Abstract
Background
Renal angiomyolipoma occurs at a high frequency in patients with tuberous sclerosis complex (TSC) and is associated with potentially life-threatening complications. Despite this frequency and severity, there are no large population-based cohort studies. Here we present baseline and follow-up data of the international TuberOus SClerosis registry to increase disease Awareness (TOSCA) with an aim to provide detailed clinical characteristics of renal angiomyolipoma among patients with TSC.
Methods
Patients of any age with a documented clinic visit for TSC within 12 months or who were newly diagnosed with TSC before participation in the registry were eligible. Data specific to renal angiomyolipoma included physical tumour characteristics (multiple, bilateral, lesion size and growing lesions), clinical signs and symptoms, and management. The effects of age, gender and genotype on the prevalence of renal angiomyolipoma were also evaluated.
Results
Renal angiomyolipoma was reported in 51.8% of patients at baseline, with higher frequency in female patients (57.8% versus 42.2%). The median age at diagnosis was 12 years. Prevalence of angiomyolipoma was higher in patients with TSC2 compared with TSC1 mutations (59.2% versus 33.3%, P < 0.01). Of the 1031 patients with angiomyolipoma at baseline, multiple lesions were reported in 88.4% and bilateral in 83.9% of patients, while the size of angiomyolipoma was >3 cm in 34.3% of patients. Most patients were asymptomatic (82%). Frequently reported angiomyolipoma-related symptoms included bleeding, pain, elevated blood pressure and impaired renal function. Embolization and mammalian target of rapamycin inhibitors were the two most common treatment modalities.
Conclusions
The TOSCA registry highlights the burden of renal angiomyolipoma in patients with TSC and shows that renal manifestations are initially asymptomatic and are influenced by gender and genotype. Furthermore, the occurrence of significant problems from angiomyolipoma in a minority of younger patients suggests that surveillance should begin in infancy or at initial diagnosis.
Keywords: mTOR Inhibitor, registry, renal angiomyolipoma, TOSCA, tuberous sclerosis complex
INTRODUCTION
Tuberous sclerosis complex (TSC) is an autosomal dominant genetic disorder resulting from inherited or sporadic germline mutations of TSC1 or TSC2 encoding hamartin and tuberin, respectively. It is characterized by hamartomatous lesions in multiple organs, including the brain, kidney, skin, heart, lungs and retina [1].
Renal problems are very frequent in patients with TSC after neurological manifestations and TSC-associated neuropsychiatric disorders and a leading cause of morbidity and mortality in these patients [2–7]. Renal manifestations include angiomyolipoma, epithelial cysts, polycystic kidney disease and renal cell carcinoma [8, 9]. The occurrence rate and clinical characteristics of renal lesions in TSC have been assessed primarily in either single- or two-centre case series [10–12] or in population-based studies with small sample sizes [8, 13, 14] with varied findings. The estimated prevalence of angiomyolipoma varied between studies and ranged from 55% to 80%. Some studies showed a higher proportion of renal angiomyolipoma in females [11, 15], whereas others have shown no gender disparity [10]. Patients with TSC2 mutations have been reported to exhibit a higher incidence and severity of angiomyolipoma compared with patients with TSC1 mutations [11, 16]. Patients with TSC-associated renal angiomyolipoma are susceptible to spontaneous life-threatening haemorrhage [4].
Despite considerable progress in the understanding of TSC and associated renal manifestations, there is a need for a large population-based cohort study to better understand clinical characteristics and natural history of renal angiomyolipoma in patients with TSC and its relationship with age, gender and genotype to target surveillance and therapy to those at greatest risk.
The TuberOus SClerosis registry to increase disease Awareness (TOSCA) has been designed to address the knowledge gaps in the natural history of TSC by collecting data from patients across many countries worldwide. The TOSCA registry has provided better insight into the overall TSC manifestations including clinical characteristics of renal angiomyolipoma [17]. In this report, we present baseline and 1-year follow-up data of the TOSCA registry with focus on the clinical characteristics of renal angiomyolipoma.
MATERIALS AND METHODS
The methods of TOSCA have been described in detail previously [18]. In short, TOSCA is a multicentre, international disease registry conducted at 170 sites across 31 countries worldwide. Between August 2012 and August 2014, patients of any age with a documented clinic visit for TSC in the preceding 12 months or newly diagnosed with TSC were enrolled.
