Abstract
Inside the human host, Leishmania infection starts with phagocytosis of infective promastigotes by macrophages. In order to survive, Leishmania has developed several strategies to manipulate macrophage functions. Among these strategies, Leishmania as a source of bioactive lipids has been poorly explored. Herein, we assessed the biosynthesis of polyunsaturated fatty acid metabolites by infective and noninfective stages of Leishmania and further explored the role of these metabolites in macrophage polarization. The concentration of docosahexaenoic acid metabolites, precursors of proresolving lipid mediators, was increased in the infective stage of the parasite compared with the noninfective stage, and cytochrome P450-like proteins were shown to be implicated in the biosynthesis of these metabolites. The treatment of macrophages with lipids extracted from the infective forms of the parasite led to M2 macrophage polarization and blocked the differentiation into the M1 phenotype induced by IFN-γ. In conclusion, Leishmania polyunsaturated fatty acid metabolites, produced by cytochrome P450-like protein activity, are implicated in parasite/host interactions by promoting the polarization of macrophages into a proresolving M2 phenotype.
Keywords: cytochrome P450, infection, macrophages, ω-3, metabolism, arachidonic acid, eicosanoids
Leishmaniasis is a plague affecting millions of people around the world, most notably in developing countries but also in the Mediterranean. It is the second-most lethal parasitic infection worldwide, with more than 20,000 annual deaths (http://www.who.int/leishmaniasis/en). This disease is supported by the intracellular development of a protozoan parasite belonging to the Leishmania genus. Metacyclic promastigotes are transmitted by the Phlebotominae sandfly during a blood meal on mammals, where they infect macrophages prior to differentiating into amastigote forms and proliferating inside the host cell. Procyclic promastigotes differentiate into metacyclic promastigotes during metacyclogenesis, a process that is triggered by an environmental pH decrease from neutral to acidic pH inside the sandfly vector (1, 2). Metacyclogenesis involves important morphological and biochemical modifications, and approximately 300 genes have been described to be specifically regulated according to the parasite stage (3–6). Macrophages are the primary replication site for Leishmania and the main effector cells to fight it (7). Leishmania infection leads to the development of macrophages that overexpress anti-inflammatory molecules such as interleukin (IL) 10 and transforming growth factor β and the induction of proinflammatory cytokine production such as TNF-α, IL-1β, and IL-6 (8). M2 macrophages control disease severity and protect the host from the detrimental effect of an excessive type 1 T helper cell response, making “symbiotic” survival between the host and parasite more likely (7, 9, 10). In order to survive in the macrophage, Leishmania has to prevent or inhibit a variety of intracellular mechanisms of parasite elimination. Among the strategies developed by the parasite for manipulating macrophage functions (7, 9, 10), Leishmania as a source of bioactive lipids has been poorly explored (11), despite the fact that very close protozoan pathogens, Trypanosoma sp., are known to produce a number of oxylipins (12). Herein, we explore the biosynthesis of PUFA metabolites in Leishmania infantum during infectivity acquisition and their putative role in the interaction with macrophages that trigger a minimal proinflammatory response in the host for the benefit of the parasite.
METHODS
Ethics statement
Male C57Bl/6J mice (6–8 weeks; Janvier Labs, Saint-Quentin-Fallavier, France) were used to produce primary cultures of macrophages. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the European Council and were approved by the Animal Care and Ethics Committee of US006/CREFE (CEEA-122).
Leishmania culture conditions
The Leishmania species used in this study was Leishmania infantum (MHOM/MA/67/ITMAP-263). Procyclic promastigotes were cultured in the log phase in RPMI 1640 medium (VWR, Fontenay-sous-Bois, France) supplemented with 10% FCS (VWR), 2 mM l-glutamine (Thermo Fisher Scientific, Illkirch-Graffenstaden, France), 25 mM HEPES (Thermo Fisher Scientific), 100 U/ml penicillin (Thermo Fisher Scientific), 100 µg/ml streptomycin (Thermo Fisher Scientific), and 50 µg/ml geneticin (Sigma-Aldrich, Lyon, France), pH 7.7, at 24°C.
Cell culture experiments
CHO cells (CCL-61; ATCC, Molsheim, France) were maintained in Ham’s F12 Nutrient Mixture (Thermo Fisher Scientific) supplemented with 5% FCS and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin) at 37°C in 5% CO2.
Bone marrows were isolated from murine femurs. Cells were cultured in RPMI 1640 supplemented with 20% FBS (VWR), 100 U/ml penicillin, and 100 µg/ml streptomycin and supplemented with 20 ng/ml mouse macrophage colony-stimulating factor (R&D Systems, Abingdon, UK) for 7 days.
Induction of Leishmania metacyclogenesis
Procyclic promastigotes in the log phase were centrifuged at 900 g for 10 min. Cells were then suspended in RPMI 1640 complete medium (pH 5.4) and incubated at 24°C for 24 h to obtain metacyclic promastigotes.
Inhibition of lipid metabolism by different inhibitors
Metacyclic promastigotes (2 × 106 promastigotes/ml in RPMI 1640 complete medium, pH 5.4) were treated with either 100 µM nordihydroguaiaretic acid (NDGA; Alfa Aesar, Karlsruhe, Germany), 100 µM 17-octadecynoic acid (17-ODYA; Cayman Chemicals, Ann Arbor, MI), 10 µM clotrimazole (Sigma-Aldrich), or 10 µM tioconazole (Sigma-Aldrich) for 24 h at 24°C.
Leishmania oxylipin extraction
Parasites were harvested by centrifugation at 900 g for 10 min and then washed three times with PBS (Sigma-Aldrich). Pellets of 1 × 108 parasites in 200 µl PBS were snap-frozen and stored in liquid nitrogen prior to lipid extraction. Thawed pellets in PBS were transferred to lysing matrix tubes containing 5 µl internal standard mixture [lipoxin A4-d5, leukotriene B4-d4, and 5-HETE-d8 at 400 ng/ml in methanol (MeOH); Cayman Chemicals] and immediately crushed with a FastPrep-24 Instrument (MP Biomedical, Santa Ana, CA). After two crush cycles (6.5 m/s, 30 s), 10 µl suspension were withdrawn for protein quantification, and 0.3 ml cold MeOH were added. Samples were centrifuged thereafter at 1,016 g for 15 min at 4°C, and the resulting supernatants were submitted to solid-phase extraction of lipids using HRX-50 mg 96-well plates (Macherey-Nagel, Hoerd, France). In brief, plates were conditioned with 2 ml MeOH and 2 ml water-MeOH (90:10; v/v). Samples were loaded at flow rate of about 1 drop per 2 s and, after complete loading, columns were washed with 2 ml water-MeOH (90:10; v/v). The columns were dried thereafter under aspiration, and lipids were eluted with 2 ml MeOH. Solvent was evaporated under N2, and samples were resuspended in 10 µl MeOH and stored at 80°C for LC/MS/MS analysis or macrophage treatments.
LC/MS/MS measurements of proinflammatory and proresolving lipids
The quantification of 6-keto-prostaglandin (PG) F1α, thromboxane B2, PGE2, PGA1, 8-iso-PGA2, PGE3, 15d-PGJ2, PGF2α, PGD2, lipoxin A4, lipoxin B4, resolvin D (RvD) 1, RvD2, 7-maresin 1 (7-MaR1), leukotriene B4, leukotriene B5, protectin Dx (PDx), 18-hydroxyeicosapentaenoic acid, 5,6-DiHETE, 15-HETE, 12-HETE, 8-HETE, 5-HETE, 13-HODE, 9-HODE, 17-hydroxydocosahexaenoic acid (HDoHE), 14-HDoHE, 14(15)-epoxyeicosatrienoic acid (EpETrE), 11(12)-EpETrE, 8(9)-EpETrE, 5(6)-EpETrE, and 5-oxoeicosatetraenoic acid (5-oxo-ETE) was performed at different stages of the life cycle of L. infantum and in stimulated macrophages. To simultaneously separate the 32 lipids of interest and the 3 deuterated internal standards, LC/MS/MS analysis was performed as previously described (13) on an Agilent LC1290 Infinity ultra-high-performance liquid chromatography system coupled to an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, Les Ulis, France) equipped with electrospray ionization operating in the negative mode. Reverse-phase ultra-high-performance liquid chromatography was performed using a ZORBAX SB-C18 column (inner diameter: 2.1 mm; length: 50 mm; particle size: 1.8 µm; Agilent Technologies) with a gradient elution.
