Abstract
A total of 511 local isolates of Bacillus thuringiensis from different geographical regions of Thailand were analyzed for the presence of the cry1A, cry1B, cry2A, cry9, and vip3A genes encoding for lepidopteran-specific toxins. PCR results revealed that 94.32% (482/511) of B. thuringiensis isolates harbored at least one of the detected genes, of which the cry1A, cry1B, cry2A, cry9, and vip3A genes were detected at frequencies of 90.61%, 89.63%, 76.32%, 40.70%, and 48.18%, respectively. Nineteen gene-combination profiles were discovered among 482 B. thuringiensis isolates, of which the most frequently detected profile contained the cry1A, cry1B, cry2A, and vip3A genes. Sixty-one isolates (12.66%), which harbored all of the detected insecticidal toxin genes, were further detected for the exochitinase (chi36) gene and chitinase activity. The results revealed that all 61 isolates contained the chi36 gene and exhibited chitinase activity. Insect bioassays showed that five isolates were highly toxic (more than 80% mortality) against second instar larvae of Spodoptera litura, of which the highest insect mortality (93%) was obtained from the B. thuringiensis isolates 225-15 and 417-1. Scanning electron microscopy revealed that the crystal morphologies of the five effective isolates were bipyramidal and cuboidal shapes. SDS-PAGE analysis of the spore–crystal mixture showed major bands of approximately 65 and 130 kDa. These five effective strains are alternative candidates for use as a microbial insecticide for the control of the S. litura pest.
Electronic supplementary material
The online version of this article (10.1007/s13205-019-1646-3) contains supplementary material, which is available to authorized users.
Keywords: Bacillus thuringiensis, Bacterial toxin, Entomopathogenic bacteria, Microbial insecticide
Introduction
Intensive use of chemical insecticides to control the outbreak of insect pests has caused major public health and environmental problems in several countries including Thailand (Negatu et al. 2016; Panuwet et al. 2012; Tawatsin et al. 2015). Microbial insecticides are attractive alternatives to chemical insecticides as the former are safe for humans and non-target organisms, and are environmentally friendly. Bacillus thuringiensis, a Gram-positive, endospore-forming bacterium has become one of the most successful microbial insecticides for the control of the larvae of insect pests and vectors belonging to the Lepidoptera, Coleoptera, and Diptera (Palma et al. 2014). The outstanding ability of this bacterium is its production of two major groups of insecticidal proteins including the Cry and Vip toxins. Cry proteins are produced during the sporulation phase and accumulated as parasporal crystalline inclusions (Palma et al. 2014). To date, 308 holotype Cry proteins have been reported under 75 major classes (Cry1–Cry75) (http://www.btnomenclature.info/). Cry proteins have been used worldwide for the control of insect pests in the form of crystal protein–spore mixture or heterologous expression in transgenic plants. The Cry1, Cry2, and Cry9 proteins were reported toxic against larvae of various lepidopteran insect pests (Herrero et al. 2016; Seifinejad et al. 2008). Vegetative insecticidal proteins (Vip) are produced during the vegetative phase of growth and are secreted into the culture medium (Palma et al. 2014). More than 30 holotype Vip proteins have been identified and separated into four classes (Vip1–Vip4) (http://www.btnomenclature.info/). The Vip1 and Vip2 proteins form a binary toxin that is highly toxic against some coleopteran insect larvae and certain hemipteran species (Chakroun et al. 2016; Palma et al. 2014). Vip3 proteins have been reported as toxic against the larvae of various lepidopteran insect pests including those reported to have decreasing susceptibility to Cry1A toxins (de Escudero et al. 2014; Zhang et al. 2015). However, very little information is known about Vip4 protein (Chakroun et al. 2016).
Cry toxins exhibit their insecticidal activity after proteolytic activation and then binding to specific receptors on the epithelial cells of the insect midgut and forming non-selective pores permeable to inorganic ions, amino acids, and sugars leading to cell disruption and insect death (Vachon et al. 2012). Even though there is no structural homology to Cry proteins, Vip3 toxins exhibited their larvicidal activity with the same sequence of events including proteolytic activation, receptor binding, and pore formation (Chakroun et al. 2016). However, Vip and Cry toxins have been reported do not share binding sites (Gouffon et al. 2011; Lemes et al. 2017). A combination of the use of toxins with diverse modes of action or different binding sites for insect pest control has been proposed as an efficient strategy to broaden the insecticidal spectrum and delay the onset of insect resistance (Gouffon et al. 2011). For example, a mixture of Cry and Vip3Aa toxins demonstrated a synergistic effect and broadened the insect spectrum (Baranek et al. 2017). In addition, the combined use of toxins by co-expression of more than one B. thuringiensis toxin gene (the pyramid strategy) has been increasingly reported in the new generation of transgenic plants to overcome the resistance of insect pests (Chen et al. 2017; Naqvi et al. 2017). Application of B. thuringiensis in microbial insecticides may be limited due to the narrow host range, low larvicidal activity to the targeted pests, and the resistance from insects after long-term exposure to a single toxin. Therefore, screening new effective isolates and screening for novel B. thuringiensis toxins have been performed continuously (Jain et al. 2017; Reyaz et al. 2017; Sauka and Benintende 2017). Since the presence of specific toxin genes correlates with the corresponding specific larvicidal activity, detection of specific toxin genes using polymerase chain reaction (PCR) were performed worldwide to screen and characterize the effective B. thuringiensis isolates (Sauka and Benintende 2017; Thammasittirong and Attathom 2008; Yu et al. 2011).
In addition to insecticidal toxins, B. thuringiensis also produces a chitinase enzyme that can hydrolyze chitin, a necessary component of the peritrophic membrane of the insect midgut (Kelkenberg et al. 2015), thus allowing bacterial toxins greater access to the receptors on epithelial cells (Sampson and Gooday 1998). Chitinase has been used as a synergistic agent to increase the larvicidal activity of B. thuringiensis toxins (González-Ponce et al. 2017; Juarez-Hernandez et al. 2015). Spodoptera litura is one of the most damaging insect pests that cause huge losses in many economically important crops in tropical and subtropical areas. In this work, we performed molecular characterization of the lepidopteran-specific toxin genes, cry1A, cry1B, cry2A, cry9, and vip3A in 511 local B. thuringiensis isolates to determine the frequency and distribution of these genes in different geographical regions of Thailand. B. thuringiensis containing all the detected genes were assayed for their toxicity against S. litura larvae. In addition, analyses of morphology and molecular mass of crystal proteins of the effective B. thuringiensis isolates were also performed.
Materials and methods
B. thuringiensis isolates and reference strains
The 511 bacterial isolates collected from soil samples from different geographical regions of Thailand, which were preliminary identified as B. thuringiensis base upon their crystal protein production were kindly provided by Prof. Dr. Tipvadee Attathom, Department of Entomology, Kasetsart University, Thailand. B. thuringiensis serovar kurstaki HD1 and serovar israelensis supplied by the Thailand Institute of Scientific and Technological Research (TISTR), the Ministry of Science and Technology (MOST), Thailand, were used as reference strains in this study. Serratia marcescens, used as a reference strain for chitinase activity assay, was supplied by the Department of Microbiology, Faculty of Liberal Arts and Science, Kasetsart University, Thailand.
