Abstract
Background
Acute kidney injury (AKI) has been extensively studied in hospital settings. Limited data exist regarding outcomes for patients with outpatient AKI who are not subsequently admitted. We investigated whether outpatient AKI, defined by a 50% increase in creatinine (Cr), is associated with increased mortality and renal events.
Methods
In this retrospective study, outpatient serum Cr values from adults receiving primary care at a health system during an 18-month exposure period were used to categorize patients into one of five groups (no outpatient AKI, outpatient AKI with recovery, outpatient AKI without recovery, outpatient AKI without repeat Cr and no Cr). Principal outcomes of all-cause mortality and renal events (50% decline in estimated glomerular filtration rate to <30 mL/min/1.73 m2) were examined using Cox proportional hazards models.
Results
Among 384 869 eligible patients, 51% had at least one Cr measured during the exposure period. Outpatient AKI occurred in 1.4% of patients while hospital AKI occurred in only 0.3% of patients. The average follow-up was 5.3 years. Outpatient AKI was associated with an increased risk of all-cause mortality {adjusted hazard ratio [aHR] 1.90 [95% confidence interval (CI) 1.76–2.06]} and results were consistent across all AKI groups. Outpatient AKI was also associated with an increased risk of renal events [aHR 1.33 (95% CI 1.11–1.59)], even among those who recovered.
Conclusions
Outpatient AKI is more prevalent than inpatient AKI and is a risk factor for all-cause mortality and renal events, even among those who recover kidney function. Further research is necessary to determine risk factors and identify strategies for preventing outpatient AKI.
Keywords: acute kidney injury, albuminuria, chronic kidney disease, epidemiology, mortality
INTRODUCTION
Acute kidney injury (AKI) has been extensively studied in hospital settings, both when present on admission and when AKI develops during an admission. Incidence rates and risk factors have been defined for hospital-acquired AKI [1, 2] and multiple large observational studies have documented its association with chronic kidney disease (CKD), hypertension, hospital length of stay and mortality [3–8]. Community-acquired AKI, a term used to describe AKI present on hospital admission, is believed to represent about a quarter of AKI events observed in the hospital, and its incidence appears to be increasing [9, 10]. Community-acquired AKI, like hospital-acquired AKI, is associated with a higher incidence of CKD and mortality [11, 12], although studies are conflicting regarding which has a higher risk [10, 13].
Little is known about outpatient AKI, that is, AKI in patients not subsequently admitted in the period after an increase in creatinine (Cr). The few studies that have addressed this issue have found that outpatient AKI may be common and associated with increased mortality and CKD [10, 14–17]. To the best of our knowledge, there has been no comprehensive study analyzing patients with and without outpatient AKI and comparing those who recover with those who do not.
The purpose of this retrospective cohort study was to determine whether outpatient AKI, with or without recovery, is associated with an increased risk of mortality, renal events and other secondary outcomes, including hospitalization, hospital AKI and recurrent outpatient AKI.
MATERIALS AND METHODS
Data source
Patients were identified from the electronic health record (EHR) of Fairview Health Services, a large health system in Minnesota that serves the Twin Cities metro area and surrounding communities. Fairview, the primary affiliate of the University of Minnesota, operates six hospitals and medical centers and more than 40 primary care clinics and 55 specialty clinics. The EHR incorporates all inpatient and outpatient documentation, labs, imaging and billing. To capture patient death, the EHR has been linked to the Minnesota Death Index. The Institutional Review Board at the University of Minnesota approved this study and the study adhered to the Declaration of Helsinki.
Cohort definition
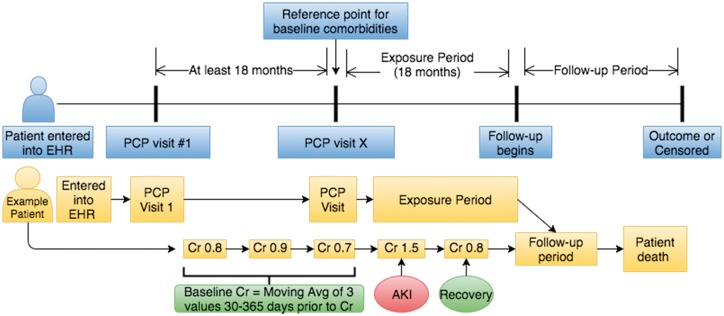
The cohort included all adult patients receiving primary care through Fairview Health Services (see Figure 1). Those with at least two primary care provider (PCP) visits separated by at least 18 months were included. The period between these visits (the ‘baseline period’) was used to establish baseline Cr values, vitals, labs and comorbidities. Patients were excluded if they were <18 years old at the second qualifying PCP visit. After the baseline period, an 18-month exposure period was used to capture outpatient serum Cr values, from which the following subgroups were defined: no outpatient AKI (reference group), outpatient AKI with recovery, outpatient AKI without recovery, outpatient AKI without a repeat Cr and no Cr checked during the exposure period. AKI events with a hospital admission in the subsequent 7 days were excluded, as were Cr values obtained in the emergency room. AKI at hospital admission or during hospitalization was collected and analyzed separately. Patients who died during the exposure period were excluded. Patients with a diagnosis of end-stage renal disease or with a kidney transplant before the end of the exposure period were also excluded. After the 18-month exposure period, a follow-up period began and continued until the outcome of interest or a censoring event occurred. Censoring occurred at the time of the last data point in the EHR for each patient.