LC/MS/MS measurements of epoxy and hydroxyl PUFAs
The detection of oxylipins was adapted from Rund et al. (14) and performed using an Agilent 6460 triple quadrupole mass spectrometer equipped with an Agilent 1290 Infinity HPLC system and ZORBAX Eclipse Plus C18 column (inner diameter: 2.1 mm; length: 150 mm; particle size: 1.8 µm). For HPLC separation, the mobile phase A was 0.1% acetic acid in water (v/v), and the mobile phase B was acetonitrile, MeOH, and acetic acid (800/150/1; v/v/v). Oxylipins were separated using an optimized 32.2-min gradient of 0.0–1.0 min, 26% B; 1.0–1.5 min, 35% B; 1.5–8.0 min, 49.5% B; 8.0–16.0 min, 59.8% B; 16.0–25.1 min, 72% B; 25.1–27.6 min, 98% B; 27.6–29.7 min, 98% B; 29.7–29.8 min, 26% B; and 29.8–32.2 min, 26% B. The flow rate was set at 0.35 ml/min, and the column temperature was set at 40°C. Electrospray ionization was used in the negative mode. The MS parameters were as follows: source temperature, 325°C; desolvatation temperature, 350°C; cone gas flow, 10 l/min; desolvatation gas flow, 12 l/min; nebulizer pressure, 30 psi; capillary voltage, 3,000 V; and nozzle voltage, 2,000 V. Data were acquired in dynamic multiple reaction monitoring mode with optimized conditions (ion optics and collision energy). Peak detection and integration analysis were done using MassHunter quantitative analysis software (Agilent Technologies).
Leishmania fatty acid extraction
Parasites were harvested by centrifugation at 900 g for 10 min and then washed three times with PBS (Sigma-Aldrich). Pellets of 1 × 108 parasites in 200 µl PBS were snap-frozen and stored in liquid nitrogen prior to lipid extraction. Thawed pellets in PBS were transferred to lysing matrix tubes with 1 ml MeOH-EGTA (5 mM). After homogenization, 75% of the sample was extracted according to Bligh and Dyer (15) in dichloromethane-MeOH-water (2.5:2.5:2.1; v/v/v) in the presence of the internal standard heptadecanoate acid (2 μg) for the free fatty acid profiling. The lipid extract was then directly methylated in 1 ml 14% boron trifluoride MeOH solution (Sigma-Aldrich) and 1 ml heptane at room temperature for 10 min. After the addition of 1 ml water to the crude, methylated free fatty acids were extracted with 3 ml heptane, evaporated to dryness, and dissolved in 20 μl ethyl acetate. In addition, 25% of the crushed parasite was extracted in the presence of 2 μg glyceryl triheptadecanoate to profile the total fatty acids. The lipid extract was hydrolyzed in potassium hydroxide (0.5 M in MeOH) at 50°C for 30 min and transmethylated in 1 ml 14% boron trifluoride MeOH solution and 1 ml heptane at 80°C for 1 h. After the addition of 1 ml water to the crude, methylated total fatty acids were extracted with 3 ml heptane, evaporated to dryness, and dissolved in 20 μl ethyl acetate.
GC with flame ionization detection of total and free fatty acids
Methylated free or total fatty acids (1 μl) were analyzed by GC/LC (16) on a Clarus 600 Perkin Elmer system using FAMEWAX RESTEK fused silica capillary columns (inner diameter: 0.32 mm; length: 30 m; film thickness: 0.25 μm). The oven temperature was programmed from 110 to 220°C at a rate of 2°C/min, and the carrier gas was hydrogen (0.5 bar). The injector and detector were set at 225 and 245°C, respectively. Peak detection and integration analysis were done using Azur software.
Leishmania RNA extraction
RNA extraction from 2 × 107 procyclic promastigotes and metacyclic promastigotes was performed with the TRIzol + RNA extraction kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
RT-qPCR
RNA (1 μg) was reverse-transcribed using the SuperScript First-Strand Synthesis System (Thermo Fisher Scientific) according to the manufacturer’s recommendations. Transcriptional levels of specific mRNAs were determined by quantitative PCR (qPCR) using the SYBR Green PCR Master Mix and performed on a LightCycler 480 (Roche Diagnostics, Meylan, France). Primers (Eurofins Genomics, Les Ullis, France) used for qPCR are listed in Table 1 for cytochrome P450 (CYP). Primers (Eurofins Genomics) used to characterize macrophage phenotype were edited by Accarias et al. (17). Expression ratios were calculated according to the expression level of the housekeeping gene LinJ.36.0990 coding for 40S ribosomal protein S18 (18) and RLP19 in the parasite and macrophage, respectively.
TABLE 1.
Primers for RT-qPCR
| Gene ID on GeneDBa | 5′-3′ | ||
| LinJ.36.0990 | 40S S18 | Forward | GCGCAAGGTGCCGTTCGCG |
| Reverse | GCTCCACGTCGATCCCGGC | ||
| LinJ.27.0090 | CYP1 | Forward | GCCTGCTGCTGTCGCATGCG |
| Reverse | TTCGCGTCAACCGCGTCGAGC | ||
| LinJ.30.3610 | CYP2 | Forward | CGGACGCTGAGCCGCACTG |
| Reverse | GCCATGGCGTGGTCCGACG | ||
| LinJ.34.3110 | CYP3 | Forward | GCGAAGAAGGCGAGGCGCTG |
| Reverse | AGGTAGCCGAGCTCCTGGCC |
Leishmania CYP cloning
DNA was extracted from 2 × 107 procyclic promastigotes with the Wizard Genomic DNA Purification Kit (Promega, Charbonnières-les-Bains, France) according to the manufacturer’s instructions for CYP2 and CYP3. L. infantum JPCM5 genome chromosome 27 (CYP1) was purchased from CliniSciences (Nanterre, France) cloned in the pUC57 vector with XbaI/NotI used as the cloning site. Primers (Eurofins Genomics) used for CYP cloning were 5′-CACCTTCTCAGCCTTGGGTTCCA-3′ and 5′-ATGGCAGCGTTTAGTCGTCTCC-3′ for CYP2 and 5′-CCCGCGTACGGCGGCTTCTTT-3′ and 5′-ATGGCCCCCACTGTCTCGCCA-3′ for CYP3. CYP-amplified inserts were subcloned in pCI-Neo (Promega) between XbaI and NotI restriction sites downstream to the constitutive promoter cytomegalovirus. Plasmid construction was verified by sequence alignment with a reference sequence from GenBank.
Transfection assay in the CHO cell line
Twenty-four hours before transfection, CHO cells were seeded in 24-well plates at 50% confluence in Opti-MEM medium. Transfections were carried out using Gene Juice transfection reagent (Merck, Kenilworth, NJ) with 500 ng of empty pCI-neo plasmid (Promega) containing CYP1, CYP2, or CYP3. Following an overnight incubation, culture medium was removed and replaced. Twenty-four hours thereafter cells were placed in HBSS (200 µl) containing vehicle (HBSS), 10 µM arachidonic acid (AA), or 10 µM DHA for 10 min at room temperature. Cells and HBSS were then collected for lipid quantification.
Bone marrow macrophage stimulation
Macrophage progenitors were isolated from the femurs of C57BL6 WT mice. Cells were differentiated and maintained in culture with 20 ng/ml macrophage colony-stimulating factor for 7 days before use. Bone marrow-derived monocytes were stimulated for 24 h with oxylipin extracted from procyclic or metacyclic promastigotes (10 µl in MeOH). For some experiments, monocytes were also incubated with oxylipin extracted from Leishmania or vehicle (MeOH) and costimulated with 20 ng/ml IFN for 24 h. Cells were collected in HBSS and submitted to lipidomic analysis. For transcriptional analysis, cells were lysed in TRIzol, and mRNA was collected according to the manufacturer’s instructions.
