Abstract
Purpose
This study aimed to evaluate the effects of i) the extent of peri-implant bone defects and ii) the application of bone cement on implant stability with respect to the measurement direction.
Methods
In 10 bovine rib bones, 4 implant osteotomies with peri-implant bone defects of various widths were prepared: i) no defect (D0), ii) a 2-mm-wide defect (D2), iii) a 4-mm-wide defect (D4), and iv) a 8-mm-wide defect (D8). The height of all defects was 10 mm. Implant stability quotient (ISQ) values and Periotest values (PTVs) were measured after implant placement and bone cement application.
Results
With increasing defect width, decreased ISQs and increased PTVs were observed. Statistically significant differences were found between groups D0 and D8, D0 and D4, and D2 and D8. Prior to bone cement application, inconsistent PTVs were found in group D8 depending on the measurement direction. Bone cement increased the implant stability.
Conclusion
Peri-implant bone deficits measuring around 50% of the implant surface compromised implant stability. Clinically, PTVs should be cautiously interpreted in implants with large peri-implant defects due to inconsistent recordings with respect to the measurement direction.
Keywords: Alveolar bone loss, Anatomic models, Bone transplantation, Dental implants
Graphical Abstract
INTRODUCTION
Mechanical engagement between implant threads and host bone contributes to primary implant stability after implant placement [1]. The host bone adjacent to the implant surface undergoes remodeling over time, resulting in secondary implant stability, which is referred to as osseointegration [2]. Although some studies demonstrated that secondary stability could be achieved even without primary stability [3,4], good primary stability is generally regarded as a prerequisite for successful osseointegration because it prevents fibrous tissue formation between the implant surface and host bone [5].
Good primary stability also has other advantages, such as reducing the need for surgical interventions and expediting the restoration of esthetic and masticatory functions. For instance, a lack of primary stability requires submerged healing, followed by secondary uncovering surgery after a proper healing period. Moreover, immediate or early delivery of a prosthesis with or without loading requires a certain level of primary stability [6,7].
Various methods, such as cutting torque resistance analysis, the reverse torque test, the percussion test, the impact hammer method, and resonance frequency analysis, can be used to measure implant stability [1]. Periotest values (PTVs) and implant stability quotient (ISQ) values are the most commonly used parameters because of their clinical correlations. PTVs range from −8 to 50, and the classification of the range is defined as follows: i) −8 to 0: good osseointegration, ii) 1 to 9: a borderline implant requiring clinical examination, and iii) ≥10: insufficient osseointegration [8]. The ISQ (range: 0–100) is a conversion of the resonance frequency value (3,500 to 8,500 Hz) [9]. Generally, ISQ values are interpreted as follows: i) >70: high stability, ii) 60–69: medium stability, and iii) <60: low stability. Specifically, implants with an ISQ value <60 should be carefully monitored, a traditional loading protocol should be applied for implants with an ISQ value of 60–65, and early loading is considered feasible if the ISQ is >65 [10,11,12].
Implant stability can be affected by the extent and the location of peri-implant bone defects [13,14]. Peri-implant bone deficiency over a certain threshold may hinder proper primary stability. Peri-implant bone deficiency is generally compensated using bone augmentation, and implant stability may increase immediately after augmentation in cases of an extraction socket defect [15] and over time due to bone consolidation [16]. Moreover, an important point to consider is that stability measurements are generally made in a specific direction, mainly the buccal aspect, regardless of the location of the bone defect. This is due to presence of adjacent structures (the tooth, implant, and other anatomical structures) and the inappropriate angulation of the other aspects (mesial, distal, and lingual aspects). Information regarding the influence of the measurement direction on implant stability is scarce in the literature.
Therefore, the aim of the present study was to evaluate the effect of the extent of peri-implant bone defects on implant stability (evaluated using PTVs and ISQ values), with respect to the measurement direction. Additionally, the effect of using bone cement to fill peri-implant defects on implant stability was evaluated.
MATERIALS AND METHODS
Implant placement and defect preparation
This study included 10 bovine rib bones with 4 dental implants placed in each of them (total number of placed implants: 40).
