Abstract
Mosquitoes infected with malaria parasites have demonstrated altered behaviour that may increase the probability of parasite transmission. Here, we examine the responses of the olfactory system in Plasmodium falciparum infected Anopheles gambiae, Plasmodium berghei infected Anopheles stephensi, and P. berghei infected An. gambiae. Infected and uninfected mosquitoes showed differential responses to compounds in human odour using electroantennography coupled with gas chromatography (GC-EAG), with 16 peaks triggering responses only in malaria-infected mosquitoes (at oocyst, sporozoite or both stages). A selection of key compounds were examined with EAG, and responses showed differences in the detection thresholds of infected and uninfected mosquitoes to compounds including lactic acid, tetradecanoic acid and benzothiazole, suggesting that the changes in sensitivity may be the reason for differential attraction and biting at the oocyst and sporozoite stages. Importantly, the different cross-species comparisons showed varying sensitivities to compounds, with P. falciparum infected An. gambiae differing from P. berghei infected An. stephensi, and P. berghei infected An. gambiae more similar to the P. berghei infected An. stephensi. These differences in sensitivity may reflect long-standing evolutionary relationships between specific Plasmodium and Anopheles species combinations. This highlights the importance of examining different species interactions in depth to fully understand the impact of malaria infection on mosquito olfactory behaviour.
Introduction
The malaria parasite, Plasmodium, alters behavioural responses of Anopheles mosquitoes, however, the degree of effect and the underlying mechanisms are yet to be fully elucidated1–4. For decades, the majority of research across multiple Anopheles-Plasmodium systems has suggested that infected mosquitoes exhibit a different behavioural profile: infected mosquitoes are more likely to bite human or animal hosts, probe more often or repeatedly after disturbance, and show greater behavioural attraction to hosts and nectar (sugar) sources than uninfected mosquitoes1,5–14. These behavioural changes are accompanied by a suite of general physiological changes15–20. Collective evidence points towards a trend for mosquitoes to show decreased host-seeking and biting activity during the early, non-transmissible oocyst stage of the parasite, and then for these traits to be increased compared to uninfected mosquitoes at the transmissible sporozoite stage1,5,8,11,13,14, although two studies did not support this, finding no changes21,22. Reduced host-seeking at the non-transmissible stage would reduce the risk of mosquito mortality prior to the possibility of transmission, while increased host-seeking activity at the transmissible stage would result in a greater opportunity for transmission. This would potentially result in changes in transmission dynamics dependant on how widespread this phenomenon is in wild populations4,23. A complementary pattern of manipulation of host volatile emissions by Plasmodium has been shown to occur in studies with Plasmodium chabaudi in a rodent model system24 and with Plasmodium falciparum in human hosts in the laboratory25,26 and field27–30, with corresponding increased mosquito attraction to infected hosts24,27,30,31.
The mechanisms behind the changes in behaviour of infected mosquitoes remain under investigation, with studies in Anopheles stephensi revealing that the same behavioural changes can be instigated through general immune compromise with the bacterium Escherichia coli1 and suggesting the behaviour may be related to insulin signalling changes taking place upon blood ingestion32. It is not yet known what other mechanisms may be involved, or how conserved these may be between different mosquito-Plasmodium systems.
Separate from the pathway, an understanding of the phenotypic effects on mosquito behaviour is also highly relevant for vector control. A laboratory study by Smallegange et al. showed, for the first time in the important malaria vector, Anopheles gambiae, that mosquitoes infected with the transmissible, sporozoite stage of P. falciparum displayed increased behavioural attraction to human volatiles8, and a study by Cator et al. demonstrated increased attraction to human odours in a windtunnel by An. stephensi infected with Plasmodium yoelii1. These behavioural data suggest that increased behavioural responses to host odour could be mediated through changes in the olfactory system, a hypothesis supported by the latter study’s results that the maxilliary palps exhibited infection-stage dependent differential responses to 1-octen-3-ol, butanoic acid and lactic acid1.
In this study, we aimed to further investigate the impact of malaria infection on the responses of infected Anopheles mosquitoes. We examined the antennal responses of An. gambiae and An. stephensi infected with P. falciparum and Plasmodium berghei to human volatile compounds, with the aim of identifying specific chemicals correlated with altered responses due to infection status. Cross infections of An. gambiae with P. berghei, which it does not naturally transmit, were also conducted to determine whether there is mosquito-parasite species-specificity.
