Abstract
Plasmacytoid dendritic cells (pDCs) are a unique subset of cells with different functional characteristics compared to classical dendritic cells. The pDCs are critical for the production of type I IFN in response to microbial and self-nucleic acids. They have an important role for host defense against viral pathogen infections. In addition, pDCs have been well studied as a critical player for breaking tolerance to self-nucleic acids that induce autoimmune disorders such as systemic lupus erythematosus. However, pDCs have an immunoregulatory role in inducing the immune tolerance by generating Tregs and various regulatory mechanisms in mucosal tissues. Here, we summarize the recent studies of pDCs that focused on the functional characteristics of gut pDCs, including interactions with other immune cells in the gut. Furthermore, the dynamic role of gut pDCs will be investigated with respect to disease status including gut infection, inflammatory bowel disease, and cancers.
Keywords: Plasmacytoid dendritic cell, Gut, Type I interferon, Immune tolerance, Host defense
INTRODUCTION
At intestinal tissues, an interplay between antigen-presenting cells (APCs) including dendritic cells (DCs) and macrophages can be critical for the maintenance of immune homeostasis (1). Classical dendritic cells (cDCs) are found throughout the gut-associated lymphoid tissues (GALTs) including the lamina propria (LP), Peyer's patches (PPs), and isolated lymphoid follicles (ILFs), as well as intestinal draining lymph nodes (LNs) such as the mesenteric lymph nodes (MLNs) (2). The cDCs fully express MHC class II and CD11c, and can be derived from committed cDC progenitors in the bone marrow (BM) and differentiate into mature cDCs in the periphery (3). When mapping the human DC lineage through 2 unbiased high-dimensional technologies, single-cell messenger RNA sequencing and cytometry by time-of-flight, pre-DCs are a group of heterogeneous populations consisting of 1 early uncommitted CD123high pre-DC subset and 2 CD45RA+CD123low lineage-committed subsets (4). Most subsets of gut cDCs can synthesize retinoic acid by expressing retinaldehyde dehydrogenase 2, which imprint gut-homing capacity for lymphocytes (5), and result in the production of mucosal IgA from B cells (6,7). Pre-cDCs, but not monocytes, can be differentiated into both CD103+CD11b+ and CD103+CD11b− cDCs in the intestinal LP (8). Differentiation of intestinal CD103+CD11b− cDCs is dependent on Batf3, IFN regulatory factor (IRF) 8, and Id2, which is developmentally associated with lymphoid tissue resident CD8α+ cDCs (9,10,11). CD103+CD11b+ intestinal cDCs are dependent on IRF4 and Notch2 (12,13).
Plasmacytoid dendritic cells (pDCs) are a rare type of immune cell that is known to secrete large quantities of type I IFNs in response to a viral infection, or self-nucleic acids through innate recognition receptors such as TLRs (14). Human pDCs lack expression of CD11c but selectively expressed BDCA2 (CD303), one of the type II C-type lectins (15). In contrast to human pDCs, mouse pDCs express intermediary CD11c and specifically express B220, Ly6C, BM stromal Ag 2, and Siglec-H (16). The functional characteristics of gut pDCs are different from typical pDCs found in the blood circulation and periphery. Gut pDCs are unable to produce type I IFNs (17), and pDCs from the liver and MLNs also suppress T cell responses and induce oral tolerance rather than produce type I IFN (18).
THE ROLE OF pDCs IN THE GUT
Gut pDCs in mucosal environments
Although both cDCs and pDCs are derived from BM cells, pDCs can be differentiated from common dendritic cell progenitors (CDPs) via a unique pathway by the constitutive expression of E2-2, which is a pDC-specific transcription factor (19). FLT3-ligand-STAT3 signaling can promote the expression of E2-2 (20). The pDC can be originated from IL-7R+ lymphoid precursor cells and the amount of IRF8 is associated with pDC commitment (21). The transcription factor Zeb2 regulates commitment to pDCs by repressing Id2 (22,23). The cDC precursors migrate from the BM to lymphatic or peripheral tissues, existing as a resident or migratory DCs. Upon microbial stimuli, these immature cDCs migrate to the LNs to develop activated phenotypes. On the other hand, pDCs are fully developed in the BM and then migrate into the thymus and secondary lymphoid organs via the blood circulation (24). Furthermore, the pDCs migrated from the BM to the small intestine via C-C chemokine receptor type 9 chemokine receptors (25). Several factors including mucosal vascular addressin cell adhesion molecule 1, β7 integrin, and CD103 are also involved in pDC trafficking to the gut (26). The pDCs can act as APCs as well as effector cells that secrete type I and type III IFNs. Once infected, pDCs secrete type I IFN, underwent morphological and functional changes, and then acted as an APC to prime naïve T cells (27). The pDCs selectively express TLR2 (28), TLR7 (29), TLR9 (30), TLR11, and TLR12 (31). The recognition of the microbial pathogen by pDC is mainly mediated by the endosomal sensors TLR7 and TLR9. TLR7 recognizes RNA viruses such as influenza, and TLR9 recognizes unmethylated CpG-rich sequences of DNA viruses such as herpes simplex virus (HSV)-1 and HSV-2 (32). Once recognized via these receptors, pDCs secrete type I IFNs through the myeloid differentiation primary response 88-IRF7 pathway and produce pro-inflammatory cytokines through the NF-κB pathway. The pDCs also detect Toxoplasma gondii profilin (31) and bacterial polysaccharide A (PSA) (28) via TLR12 and TLR2, respectively.
In addition, pDCs can also detect cytosolic DNA by the cyclic GMP-AMP synthase stimulator of interferon genes pathway to induce the production of type I IFN (33). Lastly, pDCs also express various vitamin D receptors, and vitamin D signaling can act as a natural inhibitory mechanism on pDCs (34).
The production of type I IFN from gut pDCs can be affected by the mucosal microenvironment. IL-10 expressed by activated LP DCs and macrophages, prostaglandin E2 (PGE2) by stromal cells, and TGF-β by intestinal epithelial cells can prevent PP pDCs from producing significant amounts of type I IFN by inhibiting primary signaling via TLR9 (17). In fact, the production of type I IFN from the spleen pDCs can be inhibited by IL-10, PGE2, and TGF-β in response to a treatment of CpG oligodeoxynucleotides. Furthermore, pDCs are generally resident DC subsets in the gut (35). However, pDCs can mobilize LP cDCs to the MLNs in response to TLR stimuli via TNF and type I IFN-dependent mechanisms (36).