In the TOSCA registry, general information on patient background such as demographic data, family history, genotype, vital signs, prenatal history, clinical features of TSC across all organ systems, comorbidities and rare manifestations were collected at baseline and at regular visits scheduled at a maximum interval of 1 year to ensure an ongoing data stream.
Data specific to renal angiomyolipoma included physical tumour characteristics (multiple, bilateral, lesion size and growing lesions), clinical signs and symptoms and management. The effects of age, gender and genotype on the prevalence of renal angiomyolipoma were also evaluated. Mean age of angiomyolipoma diagnosis at baseline were compared between patients with TSC1 and TSC2 mutations using Z test, while Chi-square test was used to analyze association between genotype and renal characteristics (such as history of angiomyolipoma, lesion >3 cm, growing angiomyolipoma, patients with/without signs or symptoms, or treatment received by patients) at baseline. This is an observational study, and therefore no additional clinical or laboratory assessments/interventions were performed other than those required for disease surveillance or management according to the local best practice.
As the registry is observational in nature, results are reported with descriptive statistics only. All eligible patients enrolled in the TOSCA registry were considered in the analysis. Continuous variables were evaluated quantitatively (e.g. frequency, mean, standard deviation, median, range), and categorical variables (e.g. presence/absence of a manifestation) were analysed in terms of frequency distribution at baseline and at follow-up.
This study was designed and conducted according to the Guidelines for Good Clinical Practice and ethical principles outlined in the Declaration of Helsinki [19, 20]. Written informed consents were obtained from all patients, parents or guardians prior to enrolment with prior endorsement by the local human research ethics committee.
RESULTS
Patient demographics and clinical characteristics
As of 30 September 2015 (data cut-off date for the third interim analysis), baseline data were available for 2216 patients and first follow-up visit data were available for 1911 patients. Baseline patient demographics and clinical characteristics are summarized in Table 1. Median age at inclusion was 13 years (range <1–71 years). Median age at diagnosis of TSC was 1 year (range <1–69 years). There were 144 (6.5%) patients with prenatal diagnoses. Molecular testing for genetic mutations was performed in 1000 (45.1%) patients. Of these, 644 patients (64.4%) had a TSC2 gene mutation, 197 (19.7%) had a TSC1 gene mutation, 6 patients had both TSC1 and TSC2 gene mutations and 144 (14.4%) had no mutation identified.
Table 1.
Baseline patient demographics and clinical characteristics (N = 2216)
| Characteristics | Baseline data |
|---|---|
| Age at diagnosis of TSCa, years, median (range) | 1 (<1–69) |
| Gender, n (%) | |
| Male | 1062 (47.9) |
| Female | 1154 (52.1) |
| Patients with molecular testing, n (%) | 1000 (45.1) |
| Genetic testing, n (%)b | |
| No mutation identified | 144 (14.4) |
| TSC1 mutationc | 197 (19.7) |
| TSC2 mutationc | 644 (64.4) |
| Both TSC1 and TSC2 mutations | 6 (0.6) |
| Variation type, n (%)d | |
| Pathogenic mutation | 678 (67.8) |
| Variant of unknown significance | 66 (6.6) |
| Patients with prenatal diagnosis, n (%) | 144 (6.5) |
| Patients with at least one blood relative participating in TOSCA, n (%) | 230 (10.4) |
Data available for 2216 patients.
Information on the type of mutation was missing for nine patients.
The count (n) includes six patients who had both TSC1 and TSC2 mutations.
The count (n) includes 23 patients who had both variation types.