Results
Anopheles gambiae infected with Plasmodium falciparum
EAG
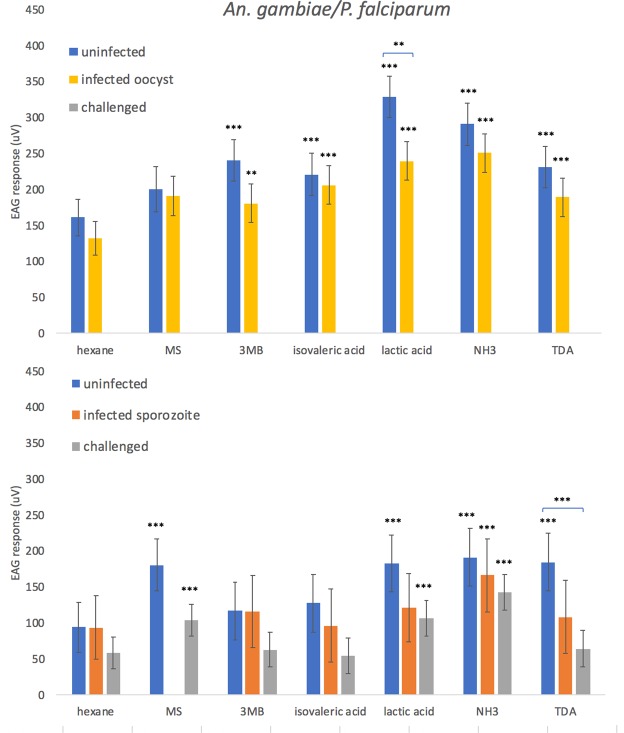
Electroantennography (EAG) is a technique by which it is possible to measure antennal receptor responses to volatile compounds. A panel of compounds known to elicit responses in host-seeking Anopheles mosquitoes33–35, including 3-methyl-1-butanol, isovaleric acid, lactic acid, ammonia, and tetradecanoic acid, were examined individually using EAG in An. gambiae infected with P. falciparum. Mosquitoes at the oocyst stage of infection (infection status was ascertained by dissection) showed a significantly lower response to lactic acid than age-matched controls (p = 0.025) (Fig. 1). Challenged mosquitoes (had fed on an infected blood meal but did not develop parasites) exhibited a significantly lower response to tetradecanoic acid at days 15–16 than the age-matched controls (p = 0.012).
Figure 1.
EAG responses (µV) of female Anopheles gambiae mosquitoes presented with an uninfected bloodmeal (uninfected) or Plasmodium falciparum infected bloodmeal which showed the presence (infected) or absence (challenged) of parasites in oocyst and sporozoite stage mosquitoes. EAG was carried out at (top) 8–10 days post challenge (oocyst stage,) or (bottom) 15–16 days post challenge (sporozoite stage). Treatments were: hexane (negative control), methyl salicylate (MS), 3-methyl-1-butanol (3MB), isovaleric acid, lactic acid, ammonia (NH3) and tetradecanoic acid (TDA). Here, compounds are tagged when significantly different from the negative control (hexane) (***p < 0.001, **p < 0.01, *p < 0.05), or, when indicated with a comparative line, as different between infection statuses. ±Standard Error bars are shown.
Generally, mosquitoes at the sporozoite stage of infection showed a lower sensitivity to the tested volatile compounds than mosquitoes at the oocyst stage of infection, with only ammonia eliciting a response significantly different from the hexane control (Fig. 1), compared to five compounds at the oocyst stage. Uninfected mosquitoes tested on days 8–10 also responded to a greater number of compounds (five) than uninfected mosquitoes tested on days 15–16 (four), showing general differences in sensitivity due to age.
GC-EAG
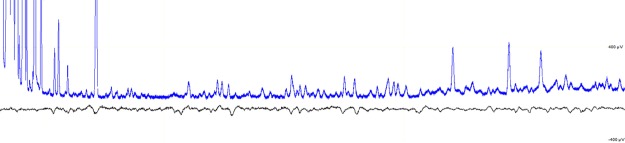
Coupled gas-chromatography-electoantennography (GC-EAG) is a technique used to detect mosquito responses to individual compounds in an odour sample. A concentrated human volatile extract, obtained using headspace collection of the feet of 17 volunteers, was simultaneously detected by flame ionisation detection (GC-FID) and passed over the antennae of individual mosquitoes to record the mosquito olfactory response (see Fig. 2 for example trace). The volatile extract was tested on female An. gambiae mosquitoes that were at the oocyst stage of infection with P. falciparum (days 8–10), at the sporozoite stage of infection (days 15–16) and on uninfected mosquitoes of the same ages (age-matched controls). Overall, 37 peaks in human foot odour were detected as EAG active for uninfected and infected mosquitoes at days 8–10 and 15–16 (Table S1) using GC-EAG. Twenty-one of these compounds were active for uninfected or both uninfected and infected mosquitoes (Table S1), however, 16 peaks were specific to infected mosquitoes. Of these, six peaks were specific to oocyst infected mosquitoes, five to sporozoite infected mosquitoes, and a further five peaks triggered responses in both oocyst and sporozoite stage mosquitoes but not the age-matched control group (Table 1). Within these 16 EAG-active peaks, 22 compounds from various chemical groups including the ketones, alkenes, aldehydes and fatty acids were identified by GC-MS (gas chromatography-mass spectrometry).
Figure 2.
Example GC-EAG trace of an An. gambiae mosquito infected with the oocyst stage of P. falciparum, showing GC detection of compounds on the top and mosquito antennal response on the bottom. Where alignment of the top and bottom peaks is consistent for at least 50% of replicates, it is considered an EAG-active mosquito response.
Table 1.
Confirmed compounds in human foot odour sample that only female An. gambiae infected with P. falciparum responded to by GC-EAG, with mosquitoes responding at the oocyst (O) or sporozoite (S) stage only, or responding at both stages (O&S).
| O | S | O&S | |||
|---|---|---|---|---|---|
| Peak | Compound | Peak | Compound | Peak | Compound |
| 13 | Styrene | 4 | Hexanal | 2 | Toluene |
| 20 | Acetophenone | 11 | 1,3-Dimethylbenzene | 6 | Unidentified |
| 25 | Benzothiazole | 30 | Verdyl acetate | 7 | 4-Hydroxy-4-methyl-2-pentanone |
| 27 | 3,4-Dimethylacetophenone | 1,4-Diacetylbenzene | 16 | Phenol | |
| 4-Ethylacetophenone | 1,3-Diacetylbenzene | 3-Ethyltoluene | |||
| 28 | 4-Ethylbenzoic acid | 34 | Dodecanoic acid | 4-Ethyltoluene | |
| 36 | 1-Tetradecanol | DEET | 21 | Nonanal | |
| 35 | 1-Hexadecene | ||||
| Tetradecanal | |||||
Where a peak consisted of multiple compounds which could not be separated, all are listed. N = 4–6 replicates for each infection status.