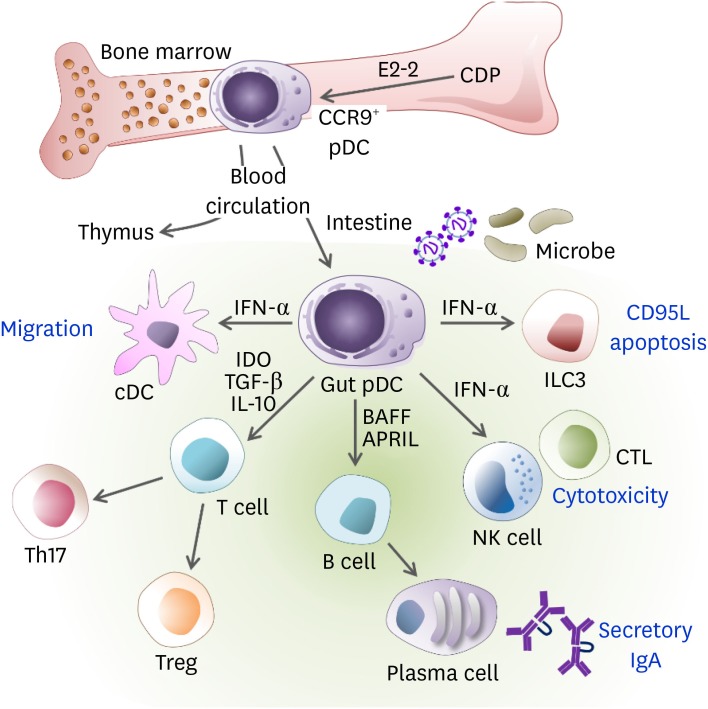
Gut pDCs are known for the induction of oral tolerance (18) rather than the production of type I IFN (17). Mucosal factors that are expressed from GALT can inhibit type I IFN secretion by pDCs, while maintaining the ability of pDCs to prime naive T cells and triggering differentiation into Tregs (37) and Th17 cells (38). Gut pDCs are effective in causing mucosal B cell responses to induce IgA production independently of T cells (39). As studies regarding the interaction between the mucosal immune system and microbiome are rapidly progressing, pDCs in GALT are also becoming the focus of increasing interest (32). The roles of gut pDCs are summarized in Fig. 1.
Figure 1. The role of pDCs in gut immunity. The pDCs can be differentiated from CDPs and IL-7R+ lymphoid precursor cells in an E2-2-dependent manner in the BM and distributed via the blood circulation to lymphoid organs such as the thymus, spleen, LNs, and peripheral tissues such as the intestine. The pDCs are recruited to the lamina propria of the small intestine in a CCR9-dependent manner. While pDCs do not migrate from the intestinal periphery to the draining MLNs, they can mobilize the lamina propria cDCs toward MLN via the production of type I IFN. During viral infections, type I IFN produced by gut pDCs induces CD95L expression on ILC3, which reduces IL-22 and then impairs barrier permeability. The pDCs activate NK cells and CD8+ T cells to enhance cytotoxicity via IFN-α. Activated pDCs produce BAFF and APRIL, which induce secretory IgA production from B cells. The pDCs are poor APCs to naïve T cells. However, the gut pDCs conditioned by microbial ligands such as PSA or TLR9 induce the generation of Tregs and Th17 cells via IDO, IL-10, and/or TGF-β.
APRIL, a proliferation-inducing ligand; BAFF, B cell activating factor; CCR9, C-C chemokine receptor type 9.
Interaction of pDCs with innate lymphoid cells (ILCs)
The interactions between ILCs and pDCs in healthy and diseased guts have not been well characterized yet. The ILC family includes classic cytotoxic NK cells as well as non-cytotoxic ILC populations consisting of 3 distinct groups (40). The distribution of human ILC subsets varies in tissues and organs (41). Group 1 ILCs, including NK cells, mostly exist in the fetal intestine and liver. ILC2s are founded in the peripheral blood, lung, and skin. ILC3s are mainly in the skin tissue, thymus, tonsils, BM, and intestine (40). Both cDCs and pDCs can activate NK cells but stimulate different functions of NK cells (42). The cDCs primarily activate NK cells to secrete IFN-γ via the production of IL-12 and IL-18, and to proliferate and survive via the production of IL-15. In contrast, pDCs trigger NK cell cytotoxicity by type I IFN (43). The production of type I IFN from pDCs can be inhibited by activating the NK cell receptor natural cytotoxicity triggering receptor 2 (44), or activated by the inhibitory NK cell receptor IRp60 (45). The ILC2 populations are low in the intestine but prevalent in the lung (40). In the lung, activation of pDCs through TLR7 suppresses ILC2-mediated airway hyper-reactivity and airway inflammation, since IFN-α increased ILC2 apoptosis (46). ILC3s play critical roles in mucosal homeostasis and gut immunity. Gut-resident ILC3s can produce IL-22 that was essential for gut barrier function (47,48). In HIV-1 infections, pDCs can make ILC3s that undergo CD95/FasL-mediated apoptosis via type I IFN (49). Therefore, sustained pDC activation and type I IFN production during chronic viral infection may possibly impair the integrity of the gut mucosal barrier by reducing intestinal ILC3 cells (50).
