Abstract
Background
There is a need for a reliable, simple diagnostic assay for typhoid fever. Available commercial serologic assays for typhoid fever have limited sensitivity and specificity. Using high-throughput immunoscreening technologies, we previously identified several immunoreactive Salmonella Typhi antigens that seem promising for possible inclusion in a new diagnostic assay: hemolysin E (HlyE), cytolethal distending toxin, S. Typhi lipopolysaccharide (LPS), and S. Typhi membrane preparation.
Methods
We assessed plasma antibody responses (immunoglobulin [Ig] M, IgA, and IgG) to these antigens by means of enzyme-linked immunosorbent assay in patients with suspected enteric fever, controls with other febrile illnesses, and healthy controls in Dhaka, Bangladesh and performed Tubex and Typhidot tests, the Widal assay, and the typhoid/paratyphoid test (TPTest) in each patient. Using machine learning methods, we identified a parsimonious serology signature to distinguish acute typhoid cases from controls and then validated our findings in an independent test cohort from Nepal of patients with culture-confirmed S. Typhi and controls with other bacteremic illnesses.
Results
We demonstrated that the use of 2 antigens (HlyE and LPS) with 1 antibody isotype (IgA) could distinguish typhoid from other invasive bacterial infections (area under the receiver operating characteristic curve [AUC], 0.95; sensitivity, 90%, specificity, 92%). Use of a single antigen (HlyE) and isotype (IgA) had an AUC of 0.93.
Conclusion
Our results suggest that development of a diagnostic assay for acute typhoid fever focused on detecting IgA responses against HlyE, with or without LPS, is warranted.
Keywords: typhoid fever, enteric fever, HlyE, LPS, Salmonella Typhi
Using machine learning methods, we identified and validated anti–hemolysin E immunoglobulin A and antilipopolysaccharide immunoglobulin A plasma responses as predictors of acute typhoid fever in 2 independent cohorts of patients in Bangladesh and Nepal (sensitivity, 90%; specificity, 92%).
Typhoid fever remains a significant public health problem, with >11 million cases each year resulting in an estimated 130000 deaths [1]. A major challenge in typhoid management and control is the lack of a reliable, simple diagnostic assay. Lack of optimal diagnostics for typhoid fever limits our ability to diagnose acute cases of typhoid fever, which is often diagnosed clinically and treated empirically. This has led to substantial overdiagnosis and the overprescribing of antityphoid antimicrobials [2, 3], which has driven emergence of antimicrobial resistance, especially fluoroquinolone resistance in Asia [4]. Misdiagnosis also often leads to delay of care for the true causative pathogen of the febrile illness, such as typhus or leptospirosis [5]. Improved diagnostics for typhoid fever would not only improve patient management and outcomes but would also extend surveillance efforts to provide reliable estimates of disease burden and allow for better targeting and evaluation of typhoid intervention strategies, especially given the recent endorsement from the World Health Organization (WHO) Strategic Advisory Group of Experts on Immunization and WHO position statement on the use of typhoid Vi conjugate vaccines [6, 7].
There are several available diagnostics for typhoid fever. Assays based on direct bacterial detection lack availability and are inherently limited by the low number of colony-forming units found in peripheral blood (median colony-forming unit count, 1/mL) [8]. An alternative approach is to identify infected patients based on a host response to the pathogen; however, current serology-based diagnostics (Widal, Tubex [IDL Biotech], and Typhidot [Reszon Diagnostics] tests) have poor sensitivity and/or specificity, especially in endemic settings [9–11]. Detecting antibodies secreted from circulating, activated-lymphocytes overcomes these limitations; however, the assay requires moderately advanced laboratory capacity and requires 24–48 hours to obtain a result [10, 12].
To address these issues, we have used several high-throughput immunoscreening technologies to identify promising Salmonella Typhi (S. Typhi) antigens for possible inclusion in next-generation serodiagnostic assays. These antigens include hemolysin E (HlyE), cytolethal distending toxin B (CdtB), S. Typhi membrane preparation (MP), and S. Typhi lipopolysaccharide (LPS) [13–15]. HlyE and CdtB are present in S. Typhi and Salmonella Paratyphi A but are rarely found in other Salmonella serovars [16]. HlyE is a pore-forming toxin that affects bacterial growth within human macrophages and contributes to the cytotoxicity and invasion of epithelial cells [17–19]. CdtB is one of the A subunits of the typhoid toxin and induces cell cycle arrest of host cells by causing DNA damage [20]. S. Typhi MP is a crude MP comprising >936 proteins, including several outer membrane proteins (eg, bacterial chaperonin [GroEl]) and a number of virulence-associated proteins (eg, PhoP-activated gene C [PagC], sensor kinase [PhoQ], phosphorylated response regulator [PhoP], and HlyE) [21]. S. Typhi LPS contains serogroup antigens O9 and O12 [22]. We produced these antigens and used a unique, well-characterized sample collection in Bangladesh to identify a parsimonious serologic signature to distinguish typhoid cases from controls, which could be adapted for use in a point-of-care diagnostic to assess antibody responses. We then validated these serologic signatures in an independent cohort in Nepal.
