Abstract
Sphingosine-1-phosphate (S1P) is a potent bioactive signaling molecule that regulates many physiological processes important for development, epithelial and endothelial barrier integrity, and the immune system, as well as for pathologies, such as autoimmune diseases, cancer, and metastasis. Most of the well-known actions of S1P are mediated by five specific G protein-coupled receptors located on the plasma membrane. Because S1P is synthesized intracellularly by two sphingosine kinase isoenzymes, we have proposed the paradigm of inside-out signaling by S1P, suggesting that S1P must be exported out of cells to interact with its receptors. While several transporters of S1P have previously been identified, spinster homologue 2 (SPNS2), a member of the large family of non-ATP-dependent organic ion transporters, has recently attracted much attention as an S1P transporter. Here, we discuss recent advances in understanding the physiological actions of SPNS2 in regulating levels of S1P and the S1P gradient that exists between the high circulating concentrations of S1P and low tissue levels that control lymphocyte trafficking. Special emphasis is on the functions of SPNS2 in inflammatory and autoimmune diseases and its recently discovered unexpected importance in metastasis.
Keywords: sphingosine kinase, sphingosine-1-phosphate receptors, spinster homologue 2, transporter
Graphical Abstract
S1P SIGNALING
The role of sphingosine-1-phosphate (S1P) as a bioactive sphingolipid metabolite was discovered in our lab almost 3 decades ago (1, 2). However, its pleiotropic actions remained enigmatic until, together with Timothy Hla, who originally cloned endothelial differentiation gene 1 as an immediate early gene induced during the differentiation of endothelial cells (3), we showed that this is a bona fide S1P receptor (S1PR1) (4, 5). This rapidly led to the discovery of a family of five specific cell-surface G protein-coupled receptors (S1PR1–5) that endow S1P with the ability to regulate many important functions (6) (Fig. 1). The activation of S1PRs leads to a plethora of downstream effects depending on the expression of S1PRs, G proteins, and cell type (7) and has been linked to the regulation of multiple physiological and pathophysiological functions, including cell growth and survival, angiogenesis and lymphangiogenesis, endothelial and epithelial barrier function, lymphocyte function and trafficking, and cancer and metastasis, to name a few (7, 8).
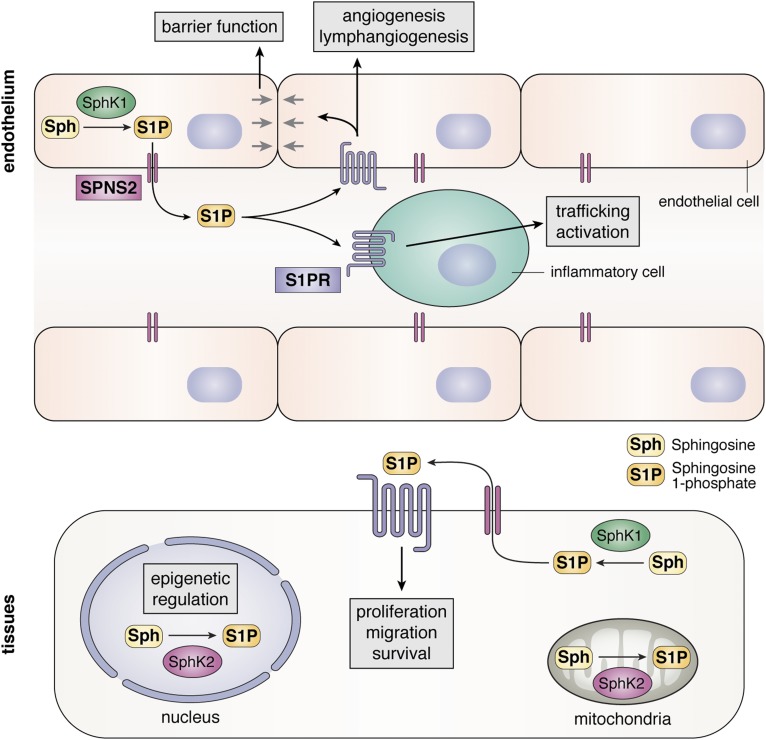
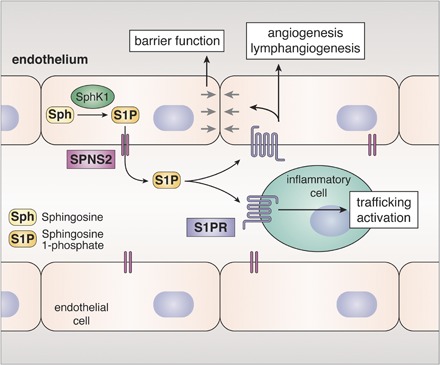
Fig. 1.