The pDCs in T cell immunity
Immune tolerance is an important process in the gut because oral tolerance discriminates the latent pathogenic microorganisms from the commensal flora. Failure of oral tolerance may induce mucosal inflammation, resulting in diseases such as inflammatory bowel diseases (IBDs) and food allergies. Gut pDCs have a tendency to induce the differentiation of CD4+Foxp3+ Tregs instead of CD4+ effector T cells via autocrine TGF-β (37). Systemic depletion of pDCs prevents induction of oral tolerance by Ag feeding (18). Unlike cDCs, pDCs are inefficient for phagocytosis and the presentation of exogenous Ags to CD4+ T cells because of the sustained MHC class II-peptide complex formation, ubiquitination, and turnover (51). Although pDCs are less efficient for induction of T cells compared to cDCs, they could still activate CD4+ helper and Tregs, as well as CD8+ cytotoxic T cells (52,53). In response to TLR stimulation, pDCs promote efficient Th17 differentiation (54). In humans, pDCs are inefficient for the activation of Tregs, but are efficient for the induction of effector T cells and support Th17 cell effector function (55,56). T cells that are primed by CD40L-activated pDCs have regulatory phenotypes, resulting in decreased IL-2 and IFN-γ production and increased IL-10 (16). The pDCs exposed to PSA from gut commensal Bacterioides fragilis do not produce proinflammatory cytokines, but instead they specifically stimulated IL-10 secretion by CD4+ T cells (28). PSA preferentially ligated to TLR2 on pDCs but not on other DC subsets. Ligation of PSA on pDCs cannot directly produce IL-10. Direct secretion of IL-10 by pDCs is dependent on TLR-9, while IL-10 secretion by myeloid DCs is dependent on TLR-2/6 (57). Furthermore, pDCs express indoleamine 2,3 dioxygenase (IDO), which is the rate-limiting step for the degradation of tryptophan into kynurenine and downstream products along the kynurenine pathway (58). IDO is essential for Treg generation by pDCs (59). Interactions of microbiota and pDCs are involved in the regulation of inflammation (60). Feeding of probiotic Bifidobacterium adolescentis increases pDCs and Treg frequencies as well as immunity towards pathogenic infections with Yersinia enterocolitica (61). Probiotic Lactobacillus gasseri increases the population of pDCs that can activate Tregs (62).
The pDCs in B cell immunity
Secretory IgA is continuously produced from the mucosal surface, which forms the first-line barrier along with the mucus to protect the mucosal surface by inhibiting attaching microbes and neutralizing toxic materials (63). B cells undergo a class-switching recombination (CSR) process to produce IgA via T cell-dependent (TD) or T cell-independent (TI) pathways mainly in the PPs as well as the LP including the ILFs (64,65). The pDCs from the MLN and PP induce a significantly higher production of TI IgA CSR by B cells compared to MLN and PP cDCs (39). However, there is minimal IgA production in pDCs isolated from the peripheral LN. The microbiota activates stromal cells to produce type I IFNs that drove the pDCs to express B cell activating factor and a proliferation-inducing ligand, thereby facilitating IgA responses in the gut. However, a recent study suggests that pDCs are not critical for non-infectious IgA responses (66). Overall, these studies suggest that various gut DC subsets including pDCs may play redundant roles in intestinal IgA homeostasis.
THE ROLE OF pDCs IN INTESTINAL DISEASES
The pDCs in gut infections
The pDCs recognize pathogens by microbial nucleic acids via TLR7 or TLR9, then produce large quantities of type I IFN. The production of type I IFN by pDC can be an important key step to protect against viral infections in the acute phase (16). The role of pDCs in enteric rotavirus infections has been relatively well studied. Rotavirus is a common pathogenic virus in children under the age of 5 years and is replicated mainly in small intestinal epithelial cells (67). Recognition of viral dsRNA or DNA by the endosomal receptor TLR7 or TLR9 is important for the production of IFN-α from pDCs. During a rotavirus infection, pDCs play a role in the activation of GALT B cells via type I IFNs (68). However, in some cases such as influenza infections, pDCs cause uncontrolled inflammation or bronchial epithelium apoptosis (69). The activation of pDCs in acute viral infections may induce different results depending on the type of virus and the route of infection (32). The role of pDCs in controlling chronic viral infections also differs depending on the type of virus. Although the level of type I IFN induced by infection of hepatitis B virus, hepatitis C virus, and human papilloma virus is low, type I IFNs appear to play an important role in the control of virus and the survival of anti-viral T cells (70). In human patients with HIV, pDCs are depleted in the circulation but accumulated in the terminal ileum showing upregulation of gut-homing receptor CD103 (71). Excessive production of IFN-α by pDCs is a typical phenomenon of HIV infection. The pDCs can induce T cell apoptosis and promote Treg development (72). In addition to T cells, pDCs can upregulate CD95 expression on ILC3s via type I IFN to induce CD95/FasL-mediated apoptosis (49).
Although pDCs have been shown to play a role in viral infections, the role of pDCs has not been well studied in bacterial infections. The pDCs in the MLN and spleen are expanded after intragastric infection of Listeria monocytogenes, which is a gram-positive intracellular bacterium causing severe food-borne gastroenteritis (73). The increased number of pDCs lasts up to 12 days after infection, in which no bacteria were detected, and returns to the basal level at day 19 of infection. In addition, the expressions of costimulatory molecules on pDCs are upregulated, contributing to interactions with T cell. The pDCs in the colon-draining LNs during a Citrobacter rodentium infection can be specifically activated, resulting in the production of C-type lectin receptor and proinflammatory cytokines (74).
The pDCs in IBD
IBDs, which includes ulcerative colitis (UC) and Crohn's disease, are chronic inflammatory diseases due to microbial violation or mucosal barrier insults mainly observed in genetically susceptible individuals (75). In patients with IBDs, pDCs significantly accumulate in the MLN and inflamed colonic mucosa. However, the secretion of IFN-α from peripheral blood pDCs in patients with flaring UC is significantly reduced, suggesting their role as tolerance inducers (76). The roles of pDCs and type I IFN have more complicated features in gut inflammation. In an acute colitis model by a single-cycle administration of dextran sulfate sodium, pDCs accumulate in inflamed areas of acute colitis (77). Selective depletion of pDCs in the pDC-ablated mice that expressed DTR under the control of the Siglec-H gene attenuates acute colitis by decreasing infiltration of inflammatory leukocytes and production of proinflammatory cytokines independent of IFN-I signaling (77). When pDCs are depleted by targeting E2-2, the pDC-specific transcription factor, pDCs are dispensable for the pathogenesis of intestinal inflammation in the experimental IBD model caused by a deficiency of the Wiskott-Aldrich syndrome protein or IL-10 (78). In contrast, several reports support the regulatory roles that type I IFN plays in intestinal injury and inflammation. GM-CSF can ameliorate colitis by increasing type I IFN production of pDC (79). Activation of TLR3 and TLR9 protects mice from experimental colitis via induction of type I IFN (80,81). Recognition of gut resident RNA viruses through TLR3 and TLR7 can regulate acute colitis via IFN-β secretion from pDCs (82). Furthermore, anti-viral drug cocktail treatments exacerbate gut inflammation. In fact, the gut virome exhibits different patterns in patients with IBD compared to healthy individuals, suggesting that the enteric virome may play a role in intestinal diseases (83). Collectively, although the role of pDCs in the intestinal inflammatory disease is controversial, pDCs have a role in gut immune homeostasis by managing the inflammatory or anti-inflammatory response in a case-dependent manner.
