Abstract
Tumor invasion along structural interphases of surrounding tumor-free tissue represents a key process during tumor progression. Much attention has been devoted to mechanisms of tumor cell migration within extracellular matrix (ECM)-rich connective tissue, however a comprehensive understanding of tumor invasion into tissue of higher structural complexity, such as muscle tissue, is lacking. Muscle invasion in cancer patients is often associated with destructive growth and worsened prognosis. Here, we review biochemical, geometrical and mechanical cues of smooth and skeletal muscle tissues and their relevance for guided invasion of cancer cells. As integrating concept, muscle-organizing ECM-rich surfaces of the epi-, peri- and endomysium provide cleft-like confined spaces along interfaces between dynamic muscle cells, which provide molecular and physical cues that guide migrating cancer cells, forming a possible contribution to cancer progression.
Keywords: Tumour invasion, Smooth/skeletal muscle tissue, Guided cell migration, Extracellular matrix, Cancer progression
1. Introduction
Invasion of cancer cells into surrounding structures, such as interstitial collagen-rich connective tissue or cell-rich muscular or fat tissue, is a key step in cancer disease progression [1]. Neoplastic invasion depends on a 4-step cyclic mechanochemical program of cell locomotion. It includes actin polymerization and cell elongation, integrin-mediated adhesion to the extracellular matrix (ECM) substrate, actomyosin contraction and cell shortening by retraction of the cell rear along the substrate [2]. Tumor cells from both mesenchymal or epithelial origin invade surrounding tissue either as single cells, chain-like files, clusters or collective strands [3]. These migration modes are adaptive and interconvertible as cells can vary the strength of cell-cell adhesion and actomyosin contractility in response to determinants of the environment. Molecular and physical tissue properties that impact cell migration include (1) the molecular ECM composition determining the engagement of selected adhesion receptors, adhesion strength and resulting downstream intracellular signaling cascades, (2) substrate stiffness leading to changes in adhesion strength by activation of mechanosensing, and (3) the dimension, structural heterogeneity and alignment of the substrate providing either barrier or guidance functions to the migrating cell body [4,5]. Tissue dimensions encountered by moving cells vary from thin one-dimensional (1D) ECM-rich linear structures, two-dimensional (2D) surfaces of basement membranes and fibrillar three-dimensional (3D) networks. Cells encounter and interpret such tissue heterogeneity, e.g. fibrillar matrix, clefts or channels, and adjust the migration strategy and speed accordingly. As an example, after downregulating cell-cell junctions, cells may detach from the cell collective and migrate as single cells [6]. Conversely, when confined by limited extracellular space, individually moving cells may become jammed and transition to collective migration [7].
Although ECM-based interstitial tissues have been well conceptualized as invasion substrates for tumors [8–11], little attention has been devoted to categorizing invasion of muscle tissue [12]. Neoplastic cell infiltration from the primary tumor into proximate muscle layers is a common and prognostically negative progression event, whereas metastasis to muscle is rare [13]. Muscle tissue provides a rich variety of ECM components, topologies and associated tissue structures including vessels and nerves, which jointly provide molecular and physical cues that guide invasion and, likely, reprogram invading cells.
We here summarize the biochemical, topological and mechanical properties of smooth and skeletal muscle tissue and translate their relevance to the appearance of cancer invasion patterns in histopathological patient samples and clinical consequences of neoplastic muscle tissue invasion. We then explore emerging results from the biophysics field that provide concepts for muscle tissue invasion and discuss possible consequences on therapeutic resistance. Together, the topologically complex musculature may guide the migration of invading malignant cells, support survival signaling by multi-ligand ECM interactions and induce genomic instability by its mechanically challenging geometry representing, overall, a cancer progression promoting environment.
2. Microanatomy and biophysical properties of muscle tissue
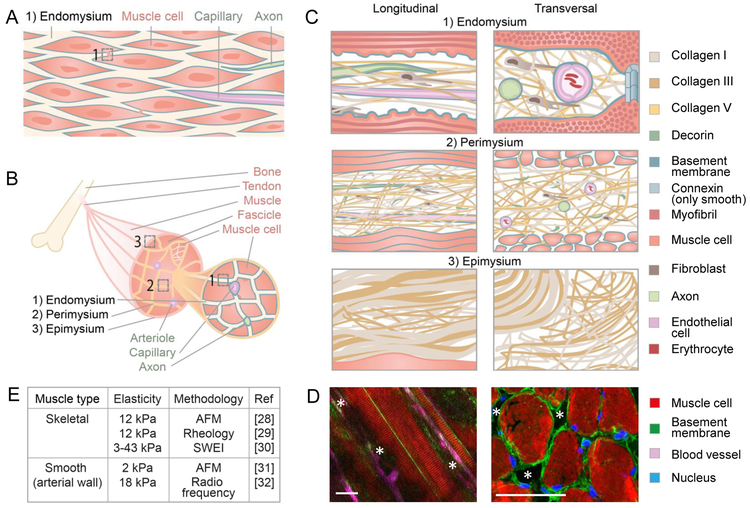
Muscle tissue is present in most body regions, where its main function is to provide mechanical stability and generate force by means of muscle contraction. Consequently, smooth and skeletal muscle tissues often interface with epithelia along body surfaces such as the gut, bladder or breast duct. As such, smooth muscle layers, together with connective tissue, form the anatomical layers submucosa, mucosa and serosa surrounding hollow organs or organ systems. The basic units of smooth muscle tissue are smooth muscle cells, spindle-shaped mononuclear cells with a length of 20–500 μm and a diameter of 5–10 μm connected by gap junctions and organized as single or multiple layers (Fig. 1A). These muscle cells (called myocytes) are covered by a basement membrane and surrounded by endomysial ECM, i.e. loose collagen-rich fibrillar meshworks which provide both tissue cohesion and flexibility of tissue movement during contraction. The endomysium is also important for the maintenance of the tissue as it accommodates blood vessels and neurons, both covered by basement membranes. Skeletal muscles contain tube-like multinucleated skeletal muscle cells (also called muscle fibers or myofibers) that span 100–30.000 μm in length and 10–100 μm in diameter [14]. Like smooth muscle cells, individual myofibers, with nanometer-range bulging ridge-groove-like ultrastructures in the cell membrane caused by the various bands in the sarcomeres [15], are surrounded by a basement membrane and embedded in collagen-rich endomysium (Fig. 1B, C1 left). In addition, however, skeletal muscle cells are arranged into higher-order fascicles which are surrounded by perimysial ECM. Several fascicles form then the muscle, eventually covered by an epimysium [16]. To integrate with other body parts, such as bones, the three mysia are mechanically interconnected with one another near the tendon [16]. Smooth and skeletal muscle cells reach lengths of up to 10–1000 times the length of a polarized, potentially neoplastic, epithelial or mesenchymal cell that typically measures around 30–150 μm [17,18], thus muscle cells provide large linear surfaces to migrating cells. Together, smooth and striated muscle tissues contain long outer and inner multi-ECM coated surfaces and interphases of particular biochemistry, geometry and stiffness.
Figure 1: Anatomy of smooth and skeletal muscle tissue.
(A) Smooth muscle tissue consisting of smooth muscle cells connected by gap junctions, covered by a basement membrane (blue) and embedded in collagen-rich endomysium that contains lymph vessels (not shown), blood vessels and axons. (B) Skeletal muscle comprising three structural hierarchical levels: a muscle consisting of muscle bundles (fascicles) which contain muscle cells covered by a basement membrane (blue). (C) The endo-, peri-, and epimysium containing collagen fibrils, fibers, and bundles, respectively, consisting of collagen I, III (and V in the endo- and perimysium only) and basement membranes surrounding muscle cells, axons and blood vessels [16,20,21,23]. All cartoons are magnifications of the numbered regions in (A) and (B). (D) Topology of skeletal muscle in the mouse represented as longitudinal (left) and perpendicular (right) view with asterisks marking anticipated channels. Asterisks, anticipated confined mysial clefts and channels. Scale bars: 50 µm. Left image modified and reprinted with permission from The Company of Biologists; [24]. (E) Elastic moduli of bulk muscle tissue obtained by different methods, including Atomic Force Microscopy (AFM) and Shear Wave Elasticity Imaging (SWEI). Measurements of arterial tissue include besides smooth muscle tissue also other structures, such as elastic fibers in other layers of the arterial wall and endothelia [28–32].
