Abstract
Sortilin 1 (Sort1) is a member of the Vps10p domain intracellular trafficking receptor family. Genetic variations of the SORT1 gene are strongly associated with plasma cholesterol levels in humans. Recent studies have linked Sort1 to regulation of cholesterol metabolism in hepatocytes and pro-inflammatory response in macrophages, but the tissue-specific roles of Sort1 in lipid metabolism have not been well defined. We developed Sort1 floxed mice and investigated the development of Western diet (WD)-induced steatosis, hepatic inflammatory response, and hyperlipidemia in hepatocyte Sort1 KO mice and myeloid cell Sort1 KO mice. Our findings suggest that hepatocyte Sort1 deficiency attenuated diet-induced hepatic steatosis and hypercholesterolemia in mice. In contrast, myeloid Sort1 deficiency did not reduce hepatic cytokine expression or plasma cholesterol levels, but exacerbated hepatic triglyceride accumulation in WD-fed mice. Finally, we showed that treating WD-fed mice with an orally bioavailable Sort1 inhibitor, AF38469, decreased plasma cholesterol and hepatic cytokine expression. AF38469 treatment did not affect diet-induced obesity or insulin resistance, but was associated with reduced hepatic VLDL secretion and higher hepatic cholesterol 7α-hydrolase expression in WD-fed mice. In conclusion, findings from this study suggest that Sort1 loss-of-function in hepatocytes contributes to lower plasma cholesterol, and pharmacological inhibition of Sort1 attenuates diet-induced hypercholesterolemia in mice.
Keywords: liver metabolism, nonalcoholic fatty liver disease, dyslipidemias, inflammation, bile acid metabolism
Obesity, diabetes, and nonalcoholic steatohepatitis (NASH) are closely associated with significantly higher risk of cardiovascular disease, which remains the leading cause of death among the general population and patients with metabolic syndromes (1). Hypercholesterolemia is a well-recognized contributor to the pathogenesis and progression of atherosclerosis (2). Liver plays a major role in regulating whole-body cholesterol metabolism, and disrupted intrahepatic cholesterol homeostasis is an important underlying cause of hypercholesterolemia. Emerging evidence also suggests that excessive cholesterol accumulates in NASH livers (3, 4) and causes mitochondrial dysfunction and oxidative stress, which sensitize hepatocytes to cytokine-induced cell death (5–8). In addition, cholesterol-laden foamy Kupffer cells were found at an early stage of NASH and showed pro-inflammatory phenotypes (9–11), suggesting that hepatic cholesterol accumulation triggers macrophage activation in a process resembling atherosclerotic plaque formation. Hepatic inflammation can further promote systemic low grade inflammation, insulin resistance, and atherosclerosis progression (1, 2). Better understanding of the complex regulation of hepatic cholesterol homeostasis is important in the prevention and treatment of liver-related and cardiovascular-related complications in patients with metabolic disorders.
Sortilin 1 (Sort1) is a transmembrane trafficking receptor that belongs to the Vps10p domain family (12). In a trafficking vesicle, the luminal domain of Sort1 binds its protein ligand, while the intracellular domain interacts with the trafficking adaptor protein complex. In many cell types, Sort1 is mainly localized to the trans-Golgi network and mediates the late endocytic compartment targeting or exocytosis. In addition, a small amount of Sort1 is located on the plasma membrane where Sort1 mediates endocytosis or cell signaling. Sort1 is universally expressed, with relatively high expression levels found in the central nervous system and adipose. Sort1 has been reported to regulate neuronal function and death and is implicated in neuron degenerative diseases (13). Sort1 has also been identified as a key component of the GLUT4 storage vesicles and may play a role in regulating insulin-stimulated glucose uptake in adipose and muscle (14–16). A number of genome-wide association studies revealed that genetic variations of the SORT1 gene were strongly associated with plasma LDL cholesterol levels in large human populations (17, 18), which has led to further inquiry of the role and mechanisms of Sort1 in regulating cholesterol metabolism in experimental models. A few studies have reported that global Sort1 KO mice under dietary or genetic hyperlipidemic conditions had lower plasma cholesterol levels (19–21), and hepatic Sort1 interacted with and regulated the cellular trafficking, secretion, or degradation of ApoB100 (19, 22), proprotein convertase subtilisin/kexin type 9 (PCSK9) (23, 24), and liver carboxylesterase 1 (21). Furthermore, Sort1 has been shown to mediate macrophage foam cell formation and cytokine production (25, 26) and smooth muscle cell-mediated vascular calcification (27), and Sort1 loss-of-function in these cell types may attenuate atherosclerosis progression independent of plasma cholesterol levels.
Given the complex pathophysiological roles of Sort1 in metabolic regulation (28, 29), studies examining the effects of tissue-specific Sort1 loss-of-function on metabolic homeostasis using conditional Sort1 KO models are needed but currently lacking. To address this knowledge gap, we developed Sort1 floxed mice and investigated the development of Western diet (WD)-induced steatosis, hepatic inflammatory response, and hyperlipidemia in the liver-specific Sort1 KO mice (L-Sort1 KO) and myeloid cell Sort1 KO mice (LysM-Sort1 KO). Our findings suggest that hepatocyte Sort1 deficiency attenuated diet-induced weight gain, hepatic triglyceride (TG) accumulation, and hypercholesterolemia in mice. In contrast, myeloid Sort1 deficiency did not reduce hepatic cytokine expression or plasma cholesterol levels, but increased hepatic TG accumulation. Finally, we showed that treating mice with an orally bioavailable Sort1 inhibitor decreased plasma cholesterol levels in WD-fed mice, which provided proof-of-concept evidence that pharmacological targeting of Sort1 may be a potential strategy to treat dyslipidemia.
MATERIALS AND METHODS
Reagents
Anti-Sort1 rabbit IgG (ab16640) was purchased from Abcam (Cambridge, MA). Actin antibody and tyloxapol were purchased from Sigma-Aldrich (St. Louis, MO). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) assay kits, a total cholesterol assay kit, and a TG assay kit were purchased from Pointe Scientific (Canton, MI). A bile acid assay kit was purchased from Diazyme Laboratories (Poway, CA). A mouse insulin ELISA kit was purchased from Thermo Fisher Scientific (Waltham, MA). The Sort1 inhibitor, AF38469, was synthesized by Artis Pharmaceutical International Ltd. (Shanghai, China).
Mice
Sort1 floxed mice on a C57BL/6N background were developed by Cyagen Biosciences (Santa Clara, CA). The targeting strategy is illustrated in Fig. 1A. The NeoR cassette was removed by crossing Sort1 floxed founders with the FLP deleter strain on a C57BL/6J background (stock #009086; Jackson Laboratory, Bar Harbor, ME). Cre-mediated recombination results in the deletion of exon 2 and exon 3 and subsequent frameshift of the Sort1 gene. L-Sort1 KO mice were generated by crossing Sort1 floxed mice with the albumin-cre deleter strain on a C57BL/6J background (stock #003574; Jackson Laboratory). LysM-Sort1 KO mice were generated by crossing Sort1 floxed mice with the LysM-cre deleter strain on a C57BL/6NJ mixed background (stock #004781; Jackson Laboratory). Littermates without the cre transgene were used as WT controls. Mice were housed in micro-isolator cages with corn cob bedding under a normal light-dark cycle. WT C57BL/6J mice were purchased from Jackson Laboratory. The standard chow diet was PicoLab Rodent Diet 20 (LabDiet, St. Louis, MO) containing 13% fat calories and no added cholesterol. WD (TD.88137) contained 42% fat calories and 0.2% cholesterol (Envigo, Denver, CO). Male C57BL/6J mice (Jackson Laboratory) were used for the AF38469 study. AF38469 was mixed with powdered WD and the estimated daily dose of ∼4 mg/kg was calculated based on daily food intake of ∼4 g per mouse (30). The control group was given powdered WD. Powdered WD was placed in a dish inside the cage and replaced every 2 days. Only male mice were used for this study. All mice were fasted overnight from 5:00 PM to 9:00 AM and euthanized. All animal protocols were approved by the Institutional Animal Care and Use Committee.
