Highlights
-
•
This is the first quantitative synthesis estimating the risk of aggressive behaviors in dementia and mild cognitive impairment.
-
•
Individuals with Alzheimer's disease have five times higher odds of aggression than healthy controls.
-
•
We found no differences in risks of aggression between mild cognitive impairment and normal population controls and between dementias of differing aetiologies.
Key Words: Alzheimer disease, mild cognitive impairment, aggressive behaviors, systematic review
Abstract
Objective
We aim to estimate the risk of perpetrating aggression in Alzheimer disease (AD) and mild cognitive impairment (MCI) by conducting a systematic review and meta-analysis of primary studies.
Methods
A systematic search was conducted in six bibliographic databases according to a preregistered protocol. Studies that reported aggressive behaviors in individuals with AD and MCI compared with healthy individuals or those with other dementia etiologies were identified. Risks of aggressive behaviors were assessed using random effects models to calculate pooled odds ratios (ORs). Publication bias was examined.
Results
In total, 17 studies involving 6,399 individuals with AD and 2,582 with MCI were identified. Compared with healthy individuals, significantly increased risks of aggressive behaviors were found in AD (OR, 4.9, 95% CI, 1.8–13.2) but not in MCI (OR, 1.8, 95% CI, 0.7–4.3). When comparing AD with MCI, the risk in AD was higher (OR, 2.6, 95% CI, 1.7–4.0). We found no differences in risk of aggressive behaviors between AD and other dementia subtypes or between amnestic and nonamnestic MCI.
Conclusion
Individuals with AD are at higher risk of manifesting aggressive behaviors than healthy individuals or those with MCI. Our findings not only underscore the necessity of treatment of aggressive behaviors in AD but also highlight the importance of preventing the transition from MCI to AD.
INTRODUCTION
Evidence increasingly suggests elevated risk of exhibiting aggressive behaviors in Alzheimer disease (AD) and mild cognitive impairment (MCI). Such aggressive behaviors are among the most frequent and disruptive behavioral complications of cognitive decline contributing to increased cost of care, hospitalization, caregiver burden, and risk of premature institutionalization. Aggressive behaviors in these conditions are associated with medication use and physical restraint.1, 2, 3 Antipsychotic use is of limited effectiveness and is associated with potentially harmful side effects, such as increased risk of stroke and death.4 Physical restraint has been associated with a multitude of adverse psychological and physical effects.5, 6 Recent work has also suggested that caregiver distress is more closely connected to aggressive behaviors than key symptoms of AD and MCI.7 Furthermore, aggressive behaviors are major contributors to the financial burden of these conditions, especially as they frequently lead to premature institutionalization.8, 9, 10, 11 The precise magnitude of the risk of aggressive behaviors in AD and MCI remains unknown, as wide variations in estimates have been reported. For example, increased odds of aggressive behaviors have been reported to vary from 2 to 11 in AD and from 0.5 to 4 in MCI.12, 13, 14, 15 To our knowledge, a quantitative analysis of primary studies has not been conducted. In this article, we used a quantitative approach to robustly estimate the risk of aggressive behaviors in AD and MCI. This could potentially aid caregivers, clinicians, and policy makers in facilitating planning of both patient care and public health policy.
METHODS
Search Strategy
We followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines with a protocol registered with PROSPERO (registration CRD42017080952). Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines were followed. Studies of the association of AD, MCI, and aggressive behaviors were sought by searches of six computer-based databases (Medline, Embase, PubMed, PsycINFO, CINAHL, and Web of Science). We used combinations of key words related to MCI (cognitive impair*, cognitive decline*, and cognitive fail*), AD (dement*, alzheimer*, frontotemporal), and aggressive behaviors (aggress*, assault*, viol*, offen*, antisocial, anti-social, dangerous*, crim*, unlawful*). These were supplemented with scanning of article reference lists and correspondence with authors. Case-control and cohort studies were included if they investigated the risk of aggressive behaviors in individuals with AD and MCI compared with individuals without these disorders. We included studies that reported aggressive behaviors using validated scales such as the Neuropsychiatric Inventory (NPI) and the Behavioral Pathology in Alzheimer's Disease Rating Scale. AD and MCI were diagnosed by validated instruments or standard clinical interview. Studies were excluded if they did not provide information that allowed for the calculation of odds ratios (ORs). In addition, studies of individuals who were inpatients or in other institutional settings were excluded to avoid probable biases associated with these samples.16
Data Extraction
A standardized form was used to extract data from the included studies. For every eligible study, the following information was extracted: numbers of individuals with and without AD/MCI by aggressive behavior status, age, sex breakdown, geographic location, year of publication, diagnostic instrument, study setting, study design, and informant. The information was recorded and coded according to a fixed protocol. Data were extracted and cross-checked by two authors (RY and AT). Discrepancies were resolved by further review, discussion among RY and AT, and consultation with SF.