The molecular composition of endo-, peri- and epimysial sheets is consistent with collagen-rich connective tissue deposited by fibroblasts, including collagens from type I, III and V that form linearized networks of fibrils, fibers and bundles (Fig. 1C). In addition, interacting proteoglycans and glycoproteins, mainly decorin, tenascin and fibronectin, provide molecular diversity and hydratic swelling. In general, the outer epimysium is of denser structure, while the inner peri- and endomysia are composed of loose collagen networks providing a nearly barrier-free matter for migrating cells, while the basement membranes provide ample ligands for cell adhesion. This high-order organization underlies slow, homeostatic tissue remodeling by fibroblasts releasing matrix metalloproteinases (MMPs) and depositing additional ECM components [16]. Taking all the connective tissue layers together, the ECM components comprise approximately 5% of the total muscle volume, but their abundance and exact composition differ according to mysial layer, muscle type and function, and age of the individual [16,19–21].
Smooth and striated muscles are highly anisotropic, ordered tissues with interstitial structures. Smooth muscle cells are organized in a staggered manner, creating linear, mildly curved spaces with a diameter of 40–80 nm [22] that bifurcate in front of every cell (Fig. 1A). The ordered arrangement of skeletal muscle into muscle fascicles and bundles comprises different interstitial topology and cellular content, including blood vessels and axons which align in parallel to these structures. Accordingly, alongside myofibers and fascicles, blood vessels and axons provide linear curved surfaces, which, because of the vicinity of these structures to each other, result in longitudinally confined channels with basement membranes interfacing with fibrillar collagens [16,23]. As an example, the panniculus carnosus muscle, a striated muscle within the deep dermis of many vertebrates including mice, contains 0.1–10 μm wide tube-like basement-membrane covered endomysial channels of roundish, cleft-like flat or polygonal cross-sections (Fig. 1D) [12]. Together, smooth and skeletal muscles contain basement membrane-coated confined linear channels filled with mysial loose collagen networks that present strong topographical cues for potentially invading cells [12,24,25].
Physical elastic properties of biological tissues, i.e. collagen, are reported by the elastic or Young’s modulus and are affected by their structural features such as crosslink status, geometry or porosity [26,27]. The elasticity of smooth and skeletal muscle tissues has been measured in the range of 2 to 43 kPa (Fig. 1E) [28–32], which is primarily due to the fibrillar ECM coats, but also the extensive filamentous stiff content in the contractile units [16]. The stiffness of muscle tissue ranges above the stiffness of connective tissue in dermis, breast or brain (below 3 kPa), but below the stiffness of cartilage (20 kPa), osteoid (30–40 kPa) or bone (15000 kPa) [33–36]. Interestingly, the mechanical properties of muscle tissue vary with contraction, which causes transient and reversible distortion, stiffening and relaxation of muscle cells and interconnected ECM [37]. As such, the elastic modulus of muscle cells increases more greatly upon contraction: from 10 kPa during relaxation to 200 kPa in fully contracted state [38]. Muscle stiffness increases linearly with muscle force, but differs between muscles based on the anatomic position, fascicle length and the degree of synchronization of motor unit activation [30]. These contractions occur either on a regular and slow basis in the range of seconds to minutes in smooth muscle tissue or as fast irregular voluntary twitches within milliseconds to seconds in skeletal muscle. In summary, muscle tissue comprises a hierarchically structured, mobile interstitium formed by linear interfaces with complex topological arrangements of moderate to high stiffness compared to other non-calcified tissues. It is important to gain an understanding of these permissive anatomical structures that potentially provide molecular and physical guidance to moving tumor cells, as tumor cell invasion into muscle tissues often has a negative impact on patient prognosis.
3. Cancer invasion into muscle tissue
In vivo, mysial interphases in smooth and skeletal muscle tissue guide, but also provide significant barriers to invading cancer cells. Consequently, the detection of muscle invasion in patient histology represents an independent prognostic parameter which influences clinical management of many cancer types.
3.1. Smooth muscle invasion
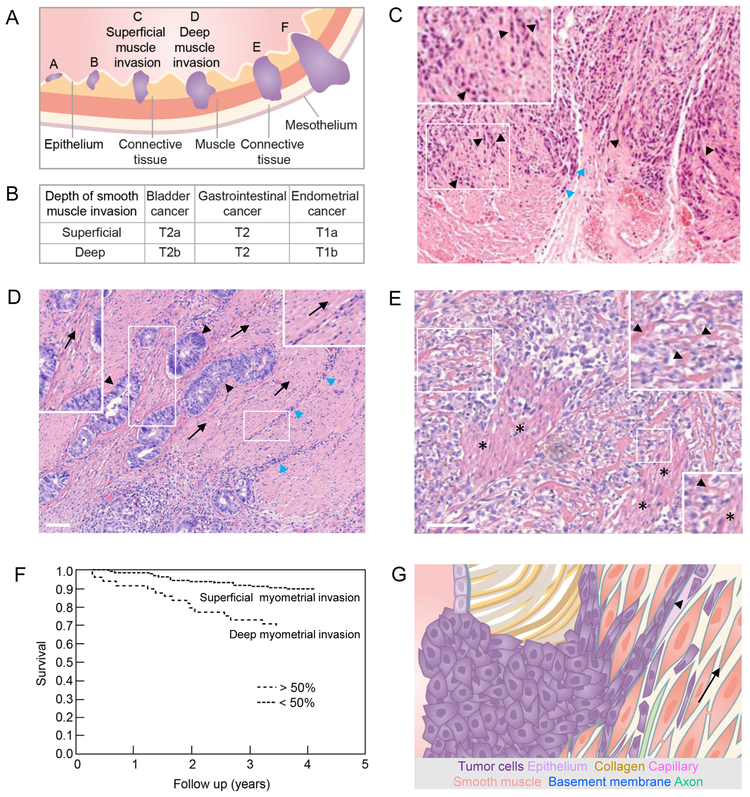
Smooth muscle invasion originates from both epithelial tumors after tumor cells cross a basement membrane or mesenchymal tumors that originate from the collagen-rich interstitium without passing a basement membrane. Tumor invasion, i.e. from bladder, stomach or uterus, occurs as a multi-step process as epithelial cancer cells first infiltrate the connective tissue which separates the epithelial and muscle layers, followed by muscle invasion (Fig. 2A, B). Tumor cells migrate either along dense outer layers of smooth muscle tissue connecting to loose collagenous tissue or infiltrate and intersperse between and along the orientation of individual muscle cells. For example, bladder tumor cells invade smooth muscle tissue in a chain-like fashion, indicative of either jammed or collective migration orienting along the linear endomysium (Fig. 2C) [39,40]. Similarly, cancer cells infiltrate the gastric wall by penetrating connective tissue mysia between smooth muscle layers where they grow and form small islands [41]. Further, colorectal cancer cells migrate in a bud-like fashion, linearly oriented along and between smooth muscle cells during early invasion, often associated with extensive immune infiltration (Fig. 2D) Whereas early steps of muscle invasion appear largely non-destructive, aggressive tumor progression leads to generalized infiltration and destruction of muscle tissue, resulting in the replacement of muscular architecture by poorly-differentiated tumor tissue and loss of function (Fig. 2E) [12,42]. Accordingly, deep infiltration of the smooth musculature by endometrial, bladder, as well as gastric cancer associates with reduced survival: when compared to relatively high three or five-year survival rates (78–95%) of patients with superficial muscle involvement, deep muscle invasion correlates with an additional reduction (15–30%) of patient survival resulting in 62–74% survival in these cancers (Fig. 2F) [43–45]. Together, neoplastic infiltration of smooth muscle is guided along ECM topologies between smooth muscle cells and is associated with reduced patient survival.
Figure 2: Overview, histopathology and impact of smooth muscle neoplastic invasion.
