Abstract
The ability for individuals to actively make decisions engages regions within the mesolimbic system and enhances memory for chosen items. In other behavioral contexts, mesolimbic engagement has been shown to enhance episodic memory by supporting consolidation. However, research has yet to investigate how consolidation may support interactions between decision-making and episodic memory. Across two studies, participants encoded items that were covered by occluder screens and could either actively decide which of two items to uncover or an item was preselected by the experimenter. In Study 1, we show that active decision-making reduces forgetting rates across an immediate and 24-hr memory test, a behavioral marker of consolidation. In Study 2, we use functional neuroimaging to characterize putative neural markers of memory consolidation by measuring interactions between the hippocampus and perirhinal cortex (PRC) during a postencoding period that reexposed participants to elements of the decision-making context without exposing them to memoranda. We show that choice-related striatal engagement is associated with increased postencoding hippocampal–PRC interactions. Finally, we show that a previous reported relationship between choicerelated striatal engagement and long-term memory is accounted for by these postencoding hippocampal–PRC interactions. Together, these findings support a model by which actively deciding to encode information enhances memory consolidation to preserve episodic memory for outcomes, a process that may be facilitated by reexposure to the original decision-making context.
INTRODUCTION
Individuals value the ability to actively make decisions and manipulate their environment (Leotti, Iyengar, & Ochsner, 2010). Although previous research has characterized the prioritization of valuable information in episodic memory (Murty & Adcock, 2017; Miendlarzewska, Bavelier, & Schwartz, 2016), these processes have not been fully characterized in the context of decision-making. Recent research has shown that the simple act of making a decision enhances episodic memory by engaging mesolimbic and hippocampal systems during choice and encoding, respectively (Murty, DuBrow, & Davachi, 2015). In parallel, animal research has shown that engagement of mesolimbic systems during encoding supports episodic memory by strengthening postencoding consolidation (Wang & Morris, 2010). However, research has yet to fully characterize how postencoding memory consolidation processes may be initiated by active decision-making. In the current study, we used behavioral and neural markers of postencoding consolidation to characterize a novel mechanism by which decision-making enhances episodic memory.
The opportunity to actively make decisions and implement agency over one’s environment engages regions associated with mesolimbic dopamine systems. When participants are given the opportunity to choose which of two gambles to partake in, there is increased engagement of the striatum and dopaminergic midbrain (Leotti & Delgado, 2011, 2014). We recently showed a parallel mechanism is engaged when individuals are given the opportunity to make decisions about what information to encode (Murty et al., 2015). In this study, we manipulated whether participants could actively decide which information to learn. Memory was enhanced for items selected by the participants versus items selected by the experimenter, and these memory enhancements were related to striatal engagement. Together, these studies suggest that the act of decision-making enhances striatal activation—a proxy of mesolimbic engagement— and episodic memory.
Rodent and human studies have shown that mesolimbic engagement enhances memory, in part, by increasing memory consolidation. Memory consolidation in this context refers to the strengthening of memory after encoding and before retrieval, often demonstrated as a resistance to forgetting over time. Behavioral studies have shown that memory enhancements of information encoded under reward, which is thought to engage mesolimbic dopamine systems, only emerge after a significant delay (Patil, Murty, Dunsmoor, Phelps, & Davachi, 2017; Murayama & Kitagami, 2014; Murayama & Kuhbandner, 2011). Furthermore, rodent studies show that reward and novelty-based memory enhancements rely on dopaminemediated consolidation (Abraham, Neve, & Lattal, 2016; Takeuchi et al., 2016; Salvetti, Morris, & Wang, 2014; Wang & Morris, 2010; Wang, Redondo, & Morris, 2010; Li, Cullen, Anwyl, & Rowan, 2003). If the same mechanism that enhances memory in these contexts also engages the mesolimbic system during decision-making, then previously identified decision-induced memory enhancements may upregulate postchoice consolidation processes.
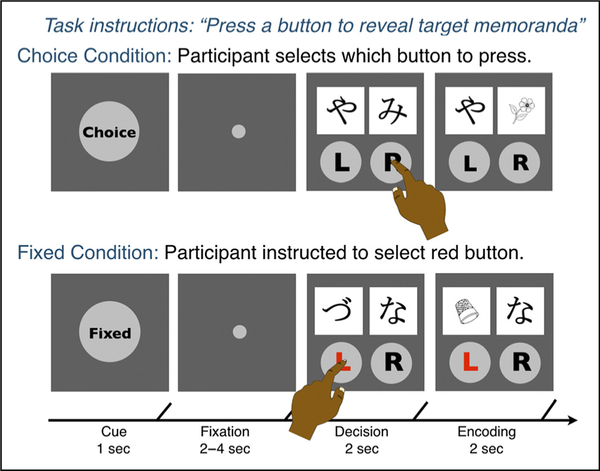
Behavioral and neuroimaging research has identified multiple methodological approaches to characterize memory consolidation in humans. Behaviorally, memory consolidation can be measured by testing memory both at immediate and delayed time points. Importantly, querying memory at both time points allows one to compute forgetting rates, which should be reduced for memories strengthened by postencoding consolidation. Neurally, memory consolidation can be characterized by relating episodic memory with measures of postencoding activity, which can either occur offline during rest or be facilitated by reexposing individuals to items that reactivate memory representations (Antony, Ferreira, Norman, & Wimber, 2017). Systems consolidation is thought to transfer information initially encoded in the hippocampus to cortical regions (Nadel, Samsonovich, Ryan, & Moscovitch, 2000; McClelland, McNaughton, & O’Reilly, 1995). Thus, one signature of this process would be increased coupling within memory networks following encoding. In line with this framework, neuroimaging studies have shown that increased functional coupling between hippocampus and category-selective regions predicts later memory performance during periods of offline rest (Murty, Tompary, Adcock, & Davachi, 2017; Schlichting & Preston, 2016; Tompary, Duncan, & Davachi, 2015; Tambini, Ketz, & Davachi, 2010). In the current study, we characterized these behavioral and neural markers of postencoding consolidation and their relationship to decision-related memory enhancements. Across two studies, participants completed a choice memory paradigm in which we manipulated whether participants could either actively decide which information to encode (Choice) or the information was selected for them (Fixed; Figure 1). During the encoding phase, object memoranda were hidden behind occluder screens, and by selecting an occluder screen, an object memorandum was revealed. In this way, the choice condition imbued participants with a sense of agency over their environment but, critically, had no effect on which memoranda were shown.