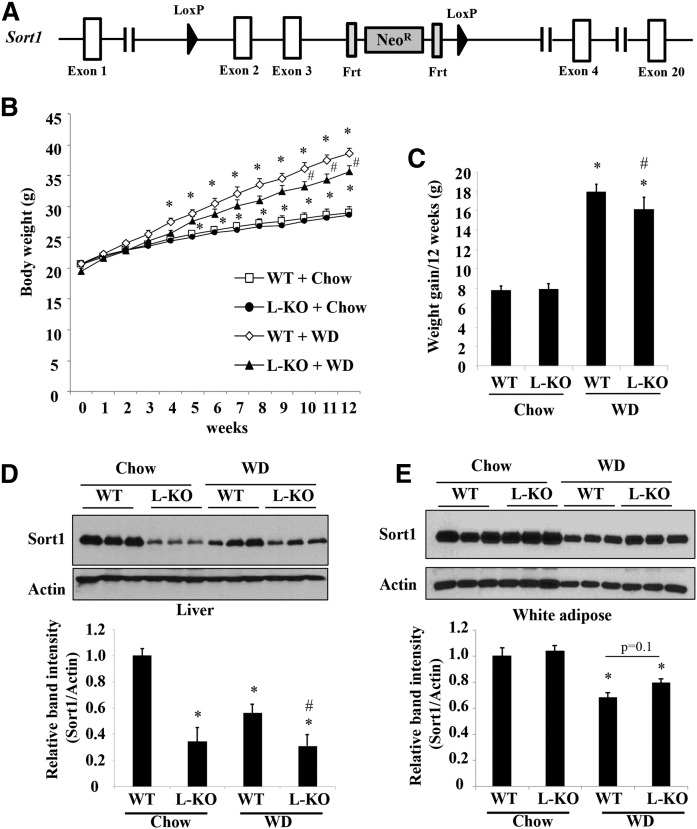
Fig. 1.
L-Sort1 KO mice fed a WD showed reduced weight gain. A: Illustration of conditional Sort1 KO strategy. B. Male 6-week-old L-Sort1 KO (L-KO) mice and WT mice were fed chow or WD for 12 weeks. Body weight was measured weekly (n = 10–12). C: Weight gain over the 12 week feeding period (n = 10–12). D, E: Representative Western blotting of Sort1 protein in liver and white adipose tissue. Relative Sort1 band intensity after normalization to actin band intensity (n = 10). Results wereare expressed as mean ± SEM. *P < 0.05 versus WT + chow. #P < 0.05 versus WT + WD.
Intraperitoneal macrophage isolation
Peritoneal macrophages were harvested 3 days after intraperitoneal injection of 500 μl of 3% thioglycollate. After euthanasia, 10 ml of ice-cold PBS were injected and PBS containing macrophages was withdrawn with a needle. Cells were plated in RPMI medium with 10% FBS, 1% penicillin/streptomycin, and 55 μM of β-mercaptoethanol. Two hours later, floating cells were removed by washing with PBS three times, and attached macrophages were lysed in RIPA buffer for Western blotting.
Glucose tolerance test
After 7 weeks of WD feeding, control and AF38469-treated mice were fasted overnight and received a single intraperitoneal injection of glucose at 2 g/kg body weight. A drop of blood was collected from a tail nick at the indicated time, and glucose was measured with a OneTouch Ultra glucometer. These mice were continued on WD or WD supplemented with AF38469 for 1 week and euthanized for tissue collection.
TG, cholesterol, and bile acid analysis
Liver lipids were extracted in chloroform:methanol (2:1; v:v), dried under nitrogen, and resuspended in isopropanol containing 1% Triton X-100. Cholesterol and TG were measured with a cholesterol assay kit (catalog #C7510) and a TG assay kit (catalog #T7532) from Pointe Scientific following the manufacturer’s instructions. Bile acids were extracted in 90% ethanol as described previously (31). Total bile acid was measured with a bile acid assay kit (catalog #DZ042A; Sekisui Diagnostics, LLC, Lexington, MA). The bile acid pool is the sum of the amount of total bile acid in liver, gallbladder, and small intestine with its content.
Measurement of VLDL secretion
Male C57BL/6J mice, at 12 weeks of age, were given powdered WD or powdered WD supplemented with AF38469 to provide an estimated daily dose of ∼4 mg/kg. After 2 weeks, mice were fasted for 6 h (9:00 AM to 3:00 PM). Tyloxapol was diluted in sterile PBS and administered to mice via tail vein injection as a single dose of 300 mg/kg. Blood was collected by tail nicking at 0 h (right before injection) and at 1.5 and 3 h post injection for TG measurement.
Western blotting
Liver lysates were prepared by placing liver homogenates in RIPA buffer containing 1% SDS and protease inhibitors on ice for 1 h followed by brief sonication. After centrifugation, supernatant was used for SDS-PAGE and immunoblotting. Densitometry was performed with ImageJ software (National Institutes of Health; https://imagej.nih.gov/ij/). Sort1 band intensity was normalized to actin band intensity and expressed as relative Sort1 band intensity.
Real-time PCR
Total RNA was purified by Trizol (Sigma-Aldrich). Reverse transcription was performed with Oligo dT primer and SuperScript III reverse transcriptase (Thermo Fisher Scientific). Real-time PCR was performed with iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Amplification of housekeeping gene 18S was used for normalization. Relative mRNA expression was calculated using the comparative CT (Ct) method and expressed as 2−ΔΔCt with the control group arbitrarily set as “1”.
Statistical analysis
Results are expressed as mean ± SEM. Statistical analysis was performed using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA). Bartlett’s test was used to determine equal variance. For equal variance, two-way ANOVA and Bonferroni’s multiple comparison post hoc test were used to calculate the P value. For unequal variance, nonpaired t-test was used to calculate the P value. P < 0.05 was considered statistically significant.
RESULTS
L-Sort1 KO mice showed reduced hepatic TG accumulation and plasma cholesterol level after WD feeding
To investigate the role of hepatocyte Sort1 deficiency in the development of fatty liver and hyperlipidemia, we fed L-Sort1 KO mice and WT littermates either chow or WD for 12 weeks. When fed a chow diet, L-Sort1 KO mice showed similar weight gain as WT controls (Fig. 1B). When fed a WD, L-Sort1 KO mice showed slower weight gain and their body weight became modestly but significantly lower than WT mice in the last 3 weeks of the 12 week feeding period (Fig. 1B, C). After tissue collection, we confirmed that liver Sort1 protein was markedly lower in L-Sort1 KO mice than WT mice, suggesting a major hepatocyte origin of liver Sort1 protein (Fig. 1D). The residual Sort1 expression detected by Western blotting likely represented nonparenchymal Sort1 in the liver (Fig. 1D). After 12 weeks of WD feeding, liver Sort1 expression was significantly reduced in WT mice, which was consistent with downregulation of hepatocyte Sort1 in fatty livers, as we reported previously (32, 33). Liver Sort1 protein was not lower in WD-fed L-Sort1 KO mice than in chow-fed L-Sort1 KO mice (Fig. 1D), suggesting that nonparenchymal Sort1 was not subjected to WD-induced downregulation. We also confirmed that adipose Sort1 expression was similar between WT and L-Sort1 KO mice and was significantly reduced upon WD feeding as previously reported (Fig. 1E) (34).