FIGURE 1.
Cohort construction with example of a patient followed by AKI definitions.
We are unaware of any validated definition of AKI strictly in the outpatient setting. For our study, outpatient AKI was defined as a 50% increase in Cr compared to baseline. Each Cr value had a moving baseline that was the average of up to the last three outpatient Cr values 30–365 days prior [18]. If no Cr value was available within 30–365 days, the most recent Cr value >365 days prior was used as the baseline. Recovery from AKI was defined as a return of the Cr to within 10% of the baseline value at any time during the exposure period. The severity of outpatient AKI was defined as Stages 1–3 based on the Kidney Disease: Improving Global Outcomes (KDIGO) definition, excluding urine output [19]. See Table 1 for all definitions.
Table 1.
AKI classification and AKI definitions for this study
| AKI by setting | |
| Outpatient AKI | AKI diagnosed and managed in the outpatient setting without hospital admission within 7 days after AKI was detected |
| Community-acquired AKI | AKI resulting in hospital admission within 7 days or present on day of admission |
| Hospital-acquired AKI | AKI occurring during hospitalization, but not present on day of admission |
| Hospital AKI | Community-acquired and hospital-acquired AKI combined |
| AKI and recovery definitions | |
| AKI | A 50% increase in Cr with a moving baseline using an average of the last three Cr values 30–365 days prior for outpatient AKI and 7–365 days for hospital AKI |
| Recovery from AKI | Recovery of Cr to within 110% of the baseline Cr after AKI |
| AKI severity (modified KDIGO classification) | |
| Stage 1 | Increase in Cr of 1.5–1.9 times the baseline Cr |
| Stage 2 | Increase in Cr of 2.0–2.9 times the baseline Cr |
| Stage 3 | Increase in Cr at least 3.0 times the baseline Cr |
| Outpatient AKI groups in our cohort | |
| No AKI | At least one Cr value present during the 18-month exposure period with no AKI occurring during this time period |
| AKI with recovery | AKI and a future Cr value during the exposure period meeting criteria for recovery as defined above |
| AKI without recovery | AKI and one or more future Cr values during the exposure period with none meeting criteria for recovery |
| AKI without repeat Cr | AKI without a repeat Cr value during the exposure period to assess for recovery |
| No Cr | No Cr value available during the exposure period |
| Proteinuria classification (adapted from KDIGO) | |
| 0 | Urine dipstick 0 or trace or urine albumin:Cr ratio or protein:Cr ratio <30 mg/g or 24-h urine protein <30 mg |
| 1 | Urine dipstick 30–100 mg/dL or urine albumin:Cr ratio or protein:Cr ratio 30–299 mg/g or 24-h urine protein 30–299 mg |
| 2 | Urine dipstick ≥300 mg/dL or urine albumin:Cr ratio or protein:Cr ratio >300 mg/g or 24-h urine protein >300 mg |
Covariate definitions
All baseline characteristics were defined using EHR data up to and including the second qualifying PCP visit. Baseline characteristics included age, gender, race and smoking status. Baseline comorbidities included diabetes, hypertension, cardiovascular disease, liver disease and any cancer diagnosis. Comorbidities were considered present if at least two International Classification of Disease codes for that condition were present during the baseline period (Supplementary data, Table S4) [20]. Baseline labs and vitals were defined using the last value before the end of the baseline period and included blood pressure (BP), estimated glomerular filtration rate (eGFR), sodium, albumin and urine protein. eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation and urine protein was classified as 0, 1 or 2 based on the KDIGO classification (Table 1) [19]. Other covariates included hospitalization, inpatient AKI and outpatient AKI during the baseline period.
Outcome measures and definitions
The principal outcomes were all-cause mortality and renal events, defined as a decrease in eGFR to <30 mL/min/1.73 m2 on at least two measurements with at least a 50% decline from the last value during the exposure period. This level of CKD with eGFR <30 mL/min/1.73 m2 was chosen since it is known to be associated with a significant increased risk of adverse outcomes [21]. Only outpatient Cr measurements were used to assess the renal outcome. Secondary outcomes included hospitalization, hospital AKI and recurrent outpatient AKI. In addition to the primary analysis, each outcome was analyzed by the severity of AKI, and all outpatient AKI groups (with recovery, without recovery, and without a repeat Cr) were combined to compare risk among those with any outpatient AKI to those with hospital AKI during the exposure period. Inpatient AKI as a covariate (exposure period) and outcome (follow-up) was defined by a 50% increase in Cr during a hospitalization compared to a known baseline 7–365 days prior to admission.
Statistical analysis
Baseline variables are reported by AKI group as the mean and standard deviation or median and the first and third quantiles for continuous variables and as the total number and percentage for categorical variables. Principal and secondary outcomes were analyzed as the time to event, with follow-up beginning at the end of the exposure period. The Kaplan–Meier method and Cox proportional hazards models were used to generate cumulative incidence curves and obtain adjusted hazard ratios (aHRs), adjusting for baseline characteristics, comorbidities, vitals, labs, hospitalization events and AKI events that occurred during the baseline period. The group without AKI was used as the reference group in all models. The models for each outcome included all covariates defined above, which were selected a priori. For covariates where data were missing, we considered ‘NA’ to be its own unique value and adjusted for this [22, 23]. We considered a P-value <0.05 to be statistically significant and 95% confidence intervals (CIs) are provided. R version 3.3.1 was used for all statistical analysis (R Foundation for Statistical Computing, Vienna, Austria).
