Abstract
Studies on the effects of longitudinal lipid trajectories on end-stage renal disease (ESRD) development and deaths among patients with chronic kidney disease (CKD) are limited. We conducted a registry-based prospective study using data from a 13-year multidisciplinary pre-ESRD care program. The final study population comprised 4,647 patients with CKD. Using group-based trajectory modeling, we dichotomized longitudinal trajectories of total cholesterol (T-CHO), triglyceride (TG), LDL cholesterol (LDL-C), and HDL cholesterol (HDL-C). Time to ESRD or death was analyzed using multiple Cox regression. At baseline, higher levels of T-CHO and LDL-C were associated with rapid progression to ESRD, whereas only HDL-C was positively associated with all-cause mortality [adjusted hazard ratio (HR), 1.20; 95% CI, 1.06–1.36; P-value, 0.005]. Compared with those with a normal T-CHO trajectory, the fully adjusted HR of patients with a high T-CHO trajectory for ESRD risk was 1.21 (P-value, 0.019). Subgroup analysis showed that a high TG trajectory was associated with a 49% increase in mortality risk in CKD patients without diabetes (P-value for interaction, 0.012). In contrast to what was observed based on baseline HDL-C, patients with a trajectory of frequent hypo-HDL cholesterolemia had higher risk of all-cause mortality (adjusted HR, 1.53; P-value, 0.014). Thus, only T-CHO, both at baseline and over the longitudinal course, demonstrated a significant potential risk of incident ESRD. The inconsistency in the observed directions of association between baseline levels and longitudinal trajectories of HDL-C warrants further research to unveil specific pathogenic mechanisms underlying the HDL-C metabolism in patients with CKD.
Keywords: lipid profile, longitudinal trajectory, chronic kidney disease, dialysis, mortality, electronic medical records
Dyslipidemia is more prevalent among chronic kidney disease (CKD) patients than among the general population, with a specific serum lipid profile of a stronger tendency toward high triglyceride (TG), high total cholesterol (T-CHO), low HDL cholesterol (HDL-C), and low or unchanged LDL cholesterol (LDL-C) levels (1–3). However, the prognostic role of dyslipidemia in CKD populations remains unclear (4). Impaired kidney function exhibits a temporal trend in both quantitative and qualitative perturbation of lipoprotein metabolism across CKD stages and different renal replacement therapies (5, 6). Quantitatively, hypertriglyceridemia mainly results from impaired TG hydrolysis because of reduced expression of LPL, hepatic lipase, and receptors involved in the clearance of TG-rich lipoproteins (2, 6). Renal dysfunction is related to reduced hepatic LCAT gene expression and activity, which is considered the major cause of low HDL-C levels (7). Qualitatively, for instance, the antioxidant and anti-inflammatory functionalities of HDL-C are compromised in patients with CKD because of a significant reduction in plasma paraoxonase and glutathione peroxidase along with a decreased concentration of LCAT (8). From the temporality perspective, dyslipidemia starts early, even before the onset of CKD stage 3, and worsens during the course of CKD (9, 10).
Despite advances in the mechanistic understanding of lipid metabolism in uremia, conclusive evidence has not been obtained because most investigations have been post hoc analyses and primarily limited to investigating the effect of a statin-induced reduction in serum LDL-C levels on cardiovascular outcomes (11, 12). A comprehensive association analysis concomitantly considering all components of the lipid profile and variable kidney outcomes is lacking. For instance, the recommendation of statin or statin/ezetimibe for patients with CKD not undergoing renal replacement therapy in the most recent Kidney Disease: Improving Global Outcomes (KDIGO) guideline was largely driven by the results of a single randomized trial: the Study of Heart and Renal Protection (SHARP) (12). Additionally, the disconnectedness between initiation of lipid-lowering therapy and measured LDL-C level appears to complicate the consideration of routine baseline lipid profile screening (12).
In CKD care, information regarding the relationship between dyslipidemia and CKD progression remains elusive. Although studies on general healthy populations have consistently demonstrated an inverse relationship between hyperlipidemia and CKD incidence (13–17), the association between dyslipidemia and CKD progression is not consistent among studies conducted in CKD populations (18, 19). For instance, apoB, LDL-C, and T-CHO have been associated with CKD progression (20–22). Conversely, in the Modification of Diet in Renal Disease (MDRD) study comprising 855 patients with an average follow-up period of 2.27 years, low HDL-C and apoA-1 levels were the only independent lipid risk factors for the rapid progression of renal disease; however, this observation was contradicted in a case-control study of 138 patients with CKD in France, which showed no association of hypertriglyceridemia and low HDL-C level with end-stage renal disease (ESRD) progression (23). Moreover, in a recent study by the Chronic Renal Insufficiency Cohort Study research group comprising 3,939 CKD patients with a mean age of 58.2 years, none of the tested serum lipids and lipoproteins, including T-CHO, TG, LDL-C, HDL-C, VLDL, apoA-1, apoB, and lipoprotein a, was associated with disease progression (18, 19). The inconsistency across previous studies can be attributed to low statistical power resulting from relatively moderate sample sizes and, more importantly, the use of only baseline lipid profiles for outcome prediction. Even in studies with long-term follow-up, the longitudinal trajectories of lipid profiles, which efficiently reflect the treatment efficacy of and patients’ adherence to lipid-lowering therapy, have rarely been considered. To address this knowledge gap, we prospectively evaluated the association between longitudinal lipid trajectories and the risk of progression to dialysis and mortality in a large registry-based CKD cohort.