Renal angiomyolipoma
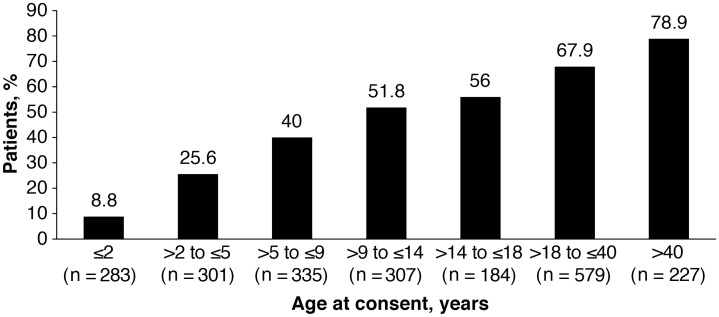
A total of 1070/2065 patients (51.8%) had renal imaging and had a history of renal angiomyolipoma. The other 151 patients had not had renal imaging. Of the 1070 patients with documented renal angiomyolipoma, 42.2% were males, and 57.8% females. The frequency at each age distribution is presented in Figure 1. The mean age at the time of diagnosis was 16.9 years [median (range) 12 years (<1–67 years)]. Of 1070 patients with a history of angiomyolipoma, angiomyolipoma was present at baseline in 1031 patients, while renal angiomyolipomas were resolved on treatment in 23 patients (2.1%) and no longer detectable in 16 patients (1.5%). In 1031 patients with renal angiomyolipoma present at baseline, 911 patients (88.4%) had multiple renal angiomyolipoma and 865 patients (83.9%) had bilateral renal angiomyolipoma. Angiomyolipoma size >3 cm was observed in 354 patients (34.3%; 68 patients aged ≤18 years and 286 aged >18 years).
FIGURE 1.
Patients with history of renal angiomyolipoma across age groups. Percentage of patients with renal angiomyolipoma in each age group was calculated considering the total number of patients in that age group as the denominator.
A repeat scan to monitor angiomyolipoma was carried out in 977 patients (44.1%) with known angiomyolipoma. Growth of angiomyolipoma was observed in 218 patients (21.1%; 100 patients aged ≤18 years and 118 aged >18 years). The mean time from the previous scan to the last assessment was 1.3 years. The occurrence rate and physical characteristics of renal angiomyolipoma at first follow-up visit were similar to that observed at baseline (Table 2).
Table 2.
Renal angiomyolipoma features according to mutation type
| Characteristics | Overall (N = 2216) |
Patients with TSC1 mutation (n = 197) |
Patients with TSC2 mutation (n = 644) |
|||
|---|---|---|---|---|---|---|
| Baseline | First follow- up visit | Baseline | First follow- up visit | Baseline | First follow- up visit | |
| History of renal angiomyolipomaa* | 1070 (51.8) | 992 (55.8) | 63 (33.3) | 61 (36.5) | 369 (59.2) | 344 (62.0) |
| Mean (range) age at diagnosis, years* | 16.9 (<1–67) | – | 23.5 (<1–60) | – | 13.2 (<1–59) | – |
| Angiomyolipoma present at the time of assessment | 1031 (96.4) | 917 (92.4) | 58 (92.1) | 60 (98.4) | 357 (96.7) | 309 (89.8) |
| Multiple | 911 (88.4) | 808 (81.5) | 41 (70.7) | 43 (70.5) | 330 (92.4) | 278 (80.8) |
| Bilateral | 865 (83.9) | 770 (77.6) | 29 (50.0) | 33 (54.1) | 308 (86.3) | 260 (75.6) |
| Angiomyolipoma size >3 cm* | 354 (34.3) | 302 (30.4) | 7 (12.0) | 10 (16.4) | 114 (31.9) | 98 (28.5) |
| Growing angiomyolipoma† | 218 (21.1) | 173 (17.4) | 7 (12.0) | 9 (14.8) | 86 (24.1) | 70 (20.3) |
| Signs and symptoms | ||||||
| None‡ | 845 (82.0) | 801 (87.4) | 52 (89.7) | 55 (91.7) | 297 (83.2) | 272 (88.0) |
| Pain not otherwise specified | 63 (6.1) | 34 (3.7) | 2 (3.4) | 2 (3.3) | 24 (6.7) | 11 (3.6) |
| Elevated blood pressure | 59 (5.7) | 47 (5.1) | 4 (6.9) | 3 (5.0) | 23 (6.4) | 20 (6.5) |
| Haemorrhage | 52 (5.0) | 14 (1.5) | 0 | 0 | 18 (5.0) | 5 (1.6) |
| Haematuria | 44 (4.3) | 30 (3.3) | 0 | 0 | 15 (4.2) | 10 (3.2) |
| Impaired renal function | 40 (3.9) | 30 (3.3) | 1 (1.7) | 1 (1.7) | 10 (2.8) | 8 (2.6) |
| Other | 33 (3.2) | 11 (1.2) | 0 | 0 | 10 (2.8) | 3 (1.0) |