Figure 1.
Choice memory encoding task.
In Study 1, we characterized behavioral markers of consolidation by testing forgetting rates for items actively selected by the participant (Choice) versus items selected by the experimenter (Fixed). In Study 2, we characterized neural markers of consolidation by measuring postencoding changes in functional coupling between the hippocampus and the perirhinal cortex (PRC). Postencoding coupling was characterized during a task state in which individuals were reexposed to elements related to the decision process (i.e., occluder screens), but not object memoranda. We selected the PRC as our cortical target given its critical role in mediating memory for object images (Staresina, Duncan, & Davachi, 2011; Graham, Barense, & Lee, 2010; Litman, Awipi, & Davachi, 2009; Awipi & Davachi, 2008; Davachi, 2006; Davachi, Mitchell, & Wagner, 2003) and prior work implicating hippocampal–PRC coupling as a neural marker for object-based memory consolidation (Vilberg & Davachi, 2013).
METHODS
Participants
Participants were recruited from the New York University and New York City communities. Informed consent was obtained for each participant in a manner approved by the University Committee on Activities Involving Human Subjects. In Study 1 (behavioral), 36 healthy, right-handed participants were paid $25 to participate. Three participants were excluded because of failure to follow task instructions (n = 1), familiarity with the stimuli (n = 1), and failure to complete the 24-hr memory recognition test (n = 1). The final sample included 33 participants (21 women; age range = 18–35 years, median age = 22 years). In Study 2 (fMRI), 24 healthy, right-handed participants were paid $50 to participate. Four participants were excluded because of failure to follow task instructions (n = 1), poor neuroimaging data quality (n = 1), and failure to complete the 24-hr memory recognition test (n = 2). Portions of these data relating to the influence of decision-making on memory and neural activations associated with memory encoding have been reported (Murty et al., 2015).
Behavioral Paradigm
Participants in both studies completed a multiphase choice memory task, which probes how arbitrary decision-making—giving individuals the opportunity to make a choice—influences episodic memory. The task consists of four phases: (1) preencoding ratings, (2) choice memory encoding, (3) postencoding ratings, and (4) a memory test.
In the preencoding ratings phase, individuals made preference ratings about 80 hiragana characters. They were instructed to indicate how much they “liked” each character on a 5-point scale. Participants had 4 sec to rate each character (2–5 sec intertrial interval). Sixty of the most neutrally related characters from the preencoding ratings phase were used as occluder screens for the choice encoding task. Next, participants completed the choice encoding phase (Figure 1), which manipulated whether individuals could actively make decisions about which information to encode. On each trial, participants were first shown a cue for 1 sec indicating the condition (i.e., choice, fixed), followed by a fixation dot for 2–4 sec, followed by a decision phase for 2 sec, and an encoding phase for 2 sec. During the decision phase, participants saw a screen with two occluder screens, which were previously rated hiragana characters, and two buttons underneath. Participants were instructed to make a button press to reveal an object covered by the occluder screen. During the encoding phase, participants were instructed to encode the previously occluded object image. In the choice condition, participants actively chose which occluder screen to remove. In the fixed condition, the participants were instructed to choose the button that was highlighted with red text. If participants took longer than 2 sec to respond, they were shown a screen indicating their response was too slow and no image was presented. Unknown to the participants, object images were preselected, so there was no relationship between decisions and the underlying object image. Following each trial, a fixation cross appeared for 3–24 sec in a manner optimized for fMRI analysis (https://surfer.nmr.mgh.harvard.edu/optseq/). Participants completed 60 choice trials and 60 fixed trials intermixed across four 8-min runs. Following this choice memory encoding phase, individuals completed the postencoding ratings task. This session was identical to the preencoding ratings task. Notably, there were no differences in the average preference ratings preversus postencoding for the hiragana characters appearing in choice and fixed trials (Study 1: t(21) = 0.13, p = .90; Study 2: t(32) = 0.64, p = .52).
Finally, participants completed the memory test phase, a self-paced recognition test for the object images presented during the choice encoding task. Participants were shown object items one at a time and had to indicate whether they previously viewed the object (yes/no) and the confidence in their response (“very sure,” “pretty sure,” “just guessing”). Trial duration was self-paced (1 sec intertrial interval). Participants completed 240 recognition memory trials including 60 objects from the choice condition, 60 objects from the fixed condition, and 120 novel/foil objects. In Study 1, recognition memory was split over two sessions (immediate memory test, 24-hr memory test; detailed below), with half of the items in each condition (choice, fixed, and novel) appearing during each test. In Study 2, the complete recognition memory test occurred after a 24-hr delay.