The pDCs in cancer
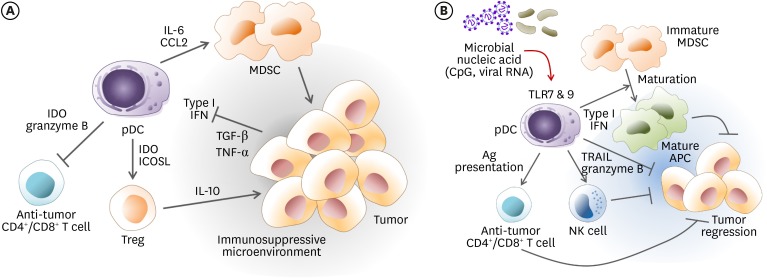
The recruitment of pDCs in peri-tumor sites is often associated with poor prognosis (Fig. 2A) since the tumor microenvironment converted pDCs into immunosuppressive cells (84). Tumor-derived TGF-β and TNF-α inhibit the production of IFN-α by tumor-associated pDCs (85,86). Tumor-infiltrating pDCs tend to induce Treg cells through the expression of IDO (59) or inducible co-stimulatory receptor ligand (87). Within tumor draining LNs, a small population of IDO+ pDCs also creates a suppressive microenvironment via constitutive activation of Tregs (88). Human CD123+ pDCs in colorectal tumor tissue and LNs may help developing Tregs and foster tumor tolerance (89). In patients with colorectal cancer, the frequency of pDCs is low, and they tend to be functionally defective (90). The pDCs are also involved in cancer progression via expression of pro-apoptotic granzyme B, which inhibits the proliferation of anti-tumor T cells (91). In contrast to NK cells, pDCs do not release the pore-forming perforin. In tumor-draining LNs, pDCs constitutively express immunosuppressive IDO enzyme (92). The lymphocyte-activation gene 3+ pDCs in melanoma produce IL-6 without induction of type I IFN. IL-6 induce the production of CCL2 that can recruit myeloid-derived suppressor cells (MDSCs) at the tumor tissue (93). The number of pDCs in blood is variable depending on the type and progressions of cancer. In patients with melanoma, circulating pDCs are significantly decreased (90). The decrease of circulating pDCs correlates to a more advanced stage of cancer progress, and recovers after complete tumor resection. In gastric carcinoma tissue, Tregs and inducible co-stimulatory receptor (ICOS)+ Tregs are the main lineage cells, whereas pDCs are the majority in peri-tumor tissue (94). Under gastric tumor conditions, pDCs may recruit ICOS+ Tregs and act as immunosuppressants. Tumor-associated pDCs are poor producers of type I IFN and favor the IL-10 induction from increased Tregs in breast (85) and ovarian cancers (95).
Figure 2. The role of pDCs in the cancer microenvironment. (A) Peritumoral pDCs confer tumor promotion by immunosuppression. Tumors can inhibit the production of type I IFN from pDCs via secretion of TGF-β and TNF-α, which confer pDCs with an immunosuppressive capacity. The pDCs derived IL-6 induces MDSCs recruitment via CCL2 and MDSCs reinforce the tumor immunosuppressive microenvironment. The pDCs directly induce the generation of Treg by expression of IDO and ICOSL. Inversely, pDCs can inhibit anti-tumor effector CD4+ T cells and cytotoxic CD8+ T cells by expression of IDO or granzyme B. (B) The immunosuppressive tumor microenvironment supported by intratumoral pDCs can be reversed. In the presence of strong stimuli by microbial ligands such as CpG or viral RNA, pDCs can be activated to produce a large amount of type I IFN via TLR7 or TLR9 signaling. Type I IFN produced by pDCs can induce the maturation of immature immunosuppressive MDSCs into mature antigen-presenting cells, which then contribute to initiate anti-tumor immunity. Activated pDCs can exert direct cytotoxicity to tumor cells by expression of TNF-related apoptosis inducing ligand and granzyme B. Alternatively, activated pDCs stimulates NK cells to kill tumor cells. The pDCs can also act as an APC to prime anti-tumor T cells.
ICOSL, inducible costimulator ligand.
When properly reprogrammed by stimuli such as TLR agonists, tumor promotion by peri- or intra-tumoral pDCs can be reversed (Fig. 2B), which can further mediate tumor rejection in a clinical setting (96). Tumor regression can be driven by restored production of type I IFN along with a cross-talk with other immune cells. Finally, pDCs can act as an APC to prime anti-tumor T cells. The pDCs isolated from human colon carcinomas have epithelial cell adhesion molecules (97). Despite deficiencies in phagocytosis, there exists an alternative mechanism of exchange for exogenous Ags between pDCs and tumor cells, which is a cell contact-dependent mechanism. This may enable pDCs to present Ag to tumor Ag-specific T cells. Furthermore, the pDCs can regulate myelopoiesis, especially CD11bhighGr-1+ MDSCs in the BM (98). When pDCs are activated by TLR9 stimulation, the production of IFN-α induces MDSC maturation and then blocks MDSC suppressive capacity (99). Activated pDCs can exert direct cytotoxicity to tumor cells by expression of TNF-related apoptosis inducing ligand and granzyme B (100). Alternatively, activated pDCs stimulate NK cells to kill tumor cells (101). Although still limited to several types of cancers besides colon cancer, modulation of pDCs by TLR ligand treatment can be a promising strategy for cancer immunotherapy.