Statistical Analysis
To synthesize the evidence from the literature, we conducted meta-analyses. Analyses were conducted in Stata 14 (StataCorp, College Station, TX). ORs with 95% confidence intervals (CIs) of the risk of aggressive behaviors in AD or MCI compared with healthy subjects were combined using meta-analysis. We also conducted comparisons between AD and MCI, AD subtypes, and MCI subtypes. The data were presented in forest plots. Random effects models, which incorporate an estimate of between-study heterogeneity into the calculation of the common effect, were used, as the heterogeneity between studies was high.17 Random effects estimates can give relatively similar weight to studies of different size.
Heterogeneity between studies was estimated using the I2 statistic, which describes the percentage of variation across studies because of heterogeneity rather than chance. I2does not inherently depend on the number of studies considered. For I2, the values of 25%, 50%, and 75% indicate low, moderate, and high levels of heterogeneity, respectively.18 Publication bias was tested by funnel plot asymmetry using the rank correlation method.19
RESULTS
Figure 1 provides details of the study selection process. The final sample consisted of 17 studies, with 6,399 individuals with AD and 2,582 persons with MCI. A large proportion of studies were conducted in the United States (9 out of 17); the rest were from Belgium, Nigeria, the United Kingdom, Tanzania, Brazil, Japan, Taiwan, and South Korea. All studies were conducted after the year 1999. The most commonly used scale for the outcomes was the NPI (9 out of 17). Details of the included studies are summarized in Table 1.
FIGURE 1.
Flow chart of the systematic search strategy.
TABLE 1.
Characteristics of Included Studies on Risk of Aggression in Alzheimer Disease and Mild Cognitive Impairment
| Study | Country | Comparisons | AD n |
MCI n |
Comparison Group (n) | Outcome | Design | Measurements | Informant | Age Range/Mean Age (Years) | Female % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baiyewu 201212 | Nigeria | AD versus MCI versus HC | 34 | 53 | 21 | Agitation/aggression | Cross-sectional | NPI | Yes | AD: 83, MCI: 81, HC: 83 |
87 |
| Lyketsos 200213 | United States | AD versus MCI versus HC | 362 | 320 | 653 | Arrest/crime for public order, driving or violent offense | Cross-sectional | NPI | Yes | 70–80 | 32 |
| Paddick 201514 | Tanzania | AD versus MCI versus HC | 78 | 46 | 172 | Agitation/aggression | Cross-sectional | NPI | Yes | AD: 85, MCI: 82, HC:a 78 | 80 |
| Tatsch 200615 | Brazil | AD versus MCI versus HC | 60 | 25 | 78 | Agitation/aggression | Cross-sectional | NPI | Yes | 70–80 | 71 |
| Gallagher 201120 | United Kingdom | AD versus MCI | 69 | 92 | Aggression | Longitudinal | BEHAVE-AD | Yes | AD: 75, MCI: 73 |
43 | |
| Mussele 201524 | Belgium | AD versus MCI | 393 | 268 | Aggression | Cross-sectional | CMAI & BEHAVE-AD | Yes | 50–97 | 62 | |
| Liljegren 201521 | United States | AD versus MCI | 545 | 243 | Criminal behaviors | Retrospective | Electronic database | No | 59–71 | 37 | |
| Lopez 200522 | United States | AD versus MCI | 427 | 228 | Aggression | Cross-sectional | Interview with psychiatrist | Yes | MCI: 70, AD: 73 |
60 | |
| Rockwood 201523 | United States | AD versus MCI | 388 | 684 | Aggression | Cross-sectional | Symptom guide based on NPI | Yes | 72 | 58 | |
| Lee 200827 | South Korea | MCI subtypes | 0 | 7 | 210 | Agitation/aggression | Cross-sectional | NPI | Yes | 70–75 | 65 |
| Edwards 200925 | United States | MCI subtypes | 50 | 56 | 328 | Aggression | Cross-sectional | Clinical interview | Yes | n/a | 53 |
| Ellison 200826 | United States | MCI subtypes | 16 | 4 | 18 | Agitation/aggression | Cross-sectional | NPI | Yes | 75 | 20 |
| Ikeda 200429 | Japan | AD versus other dementia | 21 | 60 | Agitation/aggression | Cross-sectional | NPI | Yes | 82 | 86 | |
| Lyketsos 199932 | United States | AD versus other dementia | 296 | 99 | Agitation/aggression | Cross-sectional | Patel and Hope definition | Yes | 75 | 75 | |
| Chiu 200628 | Taiwan | AD versus other dementia | 75 | 28 | Aggression | Retrospective | BEHAVE-AD | Yes | 72 | 78 | |
| Orengo 200830 | United States | AD versus other dementia | 82 | 70 | Aggression | Cross-sectional | Ryden Aggression Scale | Yes | n/a | 1 | |
| Sadak 201331 | United States | AD versus other dementia | 3338 | 239 | Agitation/aggression | Cross-sectional | NPI | Yes | 79 | 59 |
Notes: BEHAVE-AD: Behavioral Pathology in Alzheimer's Disease Rating Scale; CMAI: Cohen-Mansfield Agitation Inventory; HC: healthy individuals; n/a: not applicable; NPS: neuropsychiatric symptoms.