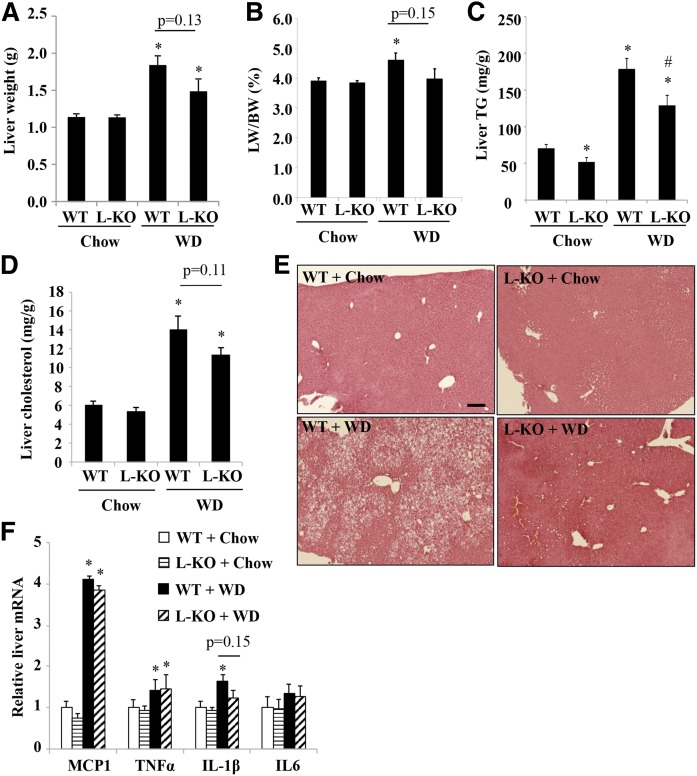
Analysis of the development of diet-induced fatty liver showed that L-Sort1 KO and WT mice fed chow showed similar liver weight; but after WD feeding, L-Sort1 KO mice tended to have reduced liver weight and liver to body weight ratio than WD-fed WT mice, although this difference was not statistically significant (Fig. 2A, B). Interestingly, L-Sort1 KO mice showed ∼25% lower hepatic TG than WT mice under both basal chow condition and WD-fed condition (Fig. 2C). Hepatic cholesterol levels were similar between chow-fed WT and L-Sort1 KO mice, and tended to be lower (P = 0.11) in WD-fed L-Sort1 KO mice than in WD-fed WT mice (Fig. 2D). H&E staining further supported reduced hepatic steatosis in WD-fed L-Sort1 KO mice (Fig. 2E). Analysis of hepatic pro-inflammatory gene expression showed that hepatic monocyte chemoattractant protein 1 (MCP1), TNFα, and interleukin (IL)-1β were induced by WD feeding to a similar extent in WT and L-Sort1 KO mice (Fig. 2F), suggesting that hepatocyte Sort1 deficiency did not have a major impact on WD-induced hepatic pro-inflammatory response.
Fig. 2.
WD-fed L-Sort1 KO mice showed reduced hepatic steatosis. Male 6-week-old L-Sort1 KO (L-KO) mice and WT mice were fed chow or WD for 12 weeks. A, B: Liver weight and liver weight/body weight ratio (LW/BW). C: Liver TG content. D:. Liver cholesterol content. E: Liver H&E staining (scale bar, 100 μm). F: Hepatic mRNA expression. Results are expressed as mean ± SEM (n = 10–12). *P < 0.05 versus WT + chow. #P < 0.05 versus WT + WD.
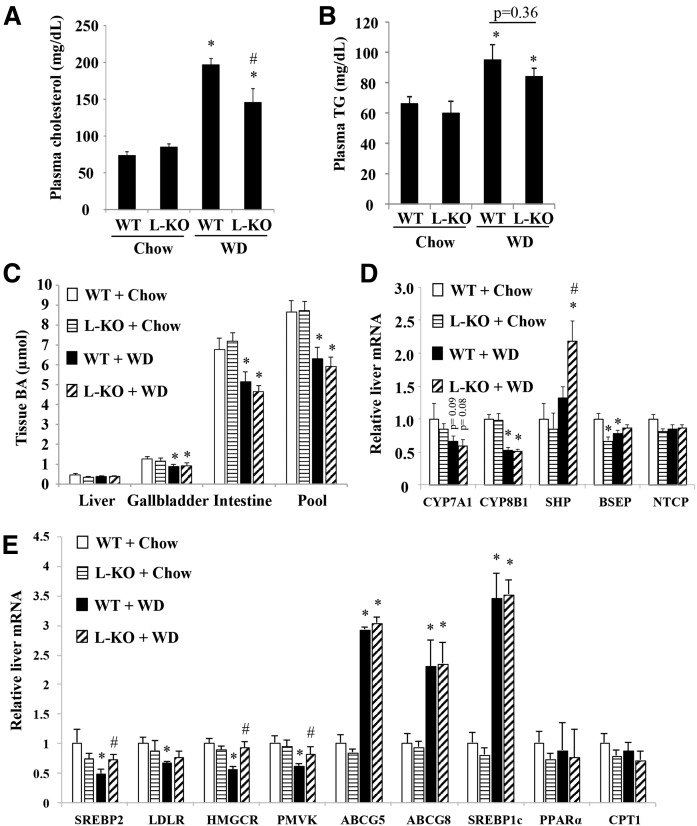
Analysis of plasma lipid levels revealed that L-Sort1 KO mice had ∼22% lower plasma cholesterol than WT mice after WD feeding (Fig. 3A), while plasma TG levels were similar between WT and L-Sort1 KO mice under chow or WD feeding (Fig. 3B). Given the previously reported link between Sort1 and bile acid metabolism (21, 31), we measured tissue bile acid content and total bile acid pool in these mice. We found that WD feeding reduced gallbladder and intestine bile acid content and total bile acid pool in both WT and L-Sort1 KO mice, but WT and L-Sort1 KO mice showed similar tissue bile acid content and bile acid pool under chow and WD conditions (Fig. 3C). Hepatic mRNA expression analysis showed that cholesterol 7α-hydroxylase (CYP7A1) and sterol 12α-hydroxylase (CYP8B1) were similar between WT and L-Sort1 KO mice under either chow or WD condition (Fig. 3D). CYP7A1 and CYP8B1 mRNA expression trended lower in WD-fed mice (Fig. 3D), which positively correlated with a smaller bile acid pool (Fig. 3C) and negatively correlated with higher hepatic cytokine expression (Fig. 2F) (35–37). Other genotype-dependent gene expression changes included higher SHP mRNA in WD-fed L-Sort1 KO mice and slightly lower BSEP mRNA in chow-fed L-Sort1 KO mice, but the underlying causes remain to be determined (Fig. 3D). Analysis of hepatic lipid metabolism genes revealed that SREBP2 and its target genes, LDLR, HMG-CoA reductase (HMGCR), and phosphomevalonate kinase, were lower in WD-fed WT mice than in chow-fed WT mice, which was presumably a result of higher hepatic cholesterol accumulation that inhibited these SREBP2 target genes (Fig. 3E). WD-fed L-Sort1 KO mice showed higher hepatic SREBP2, HMGCR, and phosphomevalonate kinase than WD-fed WT mice (Fig. 3D), which may be ascribed to relatively lower hepatic cholesterol content in these mice (Fig. 2D). Hepatic ABCG5, ABCG8, and SREBP1C were all induced by WD feeding, but were similar between WT and L-Sort1 KO mice under chow or WD conditions (Fig. 3E). The hepatic fatty acid oxidation genes, PPARα and carnitine palmitoyltransferase 1 (CPT1), were not significantly different between WT and L-Sort1 KO mice under chow or WD conditions (Fig. 3E). Consistent with the function of Sort1 as an intracellular trafficking receptor, these results suggest that liver Sort1 loss-of-function in mice likely lowered plasma cholesterol via mechanisms independent of transcriptional changes of major hepatic lipid metabolic genes.