CONCLUSION
Blood circulating peripheral pDCs are one of the main type I IFN producers in response to microbial signals to protect against viral infections. In addition, pDCs also constitute a unique cell subset that participates in the activation of autoreactive T cells via sensing of self-nucleic acids. In the intestine, however, the production of type I IFN by the gut pDCs can be regulated by interactions with the commensal microbes and mucosal environmental factors. Gut pDCs are critically involved in the maintenance of immune tolerance via generation of Tregs and secretory IgA from B cells. In intestinal diseased conditions, pDCs have contradictory roles for immune regulation or initiation of inflammation. This role of pDCs can be modulated by TLR stimuli. Further information for the role of gut pDCs can suggest novel therapeutic paradigms to control infection, inflammatory disease, and cancer in the gut.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and future Planning (NRF-2017R1A2B4002419).
Abbreviations
- APC
antigen-presenting cell
- BM
bone marrow
- cDC
classical dendritic cell
- CDP
common dendritic cell progenitor
- CSR
class-switching recombination
- DC
dendritic cell
- GALT
gut-associated lymphoid tissue
- HSV
herpes simplex virus
- IBD
inflammatory bowel disease
- ICOS
inducible co-stimulatory receptor
- IDO
indoleamine 2,3 dioxygenase
- ILC
innate lymphoid cell
- ILF
isolated lymphoid follicle
- IRF
IFN regulatory factor
- LN
lymph node
- LP
lamina propria
- MDSC
myeloid-derived suppressor cell
- MLN
mesenteric lymph node
- pDC
plasmacytoid dendritic cell
- PGE2
prostaglandin E2
- PP
Peyer's patch
- PSA
polysaccharide A
- TI
T cell-independent
- UC
ulcerative colitis
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Chang SY.
- Data curation: Won HY, Lee JY, Ryu D, Kim HT.
- Formal analysis: Won HY, Lee JY, Ryu D, Kim HT.
- Investigation: Chang SY.
- Supervision: Chang SY.
- Writing - original draft: Won HY, Lee JY, Ryu D, Kim HT, Chang SY.
- Writing - review & editing: Won HY, Lee JY, Ryu D, Kim HT, Chang SY.
References
- 1.Mann ER, Li X. Intestinal antigen-presenting cells in mucosal immune homeostasis: crosstalk between dendritic cells, macrophages and B-cells. World J Gastroenterol. 2014;20:9653–9664. doi: 10.3748/wjg.v20.i29.9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Persson EK, Scott CL, Mowat AM, Agace WW. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur J Immunol. 2013;43:3098–3107. doi: 10.1002/eji.201343740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 4.See P, Dutertre CA, Chen J, Günther P, McGovern N, Irac SE, Gunawan M, Beyer M, Händler K, Duan K, et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science. 2017;356:eaag3009. doi: 10.1126/science.aag3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Kim CH. Control of innate and adaptive lymphocytes by the RAR-retinoic acid axis. Immune Netw. 2018;18:e1. doi: 10.4110/in.2018.18.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 8.Bogunovic M, Ginhoux F, Helft J, Shang L, Hashimoto D, Greter M, Liu K, Jakubzick C, Ingersoll MA, Leboeuf M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hildner K, Edelson BT, Purtha WE, Diamond M, Matsushita H, Kohyama M, Calderon B, Schraml BU, Unanue ER, Diamond MS, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edelson BT, Kc W, Juang R, Kohyama M, Benoit LA, Klekotka PA, Moon C, Albring JC, Ise W, Michael DG, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. J Exp Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson JT, Hu Y, Liu R, Masson F, D'Amico A, Carotta S, Xin A, Camilleri MJ, Mount AM, Kallies A, et al. Id2 expression delineates differential checkpoints in the genetic program of CD8α+ and CD103+ dendritic cell lineages. EMBO J. 2011;30:2690–2704. doi: 10.1038/emboj.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satpathy AT, Briseño CG, Lee JS, Ng D, Manieri NA, Kc W, Wu X, Thomas SR, Lee WL, Turkoz M, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–948. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persson EK, Uronen-Hansson H, Semmrich M, Rivollier A, Hägerbrand K, Marsal J, Gudjonsson S, Håkansson U, Reizis B, Kotarsky K, et al. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 14.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 15.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Günther G, Johnston I, Lanzavecchia A, Nagasaka T, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 17.Contractor N, Louten J, Kim L, Biron CA, Kelsall BL. Cutting edge: Peyer's patch plasmacytoid dendritic cells (pDCs) produce low levels of type I interferons: possible role for IL-10, TGFbeta, and prostaglandin E2 in conditioning a unique mucosal pDC phenotype. J Immunol. 2007;179:2690–2694. doi: 10.4049/jimmunol.179.5.2690. [DOI] [PubMed] [Google Scholar]
- 18.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of the transcription factor e2-2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–916. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodrigues PF, Alberti-Servera L, Eremin A, Grajales-Reyes GE, Ivanek R, Tussiwand R. Distinct progenitor lineages contribute to the heterogeneity of plasmacytoid dendritic cells. Nat Immunol. 2018;19:711–722. doi: 10.1038/s41590-018-0136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Briseño CG, Grajales-Reyes GE, Haldar M, Iwata A, Kretzer NM, Kc W, Tussiwand R, Higashi Y, Murphy TL, et al. Transcription factor Zeb2 regulates commitment to plasmacytoid dendritic cell and monocyte fate. Proc Natl Acad Sci U S A. 2016;113:14775–14780. doi: 10.1073/pnas.1611408114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott CL, Soen B, Martens L, Skrypek N, Saelens W, Taminau J, Blancke G, Van Isterdael G, Huylebroeck D, Haigh J, et al. The transcription factor Zeb2 regulates development of conventional and plasmacytoid DCs by repressing Id2. J Exp Med. 2016;213:897–911. doi: 10.1084/jem.20151715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohara H, Omatsu Y, Sugiyama T, Noda M, Fujii N, Nagasawa T. Development of plasmacytoid dendritic cells in bone marrow stromal cell niches requires CXCL12-CXCR4 chemokine signaling. Blood. 2007;110:4153–4160. doi: 10.1182/blood-2007-04-084210. [DOI] [PubMed] [Google Scholar]