Anopheles stephensi infected with Plasmodium berghei
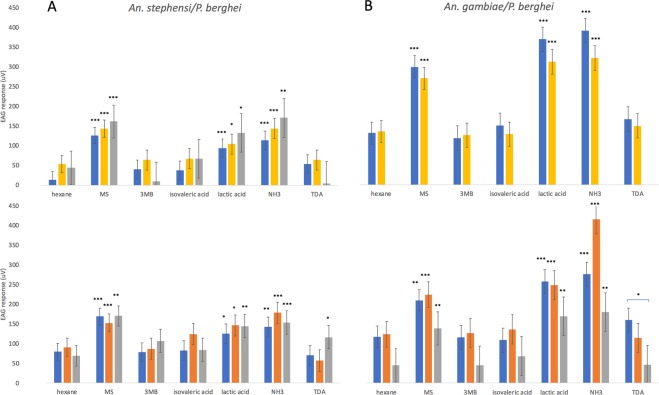
To determine whether changes were consistent in a different vector-parasite system, we investigated the EAG response of P. berghei infected An. stephensi. There were no significant differences in the level of response between infection status to 3-methyl-1-butanol, isovaleric acid, lactic acid, ammonia, and tetradecanoic acid. However, only challenged An. stephensi at days 15–16 responded to tetradecanoic acid (Fig. 3A) compared to a solvent control.
Figure 3.
EAG responses (µV) of female Anopheles stephensi (A) or Anopheles gambiae (B) mosquitoes presented with an uninfected bloodmeal (uninfected) or Plasmodium berghei infected bloodmeal which showed the presence (infected) or absence (challenged) of parasites in oocyst and sporozoite stage mosquitoes. EAG was carried out at (top) 8–10 days post challenge (oocyst stage,) or (bottom) 15–16 days post challenge (sporozoite stage). Treatments were: hexane (negative control), methyl salicylate (MS), 3-methylbutanol (3MB), isovaleric acid, lactic acid, ammonia (NH3) and tetradecanoic acid (TDA). Here, compounds are tagged when significantly different from the negative control (hexane) (***p < 0.001, **p < 0.01, *p < 0.05), or, when indicated with a comparative line, as different between infection statuses. ± Standard Error bars are shown.
A further compound selected from the literature, benzothiazole24,36, was additionally tested by EAG dose response after it also appeared as an important compound in GC-EAG experiments above (Fig. S1). Infected An. stephensi at the oocyst stage showed significantly greater responses to the highest concentration of benzothiazole (1%) than age-matched controls (p = 0.0264), and these also showed a significantly greater response to this concentration than mosquitoes at the sporozoite stage of infection (p = 0.045) (Fig. S1).
Anopheles gambiae infected with Plasmodium berghei
To determine whether there was a species-specific alteration in olfactory responses, we performed a cross infection of An. gambiae with P. berghei. We found no significant differences in EAG responses between infected and uninfected mosquitoes to any of the compounds, and overall responses to compounds compared to the control were not different between statuses (Fig. 3B). However, challenged mosquitoes at days 15–16 showed significantly lower responses to tetradecanoic acid (p = 0.0427) than age-matched controls.
Discussion
Here we have demonstrated that uninfected and P. falciparum-infected An. gambiae mosquitoes respond differently to the compounds within natural human volatile extracts when analysed by GC-EAG, with a third of the total number of compounds triggering responses only in infected mosquitoes: these included compounds also known to be emitted differentially by Plasmodium-infected hosts, such as benzothiazole, hexanal, toluene and nonanal24,26,29,30. This result suggests that infection with P. falciparum alters the olfactory sensitivity of mosquitoes to specific compounds including those known to elicit behavioural responses, such as benzothiazole and nonanal24,37. Altered sensitivity of detection could explain the increased attraction to hosts at the sporozoite stage and decreased attraction at the oocyst stage of the Plasmodium life cycle found in previous studies1,5,8,11,13.
When examining individual compounds in P. falciparum infected An. gambiae, we found direct differences in EAG responses to lactic acid between mosquitoes at the oocyst stage of infection and uninfected mosquitoes, with infected mosquitoes showing lower responses (Fig. 1). As lactic acid is a well-known mosquito attractant38, a lower response during oocyst stage infection correlates with the hypothesis that non-transmissible parasites might benefit from reduced host-seeking and the associated risk of mortality2. There also appeared to be an effect of immune challenge upon response to tetradecanoic acid, with ‘challenged’ An. gambiae (mosquitoes that had taken a P. falciparum infected bloodmeal without becoming infected) expressing a lower EAG response than age-matched controls at 15–16 days following bloodmeal exposure. This result confirms the effect of immune challenge on mosquito olfaction found in An. stephensi in a previous study1, but suggests that the specific effects on the antennal sensitivities to compounds may alter differentially dependant on both the mosquito and Plasmodium species involved.