Statistical analysis
Data are presented as means ± SEMs. GraphPad Prism version 6.0 (GraphPad, San Diego, CA) was used for statistical analysis. Multiple comparisons within groups were performed by the Kruskal-Wallis test followed by Dunn’s post hoc test. Comparisons among groups were performed by the Mann-Whitney test. Statistical significance was accepted at P < 0.05.
RESULTS
PUFA metabolite production by L. infantum varies during infectivity acquisition
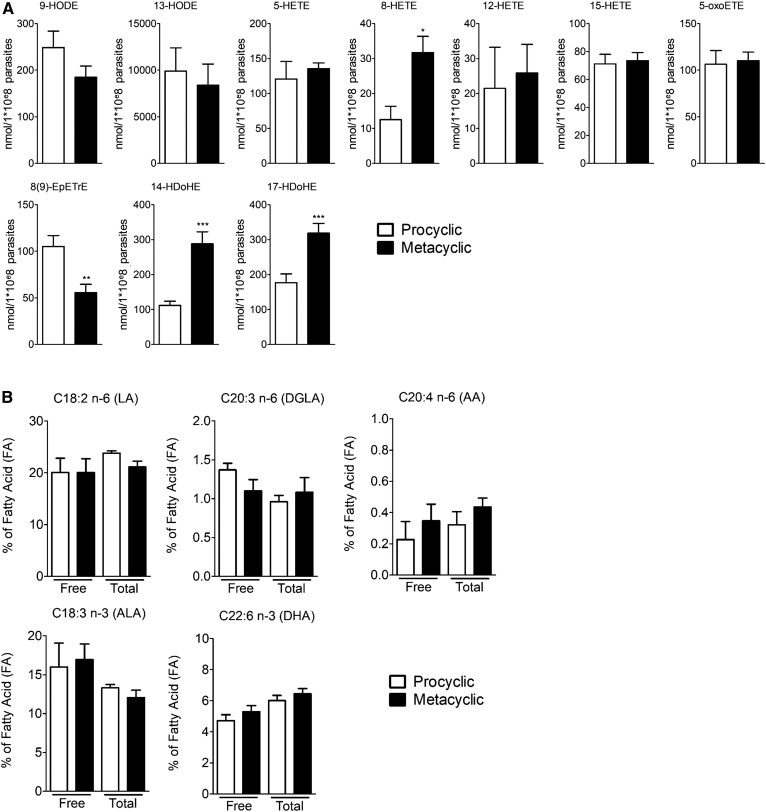
Free PUFA metabolite production during metacyclogenesis was assessed by LC/MS/MS in procyclic promastigotes (noninfective stage) and metacyclic promastigotes (infective stage) obtained in vitro by preconditioning procyclic promastigotes at acidic pH 5.4 for 24 h (19). Of the 32 PUFA metabolites assessed, 10 were detected and quantified in both the procyclic and metacyclic stages of L. infantum (Fig. 1A). These metabolites were derived from linoleic acid (LA) (9- and 13-HODE), AA [8(9)-EpETrE, 5-HETE, 8-HETE, 12-HETE, 15-HETE, and 5-oxo-ETE], and DHA (14- and 17-HDoHE). The infective form (metacyclic promastigote stage) of the parasite showed an increased concentration of 8-HETE, 14-HDoHE, and 17-HDoHE compared with the noninfective form (Fig. 1A). On the contrary, the quantity of 9-HODE and 8(9)-EpETrE was reduced during metacyclogenesis, as shown by the decreased concentration of these metabolites in the metacyclic compared with procyclic stage of the parasite (Fig. 1A). As the quantified metabolites were epoxy or hydroxyl PUFAs, we developed a method to fully quantify specifically these families (supplemental Figs. S1, S2). We confirmed the increase in the concentration of 14-HDoHE, 17-HDoHE, and 8-HETE and the decrease of 9-HODE and 8(9)-EpEtrE in metacyclic compared with procyclic promastigotes (Table 2, supplemental Figs. S1, S2). In addition, we observed an increase in the concentration of 9,10-dihydroxyoctadecenoic acid, 9,10-DiHODE, 12-HODE, 11-HETE, 4-HDoHE, 20-HDoHE, and 19(20)-epoxydocosapentaenoic acid (EpDPE) (Table 2, supplemental Figs. S1, S2). The concentration of their precursors was not different between the two stages of the parasite (Fig. 1B), suggesting that the increase in metabolite concentration is not dependent on the amounts of precursors.
Fig. 1.
PUFA metabolite production by L. infantum varies during infectivity acquisition. A: Production of PUFA metabolites assessed by LC/MS/MS on Leishmania lipid extracts from procyclic and metacyclic promastigotes. Data shown are from six experiments conducted in triplicate; means ± SEMs are shown (n = 6). *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the procyclic group. B: Percentage of main PUFAs (LA, ALA, DGLA, AA, and DHA) in free and total fatty acids assessed by GC with flame ionization detection on Leishmania lipid extracts from procyclic and metacyclic promastigotes. Data shown are from three experiments conducted in triplicate; means ± SEMs are shown (n = 3). ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid.
TABLE 2.
PUFA metabolite concentration (nmol/1 × 108 parasites) in L. infantum at the procyclic or metacyclic promastigote stage
| Metabolites | Procyclic | Metacyclic | P |
| 9,10-DiHOME | 17.68 ± 0.48 | 21.21 ± 0.69 | 0.0048 |
| 9,10-DiHODE | 10.92 ± 0.21 | 12.01 ± 0.32 | 0.0298 |
| 15,16-DiHODE | 5.83 ± 0.16 | 5.957 ± 0.12 | 0.8092 |
| 9-HODE | 396.80 ± 34.93 | 249.91 ± 34.33 | 0.0298 |
| 10-HODE | 14.24 ± 0.92 | 15.08 ± 0.75 | 0.4649 |
| 12-HODE | 17.44 ± 0.81 | 19.32 ± 0.27 | 0.0298 |
| 13-HODE | 962.11 ± 66.13 | 1,065.00 ± 67.89 | 0.3743 |
| 15-HODE | 8.11 ± 0.10 | 7.41 ± 0.41 | 0.2198 |
| 5-HETE | 26.29 ± 5.73 | 26.69 ± 4.25 | 1.0000 |
| 8-HETE | 5.26 ± 0.29 | 13.68 ± 3.60 | 0.0048 |
| 11-HETE | 3.87 ± 0.38 | 9.70 ± 0.90 | 0.0048 |
| 12-HETE | 12.61 ± 3.94 | 12.15 ± 3.27 | 1.0000 |
| 15-HETE | 43.57 ± 7.46 | 50.98 ± 1.15 | 0.7163 |
| 18-HETE | 110.30 ± 7.48 | 112.01 ± 3.34 | 0.8092 |
| 5(S)-HETrE | 0.970 ± 0.09 | 1.347 ± 0.13 | 0.1262 |
| 8(S)-HETrE | 38.12 ± 6.69 | 44.35 ± 8.47 | 0.6879 |
| 12(S)-HETrE | 12.25 ± 0.98 | 14.17 ± 0.93 | 0.1474 |
| 15(S)-HETrE | 31.26 ± 2.36 | 39.37 ± 1.83 | 0.0766 |
| 8(9)-EpETrE | 17.20 ± 2.07 | 2.91 ± 2.91 | 0.0247 |
| 4-HDoHE | 146.10 ± 2.56 | 218.80 ± 17.02 | 0.0048 |
| 7-HDoHE | 32.44 ± 2.44 | 37.94 ± 1.83 | 0.1262 |
| 8-HDoHE | 114.80 ± 16.21 | 123.70 ± 10.91 | 0.1269 |
| 10-HDoHE | 48.33 ± 3.24 | 47.18 ± 12.96 | 0.3760 |
| 11-HDoHE | 63.44 ± 11.77 | 90.39 ± 8.89 | 0.1262 |
| 13-HDoHE | 64.82 ± 3.81 | 64.05 ± 3.99 | 1.0000 |
| 14-HDoHE | 112.10 ± 6.27 | 177.00 ± 18.81 | 0.0126 |
| 16-HDoHE | 59.07 ± 3.22 | 69.35 ± 3.96 | 0.1262 |
| 17-HDoHE | 129.1 ± 13.84 | 268.5 ± 39.05 | 0.0022 |
| 20-HDoHE | 248.91 ± 24.76 | 327.50 ± 22.75 | 0.0431 |
| 7(8)-EpDPE | 38.82 ± 3.271 | 46.52 ± 2.80 | 0.0646 |
| 10(11)-EpDPE | 18.27 ± 1.89 | 24.83 ± 3.67 | 0.2403 |
| 13(14)-EpDPE | 20.62 ± 2.43 | 19.75 ± 2.29 | 0.9360 |
| 16(17)-EpDPE | 15.30 ± 2.08 | 19.19 ± 1.67 | 0.0646 |
| 19(20)-EpDPE | 56.28 ± 5.09 | 69.91 ± 1.36 | 0.0301 |
| 10,11-DiHDPE | 3.31 ± 0.29 | 3.64 ± 0.23 | 0.2598 |
| 13,14-DiHDPE | 3.56 ± 0.30 | 3.99 ± 0.20 | 0.4616 |
| 16,17-DiHDPE | 1.98 ± 0.63 | 2.22 ± 0.73 | 0.9348 |
DiHOME, dihydroxyoctadecenoic acid; HETrE, dihydroxyeicosatrienoic acid.