Median (interquartile range).
Of the 17 studies included, four provided data on aggressive behaviors in AD and MCI, with healthy elderly as the comparison group.12, 13, 14, 15 Nine studies provided data for comparison between AD and MCI,12, 13, 14, 15,20, 21, 22, 23, 24 three for comparison between amnestic and nonamnestic MCI,25, 26, 27 and five comparing AD and other types of dementia.13,28, 29, 30, 31 Of the individuals with AD, 27.8% (n = 2,321) had aggressive behaviors compared with 7.4% (n = 306) of those with MCI and 5.8% (n = 54) of the healthy elderly. Aggressive behaviors included physical aggression, verbal outbursts, agitation, and crime. The overall prevalence of aggression in AD patients was 28% in population-based studies12, 13, 14, 15,27, 29 and 23% in samples from memory clinics.20, 21, 22,24, 25, 26,28,30, 31, 32 The prevalence of aggression in MCI patients was 11% in population-based studies12, 13, 14, 15,24 and 12% in samples from memory clinics.20, 21, 22,24, 25, 26,32
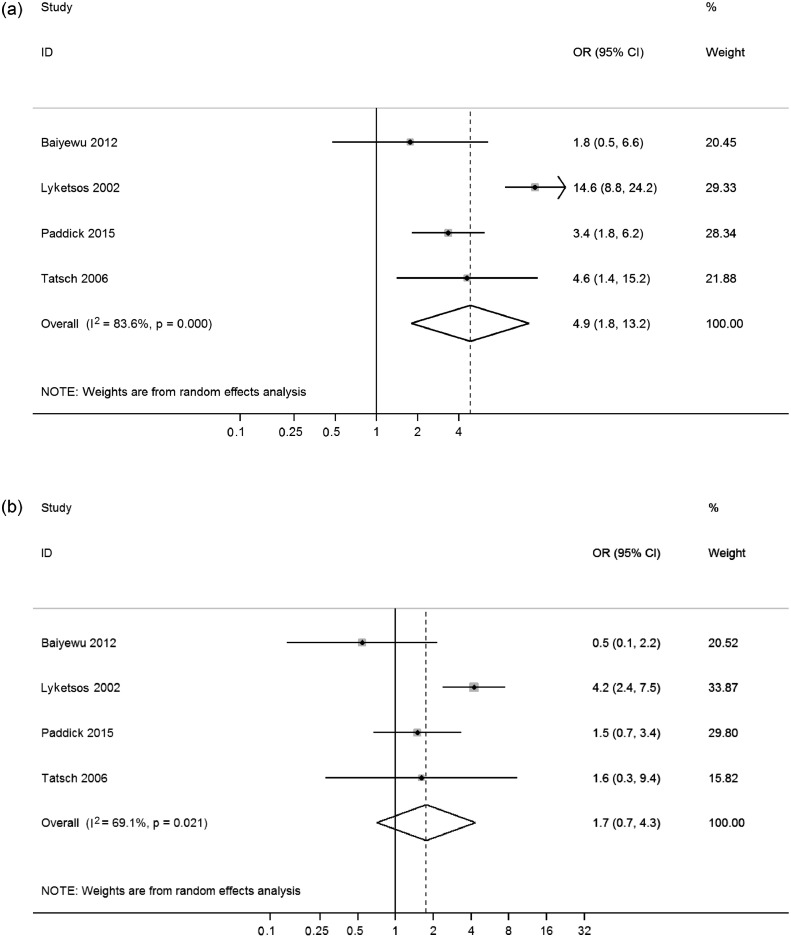
Meta-analyses showed that AD was associated with increased odds of aggressive behaviors compared with healthy elderly (OR, 4.9, 95% CI, 1.8–13.2), with high heterogeneity across studies ( = 18.3, p < 0.001, I2 = 84%) (Fig. 2a). In contrast, MCI was not significantly associated with aggressive behaviors. The overall random effects pooled OR was 1.8 (95% CI, 0.7–4.3), with moderate heterogeneity between studies ( = 9.7, p = 0.02, I2 = 69%) (Fig. 2b). The risk of aggressive behaviors in AD was higher than in MCI (OR, 2.6, 95% CI, 1.7–4.0), with moderate heterogeneity ( = 21.6, p = 0.01, I2 = 63%) (Fig. 3). Risks were similar for AD compared with other dementia subtypes, such as vascular and frontotemporal dementia (OR, 0.9, 95% CI, 0.3–2.5) (Fig. 4), and there were no significant differences in risk of aggressive behaviors when comparing amnestic and nonamnestic MCI ( = 11.0, p = 0.03) (Fig. 4).