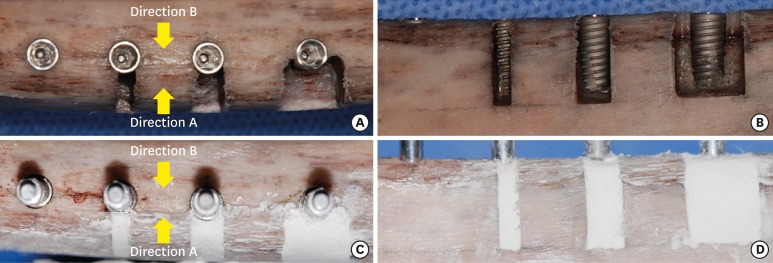
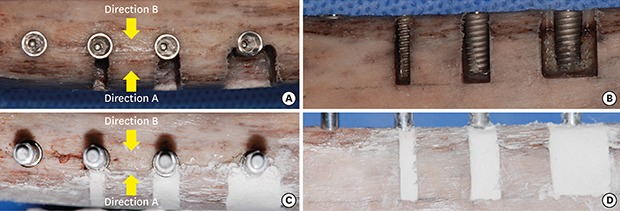
To place the bone-level implants (Ø4.0×10 mm, Luna, Shinhung Co., Seoul, Korea), osteotomies were performed according to the manufacturer's protocol. Varying sizes of box-type dehiscence defects were made on 1 side of the 3 osteotomies using a high-speed diamond bur, thus forming 4 groups: i) no dehiscence (D0), ii) a 2-mm-wide dehiscence defect (D2), iii) a 4-mm-wide dehiscence defect (D4), and iv) an 8-mm-wide dehiscence defect (D8). The height of all defects in groups D2, D4, and D8 was 10 mm. After preparing these defects, implant placement was performed with a maximum insertion torque of 25 N⋅cm (Figure 1A and B).
Figure 1. Experimental procedure for defect preparation and implant stability measurements. (A, B) Implant placement after preparing osteotomies and defects. (C, D) Bone cement application.
Measurement of ISQ values and PTVs
In order to measure ISQ values and PTVs, the rib bones were firmly fixed using a vise. Subsequently, a #7 Smartpeg was connected to the implant with hand-tightening force, and ISQ values were measured in 2 directions (Osstell Mentor®, Osstell AB, Gothenburg, Sweden): i) from the aspect with the defect (direction A), and ii) from the aspect opposite to that with the defect (direction B). The PTVs (Periotest M, Medizintechnik Gulden e. K., Modautal, Germany) were then measured from the aforementioned 2 directions after connecting a healing abutment (Ø4.5×3 mm, Luna, Shinhung Co.) Three measurements from each direction were made and averaged.
Application of bone cement
To simulate bone grafting, bone cement (Polybone, Kyungwon Medical, Cheongju, Korea) was mixed according to the manufacturer's protocol and applied to the defect area up to the level of the adjacent bone (Figure 1C and D). Once the bone cement hardened, ISQ values and PTVs were measured.
The bone cement was composed of β-tricalcium phosphate and polyphosphate (macropores: 100–500 µm. micropores: <10 µm, porosity: 60%–70%), in a blend mainly used for cranial and spinal surgery [17,18,19]. The working time of this material is about 4 minutes and hardening is completed in 7 minutes.
Statistical analyses
A statistical software program (SPSS Statistics 21.0, IBM Corp., Armonk, NY, USA) was used for all statistical analyses. The data are presented as mean±standard deviation. The Shapiro-Wilk test was applied to test the normality of the distribution. Then, the paired t-test or the Wilcoxon signed rank test was used to assess the statistical significance of differences between the measurement directions and differences before and after bone cement application. Repeated-measures analysis of variance or the Friedman test was used to assess the statistical significance of differences between groups with various defect widths, followed by the post hoc Bonferroni or Dunn test for intergroup comparisons. The level of statistical significance was set at P<0.05.
RESULTS
Implant stability in the presence of bone defects
As the defect size increased, the ISQ value decreased, irrespective of the measurement direction. These values ranged from 84.5±2.1 (group D0) to 60.3±5.9 (group D8) for direction A, and from 84.8±2.4 (group D0) to 61.2±6.2 (group D8) for direction B. An opposite tendency was observed for PTVs, which ranged from −6.2±0.3 in group D0 to −0.3±2.5 in group D8 for direction A, and from −6.4±0.5 in group D0 to 1.8±2.8 in group D8 for direction B. Statistically significant differences were found between groups D0 and D8, D0 and D4, and D2 and D8 for both measurement methods and both measurement directions (P<0.05) (Table 1).