Role of SPNS2 in inside-out signaling of S1P. SphK1 activated by cytokines phosphorylates Sph to generate intracellular S1P that is then exported from cells by SPNS2. S1P secreted from endothelial cells contributes to circulating levels of S1P, which act in an autocrine or paracrine manner by binding to S1PRs present on endothelial and inflammatory cells. Sph, sphingosine.
S1P is produced in cells by the phosphorylation of sphingosine, the backbone of all sphingolipids, by two sphingosine kinase (SphK) isoenzymes, SphK1 and SphK2. Like other bioactive mediators, levels of S1P are tightly regulated by the balance between its synthesis and degradation by S1P phosphatases and S1P lyase. SphK1 and SphK2 have some overlapping but also distinct functions due to their different intracellular localizations (Fig. 1). SphK1 is mainly cytosolic and translocated to the plasma membrane upon stimulation with growth factors and cytokines. In contrast, SphK2 localizes not only to the plasma membrane but also to the ER, mitochondria, and nucleus. This led to the discoveries that S1P produced by SphK2 in mitochondria and nuclei has important functions in these organelles to regulate respiration (9), histone acetylation, gene expression, and telomerase stability (10, 11). Importantly, however, it has now been convincingly demonstrated that S1P generated inside cells can act in a paracrine and/or autocrine manner by activating S1PRs in an inside-out signaling manner (12, 13). Thus, a robust mechanism is required to transport S1P out of cells. Several transporters have been identified that can transport S1P across the bilayer, including members of the ABC transporter family (12, 13). This review focuses on recent evidence indicating that spinster homologue 2 (SPNS2), a member of the large major facility superfamily (MFS) of non-ATP-dependent organic ion transporters, is a significant and physiologically relevant transporter of S1P. Emphasis is placed on advances in understanding the physiological actions of SPNS2 in regulating levels of S1P and the S1P gradient that exists between the high circulating concentrations of S1P and low tissue levels and lymphocyte trafficking. We also discuss recent discoveries of the roles of SPNS2 in inflammatory and autoimmune diseases, cancer, and metastasis.
SPNS2, AN S1P TRANSPORTER
The role of SPNS2 in S1P transport was first suggested from studies of zebrafish, in which mutations in the SPNS2 gene were found to induce a split heart phenotype, known as two of hearts, which mimicked the phenotype of a mutation in the S1PR2 homologue miles apart (14, 15). Both of these mutations blocked the migration of cardiac precursor cells toward the midline beneath the endoderm and could be rescued by exogenous S1P or by the expression of the human SPNS2 homologue. Several groups then showed that the overexpression of SPNS2 in mammalian cells increased the secretion of S1P (15–17), and, conversely, its down-regulation decreased the release of both S1P and dihydro-S1P (17), confirming that SPNS2 is a mammalian S1P transporter. In addition to endogenously produced S1P and dihydro-S1P, SPNS2 can also transport S1P analogues such as FTY720 phosphate, but not unphosphorylated sphingoid bases (16, 17).