FIGURE 2.
Risk of aggressive outcomes in Alzheimer disease (a) and mild cognitive impairment (b) compared with healthy individuals. ID: identification.
FIGURE 3.
Risk of aggressive outcomes in Alzheimer disease compared with mild cognitive impairment. ID: identification.
FIGURE 4.
Comparisons of risk of aggressive outcomes among dementia subtypes and mild cognitive impairment subtypes. ID: identification.
In the comparison between cases (AD and MCI) and healthy individuals, three studies provided data on the number of men and women in the AD group12, 13, 14 and four studies provided these data in the MCI group.12, 13, 14, 15 The proportion of men in each sample explained some of the differences between studies. We observed that studies with the lowest percentages of men (18% in the AD sample and 8% in the MCI sample) reported the lowest ORs in the comparisons between AD and healthy elderly (OR, 1.8, 95% CI, 0.5-6.6) and between MCI and healthy elderly (OR, 0.5, 95% CI, 0.1–2.2). In contrast, the studies with the highest percentages of men showed the highest risk. ORs were 14.6 (95% CI, 8.8-24.2) in the comparison between AD and healthy individuals and 4.2 (95% CI, 2.4-7.5) in the comparison between MCI and healthy individuals. Because of limited data, we were not able to conduct subgroup analyses to examine sex differences. However, we calculated percentages of men in the patient group and conducted metaregression testing to test whether this explained between-study variations in the odds of aggression. We found no association in the AD group (t = -0.09, p = 0.94, df = 2) and a nonsignificant association in the MCI group (t = 3.11, p = 0.09, df = 3).
In addition, we conducted subgroup analyses comparing the risk of aggression between AD and MCI by study setting. The pooled OR was 3.2 (95% CI, 2.2-4.5) in population-based studies and 2.5 (1.0-5.9) in samples from memory clinics. Because of limited data, we were not able to do subgroup analyses by study setting in other comparisons. In particular, all four studies that compared AD and MCI to healthy individuals were population-based.12, 13, 14, 15 Among the five studies comparing AD with other types of dementia, only one was population-based;29 the other four were from memory clinics. All three studies comparing amnestic MCI with nonamnestic MCI were from memory clinics.25, 26, 27
We found no evidence of publication bias in studies comparing AD and healthy individuals (t = -1.16, p = 0.37, df = 3), MCI and healthy individuals (t = -1.72, p = 0.23, df = 3), or AD and MCI (t = -0.31, p = 0.76, df = 8); those comparing MCI subtypes (t = -0.59, p = 0.66, df = 2); or those comparing AD and other dementias (t = -0.66, p = 0.56, df = 4).
CONCLUSION
Main Findings
In this systematic review and meta-analysis, we identified 17 studies involving AD and MCI—and a total of 8,981 cases—based in nine countries. Overall, we found 27.8% of individuals with AD and 7.4% with MCI exhibited aggressive behaviors. In those with AD, this equated to pooled increased odds of around five compared with healthy individuals. In those with MCI, there were slightly elevated odds of around two, but this was not statistically significant. There was moderate to high heterogeneity in risk estimates between studies, with the proportion of male participants providing one explanation. In addition, we found no clear differences in the risk of aggressive behaviors between AD and other forms of dementia or between amnestic and nonamnestic MCI.