Total DNA extraction and PCR analysis
Total DNA was extracted from B. thuringiensis isolates and B. thuringiensis serovar kurstaki HD1 following Thammasittirong and Attathom (2008). PCR was performed to screen the lepidopteran-specific toxin genes, cry1A, cry1B, cry2A, cry9 and vip3A, and to detect chi36 chitinase gene. Each 25-µl reaction contained 1.25 U Taq polymerase (RBC Bioscience, Taiwan), 1× polymerase buffer, 1.5 mM MgCl2, 200 µM dNTP, 0.2 µM of forward and reverse primers, and 1 µl (approximately 50 ng) of each total DNA. PCR amplifications were carried out using the MultiGene OptiMax Thermal Cycler (Labnet, USA) with the following thermal cycling parameters: initiation denaturation at 94 °C for 4 min; followed by 30 cycles of denaturation (94 °C for 1 min), annealing (Ta of each primer pair in Table 1, 1 min), and extension (72 °C for 1 min); and a final extension at 72 °C for 10 min. PCR products were electrophoresed through 1% agarose gel and stained with ethidium bromide. PCR products of each gene were purified using GF-1 AmbiClean Kit (Vivantis, Malaysia) and sent for sequencing (First BASE Laboratories, Malaysia). The obtained nucleotide sequences were compared to the GenBank database using BLASTN (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Table 1.
Primers used for detection of cry1A, cry1B, cry2A, cry9, vip3A, and chi36 genes
| Target gene(s) recognized | Primer name | Sequence 5′ → 3′ | Product size (bp) | T a (°C) | References |
|---|---|---|---|---|---|
| cry1Aa, cry1Ab, cry1Ac cry1Ad, cry1Ae, cry1Af, cry1Ag | Cry1A-f | ATTCGCTAGGAACCAAGC | 398 | 55 | Thammasittirong and Attathom (2008) |
| Cry1A-r | AATCCGGTCCCCATACAC | ||||
| cry1Ba, cry1Bb, cry1Bc, cry1Bd, cry1Be, cry1Bf | Cry1B-f | CAGAAACAACAGAACGACC | 921 | 57 | Thammasittirong and Attathom (2008) |
| Cry1B-r | CACTTCCCCACCATCCAT | ||||
| cry2Aa, cry2Ab, cry2Ac, cry2Ad | Cry2A-f | TACCTTTATTTGCACAGGCA | 1170 | 54 | Thammasittirong and Attathom (2008) |
| Cry2A-r | CTACCGTTTATAGTAACTCG | ||||
| cry9A, cry9B, cry9C, cry9D, cry9E | Cry9-f | CACATGAGTTTTCTTCCTAT | 440 | 54 | Thammasittirong and Attathom (2008) |
| Cry9-r | AGATACGATGCTTGTTGTAA | ||||
| vip3A | Vip3A-f | GGATTTGCCACTGGTATCAAAG | 1591 | 53 | The current work |
| Vip3A-r | TTGCTTTCCACGGCTCTA | ||||
| chi36 | Chi36-f | GATGTTAAACAGGTTCAA | 1083 | 50 | Arora et al. (2003) |
| Chi36-r | TTATTTTTGCAAGGAAAG |
Chitinase activity assays
Colloidal chitin was prepared according to Wang et al. (2014). B. thuringiensis isolates containing all the detected lepidopteran-specific genes (cry1A, cry1B, cry2A, cry9, vip3A) and chitinase gene were point inoculated on colloidal chitin agar and incubated for 96 h at 37 °C. The zone of clearance due to chitin hydrolysis and the diameters of colonies were measured. S. marcescens was used as the positive control.
Insecticidal activity assays
To obtain larvicidal activities against the second instar larvae of the common cutworm (Spodoptera litura), surface contamination bioassays were performed according to Lone et al. (2016) with modification. The second instar larvae of S. litura were supplied by the Ministry of Agriculture and Cooperatives, Thailand. A loopful of the selected B. thuringiensis isolates was inoculated into 5 ml of nutrient broth (NB) and incubated overnight at 37 °C with shaking at 100 rpm. Two percent of the seed inoculum was used to inoculate 100 ml of NB in 250 ml flask and incubated for 10 h at 37 °C with shaking at 100 rpm. A 1 ml sample of cell suspension was aliquoted and the culture supernatant was collected to obtain the secreted proteins. The remaining culture continued to incubate under the same conditions until it reached the sporulation phase at 48 h. The optical density at a wavelength of 600 nm (OD600) was measured using a spectrophotometer (GENESYS 6, Thermo Scientific, USA). The suspension mixtures containing cells, spores, and inclusion proteins at 10 OD600 units were harvested using centrifugation at 12,000×g at 4 °C for 10 min. The mixture pellets were resuspended in 1 ml of culture supernatant collected at 10 h after incubation and spread onto 2 × 2 cm2 of Ricinus communis L. leaf. NB was used as a control. After drying for approximately 1 h, each piece of leaf coating with each spore–crystal mixture was used to feed 10 larvae of S. litura in a plastic box. The treated larvae were left for 10 h and then they were fed new fresh leaves without the coating of the spore–crystal mixture. The percentage mortality was recorded after 7 days of incubation at room temperature (approximately 32 °C) and a 14 h (light): 10 h (dark) photoperiod. Three replications were performed for each B. thuringiensis isolate. B. thuringiensis serovar kurstaki HD1 was used as a reference strain.
In addition to lepidopteran insects, the selected B. thuringiensis isolates were assayed for toxicity against the larvae of a dipteran insect, Aedes aegypti, according to Thammasittirong et al. (2017). A. aegypti larvae were hatched from eggs supplied by the Ministry of Public Health, Thailand. The 10 OD600 units of suspension mixtures of each B. thuringiensis containing cells, spores, and inclusion proteins were harvested using centrifugation at 12,000×g at 4 °C for 10 min. The mixture pellets were resuspended in 10 ml of sterile distilled water. The larvicidal activity assays were performed in 48-well plates containing 1 ml of the suspension mixtures and 10 A. aegypti larvae per well. Distilled water without toxins was used as a control. B. thuringiensis serovar israelensis was used as a reference strain. In total, 100 larvae were assayed per B. thuringiensis isolate. The larvae mortality was recorded after 24 h of incubation. Three independent experiments were performed for each B. thuringiensis isolate.
Characterization of high larvicidal activity B. thuringiensis isolates
Crystal protein morphology analysis
Effective B. thuringiensis isolates were cultured on nutrient agar at 37 °C for 48 h. The sporulated cells were spread on a glass cover slip, dried overnight, and coated with carbon. The crystal morphology of each isolate was observed and imaged using a scanning electron microscope (SEM-Hitachi SU8020, Hitachi, Japan) operated at 1.5 kV.