SPNS2 IN DEVELOPMENT AND ORGAN HOMEOSTASIS
Unlike zebrafish, SPNS2 knockout mice are viable, do not exhibit cardiac defects or embryonic lethality, and are generally normal and fertile (18). However, in addition to alterations in immune cell development and trafficking, discussed at length below, SPNS2 knockouts have postnatal cochlear degeneration and retinal dysmorphogenesis (19). SPNS2 knockout mice rapidly lose endocochlear potential and auditory sensitivity after birth, accompanied by capillary abnormalities, structural changes, and secondary sensory hair loss, leading to profound deafness (19). The cochlear-specific knockout of SPNS2 recapitulates the hearing defect, suggesting that local secretion of S1P in the ear, but not systemically circulating S1P, is responsible for maintaining the cochlear structure (19). SPNS2 knockout mice are also born with their eyes open, leading to corneal ulceration and opacity that make retinal assessment by ophthalmoscopy difficult. However, vascular and retinal neurologic defects were observed in whole-animal knockouts but not when SPNS2 was specifically deleted in ear, blood, or lymph tissue, further implicating the importance of S1P signaling through local production. Rats with an insertional mutation in SPNS2 were also born with their eyes open (20). They had lower levels of S1P in amniotic fluid and in the vitreous humor of the eye, where it correlated with a loss of polarity of retinal progenitor cells and insufficient migration of neurons in the developing retina (20). The open-eye defect was corrected by an injection of S1P into the amniotic fluid, indicating that SPNS2 plays a role in regulating amniotic S1P levels and in the developing retina. Binding of S1P to S1PRs transactivates epidermal growth factor (EGF) receptor signaling through a complex mechanism causing eyelid closure in developing rats (21).
S1P/S1PR signaling has been shown to play key roles in bone homeostasis, including recruitment and differentiation; the survival of stem cells; and interactions between osteoblasts and osteoclasts (22). It was shown that the hormone calcitonin reduces the expression of SPNS2 on osteoclasts, decreasing S1P secretion. As S1P signals through S1PR3 in the osteoblast to increase osteoblast differentiation and bone formation, these observations explain how calcitonin can also function as an inhibitor of bone formation (23). Moreover, SphKs and SPNS2 were reported to be upregulated in osteoblasts and chondrocytes during differentiation and that the inhibition of S1P signaling led to reduced matrix mineralization (24). Intriguingly, patients with spondyloarthritis, which is characterized by increased mineralization and fusion of bones, also had increased serum levels of S1P, suggesting that targeting S1P signaling may have clinical benefits in this disease (24).
SPNS2 IN THE REGULATION OF CIRCULATING S1P
Erythrocytes and platelets are abundant blood cells that are the major source of S1P in the blood, yet recent observations indicate that SPNS2 is not the main S1P transporter on these cells (25, 26). This long-sought exporter has now been identified. It was recently shown that a member of the same family of transporters as SPNS2, Mfsd2b, was responsible for releasing S1P from erythrocytes and platelets and that knocking out Mfsd2b in mice reduced plasma S1P levels by ∼50% (27). However, endothelial cells can also release S1P mediated by SPNS2 (Fig. 2). Endothelial-specific knockout of SPNS2 reduced circulating S1P to the same extent as the global knockout (25, 26) and had the same lymphopenic effects (17, 28), demonstrating that SPNS2 secretes S1P from endothelial cells directly to the blood. These results also suggest that S1P from endothelial cells is important for regulating lymphocyte trafficking. In contrast, in another study, specific deletion of SPNS2 in murine hematopoietic and endothelial cells also induced lymphopenia but with decreased lymph levels and not plasma S1P (29). In a subsequent study using S1PR1 reporter mice, Fang et al. (30) found that T-cells sensed higher concentrations of S1P in the medullary cords of lymph nodes than in the T-cell zone and that the S1P transporter SPNS2 present on lymphatic endothelial cells generated this gradient. On the other hand, we observed that the lymph nodes from global SPNS2 knockout mice have aberrant lymphatic sinuses that appear collapsed, with reduced numbers of lymphocytes (17). Strikingly, although these mice had reduced levels of S1P in the blood, they had increased S1P in the lymph and lymph nodes (17). The discrepancy in reports of plasma, blood, and lymph S1P levels could be due to different methods of S1P measurement and difficulties in collecting lymph from mice. However, it is clear that SPNS2 is required for establishing the S1P gradient in the blood. Whether it has a similar role in establishing a gradient between tissue and lymph requires further study. Together, the data described above indicate that there are at least two sources of circulating S1P, one dependent on Mfsd2b in erythrocytes and platelets and another dependent on SPNS2 in endothelial cells.