Sensitivity analyses
Preplanned sensitivity analyses included the following: excluding patients without a Cr during the exposure period, adjusting for the number of Cr values measured during baseline, changing the exposure period from 18 months to 12 and 24 months, changing the exposure period to end at the date of the last Cr measurement, censoring by using the date of the last Cr measurement during follow-up and utilizing two alternative eGFR thresholds for the renal outcome (<45 and <15 mL/min/1.73 m2). A post hoc time-dependent Cox model that otherwise mirrored the primary analysis was conducted with follow-up starting at the second qualifying PCP visit and HRs were determined for the following (with the reference as no event): Cr measured, outpatient AKI, repeat Cr measured after outpatient AKI and recovery from outpatient AKI. A post hoc sensitivity analysis excluding patients that recovered from AKI ‘after’ the exposure period was also conducted.
RESULTS
Cohort characteristics
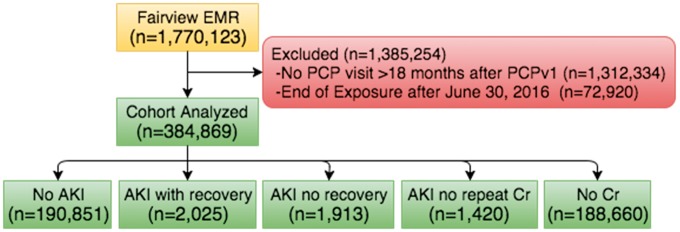
There were 1.77 million patients identified from the EHR, of which 384 869 were included in our cohort (Figure 2). Of these, nearly equal numbers of patients had no Cr measured during the exposure period (49.0%) or had at least one Cr measured and did not have outpatient AKI (49.6%). Outpatient AKI occurred in 1.4% of patients during the 18-month exposure period and in 5.9% at any time, which was more common than hospital AKI (0.3% and 1.9%, respectively). Compared with patients without outpatient AKI, those with AKI were older and more likely to be smokers, female and black (Table 2). They had more comorbidities and were more likely to have had an AKI or a hospitalization during the baseline period. On average, they had higher systolic BP and more proteinuria. Of the 5358 with outpatient AKI during the exposure period, 37.8% recovered, 35.7% did not recover and 26.5% did not have a repeat Cr. More than half (52.3%) of the patients in the latter two groups had a Cr measure qualifying them as recovered during follow-up; therefore, 70.4% of patients (3772 of 5358 with AKI) recovered to within 10% of their baseline Cr during either the exposure or follow-up period.
FIGURE 2.
Flow diagram showing all EHR patients, those excluded from our cohort and a breakdown of each AKI group.
Table 2.
Characteristics and outcomes of patients overall and by AKI group
| All | No AKI | Any outpatient AKI | AKI with recovery | AKI no recovery | AKI no repeat Cr | No Cr | |
|---|---|---|---|---|---|---|---|
| N | 384 869 | 190 851 | 5358 | 2025 | 1913 | 1420 | 188 660 |
| Stage 1 AKI, n (%) | 4310 (80.4) | 1584 (78.2) | 1556 (81.3) | 1170 (82.4) | |||
| Stage 2 AKI, n (%) | 650 (12.1) | 240 (11.9) | 277 (14.5) | 133 (9.4) | |||
| Stage 3 AKI, n (%) | 398 (7.4) | 201 (9.9) | 80 (4.2) | 117 (8.2) | |||
| Time to AKI, median (SD), days | 225 (174) | 180 (155) | 199 (171) | 364 (181) | |||
| Baseline | |||||||
| Age, mean (SD), years | 45.9 (17.2) | 52.5 (17.2) | 60.0 (17.8) | 62.1 (16.7) | 61.4 (16.7) | 55.1 (19.6) | 38.9 (14.0) |
| Male, n (%) | 165 512 (43.0) | 86 325 (45.2) | 165 512 (43.0) | 818 (40.4) | 776 (40.6) | 506 (35.6) | 77 087 (40.9) |
| Identify as black race, n (%) | 17 991 (4.7) | 9 826 (5.1) | 390 (7.3) | 133 (6.6) | 137 (7.2) | 120 (8.5) | 7775 (4.1) |
| Ever smoker, n (%) | 144 628 (37.6) | 78 931 (41.4) | 2515 (46.9) | 987 (48.7) | 885 (46.3) | 643 (45.3) | 63 182 (33.5) |
| CKD by eGFR (mL/min/1.73 m2), n (%) | |||||||
| ≥ 60 | 169 005 (85.1) | 110 537 (82.2) | 3032 (56.6) | 1079 (57.9) | 1085 (63.8) | 868 (75.0) | 55 436 (93.5) |
| 45–59 | 20 840 (10.5) | 16 892 (12.6) | 852 (15.9) | 409 (22.0) | 291 (17.1) | 152 (13.1) | 3096 (5.2) |
| 30–44 | 6585 (3.3) | 5585 (4.2) | 483 (9.0) | 231 (12.4) | 179 (10.5) | 73 (6.3) | 517 (0.9) |
| 15–29 | 1625 (0.8) | 1246 (0.9) | 232 (4.3) | 89 (4.8) | 107 (6.3) | 36 (3.1) | 147 (0.2) |
| <15 | 492 (0.2) | 278 (0.2) | 122 (2.3) | 55 (3.0) | 39 (2.3) | 28 (2.4) | 92 (0.2) |