Implications
Our study shows that the risks of exhibiting aggressive behaviors in AD are significantly higher than in healthy individuals. The high absolute risk of aggression in AD and the negative impact of these behaviors on patients themselves, caregivers, and healthcare services underscore the importance of these findings and the need for proactive management of aggression. Furthermore, they emphasize the need for prevention strategies. A recent Lancet Commission on dementia prevention estimated the total adjusted population attributable risk fraction (the percentage reduction in new cases over a given time if nine identified risk factors are completely eliminated) at 35%,33 which is likely an underestimate, as homocysteine levels and alcohol intake, which are modifiable, were not included.34, 35
Despite the high prevalence and wide range of negative outcomes in AD, currently there is a lack of effective and safe treatment options for aggressive behaviors. The most recent systematic review of antipsychotic medications for behavioral and psychological symptoms (which included but was not limited to aggression) in dementia identified 12 meta-analyses reporting modest effect sizes.36 Efficacy may be higher in hospital inpatients or those with more severe symptoms.36 Although antipsychotics may be more effective than nonpharmacologic strategies, their harms to patients is likely to be higher.37 Of particular concern is the higher risk of cerebrovascular events and death.4 In clinical practice, aggressive behaviors manifest along a severity spectrum, from aggressive resistance (usually occurring in the context of intimate care)38 to very rare extreme events such as homicide.39 Given the risks, antipsychotics should be reserved for those with the most severe symptoms.40 Nonpharmacologic interventions, including environmental and behavioral modification, are safer alternatives for less severe symptoms and, possibly, MCI.41, 42
However, these interventions can be difficult to implement, especially in nursing home settings, where staff-to-resident ratios are frequently low.43 Recent research has shown preliminary evidence of the efficacy of electroconvulsive therapy in reducing aggressive behaviors in patients with dementia,44 although future studies are warranted to confirm these findings and the use of such therapy is likely to be limited to the most difficult cases.
To develop effective preventive and treatment strategies, a deeper understanding of risk factors and underlying mechanisms will be required. Studies have indicated that depression, chronic pain, loss of family contact, social deprivation, caregiver-patient relationships, and the nursing home environment might be related to aggression in AD.45, 46, 47 Future research is required to clarify the independence, strength, and interaction of these risk factors.
We found no significant difference in aggression between AD and other forms of dementia. This might be because of limited data that restricted group comparisons. Clinical experience suggests that there could be differences between, for instance, AD and frontotemporal dementia. It has been reported that the prevalence of criminal behaviors is 37% in frontotemporal dementia and 8% in AD.21 However, we found insufficient data to investigate these different dementia presentations using meta-analytic methods. Our analyses indicate that the risk of aggression is similar in both amnestic and nonamnestic MCI even though amnestic MCI predominantly affects short-term memory, whereas nonamnestic MCI is characterized by disturbances in attention/concentration, information processing, psychomotor speed, language, and executive function.48 There were insufficient studies examining MCI subgroups of different etiology (e.g., vascular versus nonvascular) and functional status (e.g., MCI-I versus MCI-II). Additional studies and individual patient meta-analyses may facilitate evidence synthesis.
Sex and Other Factors
Among the studies providing data on the number of men and women,12, 13, 14, 15 we found that those with the highest proportion of men reported the highest prevalence of aggression. This is consistent with reports examining other disruptive behavioral problems, such as wandering, abusiveness, and social impropriety.49 Sex differences in behavioral problems associated with AD could affect treatment decisions. For instance, psychoactive medications are more likely to be used for the treatment of behavioral disturbances in men with AD than in women.49 Nevertheless, the mechanism behind the sex differences remains unknown, and future studies are needed to investigate this and potentially inform treatment strategies.
We found that the absolute prevalence of aggression in AD and MCI was similar in both population-based and memory clinic samples. This finding is not inconsistent with the possibility that cases referred to memory clinics are more likely to be deemed more cognitively impaired and behaviorally challenged15, 50 because of the way aggression is measured. In all of the population-based studies, aggression was measured with an NPI questionnaire that combined aggression and agitation, whereas aggression was measured in memory clinics with additional specific questionnaires.21, 25
In addition, we found that the absolute rate of aggression in AD was higher in memory clinic samples than population-based samples, although the rates of aggression in MCI were similar in these two settings. Furthermore, subgroup analyses by study setting demonstrated similar relative risk in the comparison between AD and MCI. Because of limited data, analyses were not conducted to explore other factors, such as diagnostic instrument, that might contribute to heterogeneity between studies.