CYP-like proteins are responsible for PUFA metabolite production in Leishmania
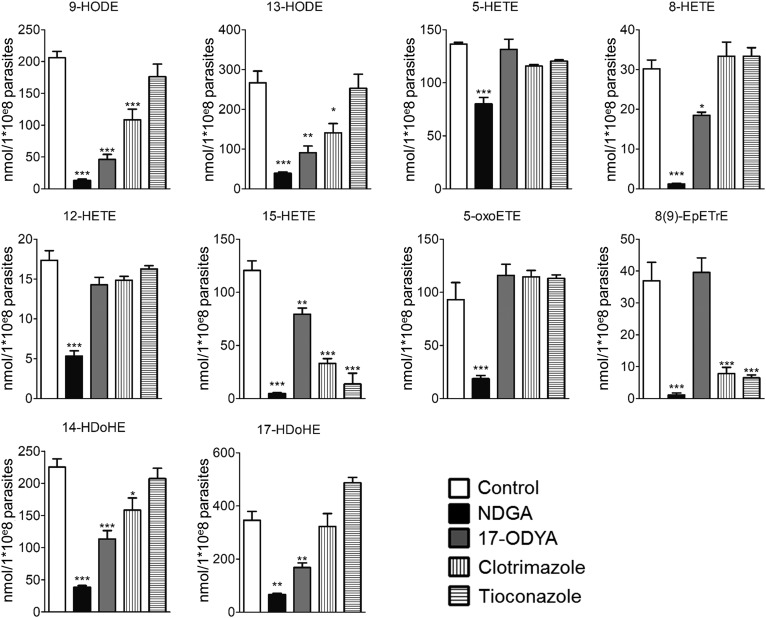
In an attempt to elucidate whether the PUFA metabolism in L. infantum results from nonenzymatic autoxidation or from an enzymatic activity, metacyclic promastigotes were treated with different enzyme inhibitors. The treatment of metacyclic promastigotes with either NDGA, 17-ODYA, mammalian CYP ω-hydroxylase inhibitor (20), or clotrimazole and tioconazole, fungal CYP-dependent 14α-demethylase inhibitors (21, 22), impaired the production of most of the PUFA metabolites (Fig. 2). NDGA was the only inhibitor that decreased the concentration of all of the metabolites tested (Fig. 2). Other inhibitors decreased differentially the concentration of the different metabolites (Fig. 2). We highlighted five different profiles: 1) 9-HODE, 13-HODE, and 14-HDoHE decreased by NDGA, 17-ODYA, and clotrimazole; 2) 5-HETE, 12-HETE, and 5-oxo-ETE decreased only by NDGA; 3) 8-HETE and 17-HDoHE decreased by NDGA and 17-ODYA; 4) 15-HETE decreased by all of the inhibitors; and 5) 8(9)-EpETrE decreased by NDGA, clotrimazole, and tioconazole.
Fig. 2.
Production of PUFA metabolites by metacyclic promastigotes is repressed by inhibitors of lipid metabolism. Production of PUFA metabolites by metacyclic promastigotes was assessed by LC/MS/MS after 24 h of treatment with 100 µM NDGA, 100 µM 17-ODYA, 10 µM clotrimazole, or 10 µM tioconazole compared with untreated metacyclic promastigotes. Data shown are from three experiments conducted in triplicate; means ± SDs are shown (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001 significantly different from control group.
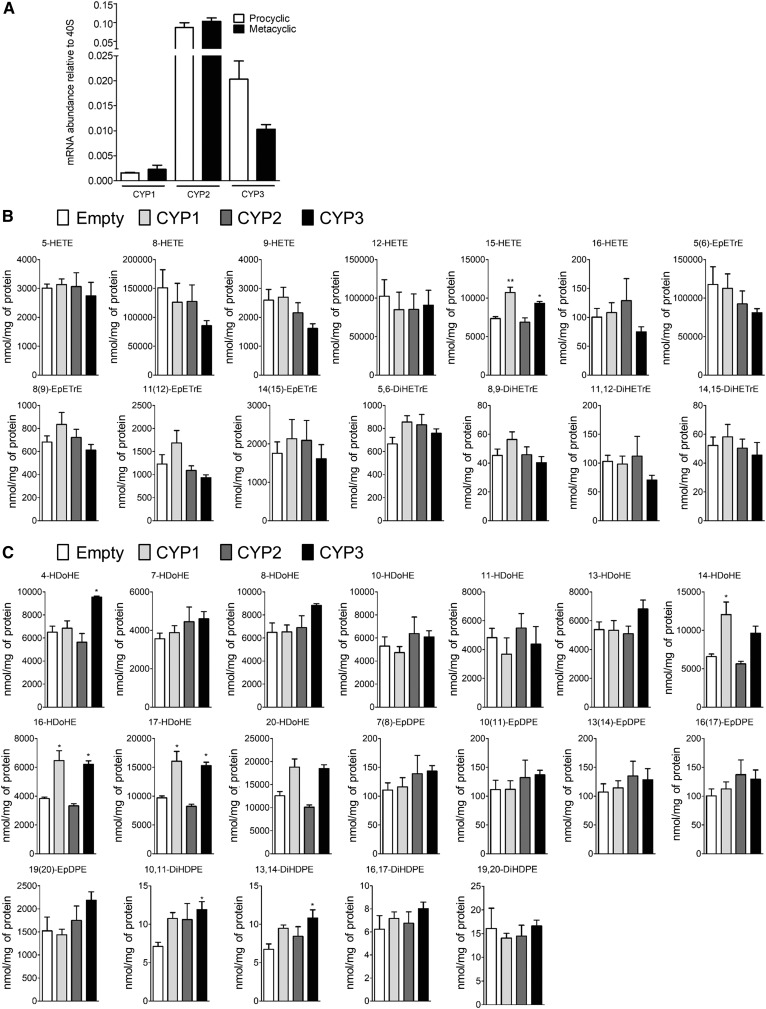
The Leishmania genome database (GeneDB; Wellcome Trust Sanger Institute, Hinxton, UK; http://www.genedb.org) annotates only three putative CYP-like proteins coded by LinJ.27.0090, LinJ.30.3610, and LinJ.34.3110 genes. These genes, conserved among species, are described here as CYP1, CYP2, and CYP3, respectively. Our data confirm the expression of these three CYP enzymes in Leishmania at the two different stages (Fig. 3A). In order to further decipher the putative role of CYP1, CYP2, and CYP3 enzymes in the biosynthesis of PUFA metabolites, CHO cells expressing Leishmania recombinant CYP1, CYP2, or CYP3 enzymes were treated with either AA or DHA as fatty acid metabolite precursors, and the quantification of epoxy and hydroxyl PUFA metabolites was performed (Fig. 3B, C). Only 15-HETE (Fig. 3B) and 4-, 14-, 16-, and 17-HDoHE (Fig. 3C) were significantly increased in CYP1- and CYP3-transfected cells compared with untransfected cells after AA (Fig. 3B) or DHA treatment (Fig. 3C). In addition, transfection by CYP3 increased the concentration of 10,11- and 13,14-dihydroxyeicosatetraenoic acid (DiHDPE) (Fig. 3C). By contrast, the transfection of CHO cells with CYP2 had no effect on PUFA metabolite synthesis (Fig. 3B, C). Thus, CYP1 and CYP3 are implicated in the biosynthesis of AA- and DHA-derived metabolites in Leishmania.