Experimental Protocol
In Study 1, participants were consented and given instructions about the study. Participants then completed the preratings task, the choice memory encoding task, and postratings task. Participants then completed the memory test for half of the stimuli presented during encoding. Participants returned approximately 24 hr later to complete a memory test for the other half of the stimuli presented during encoding. Participants were then paid and debriefed about the experiment. All sessions took place in a behavioral testing room.
In Study 2, participants were consented and given instructions outside the scanner. Once inside the scanner, participants completed the preencoding ratings, the choice memory encoding, and the postencoding ratings phase. Participants returned approximately 24 hr later to a behavioral testing room and performed the memory test for all stimuli. Participants were then paid and debriefed about the experiment.
Study 1: Analysis
To test whether individuals’ memory was above chance, we compared the percentage of objects endorsed as old for old versus new items within participants for each testing day. Next, to determine if there were choice-related memory enhancements, we compared the percentage of objects endorsed as old for choice versus fixed conditions within participants for each testing day. Finally, to determine if there was an influence of consolidation on choice memory benefits, we compared forgetting rates across the immediate and 24-hr memory test (Litman & Davachi, 2008). Forgetting was determined as a proportional difference in recognition memory across tests [(Day 1 − Day 2)/(Day 1)] for each condition separately. All comparisons were submitted to a paired t test with condition (choice, fixed) as a within-subject factor.
Study 2: fMRI Data Acquisition and Preprocessing
Functional imaging data were acquired on a Siemens Allegra 3T head-only scanner using EPI (repetition time = 2000 msec, 34 contiguous slices, voxel size = 3 mm isometric). Slices were positioned parallel to the AC–PC and include coverage of our ROIs (i.e., striatum, hippocampus, PRC). Our planned analyses focused on the pre- and postratings task. Pre- and postratings fMRI runs consisted of 308 volumes. We also collected a high-resolution T1-weighted anatomical scan (MPRAGE, voxel size = 1 mm isotropic) for use in spatial normalization. Before fMRI preprocessing, data were inspected on custom software for head motion and scanning artifacts. Data were analyzed only if they exhibited <3.0-mm motion (absolute maximum). Slice acquisitions with isolated transient noise artifacts were replaced with interpolated data from neighboring time points. fMRI preprocessing was then performed using FEAT (fMRI Expert Analysis Tool) version 6.00 as implemented in FSL version 5.0.2.1. The first four scans of each run were discarded for signal saturation. Images were skull-stripped, realigned, intensity-normalized, spatially smoothed with a 5.00-mm FWHM kernel, and subjected to a high-pass filter (Gaussian-weighted least squares straight line fitting set to 50.0 sec). fMRI images were transformed to standard space by first registering images to a subject-specific high-resolution anatomical image. We then applied a transformation matrix derived by a nonlinear transformation with a 10-mm warp resolution and 2-mm isotropic voxel resolution from the subject-specific anatomical image to an MNI standard space image, as implemented in FMRIB Nonlinear Registration Tool.
Study 2: Characterizing Pre- and Postencoding Hippocampal–PRC Network Coupling
To characterize postencoding neural markers of consolidation, we measured functional coupling between hippocampus and PRC during the “ratings task” using a “background connectivity” approach. This approach has been used in previous publications to measures low-frequency (state) changes in network interactions by removing task or trial-evoked activation related to the ratings task (Duncan, Tompary, & Davachi, 2014; Al-Aidroos, Said, & Turk-Browne, 2012). Previous work in our laboratory has used this approach to assess how postencoding changes in network coupling during an orthogonal (i.e., math) task related to subsequent memory (Tompary et al., 2015). Unlike this prior study, during the postencoding ratings task period, participants were reexposed to the occluder screens presented during encoding, and thus, signals extracted from this analysis may reflect incidental reactivation of memoranda.
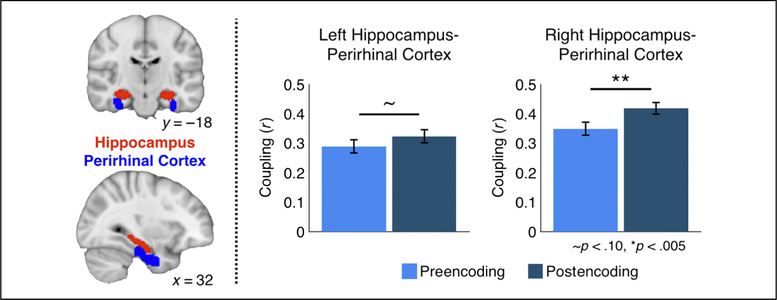
We first removed activity in response to individual events in the pre- and postencoding ratings task separately. We modeled each event using a general linear model that included separate regressors modeling hiragana characters that had appeared in (1) the choice trials, (2) the fixed trials, and (3) those that were not used in the memory encoding session, as well as their temporal derivatives. Each event was modeled with an event duration of 3 sec convolved with a double-gamma hemodynamic response function. Data were also prewhitened before analysis. We then extracted the time series from the residuals of these models from our ROIs in the hippocampus and PRC (Figure 3, left) separately for the right and left hemisphere. The hippocampus and PRC were defined using probabilistic atlases thresholded to 50% overlap. The hippocampus was defined from Harvard– Oxford Subcortical atlas (www.fmrib.ox.ac.uk/ fsl / fslview). The PRC was defined from a probabilistic atlas generated by the Memory Modulation Lab (Ritchey, McCullough, Ranganath, & Yonelinas, 2017). Simple regressions were run in MATLAB (The MathWorks) for ROI pairs of interest for each participant individually.