| Diabetes mellitus, n (%) | 28 574 (7.4) | 24 219 (12.7) | 1596 (29.8) | 718 (35.5) | 587 (30.7) | 291 (20.5) | 2759 (1.5) |
| Hypertension, n (%) | 89 370 (23.2) | 73 903 (38.7) | 3200 (59.7) | 1340 (66.2) | 1184 (61.9) | 676 (47.6) | 12 267 (6.5) |
| Cardiovascular disease, n (%) | 23 806 (6.2) | 19 513 (10.2) | 1357 (25.3) | 674 (33.3) | 462 (24.2) | 221 (15.6) | 2936 (1.6) |
| Liver disease, n (%) | 5236 (1.4) | 4082 (2.1) | 299 (5.6) | 155 (7.7) | 94 (4.9) | 50 (3.5) | 855 (0.5) |
| Cancer, n (%) | 10 464 (2.7) | 8131 (4.3) | 401 (7.5) | 179 (8.8) | 150 (7.8) | 72 (5.1) | 1932 (1.0) |
| BP, mean (SD), mmHg | |||||||
| Systolic | 120 (110 to 130) | 122 (112 to 134) | 126 (114 to 138) | 126 (114 to 138) | 128 (115 to 140) | 124 (112 to 137) | 118 (108 to 126) |
| Diastolic | 74 (68–80) | 75 (68–82) | 74 (66–81) | 72 (64–80) | 74 (66–82) | 74 (68–82) | 72 (66–80) |
| Albumin, median (Q1–Q3), g/dL | 4.2 (3.9–4.4) | 4.2 (3.9–4.4) | 4 (3.7–4.3) | 4 (3.7–4.3) | 4 (3.6–4.2) | 4 (3.7–4.3) | 4.2 (4–4.5) |
| Sodium, median (Q1–Q3), mEq/L | 140 (139–142) | 140 (138–142) | 140 (138–142) | 140 (138–142) | 140 (138–142) | 140 (138–142) | 140 (139–142) |
| Proteinuria, n (%) | 21 832 (5.7) | 13 976 (7.3) | 1060 (19.8) | 476 (23.5) | 378 (19.8) | 206 (14.5) | 6796 (3.6) |
| Hospitalized, n (%) | 19 362 (5.0) | 14 275 (7.5) | 1185 (22.1) | 583 (28.8) | 393 (20.5) | 209 (14.7) | 3902 (2.1) |
| Hospitalized with AKI, n (%) | 811 (0.2) | 628 (0.3) | 112 (2.1) | 72 (3.6) | 28 (1.5) | 12 (0.8) | 71 (0) |
| Outpatient AKI, n (%) | 5363 (1.4) | 4147 (2.2) | 627 (11.7) | 328 (16.2) | 198 (10.4) | 101 (7.1) | 589 (0.3) |
| No Cr values during baseline, median (Q1–Q3) | 8 (7–13) | 8 (7–13) | 10 (7–17) | 11 (8–20) | 9 (7–16) | 9 (7–13) | 8 (6–11) |
| Occurred during follow-up | |||||||
| Death, n (%) | 12 143 (3.2) | 9369 (4.9) | 959 (17.9) | 424 (20.9) | 341 (17.8) | 194 (13.7) | 1815 (1.0) |
| Reach eGFR threshold (mL/min/1.73 m2), n (%) | |||||||
| <30 | 1744 (0.5) | 1592 (0.8) | 152 (2.8) | 81 (4.0) | 57 (3.0) | 14 (1.0) | 0 (0) |
| <45 | 2354 (0.6) | 2181 (1.1) | 173 (3.2) | 94 (4.6) | 64 (3.3) | 15 (1.1) | 0 (0) |
| <15 | 713 (0.2) | 619 (0.3) | 94 (1.8) | 41 (2.0) | 43 (2.2) | 10 (0.7) | 0 (0) |
| eGFR 50% decline, n (%) | 2551 (0.7) | 2370 (1.2) | 181 (3.4) | 102 (5.0) | 64 (3.3) | 15 (1.1) | 0 (0) |
| Hospitalized, n (%) | 46 358 (12) | 32 941 (17.3) | 1920 (35.9) | 776 (38.3) | 755 (39.5) | 390 (27.5) | 11 496 (6.1) |
| Hospitalized with AKI, n (%) | 5664 (1.5) | 4377 (2.3) | 452 (8.4) | 229 (11.3) | 159 (8.3) | 64 (4.5) | 835 (0.4) |
| Recurrent outpatient AKI, n (%) | 14 405 (3.7) | 10 632 (5.6) | 1100 (20.5) | 409 (20.2) | 414 (21.6) | 277 (19.5) | 2673 (1.4) |
| Last eGFR, median (Q1– Q3), mL/min/1.73 m2 | 80.8 (68–94.4) | 78.4 (64.9–92.4) | 69.7 (52.0–89.7) | 62.4 (42.4–82.4) | 62.4 (42.2–85.5) | 81.3 (62.1–98.5) | 84.9 (73.7–97.6) |
| AKI recovery after exposure period, n (%) | NA | NA | NA | NA | 983 (51.4) | 764 (53.8) | NA |
| Occurred at any time | |||||||
| Hospital AKI, n (%) | 7284 (1.9) | 5627 (3.0) | 698 (13.0) | 370 (18.3) | 244 (12.8) | 84 (5.9) | 959 (0.5) |
| Outpatient AKI, n (%) | 22 745 (5.9) | 14 174 (7.4) | 100% by definition | 100% by definition | 100% by definition | 100% by definition | 3213 (1.7) |
The average follow-up was 5.3 years. Overall mortality in the cohort was 3.2%. Mortality was lowest in those without a Cr measured (1%), higher in patients without outpatient AKI (4.9%) and highest in those with outpatient AKI (17.9%). Patients that recovered had the highest mortality (20.9%), followed by those without recovery (17.8%) and those without a repeat Cr (13.7%). Renal events occurred during follow-up in 0.8% of patients without AKI and in 4.0, 3.0 and 1.0% in patients with outpatient AKI with recovery, without recovery and without a repeat Cr, respectively. Secondary outcomes including hospitalization, hospital AKI and outpatient AKI had a similar pattern among the five groups (Table 2).
