Abstract
Concentrations of antiretrovirals in hair are associated with virologic outcomes in cohorts of human immunodeficiency virus (HIV)-positive individuals but have never been examined in a clinical trial. We show for the first time the predictive utility of hair antiretroviral concentrations in a large HIV treatment-naive trial (AIDS Clinical Trials Group protocol A5257).
Keywords: HIV, A5257 study, AIDS Clinical Trials Group (ACTG), hair concentrations, non-NNRTI regimens
Pharmacologic measures of antiretroviral (ARV) adherence entail measuring ARV concentrations in a biomatrix such as plasma, dried blood spots, or hair. Pharmacologic metrics may have particular utility in the interpretation of clinical trial data, where social desirability bias can inflate self-reported adherence. Indeed, in preexposure prophylaxis (PrEP) trials, pharmacologic measures were critical to study interpretation [1], and many PrEP demonstration projects have subsequently incorporated drug concentration monitoring into their design. Short half-life plasma ARV measures have proven inconsistent in predicting virologic outcomes when used for therapeutic drug monitoring during human immunodeficiency virus (HIV) treatment, but the use of longer-term pharmacologic adherence metrics in HIV treatment trials has lagged behind prevention trials.
Concentrations of ARVs in hair have been used to monitor adherence over longer periods of time than plasma concentrations, which are susceptible to intraindividual variability [2] and “white coat” effects. Hair measures are analogous to using hemoglobin A1c to assess long-term glucose control. Moreover, hair collection is easy and avoids phlebotomy, and hair can be stored and shipped at room temperature without biohazardous precautions [3]. However, although hair ARV concentrations are associated with virologic outcomes in observational cohorts [4, 5], hair-based metrics of ARV adherence have not been examined in an HIV treatment trial to date.
AIDS Clinical Trials Group (ACTG) protocol A5257, a phase 3 study comparing 3 nonnucleoside reverse transcriptase inhibitor-sparing combination regimens for HIV-1 treatment-naive individuals [6], was the first HIV treatment trial to incorporate hair collection into its design. The incorporation of hair measures into a randomized clinical trial with frequent, consistent viral load monitoring provides additional power over observational cohorts (where regimens change and viral loads are monitored less frequently) in analyzing the predictive utility of ARV concentrations in hair for predicting virologic failure (VF). This analysis examines the relationship between hair ARV concentrations and virologic response in A5257.
METHODS
Parent Study and Primary Outcome
Protocol A5257 randomized 1809 treatment-naive adults to 1 of 3 regimens, atazanavir (ATV)/ritonavir (RTV), darunavir (DRV)/RTV, or raltegravir (RAL), each in combination with tenofovir disoproxil fumarate/emtricitabine. Participants were followed for 96 weeks after randomization of the last participant. The primary endpoint was VF defined as a confirmed plasma HIV-1 RNA >1000 copies/mL at or after 16 weeks and before 24 weeks, or >200 copies/mL at or after 24 weeks. The trial opened to enrollment in May 2009; hair sampling was initiated in August 2010; and the study was completed in June 2013 with the primary results published [6].
Hair Collection and Assays for the Hair Substudy
A5257 collected small hair samples at weeks 8, 16, and 24 and then quarterly using previously described methods [3]. Briefly, from all participants with scalp hair and who consented for hair collection, 20–30 strands of hair were cut from the head. The distal end of the hair sample was marked with a small piece of tape to denote directionality, and the hair was stored in aluminum foil at room temperature. After trial completion, all hair samples were analyzed at the University of California–San Francisco (UCSF) Hair Analytical Laboratory using methods peer-reviewed and approved by the National Institutes of Health, Division of AIDS’ Clinical Pharmacology and Quality Assurance Program [7].
Sample preparation involves extracting the relevant ARV from hair with an organic solvent and analysis by liquid-chromatography–tandem mass spectrometry (LC-MS/MS) [8]. These methods have been validated from 0.01 to 4.0 ng/mg hair for RTV, 0.05 to 20 ng/mg hair for ATV, and 0.02 to 8.0 ng/mg for RAL and DRV with good linearity (R2 >0.99) and reproducibility (coefficient of variation <15%) for all drugs.
Data Analysis
The primary endpoint of this analysis was A5257 protocol-defined VF. Multivariable proportional hazards regression modeled VF in terms of hair concentration as a time-varying predictor. Covariates included in multivariable models were age, sex, race/ethnicity, pretreatment viral load, and education level. Analyses were performed by specific ARV, and a common hair ARV concentration effect was modeled across drugs in terms of association with VF by taking the log of the most recently measured hair concentration divided by the within-arm median.