Figure 3.
Encoding increases functional coupling between hippocampal–PRC. ROIs were defined in the hippocampus and PRC (left). Functional coupling between the hippocampus and PRC was increased postencoding in the right hemisphere ( p < .005), and a similar trend was seen in the left hemisphere ( p < .10). Error bars represent SEM.
Study 2: Analysis
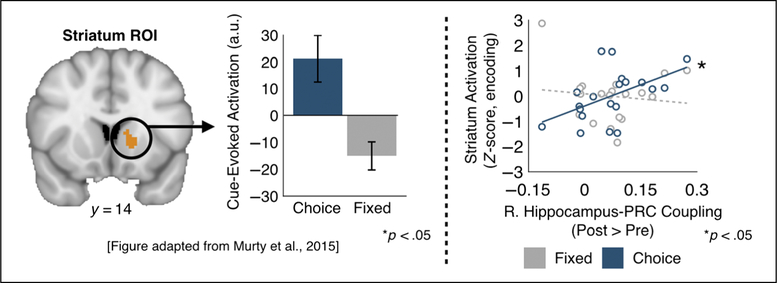
To determine whether there were differences in functional coupling between the hippocampus and PRC after encoding, we compared r scores from the pre-and post-encoding ratings task. Functional coupling from each ROI pair was compared using a paired t test with the ratings session (Post, Pre) as a within-subject factor, corrected for multiple comparisons across the two pairs of ROIs. In our prior work, we found there was greater engagement of the striatum on choice versus fixed trials during encoding (Murty et al., 2015). To determine whether there were relationships between this choice-related striatal activity and postencoding functional coupling, we ran correlations between striatal activation from the memory encoding phase and hippocampal–PRC coupling (Post > Pre). The striatum ROI was functionally defined from a contrast of cues indicating choice trials versus fixed trials during encoding (Figure 4, p < .05 whole-brain corrected, peak MNI coordinates x, y, z (−16, 14, 2); cluster size = 77 voxels). To minimize multiple comparisons, we only ran this analysis on pairs of hippocampal–PRC ROIs that showed significant differences in comparisons of post- and preencoding functional coupling. Striatal activations were extracted in response to the cues indicating choice and fixed trials during memory encoding ( p < .05, whole-brain corrected; Murty et al., 2015). Differences in correlations across conditions were tested using a Fisher r-to-z transform. Finally, we ran a series of analysis to investigate how postencoding functional coupling was related to 24-hr memory. First, we tested whether differences in postencoding functional coupling (Post > Pre) was related to 24-hr recognition memory for the choice condition using a simple regression. Then, to determine if our previously reported relationship between choice striatal activation and 24-hr memory was explained by differences in hippocampal–PRC functional coupling, we ran a mediation analysis using the function on R. Significance was tested using a nonparametric bootstrapping method, and we report on the average causal mediation effect.
Figure 4.
Choice-related striatal activation during encoding is associated with increased functional coupling between hippocampal–PRC after encoding. The striatum ROI was defined from a contrast of choice versus fixed cues during the encoding phase (left). Striatal activation during the choice encoding condition (blue) was significantly related to increase right hippocampal– PRC coupling postencoding ( p < .05; right). No such relationship was seen for the fixed encoding condition (gray; right). Error bars represent the SEM. Solid lines indicate significance.
Significance Testing
Before submitting data to parametric statistical testing, data were submitted to a Lilliefors test for nonnormality. In all cases, the null hypothesis that the sample comes from a normal distribution could not be rejected ( p > .10). For all tests, significance was determined at a value of p < .05, and trends were determined at a level of p < .10. Effect sizes are reported using Cohen’s d, t tests, and r values for regressions. Notably, for paired t tests, we calculated effect sizes based on two-sample t tests, as to not overinflate our estimations.
RESULTS
Study 1
In Study 1, we compared differences in memory performance for objects in the choice versus fixed conditions across an immediate and 24-hr memory test. By comparing forgetting rates, we could determine if postencoding processes preferentially strengthened memory for chosen items.
Object Memory Performance
Participants’ memory was tested immediately and after a ~24-hr delay for the objects appearing in the choice and fixed conditions during encoding. Participants memory performance was significantly above chance both during the immediate memory test (Hits > False Alarm; choice: t(32) = 19.7, p < .001, d = 5.67; fixed: t(32): 18.9, p < .001, d = 7.49; Table 1) and delayed memory test (Hits > False Alarm; choice: t(32) = 14.1, p < .001, d = 3.35; fixed: t(32): 10.5, p < .001, d = 2.66; Table 1). Participants showed greater memory for objects in the choice versus fixed condition during the immediate memory test (Choice > Fixed; t(32) = 3.06, p = .005, d = 0.28; Table 1) and delayed memory test (Choice > Fixed, t(32) = 4.38, p < .001, d = 0.49; Table 1).