Fig. 3.
CYP-like proteins are responsible for PUFA metabolite production in Leishmania. A: mRNA expression of CYP1, CYP2, and CYP3 genes in procyclic and metacyclic promastigotes determined by RT-qPCR. Data shown are from five experiments conducted in triplicate; means ± SEMs are shown (n = 5). B: Concentration of AA-derived metabolites in CHO cells expressing recombinant CYP1, CYP2, or CYP3 determined after cell media supplementation with AA. Data shown are from six experiments conducted in duplicate; means ± SEMs are shown (n = 6). *P < 0.05 and **P < 0.01 significantly different from the empty group. C: Concentration of DHA-derived metabolites in CHO cells expressing recombinant CYP1, CYP2, or CYP3 determined after cell media supplementation with DHA. Data shown are from six experiments conducted in duplicate; means ± SEMs are shown (n = 6). *P < 0.05 significantly different from the empty group.
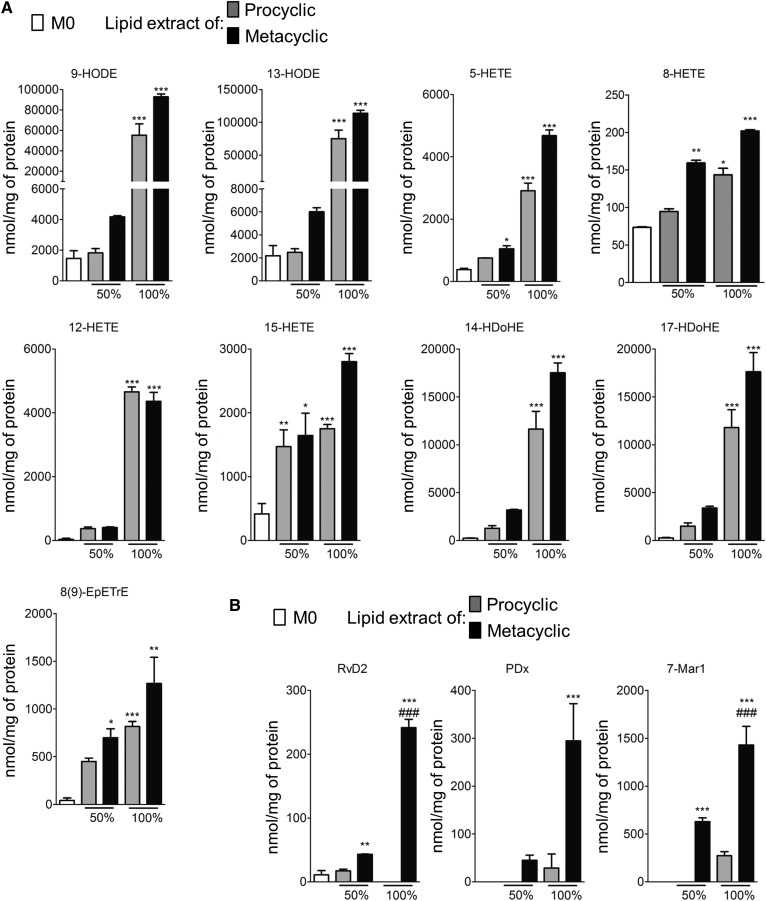
Lipids extracted from Leishmania promote the synthesis of bioactive lipids in macrophages
To further explore the role of PUFA metabolites in the parasite/host interaction, lipids extracted from procyclic and metacyclic promastigotes were assessed for their ability to induce the synthesis of lipid mediators in mouse bone marrow-derived macrophages (BMDMs). PUFA metabolites quantified in the parasite alone [9- and 13-HODE; 5-, 8-, 12-, and 15-HETE; 8(9)-EpETrE; and 14- and 17-HDoHE] were increased in treated macrophages (Fig. 4). Treatment with the parasite lipid extracts did not modify the production of PGs such as PGD2, 15d-PGJ2, PGE2, PGF2α, PGE3, and 8-iso-PGA2 or thromboxane B2, lipoxin A4, and 14(15)-EpETrE in BMDMs (supplemental Fig. S2). In contrast, the synthesis of the specialized proresolving mediators (SPMs) RvD2, PDx, and 7-MaR1 was significantly higher in BMDMs treated with metacyclic lipid extracts than those treated with procyclic lipid extracts (Fig. 4B).
Fig. 4.
Lipids extracted from Leishmania promote the synthesis of lipid mediators by BMDMs. A: Production of PUFA metabolites assessed by LC/MS/MS in BMDMs treated with 0% (M0), 50%, or 100% of lipids extracted from 1 × 108 procyclic and metacyclic promastigotes. Data shown correspond to three experiments conducted in triplicate; means ± SEMs are shown (n = 3). *P < 0.05, **P < 0.01, and ***P < 0.001 significantly different from the M0 group. B: Production of proresolving mediators assessed by LC/MS/MS in BMDMs treated with 0% (M0), 50%, or 100% of lipids extracted from 1 × 108 procyclic and metacyclic promastigotes. Data shown are from three experiments conducted in triplicate; means ± SEMs are shown (n = 3). **P < 0.05 and ***P < 0.01 significantly different from the M0 group and ###P < 0.001 significantly different from the lipid extracts of the procyclic group.