Limitations
A number of limitations should be noted. First, the majority of included studies used the Behavioral Pathology in Alzheimer's Disease Rating Scale, the Cohen-Mansfield Agitation Inventory, or the NPI to identify aggressive behaviors. These instruments capture information from only the preceding 2–4 weeks. Therefore, any calculations based on them are underestimates of the absolute risk of aggression throughout the course of a cognitive disorder, although any underestimation of relative risks will depend on whether the time at risk in the comparison group is similar. Second, we were unable to distinguish aggression from agitation in 9 out of 17 studies, as the recording instruments (such as the NPI) combined these two behaviors. Likewise, we could not separately examine risks of physical and verbal aggression. In addition, one included study suggested that AD was related to criminal behaviors, which is consistent with a previous study reporting dementia to be prevalent (7%) in older mentally disordered offenders.52 However, more studies examining the link between dementia and crime are necessary. As these outcomes are of varying severity and lead to different consequences, in terms of treatment, security management, caregiver training, and protection, it is important to know the risk of these behaviors separately. Third, risks were based on behaviors that were reported by an informant, and it is possible that some behaviors might not be witnessed or recalled. This suggests that our reported risks are likely to be underestimates. Fourth, caution should be taken in interpreting the estimates, as there was substantial heterogeneity between studies. Future studies are needed to confirm whether severity or etiology of cognitive impairment—or psychiatric comorbidity such as depressive or psychotic symptoms—could alter the risk of aggression in AD.51 Finally, aggression estimates from the included studies may not generalize to other populations. Some studies included individuals with low levels of education or literacy,12, 27,28 and one was conducted in mostly male veterans.30 In addition, two investigations examined patients seen at tertiary memory assessment centers and may not reflect those seen in other care settings.21.25
In summary, this meta-analysis reports a fivefold increase in the odds of aggressive behaviors in individuals with AD compared with healthy individuals. Our findings not only underscore the necessity of treatment and management of aggressive behaviors in AD but also highlight the importance of preventing the transition from MCI to AD. Further research is necessary to examine the role of other risk factors for aggression, including psychiatric comorbidity and environmental characteristics, and whether more accurate risk prediction can improve outcomes.
Acknowledgments
Rongqin Yu, Ph.D., is funded by a Rubicon Research Fellowship (446-15-002) from the Netherlands Organisation for Scientific Research. Seena Fazel, M.D., is funded by the Wellcome Trust Senior Research Fellowship (202836/Z/16/Z). The funders of this research had no role in the study design, analysis, or interpretation of data or in the writing of the article or decision to submit the article for publication.
Author contributions: Rongqin Yu, Ph.D., study concept and design, acquisition of data, analysis and interpretation, writing of manuscript; Anya Topiwala, Ph.D., study concept and design, acquisition of data and interpretation, writing of manuscript; Robin Jacoby, D.M., critical revision of manuscript for important intellectual content; Seena Fazel, M.D., study supervision and critical revision of manuscript for important intellectual content. The authors report no financial disclosures and no conflicts of interest.
Rongqin Yu and Anya Topiwala contributed equally to the manuscript
References
- 1.Kuronen M, Kautiainen H, Karppi P. Antipsychotic drug use and associations with neuropsychiatric symptoms in persons with impaired cognition: a cross-sectional study. Nord J Psychiatry. 2016;70:621–625. doi: 10.1080/08039488.2016.1191537. [DOI] [PubMed] [Google Scholar]
- 2.Lövheim H, Sandman PO, Kallin K. Relationship between antipsychotic drug use and behavioral and psychological symptoms of dementia in old people with cognitive impairment living in geriatric care. Int Psychogeriatr. 2006;18:713–726. doi: 10.1017/S1041610206003930. [DOI] [PubMed] [Google Scholar]
- 3.Silwanowicz RM, Maust DT, Seyfried LS. Management of older adults with dementia who present to emergency services with neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2017;32:1233–1240. doi: 10.1002/gps.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 5.Barnett R, Stirling C, Pandyan AD. A review of the scientific literature related to the adverse impact of physical restraint: gaining a clearer understanding of the physiological factors involved in cases of restraint-related death. Med Sci Law. 2012;52:137–142. doi: 10.1258/msl.2011.011101. [DOI] [PubMed] [Google Scholar]
