life Sciences Applications. ChemSusChem 2011, 4, 848–863. [DOI] [PubMed] [Google Scholar]
- (28).Yang W; Ratinac KR; Ringer SR; Thordarson P; Gooding JJ; Braet F Carbon Nanomaterials in Biosensors: Should You Use Nanotubes or Graphene. Angew. Chemie - Int. Ed 2010, 49, 2114–2138. [DOI] [PubMed] [Google Scholar]
- (29).Kauffman DR; Star A Graphene versus Carbon Nanotubes for Chemical Sensor and Fuel Cell Applications. Analyst 2010, 135, 2790–2797. [DOI] [PubMed] [Google Scholar]
- (30).Wei N; Liu Y; Xie H; Wei F; Wang S; Peng LM Carbon Nanotube Light Sensors with Linear Dynamic Range of over 120 DB. Appl. Phys. Lett 2014, 105, 073107. [Google Scholar]
- (31).Yang N; Chen X; Ren T; Zhang P; Yang D Carbon Nanotube Based Biosensors. Sensors Actuators, B Chem 2015, 207, 690–715. [Google Scholar]
- (32).Llobet E Gas Sensors Using Carbon Nanomaterials: A Review. Sensors Actuators, B Chem 2013, 179, 32–45. [Google Scholar]
- (33).Ellis JE; Star A Carbon Nanotube Based Gas Sensors toward Breath Analysis. Chempluschem 2016, 81, 1248–1265. [DOI] [PubMed] [Google Scholar]
- (34).Wang Y; Yeow JTW A Review of Carbon Nanotubes-Based Gas Sensors. J. Sensors 2009, 2009, 1–24. [Google Scholar]
- (35).Fennell JF; Liu SF; Azzarelli JM; Weis JG; Rochat S; Mirica KA; Ravnsbaek JB; Swager TM Nanowire Chemical/Biological Sensors: Status and a Roadmap for the Future. Angew. Chemie Int. Ed 2016, 55, 1266–1281. [DOI] [PubMed] [Google Scholar]
- (36).Bondavalli P; Legagneux P; Pribat D Carbon Nanotubes Based Transistors as Gas Sensors: State of the Art and Critical Review. Sensors Actuators, B Chem 2009, 140, 304–318. [Google Scholar]
- (37).Boyd A; Dube I; Fedorov G; Paranjape M; Barbara P Gas Sensing Mechanism of Carbon Nanotubes: From Single Tubes to High-Density Networks. Carbon N. Y 2014, 69, 417–423. [Google Scholar]
- (38).Zhao J; Buldum A; Han J; Lu JP Gas Molecule Adsorption in Carbon Nanotubes and Nanotube Bundles. Nanotechnology 2002, 13, 195–200. [Google Scholar]
- (39).Collins PG; Bradley K; Ishigami M; Zettl A Extreme Oxygen Sensitivity of Electronic Properties of Carbon Nanotubes. 2000, 287, 1801–1804. [DOI] [PubMed] [Google Scholar]
- (40).Kang D; Park N; Ko J; Bae E; Park W Oxygen-Induced p-Type Doping of a Long Individual Single-Walled Carbon Nanotube. Nanotechnology 2005, 16, 1048–1052. [Google Scholar]
- (41).Novak JP; Snow ES; Houser EJ; Park D; Stepnowski JL; McGill RA Nerve Agent Detection Using Networks of Single-Walled Carbon Nanotubes. Appl. Phys. Lett 2003, 83, 4026–4028. [Google Scholar]
- (42).Peng G; Tisch U; Haick H Detection of Nonpolar Molecules by Means of Carrier Scattering in Random Networks of Carbon Nanotubes: Toward Diagnosis of Diseases via Breath Samples. Nano Lett 2009, 9, 1362–1368. [DOI] [PubMed] [Google Scholar]
- (43).Lee CY; Baik S; Zhang J; Masel RI; Strano MS Charge Transfer from Metallic Single-Walled Carbon Nanotube Sensor Arrays. J. Phys. Chem. B 2006, 110, 11055–11061. [DOI] [PubMed] [Google Scholar]
- (44).Udomvech A; Kerdcharoen T; Osotchan T First Principles Study of Li and Li+ Adsorbed on Carbon Nanotube: Variation of Tubule Diameter and Length. Chem. Phys. Lett 2005, 406, 161–166. [Google Scholar]
- (45).Zanolli Z; Leghrib R; Felten A; Pireaux J-JJ; Llobet E; Charlier J-CC Gas Sensing with Au-Decorated Carbon Nanotubes. ACS Nano 2011, 5, 4592–4599. [DOI] [PubMed] [Google Scholar]
- (46).Cao C; Kemper AF; Agapito L; Zhang J-W; He Y; Rinzler A; Cheng H-P; Zhang X-G; Rocha AR; Sanvito S Nonequilibrium Green’s Function Study of Pd4-Cluster-Functionalized Carbon Nanotubes as Hydrogen Sensors. Phys. Rev. B 2009, 79, 075127. [Google Scholar]
- (47).Wang F; Gu H; Swager TM Carbon Nanotube/Polythiophene Chemiresistive Sensors for Chemical Warfare Agents. J. Am. Chem. Soc 2008, 130, 5392–5393. [DOI] [PubMed] [Google Scholar]
- (48).Star A; Han TR; Joshi V; Gabriel JCP; Grüner G Nanoelectronic Carbon Dioxide Sensors. Adv. Mater 2004, 16, 2049–2052. [Google Scholar]
- (49).Star A; Joshi V; Skarupo S; Thomas D; Gabriel J-CP Gas Sensor Array Based on Metal-Decorated Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 21014–21020. [DOI] [PubMed] [Google Scholar]
- (50).Zhang T; Mubeen S; Myung NV; Deshusses MA Recent Progress in Carbon Nanotube-Based Gas Sensors. Nanotechnology 2008, 19, 332001. [DOI] [PubMed] [Google Scholar]
- (51).Roberts ME; LeMieux MC; Bao Z Sorted and Aligned Single-Walled Carbon Nanotube Networks for Transistor-Based Aqueous Chemical Sensors. ACS Nano 2009, 3, 3287–3293. [DOI] [PubMed] [Google Scholar]
- (52).Star A; Gabriel JCP; Bradley K; Grüner G Electronic Detection of Specific Protein Binding Using Nanotube FET Devices. Nano Lett 2003, 3, 459–463. [Google Scholar]
- (53).Heller I; Janssens AM; Männik J; Minot ED; Lemay SG; Dekker C; Mannik J; Minot ED; Lemay SG; Dekker C; et al. Identifying the Mechanism of Biosensing with Carbon Nanotube Transistors. Nano Lett 2008, 8, 591–595. [DOI] [PubMed] [Google Scholar]
- (54).Rao AM; Eklund PC; Bandow S; Thess A; Smalley RE Evidence for Charge Transfer in Doped Carbon Nanotube Bundles from Raman Scattering. Nature 1997, 388, 257–259. [Google Scholar]
- (55).Hatting B Optical and Vibrational Properties of Doped Carbon Nanomaterials Ph.D. Dissertation, Freie Universitat Berlin, 2016. [Google Scholar]
- (56).Monteiro FH; Larrude DG; Maia Da Costa MEH; Freire FL Estimating the Boron Doping Level on Single Wall Carbon Nanotubes Using Raman Spectroscopy. Mater. Lett 2013, 92, 224–226. [Google Scholar]
- (57).Panchakarla LS; Govindaraj A; Rao CNR Nitrogen- and Boron-Doped Double-Walled Carbon Nanotubes. ACS Nano 2007, 1, 494–500. [DOI] [PubMed] [Google Scholar]
- (58).Lyu SC; Han JH; Shin KW; Sok JH Synthesis of Boron-Doped Double-Walled Carbon Nanotubes by the Catalytic Decomposition of Tetrahydrofuran and Triisopropyl Borate. Carbon N. Y 2011, 49, 1532–1541. [Google Scholar]
- (59).Voggu R; Rout CS; Franklin AD; Fisher TS; Rao CNR Extraordinary Sensitivity of the Electronic Structure and Properties of Single-Walled Carbon Nanotubes to Molecular Charge-Transfer. J. Phys. Chem. C 2008, 112, 13053–13056. [Google Scholar]
- (60).Shin H-J; Kim SM; Yoon S-M; Benayad A; Kim KK; Kim SJ; Park HK; Choi J-Y; Lee YH Tailoring Electronic Structures of Carbon Nanotubes by Solvent with Electron-Donating and -Withdrawing Groups. J. Am. Chem. Soc 2008, 130, 2062–2066. [DOI] [PubMed] [Google Scholar]
- (61).Yim W-L; Gong XG; Liu Z-F Chemisorption of NO 2 on Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 9363–9369. [Google Scholar]
- (62).Ricca A; Bauschlicher CW The Adsorption of NO2 on (9,0) and (10,0) Carbon Nanotubes. Chem. Phys 2006, 323, 511–518. [Google Scholar]
- (63).Zhang Y; Suc C; Liu Z; Li J Carbon Nanotubes Functionalized by NO2: Coexistence of Charge Transfer and Radical Transfer. J. Phys. Chem. B 2006, 110, 22462–22470. [DOI] [PubMed] [Google Scholar]
- (64).Soylemez S; Yoon B; Toppare L; Swager TM Quaternized Polymer-Single-Walled Carbon Nanotube Scaffolds for a Chemiresistive Glucose Sensor. ACS Sensors 2017, 2, 1123–1127. [DOI] [PubMed] [Google Scholar]
- (65).Li C; Thostenson ET; Chou TW Dominant Role of Tunneling Resistance in the Electrical Conductivity of Carbon Nanotube-Based Composites. Appl. Phys. Lett 2007, 91. [Google Scholar]
- (66).Hu N; Karube Y; Yan C; Masuda Z; Fukunaga H Tunneling Effect in a Polymer/Carbon Nanotube Nanocomposite Strain Sensor. Acta Mater 2008, 56, 2929–2936. [Google Scholar]
- (67).Ponnamma D; Sadasivuni KK; Strankowski M; Guo Q; Thomas S Synergistic Effect of Multi Walled Carbon Nanotubes and Reduced Graphene Oxides in Natural Rubber for Sensing Application. Soft Matter 2013, 9, 10343–10353. [Google Scholar]
- (68).Liu SF; Moh LCH; Swager TM Single-Walled Carbon Nanotube–Metalloporphyrin Chemiresistive Gas Sensor Arrays for Volatile Organic Compounds. Chem. Mater 2015, 27, 3560–3563. [Google Scholar]
- (69).Wang HC; Li Y; Yang MJ Sensors for Organic Vapor Detection Based on Composites of Carbon Nonotubes Functionalized with Polymers. Sensors Actuators, B Chem 2007, 124, 360–367. [Google Scholar]
- (70).Niu L; Luo Y; Li Z A Highly Selective Chemical Gas Sensor Based on Functionalization of Multi-Walled Carbon Nanotubes with Poly(Ethylene Glycol). Sensors Actuators, B Chem 2007, 126, 361–367. [Google Scholar]
- (71).Wei C; Dai L; Roy A; Tolle TB Multifunctional Chemical Vapor Sensors of Aligned Carbon Nanotube and Polymer Composites. J. Am. Chem. Soc 2006, 128, 1412–1413. [DOI] [PubMed] [Google Scholar]
- (72).Philip B; Abraham JK; Chandrasekhar A Carbon Nanotube / PMMA Composite Thin Films for Gas-Sensing Applications. Smart Mater. Struct 2003, 935, 1–6. [Google Scholar]
- (73).Ishihara S; O’Kelly CJ; Tanaka T; Kataura H; Labuta J; Shingaya Y; Nakayama T; Ohsawa T; Nakanishi T; Swager TM Metallic versus Semiconducting SWCNT Chemiresistors: A Case for Separated SWCNTs Wrapped by a Metallosupramolecular Polymer. ACS Appl. Mater. Interfaces 2017, 9, 38062–38067. [DOI] [PubMed] [Google Scholar]
- (74).Lobez JM; Swager TM Radiation Detection: Resistivity Responses in Functional Poly(Olefin Sulfone)/Carbon Nanotube Composites. Angew. Chemie - Int. Ed 2010, 49, 95–98. [DOI] [PubMed] [Google Scholar]
- (75).Zeininger L; He M; Hobson ST; Swager TM Resistive and Capacitive γ-Ray Dosimeters Based On Triggered Depolymerization in Carbon Nanotube Composites. ACS Sensors 2018, 3, 976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Peng N; Zhang Q; Chow CL; Tan OK; Marzari N Sensing Mechanisms for Carbon Nanotube Based NH 3 Gas Detection. Nano 2009, 9, 1–7. [DOI] [PubMed] [Google Scholar]
- (77).Bradley K; Gabriel JCP; Star A; Grüner G Short-Channel Effects in Contact-Passivated Nanotube Chemical Sensors. Appl. Phys. Lett 2003, 83, 3821–3823. [Google Scholar]
- (78).Liu X; Luo Z; Han S; Tang T; Zhang D; Zhou C Band Engineering of Carbon Nanotube Field-Effect Transistors via Selected Area Chemical Gating. Appl. Phys. Lett 2005, 86, 1–3. [Google Scholar]
- (79).Zhang J; Boyd A; Tselev A; Paranjape M; Barbara P Mechanism of NO2 Detection in Carbon Nanotube Field Effect Transistor Chemical Sensors. Appl. Phys. Lett 2006, 88, 123112. [Google Scholar]
- (80).Salehi-Khojin A; Khalili-Araghi F; Kuroda MA; Lin KY; Leburton J-P; Masel RI On the Sensing Mechanism in Carbon Nanotube Chemiresistors. ACS Nano 2011, 5, 153–158. [DOI] [PubMed] [Google Scholar]
- (81).Kim Y; Lee S; Choi HH; Noh J-S; Lee W Detection of a Nerve Agent Simulant Using Single-Walled Carbon Nanotube Networks: Dimethyl-Methyl-Phosphonate. Nanotechnology 2010, 21, 495501. [DOI] [PubMed] [Google Scholar]
- (82).Maiti A; Ricca A Metal-Nanotube Interactions - Binding Energies and Wetting Properties. Chem. Phys. Lett 2004, 395, 7–11. [Google Scholar]
- (83).Zhu W; Kaxiras E The Nature of Contact between Pd Leads and Semiconducting Carbon Nanotubes. Nano Lett 2006, 6, 1415–1419. [DOI] [PubMed] [Google Scholar]
- (84).Zhang M; Brooks LL; Chartuprayoon N; Bosze W; Choa YH; Myung NV Palladium/Single-Walled Carbon Nanotube Back-to-Back Schottky Contact-Based Hydrogen Sensors and Their Sensing Mechanism. ACS Appl. Mater. Interfaces 2014, 6, 319–326. [DOI] [PubMed] [Google Scholar]
- (85).Karousis N; Tagmatarchis N; Tasis D Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev 2010, 110, 5366–5397. [DOI] [PubMed] [Google Scholar]
- (86).Wei L; Lu D; Wang J; Wei H; Zhao J; Geng H; Zhang Y Highly Sensitive Detection of Trinitrotoluene in Water by Chemiresistive Sensor Based on Noncovalently Amino Functionalized Single-Walled Carbon Nanotube. Sensors Actuators, B Chem 2014, 190, 529–534. [Google Scholar]
- (87).Zhao Y-L; Hu L; Stoddart JF; Gruner G Pyrenecyclodextrin-Decorated Single-Walled Carbon Nanotube Field-Effect Transistors as Chemical Sensors. Adv. Mater 2008, 20, 1910–1915. [Google Scholar]
- (88).Lerner MB; Kybert N; Mendoza R; Villechenon R; Bonilla Lopez MA; Charlie Johnson AT Scalable, Non-Invasive Glucose Sensor Based on Boronic Acid Functionalized Carbon Nanotube Transistors. Appl. Phys. Lett 2013, 102, 183113. [Google Scholar]
- (89).Frazier KM; Swager TM Robust Cyclohexanone Selective Chemiresistors Based on Single-Walled Carbon Nanotubes. Anal. Chem 2013, 85, 7154–7158. [DOI] [PubMed] [Google Scholar]
- (90).Zhu R; Azzarelli JM; Swager TM Wireless Hazard Badges to Detect Nerve-Agent Simulants. Angew. Chemie - Int. Ed 2016, 55, 9662–9666. [DOI] [PubMed] [Google Scholar]
- (91).Weis JG; Ravnsbæk JB; Mirica KA; Swager TM Employing Halogen Bonding Interactions in Chemiresistive Gas Sensors. ACS Sensors 2016, 1, 115–119. [Google Scholar]
- (92).Im J; Sterner E; Swager T Integrated Gas Sensing System of SWCNT and Cellulose Polymer Concentrator for Benzene, Toluene, and Xylenes. Sensors 2016, 16, 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Liu SF; Petty AR; Sazama GT; Swager TM Single-Walled Carbon Nanotube/Metalloporphyrin Composites for the Chemiresistive Detection of Amines and Meat Spoilage. Angew. Chemie Int. Ed 2015, 54, 6554–6557. [DOI] [PubMed] [Google Scholar]
- (94).Liu SF; Lin S; Swager TM An Organocobalt-Carbon Nanotube Chemiresistive Carbon Monoxide Detector. ACS Sensors 2016, 1, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Lin S; Swager TM Carbon Nanotube Formic Acid Sensors Using a Nickel Bis(Ortho - Diiminosemiquinonate) Selector. ACS Sensors 2018, 3, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Esser B; Schnorr JM; Swager TM Selective Detection of Ethylene Gas Using Carbon Nanotube-Based Devices: Utility in Determination of Fruit Ripeness. Angew. Chemie - Int. Ed 2012, 51, 5752–5756. [DOI] [PubMed] [Google Scholar]
- (97).Wang F; Yang Y; Swager TM Molecular Recognition for High Selectivity in Carbon Nanotube/Polythiophene Chemiresistors. Angew. Chemie - Int. Ed 2008, 47, 8394–8396. [DOI] [PubMed] [Google Scholar]
- (98).Zheng M; Jagota A; Semke ED; Diner BA; McLean RS; Lustig SR; Richardson RE; Tassi NG DNA-Assisted Dispersion and Separation of Carbon Nanotubes. Nat. Mater 2003, 2, 338–342. [DOI] [PubMed] [Google Scholar]
- (99).Star A; Joshi V; Han TR; Altoé MVP; Grüner G; Stoddart JF Electronic Detection of the Enzymatic Degradation of Starch. Org. Lett 2004, 6, 2089–2092. [DOI] [PubMed] [Google Scholar]
- (100).Star A; Steuerman DW; Heath JR; Stoddart JF Starched Carbon Nanotubes. Angew. Chemie - Int. Ed 2002, 41, 2508–2512. [DOI] [PubMed] [Google Scholar]
- (101).Cella LN; Chen W; Myung NV; Mulchandani A Single-Walled Carbon Nanotube-Based Chemiresistive Affinity Biosensors for Small Molecules: Ultrasensitive Glucose Detection. J. Am. Chem. Soc 2010, 132, 5024–5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Zhang M; Gorski W Electrochemical Sensing Platform Based on the Carbon Nanotubes/Redox Mediators-Biopolymer System. J. Am. Chem. Soc 2005, 127, 2058–2059. [DOI] [PubMed] [Google Scholar]
- (103).Star A; Liu Y; Grant K; Ridvan L; Stoddart JF; Steuerman DW; Diehl MR; Boukai A; Heath JR Noncovalent Side-Wall Functionalization of Single-Walled Carbon Nanotubes. Macromolecules 2003, 36, 553–560. [Google Scholar]
- (104).Tang BZ; Xu H Preparation, Alignment, and Optical Properties of Soluble Poly(Phenylacetylene)-Wrapped Carbon Nanotubes. Macromolecules 1999, 32, 2569–2576. [Google Scholar]
- (105).Steuerman DW; Star A; Narizzano R; Choi H; Ries RS; Nicolini C; Stoddart JF; Heath JR Interactions between Conjugated Polymers and Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2002, 106, 3124–3130. [Google Scholar]
- (106).Fennell J; Hamaguchi H; Yoon B; Swager T Chemiresistor Devices for Chemical Warfare Agent Detection Based on Polymer Wrapped Single-Walled Carbon Nanotubes. Sensors 2017, 17, 982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Ishihara S; Azzarelli JM; Krikorian M; Swager TM Ultratrace Detection of Toxic Chemicals: Triggered Disassembly of Supramolecular Nanotube Wrappers. J. Am. Chem. Soc 2016, 138, 8221–8227. [DOI] [PubMed] [Google Scholar]
- (108).Chen RJ; Bangsaruntip S; Drouvalakis KA; Wong Shi Kam N; Shim M; Li Y; Kim W; Utz PJ; Dai H Noncovalent Functionalization of Carbon Nanotubes for Highly Specific Electronic Biosensors. Proc. Natl. Acad. Sci 2003, 100, 4984–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).So HM; Won K; Kim YH; Kim BK; Ryu BH; Na PS; Kim H; Lee JO Single-Walled Carbon Nanotube Biosensors Using Aptamers as Molecular Recognition Elements. J. Am. Chem. Soc 2005, 127, 11906–11907. [DOI] [PubMed] [Google Scholar]
- (110).Kong J; Chapline MG; Dai H; Nanotubes FC; Sensors MH Functionalized Carbon Nanotubes for Molecular Hydrogen Sensors. Adv. Mater 2001, 13, 1384–1386. [Google Scholar]
- (111).Sun Y; Wang HH High-Performance, Flexible Hydrogen Sensors That Use Carbon Nanotubes Decorated with Palladium Nanoparticles. Adv. Mater 2007, 19, 2818–2823. [Google Scholar]