Fig. 3.
WD-fed L-Sort1 KO mice showed lowered plasma cholesterol and hepatic bile acid levels. Male 6-week-old L-Sort1 KO (L-KO) mice and WT mice were fed chow or WD for 12 weeks. A: Plasma cholesterol. B: Plasma TG. C: Tissue bile acid (BA) content and total bile acid pool size. D: Hepatic mRNA expression. Results are expressed as mean ± SEM (n = 10–12). *P < 0.05 versus WT + chow. #P < 0.05 versus WT + WD.
Macrophage Sort1 KO did not attenuate WD-induced liver cytokine expression, but worsened WD-induced hepatic steatosis in mice
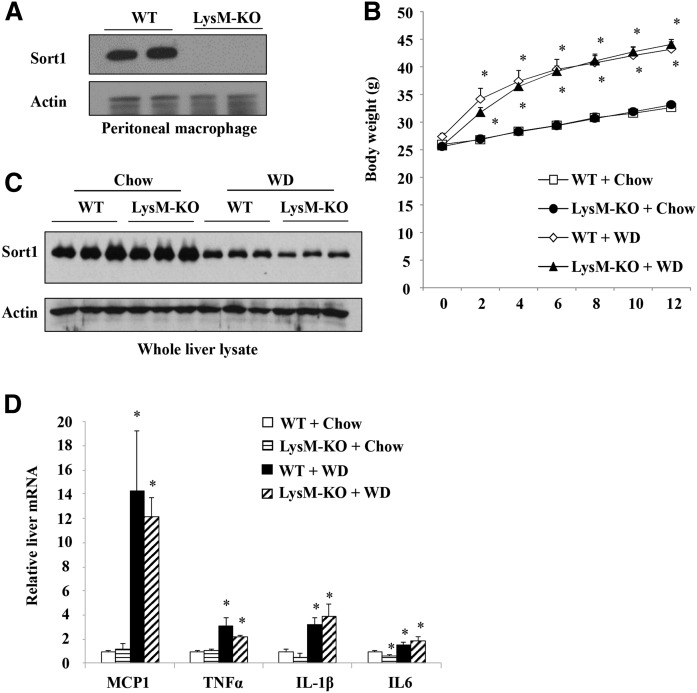
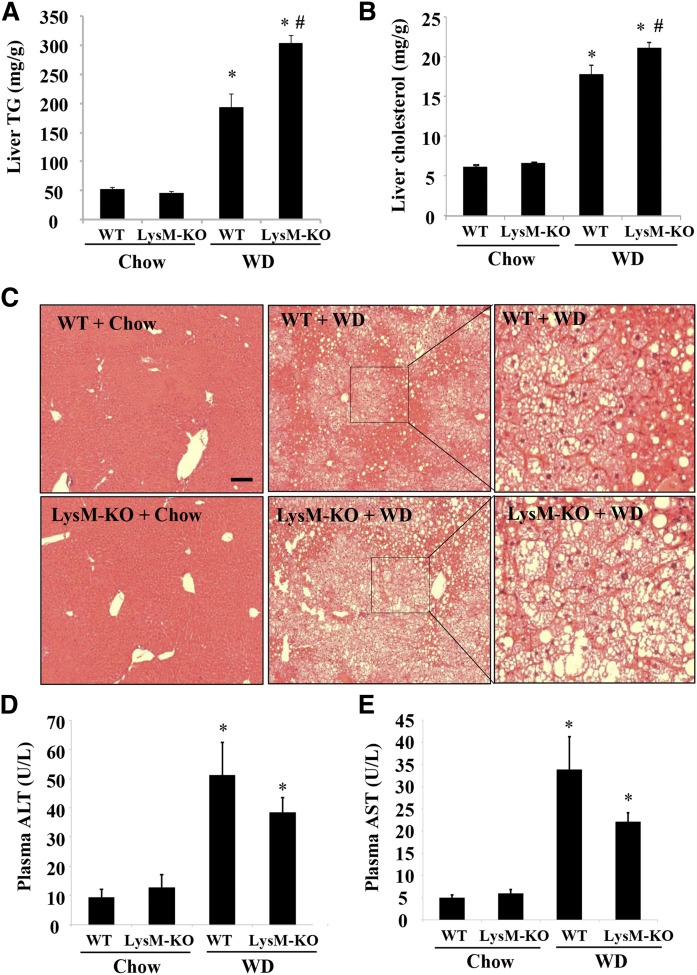
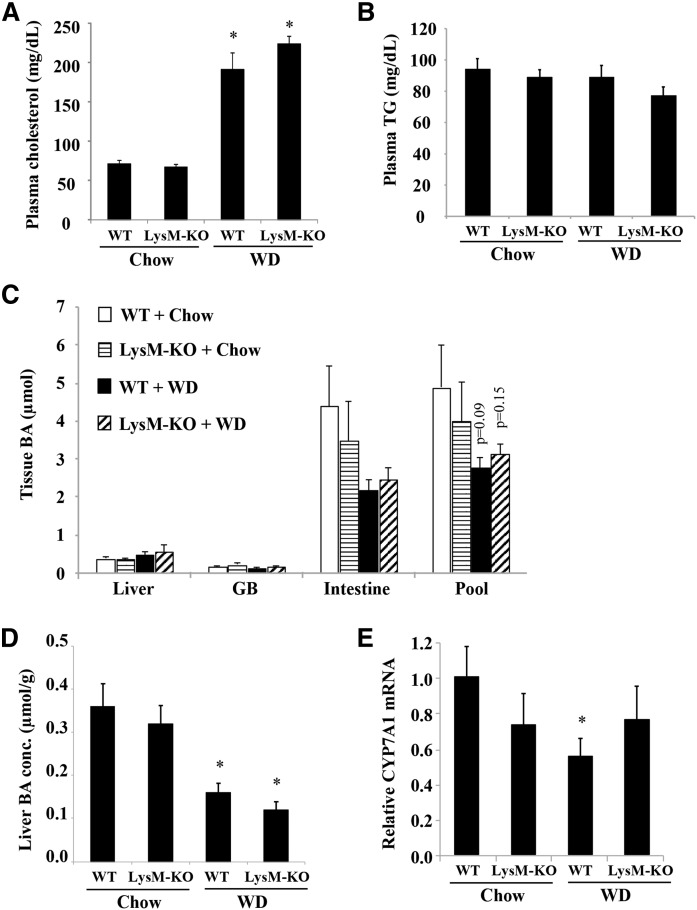
Recent studies have reported that Sort1 deficiency in macrophages attenuated macrophage activation and atherosclerosis in mice (25, 26). Because macrophages play important roles in hepatic inflammatory response during the development of fatty liver disease (9–11), we next examined the effects of myeloid Sort1 deficiency on WD-induced hepatic pro-inflammatory response and lipid metabolism in LysM-Sort1 KO mice. We first confirmed that peritoneal macrophages isolated from LysM-Sort1 KO mice lacked Sort1 expression (Fig. 4A). LysM-Sort1 KO mice showed similar weight gain compared with WT mice under chow or 12 week WD feeding conditions (Fig. 4B). KO of macrophage Sort1 did not affect total liver Sort1 protein abundance, and WD feeding significantly reduced total liver Sort1 in both WT and LysM-Sort1 KO mice, which was presumably a result of hepatocyte Sort1 downregulation (Fig. 4C). Under the chow condition, hepatic MCP1, TNFα, and IL-1β were similar in WT and LysM-Sort1 KO mice, while IL-6 mRNA was significantly lower in LysM-Sort1 KO mice than in WT mice (Fig. 4D). WD feeding increased hepatic chemokine MCP1 mRNA and inflammatory cytokines, TNFα, IL-1β, and IL-6, to comparable levels in WT and LysM-Sort1 KO mice (Fig. 4D). Unexpectedly, LysM-Sort1 KO mice showed ∼50% higher hepatic TG accumulation than WT mice upon WD feeding (Fig. 5A). Hepatic total cholesterol was significantly higher in LysM-Sort1 KO mice than in WT mice under WD condition (Fig. 5B). Worsened hepatic steatosis in WD-fed LysM-Sort1 KO mice was also histologically evident in H&E staining (Fig. 5C). Plasma AST and ALT levels were not significantly different between WT and LysM-Sort1 KO mice under either chow or WD condition (Fig. 5D, E). Plasma cholesterol and TG levels were similar in WT and LysM-Sort1 KO mice under chow and WD conditions (Fig. 6A, B). Bile acid pool size, hepatic bile acid concentration, and CYP7A1 mRNA were not significantly different between WT and LysM-Sort1 KO mice under chow or WD conditions (Fig. 6C–E). In summary, these results suggest that deletion of Sort1 in myeloid cells does not attenuate hepatic pro-inflammatory response but promotes hepatic TG and cholesterol accumulation in WD-fed mice. In addition, myeloid Sort1 KO did not appear to alter hepatic bile acid concentration or bile acid pool size.