Table 1. ISQ values and PTVs before and after bone cement application (mean±standard deviation).
| Variables | ISQ values | PTVs | |||
|---|---|---|---|---|---|
| Direction A | Direction B | Direction A | Direction B | ||
| Before bone cement application | |||||
| D0 | 84.5±2.1 | 84.8±2.4 | −6.2±0.3 | −6.4±0.5 | |
| D2 | 81.8±2.1 | 81.9±2.1 | −5.8±0.6 | −5.6±0.6 | |
| D4 | 73.9±3.1a) | 74.0±3.1a) | −3.0±1.6a) | −1.5±3.1a) | |
| D8 | 60.3±5.9a,b) | 61.2±6.2a,b) | −0.3±2.5a) | 1.8±2.8a,b,c) | |
| After bone cement application | |||||
| D0 | - | - | - | - | |
| D2 | 83.6±1.6d) | 84.0±1.8c,d) | −6.3±0.7d) | −6.1±0.6d) | |
| D4 | 79.7±2.6b,d) | 79.6±2.8b,c,d) | −5.1±1.1b,d) | −4.1±2.3d) | |
| D8 | 78.2±2.3b,d) | 77.9±2.1b,c,d) | −3.1±1.4b,d,e) | −2.3±1.4b,c,d,e) | |
D0: group with no dehiscence; D2: group with a 2-mm-wide dehiscence defect; D4: group with a 4-mm-wide dehiscence defect; D8: group with a 8-mm-wide dehiscence defect; Direction A: from the aspect with the defect; Direction B: from the opposite aspect of the defect. To analyze the directions before bone cement application, the paired t-test was used in groups D0 and D8 for ISQ values, and in groups D0, D2, and D4 for PTV (the Wilcoxon signed rank test was used for the other comparisons). To analyze the directions after bone cement application, the paired t-test was used for all comparisons. The Friedman test was used to assess intergroup statistical differences before bone cement application, and repeated-measures analysis of variance was used to assess intergroup statistical difference after bone cement application. The Wilcoxon signed rank test was used to analyze intragroup statistical differences in groups D2 (ISQ value in direction A) and D8 (ISQ value in direction B, PTV in direction A), and the paired t-test was used for the other intragroup comparisons.
ISQ: implant stability quotient, PTV: Periotest value.
a)Statistically significant difference compared to D0; b)Statistically significant difference compared to D2; c)Statistically significant difference compared to direction A; d)Statistically significant difference between before and after bone cement application; e)Statistically significant difference compared to D4.
In terms of the measurement direction, there was no statistically significant difference in any of the groups when ISQ values were measured. However, there was a statistically significant difference in the D8 group (P<0.001) and a borderline significant difference in the D4 group (P=0.058) when PTVs were measured (Table 1).
Implant stability after bone cement application
Once the bone cement hardened, increased ISQ values and decreased PTVs were observed compared to the values obtained without bone cement. With increasing defect size, ISQ values decreased and PTVs decreased. The ISQ values ranged from 84.0±1.8 (direction B in group D2) to 77.9±2.1 (direction B in group D8), and there were statistically significant differences between groups D2 and D4 and between groups D2 and D8 (P<0.05) in both directions. The PTVs for direction A were −6.3±0.7 in group D2, −5.1±1.1 in group D4, and −3.1±1.4 in group D8, with statistically significant differences in all intergroup comparisons (P<0.05). The PTVs for direction B showed the same trends as direction A, with statistically significant differences between groups D2 and D8 and between groups D4 and D8 (P<0.05) (Table 1).
The ISQ values demonstrated a statistically significant difference between directions A and B in all groups, although the differences were numerically small (P<0.05). The PTVs only showed a statistically significant difference between the 2 directions in the D8 group (P<0.05) (Table 1).
Changes of implant stability before and after bone cement application
Larger defects were associated with greater increases in both ISQ values and PTVs. In group D8, the ISQ values increased by 17.9±4.3 and 16.7±4.9, and the PTVs increased by −2.8±2.4 and −4.1±2.1 for directions A and B, respectively. All changes between before and after bone cement application were statistically significant (P<0.05).