Outcomes
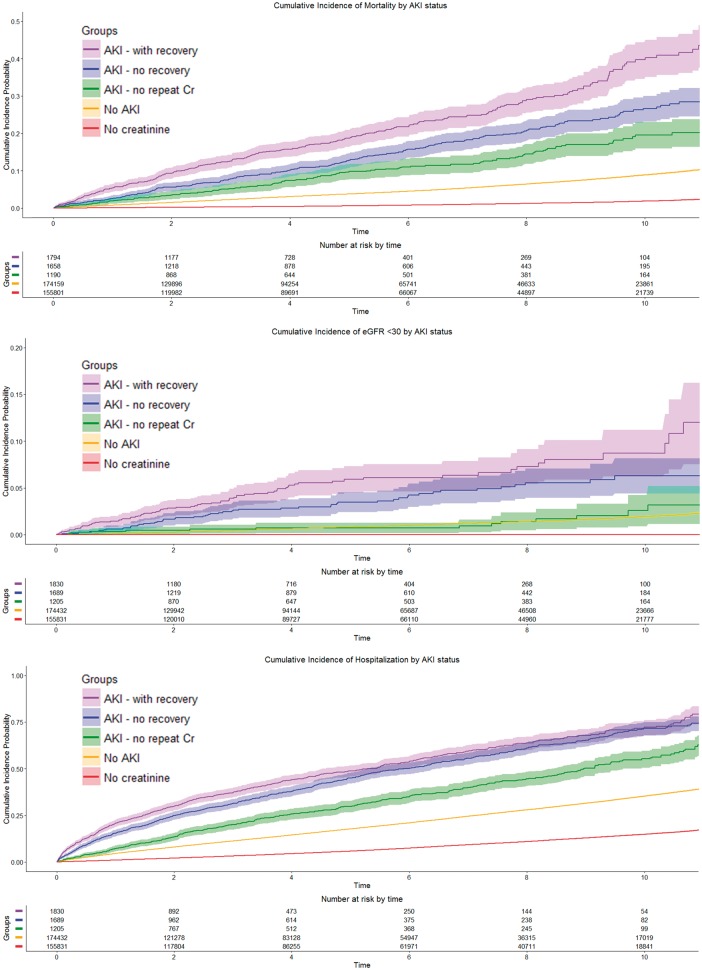
Figure 3 shows the unadjusted cumulative incidence curves for mortality, renal events and hospitalization by outpatient AKI group. Table 3 shows the aHRs for the principal and secondary outcomes by AKI group and by AKI severity. Outpatient AKI was associated with an increased risk for mortality, including those with recovery [aHR 2.15 (95% CI 1.91–2.41)], with no recovery [aHR 1.71 (95% CI 1.51–1.95)] and with no repeat Cr [aHR 1.77 (95% CI 1.49–2.12)]. In a post hoc time-dependent analysis, the association between outpatient AKI and mortality was consistent [aHR 1.55 (95% CI 1.28–1.88)]. Measurement of Cr after outpatient AKI was associated with lower mortality [aHR 0.55 (95% CI 0.41–0.74)], but having a repeat Cr qualifying as recovery was not associated with a further reduction in mortality [aHR 1.19 (95% CI 0.87–1.63)].
FIGURE 3.
Cumulative incidence curves by AKI group for mortality, incident CKD and hospitalization.
Table 3.