Determination of molecular mass of proteins
A loopful of each effective B. thuringiensis isolate was inoculated into 5 ml of NB and incubated overnight at 37 °C with shaking at 100 rpm. Two percent of the seed inoculum was used to inoculate 50 ml of NB in 250 ml flask and incubated for 48 h at 37 °C with shaking at 100 rpm. After incubation, the absorbance at OD600 was measured and the culture medium containing the spore–crystal mixture with an OD600 of one was centrifuged at 13,000×g at 4 °C for 10 min using a MX-307 centrifuge (Tomy, Japan). The pellet of the spore–crystal mixture was resuspended in 70 µl of diH2O and mixed with 30 µl of sample buffer (62.5 mM Tris hydrochloride, pH 6.8, 2% SDS, 5% 2-mercaptoethanol, 10% glycerol, and 0.002% bromophenol blue), then boiled for 5 min. After centrifugation at 13,000×g for 20 min, a 10 µl of sample was separated by sodium dodecyl sulfate (10% w/v) polyacrylamide gel electrophoresis (SDS−PAGE) and stained with Coomassie Brilliant Blue R-250 (Bio-Rad, USA). The molecular masses of the resolved proteins were estimated by comparison with the prestained protein standard (BLUeye prestained protein ladder, GeneDireX Inc., Taiwan). Inclusion proteins of B. thuringiensis serovar kurstaki HD1 and B. thuringiensis serovar israelensis were used as references for protein molecular weight sizes.
Results
Frequency and distribution of lepidopteran-specific toxin genes
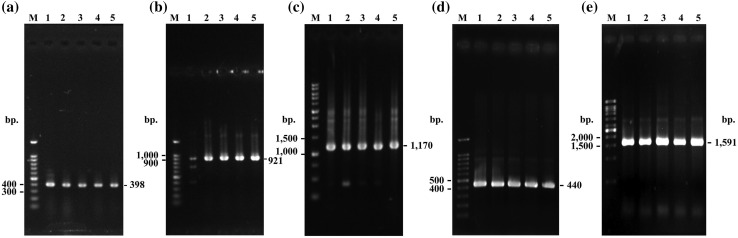
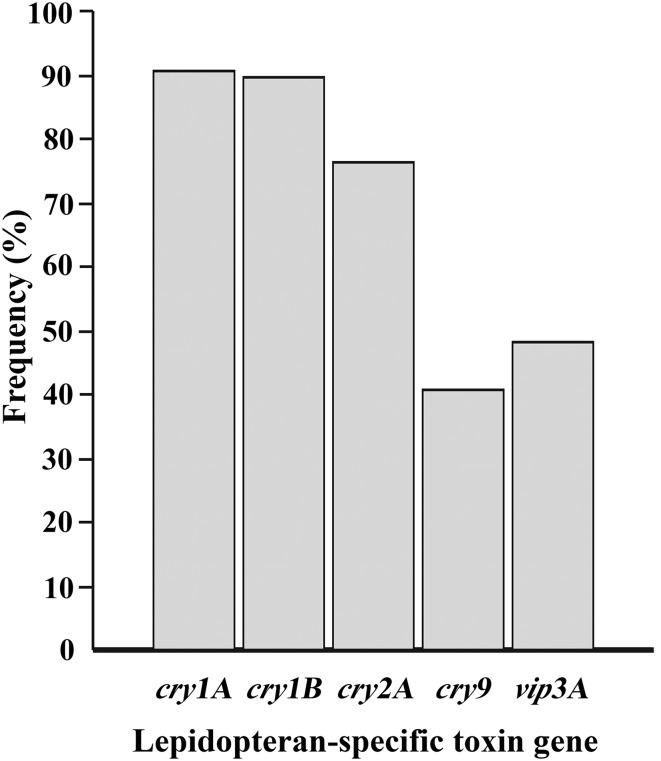
We investigated lepidopteran-specific toxin genes (cry1A, cry1B, cry2A, cry9, and vip3A) encoding for their corresponding lepidopteran-specific toxins in 511 local B. thuringiensis isolates using PCR-based detection. The amplification products of target genes were analyzed using agarose gel electrophoresis (Fig. 1). The PCR products were confirmed as their corresponding genes using DNA sequencing and NCBI-BLAST analysis. B. thuringiensis isolates that showed the amplification products of desired sizes were consider positive for respective genes. The results showed that 94.32% (482/511) of the B. thuringiensis isolates contained at least one of the detected genes, whereas only 5.68% (29/511) did not show any of the PCR products of the detected genes. The cry1A, cry1B, and cry2A genes were detected with high frequencies of 90.61% (463/511 isolates), 89.63% (458/511), and 76.32% (390/511 isolates), respectively (Fig. 2), whereas the cry9 and vip3A genes were detected with medium frequency at 40.70% (208/511 isolates) and 47.55% (243/511 isolates), respectively.
Fig. 1.
PCR amplification products of indigenous B. thuringiensis a cry1A, b cry1B, c cry2A, d cry9, e vip3A; (Lane M: DNA Marker, 1: B. thuringiensis 225-15, 2: B. thuringiensis 349-4, 3: B. thuringiensis 417-1, 4: B. thuringiensis 831-2, 5: B. thuringiensis 834-1)
Fig. 2.
Insecticidal toxin gene composition of B. thuringiensis in Thailand
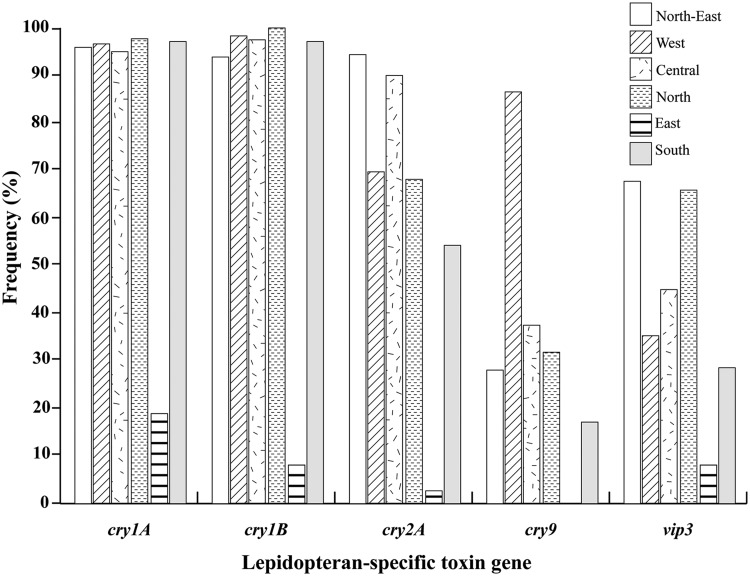
The cry1A and cry1B genes were detected at high frequency (more than 90%) in B. thuringiensis from every region of Thailand except the East (Fig. 3), where it was found in less than 20% of isolates. The cry2 gene was highly distributed in the North-East and Central regions (more than 80%) but moderately in the North, South, and West regions. The cry9 and vip3A genes were detected at moderate to low frequency in almost every region except for the West, where cry9 was present at a high frequency (more than 80%).
Fig. 3.
Insecticidal toxin gene distribution in different regions of Thailand
Among the 482 isolates harboring insecticidal toxin genes, 19 gene combination profiles were observed in these isolates (Table 2). There were 471 B. thuringiensis isolates (97.72%) that harbored more than one lepidopteran-specific gene, whereas only 11 isolates (2.28%) contained only one of the detected genes. The most frequent pattern contained the cry1A, cry1B, cry2A, and vip3A genes in 118 isolates (24.48%), whereas 61 B. thuringiensis isolates (12.66%) contained all the detected genes.