DISCUSSION
The present study evaluated the effects of the extent of peri-implant bone defects and bone cement on implant stability with respect to the measurement direction. The results revealed that i) narrow peri-implant bone defects (group D2) did not affect implant stability; ii) wide peri-implant bone defects above a certain level (group D8) jeopardized implant stability; iii) the PTVs of implants with wide peri-implant bone defects were inconsistent and depended on the measurement direction, unlike the ISQ values (group D8); and iv) application of bone cement significantly increased implant stability, with a greater increase in the groups with larger defects (D4 and D8).
Various forms of bone defects are simultaneously treated with implant placement. However, these bone deficits may affect the primary stability. Lack of primary stability may jeopardize or delay secondary stability. The present study used box-type bony dehiscence to simulate peri-implant bone deficiencies. In group D2, with narrow bony dehiscence defects, good implant stability was achieved because most of the implant surface was embedded in the bone. However, there was a tendency for the implant stability to decrease in group D4, where approximately 25% of the implant surface was not embedded in the bone. Despite this tendency, implant stability in group D4 was still clinically acceptable (ISQ: 73.9–74.0, PTV: −3.0 to −1.5). In group D8, where approximately 50% of the implant surface was not embedded in the bone, implant stability demonstrated a statistically significant drop as compared to that in groups D0 and D2, resulting in unacceptable ISQ values and PTVs for early loading, as 3 specimens from group D8 demonstrated ISQ values <60. A similar significant decrease was demonstrated in a previous study. In a formalin-fixed cadaver model, the removal of peri-implant bone along up to a 180º perimeter in the presence of a 6-mm-long dehiscence led to a significant decrease of implant stability, compared to what was observed before bone removal [13]. This observation indicates that above a certain threshold of bone deficiency around the implant, primary stability is hampered.
A particularly remarkable finding was the difference in PTVs in the presence of peri-implant defects according to the measurement direction, which has rarely been reported in previous studies, especially in clinical studies [1,2,7,13,15,16,20,21,22,23]. In groups D4 (P=0.058) and D8 (P<0.001), the PTV measured from the aspect of the defect (direction A) was lower than that measured from the opposite direction (direction B). This may be derived from the principle of stability measurements made using Periotest. This device delivers several impacts to the implant abutment to measure the contact time between the test object and tapping rod, and then converts the measurement to PTVs [1]. When PTVs were measured in direction A, there was a bony wall supporting the implant from the other side, but there was limited support for the implant when PTVs were measured in direction B. Thus, the impact in direction B from the Periotest device may have delivered a dislodging force to the implant, possibly contributing to the different PTVs between the 2 directions. Even though the ISQ values were constant in groups D4 and D8 irrespective of the directions, this finding leaves room for discussion regarding which value is clinically more trustworthy. Moreover, in a clinical setting, clinicians can only measure PTVs from the buccal aspect.
In the present study, bone cement was used to simulate bone grafting and subsequent consolidation. After hardening of the bone cement, the ISQ values and PTVs showed a statistically significant increase, with varying amounts. Especially in the D8 group, the ISQ values and PTVs were approximately 77–78 and −3 to −2, respectively, which were suitable for loading. Previously, the effect of bone cement on achieving greater implant stability in circumferential peri-implant defects was tested in vitro [23]. In that study, circumferential defects with depths of 2.5 mm and 5 mm were used. The mean ISQ value changed from 69.42 to 73.72 in the group with 2.5-mm-deep defects and from 57.43 to 67.88 in the group with 5.0-mm-deep defects. Considering those findings, it should be clinically emphasized that sufficient healing time for new bone formation around an implant should be provided before prosthetic loading in cases with large peri-implant defects. However, the duration of healing may be case-specific due to variations in individual healing potential, bone quality, and material characteristics.
A limitation of the present study was the relatively thick cortical bone of the rib bone (approximately 3–4 mm). In a human cadaveric study, the mean thickness of the buccal and lingual cortical bone ranged from 1.04 to 2.06 mm and from 1.36 to 2.39 mm, respectively [24]. In a computed tomography study, the cortical bone plate was measured as between 1.3 and 2.0 mm in premolar and molar areas [25]. Therefore, the ISQ values and PTVs in the present study may not correspond to the values from human alveolar bone. Moreover, even though the hardening of bone cement material is a convenient way to simulate bone graft material consolidation, it should be noted that healing dynamics in vivo are more complex, meaning that this study should be further supported by clinical studies. Lastly, the quality of the regenerated bone on the implant surface may be different from that of bone cement.