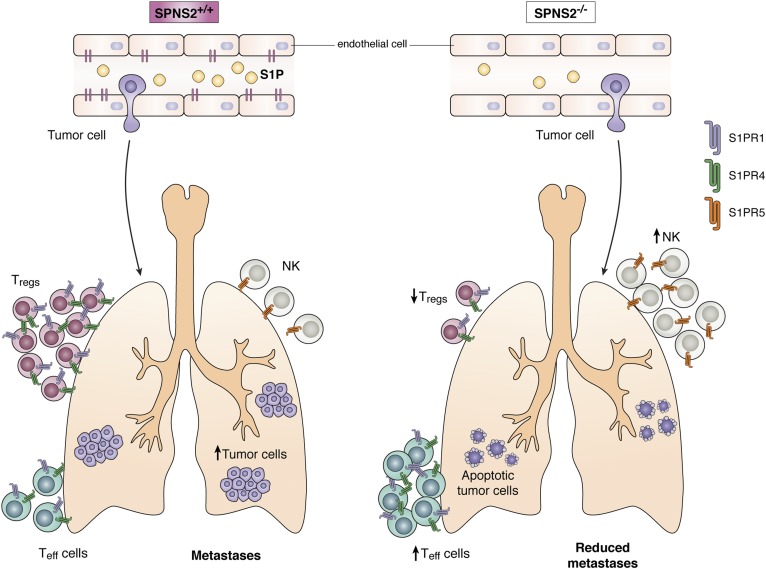
Fig. 2.
Role of SPNS2 in metastasis. Deficiency of SPNS2 in mice leads to enhanced tumor cell killing by Teff and NK cells accompanied by reduced immunosuppressive Tregs, resulting in the attenuation of pulmonary metastases. Teff, effector T-cell; Treg, regulatory T-cell.
SPNS2 IN INFLAMMATION AND THE IMMUNE SYSTEM
In their pioneering work, Cyster and Schwab (31) demonstrated that lymphocyte trafficking is mediated by S1PR1 and regulated by the gradient of S1P, that is, higher concentrations in circulation than in tissues. The role of S1PR1 signaling in trafficking and functions of many types of immune cells has been well established and extensively reviewed, and the reader is referred to reviews on this topic (31, 32). Thus, it is not surprising that growing evidence points to a key role of SPNS2 that regulates levels of S1P in the blood in immune cell trafficking and functions. Several studies have convincingly demonstrated that mice with a global (17, 18, 28) or endothelial-specific deletion of SPNS2 (25, 26, 29) are lymphopenic and exhibit decreased numbers of T- and B-cells in the blood, suggesting that SPNS2 functions in endothelial cells to establish the S1P gradient required for T- and B-cells to egress from their respective primary lymphoid organs. Redistribution of lymphocytes in mice lacking SPNS2 was not dependent on hematopoietic cells, as shown by bone marrow adoptive transfer experiments (28). While a major focus of research on SPNS2 has been on S1P-mediated regulation of immune cell trafficking, it has been shown that S1P secreted from lymph endothelial cells by SPNS2 not only promotes trafficking but also survival of naive T-cells through S1PR1-dependent mitochondrial maintenance (33). Indeed, the loss of SPNS2 has a profound effect on inflammatory responses. The deletion of SPNS2 impaired humoral immune responses to immunization (18), reduced sensitivity to antigen-induced airway inflammation and hypersensitivity, and attenuated delayed-type contact hypersensitivity (28). The global SPNS2 knockout mice are also less susceptible to chemically induced colitis and have attenuated collagen-induced arthritis and experimental autoimmune encephalomyelitis (28), a model that mimics the hallmarks of human multiple sclerosis. These studies indicate important roles for SPNS2-mediated S1P transport in the initiation and development of a wide variety of adaptive immune-related pathologies.