(A) Hollow organs such as bladder, gastrointestinal tract and uterus lined by epithelium, connective tissue and smooth muscle layers (muscularis mucosa and muscularis propria) when invaded by tumors (purple) at their onset (A,B), during superficial (C) and deep muscle invasion (D), and when ultimately reaching connective and fatty tissue of nearby organs (E,F). (B) Tumor - Lymph Node - Metastasis (TNM) staging of primary tumors from the indicated neoplasias as defined by the American Joint Committee on Cancer, with T1 defining the smallest, and T4 defining the greatest local extent of the primary tumor. The depth of muscle invasion differentiates tumors into different T-sub-stages, depending on the tumor type [98]. (C) Bladder cancer cell invasion (purple) in chain-like fashion into surrounding smooth muscle tissue (black arrowheads and inset) or along connective tissue (blue arrowheads). Image reprinted with permission from Springer Nature; [40]. (D) Colorectal cancer bud formation (black arrowheads) and immune infiltration (blue arrowheads) along the orientation (arrows; also see insets) of the smooth muscle layer of the colon. (E) Muscle destruction in the anus caused by advanced colorectal cancer disease (also see insets; arrowheads point to remnant muscle structures; asterisks indicate partly remaining muscle). (F) Deep cancer invasion of the myometrium surrounding the uterus is associated with significantly (P <0.0001) decreased overall survival and thus serves as a prognostic parameter. Two hundred and two patients with <50% myometrial invasion and 49 patients with myometrial invasion exceeding 50%. Image modified and reprinted with permission from John Wiley and Sons; [45]. (G) Schematic representation of tumor cell guidance by smooth muscle tissue. Guiding structures for migrating tumor cells (in the direction of the arrow) include endomysium and basement membranes of muscle cells, axons and capillaries as well as the lumen of capillaries (arrowhead). Colored structures are identical to their annotation in Fig. 1A and C. All scale bars, 100 µm.
3.2. Skeletal muscle invasion
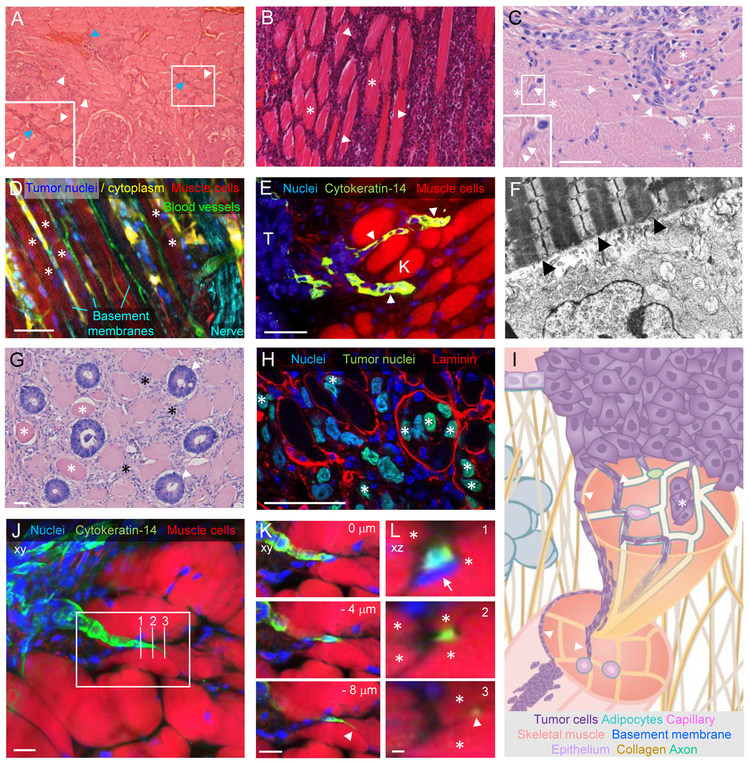
Similar to smooth muscle infiltration, progressing neoplasias from epithelial or mesenchymal origin invade skeletal muscle, whereas cardiac muscle is rarely invaded [46]. Infiltrating tumor cells predominantly align within the interstitial endo- and perimysium between and along muscle cells and fascicles in striated muscle tissue (Fig. 3A-C) [47–49]. In experimental tumor mouse models, migrating melanoma or breast cancer cells follow the outer surface of myofibers in either longitudinal or perpendicular direction in tight interaction with single skeletal muscle cells (Fig. 3D, E) [12,50]. These chain-like multicellular cancer cell files intercalating between myofibers dominate as the main collective invasion mode, likely as a consequence of space confinement [12]. A similar intimacy between adhesive tumor cell protrusions and muscle cell sarcolemma can be observed after tumor injection into the rat abdominal wall (Fig. 3F) [42]. This reflects the orientation and presumably direct interaction of moving cells with guidance structure forming basement membranes. Interestingly, besides interstitial migration along the mysial layers of muscle cells, casuistic reports indicate tumor cells infiltrating skeletal myofibers. Likely, invading colorectal cancer cells occasionally fully replace individual myofibers, while neighboring myofibers are either unaffected or dying (Fig. 3G), and intra-myofiber invasion is reproduced in an experimental mouse model where human fibrosarcoma cells have entered mouse skeletal muscle (Fig. 3H). This phenomenon was also observed in 5% of patients suffering from Ewing’s sarcoma, a rare cancer of the bone, as well as in isolated cases of breast and lung carcinoma, leukemia, meningioma and bronchial cancer [51]. To enter muscle cells, tumor cells might have penetrated through traumatized areas of the sarcolemma, or been actively phagocytosed by or fused with muscle cells, followed by a combination of tumor movement and proliferation along the actomyosin scaffold inside the myofiber.
Figure 3: Histopathology of cancer invasion into skeletal muscle tissue.
Examples of skeletal muscle invasion by different human (A,B,C,G) and experimental animal (D,E,F,H) neoplasias. (A) Thyroid cancer cell migration (purple) along the endomysium (white arrowheads) and perimysium (blue arrowheads; also see inset) of skeletal muscle (pink) while muscle integrity remains intact. Image reprinted with permission from Springer Nature; [47]. (B) Non-Hodgkin lymphoma (purple; white arrowheads) infiltrating the endomysium of skeletal muscle tissue (pink; asterisks). Image reprinted with permission from SAGE Publications; [48]. (C) Collective and single muscle invasion of thyroid cancer cells (arrowheads) guided by intact muscle cells (asterisks). See inset for an example of single cell migration between 2 muslce fibers. (D,E,F) High resolution examples of neoplastic invasion along skeletal muscle cells in animal models. (D) Fluorescently labeled murine B16F10 melanoma cells injected into the deep dermis of C57/Bl6 J mice invading into the hypodermis along the pre-existing architecture of the panniculus carnosus muscle (asterisks). Image reprinted with the permission from Landes Bioscience; [12]. (E) Cancer cells, expressing cytokeratin-14 (K) as a basal epithelial marker, migrating as cellular streams (arrowheads) between muscle cells in a transgenic spontaneous breast cancer mouse model. Nuclei on the left side of the image depict cytokeratin-14 negative non-invading tumor cells (T). Image reprinted with permission from Elsevier; [50]. (F) Rat Rd/3 tumor cells injected into rat abdominal wall invading skeletal muscle 6 days post injection. Numerous cytoplasmic processes of a tumor cell (bottom) closely appose to the muscle cell (arrowheads). Imaging by transmission electron microscopy; magnification: 12.600x. Image reprinted with permission from John Wiley and Sons; [42]. (G,H) Intra-muscle fiber invasion by neoplastic cells. (G) Apparent replacement of abdominal muscle cells by malignant glands from colorectal cancer (arrowheads), in the direct vicinity to dystrophic muscle cells (asterisks). (H) Infiltration of laminin-marked panniculus carnosus skeletal muscle fibers by fluorescently labelled human HT1080 fibrosarcoma cells (asterisks) in nude mice. (I) Principles of skeletal muscle invasion. Guidance of tumor cell migration along the endo-, peri- and epimysium and basement membranes sheathing skeletal muscle cells, axons and capillaries (arrowheads), as well as inside muscle cells (asterisk). Colored structures as indicated and identical to their annotation in Fig. 1B and C. (J-L) Guidance of breast cancer invasion by skeletal muscle cells. File-like strand invasion of cytokeratin-14 positive breast cancer cells between skeletal muscle fibers in a transgenic spontaneous breast cancer mouse model (as described in [50]; image set provided by K. Cheung and A. Ewald). (J) Z-projection of whole tumor invasion field into muscle, with rectangle depicting zone for zoom in (K) and three vertical lines for xz projections in (L). (K) A single strand with a highly protrusive leader cell (arrowhead) migrates between individual muscle cells; single cropped z-sections with indicated distances between each other. (L) Orthogonal xz views of breast cancer cell migration (green) being progressively guided by individual muscle cells (asterisks), even on the level of the protrusion in section 3 (white arrowhead). Also note strongly deformed nuclear cross section in section 1 (arrow). Scale bars, 50 µm (C,D,E,G,H); 10 µm (J,K,L); 1 µm (L).