RESULTS
Participants in A5257 Hair Substudy
Hair and viral load data were available for 2192 person-visits among 599 A5257 participants. Hair collection was not offered at all person-visits because hair collection was added to the protocol after study launch. Among person-visits where hair collection was offered, the overall participation rate was 62%. Participation in the hair substudy did not differ by treatment arm, and there were no differences in VF rates among those who did and did not participate. Of the 599 participants, 32% were female, and median age was 38 years (range, 18–76); 48% were white, 33% black, 17% Latino, 2% other; 28% had baseline HIV-1 RNA levels >100000 copies/mL; and 60% had some college education (with 40% high school education or less). The median duration of follow-up for hair substudy participants was 124 weeks.
Relationship Between Hair ARV Concentrations and VF
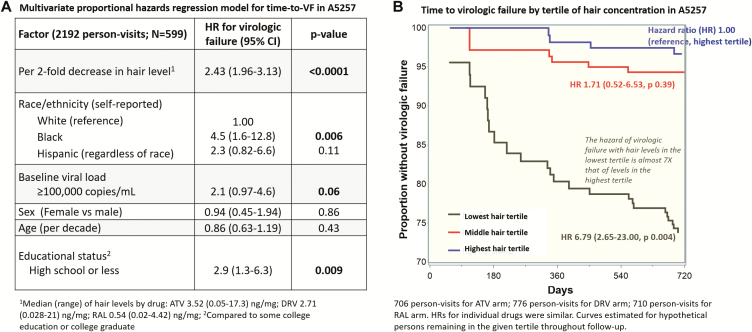
A multivariate proportional hazards regression model showed that lower hair ARV concentrations strongly predicted a higher risk of VF (hazards ratio [HR], 2.43; 95% confidence interval, 1.96–3.13; P < .0001) for every 2-fold decrease in hair concentration, which remained consistent for each drug individually, even with adjustment for age, sex, race/ethnicity, pretreatment viral load, and education. Being black, having a pretreatment viral load >100000 copies/mL, and having a lower educational level were each independently associated with VF (Figure 1A).
Figure 1.
Relationship between hair antiretroviral concentrations and time to virologic failure in A5257 study in multivariate models (Panel 1A) and in Kaplan Meier plot (Panel 1B). Abbreviations: ATV, atazanavir; CI, confidence interval; DRV, darunavir; HR, hazards ratio; RAL, raltegravir.
The overall incidence of VF by 96 weeks in A5257 was 16.3% across all arms [6]. Rates of VF among participants who underwent hair ARV concentration testing were 26%, 6%, and 3% at 2 years for those with hair concentrations in the lowest, middle, and highest tertiles, respectively. The HR of VF with hair ARV concentrations in the lowest tertile was 6.8 times that with concentrations in the highest tertile (Figure 1B). Similar HRs were observed for the effects of hair concentrations on VF for males (1385 person-visits) and females (807 person-visits).
DISCUSSION
We demonstrate for the first time that higher hair ARV concentrations, an index of long-term exposure, predict a significantly decreased risk of VF in a randomized HIV treatment-naive trial. Although hair ARV concentrations had been shown to be associated with virologic response in observational cohorts [4], the frequency of viral load monitoring in this treatment trial (with viral load measurements at weeks 8, 16, and every 12 weeks thereafter) provides additional information over the cohort setting for hair concentrations predicting VF. The strong relationship between hair ARV concentrations and subsequent virologic response in A5257 strengthens the evidence for the utility of this novel long-term adherence metric for HIV treatment monitoring.
Despite the increasing use of objective biomarkers of ARV adherence using drug levels in PrEP rollout or demonstration projects, the use of pharmacologic measures to assess adherence in the context of HIV treatment is rare. Virologic failure is the most common way to diagnose low adherence to antiretroviral therapy (ART). However, by the time VF has developed on ART, opportunities for adherence intervention have been lost. The present study suggests useful algorithms in the clinical setting to enhance treatment outcomes. For example, in the setting of long-term treatment, VF with low hair ARV concentrations suggests nonadherence; VF with high ARV concentrations suggests and should trigger testing for viral resistance. As another example, hair ARV concentrations determined soon after regimen initiation may identify patients at risk for early treatment failure (or failure to initially respond); low hair concentrations can be detected prior to the lack of initial viral load decay, triggering early adherence intervention. Finally, since ARV concentrations in hair have been shown to increase in individuals after implementation of adherence interventions [9], an additional utility of hair monitoring could be to assess the efficacy of such interventions.