In conclusion, a wide peri-implant bone deficit (over 50% of the implant surface) could compromise the primary stability of the implant. In terms of the measurement direction, Periotest showed inconsistent values for implants with peri-implant defects above a certain threshold. Bone grafting using bone cement contributed to an increase in the implant stability.
Footnotes
- Conceptualization: Ji-Youn Hong.
- Data curation: Ji-Youn Hong.
- Formal analysis: Hyunjin Yim, Yeek Herr.
- Investigation: Hyun-Chang Lim.
- Methodology: Hyun-Chang Lim, Hyunjin Yim, Jong-Hyuk Chung.
- Project administration: Seung-Il Shin, Jong-Hyuk Chung, Seung-Yun Shin.
- Supervision: Jong-Hyuk Chung, Yeek Herr, Seung-Yun Shin.
- Validation: Ji-Youn Hong, Seung-Yun Shin.
- Writing - original draft: Seung-Yun Shin.
- Writing - review & editing: Hyun-Chang Lim, Hyunjin Yim, Seung-Il Shin, Yeek Herr.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Atsumi M, Park SH, Wang HL. Methods used to assess implant stability: current status. Int J Oral Maxillofac Implants. 2007;22:743–754. [PubMed] [Google Scholar]
- 2.Bischof M, Nedir R, Szmukler-Moncler S, Bernard JP, Samson J. Implant stability measurement of delayed and immediately loaded implants during healing. Clin Oral Implants Res. 2004;15:529–539. doi: 10.1111/j.1600-0501.2004.01042.x. [DOI] [PubMed] [Google Scholar]
- 3.Jung UW, Kim S, Lee IK, Kim MS, Lee JS, Kim HJ. Secondary stability of microthickness hydroxyapatite-coated dental implants installed without primary stability in dogs. Clin Oral Implants Res. 2014;25:1169–1174. doi: 10.1111/clr.12226. [DOI] [PubMed] [Google Scholar]
- 4.Rea M, Lang NP, Ricci S, Mintrone F, González González G, Botticelli D. Healing of implants installed in over- or under-prepared sites--an experimental study in dogs. Clin Oral Implants Res. 2015;26:442–446. doi: 10.1111/clr.12390. [DOI] [PubMed] [Google Scholar]
- 5.Lioubavina-Hack N, Lang NP, Karring T. Significance of primary stability for osseointegration of dental implants. Clin Oral Implants Res. 2006;17:244–250. doi: 10.1111/j.1600-0501.2005.01201.x. [DOI] [PubMed] [Google Scholar]
- 6.Atieh MA, Alsabeeha NH, Payne AG, de Silva RK, Schwass DS, Duncan WJ. The prognostic accuracy of resonance frequency analysis in predicting failure risk of immediately restored implants. Clin Oral Implants Res. 2014;25:29–35. doi: 10.1111/clr.12057. [DOI] [PubMed] [Google Scholar]
- 7.Baltayan S, Pi-Anfruns J, Aghaloo T, Moy PK. The predictive value of resonance frequency analysis measurements in the surgical placement and loading of endosseous implants. J Oral Maxillofac Surg. 2016;74:1145–1152. doi: 10.1016/j.joms.2016.01.048. [DOI] [PubMed] [Google Scholar]
- 8.Schulte W, Lukas D. Periotest to monitor osseointegration and to check the occlusion in oral implantology. J Oral Implantol. 1993;19:23–32. [PubMed] [Google Scholar]
- 9.Meredith N, Alleyne D, Cawley P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin Oral Implants Res. 1996;7:261–267. doi: 10.1034/j.1600-0501.1996.070308.x. [DOI] [PubMed] [Google Scholar]
- 10.Bornstein MM, Hart CN, Halbritter SA, Morton D, Buser D. Early loading of nonsubmerged titanium implants with a chemically modified sand-blasted and acid-etched surface: 6-month results of a prospective case series study in the posterior mandible focusing on peri-implant crestal bone changes and implant stability quotient (ISQ) values. Clin Implant Dent Relat Res. 2009;11:338–347. doi: 10.1111/j.1708-8208.2009.00148.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigo D, Aracil L, Martin C, Sanz M. Diagnosis of implant stability and its impact on implant survival: a prospective case series study. Clin Oral Implants Res. 2010;21:255–261. doi: 10.1111/j.1600-0501.2009.01820.x. [DOI] [PubMed] [Google Scholar]