SPNS2 also plays a role in innate immunity, as SPNS2 knockouts have reduced IFN-γ responses to infections due to the inability of natural killer (NK) cells to egress from lymph nodes (30). Interestingly, S1P signaling in this case was linked to the activation of S1PR5, an observation that is consistent with previous studies indicating that S1PR5 is required for NK cells to mobilize to inflamed organs (34).
SPNS2 IN CANCER
S1P signaling has been linked to many cellular processes important for cancer progression, including cell growth and survival, metastasis, and chemoresistance (35–37). Several recent studies suggest that SPNS2 may also be involved in these S1P-mediated effects. Overexpression of SPNS2 in nonsmall cell lung carcinoma cells increased S1P secretion but also led to increased apoptosis and decreased cell migration. Moreover, SPNS2 mRNA was downregulated in a small sample of human lung tumors (38). These counterintuitive results could be explained by the need of these cancer cells for internal S1P signaling, dysregulated signaling through opposing S1PRs, or both. Conversely, SPNS2 was required for growth factor-induced lamellipodia formation and motility in endothelial cells (39). This was dependent on SphK1 activation, the generation of S1P, and SPNS2-mediated extracellular transport of S1P and its inside-out signaling via S1PR1 (39). Similarly, SPNS2 was associated with increased EGF-mediated invasion of HeLa cells (40). This study also showed that EGF stimulated SPNS2-dependent signaling through S1PR2 to promote invasion. However, in a twist on the classic inside-out S1P signaling model, this effect could not be blocked by an extracellular S1P neutralizing antibody, suggesting a novel intracellular action for SPNS2 to secrete S1P into an intracellular compartment such as endosomes, leading to the activation of S1PR2 (40). Interestingly, in HeLa cells, the S1P-S1PR5 signaling axis promotes the mitotic transition, and although SPNS2 was required for this, it was not clear whether this was due to secreted S1P (41). In contrast, the same S1P neutralizing antibody was shown to effectively block the progrowth SphK1-SPNS2-S1PR1-hypoxia-inducible factor 2α signaling cascade in renal carcinoma cells (42).
While there is some evidence that suggests a role for SPNS2 in cancer cells themselves, the importance of host SPNS2 in metastasis was recently highlighted by a massive screen of more than 800 randomly selected knockout mice for host genes that regulate susceptibility to metastatic colonization of the lung (43). This impressive screen involved injecting syngeneic melanoma cells into the tail veins of knockout mice and their wild-type littermates and then determining the number of lung nodules. Interestingly, the strongest suppressor of metastatic colonization was the knockout of the SPNS2 gene (43).
The suppression of metastases was not due to a decrease in the ability of the cancer cells to enter the lung but rather rapid apoptosis of the cancer cells in the lung, indicating a hostile microenvironment in the knockout animals. The deletion of SPNS2, either globally or in a lymphatic endothelial cell-specific manner, created lymphopenia. At first glance, it seems puzzling that decreasing lymphocytes in the lung would weaken metastatic colonization. However, it was demonstrated that despite a general decrease in T-cells, the ratio of effector T-cells to immunosuppressive regulatory T-cells was increased, and there also was an increase in NK cells in the lungs of SPNS2-deficient mice (Fig. 2). In addition, CD4+ and CD8+ T-cells from SPNS2 knockout animals had higher T-cell activities. Further, in vivo depletion studies demonstrated that CD8+ T-cells and NK cells contribute to the reduced pulmonary metastatic burden. This work represents a novel approach for studying the complicated role of host tissue in cancer metastasis and strongly suggests that SPNS2 could be an effective target for reducing metastases after primary tumor resection by increasing the efficacy of immunotherapy to kill the cancer cells.