- 25.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, Förster R. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc Natl Acad Sci U S A. 2007;104:6347–6352. doi: 10.1073/pnas.0609180104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clahsen T, Pabst O, Tenbrock K, Schippers A, Wagner N. Localization of dendritic cells in the gut epithelium requires MAdCAM-1. Clin Immunol. 2015;156:74–84. doi: 10.1016/j.clim.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Lombardi VC, Khaiboullina SF. Plasmacytoid dendritic cells of the gut: relevance to immunity and pathology. Clin Immunol. 2014;153:165–177. doi: 10.1016/j.clim.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dasgupta S, Erturk-Hasdemir D, Ochoa-Reparaz J, Reinecker HC, Kasper DL. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. 2014;15:413–423. doi: 10.1016/j.chom.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 30.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. Toll-like receptor 9-mediated recognition of Herpes simplex virus-2 by plasmacytoid dendritic cells. J Exp Med. 2003;198:513–520. doi: 10.1084/jem.20030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koblansky AA, Jankovic D, Oh H, Hieny S, Sungnak W, Mathur R, Hayden MS, Akira S, Sher A, Ghosh S. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii . Immunity. 2013;38:119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swiecki M, Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat Rev Immunol. 2015;15:471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karthaus N, van Spriel AB, Looman MW, Chen S, Spilgies LM, Lieben L, Carmeliet G, Ansems M, Adema GJ. Vitamin D controls murine and human plasmacytoid dendritic cell function. J Invest Dermatol. 2014;134:1255–1264. doi: 10.1038/jid.2013.501. [DOI] [PubMed] [Google Scholar]
- 35.Yrlid U, Cerovic V, Milling S, Jenkins CD, Zhang J, Crocker PR, Klavinskis LS, MacPherson GG. Plasmacytoid dendritic cells do not migrate in intestinal or hepatic lymph. J Immunol. 2006;177:6115–6121. doi: 10.4049/jimmunol.177.9.6115. [DOI] [PubMed] [Google Scholar]
- 36.Yrlid U, Milling SW, Miller JL, Cartland S, Jenkins CD, MacPherson GG. Regulation of intestinal dendritic cell migration and activation by plasmacytoid dendritic cells, TNF-alpha and type 1 IFNs after feeding a TLR7/8 ligand. J Immunol. 2006;176:5205–5212. doi: 10.4049/jimmunol.176.9.5205. [DOI] [PubMed] [Google Scholar]
- 37.Uto T, Takagi H, Fukaya T, Nasu J, Fukui T, Miyanaga N, Arimura K, Nakamura T, Choijookhuu N, Hishikawa Y, et al. Critical role of plasmacytoid dendritic cells in induction of oral tolerance. J Allergy Clin Immunol. 2018;141:2156–2167.e9. doi: 10.1016/j.jaci.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Bonnefoy F, Couturier M, Clauzon A, Rémy-Martin JP, Gaugler B, Tiberghien P, Chen W, Saas P, Perruche S. TGF-beta-exposed plasmacytoid dendritic cells participate in Th17 commitment. J Immunol. 2011;186:6157–6164. doi: 10.4049/jimmunol.1002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tezuka H, Abe Y, Asano J, Sato T, Liu J, Iwata M, Ohteki T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity. 2011;34:247–257. doi: 10.1016/j.immuni.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Juelke K, Romagnani C. Differentiation of human innate lymphoid cells (ILCs) Curr Opin Immunol. 2016;38:75–85. doi: 10.1016/j.coi.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Simoni Y, Fehlings M, Kløverpris HN, McGovern N, Koo SL, Loh CY, Lim S, Kurioka A, Fergusson JR, Tang CL, et al. Human innate lymphoid cell subsets possess tissue-type based heterogeneity in phenotype and frequency. Immunity. 2017;46:148–161. doi: 10.1016/j.immuni.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferlazzo G, Münz C. Dendritic cell interactions with NK cells from different tissues. J Clin Immunol. 2009;29:265–273. doi: 10.1007/s10875-009-9283-y. [DOI] [PubMed] [Google Scholar]
- 43.Swann JB, Hayakawa Y, Zerafa N, Sheehan KC, Scott B, Schreiber RD, Hertzog P, Smyth MJ. Type I IFN contributes to NK cell homeostasis, activation, and antitumor function. J Immunol. 2007;178:7540–7549. doi: 10.4049/jimmunol.178.12.7540. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs A, Cella M, Kondo T, Colonna M. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood. 2005;106:2076–2082. doi: 10.1182/blood-2004-12-4802. [DOI] [PubMed] [Google Scholar]
- 45.Ju X, Zenke M, Hart DN, Clark GJ. CD300a/c regulate type I interferon and TNF-alpha secretion by human plasmacytoid dendritic cells stimulated with TLR7 and TLR9 ligands. Blood. 2008;112:1184–1194. doi: 10.1182/blood-2007-12-127951. [DOI] [PubMed] [Google Scholar]
- 46.Maazi H, Banie H, Aleman Muench GR, Patel N, Wang B, Sankaranarayanan I, Bhargava V, Sato T, Lewis G, Cesaroni M, et al. Activated plasmacytoid dendritic cells regulate type 2 innate lymphoid cell-mediated airway hyperreactivity. J Allergy Clin Immunol. 2018;141:893–905.e6. doi: 10.1016/j.jaci.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 47.Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention JJ, Thiam K, Cerf-Bensussan N, Mandelboim O, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–970. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Cheng L, Zhao J, Li G, Zhang L, Chen W, Nie W, Reszka-Blanco NJ, Wang FS, Su L. Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. J Clin Invest. 2015;125:3692–3703. doi: 10.1172/JCI82124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo X, Fu YX. The tragic fate of group 3 innate lymphoid cells during HIV-1 infection. J Clin Invest. 2015;125:3430–3432. doi: 10.1172/JCI83823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 52.Mouriès J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Gregorio JD, Iwahori T, Zhang X, Choi O, Tolentino LL, Prestwood T, Carmi Y, Engleman EG. A distinct subset of plasmacytoid dendritic cells induces activation and differentiation of B and T lymphocytes. Proc Natl Acad Sci U S A. 2017;114:1988–1993. doi: 10.1073/pnas.1610630114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guéry L, Dubrot J, Lippens C, Brighouse D, Malinge P, Irla M, Pot C, Reith W, Waldburger JM, Hugues S. Ag-presenting CpG-activated pDCs prime Th17 cells that induce tumor regression. Cancer Res. 2014;74:6430–6440. doi: 10.1158/0008-5472.CAN-14-1149. [DOI] [PubMed] [Google Scholar]