Table 2.
Insecticidal toxin gene combination profiles of B. thuringiensis in Thailand
| Profile | Gene composition | No. of isolate | % |
|---|---|---|---|
| 1 | cry1A + cry1B + cry2 + vip3A | 118 | 24.48 |
| 2 | cry1A + cry1B + cry2 | 94 | 19.50 |
| 3 | cry1A + cry1B + cry2 + cry9 | 88 | 18.26 |
| 4 | cry1A + cry1B + cry2 + cry9 + vip3A | 61 | 12.66 |
| 5 | cry1A + cry1B + cry9 | 41 | 8.51 |
| 6 | cry1A + cry2 | 23 | 4.77 |
| 7 | cry1A + cry1B + cry9 + vip3A | 11 | 2.28 |
| 8 | cry1A + cry1B + vip3A | 11 | 2.28 |
| 9 | cry1B + cry2 + cry9 | 7 | 1.45 |
| 10 | cry1B + cry2 | 6 | 1.24 |
| 11 | cry1A | 5 | 1.04 |
| 12 | cry2 | 5 | 1.04 |
| 13 | cry1A + cry2 + cry9 | 4 | 0.83 |
| 14 | cry1A + cry2 + vip3A | 2 | 0.41 |
| 15 | cry1A + cry9 | 2 | 0.41 |
| 16 | cry1B + cry2 + vip3A | 1 | 0.21 |
| 17 | cry1B + cry9 | 1 | 0.21 |
| 18 | cry1A + cry1B | 1 | 0.21 |
| 19 | cry1B | 1 | 0.21 |
| Total | 482 | 100 |
Chitinase assays
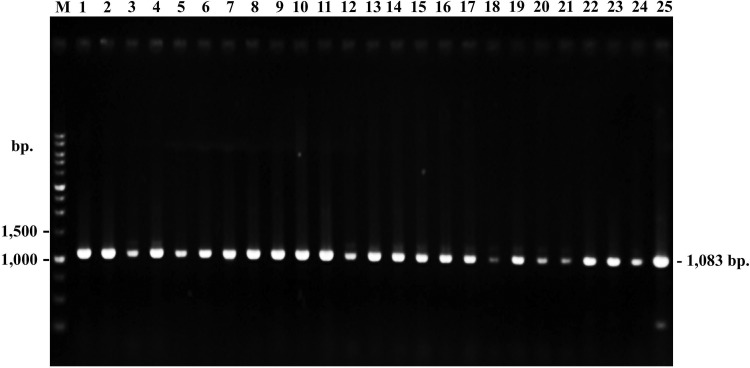
Sixty-one isolates containing all the detected genes (cry1A, cry1B, cry2A, cry9 and vip3A) were further detected for the chi36 gene encoding exochitinase. Amplification products of the target gene were analyzed using agarose gel electrophoresis (Fig. 4). The results revealed that all 61 isolates contained the chi36 gene as they provided amplicons of the expected size (1083 bp). In addition, the exochitinase activity of 61 isolates was analyzed by measuring the hydrolysis zones around their colonies, for which the highest activity was obtained from the B. thuringiensis 312-4 (Table S1 and Fig. S1).
Fig. 4.
Examples of PCR amplification products of chi36 gene in B. thuringiensis (Lane M: DNA Marker, 1–25: B. thuringiensis isolates)
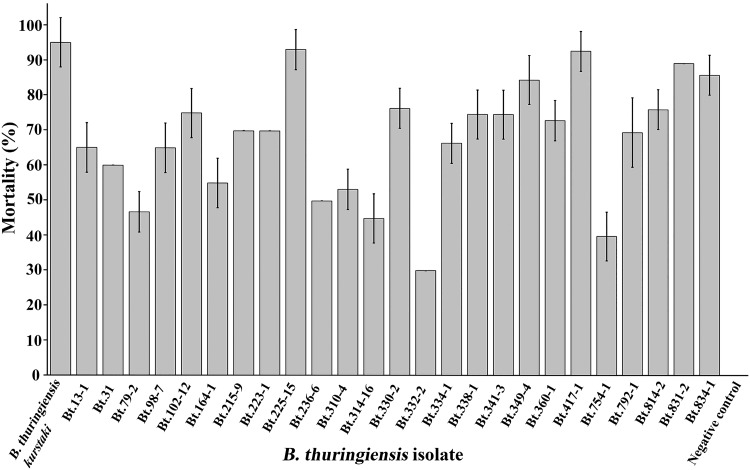
Insecticidal activity against Spodoptera litura
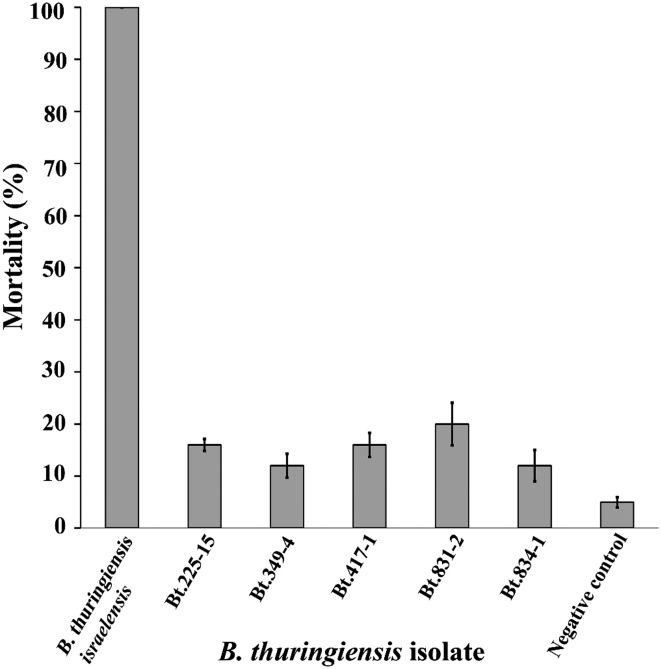
Twenty-five isolates of B. thuringiensis containing cry1A, cry1B, cry2, cry9, vip3A, and chi36 genes were selected for further larvicidal activity assays against second instar larvae of S. litura. The results revealed that all the isolates displayed larvicidal activity with different level of toxicities (Fig. 5) in a range between 30 and 93% mortality. It is interesting to note that five isolates (B. thuringiensis 225-15, 349-4, 417-1, 831-2, and 834-1) showed high larvicidal activity that caused more than 80% mortality of S. litura larvae which was comparable to that of the reference strain B. thuringiensis serovar kurstaki HD1.
Fig. 5.