- (112).Mubeen S; Zhang T; Chartuprayoon N; Rheem Y; Mulchandani A; Myung NV; Deshusses MA Sensitive Detection of H 2 S Using Gold Nanoparticle Decorated Single-Walled Carbon Nanotubes. Anal. Chem 2010, 82, 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Lu G; Ocola LE; Chen J Room-Temperature Gas Sensing Based on Electron Transfer between Discrete Tin Oxide Nanocrystals and Multiwalled Carbon Nanotubes. Adv. Mater 2009, 21, 2487–2491. [Google Scholar]
- (114).Mann JA; Rodríguez-López J; Abruña HD; Dichtel WR Multivalent Binding Motifs for the Noncovalent Functionalization of Graphene. J. Am. Chem. Soc 2011, 133, 17614–17617. [DOI] [PubMed] [Google Scholar]
- (115).Cao M; Fu A; Wang Z; Liu J; Kong N; Zong X; Liu H; Gooding JJ Electrochemical and Theoretical Study of Pi-Pi Stacking Interactions between Graphitic Surfaces and Pyrene Derivatives. J. Phys. Chem. C 2014, 118, 2650–2659. [Google Scholar]
- (116).Huang J; Ng AL; Piao Y; Chen C-F; Green A. a; Sun C-F; Hersam MC; Lee CS; Wang Y Covalently Functionalized Double-Walled Carbon Nanotubes Combine High Sensitivity and Selectivity in the Electrical Detection of Small Molecules. J. Am. Chem. Soc 2013, 135, 2306–2312. [DOI] [PubMed] [Google Scholar]
- (117).Wang F; Swager TM Diverse Chemiresistors Based upon Covalently Modified Multiwalled Carbon Nanotubes. J. Am. Chem. Soc 2011, 133, 11181–11193. [DOI] [PubMed] [Google Scholar]
- (118).Zhang W; Sprafke JK; Ma M; Tsui EY; Sydlik SA; Rutledge GC; Swager TM Modular Functionalization of Carbon Nanotubes and Fullerenes. J. Am. Chem. Soc 2009, 131, 8446–8454. [DOI] [PubMed] [Google Scholar]
- (119).Zhang W; Swager TM Functionalization of Single-Walled Carbon Nanotubes and Fullerenes via a Dimethyl Acetylenedicarboxylate-4-Dimethylaminopyridine Zwitterion Approach. J. Am. Chem. Soc 2007, 129, 7714–7715. [DOI] [PubMed] [Google Scholar]
- (120).Weizmann Y; Chenoweth DM; Swager TM DNA-CNT Nanowire Networks for DNA Detection. J. Am. Chem. Soc 2011, 133, 3238–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Setaro A; Adeli M; Glaeske M; Przyrembel D; Bisswanger T; Gordeev G; Maschietto F; Faghani A; Paulus B; Weinelt M; et al. Preserving π-Conjugation in Covalently Functionalized Carbon Nanotubes for Optoelectronic Applications. Nat. Commun 2017, 8, 14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (122).Schnorr JM; van der Zwaag D; Walish JJ; Weizmann Y; Swager TM Sensory Arrays of Covalently Functionalized Single-Walled Carbon Nanotubes for Explosive Detection. Adv. Funct. Mater 2013, 23, 5285–5291. [Google Scholar]
- (123).Gao C; He H; Zhou L; Zheng X; Zhang Y Scalable Functional Group Engineering of Carbon Nanotubes by Improved One-Step Nitrene Chemistry. 2009, No. 1, 360–370. [Google Scholar]
- (124).Dionisio M; Schnorr JM; Michaelis VK; Griffin RG; Swager TM; Dalcanale E Cavitand-Functionalized SWCNTs for N-Methylammonium Detection. J. Am. Chem. Soc 2012, 134, 6540–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Paoletti C; He M; Salvo P; Melai B; Calisi N; Mannini M; Cortigiani B; Bellagambi FG; Swager TM; Di Francesco F; et al. Room Temperature Amine Sensors Enabled by Sidewall Functionalization of Single-Walled Carbon Nanotubes. RSC Adv 2018, 8, 5578–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).He M; Swager TM Covalent Functionalization of Carbon Nanomaterials with Iodonium Salts. Chem. Mater 2016, 28, 8542–8549. [Google Scholar]
- (127).Savagatrup S; Schroeder V; He X; Lin S; He M; Yassine O; Salama KN; Zhang X-X; Swager TM Bio-Inspired Carbon Monoxide Sensors with Voltage-Activated Sensitivity. Angew. Chemie Int. Ed 2017, 56, 14066–14070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Lerner MB; Dailey J; Goldsmith BR; Brisson D; Charlie Johnson AT Detecting Lyme Disease Using Antibody-Functionalized Single-Walled Carbon Nanotube Transistors. Biosens. Bioelectron 2013, 45, 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Lerner MB; D’Souza J; Pazina T; Dailey J; Goldsmith BR; Robinson MK; Johnson ATC Hybrids of a Genetically Engineered Antibody and a Carbon Nanotube Transistor for Detection of Prostate Cancer Biomarkers. ACS Nano 2012, 6, 5143–5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Eder D Carbon Nanotube - Inorganic Hybrids. Chem. Rev 2010, 110, 1348–1385. [DOI] [PubMed] [Google Scholar]
- (131).Cargnello M; Grzelczak M; Rodríguez-González B; Syrgiannis Z; Bakhmutsky K; La Parola V; Liz-Marzán LM; Gorte RJ; Prato M; Fornasiero P Multiwalled Carbon Nanotubes Drive the Activity of Metal@oxide Core-Shell Catalysts in Modular Nanocomposites. J. Am. Chem. Soc 2012, 134, 11760–11766. [DOI] [PubMed] [Google Scholar]
- (132).Liang YX; Chen YJ; Wang JH Low-Resistance Gas Sensors Fabricated from Multiwalled Carbon Nanotubes Coated with a Thin Tin Oxide Layer. Appl. Phys. Lett 2004, 85, 666–668. [Google Scholar]
- (133).Chen Y; Zhu C; Wang T The Enhanced Ethanol Sensing Properties of Multi-Walled Carbon Nanotubes/SnO2 Core/Shell Nanostructures. Nanotechnology 2006, 17, 3012–3017. [Google Scholar]
- (134).Vinoth V; Wu JJ; Asiri AM; Anandan S Simultaneous Detection of Dopamine and Ascorbic Acid Using Silicate Network Interlinked Gold Nanoparticles and Multi-Walled Carbon Nanotubes. Sensors Actuators, B Chem 2015, 210, 731–741. [Google Scholar]
- (135).Ragupathy D; Gopalan AI; Lee KP Electrocatalytic Oxidation and Determination of Ascorbic Acid in the Presence of Dopamine at Multiwalled Carbon Nanotube-Silica Network-Gold Nanoparticles Based Nanohybrid Modified Electrode. Sensors Actuators, B Chem 2010, 143, 696–703. [Google Scholar]
- (136).Ding M; Sorescu DC; Star A Photoinduced Charge Transfer and Acetone Sensitivity of Single-Walled Carbon Nanotube-Titanium Dioxide Hybrids. J. Am. Chem. Soc 2013, 135, 9015–9022. [DOI] [PubMed] [Google Scholar]
- (137).Ellis JE; Green U; Sorescu DC; Zhao Y; Star A Indium Oxide-Single-Walled Carbon Nanotube Composite for Ethanol Sensing at Room Temperature. J. Phys. Chem. Lett 2015, 6, 712–717. [DOI] [PubMed] [Google Scholar]
- (138).Peng G; Wu S; Ellis JE; Xu X; Xu G; Yu C; Star A Single-Walled Carbon Nanotubes Templated CuO Networks for Gas Sensing. J. Mater. Chem. C 2016, 4, 6575–6580. [Google Scholar]
- (139).Zhao B; Hu H; Haddon RC Synthesis and Properties of a Water-Soluble Single-Walled Carbon Nanotube–Poly(m-Aminobenzene Sulfonic Acid) Graft Copolymer. Adv. Funct. Mater 2004, 14, 71–76. [Google Scholar]
- (140).Bekyarova E; Davis M; Burch T; Itkis ME; Zhao B; Sunshine S; Haddon RC Chemically Functionalized Single-Walled Carbon Nanotubes as Ammonia Sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar]
- (141).Zhang T; Mubeen S; Bekyarova E; Yoo BY; Haddon RC; Myung NV; Deshusses MA Poly(m-Aminobenzene Sulfonic Acid) Functionalized Single-Walled Carbon Nanotubes Based Gas Sensor. Nanotechnology 2007, 18, 165504. [Google Scholar]
- (142).Bekyarova E; Kalinina I; Sun X; Shastry T; Worsley K; Chi X; Itkis ME; Haddon RC Chemically Engineered Single-Walled Carbon Nanotube Materials for the Electronic Detection of Hydrogen Chloride. Adv. Mater 2010, 22, 848–852. [DOI] [PubMed] [Google Scholar]
- (143).Guo X; Small JP; Klare JE; Wang Y; Purewal MS; Tam IW; Hong BH; Caldwell R; Huang L; O’brien S; et al. Covalently Bridging Gaps in Single-Walled Carbon Nanotubes with Conducting Molecules. Science 2006, 311, 356–359. [DOI] [PubMed] [Google Scholar]
- (144).Guo X; Gorodetsky AA; Hone J; Barton JK; Nuckolls C Conductivity of a Single DNA Duplex Bridging a Carbon Nanotube Gap. Nat. Nanotechnol 2008, 3, 163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (145).Weizmann Y; Chenoweth DM; Swager TM Addressable Terminally Linked DNA-CNT Nanowires. J. Am. Chem. Soc 2010, 132, 14009–14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (146).Weizmann Y; Lim J; Chenoweth DM; Swager TM Regiospecific Synthesis of Au-Nanorod/SWCNT/Au-Nanorod Heterojunctions. Nano Lett 2010, 10, 2466–2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (147).Wilson NR; Macpherson JV Carbon Nanotube Tips for Atomic Force Microscopy. Nat. Nanotechnol 2009, 4, 483–491. [DOI] [PubMed] [Google Scholar]
- (148).Dai H; Hafner JH; Rinzler AG; Colbert DT; Smalley RE Nanotubes as Nanoprobes in Scanning Probe Microscopy. Nature 1996, 384, 147–150. [Google Scholar]
- (149).Jariwala D; Sangwan VK; Lauhon LJ; Marks TJ; Hersam MC Carbon Nanomaterials for Electronics, Optoelectronics, Photovoltaics, and Sensing. Chem. Soc. Rev 2013, 42, 2824–2860. [DOI] [PubMed] [Google Scholar]
- (150).Goldsmith BR; Coroneus JG; Kane AA; Weiss GA; Collins PG Monitoring Single-Molecule Reactivity on a Carbon Nanotube. Nano Lett 2008, 8, 189–194. [DOI] [PubMed] [Google Scholar]
- (151).Sorgenfrei S; Chiu C; Gonzalez RL; Yu Y-J; Kim P; Nuckolls C; Shepard KL Label-Free Single-Molecule Detection of DNA-Hybridization Kinetics with a Carbon Nanotube Field-Effect Transistor. Nat. Nanotechnol 2011, 6, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (152).Vernick S; Trocchia SM; Warren SB; Young EF; Bouilly D; Gonzalez RL; Nuckolls C; Shepard KL Electrostatic Melting in a Single-Molecule Field-Effect Transistor with Applications in Genomic Identification. Nat. Commun 2017, 8, 15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Shim S-B; Imboden M; Mohanty P; Heer WA; Bachtold A Synchronized Oscillation in Coupled Nanomechanical Oscillators. Science 2007, 316, 95–99. [DOI] [PubMed] [Google Scholar]
- (154).Chaste J; Eichler A; Moser J; Ceballos G; Rurali R; Bachtold A A Nanomechanical Mass Sensor with Yoctogram Resolution. Nat. Nanotechnol 2012, 7, 301–304. [DOI] [PubMed] [Google Scholar]
- (155).Lee CY; Choi W; Han J-H; Strano MS Coherence Resonance in a Single-Walled Carbon Nanotube Ion Channel. Science 2010, 329, 1320–1324. [DOI] [PubMed] [Google Scholar]
- (156).Peng R; Tang XS; Li D Detection of Individual Molecules and Ions by Carbon Nanotube-Based Differential Resistive Pulse Sensor. Small 2018, 14, 1800013. [DOI] [PubMed] [Google Scholar]
- (157).Feldman AK; Steigerwald ML; Guo X; Nuckolls C Molecular Electronic Devices Based on Single-Walled Carbon Nanotube Electrodes. Acc. Chem. Res 2008, 41, 1731–1741. [DOI] [PubMed] [Google Scholar]
- (158).Gao XPA; Zheng G; Lieber CM Subthreshold Regime Has the Optimal Sensitivity for Nanowire FET Biosensors. Nano Lett 2010, 10, 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Ishikawa FN; Curreli M; Olson CA; Liao H-I; Sun R; Roberts RW; Cote RJ; Thompson ME; Zhou C Importance of Controlling Nanotube Density for Highly Sensitive and Reliable Biosensors Functional in Physiological Conditions. ACS Nano 2010, 4, 6914–6922. [DOI] [PubMed] [Google Scholar]
- (160).Okuda S; Okamoto S; Ohno Y; Maehashi K; Inoue K; Matsumoto K Horizontally Aligned Carbon Nanotubes on a Quartz Substrate for Chemical and Biological Sensing. J. Phys. Chem. C 2012, 116, 19490–19495. [Google Scholar]
- (161).Abe M; Murata K; Ataka T; Matsumoto K Comparison of Sensitivities of Carbon Nanotube Field-Effect Transistor Biosensors with and without Top Metal Gate. J. Appl. Phys 2008, 104, 104304. [Google Scholar]
- (162).Shulaker MM; Hills G; Park RS; Howe RT; Saraswat K; Wong H-SP; Mitra S Three-Dimensional Integration of Nanotechnologies for Computing and Data Storage on a Single Chip. Nature 2017, 547, 74–78. [DOI] [PubMed] [Google Scholar]
- (163).Wu J; Antaris A; Gong M; Dai H Top-Down Patterning and Self-Assembly for Regular Arrays of Semiconducting Single-Walled Carbon Nanotubes. Adv. Mater 2014, 26, 6151–6156. [DOI] [PubMed] [Google Scholar]
- (164).Li X; Zhang L; Wang X; Shimoyama I; Sun X; Seo WK; Dai H Langmuir-Blodgett Assembly of Densely Aligned Single-Walled Carbon Nanotubes from Bulk Materials. J. Am. Chem. Soc 2007, 129, 4890–4891. [DOI] [PubMed] [Google Scholar]
- (165).Brady GJ; Jinkins KR; Arnold MS Channel Length Scaling Behavior in Transistors Based on Individual versus Dense Arrays of Carbon Nanotubes. J. Appl. Phys 2017, 122, 124506. [Google Scholar]
- (166).Lin C-T; Lee C-Y; Chin T-S; Xiang R; Ishikawa K; Shiomi J; Maruyama S Anisotropic Electrical Conduction of Vertically-Aligned Single-Walled Carbon Nanotube Films. Carbon N. Y 2011, 49, 1446–1452. [Google Scholar]
- (167).Cao Q; Rogers JA Random Networks and Aligned Arrays of Single-Walled Carbon Nanotubes for Electronic Device Applications. Nano Res 2008, 1, 259–272. [Google Scholar]
- (168).Li P; Xue W Selective Deposition and Alignment of Single-Walled Carbon Nanotubes Assisted by Dielectrophoresis: From Thin Films to Individual Nanotubes. Nanoscale Res. Lett 2010, 5, 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Biswas C; Lee SY; Ly TH; Ghosh A; Dang QN; Lee YH Chemically Doped Random Network Carbon Nanotube P–n Junction Diode for Rectifier. ACS Nano 2011, 5, 9817–9823. [DOI] [PubMed] [Google Scholar]
- (170).Kong J; Soh HT; Cassell AM; Quate CF; Dai H Synthesis of Individual Single-Walled Carbon Nanotubes on Patterned Silicon Wafers. Nature 1998, 395, 878–881. [Google Scholar]
- (171).Silva GO; Michael ZP; Bian L; Shurin GV; Mulato M; Shurin MR; Star A Nanoelectronic Discrimination of Nonmalignant and Malignant Cells Using Nanotube Field-Effect Transistors. ACS Sensors 2017, 2, 1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Abe M; Murata K; Kojima A; Ifuku Y; Shimizu M; Ataka T; Matsumoto K Quantitative Detection of Protein Using a Top-Gate Carbon Nanotube Field Effect Transistor. J. Phys. Chem. C 2007, 111, 8667–8670. [Google Scholar]
- (173).White HS; Kittlesen GP; Wrighton MS Chemical Derivatization of an Array of Three Gold Microelectrodes with Polypyrrole: Fabrication of a Molecule-Based Transistor. J. Am. Chem. Soc 1984, 106, 5375–5377. [Google Scholar]
- (174).Yanagi K; Kanda S; Oshima Y; Kitamura Y; Kawai H; Yamamoto T; Takenobu T; Nakai Y; Maniwa Y Tuning of the Thermoelectric Properties of One-Dimensional Material Networks by Electric Double Layer Techniques Using Ionic Liquids. Nano Lett 2014, 14, 6437–6442. [DOI] [PubMed] [Google Scholar]
- (175).Bisri SZ; Shimizu S; Nakano M; Iwasa Y Endeavor of Iontronics: From Fundamentals to Applications of Ion-Controlled Electronics. Adv. Mater 2017, 29, 1607054. [DOI] [PubMed] [Google Scholar]
- (176).Du H; Lin X; Xu Z; Chu D Electric Double-Layer Transistors: A Review of Recent Progress. J. Mater. Sci 2015, 50, 5641–5673. [Google Scholar]
- (177).Son BH; Park J-Y; Lee S; Ahn YH Suspended Single-Walled Carbon Nanotube Fluidic Sensors. Nanoscale 2015, 7, 15421–15426. [DOI] [PubMed] [Google Scholar]
- (178).Rosenblatt S; Yaish Y; Park J; Gore J; Sazonova V; McEuen PL High Performance Electrolyte Gated Carbon Nanotube Transistors. Nano Lett 2002, 2, 869–872. [Google Scholar]
- (179).Lu C; Fu Q; Huang S; Liu J Polymer Electrolyte-Gated Carbon Nanotube Field-Effect Transistor. Nano Lett 2004, 4, 623–627. [Google Scholar]
- (180).Qi P; Javey A; Rolandi M; Wang Q; Yenilmez E; Dai H Miniature Organic Transistors with Carbon Nanotubes as Quasi-One-Dimensional Electrodes. J. Am. Chem. Soc 2004, 126, 11774–11775. [DOI] [PubMed] [Google Scholar]
- (181).He Y; Zhang J; Zhao J Electron Transport and CO Sensing Characteristics of Fe(II) Porphyrin with Single-Walled Carbon Nanotube Electrodes. J. Phys. Chem. C 2014, 118, 18325–18333. [Google Scholar]
- (182).Soylemez S; Yoon B; Toppare L; Swager TM Quaternized Polymer–Single-Walled Carbon Nanotube Scaffolds for a Chemiresistive Glucose Sensor. ACS Sensors 2017, 2, 1123–1127. [DOI] [PubMed] [Google Scholar]
- (183).Dionisio M; Schnorr JM; Michaelis VK; Griffin RG; Swager TM; Dalcanale E Cavitand-Functionalized SWCNTs for N -Methylammonium Detection. J. Am. Chem. Soc 2012, 134, 6540–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (184).Chen Y; Mun SC; Kim J A Wide Range Conductometric PH Sensor Made With Titanium Dioxide/Multiwall Carbon Nanotube/Cellulose Hybrid Nanocomposite. IEEE Sens. J 2013, 13, 4157–4162. [Google Scholar]
- (185).Kwon J-H; Lee K-S; Lee Y-H; Ju B-K Single-Wall Carbon Nanotube-Based PH Sensor Fabricated by the Spray Method. Electrochem. Solid-State Lett 2006, 9, H85. [Google Scholar]
- (186).Tang R; Shi Y; Hou Z; Wei L Carbon Nanotube-Based Chemiresistive Sensors. Sensors 2017, 17, 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Mirica KA; Azzarelli JM; Weis JG; Schnorr JM; Swager TM Rapid Prototyping of Carbon-Based Chemiresistive Gas Sensors on Paper. Proc. Natl. Acad. Sci 2013, 110, E3265–E3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (188).Azzarelli JM; Mirica KA; Ravnsbæk JB; Swager TM Wireless Gas Detection with a Smartphone via Rf Communication. Proc. Natl. Acad. Sci 2014, 111, 18162–18166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (189).Zhu R; Desroches M; Yoon B; Swager TM Wireless Oxygen Sensors Enabled by Fe(II)-Polymer Wrapped Carbon Nanotubes. ACS Sensors 2017, 2, 1044–1050. [DOI] [PubMed] [Google Scholar]