Fig. 4.
WD-fed LysM-Sort1 KO mice did not show reduced hepatic pro-inflammatory marker gene expression. A: Western blotting of Sort1 protein in isolated intraperitoneal macrophages from WT and LysM-Sort1 KO (LysM-KO) mice. B: Body weight of male 12-week-old LysM-KO mice and WT mice that were fed chow or WD for 12 weeks. C: Western blotting of Sort1 in total liver lysate. D: Hepatic mRNA expression. Results are expressed as mean ± SEM (n = 5–10). *P < 0.05 versus WT + chow. #P < 0.05 versus WT + WD.
Fig. 5.
WD-fed LysM-Sort1 KO mice showed worsened hepatic TG accumulation. Male 12-week-old LysM-Sort1 KO (LysM-KO) mice and WT mice were fed chow or WD for 12 weeks. A: Liver TG content. B: Liver total cholesterol content. C: Liver H&E staining (scale bar, 100 μm). D, E: Plasma ALT and AST concentrations. Results are expressed as mean ± SEM n = 5–10). *P < 0.05 versus WT + chow. #P < 0.05 versus WT + WD.
Fig. 6.
LysM-Sort1 KO mice did not show altered plasma cholesterol or bile acid pool. Male 12-week-old LysM-Sort1 KO (LysM-KO) mice and WT mice were fed chow or WD for 12 weeks. A, B: Plasma cholesterol and TG concentration. C: Total organ bile acid (BA) content and bile acid pool. D: Liver bile acid concentration. The P values shown are comparisons to the WT + Chow group. E: Relative liver CYP7A1 mRNA expression. Results are expressed as mean ± SEM (n = 5–10). *P < 0.05 versus WT + chow.
Sort1 inhibitor reduced plasma cholesterol levels, hepatic VLDL secretion, and hepatic pro-inflammatory cytokines in WD-fed mice
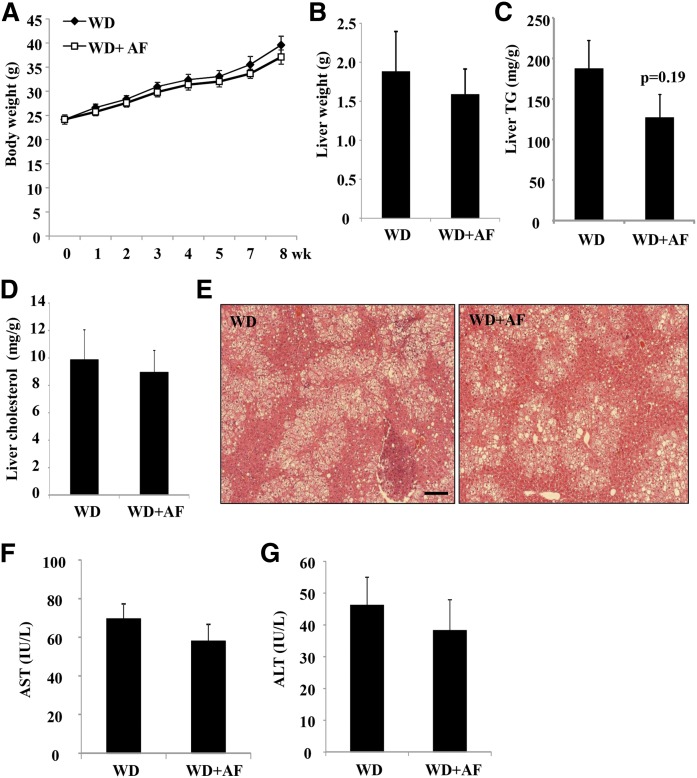
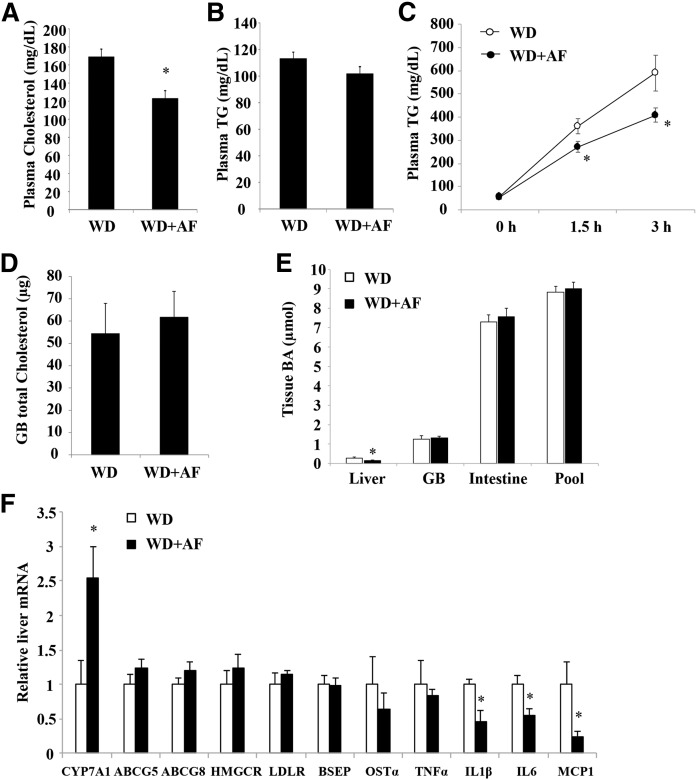
Recently, an orally bioavailable Sort1 inhibitor, AF38469, with an IC50 value of ∼330 nM has been identified (38). To determine whether pharmacological inhibition of Sort1 with AF38469 might improve lipid homeostasis and inflammation, we fed mice WD supplemented with AF38469 to achieve an estimated 4 mg/kg daily dose, which was in line with the published pharmacokinetic data (38). AF38469 treatment did not affect the body weight gain or liver weight over a period of 8 weeks (Fig. 7A, B). Hepatic TG and cholesterol levels were not significantly different between the treated group and the controls, although we noted that hepatic TG trended ∼30% lower in the AF38469-treated mice (P = 0.19) (Fig. 7C–E). Plasma AST and ALT levels were similar between the two groups, suggesting that AF38469 treatment for 8 weeks was not associated with hepatotoxicity (Fig. 7F, G). Analysis of plasma lipid parameters revealed that AF38469 significantly decreased plasma cholesterol by ∼30% (Fig. 8A), but did not affect plasma TG levels (Fig. 8B). To gain additional insights about the cholesterol lowering effect of AF38469, we first measured hepatic VLDL secretion because previous genetic studies suggested a role of hepatic Sort1 in regulating hepatic VLDL secretion (19, 22). Interestingly, AF38469-treated mice showed significantly reduced hepatic VLDL secretion (Fig. 8C). Biliary cholesterol secretion and bile acid synthesis are two major cholesterol elimination mechanisms that can impact circulating cholesterol levels. AF38469 treatment did not affect total gallbladder cholesterol content (Fig. 8D). Analysis of bile acid metabolism revealed that AF38469-treated mice showed significantly reduced hepatic bile acid levels (Fig. 8E), while gallbladder bile acid, intestinal bile acids, and the total bile acid pool size were unaltered (Fig. 8E). Analysis of the hepatic gene expression profile found that hepatic CYP7A1 was significantly induced, while other cholesterol metabolism genes (ABCG5, ABCG8, HMGCR, LDLR) were unaltered in AF38469-treated mice (Fig. 8F). Hepatic mRNA expression of chemokine MCP1 and the pro-inflammatory cytokines, IL-1β and IL-6, but not TNFα, was significantly lower in AF38469-treated mice (Fig. 8F). Lower hepatic cytokines may provide a possible explanation for higher CYP7A1 mRNA expression in AF38469-treated mice.