| Treatment | ||||||
| Patients received treatment† | 309 (28.9) | 272 (27.4) | 8 (12.7) | 5 (8.2) | 99 (26.8) | 84 (24.4) |
| Type of treatment | ||||||
| Embolizationb | 142 (46.0) | 8 (2.9) | 2 (25.0) | 0 | 41 (41.4) | 2 (2.4) |
| mTOR inhibitorb | 134 (43.4) | 35 (12.9) | 3 (37.5) | 0 | 51 (51.5) | 8 (9.5) |
| Nephrectomyb | 62 (20.1) | 5 (1.8) | 3 (37.5) | 0 | 22 (22.2) | 1 (1.2) |
| Resectionb | 20 (6.5) | 1 (0.4) | 1 (12.5) | 0 | 5 (5.1) | 0 |
| Dialysisc | 3 (1.0) | 1 (0.4) | 0 | 0 | 1 (1.0) | 1 (1.2) |
| Other | 13 (4.2) | 1 (0.4) | 0 | 0 | 3 (3.0) | 0 |
Values are presented as n (%), unless otherwise specified.
TSC1 vs TSC2 at baseline: *P < 0.01; †P < 0.05; ‡P = 0.77.
Percentage were calculated based on number of patients with at least one renal imaging.
Used alone or in combination with other treatment modalities; at baseline in overall population, embolization as single agent was used in 102 of 142 patients; mTOR inhibitors as single agent were used in 87 of 134 patients; nephrectomy as single modality was used in 34 of 62 patients and resection as single modality was used in 11 of 20 patients.
Dialysis was used only in combination with other treatment modalities.
The majority of the patients with renal angiomyolipoma were asymptomatic at baseline (845 patients, 82.0%) and at first follow-up visit (801 patients, 87.4%). Among the symptomatic patients, the most common symptoms reported at baseline and at first follow-up visit were pain (6.1% and 3.7%), elevated blood pressure (5.7% and 5.1%), haemorrhage (5.0% and 1.5%), microscopic haematuria (4.3% and 3.3%) and impaired renal function (3.9% and 3.3%), respectively (Table 2).
Overall, renal angiomyolipomas were treated in 309 patients (28.9%). However, the percentage of patients requiring treatment increased progressively with age to 48.6% by age >40 years (Table 3). The most common treatment modalities (either as monotherapy or in combination with other treatment modalities) were embolization (46.0%) and mammalian target of rapamycin (mTOR) inhibitors (43.4%) (Table 2). The most common treatment modality in patients aged ≤18 years was mTOR inhibitors whereas embolization was most common in patients aged >18 years (Table 3). The most common treatment modality at first follow-up visit was mTOR inhibitors (Table 2).
Table 3.
Treatment modalities according to age
| Treatment modalities | Age at consent for patients with history of renal angiomyolipoma, n = 1070 |
||||||
|---|---|---|---|---|---|---|---|
| ≤2 (n = 25) | >2 to ≤5 (n = 77) | >5 to ≤9 (n = 134) | >9 to ≤14 (n = 159) | >14 to ≤18 (n = 103) | >18 to ≤40 (n = 393) | >40 (n = 179) | |
| Patients received treatment, n (%) | 1 (4.0) | 1 (1.3) | 11 (8.2) | 24 (15.1) | 27 (26.0) | 158 (40.2) | 87 (48.6) |
| Type of treatmenta, n (%) | |||||||
| Dialysis | 0 | 0 | 0 | 0 | 0 | 2 (1.3) | 1 (1.1) |
| Embolization | 0 | 0 | 0 | 2 (8.3) | 7 (25.9) | 83 (52.5) | 50 (57.5) |
| Nephrectomy | 0 | 0 | 0 | 2 (8.3) | 3 (11.1) | 29 (18.4) | 28 (32.2) |
| Resection | 0 | 0 | 1 (9.1) | 1 (4.2) | 3 (11.1) | 10 (6.3) | 5 (5.7) |
| mTOR inhibitor | 1 (100.0) | 1 (100.0) | 11 (100.0) | 17 (70.8) | 16 (59.3) | 67 (42.4) | 21 (24.1) |
| Others | 0 | 0 | 0 | 0 | 0 | 9 (5.7) | 4 (4.6) |
Used alone or in combination with other treatment modalities.