- 55.Yu CF, Peng WM, Oldenburg J, Hoch J, Bieber T, Limmer A, Hartmann G, Barchet W, Eis-Hübinger AM, Novak N. Human plasmacytoid dendritic cells support Th17 cell effector function in response to TLR7 ligation. J Immunol. 2010;184:1159–1167. doi: 10.4049/jimmunol.0901706. [DOI] [PubMed] [Google Scholar]
- 56.Hubo M, Jonuleit H. Plasmacytoid dendritic cells are inefficient in activation of human regulatory T cells. PLoS One. 2012;7:e44056. doi: 10.1371/journal.pone.0044056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konieczna P, Groeger D, Ziegler M, Frei R, Ferstl R, Shanahan F, Quigley EM, Kiely B, Akdis CA, O'Mahony L. Bifidobacterium infantis 35624 administration induces Foxp3 T regulatory cells in human peripheral blood: potential role for myeloid and plasmacytoid dendritic cells. Gut. 2012;61:354–366. doi: 10.1136/gutjnl-2011-300936. [DOI] [PubMed] [Google Scholar]
- 58.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 60.Mann ER, Bernardo D, Ng SC, Rigby RJ, Al-Hassi HO, Landy J, Peake ST, Spranger H, English NR, Thomas LV, et al. Human gut dendritic cells drive aberrant gut-specific t-cell responses in ulcerative colitis, characterized by increased IL-4 production and loss of IL-22 and IFNγ. Inflamm Bowel Dis. 2014;20:2299–2307. doi: 10.1097/MIB.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 61.Wittmann A, Autenrieth IB, Frick JS. Plasmacytoid dendritic cells are crucial in Bifidobacterium adolescentis-mediated inhibition of Yersinia enterocolitica infection. PLoS One. 2013;8:e71338. doi: 10.1371/journal.pone.0071338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aoki-Yoshida A, Yamada K, Hachimura S, Sashihara T, Ikegami S, Shimizu M, Totsuka M. Enhancement of oral tolerance induction in do11.10 mice by Lactobacillus gasseri oll2809 via increase of effector regulatory t cells. PLoS One. 2016;11:e0158643. doi: 10.1371/journal.pone.0158643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3:63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 64.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 65.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Moro-Sibilot L, This S, Blanc P, Sanlaville A, Sisirak V, Bardel E, Boschetti G, Bendriss-Vermare N, Defrance T, Dubois B, et al. Plasmacytoid dendritic cells are dispensable for noninfectious intestinal IgA responses in vivo . Eur J Immunol. 2016;46:354–359. doi: 10.1002/eji.201545977. [DOI] [PubMed] [Google Scholar]
- 67.Cho H, Kelsall BL. The role of type I interferons in intestinal infection, homeostasis, and inflammation. Immunol Rev. 2014;260:145–167. doi: 10.1111/imr.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Deal EM, Lahl K, Narváez CF, Butcher EC, Greenberg HB. Plasmacytoid dendritic cells promote rotavirus-induced human and murine B cell responses. J Clin Invest. 2013;123:2464–2474. doi: 10.1172/JCI60945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon αβ in acute influenza infection. Nat Commun. 2014;5:3864. doi: 10.1038/ncomms4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alculumbre S, Raieli S, Hoffmann C, Chelbi R, Danlos FX, Soumelis V. Plasmacytoid pre-dendritic cells (pDC): from molecular pathways to function and disease association. Semin Cell Dev Biol. 2018:S1084-9521(17)30385-3. doi: 10.1016/j.semcdb.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Lehmann C, Jung N, Förster K, Koch N, Leifeld L, Fischer J, Mauss S, Drebber U, Steffen HM, Romerio F, et al. Longitudinal analysis of distribution and function of plasmacytoid dendritic cells in peripheral blood and gut mucosa of HIV infected patients. J Infect Dis. 2014;209:940–949. doi: 10.1093/infdis/jit612. [DOI] [PubMed] [Google Scholar]
- 72.Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, Bhardwaj N. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tam MA, Wick MJ. Differential expansion, activation and effector functions of conventional and plasmacytoid dendritic cells in mouse tissues transiently infected with Listeria monocytogenes . Cell Microbiol. 2006;8:1172–1187. doi: 10.1111/j.1462-5822.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 74.Toivonen R, Kong L, Rasool O, Lund RJ, Lahesmaa R, Hänninen A. Activation of plasmacytoid dendritic cells in colon-draining lymph nodes during Citrobacter rodentium infection involves pathogen-sensing and inflammatory pathways distinct from conventional dendritic cells. J Immunol. 2016;196:4750–4759. doi: 10.4049/jimmunol.1600235. [DOI] [PubMed] [Google Scholar]
- 75.Podolsky DK. Inflammatory bowel disease (1) N Engl J Med. 1991;325:928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 76.Baumgart DC, Metzke D, Guckelberger O, Pascher A, Grötzinger C, Przesdzing I, Dörffel Y, Schmitz J, Thomas S. Aberrant plasmacytoid dendritic cell distribution and function in patients with Crohn's disease and ulcerative colitis. Clin Exp Immunol. 2011;166:46–54. doi: 10.1111/j.1365-2249.2011.04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arimura K, Takagi H, Uto T, Fukaya T, Nakamura T, Choijookhuu N, Hishikawa Y, Yamashita Y, Sato K. Crucial role of plasmacytoid dendritic cells in the development of acute colitis through the regulation of intestinal inflammation. Mucosal Immunol. 2017;10:957–970. doi: 10.1038/mi.2016.96. [DOI] [PubMed] [Google Scholar]