- (190).Vieira SMC; Beecher P; Haneef I; Udrea F; Milne WI; Namboothiry MAG; Carroll DL; Park J; Maeng S Use of Nanocomposites to Increase Electrical “Gain” in Chemical Sensors. Appl. Phys. Lett 2007, 91, 203111. [Google Scholar]
- (191).Wong YM; Kang WP; Davidson JL; Wisitsora-At A; Soh KL A Novel Microelectronic Gas Sensor Utilizing Carbon Nanotubes for Hydrogen Gas Detection. Sensors Actuators, B Chem 2003, 93, 327–332. [Google Scholar]
- (192).Lim H; Song HJ; Lee Y; Shin HJ; Choi HC Carbon Nanotube Schottky Diode via Selective Electrochemical Metal Deposition. Langmuir 2010, 26, 1464–1467. [DOI] [PubMed] [Google Scholar]
- (193).Lim H; Hyeon SS; Shin HJ; Hee CC Lithium Ions Intercalated into Pyrene-Functionalized Carbon Nanotubes and Their Mass Transport: A Chemical Route to Carbon Nanotube Schottky Diode. J. Am. Chem. Soc 2008, 130, 2160–2161. [DOI] [PubMed] [Google Scholar]
- (194).Zhou Y; Gaur A; Hur SH; Kocabas C; Meitl MA; Shim M; Rogers JA P-Channel, n-Channel Thin Film Transistors and p-n Diodes Based on Single Wall Carbon Nanotube Networks. Nano Lett 2004, 4, 2031–2035. [Google Scholar]
- (195).Zhou C; Kong J; Yenilmez E; Dai H Modulated Chemical Doping of Individual Carbon Nanotubes. Science 2000, 290, 1552–1555. [DOI] [PubMed] [Google Scholar]
- (196).Ilani S; Donev LAK; Kindermann M; McEuen PL Measurement of the Quantum Capacitance of Interacting Electrons in Carbon Nanotubes. Nat. Phys 2006, 2, 687–691. [Google Scholar]
- (197).Liu Z; Li J; Qiu Z-J; Zhang Z-B; Zheng L-R; Zhang S-L On Gate Capacitance of Nanotube Networks. IEEE Electron Device Lett 2011, 32, 641–643. [Google Scholar]
- (198).Kiriya D; Chen K; Ota H; Lin Y; Zhao P; Yu Z; Ha T; Javey A Design of Surfactant–Substrate Interactions for Roll-to-Roll Assembly of Carbon Nanotubes for Thin-Film Transistors. J. Am. Chem. Soc 2014, 136, 11188–11194. [DOI] [PubMed] [Google Scholar]
- (199).Snow ES; Perkins FK; Houser EJ; Badescu SC; Reinecke TL Chemical Detection with a Single-Walled Carbon Nanotube Capacitor. Science 2005, 307, 1942–1945. [DOI] [PubMed] [Google Scholar]
- (200).Snow ES; Perkins FK; Robinson JA Chemical Vapor Detection Using Single-Walled Carbon Nanotubes. Chem. Soc. Rev 2006, 35, 790–798. [DOI] [PubMed] [Google Scholar]
- (201).Esen G; Fuhrer MS; Ishigami M; Williams ED Transmission Line Impedance of Carbon Nanotube Thin Films for Chemical Sensing. Appl. Phys. Lett 2007, 90, 123510. [Google Scholar]
- (202).Suehiro J; Zhou G; Hara M Fabrication of a Carbon Nanotube-Based Gas Sensor Using Dielectrophoresis and Its Application for Ammonia Detection by Impedance Spectroscopy. J. Phys. D. Appl. Phys 2003, 36, L109–L114. [Google Scholar]
- (203).Varghese OK; Kichambre PD; Gong D; Ong KG; Dickey EC; Grimes CA Gas Sensing Characteristics of Multi-Wall Carbon Nanotubes. Sensors Actuators B 2001, 81, 32–41. [Google Scholar]
- (204).Zhu Z An Overview of Carbon Nanotubes and Graphene for Biosensing Applications Nano-Micro Letters Springer Berlin Heidelberg; July 2017, p 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Li CA; Han KN; Pham X-H; Seong GH A Single-Walled Carbon Nanotube Thin Film-Based PH-Sensing Microfluidic Chip. Analyst 2014, 139, 2011–2015. [DOI] [PubMed] [Google Scholar]
- (206).Gou P; Kraut ND; Feigel IM; Bai H; Morgan GJ; Chen Y; Tang Y; Bocan K; Stachel J; Berger L; et al. Carbon Nanotube Chemiresistor for Wireless PH Sensing. Sci. Rep 2015, 4, 4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Kaempgen M; Roth S Transparent and Flexible Carbon Nanotube/Polyaniline PH Sensors. J. Electroanal. Chem 2006, 586, 72–76. [Google Scholar]
- (208).Hung S-C; Cheng N-J; Yang C-F; Lo Y-P Investigation of Extended-Gate Field-Effect Transistor PH Sensors Based on Different-Temperature-Annealed Bi-Layer MWCNTs-In2O3 Films. Nanoscale Res. Lett 2014, 9, 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (209).Matsui Y; Hoshino T; Yoshizawa M; Muguruma H NADH Sensing Using a Carbon Nanotube Electrode Reinforced with a Plasma-Polymerized Thin Film. Electrochemistry 2008, 76, 610–613. [Google Scholar]
- (210).Qu F; Yang M; Jiang J; Shen G; Yu R Amperometric Biosensor for Chorine Based on Layer-by-Layer Assembled Functionalized Carbon Nanotube and Polyaniline Multilayer Film. Anal. Biochem 2005, 344, 108–114. [DOI] [PubMed] [Google Scholar]
- (211).Wanekaya AK Applications of Nanoscale Carbon-Based Materials in Heavy Metal Sensing and Detection. Analyst 2011, 136, 4383–4391. [DOI] [PubMed] [Google Scholar]
- (212).Wang L; Wang X; Shi G; Peng C; Ding Y Thiacalixarene Covalently Functionalized Multiwalled Carbon Nanotubes as Chemically Modified Electrode Material for Detection of Ultratrace Pb 2+ Ions. Anal. Chem 2012, 84, 10560–10567. [DOI] [PubMed] [Google Scholar]
- (213).Song W; Pang P; He J; Lindsay S Optical and Electrical Detection of Single-Molecule Translocation through Carbon Nanotubes. ACS Nano 2013, 7, 689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Cao D; Pang P; Liu H; He J; Lindsay SM Electronic Sensitivity of a Single-Walled Carbon Nanotube to Internal Electrolyte Composition. Nanotechnology 2012, 23, 085203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Chopra S; McGuire K; Gothard N; Rao AM; Pham A Selective Gas Detection Using a Carbon Nanotube Sensor. Appl. Phys. Lett 2003, 83, 2280–2282. [Google Scholar]
- (216).Picaud F; Langlet R; Arab M; Devel M; Girardet C; Natarajan S; Chopra S; Rao AM Gas-Induced Variation in the Dielectric Properties of Carbon Nanotube Bundles for Selective Sensing. J. Appl. Phys 2005, 97, 114316. [Google Scholar]
- (217).Penza M; Cassano G; Aversa P; Antolini F; Cusano A; Cutolo A; Giordano M; Nicolais L Alcohol Detection Using Carbon Nanotubes Acoustic and Optical Sensors. Appl. Phys. Lett 2004, 85, 2379–2381. [Google Scholar]
- (218).Sazonova V; Yaish Y; Üstünel H; Roundy D; Arias TA; McEuen PL A Tunable Carbon Nanotube Electromechanical Oscillator. Nature 2004, 431, 284–287. [DOI] [PubMed] [Google Scholar]
- (219).Ishihara S; Labuta J; Nakanishi T; Tanaka T; Kataura H Amperometric Detection of Sub-Ppm Formaldehyde Using Single-Walled Carbon Nanotubes and Hydroxylamines: A Referenced Chemiresistive System. ACS Sensors 2017, 2, 1405–1409. [DOI] [PubMed] [Google Scholar]
- (220).Stewart S; Ivy MA; Anslyn EV The Use of Principal Component Analysis and Discriminant Analysis in Differential Sensing Routines. Chem. Soc. Rev 2014, 43, 70–84. [DOI] [PubMed] [Google Scholar]
- (221).Collins PG; Arnold MS; Avouris P Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown. Science 2001, 292, 706–709. [DOI] [PubMed] [Google Scholar]
- (222).Seidel R; Graham AP; Unger E; Duesberg GS; Liebau M; Steinhoegl W; Kreupl F; Hoenlein W; Pompe W High-Current Nanotube Transistors. Nano Lett 2004, 4, 831–834. [Google Scholar]
- (223).Zhang G; Qi P; Wang X; Lu Y; Li X; Tu R; Bangsaruntip S; Mann D; Zhang L; Dai H Selective Etching of Metallic Carbon Nanotubes by Gas-Phase Reaction. Science 2006, 314, 974–977. [DOI] [PubMed] [Google Scholar]
- (224).Zhao Q; Yao F; Wang Z; Deng S; Tong L; Liu K; Zhang J Real-Time Observation of Carbon Nanotube Etching Process Using Polarized Optical Microscope. Adv. Mater 2017, 29, 1701959. [DOI] [PubMed] [Google Scholar]
- (225).Zhang Y; Zhang Y; Xian X; Zhang J; Liu Z Sorting out Semiconducting Single-Walled Carbon Nanotube Arrays by Preferential Destruction of Metallic Tubes Using Xenon-Lamp Irradiation. J. Phys. Chem. C 2008, 112, 3849–3856. [Google Scholar]
- (226).Zhou X; Boey F; Zhang H Controlled Growth of Single-Walled Carbon Nanotubes on Patterned Substrates. Chem. Soc. Rev 2011, 40, 5221–5231. [DOI] [PubMed] [Google Scholar]
- (227).Komatsu N; Wang F A Comprehensive Review on Separation Methods and Techniques for Single-Walled Carbon Nanotubes. Materials (Basel). 2010, 3, 3818–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (228).Lei T; Pochorovski I; Bao Z Separation of Semiconducting Carbon Nanotubes for Flexible and Stretchable Electronics Using Polymer Removable Method. Acc. Chem. Res 2017, 50, 1096–1104. [DOI] [PubMed] [Google Scholar]
- (229).Chen T; Wei L; Zhou Z; Shi D; Wang J; Zhao J; Yu Y; Wang Y; Zhang Y Highly Enhanced Gas Sensing in Single-Walled Carbon Nanotube-Based Thin-Film Transistor Sensors by Ultraviolet Light Irradiation. Nanoscale Res. Lett 2012, 7, 644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (230).Tans SJ; Verschueren ARM; Dekker C Room-Temperature Transistor Based on a Single Carbon Nanotube. Nature 1998, 393, 49–52. [Google Scholar]
- (231).Mann D; Javey A; Kong J; Wang Q; Dai H Ballistic Transport in Metallic Nanotubes with Reliable Pd Ohmic Contacts. Nano Lett 2003, 3, 1541–1544. [Google Scholar]
- (232).Frazier KM; Mirica KA; Walish JJ; Swager TM Fully-Drawn Carbon-Based Chemical Sensors on Organic and Inorganic Surfaces. Lab Chip 2014, 14, 4059–4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (233).Smith MK; Martin-Peralta DG; Pivak PA; Mirica KA Fabrication of Solid-State Gas Sensors by Drawing: An Undergraduate and High School Introduction to Functional Nanomaterials and Chemical Detection. J. Chem. Educ 2017, 94, 1933–1938. [Google Scholar]
- (234).Sekitani T; Noguchi Y; Hata K; Fukushima T; Aida T; Someya T A Rubberlike Stretchable Active Matrix Using Elastic Conductors. Science 2008, 321, 1468–1472. [DOI] [PubMed] [Google Scholar]
- (235).Kim C. Bin; Jeong KB; Yang BJ; Song J-W; Ku B-C; Lee S; Lee S-K; Park C Facile Supramolecular Processing of Carbon Nanotubes and Polymers for Electromechanical Sensors. Angew. Chemie Int. Ed 2017, 56, 16180–16185. [DOI] [PubMed] [Google Scholar]
- (236).Schnorr JM; Zentner CA; Petty AR; Swager TM Formulations for Enhanced Chemiresistive Sensing. US Pat 20170212104, July 2016. [Google Scholar]
- (237).Danzer K; Currie LA Guidelines for Calibration in Analytical Chemistry. Part I. Fundamentals and Single Component Calibration (IUPAC Recommendations 1998). Pure Appl. Chem 1998, 70, 993–1014. [Google Scholar]
- (238).Harris DC Quantitative Chemical Analysis; W.H. Freeman and Co.: New York, NY, 2007. [Google Scholar]
- (239).Ammu S; Dua V; Agnihotra SR; Surwade SP; Phulgirkar A; Patel S; Manohar SK Flexible, All-Organic Chemiresistor for Detecting Chemically Aggressive Vapors. J. Am. Chem. Soc 2012, 134, 4553–4556. [DOI] [PubMed] [Google Scholar]
- (240).Li J; Lu Y; Ye Q; Cinke M; Han J; Meyyappan M Carbon Nanotube Sensors for Gas and Organic Vapor Detection. Nano Lett 2003, 3, 929–933. [Google Scholar]
- (241).Mirica KA; Weis JG; Schnorr JM; Esser B; Swager TM Mechanical Drawing of Gas Sensors on Paper. Angew. Chemie - Int. Ed 2012, 51, 10740–10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Torsi L; Magliulo M; Manoli K; Palazzo G Organic Field-Effect Transistor Sensors: A Tutorial Review. Chem. Soc. Rev 2013, 42, 8612–8628. [DOI] [PubMed] [Google Scholar]
- (243).Penza M; Cassano G; Rossi R; Rizzo A; Signore MA; Alvisi M; Lisi N; Serra E; Giorgi R Effect of Growth Catalysts on Gas Sensitivity in Carbon Nanotube Film Based Chemiresistive Sensors. Appl. Phys. Lett 2007, 90, 103101. [Google Scholar]
- (244).Wang SG; Zhang Q; Yang DJ; Sellin PJ; Zhong GF Multi-Walled Carbon Nanotube-Based Gas Sensors for NH3 Detection. Diam. Relat. Mater 2004, 13, 1327–1332. [Google Scholar]
- (245).Feng X; Irle S; Witek H; Morokuma K; Vidic R; Borguet E Sensitivity of Ammonia Interaction with Single-Walled Carbon Nanotube Bundles to the Presence of Defect Sites and Functionalities. J. Am. Chem. Soc 2005, 127, 10533–10538. [DOI] [PubMed] [Google Scholar]
- (246).Robinson JA; Snow ES; Bǎdescu ŞC; Reinecke TL; Perkins FK Role of Defects in Single-Walled Carbon Nanotube Chemical Sensors. Nano Lett 2006, 6, 1747–1751. [DOI] [PubMed] [Google Scholar]
- (247).Snow AW; Perkins FK; Ancona MG; Robinson JT; Snow ES; Foos EE Disordered Nanomaterials for Chemielectric Vapor Sensing: A Review. IEEE Sens. J 2015, 15, 1301–1320. [Google Scholar]
- (248).Fu D; Lim H; Shi Y; Dong X; Mhaisalkar SG; Chen Y; Moochhala S; Li L Differentiation of Gas Molecules Using Flexible and All-Carbon Nanotube Devices. J. Phys. Chem. C 2008, 112, 650–653. [Google Scholar]
- (249).Zhao W; Fam DWH; Yin Z; Sun T; Tan HT; Liu W; Tok AIY; Boey YCF; Zhang H; Hng HH; et al. A Carbon Monoxide Gas Sensor Using Oxygen Plasma Modified Carbon Nanotubes. Nanotechnology 2012, 23, 425502. [DOI] [PubMed] [Google Scholar]
- (250).Rigoni F; Tognolini S; Borghetti P; Drera G; Pagliara S; Goldoni A; Sangaletti L Enhancing the Sensitivity of Chemiresistor Gas Sensors Based on Pristine Carbon Nanotubes to Detect Low-Ppb Ammonia Concentrations in the Environment. Analyst 2013, 138, 7392–7399. [DOI] [PubMed] [Google Scholar]
- (251).Quang NH; Van Trinh M; Lee B-H; Huh J-S Effect of NH3 Gas on the Electrical Properties of Single-Walled Carbon Nanotube Bundles. Sensors Actuators B Chem 2006, 113, 341–346. [Google Scholar]
- (252).Saito R; Fujita M; Dresselhaus G; Dresselhaus MS Electronic Structure of Chiral Graphene Tubules. Appl. Phys. Lett 1992, 60, 2204–2206. [DOI] [PubMed] [Google Scholar]
- (253).Yang L; Han J Electronic Structure of Deformed Carbon Nanotubes. Phys. Rev. Lett 2000, 85, 154–157. [DOI] [PubMed] [Google Scholar]
- (254).Berber S; Kwon Y-K; Tomanek D Unusually High Thermal Conductivity of Carbon Nanotubes. Phys. Rev. Lett 2000, 84, 4613–4616. [DOI] [PubMed] [Google Scholar]
- (255).Lu JP Elastic Properties of Carbon Nanotubes and Nanoropes. 1997, 1297–1300. [Google Scholar]
- (256).Nardelli M; Yakobson B; Bernholc J Brittle and Ductile Behavior in Carbon Nanotubes. Phys. Rev. Lett 1998, 81, 4656–4659. [Google Scholar]
- (257).Yakobson BI; Campbell MP; Brabec CJ; Bernholc J High Strain Rate Fracture and C-Chain Unraveling in Carbon Nanotubes. Comput. Mater. Sci 1997, 8, 341–348. [Google Scholar]
- (258).Iijima S; Brabec C; Maiti A; Bernholc J Structural Flexibility of Carbon Nanotubes. J. Chem. Phys 1996, 104, 2089–2092. [Google Scholar]
- (259).Overney G; Zhong W; Tomanek D Structural Rigidity and Low-Frequency Vibrational-Modes of Long Carbon Tubules. Zeitschrift Fur Phys. D-Atoms Mol. Clust 1993, 27, 93–96. [Google Scholar]
- (260).Bernholc J; Brabec C; Buongiorno Nardelli M; Maiti a.; Roland C; Yakobson BI Theory of Growth and Mechanical Properties of Nanotubes. Appl. Phys. A Mater. Sci. Process 1998, 67, 39–46. [Google Scholar]
- (261).Thostenson ET; Ren Z; Chou T-W Advances in the Science and Technology of Carbon Nanotubes and Their Composites: A Review. Compos. Sci. Technol 2001, 61, 1899–1912. [Google Scholar]
- (262).Dresselhaus MS; Dresselhaus G; Avouris P Carbon Nanotubes; Topics in Applied Physics; Springer Berlin Heidelberg, 2001; Vol. 80. [Google Scholar]
- (263).Shokrieh MM; Rafiee R A Review of the Mechanical Properties of Isolated Carbon Nanotubes and Carbon Nanotube Composites. Mech. Compos. Mater 2010, 46, 155–172. [Google Scholar]
- (264).Ruoff RS; Lorents DC Mechanical and Thermal Properties of Carbon Nanotubes. Carbon N. Y 1995, 33, 925–930. [Google Scholar]
- (265).Grimme S; Antony J; Schwabe T; Mück-Lichtenfeld C Density Functional Theory with Dispersion Corrections for Supramolecular Structures, Aggregates, and Complexes of (Bio)Organic Molecules. Org. Biomol. Chem 2007, 5, 741–758. [DOI] [PubMed] [Google Scholar]
- (266).Du AJ; Smith SC Van Der Waals-Corrected Density Functional Theory: Benchmarking for Hydrogen-Nanotube and Nanotube-Nanotube Interactions. Nanotechnology 2005, 16, 2118–2123. [DOI] [PubMed] [Google Scholar]
- (267).Ramirez J; Mayo ML; Kilina S; Tretiak S Electronic Structure and Optical Spectra of Semiconducting Carbon Nanotubes Functionalized by Diazonium Salts. Chem. Phys 2013, 413, 89–101. [Google Scholar]
- (268).Demichelis R; Noël Y; D’Arco P; Rérat M; Zicovich-Wilson CM; Dovesi R Properties of Carbon Nanotubes: An Ab Initio Study Using Large Gaussian Basis Sets and Various DFT Functionals. J. Phys. Chem. C 2011, 115, 8876–8885. [Google Scholar]
- (269).Peng S; Cho K Chemical Control of Nanotube Electronics. Nanotechnology 2000, 11, 57–60. [Google Scholar]
- (270).Santucci S; Picozzi S; Di Gregorio F; Lozzi L; Cantalini C; Valentini L; Kenny JM; Delley B NO2 and CO Gas Adsorption on Carbon Nanotubes: Experiment and Theory. J. Chem. Phys 2003, 119, 10904–10910. [Google Scholar]
- (271).Chang H; Lee J. Do; Lee SM; Lee YH Adsorption of NH3 and NO2 Molecules on Carbon Nanotubes. Appl. Phys. Lett 2001, 79, 3863–3865. [Google Scholar]
- (272).Sivasathya S; Thiruvadigal DJ; Mathi Jaya S Electron Transport through Metallic Single Wall Carbon Nanotubes with Adsorbed NO2 and NH3 Molecules: A First-Principles Study. Chem. Phys. Lett 2014, 609, 76–81. [Google Scholar]