Larvicidal activity of B. thuringiensis against S. litura larvae. Error bar indicates ± standard error for three experiments
Characterization of high lepidopteran-toxic B. thuringiensis isolates
SEM analysis of crystal morphologies
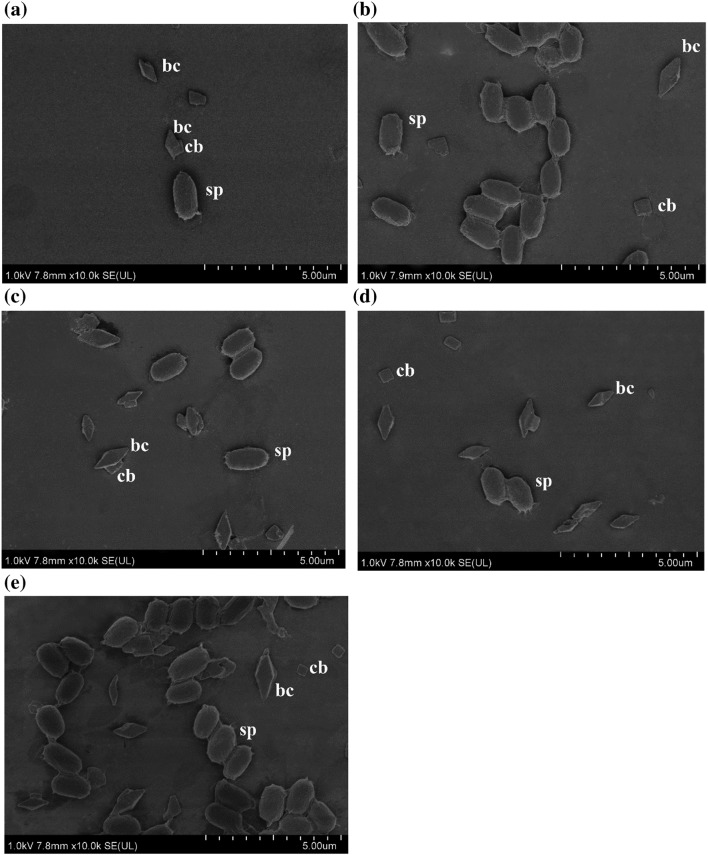
Crystal protein morphologies of five effective isolates (B. thuringiensis 225-15, 349-4, 417-1, 831-2, and 834-1) were characterized using SEM. The SEM images revealed that the bipyramidal and cuboidal crystal proteins were present in all isolates (Fig. 6).
Fig. 6.
Electron micrographs of crystals and spores of B. thuringiensis a B. thuringiensis 225-15, b B. thuringiensis 349-4, c B. thuringiensis 417-1, d B. thuringiensis 831-2 e B. thuringiensis 834-1. sp spore, bc bipyramidal crystal, cb cuboidal crystal
SDS-PAGE analysis
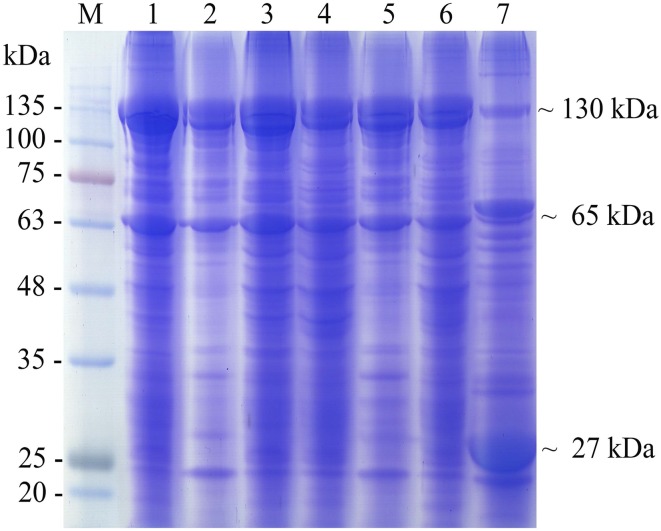
The molecular mass of the protein compositions of the crystal proteins from the five effective isolates was determined using SDS-PAGE analysis. SDS-PAGE investigation of the spore–crystal mixture revealed that all isolates contained major bands with molecular mass of approximately 130 and 65 kDa that were similar to those of B. thuringiensis serovar kurstaki HD1, which was used as a reference (Fig. 7), whereas the protein pattern of B. thuringiensis serovar israelensis showed major bands at approximately 27, 70, and 130 kDa.
Fig. 7.
SDS-PAGE of spore–crystal mixture of B. thuringiensis; (Lane M: prestained protein ladder, 1: B. thuringiensis serovar kurstaki, 2: B. thuringiensis 225-15, 3: B. thuringiensis 349-4, 4: B. thuringiensis 417-1, 5: B. thuringiensis 831-2, 6: B. thuringiensis 834-1, 7: B. thuringiensis serovar israelensis)
Insecticidal activity against Aedes aegypti
The larvicidal activity of five isolates (B. thuringiensis 225-15, 349-4, 417-1, 831-2, and 834-1), which were highly toxic to S. litura larvae, was assayed against a non-target organism, A. aegypti larvae (a dipteran insect). The bioassay results demonstrated that these isolates showed very low toxicity (less than 20% mortality) against second instar larvae of A. aegypti compared with the reference strain B. thuringiensis serovar israelensis that provided 100% mortality (Fig. 8).
Fig. 8.
Larvicidal activity of B. thuringiensis against A. aegypti larvae. Error bar indicates ± standard error for three experiments
Discussion
Isolation and characterization of novel B. thuringiensis strains may provide known or novel insecticidal proteins with higher larvicidal activity or broaden the insect spectrum. Therefore, analysis for native effective B. thuringiensis isolates was intensively performed, leading to an increase in the B. thuringiensis collection worldwide. We previously analyzed the cry gene composition in 134 B. thuringiensis isolates from 6 geographical regions of Thailand and reported that the cry1A and cry1B genes were found at moderate frequencies of approximately 50%. cry2A was found at high frequency of 80.6%, whereas 37.3% of B. thuringiensis isolates contained cry9 gene (Thammasittirong and Attathom 2008). In the present study, a higher number of local B. thuringiensis isolates (511 isolates from the same geographical regions of Thailand) were analyzed for the presence of the cry1A, cry1B, cry2A, cry9, and the additional vip3A genes encoding for lepidopteran-specific toxins. The isolated B. thuringiensis isolates from the same soil sample those shared the same morphology of inclusion proteins, protein pattern and the insecticidal toxin gene profile were considered as twin strains. The twin strains were excluded from this analysis to obtain a real estimate of the genetic diversity of the sampled areas. The results revealed that the cry2A and cry9 genes were detected with almost similar frequencies to the previous study, with the exceptions being cry1A and cry1B whose frequencies (approximately 90%) were higher in this B. thuringiensis collection. The results showed that even using the same primers and conditions for PCR detection and the same geographical regions of samples, differences in gene frequencies could be obtained.
The cry1A gene was detected at high frequency in our B. thuringiensis collection comparable with those of B. thuringiensis in Argentina and Egypt (Table 3). It was interesting to note that cry1B was not present in B. thuringiensis from Argentina and Iran and was found at low-medium frequency in China and Egypt compared with the high frequency in our collection (Table 3). The cry2A gene was detected with high frequencies in our collection and in B. thuringiensis from Argentina and China; however, this gene was detected at medium frequency in Iran and Egypt (Table 3). The cry9 gene was detected with low frequency in Argentina, Iran, and China compared with medium frequency in our collection. Finally, the vip3A gene was detected in our collection at a frequency comparable to those of India and China; however, higher frequencies of vip3A were reported for the B. thuringiensis collections from Argentina and Iran (Table 3). This information confirmed that the frequencies of the cry and vip genes varied depending on each B. thuringiensis collection. The diversity of B. thuringiensis in each collection may provide novel B. thuringiensis isolates with higher larvicidal activity or broaden the target insects suitable for use in combination with or as an alternative to the existing B. thuringiensis strains.