Fig. 7.
AF38469 treatment did not affect WD-induced weight gain or hepatic steatosis in mice. Male 12-week-old C57BL/6J mice were fed either WD or WD supplemented with AF38469 (AF) for 8 weeks. A: Body weight. B: Liver weight. C: Liver TG content. D. Liver cholesterol content. E: H&E staining (scale bar, 100 μm). F, G: Plasma AST and ALT. Results are expressed as mean ± SEM (n = 5).
Fig. 8.
AF38469 treatment decreased plasma cholesterol and hepatic VLDL secretion in WD-fed mice. A, B, D–F: Male 12-week-old C57BL/6J mice were fed either WD or WD supplemented with AF38469 (AF) for 8 weeks. Plasma cholesterol (A), TG (B), gallbladder (GB) total cholesterol content (D), tissue total bile acid content and bile acid pool (E), and hepatic mRNA expression (E) are shown. C: Male 12-week-old C57BL/6J mice were fed either WD or WD supplemented with AF38469 (AF) for 2 weeks. A VLDL secretion assay was performed as described in the Materials and Methods. Results are expressed as mean ± SEM (n = 5). *P < 0.05 versus WD-fed mice.
Sort1 inhibitor did not affect fasting plasma glucose and glucose tolerance in WD-fed mice
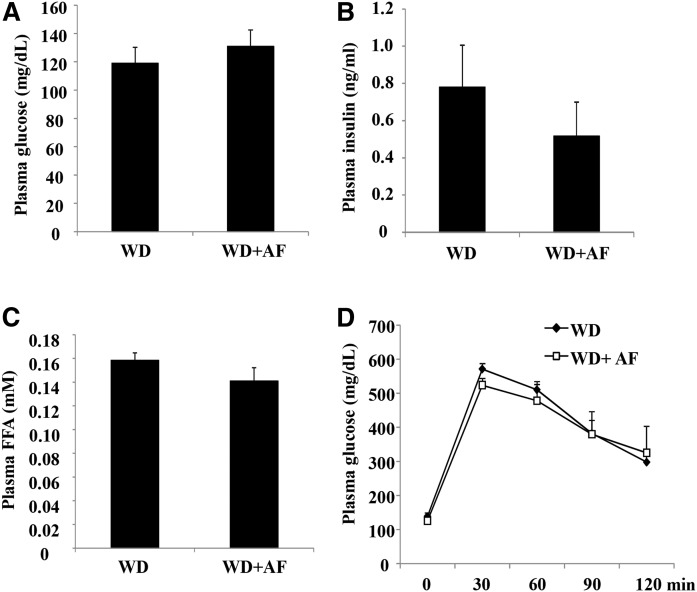
To gain a more comprehensive understanding of the metabolic effects of pharmacological Sort1 inhibition, we next evaluated the effect of AF38469 on insulin sensitivity in WD-fed mice. AF38469 treatment did not affect fasting plasma glucose or fasting insulin concentration (Fig. 9A, B). In addition, AF38469 treatment did not affect fasting plasma free fatty acid concentration (Fig. 9C) or glucose tolerance in a glucose tolerance test (Fig. 9D). These results suggest that AF38469 did not affect insulin sensitivity in WD-fed mice.
Fig. 9.
AF38469 treatment did not affect fasting glucose or glucose tolerance in WD-fed mice. Male 12-week-old C57BL/6J mice were fed either WD or WD supplemented with AF38469 (AF) for 8 weeks. A: Fasting plasma glucose levels. B: Fasting plasma insulin levels. C: Plasma FFA levels. D: Glucose tolerance test. Results are expressed as mean ± SEM (n = 5). *P < 0.05 versus WD-fed mice.
DISCUSSION
In this study, we employed novel tissue-specific Sort1 KO mouse models and obtained new insights on the differential effects of tissue-specific Sort1 loss-of-function on WD-induced hepatic steatosis, hepatic pro-inflammatory response, and hyperlipidemia. A major finding is that hepatocyte Sort1 deletion was sufficient to lower plasma cholesterol in WD-fed mice. The magnitude of plasma cholesterol reduction in WD-fed L-Sort1 KO mice was in line with ∼20–30% plasma cholesterol reduction in WD-fed global Sort1 KO mice and global Sort1/LDLR double KO mice reported previously (19, 20). This implies that hepatocyte Sort1 deficiency may contribute significantly to plasma cholesterol reduction in mice, and genetic variations that alter hepatic Sort1 function may act as a potential link between SORT1 and plasma cholesterol levels in humans. Sort1 has been suggested to modulate several hepatic pathways that may influence hepatic and plasma lipid levels, including ApoB metabolism (19, 22), intracellular cholesterol mobilization (21), and lipoprotein uptake (23, 24). However, the relative contributions of these cellular pathways in mediating the lipid lowering effects of hepatocyte Sort1 deletion are less clear. Our observations in L-Sort1 KO mice were consistent with a previous report that WD-fed global Sort1/LDLR double KO mice showed lower hepatic lipid content and plasma cholesterol than LDLR KO mice (19), suggesting that LDLR-mediated lipoprotein uptake was not likely required for lipid reduction in Sort1 KO mice (23, 24). We found that hepatocyte Sort1 deletion reduced hepatic TG content, which may contribute to lower plasma lipids. Notably, the baseline hepatic TG content was already ∼25% lower in L-Sort1 KO mice than in WT mice, although the two groups showed similar body weight under chow condition. Upon WD feeding, hepatocyte Sort1 deletion reduced WD-induced weight gain, which may further contribute to reduced hepatic steatosis under WD-fed conditions. A limitation of this study was that food intake, fat absorption, and energy expenditure were not determined during the feeding study. The mechanisms by which liver Sort1 KO attenuates WD-induced weight gain remain to be investigated in future studies.