METHODS
Ethics Statement
This study was approved by the research and ethical review committees and/or institutional review board for human subjects research of the International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh (icddr,b), the Nepal Health Research Council (Kathmandu, Nepal), Massachusetts General Hospital, and Stanford University. The study was conducted per the principles expressed in the Declaration of Helsinki/Belmont Report. Written informed consent was obtained from all individuals or their guardians before study participation.
Study Subject Selection and Sample Collection
Individuals presenting to the icddr,b Dhaka hospital or Mirpur field site (Dhaka, Bangladesh) or the Dhulikhel Hospital (Kavrepalanchowk, Nepal) with clinical symptoms of enteric fever were eligible for enrollment if they were 1–59 years of age (>1 year of age for Nepal cohort) and had a self-reported fever of 3–7 days duration without an obvious focus of infection or alternate diagnosis. Venous blood was collected from participants at enrollment before the start of antimicrobial therapy. Bacteremia was confirmed by blood culture using a BacT/Alert or Bactec 9050 automated system (BD Diagnostics) with identification of isolates by standard culture and biochemical tests [23, 24].
Immunodiagnostic Assays
The Widal assay and typhoid/paratyphoid test (TPTest) were performed as described elsewhere [10] and the commercial assays, Tubex and Typhidot, were performed according to the manufacturers’ instructions [10]. For enzyme-linked immunosorbent assays, the following antigens were used: S. Typhi LPS, MP, HlyE, and CdtB. Source and preparation of antigens are described elsewhere [12, 25, 26], with modifications and details listed in the Supplementary Methods. Microplates were coated with LPS (2.5 µg/mL), MP (2 µg/mL), HlyE (1 µg/mL), or CdtB (2.5 µg/mL), and plasma was added at a dilution of 1:500 except for measurement of IgG responses to HlyE for which a 1:20000 dilution of plasma was used. Bound antibodies were detected with anti-human immunoglobulin (Ig) G, IgA, and IgM conjugated with horseradish peroxidase (Jackson ImmunoResearch), and peroxidase activity was measured with o-phenylenediamine. To compare across plates, the sample readings were divided by the readings of an in-house pooled standard, multiplied by 100, and results were expressed as enzyme-linked immunosorbent assay units.
Statistical Analyses and Latent Class Modeling
For the primary analyses, we used the Bangladeshi samples as our training data set and compared the distribution of anti-HlyE, LPS, CdtB, and MP values for IgA, IgM, and IgG in this training cohort. We first evaluated responses for each antigen–antibody isotype combination in the Bangladesh cohort. We then assessed the classification accuracy of antigen–antibody isotype combinations in this cohort by 3 supervised learning methods: support vector machines, random forests, and partial least squares regression. To do this in the absence of a reference standard for diagnosing acute typhoid fever, we probabilistically assigned individuals to typhoid or control status according to their results by 5 tests (blood culture, Widal assay, Typhidot and Tubex tests, and TPTest) and clinical status (known healthy controls or confirmed alternative diagnoses). All individuals underwent all 5 tests, except for blood cultures in healthy individuals.
We previously reported diagnostic test results in detail for this training cohort [10], and the test characteristics for this analysis were derived using Bayesian latent class analysis [10, 27]. We generated 1000 training sets in which each sample was probabilistically classified as a case or control, according to their results on the 5 tests, by applying Bayes rule using the sensitivity and specificity for each test together with estimated prevalence from the prior latent class analysis. We then performed the 3 supervised learning algorithms on each set to identify the most predictive antigen-antibody isotypes, assessed by variable importance ranking.
We repeated this for the 1000 training sets and calculated average variable importance across all sets. Using the top 4 antigen-antibody isotype combinations in this training set, we compared the distribution of antibody responses in culture-confirmed cases and controls by Wilcoxon rank sum test. We then created logistic regression models using log-normalized Bangladeshi data to identify the optimal antibody signature to identify patients with acute typhoid, performed backward selection for significant variables, and evaluated the full model and submodels using the log-normalized data from Nepal. We assessed accuracy according to the area under the receiver operator characteristic curve (AUC). All analyses were performed in R software version 3.2.4 (R Foundation for Statistical Computing, Vienna; available at: https://www.R-project.org/).
RESULTS
Characteristics of Study Participants
Of the 127 Bangladeshi study participants with suspected enteric fever, controls with other febrile illness, and healthy controls, 59 (46.5%) were male, and the median age was 13 years (interquartile range [IQR], 5–27 years) (Table 1). For the Nepalese cohort (n = 126), the median age was 22.5 (IQR, 16–36), and 61 participants (48%) were male (Table 1).