Table 3.
Insecticidal toxin gene detection in different countries
| Country of Bt collection | Gene frequency (%) | No. of isolate | References | ||||
|---|---|---|---|---|---|---|---|
| cry1A | cry1B | cry2A | cry9 | vip3A | |||
| Thailand | 90.61 | 89.63 | 76.32 | 40.70 | 48.18 | 511 | This study |
| Argentina | 92.5 | 0 | 92.5 | 2.5 | 91.3 | 268 | Sauka and Benintende (2017) |
| Iran | 44.29 | 0 | 54.29 | 25.71 | 82.6 | 70 | Seifinejad et al. (2008) |
| Egypt | 83.33(cry1) | 38.89 | 55.55 | nt | 0 | 18 | Salama et al. (2015) |
| India | nt | nt | nt | nt | 43.18 | 86 | Lone et al. (2016) |
| China | nt | nt | nt | nt | 67.4 | 2134 | Yu et al. (2011) |
| China | 67.7 | 12.9 | 70.0 | 15.5 | nt | 310 | Wang et al. (2003) |
nt not test
We previously reported that the cry1A, cry1B, and cry2 genes were found in effective isolates at high frequencies and suggested that these toxins may contribute to high larvicidal activity against lepidopteran insects (Thammasittirong and Attathom 2008). In the current work, we observed higher frequencies of cry1A and cry1B in B. thuringiensis from every area except for B. thuringiensis from the East region. The results suggest that we might obtain a high number of B. thuringiensis isolates containing larvicidal activity against lepidopteran insect larvae. The results showed high frequencies of gene combinations in our B. thuringiensis collection, as 19 gene profiles were observed which were higher than that reported in Argentina where 10 gene combination profiles were reported (Sauka and Benintende 2017). However, higher numbers of cry gene combination profiles (43 profiles) were reported for cry1 subclasses of B. thuringiensis from China (Wang et al. 2003). The difference in gene combination profiles may be attributable to differences in the numbers of isolates in each B. thuringiensis collection, the type of the detected genes, and the diversity of B. thuringiensis isolates in the collection. Our results showed that cry1A was usually found with cry1B and cry2, with combination frequencies of 88.17% and 80.91%, respectively. High associations between cry1 and cry2 have been commonly reported in various B. thuringiensis strains including B. thuringiensis serovar kurstaki HD1 and various B. thuringiensis isolates (Rangeshwaran et al. 2014; Sauka and Benintende 2017; Wang et al. 2003).
As previous reports have shown that the larvicidal activity of Cry toxins was improved by the chitinase enzyme (Chen et al. 2015; Ding et al. 2008), the 61 isolates of B. thuringiensis containing all the detected genes in the current work were therefore detected for the exochitinase gene and chitinase activity. Exochitinase gene and chitinase activities were detected in all 61 isolates; the exochitinase may synergize the larvicidal activity of insecticidal toxins from these isolates. The Cry1B, Cry2, and Vip3A toxins have been reported as toxic toward S. litura larvae (Herrero et al. 2016; Lu et al. 2013; Reyaz et al. 2017), whereas Cry1Ab and Cry1Ac were reported as non-toxic against S. litura larvae (Lu et al. 2013). In addition, Cry9Ea1, which is a member of the cry9 genes that presented at a low level in our B. thuringiensis collection, were reported as non-toxic against S. litura larvae (Wasano et al. 2005). Out of the 61 isolates containing all the detected genes and chitinase activity, 25 isolates were selected for larvicidal activity assay against S. litura larvae. The result revealed that only five isolates (B. thuringiensis 225-15, 349-4, 417-1, 831-2, and 834-1) showed high larvicidal toxicity (more than 80%) to S. litura larvae, implying that insecticidal activity may not rely only on the presence of the detected toxins and the chitinase enzyme but also on toxins, which were not detected in this work or the expression level of the protein toxins.
The SEM images showed bipyramidal and cuboidal crystal morphologies of B. thuringiensis 225-15, 349-4, 417-1, 831-2, and 834-1 that were similar to most of the B. thuringiensis samples in Argentina, of which 88.8% of isolates containing cry1 and cry2 genes exhibited bipyramidal and cuboidal crystals (Sauka and Benintende 2017). The inclusion morphologies of B. thuringiensis serovar kurstaki HD1 have been reported to be bipyramidal and cuboidal structures (Monnerat et al. 2007; Patel et al. 2012; Zorzetti et al. 2017); however, some publications reported that this strain contained bipyramidal, cuboidal, and spherical structures (Azizoglu et al. 2015; Yılmaz et al. 2012). Molecular mass analysis using SDS-PAGE showed the major bands of five effective isolate at approximately 130 and 65 kDa proteins, which were consistent with the 130 and 65 kDa proteins of the bipyramidal crystals of Cry1 proteins and the cuboidal crystals of Cry2 proteins, respectively (Azizoglu et al. 2015; Patel et al. 2012; Zorzetti et al. 2017). Even Cry2 toxins which form cuboidal inclusion have been reported to exhibit dual toxicity against both lepidopteran and dipteran insects (Ribeiro et al. 2017). In this work, very low toxicity against A. aegypti larvae was observed, this may be due to the five effective B. thuringiensis isolates contain Cry2 toxin which do not show larvicidal activity to A. aegypti larvae. The Cry2Aa was reported to exhibit the toxicity to lepidopteran and dipteran insects including A. aegypti, whereas a closely related Cry2Ab toxin was shown to contain toxicity to lepidopteran insects and mosquitocidal activity against Anopheles gambiae but not A. aegypti larvae (McNeil and Dean 2011). Low toxicity against A. aegypti larvae implies that the five effective isolates exhibit their larvicidal activities with high specificity.