Relationship of renal angiomyolipoma with mutation type
Significantly more patients with TSC2 mutations had renal angiomyolipoma at baseline compared with those with a TSC1 mutation (59.2% versus 33.3%, P < 0.01). The mean age at diagnosis of renal angiomyolipoma in patients with a TSC2 mutation was 13.2 years (range <1-59 years), which was significantly lower than those with a TSC1 mutation (23.5 years, range <1-60 years; P < 0.01). Furthermore, significantly greater percentage of patients with a TSC2 mutation compared with those with a TSC1 mutation had an angiomyolipoma >3 cm in size (31.9% versus 12.0%, P < 0.01) or a growing angiomyolipoma (24.1% versus 12.0%, P < 0.05; Table 2). The age range of patients with TSC1 and TSC2 mutations was similar (Supplementary data, Table S1).
Similar to the overall population, renal angiomyolipoma were asymptomatic in most patients with TSC1 (89.7%) and TSC2 (83.2%) mutations, with no differences between the groups (P = 0.77). More patients with a TSC2 mutation required one or more treatment than those with TSC1 mutation (26.8% versus 12.7%, P < 0.05). However, most common treatment modalities did not differ based on gene mutation (Table 2).
Other renal manifestations
The other renal features reported at baseline and at first follow-up visit include multiple renal cysts (24.2% and 28.3%), polycystic kidney disease (proven TSC2/PKD1 mutation; 3.4% and 4%), renal malignancy (1.1% and 0.7%) and impaired renal function (non-angiomyolipoma-related; 1.9% and 2.2%), respectively. Compared with patients with a TSC1 mutation, those with TSC2 mutations had a higher occurrence of multiple renal cysts (33.4% versus 13.7%) and polycystic kidney disease (4.5% versus 0%).
DISCUSSION
The TOSCA study represents the largest cohort of TSC patients, with data accrued from 170 sites across 31 countries worldwide. The study showed several notable findings. Renal angiomyolipoma were reported in 51.8% of patients in the TOSCA cohort, which was lower than that observed in other studies [8, 10–13]. A probable reason for the lower rates of renal angiomyolipoma observed in this study was the younger median age of the TOSCA cohort. As shown in Figure 1, there is markedly increasing prevalence in the adult age range mirroring that of the published series rates of 55–80% [11, 15]. In a retrospective cohort study of 170 patients with TSC, a significant association between advancing age and the incidence of renal angiomyolipoma was reported [11]. Furthermore, the presence of renal angiomyolipoma was unknown for 151 patients (6.8%) suggesting no renal imaging was performed in these patients to confirm or exclude the presence of renal angiomyolipoma. However, it is important to note that the total number of patients enrolled in this study was considerably larger than in previous studies.
A striking finding in our study was the occurrence of angiomyolipoma in very young children and the need for treatment as early as 2 years of age or younger (Table 3 and Figure 1). In the TOSCA cohort the earliest age of diagnosis of angiomyolipoma was <2 years. This compares with other studies, which reported an estimated average age of onset to be between 7.2 and 11.1 years [10–12]. The proportion of patients receiving treatment for angiomyolipoma increased progressively with increasing age. About 15% of patients received treatment by age 14, 27% by age 18, 40% by age 40 and 49% by age >40.
The number of patients with haematuria and hypertension in the TOSCA cohort was low compared with those reported in TSC patients in other studies [21–23]. This low incidence of signs and symptoms could be explained by the high number of young patients enrolled in the study and also by the preemptive treatment of renal angiomyolipoma in patients who were under on-going surveillance (not lapsed from follow-up). On-going surveillance is recommended in patients with asymptomatic tumours per international TSC guidelines [24]. Chopra et al. evaluated adherence to surveillance guideline recommendations in an Australian TSC cohort and compared it among adults and pediatric patients [25]. The study showed that there was a significantly lower rate of adherence to surveillance guidelines in adult patients than in pediatric patients. This highlights the need for a focused transition plan for TSC patients transferred to adult care.