Table 1.
Characteristics of Participants from Study Cohorts in Bangladesh and Nepal
| Characteristic | Bangladesh (n = 127) | Nepal (n = 126) |
|---|---|---|
| Age, median (IQR), y | 13 (5–27) | 22.5 (16–36) |
| Female sex, No. (%) | 68 (54) | 65 (52) |
| Suspected enteric fever, No. | 92 | 126 |
| Salmonella Typhi blood culture positive | 28 | 77 |
| S. Typhi blood culture negative | 64 | 0 |
| Healthy endemic zone controls, No. | 20 | 0 |
| Other febrile disease, No. | 15 | 49 |
| Visceral leishmaniasis | 5 | 0 |
| Tuberculosis | 7 | 0 |
| Malaria | 3 | 0 |
| Staphylococcus aureus bacteremia | 0 | 19 |
| Escherichia coli bacteremia | 0 | 15 |
| Streptococcal bacteremia | 0 | 7 |
| Enterococcus bacteremia | 0 | 5 |
| Other bacteremia (Acinetobacter, Klebsiella) | 0 | 3 |
Abbreviation: IQR, interquartile range.
Grouping of Study Participants
Of the 127 study participants in the Bangladesh cohort, 92 were suspected to have enteric fever, and 28 of these participants were blood culture positive for S. Typhi (Table 1). We also added the following 2 cohorts for comparison: healthy endemic zone controls (n = 20) and febrile patients with other confirmed infectious diseases (visceral leishmaniasis, tuberculosis, and malaria; n = 15). The 126 Nepalese patients with suspected enteric fever were divided into 2 cohorts: positive blood culture for S. Typhi (n = 77) and positive blood culture for other bacteria (Escherichia coli, Staphylococcus aureus, Streptococcus, Klebsiella, Acinetobacter, or Enterococcus) (n = 49) (Table 1).
Distribution of Anti–S. Typhi Plasma Responses in Bangladeshis
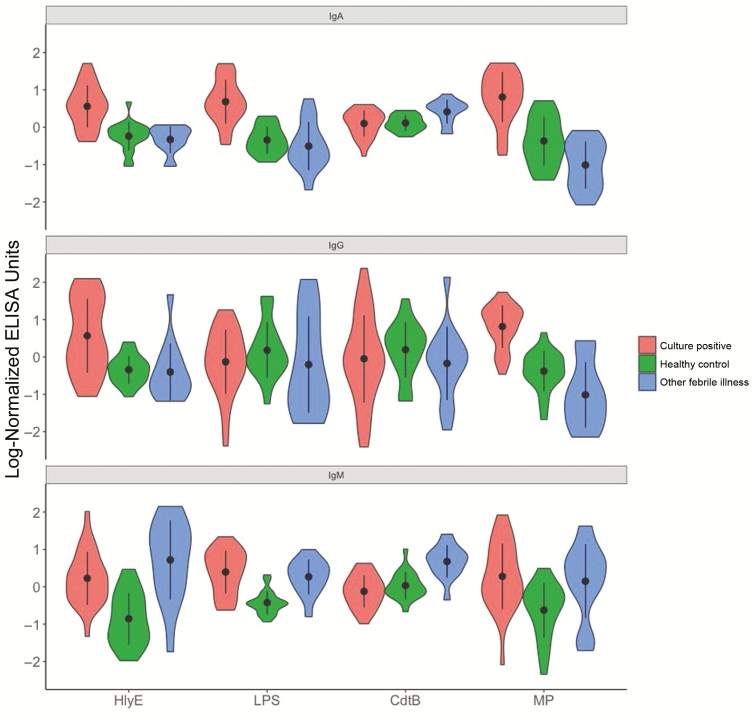
We measured IgG, IgA, and IgM plasma antibody responses targeting HlyE, CdtB, S. Typhi LPS, and S. Typhi MP. The distribution of antibody responses by isotype to the various antigens in blood culture positive individuals (cases) and healthy controls and those with other febrile illnesses is plotted in Figure 1. There is a clear distinction between case patients and controls for anti-HlyE and MP IgA and IgG responses and LPS IgA responses. There was nonspecific immunoreactivity to IgM for all antigens, and anti-CdtB responses for all isotypes did not perform well as a biomarker for S. Typhi infection. To illustrate how various antigen-antibody isotype combinations performed relative to historical and improved antibody-based tests for typhoid fever, we also plotted the distribution of the antibody responses by isotype and group to the various antigens with groupings based on the results of blood culture and Widal assay (Supplementary Figures 1A and 2) and blood culture and TPTest (Supplementary Figure 1B).
Figure 1.
Distribution of anti–Salmonella Typhi plasma responses in Bangladeshis by group. Violin plots of log-normalized immunoglobulin (Ig) M, IgA, and IgG plasma responses against hemolysin E (HlyE), lipopolysaccharide (LPS), cytolethal distending toxin (CdtB), and membrane preparation (MP) in patients with positive blood cultures, healthy controls, and those with other febrile illnesses. Abbreviation: ELISA, enzyme-linked immunosorbent assay.