Conclusion
The frequencies and distribution of insecticidal toxin genes were analyzed in 511 local B. thuringiensis isolates. The results showed that the frequencies of insecticidal toxin genes depended on the diversity of the B. thuringiensis isolates in the collection. Five isolates containing all the detected insecticidal toxin genes and chitinase enzyme displayed high toxicity and specificity against S. litura. These five effective isolates are alternative candidates for use in combination or as alternatives to the existing B. thuringiensis strains for controlling outbreaks of S. litura.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1. Chitinase activity assay on colloidal chitin agar a): S. marcescens, b): B. thuringiensis serovar kurstaki, c): B. thuringiensis 314-2, d): B. thuringiensis 225-15, e): B. thuringiensis 349-4, f): B. thuringiensis 417-1, g): B. thuringiensis 831-2, h): B. thuringiensis 834-1. Table S1. Chitinase activity assay of B. thuringiensis isolates (DOCX 1977 KB)
Acknowledgements
This work was financially supported by the Kasetsart University Research and Development Institute (KURDI) and partially supported by the Research Promotion and Technology Transfer Center (RPTTC), and the Department of Microbiology (Grant year 2018) Faculty of Liberal Arts and Science, Kasetsart University, Nakhon Pathom, Thailand.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Arora N, Ahmad T, Rajagopal R, Bhatnagar RK. A constitutively expressed 36 kDa exochitinase from Bacillus thuringiensis HD-1. Biochem Biophys Res Commun. 2003;307:620–625. doi: 10.1016/S0006-291X(03)01228-2. [DOI] [PubMed] [Google Scholar]
- Azizoglu U, Yılmaz S, Ayvaz A, Karabörklü S. Effects of Bacillus thuringiensis subsp. kurstaki HD1 spore-crystal mixture on the adults of egg parasitoid Trichogramma evanescens (Hymenoptera: Trichogrammatidae) Biotechnol Biotechnol Equip. 2015;29:653–658. doi: 10.1080/13102818.2015.1038303. [DOI] [Google Scholar]
- Baranek J, Konecka E, Kaznowski A. Interaction between toxin crystals and vegetative insecticidal proteins of Bacillus thuringiensis in lepidopteran larvae. Biocontrol. 2017;62:649–658. doi: 10.1007/s10526-017-9828-6. [DOI] [Google Scholar]
- Chakroun M, Banyuls N, Bel Y, Escriche B, Ferre J. Bacterial vegetative insecticidal proteins (Vip) from entomopathogenic bacteria. Microbiol Mol Biol Rev. 2016;80:329–350. doi: 10.1128/MMBR.00060-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jiang H, Cheng Q, Chen J, Wu G, Kumar A, Sun M, Liu Z. Enhanced nematicidal potential of the chitinase pachi from Pseudomonas aeruginosa in association with Cry21Aa. Sci Rep. 2015;5:14395. doi: 10.1038/srep14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WB, Lu GQ, Cheng HM, Liu CX, Xiao YT, Xu C, Shen ZC, Soberón M, Bravo A, Wu KM. Transgenic cotton co-expressing chimeric Vip3AcAa and Cry1Ac confers effective protection against Cry1Ac-resistant cotton bollworm. Transgenic Res. 2017;26:763–774. doi: 10.1007/s11248-017-0048-8. [DOI] [PubMed] [Google Scholar]
- de Escudero IR, Banyuls N, Bel Y, Maeztu M, Escriche B, Munoz D, Caballero P, Ferre J. A screening of five Bacillus thuringiensis Vip3A proteins for their activity against lepidopteran pests. J Invertebr Pathol. 2014;117:51–55. doi: 10.1016/j.jip.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Ding X, Luo Z, Xia L, Gao B, Sun Y, Zhang Y. Improving the insecticidal activity by expression of a recombinant cry1Ac gene with chitinase-encoding gene in acrystalliferous Bacillus thuringiensis. Curr Microbiol. 2008;56:442–446. doi: 10.1007/s00284-008-9112-1. [DOI] [PubMed] [Google Scholar]
- González-Ponce KS, Casados-Vázquez LE, Salcedo-Hernández R, Bideshi DK, del Rincón-Castro MC, Barboza-Corona JE. Recombinant Bacillus thuringiensis subsp. kurstaki HD73 strain that synthesizes Cry1Ac and chimeric ChiA74∆sp chitinase inclusions. Arch Microbiol. 2017;199:627–633. doi: 10.1007/s00203-017-1339-4. [DOI] [PubMed] [Google Scholar]
- Gouffon C, Van Vliet A, Van Rie J, Jansens S, Jurat-Fuentes JL. Binding sites for Bacillus thuringiensis Cry2Ae toxin on heliothine brush border membrane vesicles are not shared with Cry1A, Cry1F, or Vip3A Toxin. Appl Environ Microbiol. 2011;77:3182–3188. doi: 10.1128/AEM.02791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero S, Bel Y, Hernández-Martínez P, Ferré J. Susceptibility, mechanisms of response and resistance to Bacillus thuringiensis toxins in Spodoptera spp. Curr Opin Insect Sci. 2016;15:89–96. doi: 10.1016/j.cois.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Jain D, Sunda SD, Sanadhya S, Nath DJ, Khandelwal SK. Molecular characterization and PCR-based screening of cry genes from Bacillus thuringiensis strains. 3 Biotech. 2017;7:4. doi: 10.1007/s13205-016-0583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez-Hernandez EO, Casados-Vazquez LE, del Rincon-Castro MC, Salcedo-Hernandez R, Bideshi DK, Barboza-Corona JE. Bacillus thuringiensis subsp. israelensis producing endochitinase ChiA74Deltasp inclusions and its improved activity against Aedes aegypti. J Appl Microbiol. 2015;119:1692–1699. doi: 10.1111/jam.12962. [DOI] [PubMed] [Google Scholar]
- Kelkenberg M, Odman-Naresh J, Muthukrishnan S, Merzendorfer H. Chitin is a necessary component to maintain the barrier function of the peritrophic matrix in the insect midgut. Insect Biochem Mol Biol. 2015;56:21–28. doi: 10.1016/j.ibmb.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Lemes ARN, Figueiredo CS, Sebastião I, Marques da Silva L, da Costa Alves R, de Siqueira HÁA, Lemos MVF, Fernandes OA, Desidério JA. Cry1Ac and Vip3Aa proteins from Bacillus thuringiensis targeting Cry toxin resistance in Diatraea flavipennella and Elasmopalpus lignosellus. from sugarcane. Peer J. 2017;5:e2866. doi: 10.7717/peerj.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lone SA, Yadav R, Malik A, Padaria JC. Molecular and insecticidal characterization of Vip3A protein producing Bacillus thuringiensis strains toxic against Helicoverpa armigera (Lepidoptera: Noctuidae) Can J Microbiol. 2016;62:179–190. doi: 10.1139/cjm-2015-0328. [DOI] [PubMed] [Google Scholar]
- Lu Q, Cao G, Zhang L, Liang G, Gao X, Zhang Y, Guo Y. The binding characterization of Cry insecticidal proteins to the brush border membrane vesicles of Helicoverpa armigera, Spodoptera exigua, Spodoptera litura and Agrotis ipsilon. J Integr Agric. 2013;12:1598–1605. doi: 10.1016/S2095-3119(13)60427-X. [DOI] [Google Scholar]
- McNeil BC, Dean DH. Bacillus thuringiensis Cry2Ab is active on Anopheles mosquitoes: single D block exchanges reveal critical residues involved in activity. FEMS Microbiol Lett. 2011;325:16–21. doi: 10.1111/j.1574-6968.2011.02403.x. [DOI] [PubMed] [Google Scholar]