- (273).Sadrzadeh A; Farajian AA; Yakobson BI Electron Transport of Nanotube-Based Gas Sensors: An Ab Initio Study. Appl. Phys. Lett 2008, 92, 022103. [Google Scholar]
- (274).Seo K; Park KA; Kim C; Han S; Kim B; Lee YH Chirality- and Diameter-Dependent Reactivity of NO 2 on Carbon Nanotube Walls. J. Am. Chem. Soc 2005, 127, 15724–15729. [DOI] [PubMed] [Google Scholar]
- (275).Eid KM; Kamel MA; Osman HM; Ismail GH Effect of Tubular Chiralities and Diameters of Single Carbon Nanotubes on Gas Sensing Behavior : A DFT Analysis. J. Surf. Eng. Mater. Adv. Technol 2014, 4, 66–74. [Google Scholar]
- (276).Chen Z; Nagase S; Hirsch A; Haddon RC; Thiel W; Schleyer PVR Side-Wall Opening of Single-Walled Carbon Nanotubes (SWCNTs) by Chemical Modification: A Critical Theoretical Study. Angew. Chemie - Int. Ed 2004, 43, 1552–1554. [DOI] [PubMed] [Google Scholar]
- (277).Bettinger HF Effects of Finite Carbon Nanotube Length on Sidewall Addition of Fluorine Atom and Methylene. Org. Lett 2004, 6, 731–734. [DOI] [PubMed] [Google Scholar]
- (278).Baldoni M; Sgamellotti A; Mercuri F Finite-Length Models of Carbon Nanotubes Based on Clar Sextet Theory. Org. Lett 2007, 9, 4267–4270. [DOI] [PubMed] [Google Scholar]
- (279).Clar E Polycyclic Hydrocarbons; Springer Berlin Heidelberg, 1964; Vol. 2. [Google Scholar]
- (280).Groß L; Bahlke MP; Steenbock T; Klinke C; Herrmann C Modeling Adsorbate-Induced Property Changes of Carbon Nanotubes. J. Comput. Chem 2017, 38, 861–868. [DOI] [PubMed] [Google Scholar]
- (281).Matsuo Y; Tahara K; Nakamura E Theoretical Studies on Structures and Aromaticity of Finite-Length Armchair Carbon Nanotubes. Org. Lett 2003, 5, 3181–3184. [DOI] [PubMed] [Google Scholar]
- (282).Ormsby JL; King BT Clar Valence Bond Representation of Pi-Bonding in Carbon Nanotubes. J. Org. Chem 2004, 69, 4287–4291. [DOI] [PubMed] [Google Scholar]
- (283).Baldoni M; Selli D; Sgamellotti A; Mercuri F Unraveling the Reactivity of Semiconducting Chiral Carbon Nanotubes through Finite-Length Models Based on Clar Sextet Theory. J. Phys. Chem. C 2009, 113, 862–866. [Google Scholar]
- (284).Ormsby JL; King BT The Regioselectivity of Addition to Carbon Nanotube Segments. J. Org. Chem 2007, 72, 4035–4038. [DOI] [PubMed] [Google Scholar]
- (285).Mercuri F; Sgamellotti A First-Principles Investigations on the Functionalization of Chiral and Non-Chiral Carbon Nanotubes by Diels–Alder Cycloaddition Reactions. Phys. Chem. Chem. Phys 2009, 11, 563–567. [DOI] [PubMed] [Google Scholar]
- (286).Adjizian JJ; Leghrib R; Koos AA; Suarez-Martinez I; Crossley A; Wagner P; Grobert N; Llobet E; Ewels CP Boron- and Nitrogen-Doped Multi-Wall Carbon Nanotubes for Gas Detection. Carbon N. Y 2014, 66, 662–673. [Google Scholar]
- (287).Hamadanian M; Fotooh FK Density Functional Study of Al/N Co-Doped (10,0) Zigzag Single-Walled Carbon Nanotubes as CO Sensor. Comput. Mater. Sci 2014, 82, 497–502. [Google Scholar]
- (288).Hizhnyi Y; Nedilko SG; Borysiuk V; Gubanov VA Computational Studies of Boron- and Nitrogen-Doped Single-Walled Carbon Nanotubes as Potential Sensor Materials of Hydrogen Halide Molecules HX (X = F, Cl, Br). Int. J. Quantum Chem 2015, 115, 1475–1482. [Google Scholar]
- (289).Rocha AR; Rossi M; Fazzio A; da Silva AJR Designing Real Nanotube-Based Gas Sensors. Phys. Rev. Lett 2008, 100, 176803. [DOI] [PubMed] [Google Scholar]
- (290).Azizi K; Karimpanah M Computational Study of Al- or P-Doped Single-Walled Carbon Nanotubes as NH3and NO2sensors. Appl. Surf. Sci 2013, 285, 102–109. [Google Scholar]
- (291).Tavakol H; Hassani F Adsorption of Molecular Iodine on the Surface of Sulfur-Doped Carbon Nanotubes: Theoretical Study on Their Interactions, Sensor Properties, and Other Applications. Struct. Chem 2015, 26, 151–158. [Google Scholar]
- (292).Gowri sankar PA; Udhayakumar K Electronic Properties of Boron and Silicon Doped (10, 0) Zigzag Single-Walled Carbon Nanotube upon Gas Molecular Adsorption: A DFT Comparative Study. J. Nanomater 2013, 2013, 1–12. [Google Scholar]
- (293).Vikramaditya T; Sumithra K Effect of Substitutionally Boron-Doped Single-Walled Semiconducting Zigzag Carbon Nanotubes on Ammonia Adsorption. J. Comput. Chem 2014, 35, 586–594. [DOI] [PubMed] [Google Scholar]
- (294).Talla JA First Principles Modeling of Boron-Doped Carbon Nanotube Sensors. Phys. B Condens. Matter 2012, 407, 966–970. [Google Scholar]
- (295).Zhang Y; Zhang D; Liu C Novel Chemical Sensor for Cyanides: Boron-Doped Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 4671–4674. [DOI] [PubMed] [Google Scholar]
- (296).Wang R; Zhang D; Zhang Y; Liu C Boron-Doped Carbon Nanotubes Serving as a Novel Chemical Sensor for Formaldehyde. J. Phys. Chem. B 2006, 110, 18267–18271. [DOI] [PubMed] [Google Scholar]
- (297).Hamadanian M; Khoshnevisan B; Fotooh FK; Tavangar Z Computational Study of Super Cell Al-Substituted Single-Walled Carbon Nanotubes as CO Sensor. Comput. Mater. Sci 2012, 58, 45–50. [Google Scholar]
- (298).Zhou Q; Wang C; Fu Z; Zhang H; Tang Y Adsorption of Formaldehyde Molecule on Al-Doped Vacancy-Defected Single-Walled Carbon Nanotubes: A Theoretical Study. Comput. Mater. Sci 2014, 82, 337–344. [Google Scholar]
- (299).Zhang X; Dai Z; Chen Q; Tang J A DFT Study of SO 2 and H 2 S Gas Adsorption on Au-Doped Single-Walled Carbon Nanotubes. Phys. Scr 2014, 89, 065803. [Google Scholar]
- (300).Zhang X; Gui Y; Xiao H; Zhang Y Analysis of Adsorption Properties of Typical Partial Discharge Gases on Ni-SWCNTs Using Density Functional Theory. Appl. Surf. Sci 2016, 379, 47–54. [Google Scholar]
- (301).Buasaeng P; Rakrai W; Wanno B; Tabtimsai C DFT Investigation of NH3, PH3, and AsH3adsorptions on Sc-, Ti-, V-, and Cr-Doped Single-Walled Carbon Nanotubes. Appl. Surf. Sci 2017, 400, 506–514. [Google Scholar]
- (302).Zhang X; Gong X DFT, QTAIM, and NBO Investigations of the Ability of the Fe or Ni Doped CNT to Absorb and Sense CO and NO. J. Mol. Model 2015, 21, 225. [DOI] [PubMed] [Google Scholar]
- (303).Sharafeldin IM; Allam N DFT Insights into the Electronic Properties and Adsorption of NO2 on Metal-Doped Carbon Nanotubes for Gas Sensing Applications. New J. Chem 2017, 41, 14936–14944. [Google Scholar]
- (304).Mowbray DJ; Garca-Lastra JM; Thygesen KS; Rubio A; Jacobsen KW Designing Multifunctional Chemical Sensors Using Ni and Cu Doped Carbon Nanotubes. Phys. status solidi 2010, 247, 2678–2682. [Google Scholar]
- (305).Cui S; Pu H; Mattson EC; Lu G; Mao S; Weinert M; Hirschmugl CJ; Gajdardziska-Josifovska M; Chen J Ag Nanocrystal as a Promoter for Carbon Nanotube-Based Room-Temperature Gas Sensors. Nanoscale 2012, 4, 5887–5894. [DOI] [PubMed] [Google Scholar]
- (306).Charlier J-C; Arnaud L; Avilov IV; Delgado M; Demoisson F; Espinosa EH; Ewels CP; Felten A; Guillot J; Ionescu R; et al. Carbon Nanotubes Randomly Decorated with Gold Clusters: From Nano 2 Hybrid Atomic Structures to Gas Sensing Prototypes. Nanotechnology 2009, 20, 375501. [DOI] [PubMed] [Google Scholar]
- (307).Singh NB; Bhattacharya B; Mondal R; Sarkar U Nickel Cluster Functionalised Carbon Nanotube for CO Molecule Detection: A Theoretical Study. Mol. Phys 2016, 114, 671–680. [Google Scholar]
- (308).Singh NB; Bhattacharya B; Sarkar U Nickel Decorated Single-Wall Carbon Nanotube as CO Sensor. Soft Nanosci. Lett 2013, 3, 9–11. [Google Scholar]
- (309).Yoosefian M; Raissi H; Mola A The Hybrid of Pd and SWCNT (Pd Loaded on SWCNT) as an Efficient Sensor for the Formaldehyde Molecule Detection: A DFT Study. Sensors Actuators, B Chem 2015, 212, 55–62. [Google Scholar]
- (310).Li L; Zhang G; Chen L; Bi HM; Shi KY Ni(NiO)/Single-Walled Carbon Nanotubes Composite: Synthesis of Electro-Deposition, Gas Sensing Property for NO Gas and Density Functional Theory Calculation. Mater. Res. Bull 2013, 48, 504–511. [Google Scholar]
- (311).Liu SF; Lin S; Swager TM An Organocobalt–Carbon Nanotube Chemiresistive Carbon Monoxide Detector. ACS Sensors 2016, 1, 354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (312).Mittal M; Kumar A Carbon Nanotube (CNT) Gas Sensors for Emissions from Fossil Fuel Burning. Sensors Actuators, B Chem 2014, 203, 349–362. [Google Scholar]
- (313).Timmer B; Olthuis W; Van Den Berg A Ammonia Sensors and Their Applications - A Review. Sensors Actuators, B Chem 2005, 107, 666–677. [Google Scholar]
- (314).Michaels RA Emergency Planning and the Acute Toxic Potency of Inhaled Ammonia. Environ. Health Perspect 1999, 107, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (315).Behera SN; Sharma M; Aneja VP; Balasubramanian R Ammonia in the Atmosphere: A Review on Emission Sources, Atmospheric Chemistry and Deposition on Terrestrial Bodies. Environ. Sci. Pollut. Res 2013, 20, 8092–8131. [DOI] [PubMed] [Google Scholar]
- (316).OSHA. Occupational Safety and Health Standards. 1910.1000 TABLE Z-1, 1993. [Google Scholar]
- (317).Environmental Protection Agency. Aquatic Life Ambient Water Quality Criteria for Ammonia – Freshwater (EPA-822-R-13–001). 2013, 255. [Google Scholar]
- (318).Bradley K; Gabriel J-CP; Briman M; Star A; Grüner G Charge Transfer from Ammonia Physisorbed on Nanotubes. Phys. Rev. Lett 2003, 91, 218301. [DOI] [PubMed] [Google Scholar]
- (319).Jones FE; Talin AA; Léonard F; Dentinger PM; Clift WM Effect of Electrode Material on Transport and Chemical Sensing Characteristics of Metal/Carbon Nanotube Contacts. J. Electron. Mater 2006, 35, 1641–1646. [Google Scholar]
- (320).Jang YT; Moon S Il; Ahn JH; Lee YH; Ju BK A Simple Approach in Fabricating Chemical Sensor Using Laterally Grown Multi-Walled Carbon Nanotubes. Sensors Actuators, B Chem 2004, 99, 118–122. [Google Scholar]
- (321).Jung HY; Jung SM; Kim J; Suh JS Chemical Sensors for Sensing Gas Adsorbed on the Inner Surface of Carbon Nanotube Channels. Appl. Phys. Lett 2007, 90, 153114. [Google Scholar]
- (322).Han J-W; Kim B; Li J; Meyyappan M A Carbon Nanotube Based Ammonia Sensor on Cellulose Paper. RSC Adv 2014, 4, 549–553. [Google Scholar]
- (323).Goldoni A; Larciprete R; Petaccia L; Lizzit S Single-Wall Carbon Nanotube Interaction with Gases: Sample Contaminants and Environmental Monitoring. J. Am. Chem. Soc 2003, 125, 11329–11333. [DOI] [PubMed] [Google Scholar]
- (324).Salehi-Khojin A; Khalili-Araghi F; Kuroda MA; Lin KY; Leburton J-P; Masel RI On the Sensing Mechanism in Carbon Nanotube Chemiresistors. ACS Nano 2011, 5, 153–158. [DOI] [PubMed] [Google Scholar]
- (325).Cantalini C; Valentini L; Lozzi L; Armentano I; Kenny JM; Santucci S NO2 Gas Sensitivity of Carbon Nanotubes Obtained by Plasma Enhanced Chemical Vapor Deposition. Sensors Actuators, B Chem 2003, 93, 333–337. [Google Scholar]
- (326).Penza M; Cassano G; Rossi R; Alvisi M; Rizzo A; Signore MA; Dikonimos T; Serra E; Giorgi R Enhancement of Sensitivity in Gas Chemiresistors Based on Carbon Nanotube Surface Functionalized with Noble Metal (Au, Pt) Nanoclusters. Appl. Phys. Lett 2007, 90, 88–91. [Google Scholar]
- (327).Penza M; Rossi R; Alvisi M; Cassano G; Serra E Functional Characterization of Carbon Nanotube Networked Films Functionalized with Tuned Loading of Au Nanoclusters for Gas Sensing Applications. Sensors Actuators, B Chem 2009, 140, 176–184. [Google Scholar]
- (328).Lee K; Scardaci V; Kim H-Y; Hallam T; Nolan H; Bolf BE; Maltbie GS; Abbott JE; Duesberg GS Highly Sensitive, Transparent, and Flexible Gas Sensors Based on Gold Nanoparticle Decorated Carbon Nanotubes. Sensors Actuators B Chem 2013, 188, 571–575. [Google Scholar]
- (329).Cui S; Pu H; Lu G; Wen Z; Mattson EC; Hirschmugl C; Gajdardziska-Josifovska M; Weinert M; Chen J Fast and Selective Room-Temperature Ammonia Sensors Using Silver Nanocrystal-Functionalized Carbon Nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 4898–4904. [DOI] [PubMed] [Google Scholar]
- (330).Nguyen L; Phan P; Duong H; Nguyen C; Nguyen L Enhancement of NH3 Gas Sensitivity at Room Temperature by Carbon Nanotube-Based Sensor Coated with Co Nanoparticles. Sensors 2013, 13, 1754–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (331).Yoon B; Liu SF; Swager TM Surface-Anchored Poly(4-Vinylpyridine)–Single-Walled Carbon Nanotube–Metal Composites for Gas Detection. Chem. Mater 2016, 28, 5916–5924. [Google Scholar]
- (332).Hoa ND; Quy N. Van; Cho YS; Kim D Nanocomposite of SWNTs and SnO 2 Fabricated by Soldering Process for Ammonia Gas Sensor Application. Phys. Status Solidi Appl. Mater. Sci 2007, 204, 1820–1824. [Google Scholar]
- (333).Ghaddab B; Sanchez JB; Mavon C; Paillet M; Parret R; Zahab AA; Bantignies J-L; Flaud V; Beche E; Berger F Detection of O3 and NH3 Using Hybrid Tin Dioxide/Carbon Nanotubes Sensors: Influence of Materials and Processing on Sensor’s Sensitivity. Sensors Actuators B Chem 2012, 170, 67–74. [Google Scholar]
- (334).Mubeen S; Lai M; Zhang T; Lim JH; Mulchandani A; Deshusses MA; Myung NV Hybrid Tin Oxide-SWNT Nanostructures Based Gas Sensor. Electrochim. Acta 2013, 92, 484–490. [Google Scholar]
- (335).Hoa ND; Van Quy N; Kim D Nanowire Structured SnOx-SWNT Composites: High Performance Sensor for NOx Detection. Sensors Actuators, B Chem 2009, 142, 253–259. [Google Scholar]
- (336).Rigoni F; Drera G; Pagliara S; Goldoni A; Sangaletti L High Sensitivity, Moisture Selective, Ammonia Gas Sensors Based on Single-Walled Carbon Nanotubes Functionalized with Indium Tin Oxide Nanoparticles. Carbon N. Y 2014, 80, 356–363. [Google Scholar]
- (337).Du N; Zhang H; Chen B; Xiangyang M; Liu Z; Wu J; Yang D Porous Indium Oxide Nanotubes: Layer-by-Layer Assembly on Carbon-Nanotube Templates and Application for Room-Temperature NH3 Gas Sensors. Adv. Mater 2007, 19, 1641–1645. [Google Scholar]
- (338).Choi S-W; Kim J; Byun YT Highly Sensitive and Selective NO 2 Detection by Pt Nanoparticles-Decorated Single-Walled Carbon Nanotubes and the Underlying Sensing Mechanism. Sensors Actuators B Chem 2017, 238, 1032–1042. [Google Scholar]
- (339).Bekyarova E; Kalinina I; Itkis ME; Beer L; Cabrera N; Haddon RC Mechanism of Ammonia Detection by Chemically Functionalized Single-Walled Carbon Nanotubes: In Situ Electrical and Optical Study of Gas Analyte Detection. J. Am. Chem. Soc 2007, 129, 10700–10706. [DOI] [PubMed] [Google Scholar]
- (340).Zhang T; Nix MB; Yoo BY; Deshusses MA; Myung NV Electrochemically Functionalized Single-Walled Carbon Nanotube Gas Sensor. Electroanalysis 2006, 18, 1153–1158. [Google Scholar]
- (341).Ding M; Tang Y; Gou P; Reber MJ; Star A Chemical Sensing with Polyaniline Coated Single-Walled Carbon Nanotubes. Adv. Mater 2011, 23, 536–540. [DOI] [PubMed] [Google Scholar]
- (342).Tian X; Wang Q; Chen X; Yang W; Wu Z; Xu X; Jiang M; Zhou Z Enhanced Performance of Core-Shell Structured Polyaniline at Helical Carbon Nanotube Hybrids for Ammonia Gas Sensor. Appl. Phys. Lett 2014, 105, 203109. [Google Scholar]
- (343).Li Y; Wang H; Yang M N-Type Gas Sensing Characteristics of Chemically Modified Multi-Walled Carbon Nanotubes and PMMA Composite. Sensors Actuators B Chem 2007, 121, 496–500. [Google Scholar]
- (344).Datta K; Ghosh P; More MA; Shirsat MD; Mulchandani A Controlled Functionalization of Single-Walled Carbon Nanotubes for Enhanced Ammonia Sensing: A Comparative Study. J. Phys. D. Appl. Phys 2012, 45, 355305. [Google Scholar]
- (345).Qi P; Vermesh O; Grecu M; Javey A; Wang Q; Dai H; Peng S; Cho KJ Toward Large Arrays of Multiplex Functionalized Carbon Nanotube Sensors for Highly Sensitive and Selective Molecular Detection. Nano Lett 2003, 3, 347–351. [DOI] [PubMed] [Google Scholar]
- (346).An KH; Jeong SY; Hwang HR; Lee YH Enhanced Sensitivity of a Gas Sensor Incorporating Single-Walled Carbon Nanotube-Polypyrrole Nanocomposites. Adv. Mater 2004, 16, 1005–1009. [Google Scholar]
- (347).Liang X; Chen Z; Wu H; Guo L; He C; Wang B; Wu Y Enhanced NH3-Sensing Behavior of 2,9,16,23-Tetrakis(2,2,3,3-Tetrafluoropropoxy) Metal(II) Phthalocyanine/Multi-Walled Carbon Nanotube Hybrids: An Investigation of the Effects of Central Metals. Carbon N. Y 2014, 80, 268–278. [Google Scholar]
- (348).Kaya EN; Basova T; Polyakov M; Durmuş M; Kadem B; Hassan A Hybrid Materials of Pyrene Substituted Phthalocyanines with Single-Walled Carbon Nanotubes: Structure and Sensing Properties. RSC Adv 2015, 5, 91855–91862. [Google Scholar]
- (349).Hübert T; Boon-Brett L; Black G; Banach U Hydrogen Sensors - A Review. Sensors Actuators, B Chem 2011, 157, 329–352. [Google Scholar]
- (350).Dag S; Ozturk Y; Ciraci S; Yildirim T Adsorption and Dissociation of Hydrogen Molecules on Bare and Functionalized Carbon Nanotubes. Phys. Rev. B 2005, 72, 155404. [Google Scholar]
- (351).Krishna Kumar M; Ramaprabhu S Nanostructured Pt Functionlized Multiwalled Carbon Nanotube Based Hydrogen Sensor. J. Phys. Chem. B 2006, 110, 11291–11298. [DOI] [PubMed] [Google Scholar]
- (352).Penner RM A Nose for Hydrogen Gas: Fast, Sensitive H2Sensors Using Electrodeposited Nanomaterials. Acc. Chem. Res 2017, 50, 1902–1910. [DOI] [PubMed] [Google Scholar]