In order to examine species effects, we also performed EAG experiments on An. stephensi infected with P. berghei. In contrast to the results seen with An. gambiae and P. falciparum, An. stephensi with P. berghei oocyst-stage infection did not show different responses to lactic acid, but showed a greater response than age-matched controls to benzothiazole, a compound known to influence mosquito attraction36 that was recently found to be produced in lower quantities by P. chabaudi-infected rodent hosts, which were more attractive to An. stephensi24. The greater sensitivity towards this compound by oocyst-infected An. stephensi in our study suggests that infected mosquitoes are capable of detecting specific host-derived volatiles at lower concentrations than uninfected mosquitoes. It is unknown, however, how this sensitivity manifests behaviourally, since a prior study examining An. stephensi infected with P. berghei found no alterations in probing duration39. Alterations due to immune challenge with an infected blood meal were not seen here in An. stephensi, despite demonstration of this in a previous study with P. yoelii1.
The overall patterns of significant responses versus a solvent control shifted between infection statuses in certain species. For example, comparing oocyst-stage infections, P. falciparum-infected An. gambiae responded to a larger panel of compounds than P. berghei-infected An. stephensi, while at the sporozoite stage the former showed a reduction in the range of detected compounds, with lactic acid no longer detected by infected individuals. This shift in which compounds are detected at the sporozoite stage potentially runs counterintuitive to the hypothesis that mosquitoes will bite more if they detect compounds more strongly, and the previously observed increased behaviour at this transmissible stage in An. gambiae8. However, as An. gambiae infected at the sporozoite stage with P. falciparum still show detection of ammonia, it is possible that mosquitoes at this stage in the infection cycle rely more heavily on a select few volatile cues – this should be investigated further by focusing on behavioural responses to blends. Furthermore, as the electroantennograms with An. stephensi do not show this reduction in detection at the sporozoite stage, this is possibly an effect which varies between mosquito species and is dependent on the effect of parasite infection on the host physiology, or alternatively the effect may have been caused by variation in parasite load. Due to the logistical difficulties of artificially infecting An. gambiae with P. falciparum, the number of sporozoite infected mosquitoes tested in this study was low, and there was also an overall correlation between increased mosquito age and reduction in response which may have acted as a confounding factor.
Overall, An. gambiae infected with P. falciparum and An. stephensi infected with P. berghei both show alterations in sensitivity to different compounds, suggesting that the effect of Plasmodium infection on mosquitoes is species-specific. This may explain why some studies found no effect on vector behaviour in their Plasmodium-mosquito systems21,22. If certain combinations of mosquito and Plasmodium species do not show altered biting or attraction behaviour, it is likely that the change in olfactory responses seen here in the antennae of An. gambiae and An. stephensi, and previously in the maxilliary palps of An. stephensi1, would also not occur in those systems. In addition, there may be variation in mosquito response to infection even within the same system, given that a recent study on An. gambiae and P. falciparum found no differences in behavioural responses22. While the contrast in findings to previous behavioural studies, which showed altered responses in An. gambiae infected with P. falciparum8,12,40, could be due to different methodologies, it is also possible that genetic and environmental factors play a large role in the development of the altered behavioural phenotype. Additionally, mosquitoes and Plasmodium from varying geographical locations may show contrasting responses. The cross-infection of An. gambiae with P. berghei in our study showed a similar profile of responses to An. stephensi infected with P. berghei, but unfortunately parallel results for benzothiazole could not be presented due to a low number of replicates. Plasmodium falciparum and P. berghei have been shown previously to have different effects on the An. gambiae immune system41 and it is uncertain whether the similarities between the two P. berghei infections are due to the Plasmodium species being responsible for the majority of the olfactory alterations, irrespective of mosquito species, or if this may be an artefact due to the lower incubation temperatures used when infecting with P. berghei in both cases – it is difficult to draw direct conclusions in this case.
This study has provided more evidence indicating that malaria parasite infection can influence the physiology of Anopheles mosquitoes, in this case by altering their sensitivity to specific host-derived compounds. The differences observed between the results of each Plasmodium-Anopheles combination highlight the importance of conducting electrophysiological and by extension behavioural studies using different model systems in both the laboratory and field to fully elucidate the impact of parasite infection. Such system-specific differences are likely to result from a long evolutionary association between certain mosquitoes, parasites and hosts and hint at possible approaches for exploitation of tailored compound blends for use in vector surveillance and control strategies.
Methods
Mosquitoes and Plasmodium
Mosquito rearing
Anopheles gambiae s.s. N’guesso strain (originally obtained from Imperial College, London, UK) and Anopheles stephensi SDA 500 strain were maintained at the London School of Hygiene and Tropical Medicine (LSHTM, UK). Both species were reared at a relative humidity of 60–70% and a 12:12 light:dark photoperiod and maintained at a temperature of 27 ± 2 °C. Larvae were fed with Tetramin® (Melle, Germany) tropical fish flakes, and adults provided with 10% glucose solution, with weekly feeds of human blood (collected from volunteers at LSHTM with informed consent obtained; LSHTM ethics committee reference number 5520) using a Hemotek© artificial membrane feeding system (Hemotek Ltd, Blackburn, UK). Females for use in experimental infections were allowed to emerge in a separate cage and supplied with 10% glucose only.