Footnotes
Abbreviations:
- EGF
- epidermal growth factor
- MFS
- major facility superfamily
- NK
- natural killer
- S1P
- sphingosine-1-phosphate
- S1PR
- S1P receptor
- SphK
- sphingosine kinase
- SPNS2
- spinster homologue 2
This work was supported by National Institutes of Health Grants F31 CA220798 (M.A.M.) and R01 GM043880 (S.S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Zhang H., Desai N. N., Olivera A., Seki T., Brooker G., and Spiegel S.. 1991. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 114: 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olivera A., and Spiegel S.. 1993. Sphingosine-1-phosphate as a second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 365: 557–560. [DOI] [PubMed] [Google Scholar]
- 3.Hla T., and Maciag T.. 1990. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein coupled receptors. J. Biol. Chem. 265: 9308–9313. [PubMed] [Google Scholar]
- 4.Lee M. J., Van Brocklyn J. R., Thangada S., Liu C. H., Hand A. R., Menzeleev R., Spiegel S., and Hla T.. 1998. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 279: 1552–1555. [DOI] [PubMed] [Google Scholar]
- 5.Van Brocklyn J. R., Lee M. J., Menzeleev R., Olivera A., Edsall L., Cuvillier O., Thomas D. M., Coopman P. J. P., Thangada S., Hla T., et al. . 1998. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled orphan receptor edg-1 and intracellular to regulate proliferation and survival. J. Cell Biol. 142: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spiegel S., and Milstien S.. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4: 397–407. [DOI] [PubMed] [Google Scholar]
- 7.Rosen H., Stevens R. C., Hanson M., Roberts E., and Oldstone M. B.. 2013. Sphingosine-1-phosphate and its receptors: structure, signaling, and influence. Annu. Rev. Biochem. 82: 637–662. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S., and Milstien S.. 2011. The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11: 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strub G. M., Paillard M., Liang J., Gomez L., Allegood J. C., Hait N. C., Maceyka M., Price M. M., Chen Q., Simpson D. C., et al. . 2011. Sphingosine-1-phosphate produced by sphingosine kinase 2 in mitochondria interacts with prohibitin 2 to regulate complex IV assembly and respiration. FASEB J. 25: 600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hait N. C., Allegood J., Maceyka M., Strub G. M., Harikumar K. B., Singh S. K., Luo C., Marmorstein R., Kordula T., Milstien S., et al. . 2009. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 325: 1254–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panneer Selvam S., De Palma R. M., Oaks J. J., Oleinik N., Peterson Y. K., Stahelin R. V., Skordalakes E., Ponnusamy S., Garrett-Mayer E., Smith C. D., et al. . 2015. Binding of the sphingolipid S1P to hTERT stabilizes telomerase at the nuclear periphery by allosterically mimicking protein phosphorylation. Sci. Signal. 8: ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takabe K., and Spiegel S.. 2014. Export of sphingosine-1-phosphate and cancer progression. J. Lipid Res. 55: 1839–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishi T., Kobayashi N., Hisano Y., Kawahara A., and Yamaguchi A.. 2014. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta. 1841: 759–765. [DOI] [PubMed] [Google Scholar]
- 14.Osborne N., Brand-Arzamendi K., Ober E. A., Jin S. W., Verkade H., Holtzman N. G., Yelon D., and Stainier D. Y.. 2008. The spinster homolog, two of hearts, is required for sphingosine 1-phosphate signaling in zebrafish. Curr. Biol. 18: 1882–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawahara A., Nishi T., Hisano Y., Fukui H., Yamaguchi A., and Mochizuki N.. 2009. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 323: 524–527. [DOI] [PubMed] [Google Scholar]