Neoplastic invasion of skeletal muscle is, like smooth muscle invasion, associated with worsened cancer prognosis. In one example only, breast cancer invasion of the pectoralis major, the muscle nearest to the breast, had no prognostic value for overall outcome [52]. In contrast, squamous cell carcinoma (SCC) invasion of the extrinsic skeletal muscle of the tongue, as compared to SCC invasion not involving muscle, was significantly associated with 50% increased local recurrence, 93% increased risk of distant metastases and 55% lower disease free survival after 96 months, without significant differences in the incidence of lymphatic invasion [53]. In addition, intramyofiber invasion in Ewing’s sarcoma was associated with increased incidence of distant metastases and a 45% decrease in 4-year survival as compared to patients without intramyofiber invasion. Although the provided evidence does not directly link invasion to metastatic progression, skeletal muscle invasion hinders body function, imposes a significant surgical challenge and compromises quality of life after radical resection. Together, the topological cancer invasion patterns into smooth and skeletal muscle are strongly indicative of guidance by molecular and physical interphases along myocytes, as well as along vessels and nerves (Fig. 2G, 3I). If followed by excessive proliferation and proteolysis, such invasion zones could gradually destroy the tissue and cause functional deficits, reducing quality of life and survival.
4. Guidance principles of cancer cell migration in complex environments for cancer progression and resistance
Little work has been performed to address tissue-specific mechanisms that underlie cancer invasion into muscle tissue as the muscular 3D cellular and ECM organization are not readily recapitulated by in vitro culture. Similarly, in vivo analysis by intravital microscopy is hampered by the mobility of muscle tissue in model organisms, rendering time-resolved observations of cell functions challenging. Therefore, principles of tumor cell-muscle interaction have been deduced so far from fixed histological analyses instead. To bridge this gap, we here review and extrapolate recent progress in live tissue microscopy in the mouse with emerging molecular and biophysical concepts of guided cell migration and their implications for neoplastic invasion into musculature. Central mechanisms include adaptive adhesion systems, geometrical guidance cues and constraints, and mechanosensing of tissue stiffness and alignment in dynamic environments. These parameters jointly define type and efficacy of cell migration and likely apply to cancer invasion and survival in muscle-like environments.
4.1. Integrin-dependent and independent mechanisms of cell migration
Basement membranes and interstitial ECM components in mysia are rich in adhesion ligands that can be recognized by tumor cells through adhesion receptors. Integrins are important transmembrane adhesion receptors that connect these ECM compartments with the actin cytoskeleton to transmit force and activate intracellular signaling cascades during cell migration [54]. Integrin-mediated signaling protects cells from programmed cell death caused by the detachment from ECM (anoikis) and confers therapeutic resistance via enhanced survival [55,56]. A biphasic relationship between adhesion and cell migration speed exists with intermediate adhesion levels mediating most efficient locomotion [57]. However, in contrast to other tissues such as brain or breast tissue [58], the critical adhesive mechanisms for muscle tissue invasion remain unclear. It is expected that laminin and collagen IV in basement membrane, due to their guiding functions, as well as the abundantly present interstitial fibrillar collagens I, III and V serve as major ligands for commonly expressed adhesion receptors on invading tumor cells, such as α1β1, α2β1 and α11β1 integrins [54]. In mechanically active muscle tissue, cryptic tension-sensitive sites in the FNIII-1 domain of fibronectin could be transiently opened upon muscle contraction and serve as adhesion sites, promoting cell spreading, contractility and migration [59]. In addition, cell forces trigger the release of ECM-sequestered cytokine TGF-β, a promoter of cell spreading, contractility and migration [4]. Additional adhesion systems in muscle guided migration could include syndecan binding to glycosaminoglycans and fibrillar collagens as main ligands for discoidin domain receptors [60].
While integrin and actomyosin-mediated mechanotransduction is critical to cell migration across 2D surfaces and in 3D matrices, alternative mechanisms for force generation and transmission have been identified for migration in confining linear channels. These mechanisms include microtubule dynamics, friction generated by an actin flow-mediated pressure gradient against the channel walls, and/or polarized water and ion permeation [61–63]. Current concepts consider that under such channel-like conditions, rather than adhesion-mediated pulling on the substrate, low levels of force may be sufficient to push the cell body forward [64]. Consequently, tumor cells lacking the mysial ECM-specific integrin sets may still be able to locomote between myocytes by friction-based motility. In addition, inhibitors targeting the invasion process by inhibiting MMPs, integrins and Rho GTPases might not suffice to inhibit cancer cell invasion in muscle tissue, not unlike migration in capillaries or compliant 3D collagen tracks [27,61,65]. Whether microtubule inhibition is particularly effective in halting muscle invasion needs to be tested, e.g. by using taxol-based chemotherapy, and whether adhesive or friction-based migration mechanisms predominate in myofiber invasion is currently unknown.
4.2. Confinement induced cancer cell migration and progression
Different ECM dimensionalities, including 1D fibers, 2D surfaces or 3D tube-like channels, are present in all basic tissues throughout the body. Migrating cells sense and interpret these principal geometries and tend to follow tissue topologies by mechanical alignment or adhesive contact, termed contact guidance or haptokinesis [66,67]. Accordingly, invading tumor cells may orient themselves along muscle cells in animal tumor models (Fig. 3D,F,J) [12,42] and likely in cancer patients (Fig. 2C) [40,47], but the resolution of standard pathological histology is too low to ultimately confirm this. If low-resistance gaps or clefts between linear topographies (such as present between muscle cells) are below the cross-section of a migrating cell, yet spacious enough to accommodate the cell body [68], they facilitate guided but confined tumor cell migration by cell shape elongation initiated by a long leading protrusion (Fig. 3J-L). Accordingly, confinement-like elongation of the cell body during migration on thin 1D lines causes increased membrane and cortical tension, which activates Piezo1, a calcium-sensitive channel, and leads to changes in cellular state [69]. In addition, tumor cells have been shown to widen the linear spaces between muscle cells by a factor of about two [12]. Thus, the ECM-rich mysia between neighboring muscle cells or fascicles contain somewhat compliant clefts of flat or tube-like non-confining and confining geometry that present strong topographical cues to migrating cells.
Confinement, as provided by mysial clefts, may mechanically alter the homeostasis of the nucleus by deforming it past critical limits. Cell compression by extracellular confinement may perturb mitotic rounding and correct spindle positioning and thereby induce chromosomal missegregation and abnormal cell divisions [70]. While strong confinement may arrest mitotic progression and increase cell death, mild confinement can accelerate mitosis and induce mitotic abnormalities [70,71]. In addition, cell migration in highly confined spaces in vitro as well as in mouse dermis causes strong nuclear deformation and defects in nuclear envelope integrity [72]. Such nuclear deformation causes DNA damage and repair and repeated mechanical perturbation of the nucleus even induces genetic heterogeneity [72–74]. In addition, stiffness itself could possibly induce genetic aberrations as the number of large genomic changes and mutations in cancers scale with the stiffness of the tissue of origin [75]. Together, because muscle tissue contains confined spacing, is relatively stiff and undergoes frequent mechanical deformation, the consequences for mechanical integrity of cancer cells and their nuclei when invading the musculature remain to be identified.
4.3. Cancer cell mechanosensing of muscle-like mechanical properties
Mysial topologies, stiffness, and mechanosensing of muscle-intrinsic ECM types very likely impact how tumor cells invade. Cell contact with a non-confining in vitro substrate of muscle-like intermediate stiffness of 10 kPa is suited to support maximally efficient migration of glioma cells, consistent with intermediate adhesion and optimal traction force [76]. With higher stiffness, migration speed decreases due to adhesion strengthening and immobilization, whereas low substrate stiffness dampens migration speed due to weak traction and cell slipping [76]. This biphasic relationship between substrate rigidity and migration speed is readjusted when cells move in confinement, resulting in a near-linear increase of speed with increasing substrate stiffness, which indicates that the 3D geometry modulates both stiffness sensing and cell responses to stiffness [76]. In addition, stiffness values similar to relaxed muscle tissue induce, besides efficient migration, very efficient cell cycle progression and malignant progression by reducing levels of tumor suppressor phosphatase and tensin homolog (PTEN) and enhancing (extracellular signal–regulated kinase) ERK signaling activation [31,77,78]. Further, substrates stiffer than 5–10 kPa activate cellular mechanosensory pathways, including the relocalization of nuclear co-activators from the cytoplasm to the nucleus, including yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) [79,80]. Nuclear relocalization of YAP/TAZ on stiff substrate occurs through stretched and opened nuclear pores in adhering cells [81] and regulates transcription of genes that mediate cell growth, survival and resistance to apoptosis and anoikis [80,82]. High or deregulated YAP and TAZ activity in tumor cells is correlated with tumor progression and poor prognosis of various cancer types, including rhabdomyosarcoma and, in part, associated with resistance to chemo- and radiotherapy or targeted therapies such as epidermal growth factor receptor or mutated B-raf protein inhibitors [82,83]. Resistance to therapy is conferred by multiple molecular mechanisms including YAP/TAZ mediated activation of growth factor signaling, cell cycle progression, suppression of apoptosis, as well as altered DNA damage response followed by genomic instability [82]. Of note, stiffness sensing includes a “memory” function as cells, after priming on a stiff substrate and relocation onto soft substrate, retain nuclear YAP for days, implying that stiffness signals can persist mid- to long-term [84,85]. Taken together, mechanosignaling at intermediate muscle stiffness levels promotes tumor infiltration and proliferation in muscle, thereby assisting resistance to therapy.