Another major finding of this study is that Sort1 inhibitor exhibited a hypocholesterolemic effect in WD-fed mice. Several studies have reported improved lipid homeostasis in global Sort1 KO mice (19–21). However, whether Sort1 can serve as a potential therapeutic target has not been substantiated experimentally. This study provided proof-of-concept evidence that pharmacological inhibition of Sort1 decreased plasma cholesterol in WD-fed mice. Previous characterization studies have shown that AF38469 is a potent, selective, and orally bioavailable Sort1 inhibitor (38). Given that hepatocyte Sort1 deletion lowered plasma cholesterol in WD-fed mice, it may be assumed that the hypocholesterolemic effect of AF38469 treatment may at least be partially mediated by hepatic Sort1 inhibition. In this regard, we found that AF38469 treatment significantly decreased hepatic VLDL secretion. The hypocholesterolemic effect of AF38469 was independent of changes in diet-induced obesity in WD-fed mice. In addition, AF38469 treatment did not seem to have a major impact on insulin resistance, which is a characteristic feature of metabolic diseases and a major underlying cause of dyslipidemia. Analysis of hepatic gene expression showed that the only altered gene related to cholesterol metabolism was CYP7A1, suggesting that increased bile acid synthesis may possibly contribute partly to lower plasma cholesterol in AF38469-treated mice. This effect also resembled previous findings of higher CYP7A1 expression in high cholesterol diet-fed global Sort1 KO mice on either WT or LDLR KO background (19, 21). AF38469-treated mice showed ∼50% lower hepatic bile acids without significantly altered total bile acid pool. In addition, hepatic pro-inflammatory cytokines, IL-1β, IL-6, and MCP1, but not TNFα, were lower in AF38469-treated mice. Pro-inflammatory cytokines are strong negative regulators of CYP7A1 expression (35–37). WD-induced hepatic cytokines also correlated with lower CYP7A1 mRNA in mice. Higher CYP7A1 mRNA in AF38469-treated WD-fed mice may be due to attenuated repression by cytokines and bile acids. Interestingly, our previous study found that global Sort1 KO mice also showed significantly attenuated intrahepatic bile acid accumulation upon bile duct ligation (31), which serves as additional evidence suggesting an underlying link between Sort1 inhibition and hepatic bile acid concentration.
The identification of Sort1 regulation of macrophage function warrants further study to determine whether macrophage Sort1 deficiency attenuates hepatic pro-inflammatory response in diet-induced fatty liver disease (25, 26, 39). Findings from our studies conducted in LysM-Sort1 KO mice showed that, although chronic WD feeding elicited marked elevation of hepatic MCP1 and corresponding induction of pro-inflammatory cytokines, deletion of macrophage Sort1 did not attenuate hepatic inflammatory markers induced by WD feeding. To our surprise, LysM-Sort1 KO mice accumulated significantly more TG and cholesterol after WD feeding, which was independent of changes in WD-induced weight gain. Therefore, in contrast to hepatocyte Sort1 deficiency, myeloid Sort1 deficiency did not seem to provide beneficial effects in maintaining lipid homeostasis and reducing pro-inflammatory response in WD-fed mice. Macrophages play important roles in the pathogenesis of fatty liver disease (40). How macrophage Sort1 deficiency may alter macrophage function to promote hepatocyte lipid accumulation upon WD feeding requires further investigation and is out of the scope of the current study. It should also be noted that, due to technical challenges in generating macrophage-specific KO mice, the LysM-Sort1 KO model used to study macrophage Sort1 deletion also lacks Sort1 in other types of myeloid cells, such as neutrophils, and the functional role of Sort1 in other types of myeloid cells is currently unclear. Two independent studies have consistently reported that transplantation of Sort1-deficient macrophages to LDLR KO or ApoE KO recipient mice attenuated atherosclerosis development (25, 26). Whether LysM-Sort1 KO mice on an atherogenic-prone genetic background may be protected against atherosclerosis remains to be further tested.
It is worth mentioning that certain phenotypic changes observed in AF38469-treated mice were not observed in tissue-specific Sort1 KO mice, and vice versa. Notably, L-Sort1 KO mice showed modestly lower weight gain upon WD challenge, while AF38469 treatment lowered plasma cholesterol, but did not affect WD-induced weight gain. In addition, AF38469 treatment reduced hepatic cytokine expression and caused higher CYP7A1 expression in WD-fed mice, while neither L-Sort1 KO mice nor LysM-Sort1 KO mice showed lower hepatic cytokine mRNA or higher CYP7A1 expression upon WD feeding. Although further mechanistic studies are clearly required, differences in the model systems used in our study need to be considered in the result interpretation. AF38469 treatment likely caused partial loss of Sort1 function, while genetic KO resulted in complete loss of Sort1 function. Second, AF38469 may also target Sort1 in cells other than hepatocytes and myeloid cells. Finally, congenic loss of Sort1 in genetic KO models may lead to certain phenotypic changes that are not seen in pharmacological inhibition models.
In summary, key findings from this study showed that hepatocyte Sort1 deletion reduced hepatic steatosis and plasma cholesterol levels, while macrophage Sort1 deficiency did not attenuate hepatic pro-inflammatory response, but promoted hepatic steatosis in WD-fed mice. In addition, pharmacological inhibition of Sort1 lowered plasma cholesterol in hyperlipidemic mice, suggesting that Sort1 may be a potential therapeutic target for hyperlipidemia treatment. Additional studies are required to further dissect the tissue-specific mechanisms linking Sort1 function and cholesterol metabolism.
Footnotes
Abbreviations:
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- CYP7A1
- cholesterol 7α-hydroxylase
- HMGCR
- HMG-CoA reductase
- IL
- interleukin
- L-Sort1 KO
- liver-specific Sort1 KO
- LysM-Sort1 KO
- myeloid cell Sort1 KO
- MCP1
- monocyte chemoattractant protein 1
- NASH
- nonalcoholic steatohepatitis
- Sort1
- sortilin 1
- TG
- triglyceride
- WD
- Western diet
This work was supported in part by National Institutes of Health Grant 1R01DK102487-01 (T.L), an American Diabetes Association Junior Faculty Award (T.L.), National Center for Research Resources Grant 5P20RR021940-07, and National Institute of General Medical Sciences Grant 8 P20 GM103549-07. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Anstee Q. M., Targher G., and Day C. P.. 2013. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat. Rev. Gastroenterol. Hepatol. 10: 330–344. [DOI] [PubMed] [Google Scholar]
- 2.Lonardo A., Sookoian S., Chonchol M., Loria P., and Targher G.. 2013. Cardiovascular and systemic risk in nonalcoholic fatty liver disease - atherosclerosis as a major player in the natural course of NAFLD. Curr. Pharm. Des. 19: 5177–5192. [PubMed] [Google Scholar]
- 3.Puri P., Baillie R. A., Wiest M. M., Mirshahi F., Choudhury J., Cheung O., Sargeant C., Contos M. J., and Sanyal A. J.. 2007. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 46: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 4.Caballero F., Fernandez A., De Lacy A. M., Fernandez-Checa J. C., Caballeria J., and Garcia-Ruiz C.. 2009. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J. Hepatol. 50: 789–796. [DOI] [PubMed] [Google Scholar]
- 5.Marí M., Caballero F., Colell A., Morales A., Caballeria J., Fernandez A., Enrich C., Fernandez-Checa J. C., and García-Ruiz C.. 2006. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 4: 185–198. [DOI] [PubMed] [Google Scholar]
- 6.Matsuzawa N., Takamura T., Kurita S., Misu H., Ota T., Ando H., Yokoyama M., Honda M., Zen Y., Nakanuma Y., et al. 2007. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology. 46: 1392–1403. [DOI] [PubMed] [Google Scholar]