Figure 2.
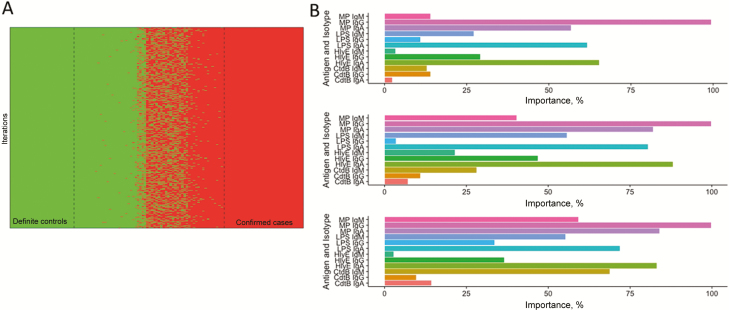
Optimal biomarker selection by probabilistic classification and machine learning methods. A, First, 1000 sets were generated in which samples were probabilistically assigned to typhoid or control status according to the results of 5 tests (ie, blood culture, Widal assay, Typhidot and Tubex tests, and typhoid/paratyphoid test [TPTest]) and clinical status (known healthy controls or confirmed alternative diagnoses).B, Machine learning algorithms were then performed on each set (from top to bottom: random forest, support vector machines, and partial least squares regression) to identify the most predictive antigen-antibody isotypes and assessed by means of variable importance ranking. Abbreviations: CdtB, cytolethal distending toxin; HlyE, hemolysin E; Ig, immunoglobulin; LPS, lipopolysaccharide, MP, membrane preparation.
Identification of Optimal Biomarker for Acute S. Typhi Infection
We used probabilistic classification (Figure 2A) and the following supervised learning methods to identify the optimal biomarker for acute S. Typhi infection: random forests, support vector machine, and partial least squares regression (Figure 2B). All 3 machine learning methods yielded similar results, with anti-MP IgG the most important predictive variable overall. The next 3 best predictors, across all 3 methods, were anti-HlyE IgA, LPS IgA, and MP IgA.
Distribution of Anti–S. Typhi Responses in the Nepalese by Cohort
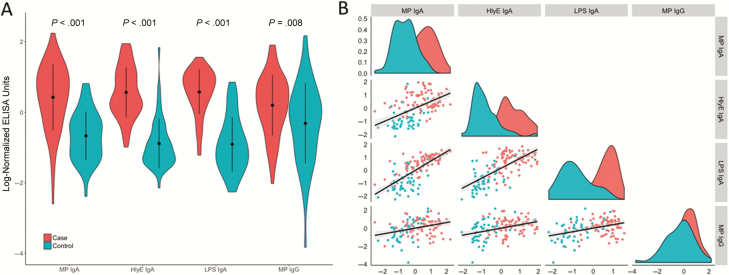
Next, we chose to look further at the top 4 antigen-antibody isotype predictors of acute typhoid infection: MP IgG, HlyE IgA, LPS IgA, and MP IgA. Using our independent Nepalese validation cohort, we confirmed that anti-HlyE, LPS, and MP IgA plasma antibodies could distinguish acute S. Typhi bacteremic individuals from those with other invasive bacteremias (P < .001 for all comparisons; Figure 3A), and we found that IgA responses to these 3 antigens were highly correlated (Figure 3B). We performed age-stratified analyses and found no difference in plasma IgA responses to LPS and MP IgA by age; however, IgA responses to HlyE increased with age among case patients in the Nepal cohort (Supplementary Figure 2). Because HlyE is present in other gram-negative bacteria (eg, E. coli), we next evaluated by cause of nontyphoidal bacteremia and found that HlyE IgA antibody responses were elevated uniquely in patients with S. Typhi bacteremia and not in those with other gram-negative bacteremias (E. coli, Klebsiella, or Acinetobacter; Table 1 and Supplementary Figure 3).
Figure 3.
Distribution of anti–Salmonella Typhi plasma responses in the Nepal cohort. A, Violin plots of immunoglobulin (Ig) A plasma responses against hemolysin E (HlyE), lipopolysaccharide (LPS), and membrane preparation (MP) and IgG plasma responses to MP in Nepalese study participants by cohort. Differences between groups were assessed using the Wilcoxon rank sum test. B, Density histograms for patients with culture-confirmed typhoid (red) and controls with other bacterial infections (teal) for each antigen, along with scatterplots and Pearson correlation of antibody responses between 2 selected antigens (column-row combinations). Abbreviation: ELISA, enzyme-linked immunosorbent assay.