- Monnerat RG, Batista AC, de Medeiros PT, Martins ÉS, Melatti VM, Praça LB, Dumas VF, Morinaga C, Demo C, Gomes ACM, Falcão R, Siqueira CB, Silva-Werneck JO, Berry C. Screening of Brazilian Bacillus thuringiensis isolates active against Spodoptera frugiperda. Plutella xylostella and Anticarsia gemmatalis. Biol Control. 2007;41:291–295. doi: 10.1016/j.biocontrol.2006.11.008. [DOI] [Google Scholar]
- Naqvi RZ, Asif M, Saeed M, Asad S, Khatoon A, Amin I, Mukhtar Z, Bashir A, Mansoor S. Development of a triple gene Cry1Ac-Cry2Ab-EPSPS construct and its expression in Nicotiana benthamiana for insect resistance and herbicide tolerance in plants. Front Plant Sci. 2017;8:55. doi: 10.3389/fpls.2017.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negatu B, Kromhout H, Mekonnen Y, Vermeulen R. Use of chemical pesticides in Ethiopia: a cross-sectional comparative study on knowledge, attitude and practice of farmers and farm workers in three farming systems. Ann Occup Hyg. 2016;60:551–566. doi: 10.1093/annhyg/mew004. [DOI] [PubMed] [Google Scholar]
- Palma L, Munoz D, Berry C, Murillo J, Caballero P. Bacillus thuringiensis toxins: an overview of their biocidal activity. Toxins (Basel) 2014;6:3296–3325. doi: 10.3390/toxins6123296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panuwet P, Siriwong W, Prapamontol T, Ryan PB, Fiedler N, Robson MG, Barr DB. Agricultural pesticide management in Thailand: situation and population health risk. Environ Sci Policy. 2012;17:72–81. doi: 10.1016/j.envsci.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel KD, Chudasama CJ, Ingle SS. Molecular characterization of Bacillus thuringiensis isolated from diverse habitats of India. J Basic Microbiol. 2012;52:437–445. doi: 10.1002/jobm.201100080. [DOI] [PubMed] [Google Scholar]
- Rangeshwaran R, Gorky A, Viswakethu V, Karkera A, Sivakumar G, Mohan M. Cry gene and plasmid profiling of Bacillus thuringiensis isolated from Indian soils. J Biol Control. 2014;28:185–191. [Google Scholar]
- Reyaz AL, Gunapriya L, Indra Arulselvi P. Molecular characterization of indigenous Bacillus thuringiensis strains isolated from Kashmir valley. 3 Biotech. 2017;7:143. doi: 10.1007/s13205-017-0756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Bergmann Morais, Martins Érica Soares, de Souza Aguiar Raimundo Wagner, Corrêa Roberto Franco Teixeira. Bacillus thuringiensis and Lysinibacillus sphaericus. Cham: Springer International Publishing; 2017. Expression of Bacillus thuringiensis Toxins in Insect Cells; pp. 99–110. [Google Scholar]
- Salama HS, Abd El-Ghany NM, Saker MM. Diversity of Bacillus thuringiensis isolates from Egyptian soils as shown by molecular characterization. J Genet Eng Biotechnol. 2015;13:101–109. doi: 10.1016/j.jgeb.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson MN, Gooday GW. Involvement of chitinases of Bacillus thuringiensis during pathogenesis in insects. Microbiology. 1998;144:2189–2194. doi: 10.1099/00221287-144-8-2189. [DOI] [PubMed] [Google Scholar]
- Sauka DH, Benintende GB. Diversity and distribution of lepidopteran-specific toxin genes in Bacillus thuringiensis strains from Argentina. Rev Argent Microbiol. 2017;49:273–281. doi: 10.1016/j.ram.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Seifinejad A, Jouzani GRS, Hosseinzadeh A, Abdmishani C. Characterization of Lepidoptera-active cry and vip genes in Iranian Bacillus thuringiensis strain collection. Biol Control. 2008;44:216–226. doi: 10.1016/j.biocontrol.2007.09.010. [DOI] [Google Scholar]
- Tawatsin A, Usavadee T, Padet S. Pesticides used in Thailand and toxic effects to human health. Med Res Arch. 2015;3:1–10. [Google Scholar]
- Thammasittirong A, Attathom T. PCR-based method for the detection of cry genes in local isolates of Bacillus thuringiensis from Thailand. J Invertebr Pathol. 2008;98:121–126. doi: 10.1016/j.jip.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Thammasittirong A, Prigyai K, Thammasittirong SNR. Mosquitocidal potential of silver nanoparticles synthesized using local isolates of Bacillus thuringiensis subsp. israelensis and their synergistic effect with a commercial strain of B. thuringiensis subsp. israelensis. Acta Tropica. 2017;176:91–97. doi: 10.1016/j.actatropica.2017.07.020. [DOI] [PubMed] [Google Scholar]
- Vachon V, Laprade R, Schwartz JL. Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J Invertebr Pathol. 2012;111:1–12. doi: 10.1016/j.jip.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Wang J, Boets A, Van Rie J, Ren G. Characterization of cry1, cry2, and cry9 genes in Bacillus thuringiensis isolates from China. J Invertebr Pathol. 2003;82:63–71. doi: 10.1016/S0022-2011(02)00202-1. [DOI] [PubMed] [Google Scholar]
- Wang K, Yan P, Cao L. Chitinase from a novel strain of Serratia marcescens JPP1 for biocontrol of aflatoxin: molecular characterization and production optimization using response surface methodology. Biomed Res Int. 2014;2014:1–8. doi: 10.1155/2014/482623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasano N, Saitoh H, Maeda M, Ohgushi A, Mizuki E, Ohba M. Cloning and characterization of a novel gene cry9Ec1 encoding lepidopteran-specific parasporal inclusion protein from a Bacillus thuringiensis serovar galleriae strain. Can J Microbiol. 2005;51:988–995. doi: 10.1139/w05-084. [DOI] [PubMed] [Google Scholar]
- Yılmaz S, Ayvaz A, Akbulut M, Azizoglu U, Karabörklü S. A novel Bacillus thuringiensis strain and its pathogenicity against three important pest insects. J Stored Prod Res. 2012;51:33–40. doi: 10.1016/j.jspr.2012.06.004. [DOI] [Google Scholar]
- Yu X, Zheng A, Zhu J, Wang S, Wang L, Deng Q, Li S, Liu H, Li P. Characterization of vegetative insecticidal protein vip genes of Bacillus thuringiensis from Sichuan Basin in China. Curr Microbiol. 2011;62:752–757. doi: 10.1007/s00284-010-9782-3. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Chen LZ, Lu Q, Zhang Y, Liang GM. Toxicity and binding analyses of Bacillus thuringiensis toxin Vip3A in Cry1Ac-resistant and -susceptible strains of Helicoverpa armigera (Hubner) J Integr Agr. 2015;14:347–354. doi: 10.1016/S2095-3119(14)60770-X. [DOI] [Google Scholar]
- Zorzetti J, Ricietto APS, Fazion FAP, Meneguim AM, Neves PMOJ, Vilas-Boas LA, Rodrigues RB, Vilas-Bôas GT. Selection and characterization of Bacillus thuringiensis (Berliner) (Eubacteriales: Bacillaceae) strains for Ecdytolopha aurantiana (Lima) (Lepidoptera: Tortricidae) control. Neotrop Entomol. 2017;46:86–92. doi: 10.1007/s13744-016-0424-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Chitinase activity assay on colloidal chitin agar a): S. marcescens, b): B. thuringiensis serovar kurstaki, c): B. thuringiensis 314-2, d): B. thuringiensis 225-15, e): B. thuringiensis 349-4, f): B. thuringiensis 417-1, g): B. thuringiensis 831-2, h): B. thuringiensis 834-1. Table S1. Chitinase activity assay of B. thuringiensis isolates (DOCX 1977 KB)