Studies have shown a gender disparity among patients with TSC-associated angiomyolipoma, with more occurrences in female patients [11, 21, 26]. A retrospective study by Rakowski et al. showed that complications due to angiomyolipoma were more common in women than in men with TSC [11]. Furthermore, about two-thirds of the patients recruited in EXIST-2, a Phase 3 study evaluating the efficacy and safety of everolimus in patients with angiomyolipoma associated with TSC or sporadic lymphangioleiomyomatosis, were women [27]. Both of these findings suggest that women with TSC are more vulnerable to developing renal complications. The gender disparity in angiomyolipoma complications raises a possible role of sex hormones in the pathogenesis of these lesions. Our study showed a similar finding with higher frequency of renal angiomyolipoma in females (57.8%) compared with males, however, this was not statistically significant. A future analysis is planned (after another years data has been collected) to ascertain if the rate of complications or treatment is higher in females.
The effect of mutation type on occurrence rate and severity of renal angiomyolipoma has been reported to be consistently greater among patients with TSC2 compared with TSC1 mutations [11, 16]. A study by Dabora et al. [16] in 224 unselected patients with TSC showed that frequency and severity of renal angiomyolipoma were significantly higher among patients with TSC2 mutations than those with TSC1 mutations (60% versus 31%, P = 0.03; mean grade 0.97 versus 0.32, P = 0.006). Our study, in a far larger cohort, confirms these findings. In TOSCA, patients with TSC2 mutations were at higher risk of developing renal angiomyolipoma than those with TSC1 mutations. The occurrence rate of multiple and bilateral angiomyolipoma lesions were also higher among those with a TSC2 mutation. In addition, bleeding complications were observed only in patients with a TSC2 gene mutation (Table 2), suggesting that the risk is higher in this group. These findings suggest that mutational analysis may help predict renal prognosis.
The treatment of renal angiomyolipoma associated with TSC is mainly focused on preventing acute events, preserving renal parenchyma and maintaining kidney function. A surprisingly high rate (34.6%) of patients had lesions >3 cm in diameter, a size where active intervention is recommended [24]. Embolization is the preferred therapy for renal angiomyolipoma presenting with acute haemorrhage, while mTOR inhibitors are the recommended first-line therapy for asymptomatic growing angiomyolipoma lesions ≥3 cm in diameter [9]. Overall, patients with renal angiomyolipoma in our study were most commonly treated with embolization followed by mTOR inhibitors. This balance may change with the expanding use of mTOR inhibitors in younger populations, an important question for this ongoing cohort study to address.
The 2012 International TSC Consensus Group recommends that nephrectomy should be avoided in patients with TSC-associated renal angiomyolipoma [24]. However, there was a high rate of nephrectomy in patients in the TOSCA cohort (20%). When treatment modalities used were stratified by age of patients, mTOR inhibitors were the most common treatment modality in patients aged ≤18 years while embolization and nephrectomy was more common in patients aged >18 years. As stated, mTOR inhibitor therapy is now the recommended first-line treatment for pre-symptomatic angiomyolipoma and our cohort data included historical treatment in older patients. Renal malignancy has been reported in about 2–4% patients with TSC [28], which is much higher than that reported in a comparable age group in the general population [29]. However, the occurrence rate of renal malignancy observed in this cohort was lower (1.1%) than that reported previously. However, we need to consider that the rate of malignancy was calculated based on patients who survived at the time of analysis.
This study had a number of limitations. First, owing to the observational nature of the registry, only data already available from routine clinical practice was collected. However, participation of a variety of centres with different specialists has helped inclusion of a large number of patients with TSC, which are representative of hospital clinical practice. Furthermore, patients were recruited through clinical centres with expertise in TSC and hence milder cases may not always be seen at these centres.
In conclusion, the TOSCA registry highlights the burden of renal angiomyolipoma in patients with TSC and provides valuable insights in understanding the characteristics, complications and treatment of renal angiomyolipoma in these patients. The data in the TOSCA registry show that renal manifestations are generally initially asymptomatic and are influenced by gender and genotype. Renal angiomyolipoma may occur in patients aged <2 years but the occurrence rate increases markedly with time, with up to 49% of patients requiring treatment interventions over 40 years of age.