Model Selection and Validation
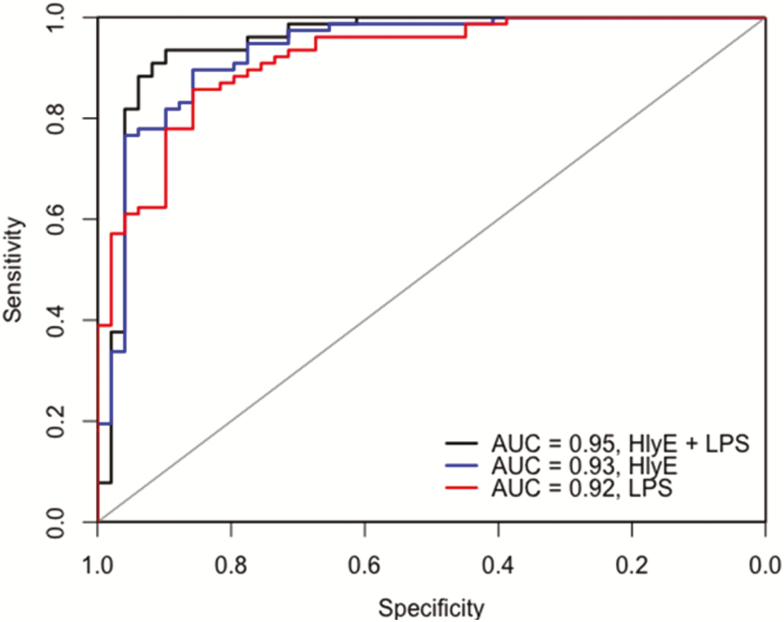
Using log-normalized Bangladeshi data, we fit logistic regression models using 3 biomarkers and their combinations: HlyE, LPS, and MP IgA antibody. We then tested these models on the log-normalized Nepal data and assessed classification accuracy in this independent cohort by AUC (Figure 4 and Table 2). Use of 2 antigens (HlyE, and LPS) with 1 antibody isotype (IgA) distinguished typhoid from other invasive bacterial infections with a sensitivity of 90% and specificity of 92% (AUC, 0.95; Figure 4). Use of a single antigen (HlyE) had an AUC of 0.93, and at a sensitivity of 90%, specificity was 87%.
Figure 4.
Assessment of sensitivity and specificity of immunoglobulin (Ig) A responses to hemolysin E (HlyE) and lipopolysaccharide (LPS). Receiver operating characteristic curves were generated from log-normalized Nepal data. The area under the receiver operating characteristic curve (AUC) is plotted for a model using 2 antigens (HlyE and LPS) and each antigen alone.
Table 2.
Diagnostic Performance of Immunoglobulin A Responses as Assessed by Area Under the Receiver Operating Characteristic Curve, Sensitivity, and Specificity in Out-of-Sample Validation
| Antigen(s) | AUC (95% CI) | Specificity (at 90% Sensitivity), % | Sensitivity (at 90% Specificity), % |
|---|---|---|---|
| HlyE | 0.93 (.88–.98) | 87 | 78 |
| MP | 0.83 (.76–.90) | 48 | 61 |
| LPS | 0.92 (.87–.97) | 77 | 68 |
| HlyE and LPS | 0.95 (.90–1.00) | 92 | 91 |
| HlyE, LPS, and MP | 0.95 (.90–.99) | 90 | 87 |
Abbreviations: AUC: area under the receiver operating characteristic curve; CI, confidence interval; HlyE, hemolysin E; LPS, lipopolysaccharide; MP, membrane preparation.
DISCUSSION
Starting with a set of immunoreactive antigens of S. Typhi identified from high-throughput screens, we applied supervised learning methods to identify optimal antigen and antibody isotype combinations to identify patients with acute typhoid fever. Using a Bangladeshi training set, we found that IgA responses targeting HlyE, LPS, and MP and IgG responses to MP had strong discriminatory value in identifying patients with typhoid during the acute phases of illness. We then performed a validation step using an independent cohort of Nepalese patients with blood culture-confirmed S. Typhi and other bacteremias to verify that these 3 antigens are promising biomarkers of typhoid fever. The IgA responses to these 3 antigens were highly correlated, and we found only incremental value in combining various target antigens or antibody isotypes.
Our findings were consistent with those of 2 earlier studies investigating serodiagnostic signatures for typhoid fever. In a study in which human challenge participants in the United Kingdom were used as a training cohort and typhoid cases and febrile controls from Nepal were used for validation, IgA responses to LPS had the highest diagnostic accuracy (AUC, 0.88) for a single antigen-antibody isotype, but HlyE was also selected for accuracy in several models [28]. In a study in Nigeria, LPS IgA, IgM and IgG responses (AUCs, 0.90–0.93) and HlyE IgG responses (AUC, 0.91) performed well in distinguishing typhoid from other infections [29]. In our current analysis in South Asia, we found that HlyE IgA (AUC 0.93) performed better than LPS IgA (AUC, 0.92) or MP IgA (AUC, 0.83).