Table 1.
Recognition Memory Performance
| Study | Hit Rate Mean (SE) |
False Alarm Rate Mean (SE) |
|---|---|---|
| Study 1: Immediate | ||
| Choice | 0.80 (0.02) | 0.13 (0.02) |
| Fixed | 0.75 (0.02) | |
| Study 1: Delayed | ||
| Choice | 0.71 (0.03) | 0.21 (.03) |
| Fixed | 0.61 (0.03) | |
| Study 2: Delayed | ||
| Choice | 0.71 (0.03) | 0.29 (.04) |
| Fixed | 0.64 (0.03) |
Forgetting Rates
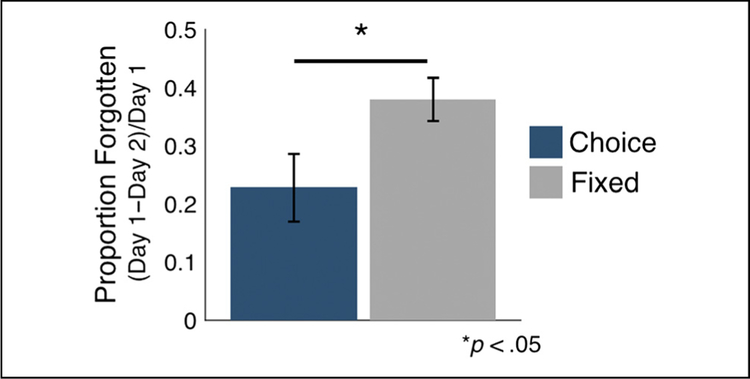
To test whether making decisions influenced memory consolidation, we examined forgetting rates by comparing memory performance across the immediate and 24-hr memory tests. Memory performance significantly declined across the 24-hr delay in the choice and fixed conditions (choice: t(32) = 3.81, p < .001, d = 0.88; fixed: t(32) = 9.69, p > .001, d = 1.11). Forgetting rates were significantly lower for objects presented in the choice versus fixed condition (Figure 2; t(32) = 2.36, p = .02, d = 0.53), suggesting that objects presented in the choice condition showed better long-term memory retention.
Figure 2.
Active decision-making during encoding reduces forgetting. Participants showed decreased forgetting across the immediate and 24-hr memory test for objects encoded in the choice condition (blue) compared with the fixed condition (gray, p < .05). Error bars represent SEM of each condition.
Study 2
In Study 2, we examined how changes in functional coupling across the hippocampus and PRC after encoding were related to 24-hr memory performance. Functional coupling was assayed using a “background connectivity” approach during the preencoding and postencoding ratings task, in which participants rated Hiragana characters that were previously used as occluder screens during the encoding phase of the experiment (see Methods: Behavioral Paradigm). Critically, object memoranda were not presented during the ratings task.
Changes in Hippocampal–PRC Coupling after Encoding
We compared differences in functional coupling between the hippocampus and PRC before and after encoding, as functional coupling between these regions has been associated with object-based memory consolidation (Vilberg & Davachi, 2013). Functional coupling was significantly greater following encoding between the right hippocampus and PRC (Figure 3; t(19) = 3.43, p = .006, corrected, d = 0.68), and increases were apparent in 70% of participants. There were no significant differences in functional coupling between the left hippocampus and PRC following encoding (Figure 3; t(19) = 1.79, p = .18, corrected, d = 0.37).
Relationships between Encoding-related Striatal Activity and Postencoding Hippocampal–PRC Interactions
In our previous study analyzing the same data set, we found that during encoding there was greater activation of the left striatum in response to choice versus fixed cues (Figure 4; Murty et al., 2015). Furthermore, the extent to which the striatum was engaged during choice trials predicted 24-hr recognition memory. These findings suggested a relationship between striatal engagement and decision-related memory benefits.
Here, we tested whether striatal activation during encoding was related to postencoding changes in functional coupling between right hippocampus and PRC. We found that striatal activation in response to choice cues was positively associated with increased postencoding coupling of the right hippocampus and PRC (Figure 4; r(18) = .51, p = .02). There was no such relationship with striatal activation in response to fixed cues (Figure 4; r(18) = −.12, p = .60), and correlations were significantly greater for striatal activations in response to choice versus fixed cues ( p = .04, Z = 2.05).
Relationships between 24-hr Choice Memory and Postencoding Hippocampal–PRC Interactions
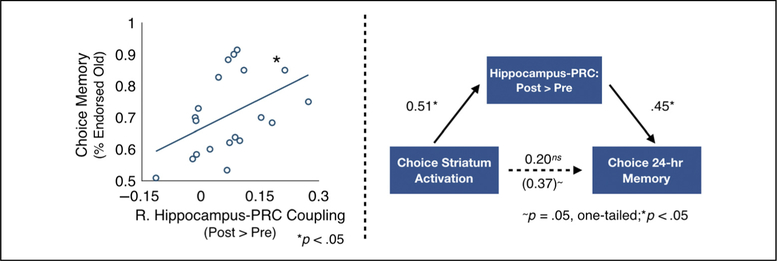
We next tested whether postencoding hippocampal–PRC interactions were related to memory for objects in the choice condition. Individuals that showed greater postencoding increases in right hippocampal–PRC coupling had better 24-hr memory for objects encoded in the choice condition, r(18) = .45, p = .046 (Figure 5). This relationship was not significant for objects encoded in the fixed condition, r(18) = .37, p = .11, but these correlations did not significantly differ from each other ( p = .55, Z = 0.59).