- (353).Su HC; Zhang M; Bosze W; Lim J-H; Myung NV Metal Nanoparticles and DNA Co-Functionalized Single-Walled Carbon Nanotube Gas Sensors. Nanotechnology 2013, 24, 505502. [DOI] [PubMed] [Google Scholar]
- (354).Sayago I; Terrado E; Lafuente E; Horrillo MC; Maser WK; Benito AM; Navarro R; Urriolabeitia EP; Martinez MT; Gutierrez J Hydrogen Sensors Based on Carbon Nanotubes Thin Films. Synth. Met 2005, 148, 15–19. [Google Scholar]
- (355).Sayago I; Terrado E; Aleixandre M; Horrillo MC; Fernández MJ; Lozano J; Lafuente E; Maser WK; Benito AM; Martinez MT; et al. Novel Selective Sensors Based on Carbon Nanotube Films for Hydrogen Detection. Sensors Actuators, B Chem 2007, 122, 75–80. [Google Scholar]
- (356).Sippel-Oakley J; Wang H-T; Kang BS; Wu Z; Ren F; Rinzler AG; Pearton SJ Carbon Nanotube Films for Room Temperature Hydrogen Sensing. Nanotechnology 2005, 16, 2218–2221. [DOI] [PubMed] [Google Scholar]
- (357).Mubeen S; Zhang T; Yoo B; Deshusses MA; Myung NV Palladium Nanoparticles Decorated Single-Walled Carbon Nanotube Hydrogen Sensor. J. Phys. Chem. C 2007, 111, 6321–6327. [Google Scholar]
- (358).Sun Y; Wang HH Electrodeposition of Pd Nanoparticles on Single-Walled Carbon Nanotubes for Flexible Hydrogen Sensors. Appl. Phys. Lett 2007, 90, 213107. [Google Scholar]
- (359).Khalap VR; Sheps T; Kane AA; Collins PG Hydrogen Sensing and Sensitivity of Palladium-Decorated Single-Walled Carbon Nanotubes with Defects. Nano Lett 2010, 10, 896–901. [DOI] [PubMed] [Google Scholar]
- (360).Rumiche F; Wang HH; Indacochea JE Development of a Fast-Response/High-Sensitivity Double Wall Carbon Nanotube Nanostructured Hydrogen Sensor. Sensors Actuators, B Chem 2012, 163, 97–106. [Google Scholar]
- (361).Randeniya LK; Martin PJ; Bendavid A Detection of Hydrogen Using Multi-Walled Carbon-Nanotube Yarns Coated with Nanocrystalline Pd and Pd/Pt Layered Structures. Carbon N. Y 2012, 50, 1786–1792. [Google Scholar]
- (362).Li X; Le Thai M; Dutta RK; Qiao S; Chandran GT; Penner RM Sub-6 Nm Palladium Nanoparticles for Faster, More Sensitive H2 Detection Using Carbon Nanotube Ropes. ACS Sensors 2017, 2, 282–289. [DOI] [PubMed] [Google Scholar]
- (363).Javey A; Guo J; Wang Q; Lundstrom M; Dai H Ballistic Carbon Nanotube Field-Effect Transistors. Nature 2003, 424, 654–657. [DOI] [PubMed] [Google Scholar]
- (364).Choi B; Lee D; Ahn J-H; Yoon J; Lee J; Jeon M; Kim DM; Kim DH; Park I; Choi Y-K; et al. Investigation of Optimal Hydrogen Sensing Performance in Semiconducting Carbon Nanotube Network Transistors with Palladium Electrodes. Appl. Phys. Lett 2015, 107, 193108. [Google Scholar]
- (365).Lu Y; Li J; Han J; Ng HT; Binder C; Partridge C; Meyyappan M Room Temperature Methane Detection Using Palladium Loaded Single-Walled Carbon Nanotube Sensors. Chem. Phys. Lett 2004, 391, 344–348. [Google Scholar]
- (366).Humayun MT; Divan R; Liu Y; Gundel L; Solomon PA; Paprotny I Novel Chemoresistive CH 4 Sensor with 10 Ppm Sensitivity Based on Multiwalled Carbon Nanotubes Functionalized with SnO 2 Nanocrystals. J. Vac. Sci. Technol. A Vacuum, Surfaces, Film 2016, 34, 01A131. [Google Scholar]
- (367).Humayun MT; Divan R; Stan L; Gupta A; Rosenmann D; Gundel L; Solomon PA; Paprotny I ZnO Functionalization of Multiwalled Carbon Nanotubes for Methane Sensing at Single Parts per Million Concentration Levels. J. Vac. Sci. Technol. B, Nanotechnol. Microelectron. Mater. Process. Meas. Phenom 2015, 33, 06FF01. [Google Scholar]
- (368).Raub JA; Mathieu-Nolf M; Hampson NB; Thom SR Carbon Monoxide Poisoning — a Public Health Perspective. Toxicology 2000, 145, 1–14. [DOI] [PubMed] [Google Scholar]
- (369).Occupational Safety and Health Administration. Occupational Safety and Health Administration, “Carbon Monoxide In Workplace Atmospheres (Direct-Reading Monitor),” OSHA Method ID-209, 1993. [Google Scholar]
- (370).Thom Stephen R.. Hyperbaric-Oxygen Theraopy For Acute Carbon Monoxide Poisoning. N Engl J Med 2002, 347, 1105–1106. [DOI] [PubMed] [Google Scholar]
- (371).Thom SR; Keim LW Carbon Monoxide Poisoning: A Review Epidemiology, Pathophysiology, Clinical Findings, and Treatment Options Including Hyperbaric Oxygen Therapy. J. Toxicol. Clin. Toxicol 1989, 27, 141–156. [DOI] [PubMed] [Google Scholar]
- (372).Swager TM Sensor Technologies Empowered by Materials and Molecular Innovations. Angew. Chemie Int. Ed 2018, 57, 4248–4257. [DOI] [PubMed] [Google Scholar]
- (373).Da Silva LB; Fagan SB; Mota R Ab Initio Study of Deformed Carbon Nanotube Sensors for Carbon Monoxide Molecules. Nano Lett 2004, 4, 65–67. [Google Scholar]
- (374).Peng S; Cho KJ Ab Initio Study of Doped Carbon Nanotube Sensors. Nano Lett 2003, 3, 513–517. [DOI] [PubMed] [Google Scholar]
- (375).Wang R; Zhang D; Sun W; Han Z; Liu C A Novel Aluminum-Doped Carbon Nanotubes Sensor for Carbon Monoxide. J. Mol. Struct. THEOCHEM 2007, 806, 93–97. [Google Scholar]
- (376).Bittencourt C; Felten A; Espinosa EHH; Ionescu R; Llobet E; Correig X; Pireaux J-JJ WO3 Films Modified with Functionalised Multi-Wall Carbon Nanotubes: Morphological, Compositional and Gas Response Studies. Sensors Actuators B Chem 2006, 115, 33–41. [Google Scholar]
- (377).Hannon A; Lu Y; Li J; Meyyappan M Room Temperature Carbon Nanotube Based Sensor for Carbon Monoxide Detection. J. Sensors Sens. Syst 2014, 3, 349–354. [Google Scholar]
- (378).Kauffman DR; Sorescu DC; Schofield DP; Allen BL; Jordan KD; Star A Understanding the Sensor Response of Metal-Decorated Carbon Nanotubes. Nano Lett 2010, 10, 958–963. [DOI] [PubMed] [Google Scholar]
- (379).Choi SW; Kim J; Lee JH; Byun YT Remarkable Improvement of CO-Sensing Performances in Single-Walled Carbon Nanotubes Due to Modification of the Conducting Channel by Functionalization of Au Nanoparticles. Sensors Actuators, B Chem 2016, 232, 625–632. [Google Scholar]
- (380).Yoosefian M; Barzgari Z; Yoosefian J Ab Initio Study of Pd-Decorated Single-Walled Carbon Nanotube with C-Vacancy as CO Sensor. Struct. Chem 2014, 25, 9–19. [Google Scholar]
- (381).Zhang Y; Cui S; Chang J; Ocola LE; Chen J Highly Sensitive Room Temperature Carbon Monoxide Detection Using SnO 2 Nanoparticle-Decorated Semiconducting Single-Walled Carbon Nanotubes. Nanotechnology 2013, 24, 025503. [DOI] [PubMed] [Google Scholar]
- (382).Leghrib R; Pavelko R; Felten A; Vasiliev A; Cané C; Gràcia I; Pireaux JJ; Llobet E Gas Sensors Based on Multiwall Carbon Nanotubes Decorated with Tin Oxide Nanoclusters. Sensors Actuators, B Chem 2010, 145, 411–416. [Google Scholar]
- (383).Wanna Y; Srisukhumbowornchai N; Tuantranont A; Wisitsoraat A; Thavarungkul N; Singjai P The Effect of Carbon Nanotube Dispersion on CO Gas Sensing Characteristics of Polyaniline Gas Sensor. J. Nanosci. Nanotechnol 2006, 6, 3893–3896. [DOI] [PubMed] [Google Scholar]
- (384).Lin Y; Kan K; Song W; Zhang G; Dang L; Xie Y; Shen P; Li L; Shi K Controllable Synthesis of Co3O4/Polyethyleneimine-Carbon Nanotubes Nanocomposites for CO and NH3 Gas Sensing at Room Temperature. J. Alloys Compd 2015, 639, 187–196. [Google Scholar]
- (385).Dong X; Fu D; Ahmed MO; Shi Y; Mhaisalkar SG; Zhang S; Moochhala S; Ho X; Rogers JA; Li LJ Heme-Enabled Electrical Detection of Carbon Monoxide at Room Temperature Using Networked Carbon Nanotube Field-Effect Transistors. Chem. Mater 2007, 19, 6059–6061. [Google Scholar]
- (386).He Y; Zhang J; Zhao J Theoretical Investigation on the Electronic Transport Properties of Iron(II) Porphyrin for CO Sensing with Single-Walled Carbon Nanotubes. Chem. Lett 2014, 43, 735–737. [Google Scholar]
- (387).Gabriel D; Deshusses M. a. Retrofitting Existing Chemical Scrubbers to Biotrickling Filters for H2S Emission Control. Proc. Natl. Acad. Sci 2003, 100, 6308–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (388).Hydrogen Sulfide. OSHA Sampling and Analytical Method 1008, Control Number T-1008-FV-01–0609-M.
- (389).Yao F; Duong DL; Lim SC; Yang SB; Hwang HR; Yu WJ; Lee IH; Güneş F; Lee YH Humidity-Assisted Selective Reactivity between NO2 and SO2 Gas on Carbon Nanotubes. J. Mater. Chem 2011, 21, 4502–4508. [Google Scholar]
- (390).Mäklin J; Mustonen T; Halonen N; Tóth G; Kordás K; Vähäkangas J; Moilanen H; Kukovecz Á; Kónya Z; Haspel H; et al. Inkjet Printed Resistive and Chemical-FET Carbon Nanotube Gas Sensors. Phys. Status Solidi Basic Res 2008, 245, 2335–2338. [Google Scholar]
- (391).Farooqui MF; Karimi MA; Salama KN; Shamim A 3D-Printed Disposable Wireless Sensors with Integrated Microelectronics for Large Area Environmental Monitoring. Adv. Mater. Technol 2017, 2, 1700051. [Google Scholar]
- (392).Zhang X; Yang B; Wang X; Luo C Effect of Plasma Treatment on Multi-Walled Carbon Nanotubes for the Detection of H2S and SO2. Sensors 2012, 12, 9375–9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (393).Zhang X; Dai Z; Wei L; Liang N; Wu X Theoretical Calculation of the Gas-Sensing Properties of Pt-Decorated Carbon Nanotubes. Sensors 2013, 13, 15159–15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Ding M; Sorescu DC; Kotchey GP; Star A Welding of Gold Nanoparticles on Graphitic Templates for Chemical Sensing. J. Am. Chem. Soc 2012, 134, 3472–3479. [DOI] [PubMed] [Google Scholar]
- (395).Dilonardo E; Penza M; Alvisi M; Di Franco C; Rossi R; Palmisano F; Torsi L; Cioffi N Electrophoretic Deposition of Au NPs on MWCNT-Based Gas Sensor for Tailored Gas Detection with Enhanced Sensing Properties. Sensors Actuators, B Chem 2016, 223, 417–428. [Google Scholar]
- (396).Fam DWH; Tok AIY; Palaniappan A; Nopphawan P; Lohani A; Mhaisalkar SG Selective Sensing of Hydrogen Sulphide Using Silver Nanoparticle Decorated Carbon Nanotubes. Sensors Actuators, B Chem 2009, 138, 189–192. [Google Scholar]
- (397).Wu H; Chen Z; Zhang J; Wu F; He C; Wang B; Wu Y; Ren Z Stably Dispersed Carbon Nanotubes Covalently Bonded to Phthalocyanine Cobalt(II) for Ppb-Level H2S Sensing at Room Temperature. J. Mater. Chem. A 2016, 4, 1096–1104. [Google Scholar]
- (398).Mendoza F; Hernández DM; Makarov V; Febus E; Weiner BR; Morell G Room Temperature Gas Sensor Based on Tin Dioxide-Carbon Nanotubes Composite Films. Sensors Actuators, B Chem 2014, 190, 227–233. [Google Scholar]
- (399).Liu H; Zhang W; Yu H; Gao L; Song Z; Xu S; Li M; Wang Y; Song H; Tang J Solution-Processed Gas Sensors Employing SnO 2 Quantum Dot/MWCNT Nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 840–846. [DOI] [PubMed] [Google Scholar]
- (400).Dai H; Xiao P; Lou Q Application of SnO2/MWCNTs Nanocomposite for SF6 Decomposition Gas Sensor. Phys. status solidi 2011, 208, 1714–1717. [Google Scholar]
- (401).Asad M; Sheikhi MH; Pourfath M; Moradi M High Sensitive and Selective Flexible H2S Gas Sensors Based on Cu Nanoparticle Decorated SWCNTs. Sensors Actuators B Chem 2015, 210, 1–8. [Google Scholar]
- (402).Asad M; Sheikhi MH Highly Sensitive Wireless H2S Gas Sensors at Room Temperature Based on CuO-SWCNT Hybrid Nanomaterials. Sensors Actuators, B Chem 2016, 231, 474–483. [Google Scholar]
- (403).Yu SYS; Yi WYW Single-Walled Carbon Nanotubes as a Chemical Sensor for SO2 Detection. IEEE Trans. Nanotechnol 2007, 6, 545–548. [Google Scholar]
- (404).Suehiro J; Zhou G; Hara M Detection of Partial Discharge in SF6 Gas Using a Carbon Nanotube-Based Gas Sensor. Sensors Actuators B Chem 2005, 105, 164–169. [Google Scholar]
- (405).Li W; Ma J-J; Liu P; Pan Z-L; He Q-Y First-Principles Study of the Adsorption Sensitivity of Ni-Doped Single-Walled Zigzag (n,0)CNTs (N=4,5,6) toward SO2 Molecules. Appl. Surf. Sci 2015, 335, 17–22. [Google Scholar]
- (406).Edokpolo B; Yu QJ; Connell D Health Risk Assessment of Ambient Air Concentrations of Benzene, Toluene and Xylene (BTX) in Service Station Environments. Int. J. Environ. Res. Public Health 2014, 11, 6354–6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Saxena Pallavi. A Review of Assessment of Benzene, Toluene, Ethylbenzene and Xylene (BTEX) Concentration in Urban Atmosphere of Delhi. Int. J. Phys. Sci 2012, 7, 850–860. [Google Scholar]
- (408).Lim SK; Shin HS; Yoon KS; Kwack SJ; Um YM; Hyeon JH; Kwak HM; Kim JY; Kim TH; Kim YJ; et al. Risk Assessment of Volatile Organic Compounds Benzene, Toluene, Ethylbenzene, and Xylene (BTEX) in Consumer Products. J. Toxicol. Environ. Heal. Part A 2014, 77, 1502–1521. [DOI] [PubMed] [Google Scholar]
- (409).Ammonia. OSHA Sampling and Analytical Method 188, Control number T-ID188-FV-02–0201-M.
- (410).Pejcic B; Crooke E; Boyd L; Doherty CM; Hill AJ; Myers M; White C Using Plasticizers to Control the Hydrocarbon Selectivity of a Poly(Methyl Methacrylate)-Coated Quartz Crystal Microbalance Sensor. Anal. Chem 2012, 84, 8564–8570. [DOI] [PubMed] [Google Scholar]
- (411).Ju JF; Syu MJ; Teng HS; Chou SK; Chang YS Preparation and Identification of β-Cyclodextrin Polymer Thin Film for Quartz Crystal Microbalance Sensing of Benzene, Toluene, and p-Xylene. Sensors Actuators, B Chem 2008, 132, 319–326. [Google Scholar]
- (412).Parsons MT; Sydoryk I; Lim A; McIntyre TJ; Tulip J; Jäger W; McDonald K Real-Time Monitoring of Benzene, Toluene, and p-Xylene in a Photoreaction Chamber with a Tunable Mid-Infrared Laser and Ultraviolet Differential Optical Absorption Spectroscopy. Appl. Opt 2011, 50, A90–A99. [DOI] [PubMed] [Google Scholar]
- (413).Rushi AD; Datta KP; Ghosh PS; Mulchandani A; Shirsat MD Selective Discrimination among Benzene, Toluene, and Xylene: Probing Metalloporphyrin-Functionalized Single-Walled Carbon Nanotube-Based Field Effect Transistors. J. Phys. Chem. C 2014, 118, 24034–24041. [Google Scholar]
- (414).Chatterjee S; Castro M; Feller JF Tailoring Selectivity of Sprayed Carbon Nanotube Sensors (CNT) towards Volatile Organic Compounds (VOC) with Surfactants. Sensors Actuators, B Chem 2015, 220, 840–849. [Google Scholar]
- (415).Son M; Cho DG; Lim JH; Park J; Hong S; Ko HJ; Park TH Real-Time Monitoring of Geosmin and 2-Methylisoborneol, Representative Odor Compounds in Water Pollution Using Bioelectronic Nose with Human-like Performance. Biosens. Bioelectron 2015, 74, 199–206. [DOI] [PubMed] [Google Scholar]
- (416).Li P; Martin CM; Yeung KK; Xue W Dielectrophoresis Aligned Single-Walled Carbon Nanotubes as PH Sensors. Biosensors 2011, 1, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (417).Takeda S; Nakamura M; Ishii A; Subagyo A; Hosoi H; Sueoka K; Mukasa K A PH Sensor Based on Electric Properties of Nanotubes on a Glass Substrate. Nanoscale Res. Lett 2007, 2, 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (418).Liao Y; Zhang C; Zhang Y; Strong V; Tang J; Li XG; Kalantar-Zadeh K; Hoek EMV; Wang KL; Kaner RB Carbon Nanotube/Polyaniline Composite Nanofibers: Facile Synthesis and Chemosensors. Nano Lett 2011, 11, 954–959. [DOI] [PubMed] [Google Scholar]
- (419).Ferrer-Anglada N; Kaempgen M; Roth S Transparent and Flexible Carbon Nanotube/Polypyrrole and Carbon Nanotube/Polyaniline PH Sensors. Phys. Status Solidi Basic Res 2006, 243, 3519–3523. [Google Scholar]
- (420).Gumpu MB; Sethuraman S; Krishnan UM; Rayappan JBB A Review on Detection of Heavy Metal Ions in Water - An Electrochemical Approach. Sensors Actuators, B Chem 2015, 213, 515–533. [Google Scholar]
- (421).Regional Screening Levels (RSLs) - Generic Tables,U.S. Environmental Protection Agency, 2018. https://www.epa.gov/risk/regional-screening-levels (accessed 0n July 31). [Google Scholar]
- (422).Musameh MM; Hickey M; Kyratzis IL Carbon Nanotube-Based Extraction and Electrochemical Detection of Heavy Metals. Res. Chem. Intermed 2011, 37, 675–689. [Google Scholar]
- (423).Li M; Gou H; Al-Ogaidi I; Wu N Nanostructured Sensors for Detection of Heavy Metals: A Review. ACS Sustain. Chem. Eng 2013, 1, 713–723. [Google Scholar]
- (424).Morton J; Havens N; Mugweru A; Wanekaya AK Detection of Trace Heavy Metal Ions Using Carbon Nanotube Modified Electrodes. Electroanalysis 2009, 21, 1597–1603. [Google Scholar]
- (425).Zhang F; Sun Y; Tian D; Shin WS; Kim JS; Li H Selective Molecular Recognition on Calixarene-Functionalized 3D Surfaces. Chem. Commun 2016, 52, 12685–12693. [DOI] [PubMed] [Google Scholar]
- (426).Kim TH; Lee J; Hong S Highly Selective Environmental Nanosensors Based on Anomalous Response of Carbon Nanotube Conductance to Mercury Ions. J. Phys. Chem. C 2009, 113, 19393–19396. [Google Scholar]
- (427).Gou P; Kraut ND; Feigel IM; Star A Rigid versus Flexible Ligands on Carbon Nanotubes for the Enhanced Sensitivity of Cobalt Ions. Macromolecules 2013, 46, 1376–1383. [Google Scholar]
- (428).Forzani ES; Li X; Zhang P; Tao N; Zhang R; Amlani I; Tsui R; Nagahara LA Tuning the Chemical Selectivity of SWNT-FETs for Detection of Heavy-Metal Ions. Small 2006, 2, 1283–1291. [DOI] [PubMed] [Google Scholar]
- (429).FAO, The Future of Food and Agriculture: Trends and Challenges. Http://Www.Fao.Org/3/a-I6583e.Pdf (Accessed on January 8, 2018); 2017.