- 12.Sennerby L. 20 Jahre Erfahrung mit der Resonanzfrequenzanalyse. Implantologie. 2013;21:21–33. [Google Scholar]
- 13.Merheb J, Coucke W, Jacobs R, Naert I, Quirynen M. Influence of bony defects on implant stability. Clin Oral Implants Res. 2010;21:919–923. doi: 10.1111/j.1600-0501.2010.01932.x. [DOI] [PubMed] [Google Scholar]
- 14.Shin SY, Shin SI, Kye SB, Hong J, Paeng JY, Chang SW, et al. The effects of defect type and depth, and measurement direction on the implant stability quotient (ISQ) Value. J Oral Implantol. 2014 doi: 10.1563/AAID-JOI-D-13-0031. [DOI] [PubMed] [Google Scholar]
- 15.Jun SH, Park CJ, Hwang SH, Lee YK, Zhou C, Jang HS, et al. The influence of bone graft procedures on primary stability and bone change of implants placed in fresh extraction sockets. Maxillofac Plast Reconstr Surg. 2018;40:8. doi: 10.1186/s40902-018-0148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S, Lee D, Shiffler K, Aghaloo T, Moy P, Pi-Anfruns J. Resonance frequency analysis of sinus augmentation by osteotome sinus floor elevation and lateral window technique. J Oral Maxillofac Surg. 2015;73:1920–1925. doi: 10.1016/j.joms.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Park JH, Choi CG, Jeon SR, Rhim SC, Kim CJ, Roh SW. Radiographic analysis of instrumented posterolateral fusion mass using mixture of local autologous bone and b-TCP (PolyBone®) in a lumbar spinal fusion surgery. J Korean Neurosurg Soc. 2011;49:267–272. doi: 10.3340/jkns.2011.49.5.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Roh SW. Anterior cervical interbody fusion using polyetheretherketone cage filled with autologous and synthetic bone graft substrates for cervical spondylosis: comparative analysis between PolyBone® and iliac bone. Neurol Med Chir (Tokyo) 2013;53:85–90. doi: 10.2176/nmc.53.85. [DOI] [PubMed] [Google Scholar]
- 19.Park YH, Kim SG, Lee JW, Yoon YH. Obliteration of temporal dorsal bulla in guinea pigs using different types of calcium phosphate. Int J Pediatr Otorhinolaryngol. 2011;75:1176–1180. doi: 10.1016/j.ijporl.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Oh JS, Kim SG, Lim SC, Ong JL. A comparative study of two noninvasive techniques to evaluate implant stability: Periotest and Osstell Mentor. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:513–518. doi: 10.1016/j.tripleo.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Rasmusson L, Stegersjö G, Kahnberg KE, Sennerby L. Implant stability measurements using resonance frequency analysis in the grafted maxilla: a cross-sectional pilot study. Clin Implant Dent Relat Res. 1999;1:70–74. doi: 10.1111/j.1708-8208.1999.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 22.Sennerby L, Meredith N. Implant stability measurements using resonance frequency analysis: biological and biomechanical aspects and clinical implications. Periodontol 2000. 2008;47:51–66. doi: 10.1111/j.1600-0757.2008.00267.x. [DOI] [PubMed] [Google Scholar]
- 23.Shin SY, Shin SI, Kye SB, Chang SW, Hong J, Paeng JY, et al. Bone cement grafting increases implant primary stability in circumferential cortical bone defects. J Periodontal Implant Sci. 2015;45:30–35. doi: 10.5051/jpis.2015.45.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katranji A, Misch K, Wang HL. Cortical bone thickness in dentate and edentulous human cadavers. J Periodontol. 2007;78:874–878. doi: 10.1902/jop.2007.060342. [DOI] [PubMed] [Google Scholar]
- 25.Deguchi T, Nasu M, Murakami K, Yabuuchi T, Kamioka H, Takano-Yamamoto T. Quantitative evaluation of cortical bone thickness with computed tomographic scanning for orthodontic implants. Am J Orthod Dentofacial Orthop. 2006;129:721.e7–721.e12. doi: 10.1016/j.ajodo.2006.02.026. [DOI] [PubMed] [Google Scholar]