Figure 5.
Changes in hippocampal–PRC coupling is related to 24-hr memory for choice items. Changes in functional coupling between the hippocampus and PRC post-versus preencoding significantly predicted 24-hr memory for objects in the choice condition ( p < .05; left). Changes in functional coupling between the hippocampus and PRC post-versus preencoding mediated the relationship between choice striatal activation and 24-hr memory for objects in the choice condition ( p = .045, one-tailed; right). Path values represent unstandardized regression coefficients. The total effect (i.e., the unmediated correlation coefficient) is represented in brackets.
In a final analysis, we test the explanatory role of hippocampal–PRC postencoding coupling in accounting for relationships between striatum and memory. In our prior study, we showed that choice-related striatal activation was also related to 24-hr choice memory, r(18) = .38, p = .049, one-tailed (as detailed in Murty et al., 2015). Here, we found that changes in hippocampal–PRC coupling significantly mediated the relationship between choice striatal activation and 24-hr choice memory ( p = .045, one-tailed, nonparametric bootstrap; Figure 5, right). Critically, the model testing a mediating role for choice memory on the relationship between striatal activation and postencoding connectivity was nonsignificant ( p = .17), and a model testing a mediating role for striatal activation on the relationship between postencoding connectivity and memory was nonsignificant ( p = .42).
DISCUSSION
In the current study, we characterized how postencoding consolidation mechanisms influence interactions between decision-making and episodic memory. We found that both behavioral and neural measures of consolidation were associated with decision-related enhancements in episodic memory. First, in a behavioral study, we showed that items participants actively decided to encode compared with items selected by the experimenter showed an enhanced resistance to forgetting over a 24-hr delay. Second, we found increased postencoding interactions between hippocampus and PRC during a task in which participants were reexposed to features of the decision-making context. Furthermore, these enhancements were associated with both choice-related striatal activation during encoding and subsequent 24-hr memory. Together, these findings support a model in which one way decision-making enhances episodic memory is by upregulating postencoding connectivity, which may in part rely on reexposure to the original decision-making context.
A variety of different affective contexts have been shown to enhance memory consolidation. Rodent and human research alike has shown that environmental threat, novelty, and reward processing all increase memory consolidation (Miendlarzewska et al., 2016; Wang & Morris, 2010; McGaugh, 2004), resulting in a resistance to forgetting for objects encoded in these contexts. Here, we show that the simple act of making an arbitrary decision, in the absence of explicit incentives, resulted in a behavioral profile consistent with greater consolidation. In our task, many other features of encoding that influence episodic memory were matched across conditions, including viewing time, motor demands, and the content of memoranda (Murty et al., 2015). However, individuals still showed a resistance to forgetting of information that was “chosen.” Giving individuals the opportunity to make decisions has been associated with both increased valuation and engagement of mesolimbic dopamine systems (Coppin et al., 2014; Leotti & Delgado, 2011, 2014; Izuma & Murayama, 2013; Izuma et al., 2010; Sharot, De Martino, & Dolan, 2009). These findings raise the interesting idea that decision-making may increase consolidation by generating an affective context and inducing consolidation mechanisms similar to those induced by reward.
In line with this interpretation, we found relationships between striatal engagement—a key node in the mesolimbic network associated with valuation—and postencoding coupling between the hippocampus and PRC. Specifically, striatal engagement during active decision-making was related to enhanced postencoding coupling of the hippocampus and PRC. Systems-level memory consolidation is thought to rely on postencoding interactions in which memory traces stored in hippocampus are distributed to cortical regions associated with the sensory content of memoranda (Nadel et al., 2000; McClelland et al., 1995). The PRC, an anterior portion of medialtemporal lobe cortex, is preferentially activated by object images (compared with other visual categories) and is important for object memory (Liang & Preston, 2017; Staresina et al., 2011; Graham et al., 2010; Ranganath, 2010; Litman et al., 2009; Awipi & Davachi, 2008; Davachi, 2006; Buckley, 2005; Davachi et al., 2003), such as those used as memoranda in this study. One prior article also demonstrated that hippocampal–PRC connectivity is a biomarker for enhanced memory consolidation for object images (Vilberg & Davachi, 2013). Thus, we propose that striatal engagement during active choice bolsters subsequent information transfer between hippocampus and cortical regions. Furthermore, we show that these processes relate to enhanced memory. Postencoding increases in hippocampal–PRC interactions predicted memory in the choice condition and accounted for our previous demonstrated relationships between mesolimbic engagement and episodic memory (Murty et al., 2015).
Although our findings are consistent with prior work investigating systems-level consolidation, notably we assayed postencoding functional coupling during an active task that may have reactivated the decision-making context. Specifically, we characterized postencoding coupling during a state in which participants were re-exposed to the characters on the occluder screens presented during encoding. Although we removed the effects of responses evoked by the presentation of the characters, re-exposure to the general decision-making context may have cued incidental recall of the object memoranda. This differs from prior work characterizing postencoding coupling during rest (Murty et al., 2017; Schlichting & Preston, 2016; Tambini et al., 2010) or an orthogonal task (Tompary et al., 2015), because the presentation of the occluder screens may have triggered incidental retrieval or intentional rehearsal. Thus, the current study is limited in the ability to disassociate whether memory benefits reflected enhanced offline reactivation or incidental cued recall. Notably, a recent model has proposed that both processes may similarly promote memory consolidation (Antony et al., 2017), which is in line with the findings from our behavioral study showing reduced forgetting over time for decision-related memoranda. However, future studies are necessary to determine if decision-making facilitates systems-level consolidation in the absence of a reminder of the decision context.