- 6.Rakhmatullina M, Taub A, Jacob T. Morbidity and mortality associated with the utilization of restraints: a review of literature. Psychiatr Q. 2013;84:499–512. doi: 10.1007/s11126-013-9262-6. [DOI] [PubMed] [Google Scholar]
- 7.Maust DT, Kales HC, McCammon RJ. Distress associated with dementia-related psychosis and agitation in relation to healthcare utilization and costs. Am J Geriatr Psychiatry. 2017;25:1074–1082. doi: 10.1016/j.jagp.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee S, Murray J, Foley B. Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry. 2003;74:1315–1316. doi: 10.1136/jnnp.74.9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilley D, Bienias J, Wilson R. Influence of behavioral symptoms on rates of institutionalization for persons with Alzheimer's disease. Psychol Med. 2004;34:1129–1135. doi: 10.1017/s0033291703001831. [DOI] [PubMed] [Google Scholar]
- 10.Luengo-Fernandez R, Leal J, Gray A. UK research spend in 2008 and 2012: comparing stroke, cancer, coronary heart disease and dementia. BMJ Open. 2015;5 doi: 10.1136/bmjopen-2014-006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaffe K, Fox P, Newcomer R. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287:2090–2097. doi: 10.1001/jama.287.16.2090. [DOI] [PubMed] [Google Scholar]
- 12.Baiyewu O, Unverzagt FW, Ogunniyi A. Behavioral symptoms in community-dwelling elderly Nigerians with dementia, mild cognitive impairment, and normal cognition. Int J Geriatr Psychiatry. 2012;27:931–939. doi: 10.1002/gps.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lyketsos CG, Lopez O, Jones B. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA. 2002;288:1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- 14.Paddick SM, Kisoli A, Longdon A. The prevalence and burden of behavioural and psychological symptoms of dementia in rural Tanzania. Int J Geriatr Psychiatry. 2015;30:815–823. doi: 10.1002/gps.4218. [DOI] [PubMed] [Google Scholar]
- 15.Tatsch MF, Bottino CM, Azevedo D. Neuropsychiatric symptoms in Alzheimer disease and cognitively impaired, nondemented elderly from a community-based sample in Brazil: prevalence and relationship with dementia severity. Am J Geriatr Psychiatry. 2006;14:438–445. doi: 10.1097/01.JGP.0000218218.47279.db. [DOI] [PubMed] [Google Scholar]
- 16.Voyer P, Verreault R, Azizah GM. Prevalence of physical and verbal aggressive behaviours and associated factors among older adults in long-term care facilities. BMC Geriatr. 2005;5:13. doi: 10.1186/1471-2318-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeks JJ, Altman DG, Bradburn MJ: Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis, in Systematic Reviews in Health Care: Meta-Analysis in Context, 2nd ed. Edited by Egger M, Smith GD, Altman DG. London, BMJ Publishing Group, 2008, pp 285–312
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 20.Gallagher D, Coen R, Kilroy D. Anxiety and behavioural disturbance as markers of prodromal Alzheimer's disease in patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2011;26:166–172. doi: 10.1002/gps.2509. [DOI] [PubMed] [Google Scholar]
- 21.Liljegren M, Naasan G, Temlett J. Criminal behavior in frontotemporal dementia and Alzheimer disease. JAMA Neurol. 2015;72:295–300. doi: 10.1001/jamaneurol.2014.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez OL, Becker JT, Sweet RA. Non-cognitive symptoms in mild cognitive impairment subjects. Neurocase. 2005;11:65–71. doi: 10.1080/13554790490896893. [DOI] [PubMed] [Google Scholar]
- 23.Rockwood K, Mitnitski A, Richard M. Neuropsychiatric symptom clusters targeted for treatment at earlier versus later stages of dementia. Int J Geriatr Psychiatry. 2015;30:357–367. doi: 10.1002/gps.4136. [DOI] [PubMed] [Google Scholar]
- 24.Van der Mussele S, Le Bastard N, Saerens J. Agitation-associated behavioral symptoms in mild cognitive impairment and Alzheimer's dementia. Aging Ment Health. 2015;19:247–257. doi: 10.1080/13607863.2014.924900. [DOI] [PubMed] [Google Scholar]
- 25.Edwards ER, Spira AP, Barnes DE. Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry. 2009;24:716–722. doi: 10.1002/gps.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellison JM, Harper DG, Berlow Y. Beyond the “C” in MCI: noncognitive symptoms in amnestic and non-amnestic mild cognitive impairment. CNS Spectr. 2008;13:66–72. doi: 10.1017/s1092852900016175. [DOI] [PubMed] [Google Scholar]
- 27.Lee KS, Cho HS, Hong CH. Differences in neuropsychiatric symptoms according to mild cognitive impairment subtypes in the community. Dement Geriatr Cogn Disord. 2008;26:212–217. doi: 10.1159/000153431. [DOI] [PubMed] [Google Scholar]
- 28.Chiu MJ, Chen TF, Yip PK. Behavioral and psychologic symptoms in different types of dementia. J Formos Med Assoc. 2006;105:556–562. doi: 10.1016/S0929-6646(09)60150-9. [DOI] [PubMed] [Google Scholar]
- 29.Ikeda M, Fukuhara R, Shigenobu K. Dementia associated mental and behavioural disturbances in elderly people in the community: findings from the first Nakayama study. J Neurol Neurosurg Psychiatry. 2004;75:146–148. [PMC free article] [PubMed] [Google Scholar]