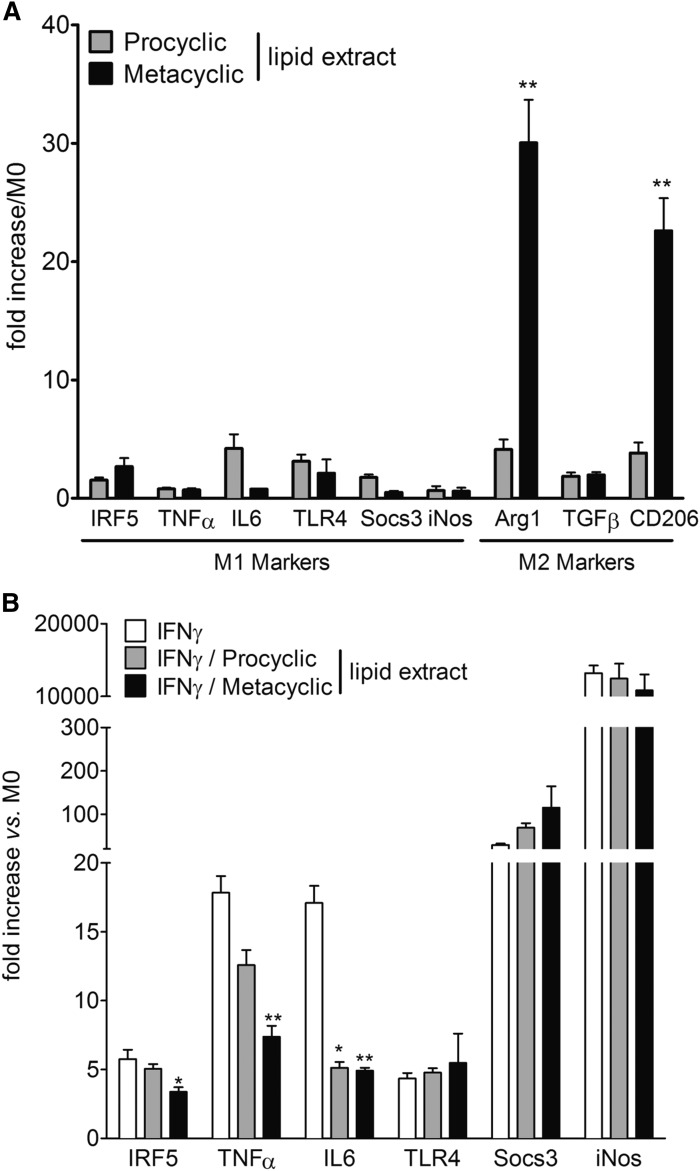
Lipids extracted from the metacyclic stage of Leishmania promote the polarization of macrophages into a proresolving M2 phenotype
M0 macrophages derived from bone marrow were treated with lipids extracted from the two stages of the parasite. Only metacyclic lipid extracts induced the transcriptional upregulation of the arginase 1 (Arg1) and CD206 genes, two bona fide markers of the alternatively activated M2 macrophage phenotype (Fig. 5A) (23). At the same time, no change in the expression level of the main markers of M1 macrophage phenotypes was observed, irrespective of parasite stages. When M0 macrophages were treated with lipids extracted from the two stages of the parasite concomitantly with INF-γ to induce M1 differentiation, only metacyclic lipid extracts decreased several M1 markers induced by IFN-γ treatment (Fig. 5B).
Fig. 5.
Lipids extracted from metacyclic promastigotes promote the polarization of macrophages into a proresolving M2 phenotype. A: mRNA expression of markers of M1 and M2 polarization quantified in BMDMs after treatment with lipid metabolites from procyclic and metacyclic promastigotes. Data shown are means of fold changes ± SEMs compared with the undifferentiated monocyte (M0) from three independent experiments conducted in triplicate (n = 3). **P < 0.01 significantly different from the procyclic lipid extract group. B: Gene expression of M1 and M2 markers in BMDMs after treatment with lipid extracts from procyclic and metacyclic promastigotes and/or IFN-γ. Data shown are means of fold changes ± SEMs compared with the undifferentiated monocyte (M0) from three independent experiments conducted in triplicate (n = 3). *P < 0.05 and **P < 0.01 significantly different from the IFN-γ group. iNOS, inducible nitric oxide synthase; IRF5, IFN regulatory factor 5; Socs3, suppressor of cytokine signaling 3.
DISCUSSION
Metacyclogenesis is known to be crucial for infectivity acquisition by promastigotes. Indeed, metacyclic promastigotes are primarily characterized by their ability to infect macrophages (6, 7, 24, 25) thanks to a higher resistance to host complement-mediated lysis, which is necessary to save time to infect macrophages. This is essentially due to the structure and size of the surface lipophosphoglycan of metacyclic promastigotes (25, 26) and the inactivation of C3b by leishmanolysin (gp63), a protein that is not present in procyclic promastigotes (4). Our study describes the ability of metacyclic promastigotes to promote their own survival by modifying the host macrophage phenotype through the synthesis of specific PUFA metabolites.
We first described the production by L. infantum of 10 different PUFA metabolites derived from LA (18:2n-6), AA (20:4n-6), and DHA (22:6n-3). The relative abundance of these different metabolites is in agreement with the abundance of their precursors (from least to most abundant: AA, dihomo-γ-linolenic acid, DHA, α-linolenic acid, and LA) (27). Contrary to its mammalian host, Leishmania is able to produce LA from oleic acid (18:1n-9) via an oleate desaturase. LA is then desaturated either to γ-linolenic acid (18:3n-6) by Δ6 desaturase activity or to α-linolenic acid (18:3n-3) by the linoleate (Δ15) desaturase. The α-linolenic acid is the major accumulated product in Leishmania (28, 29), which explains the unique DHA-AA ratio quantified in Leishmania as DHA derived from the α-isomer and AA from the γ-isomer. Independent of the amount of AA and DHA, which is similar in metacyclic promastigotes compared with procyclic promastigotes, our results highlight the overproduction of different PUFA metabolites in metacyclic promastigotes. The use of NDGA, mainly known to inhibit mammalian 5-lipoxygenase, highly repressed the synthesis of all the PUFA metabolites in L. infantum. Due to the absence of the lipoxygenase coding gene in the Leishmania genome, we further assessed whether the NDGA inhibitory effect on PUFA metabolite synthesis was dependent on its known activity as a nonspecific oxidase/reductase inhibitor or CYP inhibitor (30). Treatment with different CYP inhibitors differentially decreased the different PUFA metabolites. In mammalian cells, CYP ω-hydroxylases provide pathways for the production of bioactive metabolites from AA such as 18-, 19-, and 20-HETE mainly by the CYP4F family (31–33). Interestingly, 17-ODYA, which decreased the concentration of several metabolites in Leishmania, is a suicide inhibitor of CYP4F, indicating possible ω-hydroxylase activity present in the parasite (32). In addition, CYP2C8 and CYP2C9, characterized by their epoxygenase activity, may also produce HETE and HODE by bisallylic hydroxylation activity (34). These results, in line with previous studies, suggest that CYP enzymes may participate in the synthesis of PUFA metabolites in Leishmania. Even though there are no studies available addressing the functional involvement of CYP in PUFA metabolism in Leishmania, CYP enzymatic activities were described in trypanosomatids, including Leishmania sp. (35). A pivotal role of this enzyme in Leishmania biology is supported by the antileishmanial activity of the azole antifungals, which are known to act as CYP inhibitors (36–38). The Leishmania genome database (GeneDB, Wellcome Trust Sanger Institute, Hinxton, UK) annotates only three putative CYP-like proteins, described here as CYP1, CYP2, and CYP3. This group of proteins is annotated as membrane-associated proteins with monooxygenase activity; however, there are limited experimental data focusing on their mechanisms of action and function in the Leishmania life cycle. CYP1, classified as CYP5122A1 by the Cytochrome P450 Nomenclature Committee, has been reported to play a key role in ergosterol metabolism and to be associated with mechanisms of drug resistance in L. donovani (39) as well as in cell growth and infectivity (40). Promastigotes heterozygote for CYP1 gene deficiency show a lesser capacity to differentiate into metacyclic forms and to subsequently cause infection (40). CYP2 seems to be underregulated in L. infantum axenic amastigotes during amastigote morphogenesis (41). By expressing Leishmania recombinant CYP1, CYP2, or CYP3 enzymes in CHO cells, we demonstrated the ability of CYP1 and CYP3 to synthetize PUFA metabolites, mainly hydroxylated DHA and in particular 4-, 14-, 16-, and 17-HDoHE. The concentration of several PUFA metabolites, increased in metacyclic promastigotes, was not affected by the transfection of the different CYPs in CHO cells, demonstrating that other enzymes may be implicated in PUFA metabolite synthesis. Among them, it is important to note the increase in 19(20)-EpDPE concentration, which plays a critical role in monocyte lineage recruitment during inflammatory resolution (42).