It is of interest that our analysis identified plasma IgA responses as an excellent biomarker of acute stage typhoid in South Asia. This may reflect the relative transience of plasma IgA responses compared with IgG responses, especially in endemic areas where reinfection can occur [30–32]. IgM responses are also transient; however, they can be nonspecific (especially in endemic areas), and in our analysis, IgM responses did not provide any appreciable discriminatory value in identifying S. Typhi–infected individuals. IgG antibody levels can remain elevated for years, and endemic areas may have a high background seroprevalence of IgG antibodies, making it more difficult to interpret a single positive IgG test in the diagnosis of acute typhoid fever [11].
We found that HlyE IgA is an excellent biomarker for acute infection for both Bangladesh and Nepal cohorts. We have previously identified HlyE in high-throughput immunoscreens as an antigen that correctly identified individuals with acute typhoid or paratyphoid fever in Dhaka, Bangladesh, using plasma and antibody-in-lymphocyte supernatant, a surrogate marker of the mucosal immune response, and this finding has been confirmed by other groups [28, 29, 33]. HlyE is a pore-forming toxin that affects bacterial growth within human macrophages and contributes to the cytotoxicity and invasion of epithelial cells [17–19]. It is present in several strains of E. coli, including the nonpathogenic (K12), as well as enteroinvasive, enteroaggregative, enterotoxigenic, and Shiga toxin–producing strains [19, 34], and it is found within the Salmonella genus including the typhoidal serotypes S. Typhi and S. Paratyphi A [19]. HlyE is also present in a number of nontyphoidal Salmonella serotypes (including, but not limited to, invasive isolates of S. Schwarzengrund, Montevideo, Bredeney, and others) [18, 35, 36]. In S. Typhi, the expression of hlyE is under the control of the transcriptional regulatory protein PhoP [17, 37], a master regulator of intracellular survival of S. enterica. Even though HlyE is expressed by E. coli, our work demonstrates that HlyE IgA retained discriminatory value irrelevant of the cause of other bacteremia, including by nontyphoidal gram-negative organisms.
We also performed age-stratified analyses and found that HlyE IgA did not perform as well in the younger groups in the Nepal cohort. This may be because there were fewer young children in this cohort. Children <5 years old were more highly represented in the Bangladeshi cohort, and in this data set HlyE IgA did retain discriminatory value in young children as well as older children and adults with acute-stage typhoid. Age-based analysis is critical in deciding the optimal assay target and responses. Many diagnostic analyses focus on adults because a larger volume of blood can be collected, but adults in endemic areas may have had repetitive exposures and immune priming, altering the biomarker response. In endemic areas, a large burden of typhoid fever is borne by children, and a diagnostic assay for use in these areas must have excellent predictive value across ages.
A key challenge with identifying reliable markers of typhoid fever has been the limited sensitivity of blood culture, estimated at 40%–70% [9]. To exceed this sensitivity, a diagnostic would have to detect patients with culture-negative typhoid, but identifying such patients is challenging in the absence of more sensitive diagnostics. To overcome this challenge, we used a cohort in which multiple different serologic tests, including acute and convalescent Widal testing, were used to probabilistically classify culture-negative patients into case patients and controls (culture-positive cases were all classified as confirmed). We repeated this classification 1000 times to identify the antigen-antibody isotype combinations that most reliably identified typhoid cases. We found that these combinations performed well in detecting culture-negative typhoid.
Our study has a number of limitations. Our healthy control groups in Bangladesh included few young children, and the validation data in Nepal were predominantly from older children and adults. In addition, our validation set included only confirmed typhoid or other bacteremia cases, although our Bangladeshi training set had a wide range of patient classification types. Our data set also does not directly address the discriminatory value of serologic analysis during acute paratyphoid fever. Paratyphoid fever caused by S. Paratyphi A now accounts for up to 1 in 5 cases of enteric fever in areas of Asia; however, the previous small analysis by Charles et al [13] of HlyE responses in patients with paratyphoid fever suggests excellent performance. Our analysis also used samples collected in highly typhoid endemic areas, and findings might be different in nonendemic areas, although the discriminatory value that we found even in young immunologically naive children is reassuring and suggests that assays based on anti-HlyE IgA detection would be promising even in nonendemic areas.
Despite these limitations, our study represents one of the largest and most rigorous analyses to identify a host antibody-based biomarker profile that distinguish patients with acute typhoid fever in an area of high endemicity, irrespective of age. Our results suggest that development of a diagnostic assay focused on detecting IgA responses against HlyE in acute typhoid is warranted. The detection of LPS and MP IgA responses can also be pursued, although those antigens cannot be expressed recombinantly, and their inclusion in diagnostic assay development would require rigorous standardization to ensure reproducibility. Incorporating serologic testing into a rapid diagnostic format would be essential to achieve impact in the care of patients with suspected typhoid fever. In light of the recent WHO endorsement of incorporating typhoid conjugate vaccines in global typhoid control programs, a rapid, point-of-care, field-based diagnostic detecting anti-HlyE IgA would provide significant synergy, both in helping to identify target populations and in judging the impact of control program measures.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We would like to express our gratitude to the patients for participating in this study and the field workers and research staff at the icddr,b, Bangladesh, and Dhulikhel Hospital, Nepal for their support and effort in the sample collection and processing.