Despite these limitations, our findings extend prior literatures demonstrating that postencoding hippocampal– cortical interactions support the stabilization of memory over time (Schlichting & Preston, 2016; Tompary et al., 2015; Tambini et al., 2010). Our results suggest that regions within the mesolimbic system may promote systems-level consolidation after encoding. A growing body of rodent research has demonstrated that dopamine activation supports memory consolidation by stabilizing synaptic plasticity within the hippocampus (Li et al., 2003; Huang & Kandel, 1995). Here, we show that mesolimbic activation may stabilize memories by facilitating interactions of the hippocampus with cortical regions, either spontaneously or by re-exposure to the decision-making context. This interpretation dovetails well with prior work from our laboratory associating reward memory benefits with postencoding interactions between the hippocampus and sensory cortex (Murty et al., 2017), as well as generalization of consolidation-related memory benefits across sensory categories (Patil et al., 2017). Critically, our current findings and previous work do not directly measure mesolimbic dopamine activation but rather induce behavioral contexts that have been associated with dopamine activation in rodent and human studies. Future studies incorporating PET imaging and/or drug manipulations will be necessary to fully test this proposed mechanism.
Our findings provide a novel mechanism by which decision-making influences episodic memory via enhancing postencoding consolidation for selected objects. Although the neural mechanisms underlying episodic memory and decision-making have often been studied independently, recent efforts have begun to integrate knowledge across these fields (St-Amand, Sheldon, & Otto, 2018; Bornstein, Khaw, Shohamy, & Daw, 2017; Bornstein & Norman, 2017; Gershman & Daw, 2017; Duncan & Shohamy, 2016; Murty, FeldmanHall, Hunter, Phelps, & Davachi, 2016; Shadlen & Shohamy, 2016; Wimmer & Büchel, 2016; Gluth, Sommer, Rieskamp, & Büchel, 2015). A large focus of this literature has been to investigate how episodic memories contribute to later adaptive decision-making. Here, we provide evidence for a reciprocal interaction in which decision-making promotes subsequent episodic memory by facilitating systems-level consolidation to stabilize decision outcomes in memory. These processes represent a highly adaptive mechanism by which information that is actively acquired is given prioritization in long-term memory.
REFERENCES
- Abraham AD, Neve KA, & Lattal KM (2016). Activation of D1/5 dopamine receptors: A common mechanism for enhancing extinction of fear and reward-seeking behaviors. Neuropsychopharmacology, 41, 2072–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aidroos N, Said CP, & Turk-Browne NB (2012). Top– down attention switches coupling between low-level and high-level areas of human visual cortex. Proceedings of the National Academy of Sciences, U.S.A, 109, 14675–14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony JW, Ferreira CS, Norman KA, & Wimber M (2017). Retrieval as a fast route to memory consolidation. Trends in Cognitive Sciences, 21, 573–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awipi T, & Davachi L (2008). Content-specific source encoding in the human medial temporal lobe. Journal of Experimental Psychology: Learning, Memory, and Cognition, 34, 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein AM, Khaw MW, Shohamy D, & Daw ND (2017). Reminders of past choices bias decisions for reward in humans. Nature Communications, 8, 15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein AM, & Norman KA (2017). Reinstated episodic context guides sampling-based decisions for reward. Nature Neuroscience, 20, 997–1003. [DOI] [PubMed] [Google Scholar]
- Buckley MJ (2005). The role of the perirhinal cortex and hippocampus in learning, memory, and perception. Quarterly Journal of Experimental Psychology, 58B, 246–268. [DOI] [PubMed] [Google Scholar]
- Coppin G, Delplanque S, Bernard C, Cekic S, Porcherot C, Cayeux I, et al. (2014). Choice both affects and reflects preferences. Quarterly Journal of Experimental Psychology, 67, 1415–1427. [DOI] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology, 16, 693–700. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, & Wagner AD (2003). Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences, U.S.A, 100, 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan KD, & Shohamy D (2016). Memory states influence value-based decisions. Journal of Experimental Psychology: General, 145, 1420–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Tompary A, & Davachi L (2014). Associative encoding and retrieval are predicted by functional connectivity in distinct hippocampal area CA1 pathways. Journal of Neuroscience, 34, 11188–11198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, & Daw ND (2017). Reinforcement learning and episodic memory in humans and animals: An integrative framework. Annual Review of Psychology, 68, 101–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluth S, Sommer T, Rieskamp J, & Büchel C (2015). Effective connectivity between hippocampus and ventromedial prefrontal cortex controls preferential choices from memory. Neuron, 86, 1078–1090. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, & Lee ACH (2010). Going beyond LTM in the MTL: A synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia, 48, 831–853. [DOI] [PubMed] [Google Scholar]
- Huang YY, & Kandel ER (1995). D1/D5 receptor agonists induce a protein synthesis-dependent late potentiation in the CA1 region of the hippocampus. Proceedings of the National Academy of Sciences, U.S.A, 92, 2446–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Matsumoto M, Murayama K, Samejima K, Sadato N, & Matsumoto K (2010). Neural correlates of cognitive dissonance and choice-induced preference change. Proceedings of the National Academy of Sciences, U.S.A, 107, 22014–22019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, & Murayama K (2013). Choice-induced preference change in the free-choice paradigm: A critical methodological review. Frontiers in Psychology, 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, & Delgado MR (2011). The inherent reward of choice. Psychological Science, 22, 1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, & Delgado MR (2014). The value of exercising control over monetary gains and losses. Psychological Science, 25, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Iyengar SS, & Ochsner KN (2010). Born to choose: The origins and value of the need for control. Trends in Cognitive Sciences, 14, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, & Rowan MJ (2003). Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nature Neuroscience, 6, 526–531. [DOI] [PubMed] [Google Scholar]