Whereas these cell responses occur under tonic, i.e. stable biophysical regimes, tumor cells that invade into highly dynamic and repeatedly contracting muscle tissue are unavoidably confronted with altering mechanical stresses. Muscle contraction cycles increase tissue stiffness and may modulate the width of pre-existing tissue confinements in an oscillatory manner. Under physiological conditions, cyclic stretch-relaxation cycles of fibroblasts interacting with 2D deformable substrate cause increased migration speed [86], whereas wound closure by epithelial cell sheets, upon similar cyclic stress, is decreased [87]. How physiological contractility and stiffness changes of muscle tissue alter the biology of tumor cells, is unknown. Nevertheless, multi-parametric positive effects on cell invasiveness reinforced by muscle contraction cycles can be inferred based on known mechanocoupling and stiffening responses. However, it is expected that contraction cycles are reduced upon gradual muscle destruction during cancer progression. While the cellular and functional consequences of progressive muscle tissue infiltration on muscle function have not been studied systematically, they are expected to depend on the type muscle invaded, cancer type and tumor aggressiveness. Taken together, studies of tumor cell behaviour in defined muscle-like environments suggest that muscle tissues provide molecular and mechanical properties that may affect the mechanoresponse of invading tumor cells, leading to worsened neoplastic behaviors including growth, survival, migration, muscle destruction, and therapeutic resistance.
5. Conclusions
The above discussed considerations show that muscle invasion by cancer cells follows tissue-specific principles of guided migration along tissue topologies, which are provided by the unique microanatomy of smooth and striated muscle tissue. Mysia are part of fluid-filled interstitial spaces that drain fluid and could allow for facilitated non-destructive tumor cell migration [12,88]. In addition, oscillating stiffness and topological deformation upon muscle contraction induce kinetic mechanosensing and tumor cell reprogramming. Consequently, initial invasion takes place that may proceed non-destructively along basement membranes and vascular structures, followed over time, likely weeks to months, by destructive growth and remodeling of the musculature [12,42]. Similar to cell migration in 3D collagen matrices and microfluidic capillaries, guided cancer cell migration along mysia may be adaptive, depending on the varying dynamics, geometry, stiffness and ligand availability of the invaded subcompartment. Consequently, tumor cells constitutively undergoing mechanical challenge through muscular contractions may become more resistant to therapies due to the induction of mechanosensitive pathways, posing a number of challenges to tumor specific targeting. In addition, the mechano-challenging musculature may select for mechanoresistant cells, which have developed mechanical fitness to cope better with stressors encountered during metastatic spread. In general, the effects of muscle-specific parameters on cell responses have mostly been tested on healthy cells and the relevance on invasive and metastatic tumor cells still remains largely illusive. Therefore, key questions include whether specific cancer clones with certain genetic alterations more readily invade muscle tissue, whether infiltration into the musculature supports the capacity of tumor cells to metastasize or develop therapy resistance and how mechanical processes are involved therein.
To characterize the cellular, molecular and mechanical mechanisms of tumor infiltration into muscle and its consequence for cancer progression, improved organotypic muscle-specific in vitro and in vivo models need to be developed and applied. Valuable in vitro assays, such as multi-component live-cell culture (“organ-on-a-chip”) [89], tensile strain models [90] and 3D culture of differentiated myogenic cells isolated from native tissue [91,92], mimic defined aspects of muscle geometry and stiffness dynamics. In addition, readily available 2D or 2.5D nanodevices resemble ligand availability similar in muscle tissue, muscle-like stiffness or surface topography, while 3D synthetic or ECM-based microchannels offer mysium-like elongated channel topologies [62,93–95]. Further, considering the clinical relevance, particularly non-vascular smooth muscle culture, i.e. from murine induced pluripotent stem cells [96], will be required to address muscle-induced mechanisms of tumor cell reprogramming and worsened prognosis. However, migration characteristics in all models, such as cell deformability, elongation and migration capacity, remain to be correlated to and validated in tumor cells moving in muscle tissue in vivo. Strategies for intravital imaging of the skeletal dermal panniculus carnosus and smooth muscle layers of the bladder in mice are available and may allow analysis of cancer cell invasion [12,97]. Similar imaging strategies may be applicable for probing tumors invading major skeletal muscle groups such as the abdominal muscle. As a complimentary approach, mathematical modeling could decipher reciprocal processes between cancer cells, muscle cells and other cell types and unravel the specific contribution of muscle characteristics enabling migration. In combination, engineered and cell-based in vitro models, intravital microscopy and in silico modeling will allow to delineate the biology of tumor invasion into muscle and the efficacy of therapeutically targeting the process of neoplastic muscular infiltration.
Basement membrane coated muscle cells are embedded within linear ECM-rich mysia
Tumor cell migration is guided by linear confinement present in muscle tissue
Experimental tumor cell invasion occurs along linear ECM-coated mysial structures
Biophysical properties of muscle tissue favor tumor cell invasion and survival
Neoplastic muscle invasion is often associated with negative patient prognosis
Acknowledgements
We gratefully acknowledge C.E.E.M. Van der Zee for critical reading of the manuscript and A. Ewald, K. Cheung, B. Moore and M. Pruszko for providing fluorescence imaging on intramuscular cancer invasion, as well as the Microscopical Imaging Centre for the use of confocal microscopy.
Funding
LB is supported by a RIMLS Junior Researcher grant 2015 from the Radboud Institute of Molecular Life Sciences; PF by the European Research Council [grant number 617430-DEEPINSIGHT], NWO-Vici [grant number 918.11.626], Horizon 2020 consortium MULTIMOT [grant number 634107–2), the Cancer Genomics Center, NIH-U54 CA210184–01 and the MD Anderson Cancer Center Moon Shot program; and KW by the a grant from the Dutch Cancer Foundation KWF [grant number 11199].
Abbreviations:
- 1D
one-dimensional
- 2D
two-dimensional
- 3D
three-dimensional
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- TAZ
transcriptional coactivator with PDZ-binding motif
- YAP
yes-associated protein
Footnotes
The authors declare no competing interests.
Contributor Information
Lianne Beunk, Email: lianne.beunk@radboudumc.nl.
Kari Brown, Email: k.n.brown@student.vu.nl.
Iris Nagtegaal, Email: iris.nagtegaal@radboudumc.nl.
Peter Friedl, Email: peter.friedl@radboudumc.nl.
Katarina Wolf, Email: katarina.wolf@radboudumc.nl.