Disclaimer. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Financial support. This work was supported in parts by core grants to the icddr,b and by the government of the People’s Republic of Bangladesh, Global Affairs Canada, the Swedish International Development Cooperation Agency, the Department of International Development (UKAid), the National Institutes of Health (grant R33AI100023 to E. T. R. and R. C. C. and R01AI134814-01A1 to R. C. C.), the Fogarty International Center (training grant in Vaccine Development and Public Health TW005572 to F. K., M. H., and T. R. B. and Global Emerging Leader award K43TW010362 to T. R. B), the Robert Wood Johnson Foundation Harold Amos Medical Faculty Development Program (grant 72424 to R. C. C.), the Harvard Medical School (The Office for Diversity Inclusion and Community Partnership faculty fellowship award to R. C. C.), the Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene (J. R. A.), the Bill & Melinda Gates Foundation (J. R. A.), the George Rosenkranz Foundation (J. R. A.), and the Massachusetts General Hospital Department of Medicine (Transformative Scholars award to R. C. C.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mogasale V, Maskery B, Ochiai RL, et al. . Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Glob Health 2014; 2:e570–80. [DOI] [PubMed] [Google Scholar]
- 2. Wasihun AG, Wlekidan LN, Gebremariam SA, et al. . Diagnosis and treatment of typhoid fever and associated prevailing drug resistance in Northern Ethiopia. Int J Infect Dis 2015; 35:96–102. [DOI] [PubMed] [Google Scholar]
- 3. Andrews JR, Vaidya K, Bern C, et al. . High rates of enteric fever diagnosis and lower burden of culture-confirmed disease in peri-urban and rural Nepal. J Infect Dis 2017. doi: 10.1093/infdis/jix221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 2015; 33(suppl 3):C21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basnyat B. Typhoid versus typhus fever in post-earthquake Nepal. Lancet Glob Health 2016; 4:e516–7. [DOI] [PubMed] [Google Scholar]
- 6. Typhoid vaccines: WHO position paper—March 2018 Available at: http://apps.who.int/iris/bitstream/handle/10665/272272/WER9313.pdf?ua=1. Accessed 6 April 2018. [DOI] [PubMed]
- 7. Summary of the October 2017 meeting of the Strategic Advisory Group of Experts on Immunization Available at: http://www.who.int/immunization/policy/sage/SAGE_oct_2017_meeting_summary.pdf. Accessed 14 March 2018.
- 8. Wain J, Diep TS, Ho VA, et al. . Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol 1998; 36:1683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrews JR, Ryan ET. Diagnostics for invasive Salmonella infections: current challenges and future directions. Vaccine 2015; 33(suppl 3):C8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Islam K, Sayeed MA, Hossen E, et al. . Comparison of the performance of the TPTest, Tubex, Typhidot and Widal immunodiagnostic assays and blood cultures in detecting patients with typhoid fever in Bangladesh, including using a Bayesian latent class modeling approach. PLoS Negl Trop Dis 2016; 10:e0004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wijedoru L, Mallett S, Parry CM. Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever. Cochrane Database Syst Rev 2017; 5:CD008892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khanam F, Sheikh A, Sayeed MA, et al. . Evaluation of a typhoid/paratyphoid diagnostic assay (TPTest) detecting anti-Salmonella IgA in secretions of peripheral blood lymphocytes in patients in Dhaka, Bangladesh. PLoS Negl Trop Dis 2013; 7:e2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Charles RC, Liang L, Khanam F, et al. . Immunoproteomic analysis of antibody in lymphocyte supernatant in patients with typhoid fever in Bangladesh. Clin Vaccine Immunol 2014; 21:280–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Charles RC, Sheikh A, Krastins B, et al. . Characterization of anti-Salmonella enterica serotype Typhi antibody responses in bacteremic Bangladeshi patients by an immunoaffinity proteomics-based technology. Clin Vaccine Immunol 2010; 17:1188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris JB, Baresch-Bernal A, Rollins SM, et al. . Identification of in vivo-induced bacterial protein antigens during human infection with Salmonella enterica serovar Typhi. Infect Immun 2006; 74:5161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McClelland M, Sanderson KE, Clifton SW, et al. . Comparison of genome degradation in Paratyphi A and Typhi, human-restricted serovars of Salmonella enterica that cause typhoid. Nat Genet 2004; 36:1268–74. [DOI] [PubMed] [Google Scholar]