We also demonstrated that lipids extracted from metacyclic promastigotes did not modify the production of PGs by macrophages but were able to increase the synthesis of the SPMs RvD2, PDx, and 7-MaR1. This increase in SPMs could result from the increase of their precursors (supplemental Fig. S2), 14-HDoHE for maresin and 17-HDoHE for resolvin and protectin (43). Our results are in agreement with a previous study demonstrating a key role of RvD1 in diffuse cutaneous leishmaniasis and in intracellular L. amazonensis replication in human macrophages (44). Upon parasitic challenge, it has been shown that Leishmania actively manipulated the metabolic pathway to avoid the M1 polarization of macrophages (7). We assessed the involvement of PUFA metabolites from the parasite in this process. Lipid extracts from metacyclic promastigotes were also able to induce transcriptional upregulation of markers of the alternatively activated M2 macrophage phenotype (Fig. 5A). Our results are in agreement with the mouse model of adipose tissue inflammation, in which DHA and RvD1 intake induces a shift of the macrophage phenotype with an increase in Arg1 and CD206, concomitantly with a downregulation of TNF-α and IL-6 expression (45). Transcriptional levels of TNF-α, IL-6, and the typical transcriptional factor of the M1 phenotype induced by IFN-γ, IFN regulatory factor 5, decreased after coincubation with parasitic lipid extracts from the infective form of Leishmania. In L. major-infected mice, TNF-α inhibition is associated with the upregulation of Arg1 and CD206 without affecting inducible nitric oxide synthase expression (26). The balance in favor of Arg1 rather than inducible nitric oxide synthase represses reactive oxygen species production (46) and increases polyamine biosynthesis, which is essential for the intracellular growth of Leishmania (26, 47). Our data demonstrate that DHA- and AA-derived metabolites from metacyclic promastigotes also induce a minimal proinflammatory response in the host to favor parasite growth. Therefore, metabolites derived from DHA and specifically overrepresented in metacyclic promastigotes (14- and 17-HDoHE) could participate in building a safer environment to facilitate infection by affecting the polarization of immune response during infection.
The results presented here demonstrate the production of PUFA-derived metabolites in the Leishmania parasite. Biosynthesis of these metabolites partially depends on Leishmania CYP-like protein (CYP1 and CYP3) activity. Infectivity acquisition is accompanied by an increased production of 14- and 17-HDoHE, precursors of the proresolving lipid mediators maresin, D-series resolvin, and protectin (43). The treatment of macrophages with lipids extracted from the infective forms polarizes macrophages into an M2 phenotype. M2 macrophages produce high levels of proresolving bioactive lipids (RvD2, 7-Mar1, and PDx) and several markers of proresolving phenotypes as CD206 or Arg1, promoting the survival and proliferation of Leishmania inside the host cell.
Supplementary Material
Acknowledgments
The authors thank Jean-Luc Parrou for his advice on CYP cloning. The authors also thank Genotoul, Anexplo, US006/INSERM, the University of Toulouse, and the INSERM MetaToulLipidomics Facility-MetaboHUB ANR-11-INBS-010, where lipidomic analysis was performed.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- Arg1
- arginase
- BMDM
- bone marrow-derived macrophage
- CYP
- cytochrome P450
- DiHDPE
- dihydroxydocosapentaenoic acid
- EpDPE
- epoxydocosapentaenoic acid
- EpETrE
- epoxyeicosatrienoic acid
- HDoHE
- hydroxydocosahexaenoic acid
- IL
- interleukin
- LA
- linoleic acid
- MeOH
- methanol
- NDGA
- nordihydroguaiaretic acid
- PDx
- protectin Dx
- PG
- prostaglandin
- qPCR
- quantitative PCR
- RvD
- resolvin D
- SPM
- specialized proresolving mediator
- 5-oxo-ETE
- 5-oxoeicosatetraenoic acid
- 7-MaR1
- 7-maresin 1
- 17-ODYA
- 17-octadecynoic acid.
This work was supported by grants from Région Midi-Pyrénées (N.C.) and European Commission Marie Curie Action EPIMACASE Grant IOF-627487 (C.D.).
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Bates P. A., and Tetley L.. 1993. Leishmania mexicana: induction of metacyclogenesis by cultivation of promastigotes at acidic pH. Exp. Parasitol. 76: 412–423. [DOI] [PubMed] [Google Scholar]
- 2.Zilberstein D., and Shapira M.. 1994. The role of pH and temperature in the development of Leishmania parasites. Annu. Rev. Microbiol. 48: 449–470. [DOI] [PubMed] [Google Scholar]
- 3.Saraiva E. M., Pinto-da-Silva L. H., Wanderley J. L., Bonomo A. C., Barcinski M. A., and Moreira M. E.. 2005. Flow cytometric assessment of Leishmania spp metacyclic differentiation: validation by morphological features and specific markers. Exp. Parasitol. 110: 39–47. [DOI] [PubMed] [Google Scholar]
- 4.Mojtahedi Z., Clos J., and Kamali-Sarvestani E.. 2008. Leishmania major: identification of developmentally regulated proteins in procyclic and metacyclic promastigotes. Exp. Parasitol. 119: 422–429. [DOI] [PubMed] [Google Scholar]
- 5.Alcolea P. J., Alonso A., Gomez M. J., Postigo M., Molina R., Jimenez M., and Larraga V.. 2014. Stage-specific differential gene expression in Leishmania infantum: from the foregut of Phlebotomus perniciosus to the human phagocyte. BMC Genomics. 15: 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alcolea P. J., Alonso A., Sanchez-Gorostiaga A., Moreno-Paz M., Gomez M. J., Ramos I., Parro V., and Larraga V.. 2009. Genome-wide analysis reveals increased levels of transcripts related with infectivity in peanut lectin non-agglutinated promastigotes of Leishmania infantum. Genomics. 93: 551–564. [DOI] [PubMed] [Google Scholar]
- 7.Olivier M., and Gregory D. J.. 2008. Interactions between Leishmania and the host macrophage. In Leishmania: After the Genome. Myler P. J. and Fasel N., editors. Caister Academic Press, Norfolk, UK: 239–262. [Google Scholar]
- 8.Lefèvre L., Lugo-Villarino G., Meunier E., Valentin A., Olagnier D., Authier H., Duval C., Dardenne C., Bernad J., Lemesre J. L., et al. 2013. The C-type lectin receptors dectin-1, MR, and SIGNR3 contribute both positively and negatively to the macrophage response to Leishmania infantum. Immunity. 38: 1038–1049. [DOI] [PubMed] [Google Scholar]
- 9.Liu D., and Uzonna J. E.. 2012. The early interaction of Leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infect. Microbiol. 2: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Menezes J. P., Saraiva E. M., and da Rocha-Azevedo B.. 2016. The site of the bite: Leishmania interaction with macrophages, neutrophils and the extracellular matrix in the dermis. Parasit. Vectors. 9: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabututu Z., Martin S. K., Nozaki T., Kawazu S., Okada T., Munday C. J., Duszenko M., Lazarus M., Thuita L. W., Urade Y., et al. 2003. Prostaglandin production from arachidonic acid and evidence for a 9,11-endoperoxide prostaglandin H2 reductase in Leishmania. Int. J. Parasitol. 33: 221–228. [DOI] [PubMed] [Google Scholar]
- 12.Noverr M. C., Erb-Downward J. R., and Huffnagle G. B.. 2003. Production of eicosanoids and other oxylipins by pathogenic eukaryotic microbes. Clin. Microbiol. Rev. 16: 517–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Le Faouder P., Baillif V., Spreadbury I., Motta J. P., Rousset P., Chene G., Guigne C., Terce F., Vanner S., Vergnolle N., et al. 2013. LC-MS/MS method for rapid and concomitant quantification of pro-inflammatory and pro-resolving polyunsaturated fatty acid metabolites. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 932: 123–133. [DOI] [PubMed] [Google Scholar]
- 14.Rund K. M., Ostermann A. I., Kutzner L., Galano J. M., Oger C., Vigor C., Wecklein S., Seiwert N., Durand T., and Schebb N. H.. 2018. Development of an LC-ESI(-)-MS/MS method for the simultaneous quantification of 35 isoprostanes and isofurans derived from the major n3- and n6-PUFAs. Anal. Chim. Acta. 1037: 63–74. [DOI] [PubMed] [Google Scholar]