Supplementary Material
ACKNOWLEDGEMENTS
We thank patients and their families, investigators and staff from all the participating sites. We thank Manojkumar Patel, Novartis Healthcare Pvt. Ltd, for providing medical editorial assistance with this manuscript.
FUNDING
This study was funded by Novartis Pharma AG.
CONFLICT OF INTEREST STATEMENT
J.C.K., P.J.dV., E.B., T.C., V.C., P.C., G.B.d’A., J.C.F., M.F., C.F., C.H., S.J., R.N., F.O’C., J.Q., M.S., R.T., M.D., J.A.L., A.M., S.Y., M.P.B., B.Z. and A.C.J. received honoraria and support for the travels from Novartis. V.C. received personal fees for consulting, lecture fees and travel from Actelion, Bayer, Biogen Idec, Boehringer Ingelheim, Gilead, GSK, MSD, Novartis, Pfizer, Roche and Sanofi; grants from Actelion, Boehringer Ingelheim, GSK, Pfizer and Roche; and personal fees for developing educational material from Boehringer Ingelheim and Roche. P.J.dV. has been on the study steering group of the EXIST-1, -2 and -3 studies sponsored by Novartis, and co-principal investigator on two investigator-initiated studies part-funded by Novartis. R.N. received grant support, paid to her institution, from Eisai and lectures fees from Nutricia, Eisai, Advicenne and GW Pharma. Y.T. received personal fee from Novartis for lecture and for copyright of referential figures from the journals, and received grant from Japanese government for intractable epilepsy research. S.J. was partly financed by the EC Seventh Framework Programme (FP7/2007-2013; EPISTOP, grant agreement no. 602391), the Polish Ministerial funds for science (years 2013-2018) for the implementation of international co-financed project and the grant EPIMARKER of the Polish National Center for Research and Development No STRATEGMED3/306306/4/2016. J.C.K., P.C., C.H., J.A.L. and J.Q. received research grant from Novartis. R.M., L.D’A. and S.S. are employees of Novartis. V.S. reported no conflict of interest. The study participants have not received funds for their participation in the study. The results presented in this manuscript have not been previously published except a poster presentation at TSC International congress on 4 November 2016.
REFERENCES
- 1. Crino PB, Nathanson KL, Henske EP.. The tuberous sclerosis complex. N Engl J Med 2006; 355: 1345–1356 [DOI] [PubMed] [Google Scholar]
- 2. Shepherd CW, Gomez MR.. Mortality in the mayo clinic tuberous cclerosis complex study. Ann N Y Acad Sci 1991; 615: 375–377 [DOI] [PubMed] [Google Scholar]
- 3. Shepherd CW, Gomez MR, Lie JT. et al. Causes of death in patients with tuberous sclerosis. Mayo Clin Proc 1991; 66: 792–796 [DOI] [PubMed] [Google Scholar]
- 4. Dixon BP, Hulbert JC, Bissler JJ.. Tuberous sclerosis complex renal disease. Nephron Exp Nephrol 2011; 118: e15–e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curatolo P, Moavero R, de Vries PJ.. Neurological and neuropsychiatric aspects of tuberous sclerosis complex. Lancet Neurol 2015; 14: 733–745 [DOI] [PubMed] [Google Scholar]
- 6. Leclezio L, Jansen A, Whittemore VH. et al. Pilot validation of the tuberous sclerosis-associated neuropsychiatric disorders (TAND) checklist. Pediatr Neurol 2015; 52: 16–24 [DOI] [PubMed] [Google Scholar]
- 7. de Vries PJ, Whittemore VH, Leclezio L. et al. Tuberous sclerosis associated neuropsychiatric disorders (TAND) and the TAND Checklist. Pediatr Neurol 2015; 52: 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Callaghan FJ, Noakes MJ, Martyn CN. et al. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int 2004; 94: 853–857 [DOI] [PubMed] [Google Scholar]
- 9. Kingswood JC, Bissler JJ, Budde K, et al. Review of the Tuberous sclerosis renal guidelines from the 2012 consensus conference: current data and future study. Nephron 2016; 134: 51–58 [DOI] [PubMed] [Google Scholar]
- 10. Ewalt DH, Sheffield E, Sparagana SP. et al. Renal lesion growth in children with tuberous sclerosis complex. J Urol 1998; 160: 141–145 [PubMed] [Google Scholar]