- Liang JC, & Preston AR (2017). Medial temporal lobe reinstatement of content-specific details predicts source memory. Cortex, 91, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, Awipi T, & Davachi L (2009). Category-specificity in the human medial temporal lobe cortex. Hippocampus, 19, 308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman L, & Davachi L (2008). Distributed learning enhances relational memory consolidation. Learning & Memory, 15, 711–716. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, & O’Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102, 419–457. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27, 1–28. [DOI] [PubMed] [Google Scholar]
- Miendlarzewska EA, Bavelier D, & Schwartz S (2016). Influence of reward motivation on human declarative memory. Neuroscience & Biobehavioral Reviews, 61, 156–176. [DOI] [PubMed] [Google Scholar]
- Murayama K, & Kitagami S (2014). Consolidation power of extrinsic rewards: Reward cues enhance long-term memory for irrelevant past events. Journal of Experimental Psychology: General, 143, 15–20. [DOI] [PubMed] [Google Scholar]
- Murayama K, & Kuhbandner C (2011). Money enhances memory consolidation—but only for boring material. Cognition, 119, 120–124. [DOI] [PubMed] [Google Scholar]
- Murty VP, & Adcock RA (2017). Distinct medial temporal lobe network states as neural contexts for motivated memory formation. In Hannula DE & Duff MC (Eds.), The hippocampus from cells to systems: Structure, connectivity, and functional contributions to memory and flexible cognition (pp. 467–501). Cham, Switzerland: Springer. [Google Scholar]
- Murty VP, DuBrow S, & Davachi L (2015). The simple act of choosing influences declarative memory. Journal of Neuroscience, 35, 6255–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, FeldmanHall O, Hunter LE, Phelps EA, & Davachi L (2016). Episodic memories predict adaptive value-based decision-making. Journal of Experimental Psychology: General, 145, 548–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Tompary A, Adcock RA, & Davachi L (2017). Selectivity in postencoding connectivity with high-level visual cortex is associated with reward-motivated memory. Journal of Neuroscience, 37, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, & Moscovitch M (2000). Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus, 10, 352–368. [DOI] [PubMed] [Google Scholar]
- Patil A, Murty VP, Dunsmoor JE, Phelps EA, & Davachi L (2017). Reward retroactively enhances memory consolidation for related items. Learning & Memory, 24, 65–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C (2010). A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus, 20, 1263–1290. [DOI] [PubMed] [Google Scholar]
- Ritchey M, McCullough AM, Ranganath C, & Yonelinas AP (2017). Stress as a mnemonic filter: Interactions between medial temporal lobe encoding processes and post-encoding stress. Hippocampus, 27, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvetti B, Morris RGM, & Wang S-H (2014). The role of rewarding and novel events in facilitating memory persistence in a separate spatial memory task. Learning & Memory, 21, 61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, & Preston AR (2016). Hippocampal–medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiology of Learning and Memory, 134, 91–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadlen MN, & Shohamy D (2016). Decision making and sequential sampling from memory. Neuron, 90, 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharot T, De Martino B, & Dolan RJ (2009). How choice reveals and shapes expected hedonic outcome. Journal of Neuroscience, 29, 3760–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Amand D, Sheldon S, & Otto AR (2018). Modulating episodic memory alters risk preference during decision-making. Journal of Cognitive Neuroscience, 1–9. [DOI] [PubMed]
- Staresina BP, Duncan KD, & Davachi L (2011). Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. Journal of Neuroscience, 31, 8739–8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, et al. (2016). Locus coeruleus and dopaminergic consolidation of everyday memory. Nature, 537, 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, & Davachi L (2010). Enhanced brain correlations during rest are related to memory for recent experiences. Neuron, 65, 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompary A, Duncan K, & Davachi L (2015). Consolidation of associative and item memory is related to post-encoding functional connectivity between the ventral tegmental area and different medial temporal lobe subregions during an unrelated task. Journal of Neuroscience, 35, 7326–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, & Davachi L (2013). Perirhinal-hippocampal connectivity during reactivation is a marker for object-based memory consolidation. Neuron, 79, 1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S-H, & Morris RGM (2010). Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annual Review of Psychology, 61, 49–79. [DOI] [PubMed] [Google Scholar]
- Wang S-H, Redondo RL, & Morris RGM (2010). Relevance of synaptic tagging and capture to the persistence of long-term potentiation and everyday spatial memory. Proceedings of the National Academy of Sciences, U.S.A, 107, 19537–19542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, & Büchel C (2016). Reactivation of reward-related patterns from single past episodes supports memory-based decision making. Journal of Neuroscience, 36, 2868–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]