- (430).HLPE. Food Losses and Waste in the Context of Sustainable Food Systems. A Report by the High Level Panel of Experts on Food Security and Nutrition of the Committee on World Food Security. Hlpe Rep 2014, 1–6. [Google Scholar]
- (431).Chen H; Zuo X; Su S; Tang Z; Wu A; Song S; Zhang D; Fan C An Electrochemical Sensor for Pesticide Assays Based on Carbon Nanotube-Enhanced Acetycholinesterase Activity. Analyst 2008, 133, 1182–1186. [DOI] [PubMed] [Google Scholar]
- (432).Burg SP; Burg EA Ethylene Action and the Ripening of Fruits. Science 1965, 148, 1190–1196. [DOI] [PubMed] [Google Scholar]
- (433).Lelievre J-M; Latche A; Jones B; Bouzayen M; Pech J-C Ethylene and Fruit Ripening. Physiol. Plant 1997, 101, 727–739. [Google Scholar]
- (434).Theologis A One Rotten Apple Spoils the Whole Bushel: The Role of Ethylene in Fruit Ripening. Cell 1992, 70, 181–184. [DOI] [PubMed] [Google Scholar]
- (435).Leghrib R; Llobet E; Pavelko R; Vasiliev AA; Felten A; Pireaux JJ Gas Sensing Properties of MWCNTs Decorated with Gold or Tin Oxide Nanoparticles. Procedia Chem 2009, 1, 168–171. [Google Scholar]
- (436).Zhang Z; Huang Y; Ding W; Li G Multilayer Interparticle Linking Hybrid MOF-199 for Noninvasive Enrichment and Analysis of Plant Hormone Ethylene. Anal. Chem 2014, 86, 3533–3540. [DOI] [PubMed] [Google Scholar]
- (437).Li Y; Hodak M; Lu W; Bernholc J Selective Sensing of Ethylene and Glucose Using Carbon-Nanotube-Based Sensors: An Ab Initio Investigation. Nanoscale 2017, 9, 1687–1698. [DOI] [PubMed] [Google Scholar]
- (438).Chiu S-W; Tang K-T Towards a Chemiresistive Sensor-Integrated Electronic Nose: A Review. Sensors 2013, 13, 14214–14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (439).Son M; Lee JY; Ko HJ; Park TH Bioelectronic Nose: An Emerging Tool for Odor Standardization. Trends Biotechnol 2017, 35, 301–307. [DOI] [PubMed] [Google Scholar]
- (440).Son M; Park TH The Bioelectronic Nose and Tongue Using Olfactory and Taste Receptors: Analytical Tools for Food Quality and Safety Assessment. Biotechnol. Adv 2018, 36, 371–379. [DOI] [PubMed] [Google Scholar]
- (441).Wasilewski T; Gębicki J; Kamysz W Advances in Olfaction-Inspired Biomaterials Applied to Bioelectronic Noses. Sensors Actuators, B Chem 2018, 257, 511–537. [Google Scholar]
- (442).Wasilewski T; Gębicki J; Kamysz W Bioelectronic Nose: Current Status and Perspectives. Biosens. Bioelectron 2017, 87, 480–494. [DOI] [PubMed] [Google Scholar]
- (443).Peris M; Escuder-Gilabert LA 21st Century Technique for Food Control: Electronic Noses. Anal. Chim. Acta 2009, 638, 1–15. [DOI] [PubMed] [Google Scholar]
- (444).Ampuero S; Bosset JO The Electronic Nose Applied to Dairy Products: A Review. Sensors Actuators, B Chem 2003, 94, 1–12. [Google Scholar]
- (445).Wilson AD; Baietto M Advances in Electronic-Nose Technologies Developed for Biomedical Applications. Sensors 2011, 11, 1105–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (446).Jurs PC; Bakken GA; McClelland HE Computational Methods for the Analysis of Chemical Sensor Array Data from Volatile Analytes. Chem. Rev 2000, 100, 2649–2678. [DOI] [PubMed] [Google Scholar]
- (447).Zozulya S; Echeverri F; Nguyen T The Human Olfactory Receptor Repertoire. Genome Biol 2001, 2, RESEARCH0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (448).Shepherd GM Discrimination of Molecular Signals by the Olfactory Receptor Neuron. Neuron 1994, 13, 771–790. [DOI] [PubMed] [Google Scholar]
- (449).Jin HJ; Lee SH; Kim TH; Park J; Song HS; Park TH; Hong S Nanovesicle-Based Bioelectronic Nose Platform Mimicking Human Olfactory Signal Transduction. Biosens. Bioelectron 2012, 35, 335–341. [DOI] [PubMed] [Google Scholar]
- (450).Lim JH; Oh EH; Park J; Hong S; Park TH Ion-Channel-Coupled Receptor-Based Platform for a Real-Time Measurement of G-Protein-Coupled Receptor Activities. ACS Nano 2015, 9, 1699–1706. [DOI] [PubMed] [Google Scholar]
- (451).Kim TH; Lee SH; Lee J; Song HS; Oh EH; Park TH; Hong S Single-Carbon-Atomic-Resolution Detection of Odorant Molecules Using a Human Olfactory Receptor-Based Bioelectronic Nose. Adv. Mater 2009, 21, 91–94. [Google Scholar]
- (452).Song HS; Jin HJ; Ahn SR; Kim D; Lee SH; Kim UK; Simons CT; Hong S; Park TH Bioelectronic Tongue Using Heterodimeric Human Taste Receptor for the Discrimination of Sweeteners with Human-like Performance. ACS Nano 2014, 8, 9781–9789. [DOI] [PubMed] [Google Scholar]
- (453).Kim TH; Song HS; Jin HJ; Lee SH; Namgung S; Kim U; Park TH; Hong S “Bioelectronic Super-Taster” Device Based on Taste Receptor-Carbon Nanotube Hybrid Structures. Lab Chip 2011, 11, 2262–2267. [DOI] [PubMed] [Google Scholar]
- (454).Son M; Kim D; Ko HJ; Hong S; Park TH A Portable and Multiplexed Bioelectronic Sensor Using Human Olfactory and Taste Receptors. Biosens. Bioelectron 2017, 87, 901–907. [DOI] [PubMed] [Google Scholar]
- (455).Song HS; Kwon OS; Lee SH; Park SJ; Kim UK; Jang J; Park TH Human Taste Receptor-Functionalized Field Effect Transistor as a Human-like Nanobioelectronic Tongue. Nano Lett 2013, 13, 172–178. [DOI] [PubMed] [Google Scholar]
- (456).Lee SH; Jin HJ; Song HS; Hong S; Park TH Bioelectronic Nose with High Sensitivity and Selectivity Using Chemically Functionalized Carbon Nanotube Combined with Human Olfactory Receptor. J. Biotechnol 2012, 157, 467–472. [DOI] [PubMed] [Google Scholar]
- (457).Lee SH; Kwon OS; Song HS; Park SJ; Sung JH; Jang J; Park TH Mimicking the Human Smell Sensing Mechanism with an Artificial Nose Platform. Biomaterials 2012, 33, 1722–1729. [DOI] [PubMed] [Google Scholar]
- (458).Yoon H; Lee SH; Kwon OS; Song HS; Oh EH; Park TH; Jang J Polypyrrole Nanotubes Conjugated with Human Olfactory Receptors: High-Performance Transducers for FET-Type Bioelectronic Noses. Angew. Chemie Int. Ed 2009, 48, 2755–2758. [DOI] [PubMed] [Google Scholar]
- (459).Goldsmith BR; Mitala JJ; Josue J; Castro A; Lerner MB; Bayburt TH; Khamis SM; Jones RA; Brand JG; Sligar SG; et al. Biomimetic Chemical Sensors Using Nanoelectronic Readout of Olfactory Receptor Proteins. ACS Nano 2011, 5, 5408–5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (460).Liu Q; Zhang F; Zhang D; Hu N; Wang H; Jimmy Hsia K; Wang P Bioelectronic Tongue of Taste Buds on Microelectrode Array for Salt Sensing. Biosens. Bioelectron 2013, 40, 115–120. [DOI] [PubMed] [Google Scholar]
- (461).Qin Z; Zhang B; Hu L; Zhuang L; Hu N; Wang P A Novel Bioelectronic Tongue in Vivo for Highly Sensitive Bitterness Detection with Brain-Machine Interface. Biosens. Bioelectron 2016, 78, 374–380. [DOI] [PubMed] [Google Scholar]
- (462).Zhang W; Chen P; Zhou L; Qin Z; Gao K; Yao J; Li C; Wang P A Biomimetic Bioelectronic Tongue: A Switch for On- and Off- Response of Acid Sensations. Biosens. Bioelectron 2017, 92, 523–528. [DOI] [PubMed] [Google Scholar]
- (463).Lee M; Jung JW; Kim D; Ahn YJ; Hong S; Kwon HW Discrimination of Umami Tastants Using Floating Electrode-Based Bioelectronic Tongue Mimicking Insect Taste Systems. ACS Nano 2015, 9, 11728–11736. [DOI] [PubMed] [Google Scholar]
- (464).Park J; Lim JH; Jin HJ; Namgung S; Lee SH; Park TH; Hong S A Bioelectronic Sensor Based on Canine Olfactory Nanovesicle–carbon Nanotube Hybrid Structures for the Fast Assessment of Food Quality. Analyst 2012, 137, 3249–3254. [DOI] [PubMed] [Google Scholar]
- (465).Wei Z; Zhang W; Wang J Nickel and Copper Foam Electrodes Modified with Graphene or Carbon Nanotubes for Electrochemical Identification of Chinese Rice Wines. Microchim. Acta 2017, 184, 3441–3451. [Google Scholar]
- (466).Wang LC; Tang KT; Chiu SW; Yang SR; Kuo CT A Bio-Inspired Two-Layer Multiple-Walled Carbon Nanotube-Polymer Composite Sensor Array and a Bio-Inspired Fast-Adaptive Readout Circuit for a Portable Electronic Nose. Biosens. Bioelectron 2011, 26, 4301–4307. [DOI] [PubMed] [Google Scholar]
- (467).Chiu SW; Wu HC; Chou TI; Chen H; Tang KT A Miniature Electronic Nose System Based on an MWNT-Polymer Microsensor Array and a Low-Power Signal-Processing Chip Chemosensors and Chemoreception. Anal. Bioanal. Chem 2014, 406, 3985–3994. [DOI] [PubMed] [Google Scholar]
- (468).Kachoosangi RT; Wildgoose GG; Compton RG Carbon Nanotube-Based Electrochemical Sensors for Quantifying the ‘Heat’ of Chilli Peppers: The Adsorptive Stripping Voltammetric Determination of Capsaicin. Analyst 2008, 133, 888–895. [DOI] [PubMed] [Google Scholar]
- (469).Cost Estimates of Foodborne Illnesses, United States Department of Agriculture Economic Research Service Https://Www.Ers.Usda.Gov/Data-Products/Cost-Estimates-of-Foodborne-Illnesses/ (Accessed on July 31, 2018).
- (470).Hussain M; Dawson C Economic Impact of Food Safety Outbreaks on Food Businesses. Foods 2013, 2, 585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (471).Yam K; Takhistov P; Miltz J R : Concise Reviews / Hypotheses in Food Science Intelligent Packaging : T Package Devices. J. Food Sci 2005, 70, 1–10. [Google Scholar]
- (472).Ruiz-Capillas C; Jiménez-Colmenero F Biogenic Amines in Meat and Meat Products. Crit. Rev. Food Sci. Nutr 2004, 44, 489–499. [DOI] [PubMed] [Google Scholar]
- (473).Naila A; Flint S; Fletcher G; Bremer P; Meerdink G Control of Biogenic Amines in Food-Existing and Emerging Approaches. J. Food Sci 2010, 75, R139–R150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (474).Mehta RS; Bassette R Organoleptic, Chemical and Microbiological Changes in Ultra-High-Temperature Sterilized Milk Stored at Room Temperature. J. Food Prot 1978, 41, 806–810. [DOI] [PubMed] [Google Scholar]
- (475).Sinha RN; Tuma D; Abramson D; Muir WE Fungal Volatiles Associated with Moldy Grain in Ventilated and Non-Ventilated Bin-Stored Wheat. Mycopathologia 1988, 101, 53–60. [DOI] [PubMed] [Google Scholar]
- (476).Lee SH; Lim JH; Park J; Hong S; Park TH Bioelectronic Nose Combined with a Microfluidic System for the Detection of Gaseous Trimethylamine. Biosens. Bioelectron 2015, 71, 179–185. [DOI] [PubMed] [Google Scholar]
- (477).Lee KM; Son M; Kang JH; Kim D; Hong S; Park TH; Chun HS; Choi SS A Triangle Study of Human, Instrument and Bioelectronic Nose for Non-Destructive Sensing of Seafood Freshness. Sci. Rep 2018, 8, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (478).Lim JH; Park J; Ahn JH; Jin HJ; Hong S; Park TH A Peptide Receptor-Based Bioelectronic Nose for the Real-Time Determination of Seafood Quality. Biosens. Bioelectron 2013, 39, 244–249. [DOI] [PubMed] [Google Scholar]
- (479).Brody AL; Strupinsky ER; Kline LR Active Packaging for Food Applications; CRC Press, Boca Raton, Florida, 2001. [Google Scholar]
- (480).Mahajan R; Templeton A; Harman A; Reed RA; Chern RT The Effect of Inert Atmospheric Packaging on Oxidative Degradation in Formulated Granules. Pharm. Res 2005, 22, 128–140. [DOI] [PubMed] [Google Scholar]
- (481).Mills A Oxygen Indicators and Intelligent Inks for Packaging Food. Chem. Soc. Rev 2005, 34, 1003–1011. [DOI] [PubMed] [Google Scholar]
- (482).Ramamoorthy R; Dutta PK; Akbar SA Oxygen Sensors: Materials, Methods, Designs and Applications. J. Mater. Sci 2003, 38, 4271–4282. [Google Scholar]
- (483).Schwank JW; DiBattista M Oxygen Sensors: Materials and Applications. MRS Bull 1999, 24, 44–48. [Google Scholar]
- (484).Jouanneau S; Recoules L; Durand MJ; Boukabache A; Picot V; Primault Y; Lakel A; Sengelin M; Barillon B; Thouand G Methods for Assessing Biochemical Oxygen Demand (BOD): A Review. Water Res 2014, 49, 62–82. [DOI] [PubMed] [Google Scholar]
- (485).Collins J-A; Rudenski A; Gibson J; Howard L; O’Driscoll R Relating Oxygen Partial Pressure, Saturation and Content: The Haemoglobin–oxygen Dissociation Curve. Breathe 2015, 11, 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (486).Quaranta M; Borisov SM; Klimant I Indicators for Optical Oxygen Sensors. Bioanal. Rev 2012, 4, 115–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (487).Wang X; Wolfbeis OS Optical Methods for Sensing and Imaging Oxygen: Materials, Spectroscopies and Applications. Chem. Soc. Rev 2014, 43, 3666–3761. [DOI] [PubMed] [Google Scholar]
- (488).Bradley K; Jhi SH; Collins PG; Hone J; Cohen ML; Louie SG; Zettl A Is the Intrinsic Thermoelectric Power of Carbon Nanotubes Positive. Phys. Rev. Lett 2000, 85, 4361–4364. [DOI] [PubMed] [Google Scholar]
- (489).Rajavel K; Lalitha M; Radhakrishnan JK; Senthilkumar L; Rajendra Kumar RT Multiwalled Carbon Nanotube Oxygen Sensor: Enhanced Oxygen Sensitivity at Room Temperature and Mechanism of Sensing. ACS Appl. Mater. Interfaces 2015, 7, 23857–23865. [DOI] [PubMed] [Google Scholar]
- (490).Sumanasekera G; Adu C; Fang S; Eklund P Effects of Gas Adsorption and Collisions on Electrical Transport in Single-Walled Carbon Nanotubes. Phys. Rev. Lett 2000, 85, 1096–1099. [DOI] [PubMed] [Google Scholar]
- (491).Tchernatinsky A; Desai S; Sumanasekera GU; Jayanthi CS; Wu SY; Nagabhirava B; Alphenaar B Adsorption of Oxygen Molecules on Individual Single-Wall Carbon Nanotubes. J. Appl. Phys 2006, 99, 034306. [Google Scholar]
- (492).Ong KG; Zeng K; Grimes CA A Wireless, Passive Carbon Nanotube-Based Gas Sensor. IEEE Sens. J 2002, 2, 82–88. [Google Scholar]
- (493).Sorescu DC; Jordan KD; Avouris P Theoretical Study of Oxygen Adsorption on Graphite and the (8,0) Single-Walled Carbon Nanotube. J. Phys. Chem. B 2001, 105, 11227–11232. [Google Scholar]
- (494).Derycke V; Martel R; Appenzeller J; Avouris P Controlling Doping and Carrier Injection in Carbon Nanotube Transistors. Appl. Phys. Lett 2002, 80, 2773–2775. [DOI] [PubMed] [Google Scholar]
- (495).Kauffman DR; Shade CM; Uh H; Petoud S; Star A Decorated Carbon Nanotubes with Unique Oxygen Sensitivity. Nat. Chem 2009, 1, 500–506. [DOI] [PubMed] [Google Scholar]
- (496).Liu Y; Liu F; Pan X; Li J Protecting the Environment and Public Health from Pesticides. Environ. Sci. Technol 2012, 46, 5658–5659. [DOI] [PubMed] [Google Scholar]
- (497).Whitehorn PR; O ‘Connor S; Wackers FL; Goulson D Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Neonicotinoid Pesticide Reduces Bumble Bee Colony Growth and Queen Production. Science 2012, 336, 351–352. [DOI] [PubMed] [Google Scholar]
- (498).Relyea RA; Diecks N An Unforeseen Chain of Events : Lethal Effects of Pesticides on Frogs at Sublethal Concentrations. Ecol. Appl 2008, 18, 1728–1742. [DOI] [PubMed] [Google Scholar]
- (499).Bassil KL; Vakil C; Sanborn M; Cole DC; Kaur JS; Kerr KJ Cancer Health Effects of Pesticides. Can. Fam. Physician 2007, 53, 1704–1711. [PMC free article] [PubMed] [Google Scholar]
- (500).Bassil KL; Vakil C; Sanborn M; Cole DC; Kaur JS; Kerr KJ; Sanin LH; Cole DC; Bassil KL; Vakil C; et al. Non-Cancer Health Effects of Pesticides. Can. Fam. Physician 2007, 53, 1712–1720. [PMC free article] [PubMed] [Google Scholar]
- (501).Jurewicz J; Hanke W Prenatal and Childhood Exposure to Pesticides and Neurobehavioral Development: Review of Epidemiological Studies. Int. J. Occup. Med. Environ. Health 2008, 21, 121–132. [DOI] [PubMed] [Google Scholar]
- (502).Reddy DS; Colman E A Comparative Toxidrome Analysis of Human Organophosphate and Nerve Agent Poisonings Using Social Media. Clin. Transl. Sci 2017, 10, 225–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (503).Pimentel D; Burgess M Environmental and Economic Costs of the Application of Pesticides Primarily in the United States. Integr. Pest Manag 2014, 3, 47–71. [Google Scholar]
- (504).Gan T; Lv Z; Sun Y; Shi Z; Sun J; Zhao A Highly Sensitive and Molecular Selective Electrochemical Sensing of 6-Benzylaminopurine with Multiwall Carbon Nanotube@SnS2-Assisted Signal Amplification. J. Appl. Electrochem 2016, 46, 389–401. [Google Scholar]
- (505).Yu G; Wu W; Zhao Q; Wei X; Lu Q Efficient Immobilization of Acetylcholinesterase onto Amino Functionalized Carbon Nanotubes for the Fabrication of High Sensitive Organophosphorus Pesticides Biosensors. Biosens. Bioelectron 2015, 68, 288–294. [DOI] [PubMed] [Google Scholar]
- (506).Canevari TC; Prado TM; Cincotto FH; Machado SAS Immobilization of Ruthenium Phthalocyanine on Silica-Coated Multi-Wall Partially Oriented Carbon Nanotubes: Electrochemical Detection of Fenitrothion Pesticide. Mater. Res. Bull 2016, 76, 41–47. [Google Scholar]
- (507).Bhatt V; Joshi S; Becherer M; Lugli P Flexible, Low-Cost Sensor Based on Electrolyte Gated Carbon Nanotube Field Effect Transistor for Organo-Phosphate Detection. Sensors 2017, 17, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (508).Mugadza T; Nyokong T Electrocatalytic Oxidation of Amitrole and Diuron on Iron(II) Tetraaminophthalocyanine-Single Walled Carbon Nanotube Dendrimer. Electrochim. Acta 2010, 55, 2606–2613. [Google Scholar]
- (509).Nayak P; Anbarasan B; Ramaprabhu S Fabrication of Organophosphorus Biosensor Using ZnO Nanoparticle-Decorated Carbon Nanotube-Graphene Hybrid Composite Prepared by a Novel Green Technique. J. Phys. Chem. C 2013, 117, 13202–13209. [Google Scholar]