- 17. Faucher SP, Forest C, Béland M, Daigle F. A novel PhoP-regulated locus encoding the cytolysin ClyA and the secreted invasin TaiA of Salmonella enterica serovar Typhi is involved in virulence. Microbiology 2009; 155:477–88. [DOI] [PubMed] [Google Scholar]
- 18. Fuentes JA, Villagra N, Castillo-Ruiz M, Mora GC. The Salmonella Typhi hlyE gene plays a role in invasion of cultured epithelial cells and its functional transfer to S. Typhimurium promotes deep organ infection in mice. Res Microbiol 2008; 159:279–87. [DOI] [PubMed] [Google Scholar]
- 19. Oscarsson J, Westermark M, Löfdahl S, et al. . Characterization of a pore-forming cytotoxin expressed by Salmonella enterica serovars Typhi and Paratyphi A. Infect Immun 2002; 70:5759–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haghjoo E, Galán JE. Salmonella Typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc Natl Acad Sci U S A 2004; 101:4614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sheikh A, Khanam F, Sayeed MA, et al. . Interferon-γ and proliferation responses to Salmonella enterica serotype Typhi proteins in patients with S. Typhi bacteremia in Dhaka, Bangladesh. PLoS Negl Trop Dis 2011; 5:e1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kingsley RA, Bäumler AJ. Host adaptation and the emergence of infectious disease: the Salmonella paradigm. Mol Microbiol 2000; 36:1006–14. [DOI] [PubMed] [Google Scholar]
- 23. Talawadekar NN, Vadher PJ, Antani DU, Kale VV, Kamat SA. Chloramphenicol resistant Salmonella species isolated between 1978 and 1987. J Postgrad Med 1989; 35:79–82. [PubMed] [Google Scholar]
- 24.Cruickshank R, Duguid JP, Marmion BP, Swain RHA, eds. The Enterobacteriaceae: Salmonella. In: Medical Microbiology. 12th ed. Edinburgh, United Kingdom,: Churchill Livingstone, 1975:403–19. [Google Scholar]
- 25. Sheikh A, Bhuiyan MS, Khanam F, et al. . Salmonella enterica serovar Typhi-specific immunoglobulin A antibody responses in plasma and antibody in lymphocyte supernatant specimens in Bangladeshi patients with suspected typhoid fever. Clin Vaccine Immunol 2009; 16:1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Charles RC, Sultana T, Alam MM, et al. . Identification of immunogenic Salmonella enterica serotype Typhi antigens expressed in chronic biliary carriers of S. Typhi in Kathmandu, Nepal. PLoS Negl Trop Dis 2013; 7:e2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joseph L, Gyorkos TW, Coupal L. Bayesian estimation of disease prevalence and the parameters of diagnostic tests in the absence of a gold standard. Am J Epidemiol 1995; 141:263–72. [DOI] [PubMed] [Google Scholar]
- 28. Darton TC, Baker S, Randall A, et al. . Identification of novel serodiagnostic signatures of typhoid fever using a Salmonella proteome array. Front Microbiol 2017; 8:1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Felgner J, Jain A, Nakajima R, et al. . Development of ELISAs for diagnosis of acute typhoid fever in Nigerian children. PLoS Negl Trop Dis 2017; 11:e0005679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marmion DE, Naylor GR, Stewart IO. Second attacks of typhoid fever. J Hyg (Lond) 1953; 51:260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hornick RB, Greisman SE, Woodward TE, DuPont HL, Dawkins AT, Snyder MJ. Typhoid fever: pathogenesis and immunologic control. N Engl J Med 1970; 283:686–91. [DOI] [PubMed] [Google Scholar]
- 32. Dupont HL, Hornick RB, Snyder MJ, Dawkins AT, Heiner GG, Woodward TE. Studies of immunity in typhoid fever: protection induced by killed oral antigens or by primary infection. Bull World Health Organ 1971; 44:667–72. [PMC free article] [PubMed] [Google Scholar]
- 33. Liang L, Juarez S, Nga TV, et al. . Immune profiling with a Salmonella Typhi antigen microarray identifies new diagnostic biomarkers of human typhoid. Sci Rep 2013; 3:1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ludwig A, von Rhein C, Bauer S, Hüttinger C, Goebel W. Molecular analysis of cytolysin A (ClyA) in pathogenic Escherichia coli strains. J Bacteriol 2004; 186:5311–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Suez J, Porwollik S, Dagan A, et al. . Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS One 2013; 8:e58449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. den Bakker HC, Moreno Switt AI, Govoni G, et al. . Genome sequencing reveals diversification of virulence factor content and possible host adaptation in distinct subpopulations of Salmonella enterica. BMC Genomics 2011; 12:425, 2164-12-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Charles RC, Harris JB, Chase MR, et al. . Comparative proteomic analysis of the PhoP regulon in Salmonella enterica serovar Typhi versus Typhimurium. PLoS One 2009; 4:e6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.