- 30.Orengo CA, Khan J, Kunik ME. Aggression in individuals newly diagnosed with dementia. Am J Alzheimers Dis Other Demen. 2008;23:227–232. doi: 10.1177/1533317507313373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sadak TI, Katon J, Beck C. Key neuropsychiatric symptoms in common dementias: prevalence and implications for caregivers, clinicians, and health systems. Res Gerontol Nurs. 2013;7:44–52. doi: 10.3928/19404921-20130918-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyketsos CG, Steele C, Galik E. Physical aggression in dementia patients and its relationship to depression. Am J Psychiatry. 1999;156:66–71. doi: 10.1176/ajp.156.1.66. [DOI] [PubMed] [Google Scholar]
- 33.Livingston G, Sommerlad A, Orgeta V. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 34.Schwarzinger M, Pollock BG, Hasan OS. Contribution of alcohol use disorders to the burden of dementia in France 2008–13: a nationwide retrospective cohort study. Lancet Public Health. 2018;3:e124–e132. doi: 10.1016/S2468-2667(18)30022-7. [DOI] [PubMed] [Google Scholar]
- 35.Smith DA, Refsum H, Bottiglieri T. Homocysteine and dementia: an international consensus statement. J Alzheimers Dis. 2018;62:561–570. doi: 10.3233/JAD-171042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tampi RR, Tampi DJ, Balachandran S. Antipsychotics, antidepressants, anticonvulsants, melatonin, and benzodiazepines for behavioral and psychological symptoms of dementia: a systematic review of meta-analyses. Curr Treat Opt Psychiatry. 2017;4:55–79. [Google Scholar]
- 37.Dyer SM, Harrison SL, Laver K. An overview of systematic reviews of pharmacological and non-pharmacological interventions for the treatment of behavioral and psychological symptoms of dementia. Int Psychogeriatr. 2018;30:295–309. doi: 10.1017/S1041610217002344. [DOI] [PubMed] [Google Scholar]
- 38.Hope T, Keene J, Fairburn C. Behaviour changes in dementia. 2: are there behavioural syndromes? Int J Geriatr Psychiatry. 1997;12:1074–1078. doi: 10.1002/(sici)1099-1166(199711)12:11<1074::aid-gps696>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 39.Hiroeh U, Appleby L, Mortensen PB. Death by homicide, suicide, and other unnatural causes in people with mental illness: a population-based study. Lancet. 2001;358:2110–2112. doi: 10.1016/S0140-6736(01)07216-6. [DOI] [PubMed] [Google Scholar]
- 40.NICE: Dementia: supporting people with dementia and their carers in health and social care. (online). Available at: https://www.nice.org.uk/guidance/cg42. Accessed November 6, 2018
- 41.Gitlin LN, Kales HC, Lyketsos CG. Nonpharmacologic management of behavioral symptoms in dementia. JAMA. 2012;308:2020–2029. doi: 10.1001/jama.2012.36918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kverno KS, Black BS, Nolan MT. Research on treating neuropsychiatric symptoms of advanced dementia with non-pharmacological strategies, 1998–2008: a systematic literature review. Int Psychogeriatr. 2009;21:825–843. doi: 10.1017/S1041610209990196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janzen S, Zecevic AA, Kloseck M. Managing agitation using nonpharmacological interventions for seniors with dementia. Am J Alzheimers Dis Other Demen. 2013;28:524–532. doi: 10.1177/1533317513494444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Acharya D, Harper DG, Achtyes ED. Safety and utility of acute electroconvulsive therapy for agitation and aggression in dementia. Int J Geriatr Psychiatry. 2015;30:265–273. doi: 10.1002/gps.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JM, Chu K, Jung KH. Criminal manifestations of dementia patients: report from the national forensic hospital. Dement Geriatr Cogn Dis Extra. 2011;1:433–438. doi: 10.1159/000330929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen VT, Love AR, Kunik ME. Preventing aggression in persons with dementia. Geriatrics. 2008;63:21–26. [PubMed] [Google Scholar]
- 47.Whall AL, Colling KB, Kolanowski A. Factors associated with aggressive behavior among nursing home residents with dementia. Gerontologist. 2008;48:721–731. doi: 10.1093/geront/48.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Busse A, Hensel A, Gühne U. Mild cognitive impairment long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 49.Ott BR, Lapane KL, Gambassi G. Gender differences in the treatment of behavior problems in Alzheimer's disease. SAGE Study Group. Systemic assessment of geriatric drug use via epidemiology. Neurology. 2000;54:427–432. doi: 10.1212/wnl.54.2.427. [DOI] [PubMed] [Google Scholar]
- 50.Broadaty H, Mothakunnel A, de Vel-Palumbo M. Influence of population versus convenience sampling on sample characteristics in studies of cognitive aging. Ann Epidemiol. 2014;24:63–71. doi: 10.1016/j.annepidem.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 51.McShane R, Keene J, Fairburn C. Psychiatric symptoms in patients with dementia predict the later development of behavioural abnormalities. Psychol Med. 1998;28:1119–1127. doi: 10.1017/s0033291798007089. [DOI] [PubMed] [Google Scholar]
- 52.Fazel S, Grann M, Fairburn C. Older criminals: a descriptive study of psychiatrically examined offenders in Sweden. Int J Geriatr Psychiatry. 2002;17:907–913. doi: 10.1002/gps.715. [DOI] [PubMed] [Google Scholar]