Mosquito infection with P. falciparum
Plasmodium falciparum NF54 parasites were cultured in human serum (pooled, sterile-filtered blood from males of blood group AB) as previously described42,43. Asexual blood stage parasites were synchronized by a 5% D-sorbitol (wt/vol) treatment for 10 min at 37 °C. Synchronous ring stage parasites at 5% haematocrit were cultured to 6–10% parasitaemia, at which point sexual development was triggered via starvation-induced stress43. Trophozoite cultures were diluted two- to three-fold the following day in T25 flasks at 3–5% haematocrit such that each flask contained 0.5–2% gametocytes in a final volume of 10 ml medium. For infection, freshly drawn human whole blood was washed in RPMI and resuspended in prewarmed serum to give a packed cell volume of 40%. 14–17 day old gametocyte cultures (0.5–2% gametocytaemia, 5% haematocrit) were gently agitated for cell resuspension, transferred to prewarmed tubes and centrifuged to pellet for 5 minutes at 1800 x g at 37 °C. The parasite pellet was then mixed with an equal volume of serum to give a parasite/serum mixture that was subsequently diluted with 3–9 times its volume with fresh whole blood/serum, constituting the final infectious feed. Three to eight-day old nulliparous female mosquitoes were allowed to feed on the infectious feed via a Hemotek© (1 ml reservoirs) for 10–15 minutes. A control group of mosquitoes from the same batch was fed using only the uninfected blood/serum mixture and maintained alongside the infected batch. After blood-feeding, mosquitoes were maintained at 25 °C at ~70% relative humidity with constant access to 10% glucose.
Mosquito infection with P. berghei
Plasmodium berghei ANKA clone 234 parasites were maintained as cryopreserved stabilates or by serial blood passage in 6–8 week old female CD1 mice and regular mosquito transmission. Mosquitoes were infected with P. berghei ANKA by feeding directly on parasite-infected mice (anesthetised with Rompun and Ketaset). Briefly, hyper-reticulocytosis was induced 3 days before infection by treating mice with 200 μl intraperitoneal (i.p.) phenylhydrazine chloride (PHz; 6 mg.ml−1 in PBS; ProLabo, UK). PHz-treated mice were infected by i.p. injection of parasitized blood (approximately 200 μl of blood at 1% parasitaemia), and infections were monitored by examination of Giemsa-stained tail blood smears. At 3 days post-infection, anaesthetized mice were exposed to pots containing 50–70 starved female anopheline mosquitoes. Blood-fed mosquitoes were maintained on 10% (w/v) fructose, 0.05% (w/v) p-aminobenzoic acid at 20 °C and 60% relative humidity. Control mosquitoes of the same age were fed on uninfected mice and kept under the same conditions.
Ethics. Animal work was conducted under UK Home Office license and approval in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986 implementing European Directive 2010/63 for the protection of animals used for experimental purposes. All methods were carried out in accordance with relevant guidelines and regulations and approval was obtained from the LSHTM Animal Welfare Ethics Review Board. Animal welfare was assessed daily and animals were humanely killed upon reaching experimental or humane endpoints.
Confirmation of infection
Infection status was ascertained for all mosquitoes by light microscopy. Once the head was separated from the body, the midgut was removed and examined for the presence of oocysts after staining with mercurochrome. Salivary glands were excised to check for the presence of sporozoites. Mosquitoes were then designated as either infected (oocyst or sporozoite stage) or challenged if there were no visible oocysts or sporozoites but the mosquito had fed on an infected blood meal. Age-controlled uninfected mosquitoes were also dissected for control purposes.
Volatile collection and electrophysiology
EAG
Electroantennography was used to examine the antennal responses of infected and uninfected mosquitoes to known attractive host compounds at day 8–10 (oocyst stage) and 15–16 (sporozoite stage) post bloodmeal in An. gambiae infected with P. falciparum, An. stephensi infected with P. berghei, and An. gambiae infected with P. berghei. This approach measures the depolarisation across the antennal membrane in response to a volatile stimulus. The mosquito was prepared by removing the head after being cold-anaesthetised; the tips of the antennae were then excised using a scalpel, and the proboscis and maxilliary palps removed to prevent movement during experiments. The head was mounted on the point of a tapered glass electrode filled with Ringer’s solution (7.55 g NaCl, 0.64 g KCl, 0.22 g CaCl2, 1.73 g MgCl2, 0.86 g Na2HCO3, and 0.61 g Na3PO4 l−1 water), and the tips of the antennae positioned into a glass recording electrode (also containing Ringer’s solution). These glass electrodes were mounted on silver electrodes connected to a probe and an IDAC4, which allowed signals from the antennae to be recorded using EAD 2000 software (Syntech®, Buchenbach, Germany). Compounds were used at concentrations known to elicit EAG responses from our preliminary tests and in fitting with recommendations from the available literature44. The test compound (10 µl) was dotted onto a strip of filter paper, with hexane as a negative control and methyl salicylate as a positive control to test for responsiveness of the preparation (Table 2). A replicate consisted of each treatment tested on a single female mosquito (Table 3). All experiments were carried out in daylight, in the first 8 hours post-scotophase.
Table 2.