- 7.Wouters K., van Gorp P. J., Bieghs V., Gijbels M. J., Duimel H., Lutjohann D., Kerksiek A., van Kruchten R., Maeda N., Staels B., et al. 2008. Dietary cholesterol, rather than liver steatosis, leads to hepatic inflammation in hyperlipidemic mouse models of nonalcoholic steatohepatitis. Hepatology. 48: 474–486. [DOI] [PubMed] [Google Scholar]
- 8.Van Rooyen D. M., Larter C. Z., Haigh W. G., Yeh M. M., Ioannou G., Kuver R., Lee S. P., Teoh N. C., and Farrell G. C.. 2011. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 141: 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leroux A., Ferrere G., Godie V., Cailleux F., Renoud M. L., Gaudin F., Naveau S., Prevot S., Makhzami S., Perlemuter G., et al. 2012. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J. Hepatol. 57: 141–149. [DOI] [PubMed] [Google Scholar]
- 10.Arguello G., Balboa E., Arrese M., and Zanlungo S.. 2015. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta. 1852: 1765–1778. [DOI] [PubMed] [Google Scholar]
- 11.Walenbergh S. M., Koek G. H., Bieghs V., and Shiri-Sverdlov R.. 2013. Non-alcoholic steatohepatitis: the role of oxidized low-density lipoproteins. J. Hepatol. 58: 801–810. [DOI] [PubMed] [Google Scholar]
- 12.Hermey G. 2009. The Vps10p-domain receptor family. Cell. Mol. Life Sci. 66: 2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nykjaer A., and Willnow T. E.. 2012. Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci. 35: 261–270. [DOI] [PubMed] [Google Scholar]
- 14.Lin B. Z., Pilch P. F., and Kandror K. V.. 1997. Sortilin is a major protein component of Glut4-containing vesicles. J. Biol. Chem. 272: 24145–24147. [DOI] [PubMed] [Google Scholar]
- 15.Shi J., and Kandror K. V.. 2005. Sortilin is essential and sufficient for the formation of Glut4 storage vesicles in 3T3–L1 adipocytes. Dev. Cell. 9: 99–108. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya Y., Hatakeyama H., Emoto N., Wagatsuma F., Matsushita S., and Kanzaki M.. 2010. Palmitate-induced down-regulation of sortilin and impaired GLUT4 trafficking in C2C12 myotubes. J. Biol. Chem. 285: 34371–34381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kathiresan S., Melander O., Guiducci C., Surti A., Burtt N. P., Rieder M. J., Cooper G. M., Roos C., Voight B. F., Havulinna A. S., et al. 2008. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat. Genet. 40: 189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Myocardial Infarction Genetics Consortium, Kathiresan S., Voight B. F., Purcell S., Musunuru K., Ardissino D., Mannucci P. M., Anand S., Engert J. C., Samani N. J., et al. 2009. Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 41: 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjolby M., Andersen O. M., Breiderhoff T., Fjorback A. W., Pedersen K. M., Madsen P., Jansen P., Heeren J., Willnow T. E., and Nykjaer A.. 2010. Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab. 12: 213–223. [DOI] [PubMed] [Google Scholar]
- 20.Strong A., Ding Q., Edmondson A. C., Millar J. S., Sachs K. V., Li X., Kumaravel A., Wang M. Y., Ai D., Guo L., et al. 2012. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J. Clin. Invest. 122: 2807–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Wang Y., Matye D. J., Chavan H., Krishnamurthy P., Li F., and Li T.. 2017. Sortilin 1 modulates hepatic cholesterol lipotoxicity in mice via functional interaction with liver carboxylesterase 1. J. Biol. Chem. 292: 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musunuru K., Strong A., Frank-Kamenetsky M., Lee N. E., Ahfeldt T., Sachs K. V., Li X., Li H., Kuperwasser N., Ruda V. M., et al. 2010. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 466: 714–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsen C., Kjolby M., Nyegaard M., Mattheisen M., Lundhede J., Buttenschon H., Mors O., Bentzon J. F., Madsen P., Nykjaer A., et al. 2014. The hypercholesterolemia-risk gene SORT1 facilitates PCSK9 secretion. Cell Metab. 19: 310–318. [DOI] [PubMed] [Google Scholar]
- 24.Butkinaree C., Canuel M., Essalmani R., Poirier S., Benjannet S., Asselin M. C., Roubtsova A., Hamelin J., Marcinkiewicz J., Chamberland A., et al. 2015. Amyloid precursor-like protein 2 and sortilin do not regulate the PCSK9 convertase-mediated low density lipoprotein receptor degradation but interact with each other. J. Biol. Chem. 290: 18609–18620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel K. M., Strong A., Tohyama J., Jin X., Morales C. R., Billheimer J., Millar J., Kruth H., and Rader D. J.. 2015. Macrophage sortilin promotes LDL uptake, foam cell formation, and atherosclerosis. Circ. Res. 116: 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortensen M. B., Kjolby M., Gunnersen S., Larsen J. V., Palmfeldt J., Falk E., Nykjaer A., and Bentzon J. F.. 2014. Targeting sortilin in immune cells reduces proinflammatory cytokines and atherosclerosis. J. Clin. Invest. 124: 5317–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goettsch C., Hutcheson J. D., Aikawa M., Iwata H., Pham T., Nykjaer A., Kjolby M., Rogers M., Michel T., Shibasaki M., et al. 2016. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J. Clin. Invest. 126: 1323–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strong A., Patel K., and Rader D. J.. 2014. Sortilin and lipoprotein metabolism: making sense out of complexity. Curr. Opin. Lipidol. 25: 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goettsch C., Kjolby M., and Aikawa E.. 2018. Sortilin and its multiple roles in cardiovascular and metabolic diseases. Arterioscler. Thromb. Vasc. Biol. 38: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachmanov A. A., Reed D. R., Beauchamp G. K., and Tordoff M. G.. 2002. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 32: 435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J., Woolbright B. L., Zhao W., Wang Y., Matye D., Hagenbuch B., Jaeschke H., and Li T.. 2018. Sortilin 1 loss-of-function protects against cholestatic liver injury by attenuating hepatic bile acid accumulation in bile duct ligated mice. Toxicol. Sci. 161: 34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Matye D. J., and Li T.. 2015. Insulin resistance induces posttranslational hepatic sortilin 1 degradation in mice. J. Biol. Chem. 290: 11526–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bi L., Chiang J. Y., Ding W. X., Dunn W., Roberts B., and Li T.. 2013. Saturated fatty acids activate ERK signaling to downregulate hepatic sortilin 1 in obese and diabetic mice. J. Lipid Res. 54: 2754–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J., Chen C., Li Y., Matye D. J., Wang Y., Ding W. X., and Li T.. 2017. Inhibition of insulin/PI3K/AKT signaling decreases adipose Sortilin 1 in mice and 3T3-L1 adipocytes. Biochim. Biophys. Acta Mol. Basis Dis. 1863: 2924–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feingold K. R., Spady D. K., Pollock A. S., Moser A. H., and Grunfeld C.. 1996. Endotoxin, TNF, and IL-1 decrease cholesterol 7 alpha-hydroxylase mRNA levels and activity. J. Lipid Res. 37: 223–228. [PubMed] [Google Scholar]
- 36.Miyake J. H., Wang S. L., and Davis R. A.. 2000. Bile acid induction of cytokine expression by macrophages correlates with repression of hepatic cholesterol 7alpha-hydroxylase. J. Biol. Chem. 275: 21805–21808. [DOI] [PubMed] [Google Scholar]
- 37.Li T., Jahan A., and Chiang J. Y.. 2006. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology. 43: 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schrøder T. J., Christensen S., Lindberg S., Langgard M., David L., Maltas P. J., Eskildsen J., Jacobsen J., Tagmose L., Simonsen K. B., et al. 2014. The identification of AF38469: an orally bioavailable inhibitor of the VPS10P family sorting receptor Sortilin. Bioorg. Med. Chem. Lett. 24: 177–180. [DOI] [PubMed] [Google Scholar]
- 39.Herda S., Raczkowski F., Mittrucker H. W., Willimsky G., Gerlach K., Kuhl A. A., Breiderhoff T., Willnow T. E., Dorken B., Hopken U. E., et al. 2012. The sorting receptor Sortilin exhibits a dual function in exocytic trafficking of interferon-gamma and granzyme A in T cells. Immunity. 37: 854–866. [DOI] [PubMed] [Google Scholar]
- 40.Peterson K. R., Cottam M. A., Kennedy A. J., and Hasty A. H.. 2018. Macrophage-targeted therapeutics for metabolic disease. Trends Pharmacol. Sci. 39: 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]