- 11. Rakowski SK, Winterkorn EB, Paul E. et al. Renal manifestations of tuberous sclerosis complex: incidence, prognosis, and predictive factors. Kidney Int 2006; 70: 1777–1782 [DOI] [PubMed] [Google Scholar]
- 12. Casper KA, Donnelly LF, Chen B. et al. Tuberous sclerosis complex: renal imaging findings. Radiology 2002; 225: 451–456 [DOI] [PubMed] [Google Scholar]
- 13. Wataya-Kaneda M, Tanaka M, Hamasaki T. et al. Trends in the prevalence of tuberous sclerosis complex manifestations: an epidemiological study of 166 Japanese patients. PLoS One 2013; 8: e63910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jozwiak S, Schwartz RA, Janniger CK. et al. Usefulness of diagnostic criteria of tuberous sclerosis complex in pediatric patients. J Child Neurol 2000; 15: 652–659 [DOI] [PubMed] [Google Scholar]
- 15. Webb DW, Kabala J, Osborne JP.. A population study of renal disease in patients with tuberous sclerosis. Br J Urol 1994; 74: 151–154 [DOI] [PubMed] [Google Scholar]
- 16. Dabora SL, Jozwiak S, Franz DN. et al. Mutational analysis in a cohort of 224 tuberous sclerosis patients indicates increased severity of TSC2, compared with TSC1, disease in multiple organs. Am J Hum Genet 2001; 68: 64–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kingswood JC, d'Augeres GB, Belousova E. et al. TuberOus SClerosis registry to increase disease Awareness (TOSCA)—baseline data on 2093 patients. Orphanet J Rare Dis 2017; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kingswood JC, Bruzzi P, Curatolo P. et al. TOSCA—first international registry to address knowledge gaps in the natural history and management of tuberous sclerosis complex. Orphanet J Rare Dis 2014; 9: 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) adopts consolidated guideline on good clinical practice in the conduct of clinical trials on medicinal products for human use. Int Dig Health Legis 1997; 48: 231–234 [PubMed] [Google Scholar]
- 20.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194 [DOI] [PubMed] [Google Scholar]
- 21. Seyam RM, Bissada NK, Kattan SA. et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology 2008; 72: 1077–1082 [DOI] [PubMed] [Google Scholar]
- 22. Bissler J, Cappell K, Charles H. et al. Long-term clinical morbidity in patients with renal angiomyolipoma associated with tuberous sclerosis complex. Urology 2016; 95: 80–87 [DOI] [PubMed] [Google Scholar]
- 23. Eijkemans MJ, van der Wal W, Reijnders LJ. et al. Long-term follow-up assessing renal angiomyolipoma treatment patterns, morbidity, and mortality: an observational study in tuberous sclerosis complex patients in the Netherlands. Am J Kidney Dis 2015; 66: 638–645 [DOI] [PubMed] [Google Scholar]
- 24. Krueger DA, Northrup H, Northrup H. et al. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013; 49: 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chopra M, Lawson JA, Wilson M. et al. An Australian tuberous sclerosis cohort: are surveillance guidelines being met? J Paediatr Child Health 2011; 47: 711–716 [DOI] [PubMed] [Google Scholar]
- 26. Bhatt JR, Richard PO, Kim NS. et al. Natural History of Renal Angiomyolipoma (AML): most patients with large amls >4cm can be offered active surveillance as an initial management strategy. Eur Urol 2016; 70: 85–90 [DOI] [PubMed] [Google Scholar]
- 27. Bissler JJ, Kingswood JC, Radzikowska E. et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2013; 381: 817–824 [DOI] [PubMed] [Google Scholar]
- 28. Yang P, Cornejo KM, Sadow PM. et al. Renal cell carcinoma in tuberous sclerosis complex. Am J Surg Pathol 2014; 38: 895–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ljungberg B, Campbell SC, Cho HY. et al. The epidemiology of renal cell carcinoma. Eur Urol 2011; 60: 615–621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.