List of compounds used in EAG treatments.
| Chemical name | Supplier | % Purity | Tested concentrations (%, in hexane where diluted) |
|---|---|---|---|
| 3-Methyl-1-butanol | Sigma Aldrich | 99 | 0.00001 |
| Methyl salicylate | Sigma Aldrich | 99 | 0.01 |
| Benzothiazole | Sigma Aldrich | 96 | 1, 0.1, 0.01, 0.001, 0.0001 |
| Tetradecanoic acid | Sigma Aldrich | 99 | 0.00001 |
| Ammonium hydroxide | Sigma Aldrich | 28–30 | 28 |
| Isovaleric acid | Sigma Aldrich | 99 | 0.00001 |
| L-(+)-Lactic acid | Fisher | 90 | 90 |
Table 3.
Number of EAG replicates for each status of infection.
| Fig. | Mosquito | Parasite challenge | Oocyst control | Oocyst challenged | Oocyst infected | Sporozoite control | Sporozoite challenged | Sporozoite infected |
|---|---|---|---|---|---|---|---|---|
| 1 | An. gambiae | P. falciparum | 15 | — | 18 | 10 | 21 | 5 |
| 3 | An. gambiae | P. berghei | 14 | — | 14 | 14 | 5 | 9 |
| 3 | An. stephensi | P. berghei | 14 | 5 | 12 | 11 | 11 | 8 |
| S1 | An. stephensi | P. berghei | 12 | 5 | 11 | 10 | 7 | 7 |
Volatile collection and chemical identification
Human volatiles were collected from the feet of 17 volunteers. Volunteers placed their feet in individual 25 × 38 cm plastic roasting bags (toastabags®, Planit Products Ltd., Malvern, UK), which were sealed to be airtight. A purified airflow was then pumped into the top of the bag at 1.2 l/min, and pulled out of the bottom of the bag through a polymer filter (Porapak Q, mesh size 50/80, Supelco Analytical) at 0.8 l/min for 2 hours. Volatile odours were collected on the polymer filter, then eluted with 750 µl redistilled diethyl ether. Both feet of each volunteer were used, to give a total of 34 samples. Each sample was concentrated to 50 µl under nitrogen, and the samples were pooled. The total volume of 1.7 ml was then concentrated further to 400 µl, in order to provide a concentration representing 10 minutes of volatile collection/µl. This sample was used for all EAG work and was analysed by coupled gas chromatography-mass spectrometry (GC-MS) performed on a Micromass Autospec Ultima magnetic sector mass spectrometer, attached to an Agilent 6890 N GC (non-polar HP1 column 50 m length x 0.32 mm inner diameter x 0.52 µm film thickness, Agilent, Santa Clara, U.S.A.) equipped with a cool-on-column injector. Ionization was by electron impact (70 ev, 220 °C). The GC oven temperature was maintained at 30 °C for 5 min, then programmed at 5 °C/min to 250 °C and held for 21 min). The identity of compounds of interest was confirmed by co-injecting the odour sample and the compound to be tested onto the GC, with the aim of doubling the area of the GC peak detected.
GC-EAG
Coupled gas-chromatography-electroantennography (GC-EAG) was performed as described by Logan et al.37. Briefly, 2 µl of the pooled volunteer foot volatile collection was injected on to an HP1 column (50 m, 0.32 mm ID, 0.52 film thickness) with a cool-on column injector, and split so that half the sample was detected by the GC-FID (Chemstation, Agilent, Santa Clara, U.S.A.) and the other half was passed over the antennae of the mosquito. This formed a combined trace (Fig. 2) where mosquito responses were directly aligned with the chemicals causing them. The mosquito was prepared as above, and for the GC method, the oven started at 30 °C, was held for 1 min, then raised by 5 °C/min to 100 °C, then by 10 °C/min to 230 °C and held for 1 min. 4–6 replicates were carried out for P. falciparum infected and uninfected An. gambiae mosquitoes at day 8–10 (oocyst stage) and 15–16 days (sporozoite stage) post-bloodmeal. Conserved responses to FID peaks across replicates were aligned using a lightpad.
Statistical analysis
Several linear mixed models were analysed for each of the performed experiments. In all cases, the response variable corresponded to microvolts (µV). The data for this experiment were evaluated with a three-way analysis of variance with the main fixed factors of type, status and trt together with all interactions. The factor type has the levels of infection type (challenged, infected, control), the factor status describes the infection status (oocyst, sporozoite), and the factor trt corresponding to different chemicals and their concentration. The model fit also included the random factors of replicate and sequence, where the replicate represents a run that contained, depending on the experiment, between 6 to 32 treatment combinations (status-type-trt), and the order of these applications within a replicate were described by the factor sequence. Significance of the different factors and interactions were done with an approximate F-test where the degrees of freedom were estimated using the Kenward-Rogers method. In addition, due to this sequence of treatments, repeated measures were modelled within each replicate by considering a homogeneous autoregressive of order 1 error structure. Comparisons of predicted means of the different levels of the combination factor status-type-trt for each of the experiment were evaluated by Least Significant Difference. All models were fitted using proc mixed as implemented in SAS version 9.4 (SAS Institute, Cary, NC, USA). No departures from normality were observed and a significance level of 0.05 was considered for all tests. Significant comparisons were reported for each status-type combination where the treatments were significantly different from the control, or if there were differences in response to the same treatment across different statuses or types. Comparisons of treatments across status or type were only made when there was no difference between the means of the relevant negative controls, i.e. for control and infected oocysts responses to the negative controls were not significantly different.