- 15.Bligh E. G., and Dyer W. J.. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 16.Lillington J. M., Trafford D. J., and Makin H. L.. 1981. A rapid and simple method for the esterification of fatty acids and steroid carboxylic acids prior to gas-liquid chromatography. Clin. Chim. Acta. 111: 91–98. [DOI] [PubMed] [Google Scholar]
- 17.Accarias S., Lugo-Villarino G., Foucras G., Neyrolles O., Boullier S., and Tabouret G.. 2015. Pyroptosis of resident macrophages differentially orchestrates inflammatory responses to Staphylococcus aureus in resistant and susceptible mice. Eur. J. Immunol. 45: 794–806. [DOI] [PubMed] [Google Scholar]
- 18.Alcolea P. J., Alonso A., Gomez M. J., Sanchez-Gorostiaga A., Moreno-Paz M., Gonzalez-Pastor E., Torano A., Parro V., and Larraga V.. 2010. Temperature increase prevails over acidification in gene expression modulation of amastigote differentiation in Leishmania infantum. BMC Genomics. 11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Luz R. I., Vermeersch M., Dujardin J. C., Cos P., and Maes L.. 2009. In vitro sensitivity testing of Leishmania clinical field isolates: preconditioning of promastigotes enhances infectivity for macrophage host cells. Antimicrob. Agents Chemother. 53: 5197–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edson K. Z., and Rettie A. E.. 2013. CYP4 enzymes as potential drug targets: focus on enzyme multiplicity, inducers and inhibitors, and therapeutic modulation of 20-hydroxyeicosatetraenoic acid (20-HETE) synthase and fatty acid omega-hydroxylase activities. Curr. Top. Med. Chem. 13: 1429–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Ramamoorthy Y., Kilicarslan T., Nolte H., Tyndale R. F., and Sellers E. M.. 2002. Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab. Dispos. 30: 314–318. [DOI] [PubMed] [Google Scholar]
- 22.McLean K. J., Marshall K. R., Richmond A., Hunter I. S., Fowler K., Kieser T., Gurcha S. S., Besra G. S., and Munro A. W.. 2002. Azole antifungals are potent inhibitors of cytochrome P450 mono-oxygenases and bacterial growth in mycobacteria and streptomycetes. Microbiology. 148: 2937–2949. [DOI] [PubMed] [Google Scholar]
- 23.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., et al. 2014. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao C., Li Y., Donelson J. E., and Wilson M. E.. 2010. Proteomic examination of Leishmania chagasi plasma membrane proteins: contrast between avirulent and virulent (metacyclic) parasite forms. Proteomics Clin. Appl. 4: 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sacks D. L. 1989. Metacyclogenesis in Leishmania promastigotes. Exp. Parasitol. 69: 100–103. [DOI] [PubMed] [Google Scholar]
- 26.Schleicher U., Paduch K., Debus A., Obermeyer S., Konig T., Kling J. C., Ribechini E., Dudziak D., Mougiakakos D., Murray P. J., et al. 2016. TNF-mediated restriction of arginase 1 expression in myeloid cells triggers type 2 NO synthase activity at the site of infection. Cell Reports. 15: 1062–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouazizi-Ben Messaoud H., Guichard M., Lawton P., Delton I., and Azzouz-Maache S.. 2017. Changes in lipid and fatty acid composition during intramacrophagic transformation of Leishmania donovani complex promastigotes into amastigotes. Lipids. 52: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alloatti A., and Uttaro A. D.. 2011. Highly specific methyl-end fatty-acid desaturases of trypanosomatids. Mol. Biochem. Parasitol. 175: 126–132. [DOI] [PubMed] [Google Scholar]
- 29.Uttaro A. D. 2014. Acquisition and biosynthesis of saturated and unsaturated fatty acids by trypanosomatids. Mol. Biochem. Parasitol. 196: 61–70. [DOI] [PubMed] [Google Scholar]
- 30.Capdevila J., Gil L., Orellana M., Marnett L. J., Mason J. I., Yadagiri P., and Falck J. R.. 1988. Inhibitors of cytochrome P-450-dependent arachidonic acid metabolism. Arch. Biochem. Biophys. 261: 257–263. [DOI] [PubMed] [Google Scholar]
- 31.Hsu M. H., Savas U., Griffin K. J., and Johnson E. F.. 2007. Human cytochrome p450 family 4 enzymes: function, genetic variation and regulation. Drug Metab. Rev. 39: 515–538. [DOI] [PubMed] [Google Scholar]
- 32.Kalsotra A., and Strobel H. W.. 2006. Cytochrome P450 4F subfamily: at the crossroads of eicosanoid and drug metabolism. Pharmacol. Ther. 112: 589–611. [DOI] [PubMed] [Google Scholar]
- 33.Hardwick J. P. 2008. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem. Pharmacol. 75: 2263–2275. [DOI] [PubMed] [Google Scholar]
- 34.Bylund J., Kunz T., Valmsen K., and Oliw E. H.. 1998. Cytochromes P450 with bisallylic hydroxylation activity on arachidonic and linoleic acids studied with human recombinant enzymes and with human and rat liver microsomes. J. Pharmacol. Exp. Ther. 284: 51–60. [PubMed] [Google Scholar]
- 35.Berger B. J., and Fairlamb A. H.. 1993. Cytochrome P450 in trypanosomatids. Biochem. Pharmacol. 46: 149–157. [DOI] [PubMed] [Google Scholar]
- 36.Croft S. L., and Coombs G. H.. 2003. Leishmaniasis–current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 19: 502–508. [DOI] [PubMed] [Google Scholar]
- 37.Roberts C. W., McLeod R., Rice D. W., Ginger M., Chance M. L., and Goad L. J.. 2003. Fatty acid and sterol metabolism: potential antimicrobial targets in apicomplexan and trypanosomatid parasitic protozoa. Mol. Biochem. Parasitol. 126: 129–142. [DOI] [PubMed] [Google Scholar]
- 38.Balding P. R., Porro C. S., McLean K. J., Sutcliffe M. J., Marechal J. D., Munro A. W., and de Visser S. P.. 2008. How do azoles inhibit cytochrome P450 enzymes? A density functional study. J. Phys. Chem. A. 112: 12911–12918. [DOI] [PubMed] [Google Scholar]
- 39.Pandharkar T., Zhu X., Mathur R., Jiang J., Schmittgen T. D., Shaha C., and Werbovetz K. A.. 2014. Studies on the antileishmanial mechanism of action of the arylimidamide DB766: azole interactions and role of CYP5122A1. Antimicrob. Agents Chemother. 58: 4682–4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verma S., Mehta A., and Shaha C.. 2011. CYP5122A1, a novel cytochrome P450 is essential for survival of Leishmania donovani. PLoS One. 6: e25273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenzweig D., Smith D., Opperdoes F., Stern S., Olafson R. W., and Zilberstein D.. 2008. Retooling Leishmania metabolism: from sand fly gut to human macrophage. FASEB J. 22: 590–602. [DOI] [PubMed] [Google Scholar]
- 42.Gilroy D. W., Edin M. L., De Maeyer R. P., Bystrom J., Newson J., Lih F. B., Stables M., Zeldin D. C., and Bishop-Bailey D.. 2016. CYP450-derived oxylipins mediate inflammatory resolution. Proc. Natl. Acad. Sci. USA. 113: E3240–E3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chiang N., and Serhan C. N.. 2017. Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol. Aspects Med. 58: 114–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malta-Santos H., Andrade B. B., Zanette D. L., Costa J. M., Bozza P. T., Bandeira-Melo C., Barral A., Franca-Costa J., and Borges V. M.. 2017. Resolvin D1 drives establishment of Leishmania amazonensis infection. Sci. Rep. 7: 46363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Titos E., Rius B., Gonzalez-Periz A., Lopez-Vicario C., Moran-Salvador E., Martinez-Clemente M., Arroyo V., and Claria J.. 2011. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J. Immunol. 187: 5408–5418. [DOI] [PubMed] [Google Scholar]
- 46.Rath M., Muller I., Kropf P., Closs E. I., and Munder M.. 2014. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol. 5: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colotti G., and Ilari A.. 2011. Polyamine metabolism in Leishmania: from arginine to trypanothione. Amino Acids. 40: 269–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.