Limitations of hair ARV monitoring include the cost of LC–MS/MS-based assays, the current lack of a commercially available assay, and variable acceptability rates of hair collection in different populations. Acceptability of hair collection in this study was approximately 60%, which is consistent with acceptability rates in a study of adult men-who-have-sex-with-men in the US PrEP Demonstration Project [10]. However, when hair collection is incorporated at the start of a trial, with appropriate education of field staff and participants to “normalize” collection, rates of acceptability, both in the United States [11] and in Africa [3] and Asia [5], are much higher (>95%). Regarding cost of hair assays, our group and others are working on lower-cost methods to measure ARV concentrations in hair [12] and in developing urine-based methods to assess adherence in real time via a point-of-care assay [13]. Of note, the urine test will only assess short-term adherence, so hair levels combined with urine levels are needed to provide information on adherence patterns.
In summary, we show that ARV concentrations in hair are a strong independent predictor of virologic outcomes in a large, randomized, clinical trial of 3 ART regimens for the initial treatment of HIV. This was the first time that hair ARV concentration determinations have been incorporated into an HIV therapeutics trial, providing robust evidence that adherence monitoring using hair metrics can be used to check for impending VF. Further study is underway on whether early monitoring of hair ARV concentrations followed by targeted adherence interventions informed by this metric will reduce subsequent VF on ART.
Notes
Acknowledgments. We gratefully acknowledge the participants of the A5257 study, along with their friends and families, for taking part in the study.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH). Grant numbers from NIAID/NIH are as follows: grants UM1 AI068634, UM1 AI068636, and UM1 AI106701 for funding of the AIDS Clinical Trials Group; grants R21AI112362 and 2R01AI098472 to M. G. for the hair assays and data analysis; grants UM1 AI069439, P30 AI110527, and UL1 TR002243 to support D. W. H.; and grants TL1 TR000144 and TL1 TR001871 for the Clinical & Translational Sciences Institute at UCSF.
Potential conflicts of interest. Bristol Meyers Squibb, Gilead, Janssen, and Merck Corporations all provided study drugs for the original A5257 trial and reviewed the manuscript but did not contribute to the analysis or interpretation. R. J. L. reports grants from the Division of AIDS, NIH during the conduct of the study; personal fees and nonfinancial support from Gilead Sciences; and personal fees from Merck and Company outside the submitted work. A. N. S. reports receipt of grants from the NIH during the conduct of the study and grants from the NIH and Gilead Sciences outside the submitted work. J. S. C. reports receipt of grants from Theratechnologies outside the submitted work. All remaining authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 25th Conference on Retroviruses and Opportunistic Infections. Boston, MA, 4–7 March 2018 Abstract 24.
References
- 1. Blumenthal J, Haubrich R. Pre-exposure prophylaxis for HIV infection: how antiretroviral pharmacology helps to monitor and improve adherence. Expert Opin Pharmacother 2013; 14:1777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nettles RE, Kieffer TL, Parsons T, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis 2006; 42:1189–96. [DOI] [PubMed] [Google Scholar]
- 3. Hickey MD, Salmen CR, Tessler RA, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr 2014; 66:311–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis 2011; 52:1267–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pintye J, Bacchetti P, Teeraananchai S, et al. Brief report: lopinavir hair concentrations are the strongest predictor of viremia in HIV-infected Asian children and adolescents on second-line antiretroviral therapy. J Acquir Immune Defic Syndr 2017; 76:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lennox JL, Landovitz RJ, Ribaudo HJ, et al. ; ACTG A5257 Team. Efficacy and tolerability of 3 nonnucleoside reverse transcriptase inhibitor-sparing antiretroviral regimens for treatment-naive volunteers infected with HIV-1: a randomized, controlled equivalence trial. Ann Intern Med 2014; 161:461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther 2013; 93:479–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phung N, Kuncze K, Okochi H, et al. Development and validation of an assay to analyze atazanavir in human hair via liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2018; 32:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gwadz M, Cleland CM, Applegate E, et al. ; Heart to Heart Collaborative Research Team. Behavioral intervention improves treatment outcomes among HIV-infected individuals who have delayed, declined, or discontinued antiretroviral therapy: a randomized controlled trial of a novel intervention. AIDS Behav 2015; 19:1801–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gandhi M, Murnane PM, Bacchetti P, et al. Hair levels of preexposure prophylaxis drugs measure adherence and are associated with renal decline among men/transwomen. AIDS 2017; 31:2245–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koss CA, Hosek SG, Bacchetti P, et al. Comparison of measures of adherence to human immunodeficiency virus preexposure prophylaxis among adolescent and young men who have sex with men in the United States. Clin Infect Dis 2018; 66:213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gandhi M, Yang Q, Bacchetti P, Huang Y. Short communication: a low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses 2014; 30:25–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gandhi M, Bacchetti P, Rodriguez W, et al. Development and validation of an immunoassay for tenofovir in urine as a real-time metric of antiretroviral adherence. EClinical Medicine (Published by The Lancet) 2018. doi:10.1016/j.eclinm.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]