aHRs (95% CIs) for various outcomes by AKI group
| Mortality | eGFR <30 mL/min/1.73 m2 | Hospital AKI | Outpatient AKI | Hospitalization | |
|---|---|---|---|---|---|
| Primary analysis | |||||
| Outcomes by outpatient AKI group | |||||
| No AKI | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| AKI with recovery | 2.15 (1.91–2.41) | 1.73 (1.37–2.19) | 2.63 (2.27–2.99) | 2.37 (2.14–2.63) | 1.76 (1.63–1.89) |
| AKI no recovery | 1.71 (1.51–1.95) | 1.20 (0.91–1.57) | 1.94 (1.65–2.27) | 2.61 (2.37–2.89) | 1.77 (1.65–1.91) |
| AKI no repeat Cr | 1.77 (1.49–2.12) | 0.73 (0.43–1.25) | 1.53 (1.20–1.96) | 3.40 (3.02–3.83) | 1.51 (1.37–1.67) |
| No Cr | 0.73 (0.68–0.77) | NA | 0.53 (0.48–0.57) | 0.53 (0.50–0.55) | 0.67 (0.66–0.69) |
| Secondary analyses | |||||
| Outcomes by AKI severity | |||||
| No AKI | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Stage 1 AKI | 1.90 (1.74–2.07) | 1.34 (1.10–1.62) | 2.06 (1.85–2.30) | 2.55 (2.38–2.74) | 1.73 (1.64–1.82) |
| Stage 2 AKI | 1.92 (1.53–2.41) | 1.32 (0.86–2.03) | 2.21 (1.67–2.92) | 3.08 (2.60–3.65) | 1.73 (1.51–1.97) |
| Stage 3 AKI | 2.47 (1.61–3.80) | 0.90 (0.37–2.19) | 3.25 (2.09–5.06) | 2.64 (1.90–3.67) | 2.00 (1.58–2.54) |
| No Cr | 0.73 (0.68–0.77) | NA | 0.53 (0.49–0.57) | 0.53 (0.51–0.56) | 0.67 (0.66–0.69) |
| Any outpatient AKI versus No AKI | |||||
| No AKI | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Any outpatient AKI | 1.90 (1.76–2.06) | 1.33 (1.11–1.59) | 2.14 (1.93–2.37) | 2.75 (2.57–2.93) | 1.71 (1.63–1.79) |
| No AKI versus outpatient AKI versus hospital AKI versus both | |||||
| No AKI | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Outpatient AKI | 1.87 (1.72–2.03) | 1.37 (1.15–1.65) | 2.13 (1.91–2.37) | 2.79 (2.61–2.98) | 1.70 (1.62–1.78) |
| Hospital AKI | 2.79 (2.31–3.38) | 2.12 (1.30–3.46) | 4.30 (3.55–5.21) | 2.41 (1.98–2.94) | 2.53 (2.26–2.84) |
| Outpatient and hospital AKI | 4.42 (3.27–5.96) | 1.11 (0.55–2.26) | 4.69 (3.58–6.14) | 3.00 (2.28–3.96) | 2.93 (2.44–3.52) |
All are aHRs (Cox model). Variables in each Cox model included demographics (age, gender, race), baseline comorbidities (diabetes, hypertension, cardiovascular disease, liver disease, cancer, smoking status), baseline vitals and labs (last systolic BP, diastolic BP, sodium, albumin, urine protein and eGFR), baseline events (hospitalization, hospital AKI, outpatient AKI), recurrent outpatient AKI during exposure and total follow-up time.
Outpatient AKI with recovery was associated with an increased risk for the principal renal outcome [aHR 1.73 (95% CI 1.37–2.19)], but the AKI without recovery and AKI with no repeat Cr groups were not at increased risk for renal events in the primary analysis [aHR 1.20 (95% CI 0.91–1.57); aHR 0.73 (95% CI 0.43–1.25), respectively]. When those that recovered from outpatient AKI after the exposure period were excluded in post hoc analyses (Supplementary data, Table S3), there was an increased risk of the renal outcome in the AKI without recovery group [aHR 2.16 (95% CI 1.49–3.14)] as well as the AKI with recovery group [aHR 1.57 (95% CI 1.21–2.04)].
All categories of outpatient AKI were associated with an increased risk for secondary outcomes of hospital AKI, outpatient AKI and hospitalization (Table 3). Additionally, in secondary analyses, each stage of severity of outpatient AKI was associated with an increased risk of mortality. Stage 1 outpatient AKI was associated with an increased risk of renal events [aHR 1.34 (95% CI 1.10–1.62)] while Stages 2 and 3 were not [aHR 1.32 (95% CI 0.86–2.03); aHR 0.90 (95% CI 0.37–2.19)]. Each stage of outpatient AKI was associated with an increased risk for hospitalization, inpatient AKI and recurrent outpatient AKI. Hospital AKI was associated with an increased risk for mortality [aHR 2.79 (95% CI 2.31–3.38)], renal events [aHR 2.12 (95% CI 1.30–3.46)] and all secondary outcomes. The highest risk of mortality occurred in those with hospital and outpatient AKI [aHR 4.42 (95% CI 3.27–5.96)]. Similar results were observed for renal events and secondary outcomes.
Sensitivity analyses
Sensitivity analyses are shown in Supplementary data, Tables S1 and S2. Shortening the exposure period from 18 months to 12 months tended to attenuate the aHR slightly for each outcome in each AKI group and lengthening it to 24 months tended to increase the aHR slightly, without changing the direction or statistical significance. One exception was the 95% CI for mortality in the AKI with no repeat Cr group, which only crossed 1.0 in the 12-month exposure group. Results were consistent in sensitivity analyses for principal and secondary outcomes when excluding those without a Cr checked during the exposure period and when adjusting for the number of Cr measurements during the baseline period. For the renal outcome, results were consistent regardless of the eGFR threshold or whether the last Cr during the exposure period or last Cr during follow-up was used to start and end follow-up, respectively. In post hoc analyses, we assessed for potential inciting events by analyzing International Classification of Diseases (ICD) Revision 9/10 codes within 72 h of the Cr value that qualified as AKI. Among the 5358 patients with outpatient AKI, 1403 (26.2%) had ICD codes consistent with infection, hypotension or chronic conditions with clear links to AKI; the proportion of patients with one of these ICD codes was similar for those with recovery (30%) and those without recovery (27%) but was lower among those with no repeat Cr (19%).