- 78.Sawai CM, Serpas L, Neto AG, Jang G, Rashidfarrokhi A, Kolbeck R, Sanjuan MA, Reizis B, Sisirak V. Plasmacytoid dendritic cells are largely dispensable for the pathogenesis of experimental inflammatory bowel disease. Front Immunol. 2018;9:2475. doi: 10.3389/fimmu.2018.02475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sainathan SK, Hanna EM, Gong Q, Bishnupuri KS, Luo Q, Colonna M, White FV, Croze E, Houchen C, Anant S, et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vijay-Kumar M, Wu H, Aitken J, Kolachala VL, Neish AS, Sitaraman SV, Gewirtz AT. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis. 2007;13:856–864. doi: 10.1002/ibd.20142. [DOI] [PubMed] [Google Scholar]
- 82.Yang JY, Kim MS, Kim E, Cheon JH, Lee YS, Kim Y, Lee SH, Seo SU, Shin SH, Choi SS, et al. Enteric viruses ameliorate gut inflammation via toll-like receptor 3 and toll-like receptor 7-mediated interferon-β production. Immunity. 2016;44:889–900. doi: 10.1016/j.immuni.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 83.Norman JM, Handley SA, Baldridge MT, Droit L, Liu CY, Keller BC, Kambal A, Monaco CL, Zhao G, Fleshner P, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demoulin S, Herfs M, Delvenne P, Hubert P. Tumor microenvironment converts plasmacytoid dendritic cells into immunosuppressive/tolerogenic cells: insight into the molecular mechanisms. J Leukoc Biol. 2013;93:343–352. doi: 10.1189/jlb.0812397. [DOI] [PubMed] [Google Scholar]
- 85.Sisirak V, Vey N, Goutagny N, Renaudineau S, Malfroy M, Thys S, Treilleux I, Labidi-Galy SI, Bachelot T, Dezutter-Dambuyant C, et al. Breast cancer-derived transforming growth factor-β and tumor necrosis factor-α compromise interferon-α production by tumor-associated plasmacytoid dendritic cells. Int J Cancer. 2013;133:771–778. doi: 10.1002/ijc.28072. [DOI] [PubMed] [Google Scholar]
- 86.Terra M, Oberkampf M, Fayolle C, Rosenbaum P, Guillerey C, Dadaglio G, Leclerc C. Tumor-derived TGFβ alters the ability of plasmacytoid dendritic cells to respond to innate immune signaling. Cancer Res. 2018;78:3014–3026. doi: 10.1158/0008-5472.CAN-17-2719. [DOI] [PubMed] [Google Scholar]
- 87.Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gai XD, Song Y, Li C, Lei YM, Yang B. Potential role of plasmacytoid dendritic cells for FOXP3+ regulatory T cell development in human colorectal cancer and tumor draining lymph node. Pathol Res Pract. 2013;209:774–778. doi: 10.1016/j.prp.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 90.Legitimo A, Consolini R, Failli A, Orsini G, Spisni R. Dendritic cell defects in the colorectal cancer. Hum Vaccin Immunother. 2014;10:3224–3235. doi: 10.4161/hv.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jahrsdörfer B, Vollmer A, Blackwell SE, Maier J, Sontheimer K, Beyer T, Mandel B, Lunov O, Tron K, Nienhaus GU, et al. Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion. Blood. 2010;115:1156–1165. doi: 10.1182/blood-2009-07-235382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, Messina JL, Chandler P, Koni PA, Mellor AL. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114:280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castelli C, Triebel F, Rivoltini L, Camisaschi C. Lymphocyte activation gene-3 (LAG-3, CD223) in plasmacytoid dendritic cells (pDCs): a molecular target for the restoration of active antitumor immunity. OncoImmunology. 2014;3:e967146. doi: 10.4161/21624011.2014.967146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang XM, Liu XS, Lin XK, Yu H, Sun JY, Liu XK, Chen C, Jin HL, Zhang GE, Shi XX, et al. Role of plasmacytoid dendritic cells and inducible costimulator-positive regulatory T cells in the immunosuppression microenvironment of gastric cancer. Cancer Sci. 2014;105:150–158. doi: 10.1111/cas.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Labidi-Galy SI, Sisirak V, Meeus P, Gobert M, Treilleux I, Bajard A, Combes JD, Faget J, Mithieux F, Cassignol A, et al. Quantitative and functional alterations of plasmacytoid dendritic cells contribute to immune tolerance in ovarian cancer. Cancer Res. 2011;71:5423–5434. doi: 10.1158/0008-5472.CAN-11-0367. [DOI] [PubMed] [Google Scholar]
- 96.Le Mercier I, Poujol D, Sanlaville A, Sisirak V, Gobert M, Durand I, Dubois B, Treilleux I, Marvel J, Vlach J, et al. Tumor promotion by intratumoral plasmacytoid dendritic cells is reversed by TLR7 ligand treatment. Cancer Res. 2013;73:4629–4640. doi: 10.1158/0008-5472.CAN-12-3058. [DOI] [PubMed] [Google Scholar]
- 97.Bonaccorsi I, Morandi B, Antsiferova O, Costa G, Oliveri D, Conte R, Pezzino G, Vermiglio G, Anastasi GP, Navarra G, et al. Membrane transfer from tumor cells overcomes deficient phagocytic ability of plasmacytoid dendritic cells for the acquisition and presentation of tumor antigens. J Immunol. 2014;192:824–832. doi: 10.4049/jimmunol.1301039. [DOI] [PubMed] [Google Scholar]
- 98.Ioannou M, Alissafi T, Boon L, Boumpas D, Verginis P. In vivo ablation of plasmacytoid dendritic cells inhibits autoimmunity through expansion of myeloid-derived suppressor cells. J Immunol. 2013;190:2631–2640. doi: 10.4049/jimmunol.1201897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zoglmeier C, Bauer H, Noerenberg D, Wedekind G, Bittner P, Sandholzer N, Rapp M, Anz D, Endres S, Bourquin C. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–1775. doi: 10.1158/1078-0432.CCR-10-2672. [DOI] [PubMed] [Google Scholar]
- 100.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. J Clin Invest. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu C, Lou Y, Lizée G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang YH, Ye Y, Sikora AG, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]