References
- [1].Gupta GP, Massagué J, Cancer metastasis: Building a framework, Cell. 127 (2006) 679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- [2].Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR, Cell migration: Integrating signals from front to back, Science (80-. ). 302 (2003) 1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- [3].Friedl P, Alexander S, Cancer invasion and the microenvironment: Plasticity and reciprocity, Cell. 147 (2011) 992–1009. doi: 10.1016/j.cell.2011.11.016. [DOI] [PubMed] [Google Scholar]
- [4].van Helvert S, Storm C, Friedl P, Mechanoreciprocity in cell migration, Nat. Cell Biol. 20 (2018) 8–20. doi: 10.1038/s41556-017-0012-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Charras G, Sahai E, Physical influences of the extracellular environment on cell migration, Nat. Rev. Mol. Cell Biol 15 (2014) 813–824. doi: 10.1038/nrm3897. [DOI] [PubMed] [Google Scholar]
- [6].te Boekhorst V, Preziosi L, Friedl P, Plasticity of cell migration in vivo and in silico, Annu. Rev. Cell Dev. Biol 32 (2016) 491–526. doi: 10.1146/annurev-cellbio-111315-125201. [DOI] [PubMed] [Google Scholar]
- [7].Haeger A, Krause M, Wolf K, Friedl P, Cell jamming: Collective invasion of mesenchymal tumor cells imposed by tissue confinement, Biochim. Biophys. Acta - Gen. Subj 1840 (2014) 2386–2395. doi: 10.1016/j.bbagen.2014.03.020. [DOI] [PubMed] [Google Scholar]
- [8].Bravo-Cordero JJ, Hodgson L, Condeelis J, Directed cell invasion and migration during metastasis, Curr. Opin. Cell Biol. 24 (2012) 277–283. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wolf K, Alexander S, Schacht V, Coussens LM, von Andrian UH, van Rheenen J, Deryugina E, Friedl P, Collagen-based cell migration models in vitro and in vivo, Semin. Cell Dev. Biol. 20 (2009) 931–941. doi: 10.1016/j.semcdb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM, Matrix crosslinking forces tumor progression by enhancing integrin signaling, Cell. 139 (2009) 891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Provenzano PP, Inman DR, Eliceiri KW, Keely PJ, Matrix density-induced mechanoregulation of breast cell phenotype, signaling and gene expression through a FAK–ERK linkage, Oncogene. 28 (2009) 4326. doi: 10.1038/onc.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Weigelin B, Bakker G-J, Friedl P, Intravital third harmonic generation microscopy of collective melanoma cell invasion, IntraVital. 1 (2012) 32–43. doi: 10.4161/intv.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Surov A, Hainz M, Holzhausen H-J, Arnold D, Katzer M, Schmidt J, Spielmann RP, Behrmann C, Skeletal muscle metastases: Primary tumours, prevalence, and radiological features, Eur. Radiol. 20 (2010) 649–658. doi: 10.1007/s00330-009-1577-1. [DOI] [PubMed] [Google Scholar]
- [14].Frontera WR, Ochala J, Skeletal muscle: A brief review of structure and function, Calcif. Tissue Int. 96 (2015) 183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- [15].Zhu J, Sabharwal T, Kalyanasundaram A, Guo L, Wang G, Topographic mapping and compression elasticity analysis of skinned cardiac muscle fibers in vitro with atomic force microscopy and nanoindentation, J. Biomech 42 (2009) 2143–2150. doi: 10.1016/j.jbiomech.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gillies AR, Lieber RL, Structure and function of the skeletal muscle extracellular matrix., Muscle Nerve. 44 (2011) 318–331. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doyle AD, Wang FW, Matsumoto K, Yamada KM, One-dimensional topography underlies three-dimensional fibrillar cell migration, J. Cell Biol. 184 (2009) 481–490. doi: 10.1083/jcb.200810041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maiuri P, Rupprecht J-F, Wieser S, Ruprecht V, Bénichou O, Carpi N, Coppey M, De Beco S, Gov N, Heisenberg C-P, Lage Crespo C, Lautenschlaeger F, Le Berre M, Lennon-Dumenil A-M, Raab M, Thiam H-R, Piel M, Sixt M, Voituriez R, Actin flows mediate a universal coupling between cell speed and cell persistence, Cell. 161 (2015) 374–386. doi: 10.1016/j.cell.2015.01.056. [DOI] [PubMed] [Google Scholar]
- [19].Calvi ENC, Nahas FX, V Barbosa M, Calil JA, Ihara SSM, Juliano Y, Ferreira LM, Collagen fibers in the rectus abdominis muscle of cadavers of different age, Hernia. 18 (2014) 527–533. doi: 10.1007/s10029-014-1213-0. [DOI] [PubMed] [Google Scholar]
- [20].Duance VC, Restall DJ, Beard H, Bourne FJ, Bailey AJ, The location of three collagen types in skeletal muscle, FEBS Lett. 79 (1977) 248–252. doi: 10.1016/0014-5793(77)80797-7. [DOI] [PubMed] [Google Scholar]
- [21].Light N, Champion AE, Characterization of muscle epimysium, perimysium and endomysium collagens, Biochem. J. 219 (1984) 1017–1026. doi: 10.1042/bj2191017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Skandalakis JE, Gray’s anatomy e-dition: The anatomical basis of clinical practice, 2005. [Google Scholar]
- [23].Sanes JR, The basement membrane / basal lamina of skeletal muscle, J. Biol. Chem. 278 (2003) 12601–12604. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- [24].Weigelin B, Bakker G, Friedl P, Third harmonic generation microscopy of cells and tissue organization, J. Cell Sci. 129 (2016) 245–255. doi: 10.1242/jcs.152272. [DOI] [PubMed] [Google Scholar]
- [25].Wolf K, Friedl P, Extracellular matrix determinants of proteolytic and non-proteolytic cell migration, Trends Cell Biol. 21 (2011) 736–744. doi: 10.1016/j.tcb.2011.09.006. [DOI] [PubMed] [Google Scholar]
- [26].Yang Y, Leone LM, Kaufman LJ, Elastic moduli of collagen gels can be predicted from two-dimensional confocal microscopy, Biophys. J. 97 (2009) 2051–2060. doi: 10.1016/j.bpj.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wolf K, te Lindert M, Krause M, Alexander S, te Riet J, Willis AL, Hoffman RM, Figdor CG, Weiss SJ, Friedl P, Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force, J. Cell Biol. 201 (2013) 1069–1084. doi: 10.1083/jcb.201210152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Engler AJ, Griffin MA, Sen S, Bönnemann CG, Sweeney HL, Discher DE, Myotubes differentiate optimally on substrates with tissue-like stiffness, J. Cell Biol. 166 (2004) 877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM, Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture, Science (80-. ). 329 (2010) 1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Creze M, Nordez A, Soubeyrand M, Rocher L, Maître X, Bellin M-F, Shear wave sonoelastography of skeletal muscle: Basic principles, biomechanical concepts, clinical applications, and future perspectives, Skeletal Radiol. 47 (2018) 457–471. doi: 10.1007/s00256-017-2843-y. [DOI] [PubMed] [Google Scholar]
- [31].Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK, Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening, Curr. Biol. 19 (2009) 1511–1518. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Khamdaeng T, Luo J, Vappou J, Terdtoon P, Konofagou EE, Arterial stiffness identification of the human carotid artery using the stress–strain relationship in vivo, Ultrasonics. 52 (2012) 402–411. doi: 10.1016/j.ultras.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Butcher DT, Alliston T, Weaver VM, A tense situation: Forcing tumour progression, Nat. Rev. Cancer. 9 (2009) 108–122. doi: 10.1038/nrc2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Buxboim A, Ivanovska IL, Discher DE, Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells “feel” outside and in?, J. Cell Sci 123 (2010) 297–308. doi: 10.1242/jcs.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Janmey PA, Miller RT, Mechanisms of mechanical signaling in development and disease, J. Cell Sci. 124 (2011) 9–18. doi: 10.1242/jcs.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rho JY, Ashman RB, Turner CH, Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements, J. Biomech. 26 (1993) 111–119. doi: 10.1016/0021-9290(93)90042-D. [DOI] [PubMed] [Google Scholar]
- [37].Storm C, Pastore JJ, MacKintosh F, Lubensky T, Janmey PA, Nonlinear elasticity in biological gels, Nature. 435 (2005) 191–194. doi: 10.1038/nature03497.1. [DOI] [PubMed] [Google Scholar]