DISCUSSION
We observed that AKI in the outpatient setting is more than three times as common as AKI observed in the hospital setting and is an independent risk factor for all-cause mortality and clinically significant CKD (determined by our threshold of eGFR <30 mL/min/1.73 m2 and a minimum 50% decrease in eGFR), even among patients who recover to within 10% of their baseline Cr. There additionally appeared to be a dose–response relationship between the severity of AKI and mortality risk. While the groups of AKI without recovery and AKI without repeat Cr could represent progressive CKD, these results held for patients with near-complete recovery of their eGFR to within 10% of baseline. Compared to patients with no AKI, those with outpatient AKI had a 33% increased risk for renal events and a 90% increased risk for mortality. Recovery from outpatient AKI was not protective from this increased risk for mortality in either the primary analysis or in a time-dependent analysis; unexpectedly, patients with outpatient AKI with recovery had higher aHRs for mortality and renal events than patients with outpatient AKI without recovery. Patients with AKI without recovery may have actually had a period of rapid decline in renal function rather than an acute isolated event. It is possible that AKI poses a greater risk for adverse outcomes than a sustained decline in renal function.
Five retrospective studies have analyzed outcomes after outpatient AKI. Four of these studies were conducted in the UK and one in Canada with a variety of sampling methods, definitions and outcomes. The first was limited to patients with advanced CKD and found those with outpatient AKI had greater than double the risk for dialysis initiation and death compared with age- and gender-matched controls [16]. Two subsequent registry cohorts from the UK also found an increased risk of mortality in patients with outpatient AKI with increased risk regardless of severity [14, 17]. A larger cohort from a UK health authority registry divided patients into groups with hospital-acquired, community-acquired and outpatient AKI. They found that 5-year mortality was high in all three patient groups (67.1, 64.7 and 46.2%, respectively) [10]. However, there were no patients without AKI in the adjusted model for this cohort. Most recently, in a cohort study from Wales, mortality was 25% overall for those with AKI and was higher with hospital-acquired AKI and with increasing severity. Hospital-acquired AKI was least likely to recover and AKI detected in the community was more likely to lead to worsening renal function at 90 days [15]. These prior studies were the first to report on AKI in the outpatient setting but are limited by a lack of a control group without AKI [10, 15] and an inability to adjust for important comorbidities [15–17].
Our study is the most comprehensive analysis of outpatient AKI and is the first American cohort. Strengths are its size, length of follow-up, robust comparator arm and comprehensive clinical data allowing for adjustment of potential confounders and assessment of multiple important outcomes. Consistent results in multiple sensitivity analyses also increase confidence in the results. Outpatient AKI was a risk factor for every outcome analyzed. Outpatient AKI had nearly double the risk of mortality >5 years compared with patients without outpatient AKI, with an incidence of more than three times that of hospital AKI. Similar to prior studies that have shown hospital AKI to be a risk factor for mortality and CKD regardless of severity or whether the Cr returns to baseline [3, 5, 7], we found that even the mildest form of outpatient AKI (1.5–1.9 times the baseline Cr) and those that recover their Cr to baseline have a significantly increased risk of mortality, CKD, hospitalization and recurrent AKI.
There are several limitations to our study. First, this is a retrospective observational cohort with all the inherent limitations, including differential follow-up. We attempt to account for this by adjusting for possible confounders, including demographics, vital signs, labs and comorbidities. Second, this is a single health system database in a metropolitan area with multiple health systems and may not be representative of the general population. This problem is limited given the size and expansive nature of the health system and by selecting patients who received their primary care within the system. Third, while limited mostly to lab tests in our database, there are missing data. We account for this by using ‘missingness’ as a variable to adjust for rather than by imputation, which is limited by an assumption of randomness. Fourth, there are no established uniform definitions for outpatient AKI like those for hospital AKI. Knowing this limitation, we chose to use a 50% increase in Cr to define outpatient AKI, because it remains a significant change in eGFR regardless of the baseline Cr and is similar to previously used definitions of AKI for the outpatient setting [10]. We chose to utilize an exposure period of 18 months, which is an effective method to define comparator groups, but it is an arbitrary period of time and may result in survivorship bias and misclassification. We accounted for this by using two alternative exposure period lengths (12 and 24 months) and by conducting a time-dependent analysis, neither of which changed the overall conclusions. Finally, our definition of outpatient AKI is not consistent with the KDIGO definition, which is mainly applicable in the inpatient setting. Our definition may also capture what is sometimes known as acute kidney disease, or AKD. Our definition could classify some patients with significant variability in kidney function as having AKI. Variability in Cr has been associated with an increased risk for all-cause mortality [24]. Our definition of outpatient AKI could also capture patients with a rapid decline in renal function, although 70.4% of those with outpatient AKI were seen to eventually recover back to baseline renal function. Patients with AKI without recovery may actually have progressive CKD; if so, it may explain why this group had lower short-term mortality than those with AKI (AKI with recovery group). This study, and the few similar studies outlined above, identifies outpatient AKI as having similar risks for adverse outcomes as inpatient AKI. We plan a prospective observational study to better understand potential inciting events, assess kidney injury biomarkers and closely monitor renal function in the period after an outpatient AKI event. The results will help differentiate outpatient AKI from variability in renal function, AKD and development/progression of CKD.
The findings of this study reveal the significant consequences of AKI in the outpatient setting. This burden is managed primarily in primary care clinics and efforts should now focus on prevention, early detection and rapid intervention. Unfortunately, evidence on how and where to focus these efforts is limited. Future research is required to standardize definitions for outpatient AKI and its severity and to identify risk factors and settings in which it occurs (demographics, comorbidities, medications, illnesses and behaviors). Prospective studies are necessary to test efforts at prevention and early intervention, including the timing of referral to a specialist.