- (510).Zhang Y; Arugula MA; Wales M; Wild J; Simonian AL A Novel Layer-by-Layer Assembled Multi-Enzyme/CNT Biosensor for Discriminative Detection between Organophosphorus and Non-Organophosphrus Pesticides. Biosens. Bioelectron 2015, 67, 287–295. [DOI] [PubMed] [Google Scholar]
- (511).Wang J; Timchalk C; Lin Y Carbon Nanotube-Based Electrochemical Sensor for Assay of Salivary Cholinesterase Enzyme Activity: An Exposure Biomarker of Organophosphate Pesticides and Nerve Agents. Environ. Sci. Technol 2008, 42, 2688–2693. [DOI] [PubMed] [Google Scholar]
- (512).Wong A; Sotomayor MDPT Biomimetic Sensor Based on 5,10,15,20-Tetrakis(Pentafluorophenyl)-21H,23H-Porphyrin Iron (III) Chloride and MWCNT for Selective Detection of 2,4-D. Sensors Actuators, B Chem 2013, 181, 332–339. [Google Scholar]
- (513).Anirudhan TS; Alexander S Design and Fabrication of Molecularly Imprinted Polymer-Based Potentiometric Sensor from the Surface Modified Multiwalled Carbon Nanotube for the Determination of Lindane (γ-Hexachlorocyclohexane), an Organochlorine Pesticide. Biosens. Bioelectron 2015, 64, 586–593. [DOI] [PubMed] [Google Scholar]
- (514).Rahemi V; Garrido JMPJ; Borges F; Brett CMA; Garrido EMPJ Electrochemical Sensor for Simultaneous Determination of Herbicide MCPA and Its Metabolite 4-Chloro-2-Methylphenol. Application to Photodegradation Environmental Monitoring. Environ. Sci. Pollut. Res 2015, 22, 4491–4499. [DOI] [PubMed] [Google Scholar]
- (515).Huang B; Zhang W. De; Chen CH; Yu YX Electrochemical Determination of Methyl Parathion at a Pd/MWCNTs-Modified Electrode. Microchim. Acta 2010, 171, 57–62. [Google Scholar]
- (516).Velusamy V; Arshak K; Korostynska O; Oliwa K; Adley C An Overview of Foodborne Pathogen Detection: In the Perspective of Biosensors. Biotechnol. Adv 2010, 28, 232–254. [DOI] [PubMed] [Google Scholar]
- (517).Diarrhea: Why Children Are Still Dying and What Can Be Done; The United Nations Children’s Fund (UNICEF)/World Health Organization (WHO), 2009. [Google Scholar]
- (518).World Health Organization. Food Safety and Foodborne Illness Geneva: Fact sheet n°237 2007. [Google Scholar]
- (519).Frenzen PD; Drake A; Angulo FJ; The Emerging Infections Program Foodnet Working Group. Economic Cost of Illness Due to Escherichia Coli O157 Infections in the United States. J. Food Prot 2005, 68, 2502–2270. [DOI] [PubMed] [Google Scholar]
- (520).Mortari A; Lorenzelli L Recent Sensing Technologies for Pathogen Detection in Milk: A Review. Biosens. Bioelectron 2014, 60, 8–21. [DOI] [PubMed] [Google Scholar]
- (521).Stephen Inbaraj B; Chen BH Nanomaterial-Based Sensors for Detection of Foodborne Bacterial Pathogens and Toxins as Well as Pork Adulteration in Meat Products. J. Food Drug Anal 2016, 24, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (522).Bhardwaj J; Devarakonda S; Kumar S; Jang J Development of a Paper-Based Electrochemical Immunosensor Using an Antibody-Single Walled Carbon Nanotubes Bio-Conjugate Modified Electrode for Label-Free Detection of Foodborne Pathogens. Sensors Actuators, B Chem 2017, 253, 115–123. [Google Scholar]
- (523).Zhao G; Zhan X; Dou W A Disposable Immunosensor for Shigella Flexneri Based on Multiwalled Carbon Nanotube/Sodium Alginate Composite Electrode. Anal. Biochem 2011, 408, 53–58. [DOI] [PubMed] [Google Scholar]
- (524).Viswanathan S; Wu LC; Huang MR; Ho JAA Electrochemical Immunosensor for Cholera Toxin Using Liposomes and Poly(3,4-Ethylenedioxythiophene)-Coated Carbon Nanotubes. Anal. Chem 2006, 78, 1115–1121. [DOI] [PubMed] [Google Scholar]
- (525).Yamada K; Kim C-T; Kim J-H; Chung J-H; Lee HG; Jun S Single Walled Carbon Nanotube-Based Junction Biosensor for Detection of Escherichia Coli. PLoS One 2014, 9, e105767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (526).Yamada K; Choi W; Lee I; Cho BK; Jun S Rapid Detection of Multiple Foodborne Pathogens Using a Nanoparticle-Functionalized Multi-Junction Biosensor. Biosens. Bioelectron 2016, 77, 137–143. [DOI] [PubMed] [Google Scholar]
- (527).Balasubramanian K; Burghard M Biosensors Based on Carbon Nanotubes. Anal. Bioanal. Chem 2006, 385, 452–468. [DOI] [PubMed] [Google Scholar]
- (528).Amann A; Costello BDL; Miekisch W; Schubert J; Buszewski B; Pleil J; Ratcliffe N; Risby T The Human Volatilome: Volatile Organic Compounds (VOCs) in Exhaled Breath, Skin Emanations, Urine, Feces and Saliva. J. Breath Res 2014, 8, 034001. [DOI] [PubMed] [Google Scholar]
- (529).Kim SJ; Choi SJ; Jang JS; Cho HJ; Kim ID Innovative Nanosensor for Disease Diagnosis. Acc. Chem. Res 2017, 50, 1587–1596. [DOI] [PubMed] [Google Scholar]
- (530).Broza YY; Haick H Nanomaterial-Based Sensors for Detection of Disease by Volatile Organic Compounds. Nanomedicine 2013, 8, 785–806. [DOI] [PubMed] [Google Scholar]
- (531).Turner APF; Magan N Electronic Noses and Disease Diagnostics. Nat. Rev. Microbiol 2004, 2, 161–166. [DOI] [PubMed] [Google Scholar]
- (532).Peng G; Hakim M; Broza YY; Billan S; Abdah-Bortnyak R; Kuten A; Tisch U; Haick H Detection of Lung, Breast, Colorectal, and Prostate Cancers from Exhaled Breath Using a Single Array of Nanosensors. Br. J. Cancer 2010, 103, 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (533).Xu ZQ; Broza YY; Ionsecu R; Tisch U; Ding L; Liu H; Song Q; Pan YY; Xiong FX; Gu KS; et al. A Nanomaterial-Based Breath Test for Distinguishing Gastric Cancer from Benign Gastric Conditions. Br. J. Cancer 2013, 108, 941–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (534).Amal H; Ding L; Liu B. Bin; Tisch U; Xu ZQ; Shi DY; Zhao Y; Chen J; Sun RX; Liu H; et al. The Scent Fingerprint of Hepatocarcinoma: In-Vitro Metastasis Prediction with Volatile Organic Compounds (VOCs). Int. J. Nanomedicine 2012, 7, 4135–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (535).Mazzone PJ; Hammel J; Dweik R; Na J; Czich C; Laskowski D; Mekhail T Diagnosis of Lung Cancer by the Analysis of Exhaled Breath with a Colorimetric Sensor Array. Thorax 2007, 62, 565–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (536).Hakim M; Broza YY; Barash O; Peled N; Phillips M; Amann A; Haick H Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev 2012, 112, 5949–5966. [DOI] [PubMed] [Google Scholar]
- (537).Tisch U; Schlesinger I; Ionescu R; Nassar M; Axelrod N; Robertman D; Tessler Y; Azar F; Marmur A; Aharon-Peretz J; et al. Detection of Alzheimer’s and Parkinson’s Disease from Exhaled Breath Using Nanomaterial-Based Sensors. Nanomedicine 2013, 8, 43–56. [DOI] [PubMed] [Google Scholar]
- (538).Broza YY; Har-Shai L; Jeries R; Cancilla JC; Glass-Marmor L; Lejbkowicz I; Torrecilla JS; Yao X; Feng X; Narita A; et al. Exhaled Breath Markers for Nonimaging and Noninvasive Measures for Detection of Multiple Sclerosis. ACS Chem. Neurosci 2017, 8, 2402–2413. [DOI] [PubMed] [Google Scholar]
- (539).Novak BJ; Blake DR; Meinardi S; Rowland FS; Pontello A; Cooper DM; Galassetti PR Exhaled Methyl Nitrate as a Noninvasive Marker of Hyperglycemia in Type 1 Diabetes. Proc. Natl. Acad. Sci 2007, 104, 15613–15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (540).Konvalina G; Haick H Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res 2014, 47, 66–76. [DOI] [PubMed] [Google Scholar]
- (541).Peng G; Trock E; Haick H Detecting Simulated Patterns of Lung Cancer Biomarkers by Random Network of Single-Walled Carbon Nanotubes Coated with Nonpolymeric Organic Materials. Nano Lett 2008, 8, 3631–3635. [DOI] [PubMed] [Google Scholar]
- (542).Nakhleh MK; Amal H; Jeries R; Broza YY; Aboud M; Gharra A; Ivgi H; Khatib S; Badarneh S; Har-Shai L; et al. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano 2017, 11, 112–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (543).Owlstone medical https://www.owlstonemedical.com/science-technology/breath-biopsy/ (accessed Mar 25, 2018).
- (544).Broza YY; Mochalski P; Ruzsanyi V; Amann A; Haick H Hybrid Volatolomics and Disease Detection. Angew. Chemie - Int. Ed 2015, 54, 11036–11048. [DOI] [PubMed] [Google Scholar]
- (545).Amal H; Leja M; Funka K; Skapars R; Sivins A; Ancans G; Liepniece-Karele I; Kikuste I; Lasina I; Haick H Detection of Precancerous Gastric Lesions and Gastric Cancer through Exhaled Breath. Gut 2016, 65, 400–407. [DOI] [PubMed] [Google Scholar]
- (546).Bajtarevic A; Ager C; Pienz M; Klieber M; Schwarz K; Ligor M; Ligor T; Filipiak W; Denz H; Fiegl M; et al. Noninvasive Detection of Lung Cancer by Analysis of Exhaled Breath. BMC Cancer 2009, 9, 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (547).Calenic B; Miricescu D; Greabu M; Kuznetsov AV; Troppmair J; Ruzsanyi V; Amann A Oxidative Stress and Volatile Organic Compounds: Interplay in Pulmonary, Cardio-Vascular, Digestive Tract Systems and Cancer. Open Chem 2015, 13, 1020–1030. [Google Scholar]
- (548).Ajibola OA; Smith D; Španěl P; Ferns GA A. Effects of Dietary Nutrients on Volatile Breath Metabolites. J. Nutr. Sci 2013, 2, e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (549).Shirsat MD; Sarkar T; Kakoullis J; Myung NV; Konnanath B; Spanias A; Mulchandani A Porphyrin-Functionalized Single-Walled Carbon Nanotube Chemiresistive Sensor Arrays for VOCs. J. Phys. Chem. C 2012, 116, 3845–3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (550).Chatterjee S; Castro M; Feller JF Tailoring Selectivity of Sprayed Carbon Nanotube Sensors (CNT) towards Volatile Organic Compounds (VOC) with Surfactants. Sensors Actuators B Chem 2015, 220, 840–849. [Google Scholar]
- (551).Sarkar T; Srinives S; Sarkar S; Haddon RC; Mulchandani A Single-Walled Carbon Nanotube-Poly(Porphyrin) Hybrid for Volatile Organic Compounds Detection. J. Phys. Chem. C 2014, 118, 1602–1610. [Google Scholar]
- (552).Wang X; Ugur A; Goktas H; Chen N; Wang M; Lachman N; Kalfon-Cohen E; Fang W; Wardle BL; Gleason KK Room Temperature Resistive Volatile Organic Compound Sensing Materials Based on a Hybrid Structure of Vertically Aligned Carbon Nanotubes and Conformal OCVD/ICVD Polymer Coatings. ACS Sensors 2016, 1, 374–383. [Google Scholar]
- (553).Richardson M; Moulton K; Rabb D; Kindopp S; Pishe T; Yan C; Akpinar I; Tsoi B; Chuck A Capnography for Monitoring End-Tidal CO2 in Hospital and Pre-Hospital Settings: A Health Technology Assessment; Ottawa, 2016; Vol. 142. [PubMed] [Google Scholar]
- (554).Anderson CT; Breen PH Carbon Dioxide Kinetics and Capnography during Critical Care. Crit. Care 2000, 4, 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (555).Bryan NS; Grisham MB Methods to Detect Nitric Oxide and Its Metabolites in Biological Samples. Free Radic. Biol. Med 2007, 43, 645–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (556).Narasimhan LR; Goodman W; Patel CKN Correlation of Breath Ammonia with Blood Urea Nitrogen and Creatinine during Hemodialysis. Proc. Natl. Acad. Sci 2001, 98, 4617–4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (557).Popa C; Dutu DCA; Cernat R; Matei C; Bratu AM; Banita S; Dumitras DC Ethylene and Ammonia Traces Measurements from the Patients’ Breath with Renal Failure via LPAS Method. Appl. Phys. B Lasers Opt 2011, 105, 669–674. [Google Scholar]
- (558).Li Y; Li G; Wang X; Zhu Z; Ma H; Zhang T; Jin J Poly(Ionic Liquid)-Wrapped Single-Walled Carbon Nanotubes for Sub-Ppb Detection of CO2. Chem. Commun 2012, 48, 8222–8224. [DOI] [PubMed] [Google Scholar]
- (559).Olney D; Fuller L; Santhanam KSV A Greenhouse Gas Silicon Microchip Sensor Using a Conducting Composite with Single Walled Carbon Nanotubes. Sensors Actuators, B Chem 2014, 191, 545–552. [Google Scholar]
- (560).Kuzmych O; Allen BL; Star A Carbon Nanotube Sensors for Exhaled Breath Components. Nanotechnology 2007, 18, 375502. [Google Scholar]
- (561).Li G; Liao JM; Hu GQ; Ma NZ; Wu PJ Study of Carbon Nanotube Modified Biosensor for Monitoring Total Cholesterol in Blood. Biosens. Bioelectron 2005, 20, 2140–2144. [DOI] [PubMed] [Google Scholar]
- (562).Yang J; Lee H; Cho M; Nam J; Lee Y Nonenzymatic Cholesterol Sensor Based on Spontaneous Deposition of Platinum Nanoparticles on Layer-by-Layer Assembled CNT Thin Film. Sensors Actuators B Chem 2012, 171–172, 374–379. [Google Scholar]
- (563).Barik MA; Sarma MK; Sarkar CR; Dutta JC Highly Sensitive Potassium-Doped Polypyrrole/Carbon Nanotube-Based Enzyme Field Effect Transistor (ENFET) for Cholesterol Detection. Appl. Biochem. Biotechnol 2014, 174, 1104–1114. [DOI] [PubMed] [Google Scholar]
- (564).Canbay E; Akyilmaz E Design of a Multiwalled Carbon Nanotube-Nafion-Cysteamine Modified Tyrosinase Biosensor and Its Adaptation of Dopamine Determination. Anal. Biochem 2014, 444, 8–15. [DOI] [PubMed] [Google Scholar]
- (565).Rubianes MD; Rivas GA Enzymatic Biosensors Based on Carbon Nanotubes Paste Electrodes. Electroanalysis 2005, 17, 73–78. [Google Scholar]
- (566).Kan X; Zhou H; Li C; Zhu A; Xing Z; Zhao Z Imprinted Electrochemical Sensor for Dopamine Recognition and Determination Based on a Carbon Nanotube/Polypyrrole Film. Electrochim. Acta 2012, 63, 69–75. [Google Scholar]
- (567).Babaei A; Taheri AR Nafion/Ni(OH)2nanoparticles-Carbon Nanotube Composite Modified Glassy Carbon Electrode as a Sensor for Simultaneous Determination of Dopamine and Serotonin in the Presence of Ascorbic Acid. Sensors Actuators, B Chem 2013, 176, 543–551. [Google Scholar]
- (568).Rand E; Periyakaruppan A; Tanaka Z; Zhang DA; Marsh MP; Andrews RJ; Lee KH; Chen B; Meyyappan M; Koehne JE A Carbon Nanofiber Based Biosensor for Simultaneous Detection of Dopamine and Serotonin in the Presence of Ascorbic Acid. Biosens. Bioelectron 2013, 42, 434–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (569).Mazloum-Ardakani M; Khoshroo A High Sensitive Sensor Based on Functionalized Carbon Nanotube/Ionic Liquid Nanocomposite for Simultaneous Determination of Norepinephrine and Serotonin. J. Electroanal. Chem 2014, 717–718, 17–23. [Google Scholar]
- (570).Lin KC; Li YS; Chen SM Electrochemical Determination of Nicotinamide Adenine Dinucleotide and Hydrogen Peroxide Based on Poly(Xanthurenic Acid), Flavin Adenine Dinucleotide and Functionalized Multi-Walled Carbon Nanotubes. Sensors Actuators, B Chem 2013, 184, 212–219. [Google Scholar]
- (571).Musameh M; Wang J; Merkoci A; Lin Y Low-Potential Stable NADH Detection at Carbon-Nanotube-Modified Glassy Carbon Electrodes. Electrochem. commun 2002, 4, 743–746. [Google Scholar]
- (572).Teymourian H; Salimi A; Hallaj R Low Potential Detection of NADH Based on Fe3O4nanoparticles/Multiwalled Carbon Nanotubes Composite: Fabrication of Integrated Dehydrogenase-Based Lactate Biosensor. Biosens. Bioelectron 2012, 33, 60–68. [DOI] [PubMed] [Google Scholar]
- (573).Batra B; Lata S; Rani S; Pundir CS Fabrication of a Cytochrome c Biosensor Based on Cytochrome Oxidase/NiO-NPs/CMWCNT/PANI Modified Au Electrode. J. Biomed. Nanotechnol 2013, 9, 409–416. [DOI] [PubMed] [Google Scholar]
- (574).Boussaad S; Tao NJ; Zhang R; Hopson T; Nagahara LA In Situ Detection of Cytochrome c Adsorption with Single Walled Carbon Nanotube Device. Chem. Commun 2003, No. 13, 1502. [Google Scholar]
- (575).Lawal AT Synthesis and Utilization of Carbon Nanotubes for Fabrication of Electrochemical Biosensors. Mater. Res. Bull 2016, 73, 308–350. [Google Scholar]
- (576).Jacobs CB; Peairs MJ; Venton BJ Review: Carbon Nanotube Based Electrochemical Sensors for Biomolecules. Anal. Chim. Acta 2010, 662, 105–127. [DOI] [PubMed] [Google Scholar]
- (577).Vashist SK; Zheng D; Al-Rubeaan K; Luong JHT; Sheu FS Advances in Carbon Nanotube Based Electrochemical Sensors for Bioanalytical Applications. Biotechnol. Adv 2011, 29, 169–188. [DOI] [PubMed] [Google Scholar]
- (578).Tang H; Chen J; Yao S; Nie L; Deng G; Kuang Y Amperometric Glucose Biosensor Based on Adsorption of Glucose Oxidase at Platinum Nanoparticle-Modified Carbon Nanotube Electrode. Anal. Biochem 2004, 331, 89–97. [DOI] [PubMed] [Google Scholar]
- (579).Wang Y-T; Yu L; Zhu Z-Q; Zhang J; Zhu J-Z; Fan C Improved Enzyme Immobilization for Enhanced Bioelectrocatalytic Activity of Glucose Sensor. Sensors Actuators B Chem 2009, 136, 332–337. [Google Scholar]
- (580).Chen KJ; Lee CF; Rick J; Wang SH; Liu CC; Hwang BJ Fabrication and Application of Amperometric Glucose Biosensor Based on a Novel PtPd Bimetallic Nanoparticle Decorated Multi-Walled Carbon Nanotube Catalyst. Biosens. Bioelectron 2012, 33, 75–81. [DOI] [PubMed] [Google Scholar]
- (581).Gao M; Dai L; Wallace GG Biosensors Based on Aligned Carbon Nanotubes Coated with Inherently Conducting Polymers. Electroanalysis 2003, 15, 1089–1094. [Google Scholar]
- (582).Wang J; Musameh M Carbon-Nanotubes Doped Polypyrrole Glucose Biosensor. Anal. Chim. Acta 2005, 539, 209–213. [Google Scholar]
- (583).Pilan L; Raicopol M Highly Selective and Stable Glucose Biosensors Based on Polyaniline / Carbon Nanotubes Composites. U.P.B. Sci. Bull., Ser. B 2014, 76, 155–166. [Google Scholar]
- (584).Patolsky F; Weizmann Y; Willner I Long-Range Electrical Contacting of Redox Enzymes by SWCNT Connectors. Angew. Chemie - Int. Ed 2004, 43, 2113–2117. [DOI] [PubMed] [Google Scholar]