- [38].Lange JR, Fabry B, Cell and tissue mechanics in cell migration, Exp. Cell Res. 319 (2013) 2418–2423. doi: 10.1016/j.yexcr.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Imanishi K, Sutoh Yoneyama M, Hatakeyama S, Yamamoto H, Koie T, Saitoh H, Yamaya K, Funyu T, Nakamura T, Chikara O, Tsuboi S, Invadopodia are essential in transurothelial invasion during the muscle invasion of bladder cancer cells, Mol. Med. Rep. 9 (2014) 2159–2165. doi: 10.3892/mmr.2014.2113. [DOI] [PubMed] [Google Scholar]
- [40].Cheng L, Montironi R, Davidson DD, Lopez-Beltran A , Staging and reporting of urothelial carcinoma of the urinary bladder, Mod. Pathol 22 (2009) S70–S95. doi: 10.1038/modpathol.2009.1. [DOI] [PubMed] [Google Scholar]
- [41].Curran RC, Colour atlas of histopathology, Baillière, Tindall & Cassell, London, 1966. [Google Scholar]
- [42].Carr I, McGinty F, Norris P, The fine structure of neoplastic invasion: Invasion of liver, skeletal muscle and lymphatic vessels by the Rd/3 tumour, J. Pathol. 118 (1976) 91–99. doi: 10.1002/path.1711180205. [DOI] [PubMed] [Google Scholar]
- [43].Tilki D, Reich O, Karakiewicz PI, Novara G, Kassouf W, Ergün S, Fradet Y, Ficarra V, Sonpavde G, Stief CG, Skinner E, Svatek RS, Lotan Y, Sagalowsky AI, Shariat SF, Validation of the AJCC TNM substaging of pT2 bladder cancer: Deep muscle invasion is associated with significantly worse outcome, Eur. Urol 58 (2010) 112–117. doi: 10.1016/j.eururo.2010.01.015. [DOI] [PubMed] [Google Scholar]
- [44].Zhang WH, He D, Chen DN, Li TT, Chen XZ, Yang K, Liu K, Zhang B, Chen ZX, Zhou ZG, Hu JK, Comparison between superficial muscularis propria and deep muscularis propria infiltration in gastric cancer patients: A retrospective cohort study, Medicine (Baltimore). 95 (2016) e4165. doi: 10.1097/MD.0000000000004165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lindahl B, Ranstam J, Willén R, Five year survival rate in endometrial carcinoma stages I‐II: Influence of degree of tumour differentiation, age, myometrial invasion and DNA content, BJOG An Int. J. Obstet. Gynaecol 101 (1994) 621–625. doi: 10.1111/j.1471-0528.1994.tb13654.x. [DOI] [PubMed] [Google Scholar]
- [46].Reynen K, Frequency of primary tumors of the heart, Am. J. Cardiol 77 (1996) 107. doi: 10.1016/S0002-9149(97)89149-7. [DOI] [PubMed] [Google Scholar]
- [47].Khan ZA, Mehta S, Sumathi N, Dhiwakar M, Occult invasion of sternothyroid muscle by differentiated thyroid cancer, Eur. Arch. Oto-Rhino-Laryngology 275 (2018) 233–238. doi: 10.1007/s00405-017-4822-2. [DOI] [PubMed] [Google Scholar]
- [48].Surov A, Holzhausen H-J, Arnold D, Schmidt J, Spielmann R-P, Behrmann C, Intramuscular manifestation of non-hodgkin lymphoma and myeloma: Prevalence, clinical signs, and computed tomography features, Acta Radiol. 51 (2010) 47–51. doi: 10.3109/02841850903296678. [DOI] [PubMed] [Google Scholar]
- [49].Chandler K, Vance C, Budnick S, Muller S, Muscle invasion in oral tongue squamous cell carcinoma as a predictor of nodal status and local recurrence: Just as effective as depth of invasion?, Head Neck Pathol. 5 (2011) 359–363. doi: 10.1007/s12105-011-0296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cheung KJ, Gabrielson E, Werb Z, Ewald AJ, Collective invasion in breast cancer requires a conserved basal epithelial program, Cell. 155 (2013) 1639–1651. doi: 10.1016/j.cell.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stratton B, Askin FB, Kissane JM, Intramyofiber skeletal muscle invasion in Ewing’s sarcoma of bone: Clinicopathologic observations from the intergroup Ewing’s sarcoma study, Am. J. Pediatr. Hematol. Oncol. 4 (1982) 231–235. http://ovidsp.tx.ovid.com.ru.idm.oclc.org/sp-3.28.0a/ovidweb.cgi?WebLinkFrameset=1&S=FCGFFPIEHPDDEFJJNCFKNEMCGOIHAA00&returnUrl=ovidweb.cgi%3FMain%2BSearch%2BPage%3D1%26S%3DFCGFFPIEHPDDEFJJNCFKNEMCGOIHAA00&directlink=http%3A%2F%2Fovidsp.tx.ovid.com%2Fovft. [PubMed] [Google Scholar]
- [52].Fracchia AA, Evans JF, Eisenberg BL, Stage III carcinoma of the breast a detailed analysis, Ann. Surg. 192 (1980) 705–710. https://journals.lww.com/annalsofsurgery/Fulltext/1980/12000/Stage_III_Carcinoma_of_the_Breast_A_Detailed.2.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Liao C-T, Lee L-Y, Hsueh C, Lin C-Y, Fan K-H, Wang H-M, Hsieh C-H, Ng S-H, Lin C-H, Tsao C-K, Kang C-J, Fang T-J, Huang S-F, Chang K-P, Yang LY, Yen T-C, Clinical outcomes in pT4 tongue carcinoma are worse than in pT3 disease: How extrinsic muscle invasion should be considered?, Ann. Surg. Oncol. 24 (2017) 2570–2579. doi: 10.1245/s10434-017-5906-3. [DOI] [PubMed] [Google Scholar]
- [54].Humphries JD, Integrin ligands at a glance, J. Cell Sci. 119 (2006) 3901–3903. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Alexander S, Friedl P, Cancer invasion and resistance: Interconnected processes of disease progression and therapy failure, Trends Mol. Med. 18 (2012) 13–26. doi: 10.1016/j.molmed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- [56].Paoli P, Giannoni E, Chiarugi P, Anoikis molecular pathways and its role in cancer progression, Biochim. Biophys. Acta - Mol. Cell Res 1833 (2013) 3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- [57].Lauffenburger DA, Horwitz AF, Cell migration: A physically integrated molecular process, Cell. 84 (1996) 359–369. doi: 10.1016/S0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- [58].Gritsenko PG, Ilina O, Friedl P, Interstitial guidance of cancer invasion, J. Pathol. 226 (2012) 185–199. doi: 10.1002/path.3031. [DOI] [PubMed] [Google Scholar]
- [59].Hocking DC, Titus PA, Sumagin R, Sarelius IH, Extracellular matrix fibronectin mechanically couples skeletal muscle contraction with local vasodilation, Circ. Res. 102 (2008) 372–379. doi: 10.1161/CIRCRESAHA.107.158501. [DOI] [PubMed] [Google Scholar]
- [60].Schmidt S, Friedl P, Interstitial cell migration: Integrin-dependent and alternative adhesion mechanisms, Cell Tissue Res. 339 (2009) 83. doi: 10.1007/s00441-009-0892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Balzer EM, Tong Z, Paul CD, Hung W-C, Stroka KM, Boggs AE, Martin SS, Konstantopoulos K, Physical confinement alters tumor cell adhesion and migration phenotypes, FASEB J. 26 (2012) 4045–4056. doi: 10.1096/fj.12-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Carey SP, Rahman A, Kraning-Rush CM, Romero B, Somasegar S, Torre OM, Williams RM, Reinhart-King CA, Comparative mechanisms of cancer cell migration through 3D matrix and physiological microtracks, Am. J. Physiol. Physiol 308 (2015) C436–C447. doi: 10.1152/ajpcell.00225.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Stroka KM, Jiang H, Chen S-H, Tong Z, Wirtz D, Sun SX, Konstantopoulos K, Water permeation drives tumor cell migration in confined microenvironments, Cell. 157 (2014) 611–623. doi: 10.1016/j.cell.2014.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Hawkins RJ, Piel M, Faure-Andre G, Lennon-Dumenil AM, Joanny JF, Prost J, Voituriez R, Pushing off the walls: A mechanism of cell motility in confinement, Phys. Rev. Lett. 102 (2009) 58103–5816. doi: 10.1103/PhysRevLett.102.058103. [DOI] [PubMed] [Google Scholar]
- [65].Ilina O, Bakker G-J, Vasaturo A, Hoffman RM, Friedl P, Two-photon laser-generated microtracks in 3D collagen lattices: Principles of MMP-dependent and -independent collective cancer cell invasion, Phys. Biol 8 (2011) 15010. doi: 10.1088/1478-3975/8/1/015010. [DOI] [PubMed] [Google Scholar]
- [66].Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF, Effects of synthetic micro- and nano-structured surfaces on cell behavior, Biomaterials. 20 (1999) 573–588. doi: 10.1016/S0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- [67].Haeger A, Wolf K, Zegers MM, Friedl P, Collective cell migration: Guidance principles and hierarchies, Trends Cell Biol. 25 (2015) 556–566. doi: 10.1016/j.tcb.2015.06.003. [DOI] [PubMed] [Google Scholar]