In conclusion, outpatient AKI not requiring hospital admission was common and occurred more than three times as often as hospital AKI. Outpatient AKI was associated with an increased risk of death and renal events as well as hospitalization, hospital AKI and recurrent outpatient AKI, even in those in whom Cr recovered to within 10% of baseline. Further research is necessary to better understand the etiology and pathophysiology underlying outpatient AKI in order to develop approaches to prevent AKI events as well as reduce the risk for subsequent adverse outcomes.
FUNDING
This project was funded in part by a grant from the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant UL1TR000114.
AUTHORS’ CONTRIBUTIONS
Study concept and design was provided by M.D.L., D.P.M., S.R., D.V., R.N.F. and P.E.D. Acquisition, analysis or interpretation of data was provided by M.D.L., D.P.M., L.B., S.R., D.V., A.I., R.N.F. and P.E.D. Drafting of the manuscript was provided by M.D.L. and D.P.M. Critical revision of the manuscript for intellectual content was provided by M.D.L., D.P.M., L.B., S.R., D.V., A.I., R.N.F. and P.E.D. Statistical analysis was provided by M.D.L. and D.V. Study supervision was provided by P.E.D.
CONFLICT OF INTEREST STATEMENT
M.D.L., L.B., D.V., R.N.F, and P.E.D. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors have no conflicts of interest to disclose. The results presented in this article have not been published previously in whole or part, except in abstract format.
Supplementary Material
REFERENCES
- 1. Hoste EA, Bagshaw SM, Bellomo R. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411–1423 [DOI] [PubMed] [Google Scholar]
- 2. Uchino S, Kellum JA, Bellomo R. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005; 294: 813–818 [DOI] [PubMed] [Google Scholar]
- 3. Bucaloiu ID, Kirchner HL, Norfolk ER. et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 2012; 81: 477–485 [DOI] [PubMed] [Google Scholar]
- 4. Chertow GM, Burdick E, Honour M. et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005; 16: 3365–3370 [DOI] [PubMed] [Google Scholar]
- 5. Heung M, Steffick DE, Zivin K. et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration data. Am J Kidney Dis 2016; 67: 742–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hsu CY, Hsu RK, Yang J. et al. Elevated BP after AKI. J Am Soc Nephrol 2016; 27: 914–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones J, Holmen J, De Graauw J. et al. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 2012; 60: 402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaddourah A, Basu RK, Bagshaw SM. et al. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 2017; 376: 11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu CY, McCulloch CE, Fan D. et al. Community-based incidence of acute renal failure. Kidney Int 2007; 72: 208–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sawhney S, Fluck N, Fraser SD. et al. KDIGO-based acute kidney injury criteria operate differently in hospitals and the community-findings from a large population cohort. Nephrol Dial Transplant 2016; 31: 922–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaufman J, Dhakal M, Patel B. et al. Community-acquired acute renal failure. Am J Kidney Dis 1991; 17: 191–198 [DOI] [PubMed] [Google Scholar]
- 12. Soto K, Campos P, Pinto I. et al. The risk of chronic kidney disease and mortality are increased after community-acquired acute kidney injury. Kidney Int 2016; 90: 1090–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wonnacott A, Meran S, Amphlett B. et al. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clin J Am Soc Nephrol 2014; 9: 1007–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hobbs H, Bassett P, Wheeler T. et al. Do acute elevations of serum creatinine in primary care engender an increased mortality risk? BMC Nephrol 2014; 15: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holmes J, Rainer T, Geen J. et al. Acute kidney injury in the era of the AKI e-alert. Clin J Am Soc Nephrol 2016; 11: 2123–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lafrance JP, Djurdjev O, Levin A.. Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant 2010; 25: 2203–2209 [DOI] [PubMed] [Google Scholar]
- 17. Talabani B, Zouwail S, Pyart RD. et al. Epidemiology and outcome of community-acquired acute kidney injury. Nephrology (Carlton) 2014; 19: 282–287 [DOI] [PubMed] [Google Scholar]
- 18. Lafrance JP, Miller DR.. Defining acute kidney injury in database studies: the effects of varying the baseline kidney function assessment period and considering CKD status. Am J Kidney Dis 2010; 56: 651–660 [DOI] [PubMed] [Google Scholar]
- 19. Section 2: AKI definition. Kidney Int Suppl 2012; 2: 19–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Original Chronic Conditions Data Warehouse Chronic Condition Algorithms. Chronic conditions data warehouse, Center for Medicare and Medicaid Services. https://www.ccwdata.org/web/guest/condition-categories (12 February 2018, date last accessed)
- 21. Go AS, Chertow GM, Fan D.. et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 22. Anderson AB, Hum DPJ.. Missing data: a review of the literature In: Rossi PH, Wright JD and Anderson AB (eds). Handbook of Survey Research. New York: Academic Press, 1983, 415–492 [Google Scholar]
- 23. Chow W. A look at various estimators in logistic models in the presence of missing values. Santa Monica, CA: Rand Corp, 1979, 417–420 [Google Scholar]
- 24. Al-Aly Z, Balasubramanian S, McDonald JR.. et al. Greater variability in kidney function is associated with an increased risk of death. Kidney Int 2012; 82: 1208–1214 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.