This study and the experimental protocols herein were approved by the LSHTM ethics committee (reference numbers 6435 and 5520) and conducted according to committee guidelines and regulations.
Supplementary information
Acknowledgements
This study was funded by the BBSRC (grant BB/J008869/1). Many thanks to Patricia Aiyenuro, Shahida Begum and Andrew Blagborough for their contributions of mosquitoes, ideas and valuable time. JTD received funding from BBSRC (grant BB/M001598).
Author Contributions
N.M.S., V.A.B., J.G.L., W.T., J.P., H.H. and S.A.G. conceived and designed the study. N.M.S., V.A.B., T.M.V., V.A., F.S.R.T., M.E. and W.S. collected the data. S.A.G., J.T.D., J.C. and M.B. contributed data or analysis tools. N.M.S., V.A.B. and S.A.G. performed the analysis. N.M.S., V.A.B., J.G.L., T.M.V., W.T., R.S., H.H., J.C., M.B., J.T.D. and J.P. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
N. M. Stanczyk and V. A. Brugman contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-40074-y.
References
- 1.Cator LJ, et al. ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc. R. Soc. B-Biol. Sci. 2013;280:7. doi: 10.1098/rspb.2013.0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cator LJ, Lynch PA, Read AF, Thomas MB. Do malaria parasites manipulate mosquitoes? Trends Parasitol. 2012;28:466–470. doi: 10.1016/j.pt.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefevre T, Thomas F. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2008;8:504–519. doi: 10.1016/j.meegid.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Stanczyk NM, Mescher MC, De Moraes CM. Effects of malaria infection on mosquito olfaction and behavior: extrapolating data to the field. Curr Opin Insect Sci. 2017;20:7–12. doi: 10.1016/j.cois.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RA, Koella JC, Hurd H. The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc. R. Soc. B-Biol. Sci. 1999;266:1729–1733. doi: 10.1098/rspb.1999.0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornet S, Nicot A, Rivero A, Gandon S. Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malar J. 2013;12:8. doi: 10.1186/1475-2875-12-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornet S, Nicot A, Rivero A, Gandon S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett. 2013;16:323–329. doi: 10.1111/ele.12041. [DOI] [PubMed] [Google Scholar]
- 8.Smallegange RC, et al. Malaria Infected Mosquitoes Express Enhanced Attraction to Human Odor. PLoS One. 2013;8:3. doi: 10.1371/journal.pone.0063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson, H. M. & Read, A. F. Mosquito appetite for blood is stimulated by Plasmodium chabaudi infections in themselves and their vertebrate hosts. Malar J3, 10.1186/1475-2875-3-12 (2004). [DOI] [PMC free article] [PubMed]
- 10.Koella JC, Packer MJ. Malaria parasites enhance blood-feeding of their naturally infected vector Anopheles punctulatus. Parasitology. 1996;113(Pt 2):105–109. doi: 10.1017/S0031182000066348. [DOI] [PubMed] [Google Scholar]
- 11.Koella JC, Rieu L, Paul REL. Stage-specific manipulation of a mosquito’s host-seeking behavior by the malaria parasite Plasmodium gallinaceum. Behavioral Ecology. 2002;13:816–820. doi: 10.1093/beheco/13.6.816. [DOI] [Google Scholar]
- 12.Koella, J. C., Sorensen, F. L. & Anderson, R. The malaria parasite Plasmodium falciparum increases the frequency of multiple feeding of its mosquito vector Anopheles gambiae. Proc R Soc Lond B Biol Sci265, 10.1098/rspb.1998.0358 (1998). [DOI] [PMC free article] [PubMed]
- 13.Rossignol PA, Ribeiro JM, Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. The American journal of tropical medicine and hygiene. 1984;33:17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- 14.Rossignol PA, Ribeiro JM, Spielman A. Increased biting rate and reduced fertility in sporozoite-infected mosquitoes. The American journal of tropical medicine and hygiene. 1986;35:277–279. doi: 10.4269/ajtmh.1986.35.277. [DOI] [PubMed] [Google Scholar]
- 15.Lefevre T, et al. Malaria Plasmodium agent induces alteration in the head proteome of their Anopheles mosquito host. Proteomics. 2007;7:1908–1915. doi: 10.1002/pmic.200601021. [DOI] [PubMed] [Google Scholar]
- 16.Anderson RA, Knols BG, Koella JC. Plasmodium falciparum sporozoites increase feeding-associated mortality of their mosquito hosts Anopheles gambiae s.l. Parasitology. 2000;120(Pt 4):329–333. doi: 10.1017/S0031182099005570. [DOI] [PubMed] [Google Scholar]
- 17.Sangare I, et al. Stress dependent infection cost of the human malaria agent Plasmodium falciparum on its natural vector Anopheles coluzzii. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2014;25:57–65. doi: 10.1016/j.meegid.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Lalubin F, Deledevant A, Glaizot O, Christe P. Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. The Journal of animal ecology. 2014;83:850–857. doi: 10.1111/1365-2656.12190. [DOI] [PubMed] [Google Scholar]
- 19.Vezilier J, Nicot A, Gandon S, Rivero A. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proceedings. Biological sciences/The Royal Society. 2012;279:4033–4041. doi: 10.1098/rspb.2012.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmed AM, Hurd H. Immune stimulation and malaria infection impose reproductive costs in Anopheles gambiae via follicular apoptosis. Microbes and infection/Institut Pasteur. 2006;8:308–315. doi: 10.1016/j.micinf.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Vantaux, A. et al. Host-seeking behaviors of mosquitoes experimentally infected with sympatric field isolates of the human malaria parasite Plasmodium falciparum: no evidence for host manipulation. Frontiers in Ecology and Evolution3, 10.3389/fevo.2015.00086 (2015).