- [68].Krause M, Wolf K, Cancer cell migration in 3D tissue: Negotiating space by proteolysis and nuclear deformability, Cell Adh. Migr 9 (2015) 357–366. doi: 10.1080/19336918.2015.1061173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hung W-C, Yang JR, Yankaskas CL, Wong BS, Wu P-H, Pardo-Pastor C, Serra SA, Chiang M-J, Gu Z, Wirtz D, Valverde MA, Yang JT, Zhang J, Konstantopoulos K, Confinement sensing and signal optimization via Piezo1/PKA and Myosin II pathways, Cell Rep. 15 (2016) 1430–1441. doi: 10.1016/j.celrep.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Tse HTK, Weaver WM, Di Carlo D, Increased asymmetric and multi-daughter cell division in mechanically confined microenvironments, PLoS One. 7 (2012) e38986. doi: 10.1371/journal.pone.0038986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Cattin CJ, Düggelin M, Martinez-Martin D, Gerber C, Müller DJ, Stewart MP, Mechanical control of mitotic progression in single animal cells, Proc. Natl. Acad. Sci 112 (2015) 11258–11263. doi: 10.1073/pnas.1502029112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Denais CM, Gilbert RM, Isermann P, Mcgregor AL, te Lindert M, Weigelin B, Davidson PM, Friedl P, Wolf K, Lammerding J, Nuclear envelope rupture and repair during cancer cell migration, Science (80-. ). 352 (2016) 353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Irianto J, Xia Y, Pfeifer CR, Athirasala A, Ji J, Alvey C, Tewari M, Bennett RR, Harding SM, Liu AJ, Greenberg RA, Discher DE, DNA damage follows repair factor depletion and portends genome variation in cancer cells after pore migration, Curr. Biol. 27 (2017) 210–223. doi: 10.1016/j.cub.2016.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Raab M, Gentili M, de Belly H, Thiam H-R, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Duménil A-M, Manel N, Piel M, ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death, Science (80-. ). 352 (2016) 359–362. doi: 10.1126/science.aad7611. [DOI] [PubMed] [Google Scholar]
- [75].Irianto J, Pfeifer CR, Xia Y, Discher DE, SnapShot: Mechanosensing matrix, Cell. 165 (2016) 1820–1820. doi: 10.1016/j.cell.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Pathak A, Kumar S, Independent regulation of tumor cell migration by matrix stiffness and confinement, Proc. Natl. Acad. Sci. 109 (2012) 10334–10339. doi: 10.1073/pnas.1118073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Mouw JK, Yui Y, Damiano L, Bainer RO, Lakins JN, Acerbi I, Ou G, Wijekoon AC, Levental KR, Gilbert PM, Hwang ES, Chen Y-Y, Weaver VM, Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression, Nat. Med. 20 (2014) 360–367. doi: 10.1038/nm.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM, Tensional homeostasis and the malignant phenotype, Cancer Cell. 8 (2005) 241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [79].Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S, Role of YAP/TAZ in mechanotransduction, Nature. 474 (2011) 179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- [80].Panciera T, Azzolin L, Cordenonsi M, Piccolo S, Mechanobiology of YAP and TAZ in physiology and disease, Nat. Rev. Mol. Cell Biol. 18 (2017) 758–770. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Elosegui-Artola A, Andreu I, Beedle AEM, Lezamiz A, Uroz M, Kosmalska AJ, Oria R, Kechagia JZ, Rico-Lastres P, Le Roux A-L, Shanahan CM, Trepat X, Navajas D, Garcia-Manyes S, Roca-Cusachs P, Force triggers YAP nuclear entry by regulating transport across nuclear pores, Cell. 171 (2017) 1397–1410.e14. doi: 10.1016/j.cell.2017.10.008. [DOI] [PubMed] [Google Scholar]
- [82].Kim HM, Kim J, Role of YAP/TAZ transcriptional regulators in resistance to anti-cancer therapies, Cell. Mol. Life Sci. 74 (2017) 1457–1474. doi: 10.1007/s00018-016-2412-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wackerhage H, Del Re DP, Judson RN, Sudol M, Sadoshima J, The Hippo signal transduction network in skeletal and cardiac muscle, Sci. Signal 7 (2014) re4. doi: 10.1126/scisignal.2005096. [DOI] [PubMed] [Google Scholar]
- [84].Nasrollahi S, Walter C, Loza AJ, Schimizzi GV, Longmore GD, Pathak A, Past matrix stiffness primes epithelial cells and regulates their future collective migration through a mechanical memory, Biomaterials. 146 (2017) 146–155. doi: 10.1016/j.biomaterials.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yang C, Tibbitt MW, Basta L, Anseth KS, Mechanical memory and dosing influence stem cell fate, Nat. Mater 13 (2014) 645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Huang C, Miyazaki K, Akaishi S, Watanabe A, Hyakusoku H, Ogawa R, Biological effects of cellular stretch on human dermal fibroblasts, J. Plast. Reconstr. Aesthetic Surg 66 (2013) e351–e361. doi: 10.1016/j.bjps.2013.08.002. [DOI] [PubMed] [Google Scholar]
- [87].Desai LP, White SR, Waters CM, Cyclic mechanical stretch decreases cell migration by inhibiting phosphatidylinositol 3-kinase- and focal adhesion kinase-mediated JNK1 activation, J. Biol. Chem. 285 (2010) 4511–4519. doi: 10.1074/jbc.M109.084335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Benias PC, Wells RG, Sackey-Aboagye B, Klavan H, Reidy J, Buonocore D, Miranda M, Kornacki S, Wayne M, Carr-Locke DL, Theise ND, Structure and distribution of an unrecognized interstitium in human tissues, Sci. Rep 8 (2018) 4947. doi: 10.1038/s41598-018-23062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Hsu T-H, Kao Y-L, Lin W-L, Xiao J-L, Kuo P-L, Wu C-W, Liao W-Y, Lee C-H, The migration speed of cancer cells influenced by macrophages and myofibroblasts co-cultured in a microfluidic chip, Integr. Biol 4 (2012) 177–182. doi: 10.1039/C2IB00112H. [DOI] [PubMed] [Google Scholar]
- [90].Huang J-W, Pan H-J, Yao W-Y, Tsao Y-W, Liao W-Y, Wu C-W, Tung Y-C, Lee C-H, Interaction between lung cancer cell and myofibroblast influenced by cyclic tensile strain, Lab Chip. 13 (2013) 1114–1120. doi: 10.1039/C2LC41050H. [DOI] [PubMed] [Google Scholar]
- [91].Falcone S, Roman W, Hnia K, Gache V, Didier N, Lainé J, Auradé F, Marty I, Nishino I, Charlet-Berguerand N, Romero NB, Marazzi G, Sassoon D, Laporte J, Gomes ER, N-WASP is required for Amphiphysin-2/BIN1-dependent nuclear positioning and triad organization in skeletal muscle and is involved in the pathophysiology of centronuclear myopathy, EMBO Mol. Med. 6 (2014) 1455–1475. doi: 10.15252/emmm.201404436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Juhas M, Ye J, Bursac N, Design, evaluation, and application of engineered skeletal muscle, Methods. 99 (2016) 81–90. doi: 10.1016/j.ymeth.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lamers E, Walboomers XF, Domanski M, te Riet J, van Delft FCMJM, Luttge R, Winnubst LAJA, Gardeniers HJGE, Jansen JA, The influence of nanoscale grooved substrates on osteoblast behavior and extracellular matrix deposition, Biomaterials. 31 (2010) 3307–3316. doi: 10.1016/j.biomaterials.2010.01.034. [DOI] [PubMed] [Google Scholar]
- [94].Tong Z, Balzer EM, Dallas MR, Hung W-C, Stebe KJ, Konstantopoulos K, Chemotaxis of cell populations through confined spaces at single-cell resolution, PLoS One. 7 (2012) e29211. doi: 10.1371/journal.pone.0029211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rolli CG, Seufferlein T, Kemkemer R, Spatz JP, Impact of tumor cell cytoskeleton organization on invasiveness and migration: A microchannel-based approach, PLoS One. 5 (2010) e8726. doi: 10.1371/journal.pone.0008726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yoshida A, Chitcholtan K, Evans JJ, Nock V, Beasley SW, In vitro tissue engineering of smooth muscle sheets with peristalsis using a murine induced pluripotent stem cell line, J. Pediatr. Surg. 47 (2012) 329–335. doi: 10.1016/j.jpedsurg.2011.11.027. [DOI] [PubMed] [Google Scholar]
- [97].Sano T, Kobayashi T, Negoro H, Sengiku A, Hiratsuka T, Kamioka Y, Liou LS, Ogawa O, Matsuda M, Intravital imaging of mouse urothelium reveals activation of extracellular signal‐regulated kinase by stretch‐induced intravesical release of ATP, 4 (2016) e12033. doi: 10.14814/phy2.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, AJCC cancer staging manual, 6th ed., Springer, 2002. [Google Scholar]