- 16.Hisano Y., Kobayashi N., Kawahara A., Yamaguchi A., and Nishi T.. 2011. The sphingosine 1-phosphate transporter, SPNS2, functions as a transporter of the phosphorylated form of the immunomodulating agent FTY720. J. Biol. Chem. 286: 1758–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagahashi M., Kim E. Y., Yamada A., Ramachandran S., Allegood J. C., Hait N. C., Maceyka M., Milstien S., Takabe K., and Spiegel S.. 2013. Spns2, a transporter of phosphorylated sphingoid bases, regulates their blood and lymph levels, and the lymphatic network. FASEB J. 27: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nijnik A., Clare S., Hale C., Chen J., Raisen C., Mottram L., Lucas M., Estabel J., Ryder E., Adissu H., et al. . 2012. The role of sphingosine-1-phosphate transporter spns2 in immune system function. J. Immunol. 189: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Ingham N., Kelly J., Jadeja S., Goulding D., Pass J., Mahajan V. B., Tsang S. H., Nijnik A., Jackson I. J., et al. . 2014. Spinster homolog 2 (spns2) deficiency causes early onset progressive hearing loss. PLoS Genet. 10: e1004688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang C., Bian G., Ren P., Xiang J., Song J., Yu C., Zhang Q., Liu L., Chen K., Liu F., et al. . 2018. S1P transporter SPNS2 regulates proper postnatal retinal morphogenesis. FASEB J. 32: 3597–3613. [DOI] [PubMed] [Google Scholar]
- 21.Bian G., Yu C., Liu L. Fang C. Chen K. Ren P. Zhang Q. Liu F. Zhang K. Xue Q., et al. . 2018. Sphingosine 1-phosphate stimulates eyelid closure in the developing rat by stimulating EGFR signaling. Sci. Signal. 11: eaat1470. [DOI] [PubMed] [Google Scholar]
- 22.Sartawi Z., Schipani E., Ryan K. B., and Waeber C.. 2017. Sphingosine 1-phosphate (S1P) signalling: role in bone biology and potential therapeutic target for bone repair. Pharmacol. Res. 125: 232–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller J., Catala-Lehnen P., Huebner A. K., Jeschke A., Heckt T., Lueth A., Krause M., Koehne T., Albers J., Schulze J., et al. . 2014. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat. Commun. 5: 5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bougault C., El Jamal A., Briolay A., Mebarek S., Boutet M. A., Garraud T., Le Goff B., Blanchard F., Magne D., and Brizuela L.. 2017. Involvement of sphingosine kinase/sphingosine 1-phosphate metabolic pathway in spondyloarthritis. Bone. 103: 150–158. [DOI] [PubMed] [Google Scholar]
- 25.Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T., Sunden Y., Arai Y., Moriwaki K., Ishida J., et al. . 2012. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hisano Y., Kobayashi N., Yamaguchi A., and Nishi T.. 2012. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One. 7: e38941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vu T. M., Ishizu A. N., Foo J. C., Toh X. R., Zhang F., Whee D. M., Torta F., Cazenave-Gassiot A., Matsumura T., Kim S., et al. . 2017. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature. 550: 524–528. [DOI] [PubMed] [Google Scholar]