- 22.Nguyen PL, et al. No evidence for manipulation of Anopheles gambiae, An. coluzzii and An. arabiensis host preference by Plasmodium falciparum. Sci Rep. 2017;7:9415. doi: 10.1038/s41598-017-09821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cator LJ, Lynch PA, Thomas MB, Read AF. Alterations in mosquito behaviour by malaria parasites: potential impact on force of infection. Malar J. 2014;13:11. doi: 10.1186/1475-2875-13-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Moraes CM, et al. Malaria-induced changes in host odors enhance mosquito attraction. Proc Natl Acad Sci USA. 2014;111:11079–11084. doi: 10.1073/pnas.1405617111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Boer JG, et al. Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci Rep. 2017;7:9283. doi: 10.1038/s41598-017-08978-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaber, C. L. et al. Breathprinting reveals malaria-associated biomarkers and mosquito attractants. J Infect Dis, 10.1093/infdis/jiy072 (2018). [DOI] [PMC free article] [PubMed]
- 27.Lacroix R, Mukabana WR, Gouagna LC, Koella JC. Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol. 2005;3:e298. doi: 10.1371/journal.pbio.0030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busula, A. O. et al. Gametocytemia and attractiveness of Plasmodium falciparum–infected Kenyan children to Anopheles gambiae mosquitoes. J Infect Dis, 10.1093/infdis/jix214 (2017). [DOI] [PubMed]
- 29.De Moraes, C. M. et al. Volatile biomarkers of symptomatic and asymptomatic malaria infection in humans. Proc Natl Acad Sci USA (2018). [DOI] [PMC free article] [PubMed]
- 30.Robinson, A. et al. Plasmodium-associated changes in human odor attract mosquitoes. Proc Natl Acad Sci USA (2018). [DOI] [PMC free article] [PubMed]
- 31.Busula AO, Verhulst NO, Bousema T, Takken W, de Boer JG. Mechanisms of Plasmodium-Enhanced Attraction of Mosquito Vectors. Trends Parasitol. 2017;33:961–973. doi: 10.1016/j.pt.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Cator LJ, et al. Immune response and insulin signalling alter mosquito feeding behaviour to enhance malaria transmission potential. Sci Rep. 2015;5:11947. doi: 10.1038/srep11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smallegange RC, Qiu YT, Bukovinszkine-Kiss G, Van Loon JJ, Takken W. The effect of aliphatic carboxylic acids on olfaction-based host-seeking of the malaria mosquito Anopheles gambiae sensu stricto. J Chem Ecol. 2009;35:933–943. doi: 10.1007/s10886-009-9668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smallegange RC, Qiu YT, van Loon JJ, Takken W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) Chem Senses. 2005;30:145–152. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- 35.Verhulst NO, et al. Cultured skin microbiota attracts malaria mosquitoes. Malar J. 2009;8:302. doi: 10.1186/1475-2875-8-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu YT, et al. Behavioural and electrophysiological responses of the malaria mosquito Anopheles gambiae Giles sensu stricto (Diptera: Culicidae) to human skin emanations. Medical and veterinary entomology. 2004;18:429–438. doi: 10.1111/j.0269-283X.2004.00534.x. [DOI] [PubMed] [Google Scholar]
- 37.Logan JG, et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J Chem Ecol. 2008;34:308–322. doi: 10.1007/s10886-008-9436-0. [DOI] [PubMed] [Google Scholar]
- 38.Acree F, Turner RB, Gouck HK, Beroza M, Smith N. L-Lactic Acid: A Mosquito Attractant Isolated from Humans. Science. 1968;161:1346. doi: 10.1126/science.161.3848.1346. [DOI] [PubMed] [Google Scholar]
- 39.Li X, Sina B, Rossignol PA. Probing behaviour and sporozoite delivery by Anopheles stephensi infected with Plasmodium berghei. Medical and veterinary entomology. 1992;6:57–61. doi: 10.1111/j.1365-2915.1992.tb00036.x. [DOI] [PubMed] [Google Scholar]
- 40.Wekesa JW, Copeland RS, Mwangi RW. Effect of Plasmodium Falciparum on Blood Feeding Behavior of Naturally Infected Anopheles Mosquitoes in Western Kenya. The American journal of tropical medicine and hygiene. 1992;47:484–488. doi: 10.4269/ajtmh.1992.47.484. [DOI] [PubMed] [Google Scholar]
- 41.Dong Y, et al. Anopheles gambiae Immune Responses to Human and Rodent Plasmodium Parasite Species. PLoS Pathog. 2006;2:e52. doi: 10.1371/journal.ppat.0020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ljungstöm, I., Moll, K., Perlmann, H., Scherf, A. & Wahlgren, M. Methods in Malaria Research (2008).
- 43.Fivelman QL, et al. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Molecular and Biochemical parasitology. 2007;154:119–123. doi: 10.1016/j.molbiopara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Verhulst NO, et al. Composition of human skin microbiota affects attractiveness to malaria mosquitoes. PLoS One. 2011;6:12. doi: 10.1371/journal.pone.0028991. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.