- (585).Tian K; Prestgard M; Tiwari A A Review of Recent Advances in Nonenzymatic Glucose Sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [DOI] [PubMed] [Google Scholar]
- (586).Lin K-C; Lin Y-C; Chen S-M A Highly Sensitive Nonenzymatic Glucose Sensor Based on Multi-Walled Carbon Nanotubes Decorated with Nickel and Copper Nanoparticles. Electrochim. Acta 2013, 96, 164–172. [Google Scholar]
- (587).Gougis M; Tabet-Aoul A; Ma D; Mohamedi M Laser Synthesis and Tailor-Design of Nanosized Gold onto Carbon Nanotubes for Non-Enzymatic Electrochemical Glucose Sensor. Sensors Actuators, B Chem 2014, 193, 363–369. [Google Scholar]
- (588).Baghayeri M; Amiri A; Farhadi S Development of Non-Enzymatic Glucose Sensor Based on Efficient Loading Ag Nanoparticles on Functionalized Carbon Nanotubes. Sensors Actuators, B Chem 2016, 225, 354–362. [Google Scholar]
- (589).Besteman K; Lee JO; Wiertz FGM; Heering HA; Dekker C Enzyme-Coated Carbon Nanotubes as Single-Molecule Biosensors. Nano Lett 2003, 3, 727–730. [Google Scholar]
- (590).Lee D; Cui T Low-Cost, Transparent, and Flexible Single-Walled Carbon Nanotube Nanocomposite Based Ion-Sensitive Field-Effect Transistors for PH/Glucose Sensing. Biosens. Bioelectron 2010, 25, 2259–2264. [DOI] [PubMed] [Google Scholar]
- (591).Wang J; Liu G; Jan MR Ultrasensitive Electrical Biosensing of Proteins and DNA: Carbon-Nanotube Derived Amplification of the Recognition and Transduction Events. J. Am. Chem. Soc 2004, 126, 3010–3011. [DOI] [PubMed] [Google Scholar]
- (592).Fu D; Li L-J Label-Free Electrical Detection of DNA Hybridization Using Carbon Nanotubes and Graphene. Nano Rev 2010, 1, 5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (593).He P; Dai L Aligned Carbon Nanotube–DNA Electrochemical Sensors. Chem. Commun 2004, No. 3, 348–349. [DOI] [PubMed] [Google Scholar]
- (594).Star A; Tu E; Niemann J; Gabriel J-CP; Joiner CS; Valcke C Label-Free Detection of DNA Hybridization Using Carbon Nanotube Network Field-Effect Transistors. Proc. Natl. Acad. Sci 2006, 103, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (595).Gui E-L; Li L-J; Lee PS; Lohani A; Mhaisalkar SG; Cao Q; Kang SJ; Rogers JA; Tansil NC; Gao Z Electrical Detection of Hybridization and Threading Intercalation of Deoxyribonucleic Acid Using Carbon Nanotube Network Field-Effect Transistors. Appl. Phys. Lett 2006, 89, 232104. [Google Scholar]
- (596).Dong X; Lau CM; Lohani A; Mhaisalkar SG; Kasim J; Shen Z; Ho X; Rogers JA; Li LJ Electrical Detection of Femtomolar DNA via Gold-Nanoparticle Enhancement in Carbon-Nanotube-Network Field-Effect Transistors. Adv. Mater 2008, 20, 2389–2393. [Google Scholar]
- (597).Gui EL; Li L-J; Zhang K; Xu Y; Dong X; Ho X; Lee PS; Kasim J; Shen ZX; Rogers JA; et al. DNA Sensing by Field-Effect Transistors Based on Networks of Carbon Nanotubes. J. Am. Chem. Soc 2007, 129, 14427–14432. [DOI] [PubMed] [Google Scholar]
- (598).Kelley SO; Barton JK Electron Transfer between Bases in Double Helical DNA. Science 1999, 283, 375–381. [DOI] [PubMed] [Google Scholar]
- (599).Fainberg A Explosives Detection for Aviation Security. Science 1992, 255, 1531–1537. [DOI] [PubMed] [Google Scholar]
- (600).Noort D; Benschop HP; Black RM Biomonitoring of Exposure to Chemical Warfare Agents: A Review. Toxicol. Appl. Pharmacol 2002, 184, 116–126. [PubMed] [Google Scholar]
- (601).Devi S Life after Death—surviving the Attacks on Civilians in Syria. Lancet 2018, 391, 1009–1011. [DOI] [PubMed] [Google Scholar]
- (602).Szinicz L History of Chemical and Biological Warfare Agents. Toxicology 2005, 214, 167–181. [DOI] [PubMed] [Google Scholar]
- (603).Jang YJ; Kim K; Tsay OG; Atwood DA; Churchill DG Update 1 of: Destruction and Detection of Chemical Warfare Agents. Chem. Rev 2015, 115, PR1–PR76. [DOI] [PubMed] [Google Scholar]
- (604).Kolakowski BM; Mester Z Review of Applications of High-Field Asymmetric Waveform Ion Mobility Spectrometry (FAIMS) and Differential Mobility Spectrometry (DMS). Analyst 2007, 132, 842–864. [DOI] [PubMed] [Google Scholar]
- (605).Joo BS; Huh JS; Lee DD Fabrication of Polymer SAW Sensor Array to Classify Chemical Warfare Agents. Sensors Actuators, B Chem 2007, 121, 47–53. [Google Scholar]
- (606).Liu G; Lin Y Electrochemical Sensor for Organophosphate Pesticides and Nerve Agents Using Zirconia Nanoparticles as Selective Sorbents. Anal. Chem 2005, 77, 5894–5901. [DOI] [PubMed] [Google Scholar]
- (607).Braue EH; Pannella MG FT-IR Analysis of Chemical Warfare Agents. Mikrochim. Acta 1988, 94, 11–16. [Google Scholar]
- (608).Zhou Q; Swager TM Methodology for Enhancing the Sensitivity of Fluorescent Chemosensors: Energy Migration in Conjugated Polymers. J. Am. Chem. Soc 1995, 117, 7017–7018. [Google Scholar]
- (609).Yang JS; Swager TM Fluorescent Porous Polymer Films as TNT Chemosensors: Electronic and Structural Effects. J. Am. Chem. Soc 1998, 120, 11864–11873. [Google Scholar]
- (610).Yang J-S; Swager TM Porous Shape Persistent Fluorescent Polymer Films: An Approach to TNT Sensory Materials. J. Am. Chem. Soc 1998, 120, 5321–5322. [Google Scholar]
- (611).Weis JG; Swager TM Thiophene-Fused Tropones as Chemical Warfare Agent-Responsive Building Blocks. ACS Macro Lett 2015, 4, 138–142. [DOI] [PubMed] [Google Scholar]
- (612).Germain ME; Knapp MJ Optical Explosives Detection: From Color Changes to Fluorescence Turn-On. Chem. Soc. Rev 2009, 38, 2543–2555. [DOI] [PubMed] [Google Scholar]
- (613).Colovic MB; Krstic DZ; Lazarevic-Pasti TD; Bondzic AM; Vasic VM Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol 2013, 11, 315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (614).Wismer T Chemical Warfare Agents and Risks to Animal Health; Elsevier Inc., 2015. [Google Scholar]
- (615).Wang Y; Yang Z; Hou Z; Xu D; Wei L; Kong ESW; Zhang Y Flexible Gas Sensors with Assembled Carbon Nanotube Thin Films for DMMP Vapor Detection. Sensors Actuators, B Chem 2010, 150, 708–714. [Google Scholar]
- (616).Robinson JA; Snow ES; Perkins FK Improved Chemical Detection Using Single-Walled Carbon Nanotube Network Capacitors. Sensors Actuators, A Phys 2007, 135, 309–314. [Google Scholar]
- (617).Lee CY; Sharma R; Radadia AD; Masel RI; Strano MS On-Chip Micro Gas Chromatograph Enabled by a Noncovalently Functionalized Single-Walled Carbon Nanotube Sensor Array. Angew. Chemie - Int. Ed 2008, 47, 5018–5021. [DOI] [PubMed] [Google Scholar]
- (618).Cattanach K; Kulkarni RD; Kozlov M; Manohar SK Flexible Carbon Nanotube Sensors for Nerve Agent Simulants. Nanotechnology 2006, 17, 4123–4128. [DOI] [PubMed] [Google Scholar]
- (619).Chuang PK; Wang LC; Kuo CT Development of a High Performance Integrated Sensor Chip with a Multi-Walled Carbon Nanotube Assisted Sensing Array. Thin Solid Films 2013, 529, 205–208. [Google Scholar]
- (620).Kumar D; Jha P; Chouksey A; Rawat JSBS; Tandon RP; Chaudhury PK 4-(Hexafluoro-2-Hydroxy Isopropyl)Aniline Functionalized Highly Sensitive Flexible SWCNT Sensor for Detection of Nerve Agent Simulant Dimethyl Methylphosphonate. Mater. Chem. Phys 2016, 181, 487–494. [Google Scholar]
- (621).Kong L; Wang J; Fu X; Zhong Y; Meng F; Luo T; Liu J P-Hexafluoroisopropanol Phenyl Covalently Functionalized Single-Walled Carbon Nanotubes for Detection of Nerve Agents. Carbon N. Y 2010, 48, 1262–1270. [Google Scholar]
- (622).Wei L; Shi D; Ye P; Dai Z; Chen H; Chen C; Wang J; Zhang L; Xu D; Wang Z; et al. Hole Doping and Surface Functionalization of Single-Walled Carbon Nanotube Chemiresistive Sensors for Ultrasensitive and Highly Selective Organophosphor Vapor Detection. Nanotechnology 2011, 22, 425501. [DOI] [PubMed] [Google Scholar]
- (623).Khan MAK; Kerman K; Petryk M; Kraatz HB Noncovalent Modification of Carbon Nanotubes with Ferrocene-Amino Acid Conjugates for Electrochemical Sensing of Chemical Warfare Agent Mimics. Anal. Chem 2008, 80, 2574–2582. [DOI] [PubMed] [Google Scholar]
- (624).Diakowski PM; Xiao Y; Petryk MWP; Kraatz H-B Impedance Based Detection of Chemical Warfare Agent Mimics Using Ferrocene-Lysine Modified Carbon Nanotubes. Anal. Chem 2010, 82, 3191–3197. [DOI] [PubMed] [Google Scholar]
- (625).Xiao Y; Petryk M; Diakowski PM; Kraatz H-B Covalent Modification of Carbon Nanotubes with Ferrocene-Lysine Derivative for Electrochemical Sensors In Proceedings of SPIE - The International Society for Optical Engineering; Fountain AW III, Gardner PJ, Eds.; 2009; Vol. 7304, p 73040R. [Google Scholar]
- (626).Shankar A; Mittal J; Jagota A Binding between DNA and Carbon Nanotubes Strongly Depends upon Sequence and Chirality. Langmuir 2014, 30, 3176–3183. [DOI] [PubMed] [Google Scholar]
- (627).Staii C; Johnson AT; Chen M; Gelperin A DNA-Decorated Carbon Nanotubes for Chemical Sensing. Nano Lett 2005, 5, 1774–1778. [DOI] [PubMed] [Google Scholar]
- (628).Zuniga C; Rinaldi M; Khamis SM; Johnson AT; Piazza G Nanoenabled Microelectromechanical Sensor for Volatile Organic Chemical Detection. Appl. Phys. Lett 2009, 94, 8–11. [Google Scholar]
- (629).Kybert NJ; Lerner MB; Yodh JS; Preti G; Johnson ATC Differentiation of Complex Vapor Mixtures Using Versatile DNA-Carbon Nanotube Chemical Sensor Arrays. ACS Nano 2013, 7, 2800–2807. [DOI] [PubMed] [Google Scholar]
- (630).Khamis SM; Jones RA; Johnson ATC; Preti G; Kwak J; Gelperin A DNA-Decorated Carbon Nanotube-Based FETs as Ultrasensitive Chemical Sensors: Discrimination of Homologues, Structural Isomers, and Optical Isomers. AIP Adv 2012, 2, 022110. [Google Scholar]
- (631).Liu Y; Chen CL; Zhang Y; Sonkusale SR; Wang ML; Dokmeci MR SWNT Based Nanosensors for Wireless Detection of Explosives and Chemical Warfare Agents. IEEE Sens. J 2013, 13, 202–210. [Google Scholar]
- (632).Liu G; Lin Y Biosensor Based on Self-Assembling Acetylcholinesterase on Carbon Nanotubes for Flow Injection/Amperometric Detection of Organophosphate Pesticides and Nerve Agents. Anal. Chem 2006, 78, 835–843. [DOI] [PubMed] [Google Scholar]
- (633).Delalande M; Clavaguera S; Toure M; Carella A; Lenfant S; Deresmes D; Vuillaume D; Simonato J-P Chemical Functionalization of Electrodes for Detection of Gaseous Nerve Agents with Carbon Nanotube Field-Effect Transistors. Chem. Commun 2011, 47, 6048. [DOI] [PubMed] [Google Scholar]
- (634).Jaynes GD; Williams RM Chemical and Biological TERRORISM: Research and Development to Improve Civilian Medical Response; NATIONAL ACADEMY PRESS: Washington, DC 20418, 1999. [PubMed] [Google Scholar]
- (635).Guo Y-L; Kennedy TP; Michael JR; Sciuto AM; Ghio AJ; Adkinson NF Jr.; Gurtner GH Mechanism of Phosgene-Induced Lung Toxicity: Role of Arachidonate Mediators. J. Appl. Physiol 1990, 69, 1615–1622. [DOI] [PubMed] [Google Scholar]
- (636).Evans RD Chlorine : State of the Art. Lung 2004, 183, 151–167. [DOI] [PubMed] [Google Scholar]
- (637).Borak J; Diller WF Phosgene Exposure: Mechanisms of Injury and Treatment Strategies. J. Occup. Environ. Med 2001, 43, 110–119. [DOI] [PubMed] [Google Scholar]
- (638).Wongwiriyapan W; Honda S; Konishi H; Mizuta T; Ikuno T; Ito T; Maekawa T; Suzuki K; Ishikawa H; Oura K; et al. Single-Walled Carbon Nanotube Thin-Film Sensor for Ultrasensitive Gas Detection. Jpn. J. Appl. Phys 2005, 44, L482–L484. [Google Scholar]
- (639).Li J; Lu Y; Meyyappan M Nano Chemical Sensors with Polymer-Coated Carbon Nanotubes. IEEE Sens. J 2006, 6, 1047–1051. [Google Scholar]
- (640).Lu Y; Partridge C; Meyyappan M; Li J A Carbon Nanotube Sensor Array for Sensitive Gas Discrimination Using Principal Component Analysis. J. Electroanal. Chem 2006, 593, 105–110. [Google Scholar]
- (641).Lu Y; Meyyappan M; Li J Fabrication of Carbon-Nanotube-Based Sensor Array and Interference Study. J. Mater. Res 2011, 26, 2017–2023. [Google Scholar]
- (642).Gohier A; Chancolon J; Chenevier P; Porterat D; Mayne-L’Hermite M; Reynaud C Optimized Network of Multi-Walled Carbon Nanotubes for Chemical Sensing. Nanotechnology 2011, 22, 105501. [DOI] [PubMed] [Google Scholar]
- (643).Lee CY; Strano MS Amine Basicity (p K b) Controls the Analyte Binding Energy on Single Walled Carbon Nanotube Electronic Sensor Arrays. J. Am. Chem. Soc 2008, 130, 1766–1773. [DOI] [PubMed] [Google Scholar]
- (644).Popa A; Li J; Samia ACS Hybrid Platinum Nanobox/Carbon Nanotube Composites for Ultrasensitive Gas Sensing. Small 2013, 9, 3928–3933. [DOI] [PubMed] [Google Scholar]
- (645).Choi SW; Kim BM; Oh SH; Byun YT Selective Detection of Chlorine at Room Temperature Utilizing Single-Walled Carbon Nanotubes Functionalized with Platinum Nanoparticles Synthesized via Ultraviolet Irradiation. Sensors Actuators, B Chem 2017, 249, 414–422. [Google Scholar]
- (646).Sharma AK; Mahajan A; Bedi RK; Kumar S; Debnath AK; Aswal DK Non-Covalently Anchored Multi-Walled Carbon Nanotubes with Hexa-Decafluorinated Zinc Phthalocyanine as Ppb Level Chemiresistive Chlorine Sensor. Appl. Surf. Sci 2018, 427, 202–209. [Google Scholar]
- (647).Thom SR Hyperbaric-Oxygen Therapy For Acute Carbon Monoxide Poisoning. N Engl J Med 2002, 347, 1105–1106. [DOI] [PubMed] [Google Scholar]
- (648).Stephen R. Thom; Keim LW Carbon Monoxide Poisoning: A Review Epidemiology, Pathophysiology, Clinical Findings, and Treatment Options Including Hyperbaric Oxygen Therapy. Clin. Toxicol 1989, 27, 141–156. [DOI] [PubMed] [Google Scholar]
- (649).Baud FJ Cyanide: Critical Issues in Diagnosis and Treatment. Hum. Exp. Toxicol 2007, 26, 191–201. [DOI] [PubMed] [Google Scholar]
- (650).Srivastava A; Sharma V; Kaur K; Khan MS; Ahuja R; Rao VK Electron Transport Properties of a Single-Walled Carbon Nanotube in the Presence of Hydrogen Cyanide: First-Principles Analysis. J. Mol. Model 2015, 21, 173. [DOI] [PubMed] [Google Scholar]
- (651).Yari A; Sepahvand R Highly Sensitive Carbon Paste Electrode with Silver-Filled Carbon Nanotubes as a Sensing Element for Determination of Free Cyanide Ion in Aqueous Solutions. Microchim. Acta 2011, 174, 321–327. [Google Scholar]
- (652).Schlegelmilch J; Petkova EP; Martinez S; Redlener IE Acts of Terrorism and Mass Violence Targeting Schools : Analysis and Implications for Preparedness in the USA. J. Bus. Contin. Emer. Plan 2017, 10, 280–289. [PubMed] [Google Scholar]
- (653).Shvetsov A; Shvetsova S; Kozyrev VA; Spharov VA; Sheremet NM The “Car-Bomb” as a Terrorist Tool at Metro Stations, Railway Terminals and Airports. J. Transp. Secur 2017, 10, 31–43. [Google Scholar]
- (654).Woods LM; Bădescu ŞC; Reinecke TL; Bǎdescu ŞC; Reinecke TL Adsorption of Simple Benzene Derivatives on Carbon Nanotubes. Phys. Rev. B 2007, 75, 155415. [Google Scholar]
- (655).Star A; Han T-R; Gabriel J-CP; Bradley K; Grüner G Interaction of Aromatic Compounds with Carbon Nanotubes: Correlation to the Hammett Parameter of the Substituent and Measured Carbon Nanotube FET Response. Nano Lett 2003, 3, 1421–1423. [Google Scholar]
- (656).Ruan W; Li Y; Tan Z; Liu L; Jiang K; Wang Z In Situ Synthesized Carbon Nanotube Networks on a Microcantilever for Sensitive Detection of Explosive Vapors. Sensors Actuators, B Chem 2013, 176, 141–148. [Google Scholar]
- (657).Liu Y; Li X; Dokmeci MR; Wang ML Carbon Nanotube Sensors Integrated inside a Microfluidic Channel for Water Quality Monitoring In Proc. of SPIE; San Diego, California, 2011; Vol. 7981, p 79811I. [Google Scholar]
- (658).Chen P-C; Sukcharoenchoke S; Ryu K; Gomez de Arco L; Badmaev A; Wang C; Zhou C 2,4,6-Trinitrotoluene (TNT) Chemical Sensing Based on Aligned Single-Walled Carbon Nanotubes and ZnO Nanowires. Adv. Mater 2010, 22, 1900–1904. [DOI] [PubMed] [Google Scholar]
- (659).Kumar D; Jha P; Chouksey A; Tandon RP; Chaudhury PK; Rawat JS Flexible Single Walled Nanotube Based Chemical Sensor for 2,4-Dinitrotoluene Sensing. J. Mater. Sci. Mater. Electron 2018, 29, 6200–6205. [Google Scholar]
- (660).Kim TH; Lee BY; Jaworski J; Yokoyama K; Chung WJ; Wang E; Hong S; Majumdar A; Lee SW Selective and Sensitive TNT Sensors Using Biomimetic Polydiacetylene-Coated CNT-FETs. ACS Nano 2011, 5, 2824–2830. [DOI] [PubMed] [Google Scholar]
- (661).Zhang Y; Xu M; Bunes BR; Wu N; Gross DE; Moore JS; Zang L Oligomer-Coated Carbon Nanotube Chemiresistive Sensors for Selective Detection of Nitroaromatic Explosives. ACS Appl. Mater. Interfaces 2015, 7, 7471–7475. [DOI] [PubMed] [Google Scholar]
- (662).Hrapovic S; Majid E; Liu Y; Male K; Luong JHT Metallic Nanoparticle-Carbon Nanotube Composites for Electrochemical Determination of Explosive Nitroaromatic Compounds. Anal. Chem 2006, 78, 5504–5512. [DOI] [PubMed] [Google Scholar]
- (663).Li J; Feng H; Feng Y; Liu J; Liu Y; Jiang J; Qian D A Glassy Carbon Electrode Modified with β-Cyclodextin, Multiwalled Carbon Nanotubes and Graphene Oxide for Sensitive Determination of 1,3-Dinitrobenzene. Microchim. Acta 2014, 181, 1369–1377. [Google Scholar]
- (664).Lai H; Leung A; Magee M; Almirall JR Identification of Volatile Chemical Signatures from Plastic Explosives by SPME-GC/MS and Detection by Ion Mobility Spectrometry. Anal. Bioanal. Chem 2010, 396, 2997–3007. [DOI] [PubMed] [Google Scholar]