- 28.Donoviel M. S., Hait N. C., Ramachandran S., Maceyka M., Takabe K., Milstien S., Oravecz T., and Spiegel S.. 2015. Spinster 2, a sphingosine-1-phosphate transporter, plays a critical role in inflammatory and autoimmune diseases. FASEB J. 29: 5018–5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendoza A., Breart B., Ramos-Perez W. D., Pitt L. A., Gobert M., Sunkara M., Lafaille J. J., Morris A. J., and Schwab S. R.. 2012. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Reports. 2: 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang V., Chaluvadi V. S., Ramos-Perez W. D., Mendoza A., Baeyens A., Rivera R., Chun J., Cammer M., and Schwab S. R.. 2017. Gradients of the signaling lipid S1P in lymph nodes position natural killer cells and regulate their interferon-gamma response. Nat. Immunol. 18: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cyster J. G., and Schwab S. R.. 2012. Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30: 69–94. [DOI] [PubMed] [Google Scholar]
- 32.Maceyka M., and Spiegel S.. 2014. Sphingolipid metabolites in inflammatory disease. Nature. 510: 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendoza A., Fang V., Chen C., Serasinghe M., Verma A., Muller J., Chaluvadi V. S., Dustin M. L., Hla T., Elemento O., et al. . 2017. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature. 546: 158–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walzer T., Chiossone L., Chaix J., Calver A., Carozzo C., Garrigue-Antar L., Jacques Y., Baratin M., Tomasello E., and Vivier E.. 2007. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat. Immunol. 8: 1337–1344. [DOI] [PubMed] [Google Scholar]
- 35.Kunkel G. T., Maceyka M., Milstien S., and Spiegel S.. 2013. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 12: 688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pyne N. J., McNaughton M., Boomkamp S., MacRitchie N., Evangelisti C., Martelli A. M., Jiang H. R., Ubhi S., and Pyne S.. 2016. Role of sphingosine 1-phosphate receptors, sphingosine kinases and sphingosine in cancer and inflammation. Adv. Biol. Regul. 60: 151–159. [DOI] [PubMed] [Google Scholar]
- 37.Ogretmen B. 2018. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer. 18: 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bradley E., Dasgupta S., Jiang X., Zhao X., Zhu G., He Q., Dinkins M., Bieberich E., and Wang G.. 2014. Critical role of Spns2, a sphingosine-1-phosphate transporter, in lung cancer cell survival and migration. PLoS One. 9: e110119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu P., Ebenezer D. L., Berdyshev E. V., Bronova I. A., Shaaya M., Harijith A., and Natarajan V.. 2016. Role of sphingosine kinase 1 and S1P transporter Spns2 in HGF-mediated lamellipodia formation in lung endothelium. J. Biol. Chem. 291: 27187–27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adada M. M., Canals D., Jeong N., Kelkar A. D., Hernandez-Corbacho M., Pulkoski-Gross M. J., Donaldson J. C., Hannun Y. A., and Obeid L. M.. 2015. Intracellular sphingosine kinase 2-derived sphingosine-1-phosphate mediates epidermal growth factor-induced ezrin-radixin-moesin phosphorylation and cancer cell invasion. FASEB J. 29: 4654–4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Andrieu G., Ledoux A. Branka S. Bocquet M. Gilhodes J. Walzer T. Kasahara K. Inagaki M. Sabbadini R. A. Cuvillier O., et al. . 2017. Sphingosine 1-phosphate signaling through its receptor S1P5 promotes chromosome segregation and mitotic progression. Sci. Signal. 10: eaah4007. [DOI] [PubMed] [Google Scholar]
- 42.Bouquerel P., Gstalder C., Muller D., Laurent J., Brizuela L., Sabbadini R. A., Malavaud B., Pyronnet S., Martineau Y., Ader I., et al. . 2016. Essential role for SphK1/S1P signaling to regulate hypoxia-inducible factor 2alpha expression and activity in cancer. Oncogenesis. 5: e209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Weyden L., Arends M. J., Campbell A. D., Bald T., Wardle-Jones H., Griggs N., Velasco-Herrera M. D., Tuting T., Sansom O. J., Karp N. A., et al. . 2017. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature. 541: 233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]