METHODS
Study population
Taiwan’s National Health Insurance launched the Project of Integrated Care of CKD in 2002, initially targeting patients with an estimated glomerular filtration rate (eGFR) below 60 ml/min/1.73 m2 or proteinuria [urine protein-to-creatinine ratio (UPCR) >1 g/g creatinine]. In 2007, the program used a multidisciplinary approach to focus on CKD stages 3b to 5 (24, 25). China Medical University Hospital (CMUH), a tertiary medical center in central Taiwan, joined the program in 2003 and prospectively enrolled consecutive patients with CKD who were willing to participate (25, 26). CKD diagnosis was based on the criteria of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines for CKD (25, 27). The patients in the program were regularly followed-up in the outpatient department. Biochemical markers of renal injury, including serum creatinine, blood urea nitrogen, and spot UPCR, were measured on at least one occasion every 3 months. In addition, T-CHO and TG were measured at least annually under the care quality-based National Health Insurance reimbursement policy, whereas the frequency of LDL-C and HDL-C measurements was based on the clinician’s discretion given an individual’s underlying risk for CVD. Since 2003, CMUH has employed electronic medical records (EMRs) for care management; therefore, we integrated the data of the CMUH pre-ESRD program with the CMUH EMRs containing laboratory tests, medications, special procedures, and admission records (25). All enrolled patients were followed-up until initiation of long-term renal replacement therapy (hemodialysis, peritoneal dialysis, or transplantation), loss to follow-up, death, or until December 31, 2015, whichever occurred first.
In the present study, we included only those participants of the pre-ESRD program who were aged 20–89 years, had no history of dialysis, and for whom there were at least two measurements of serum T-CHO and TG over the follow-up period. A total of 36,451 records of T-CHO and 40,445 records of TG were included. We then excluded participants for whom the time interval between baseline and the last T-CHO and TG measurements was less than 6 months. Further, we excluded patients under dialysis or with only a single measurement of T-CHO or TG; ultimately, a total of 4,647 participants were included in the analysis (supplemental Fig. S1). From the primary study population, we further identified patients who had at least two measurements of serum LDL-C or HDL-C levels to form our secondary populations comprising 2,846 and 2,118 participants, respectively. The study was approved by the Big Data Center of CMUH and the Research Ethical Committee/Institutional Review Board of China Medical University (CMUH105-REC3-068).
Determination of serum lipid levels and kidney function
Fasting plasma lipid and lipoprotein cholesterol levels were measured using specific diagnostic kits by following the manufacturers’ instructions. Serum levels of T-CHO, HDL-C, LDL-C, and TG were measured using an LX-20 system (Beckman Coulter) prior to 2007 and then using the UniCel® DxC 800 system (Beckman Coulter). All lipid components were determined using enzymatic timed-endpoint methods with commercially available kits (SYNCHRON® system reagents; Beckman Coulter). T-CHO is hydrolyzed by cholesterol esterase to free cholesterol. After deacylation, the oxidation of free cholesterol is catalyzed by cholesterol oxidase, yielding hydrogen peroxide (H2O2), which couples with 4-aminoantipyrin to form a colored quinoneimine. Similarly, TG is hydrolyzed to glycerol and free fatty acids by LPL. Glycerol is then oxidized by glycerophosphate oxidase into H2O2, which further forms a red quinoneimine dye through oxidative coupling with 4-aminoantipyrin by peroxidase. LDL-C level is directly measured using two selective detergents to eventually solubilize LDL-C, which allows conventional coupling of LDL-C with a chromogenic dye for color formation. HDL-C is also measured on the basis of selective detergent methodology. The LDL-C levels calculated using the Friedewald formula (LDL-C = T-CHO − HDL-C − TG/5) were also considered (28). The baseline serum level of a given lipid component was defined as the average of the measured values up to 12 months before pre-ESRD enrollment. Binary variables (high vs. normal) for each baseline lipid component were defined by the cutoff values suggested by the National Cholesterol Education Program-Adult Treatment Panel III (NCEP ATP III) criteria (29). The cutoff values for each lipid were as follows: T-CHO, 200 mg/dl; TG, 200 mg/dl; LDL-C, 130 mg/dl; and HDL-C, 40 mg/dl. The eGFR was estimated using the abbreviated MDRD equation [eGFR = 186 × creatinine−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female)] (30). The serum creatinine level at enrollment was used to define the baseline eGFR and the corresponding CKD stages by using the following cutoff values: >90, 60–89.9, 30–59.9, 15–29.9, and <15 ml/min/1.73 m2. All lipid measurements of the enrolled participants were considered until the study endpoints. For instance, the quarterly average T-CHO level was calculated if the patient had received more than one T-CHO measurement in a 3 month period, and the individual’s T-CHO trajectory was modeled based on quarterly average T-CHO measures. The same approach was applied to model the trajectories of HDL-C, TG, LDL-C, and eGFR. Random spot urine dipstick and UPCR measurements were used to quantify proteinuria. Proteinuria was defined as urine proteinuria >0.5 g/day from random spot urine in at least two of three consecutive urine examinations.
Other variables
Sociodemographic variables collected during the enrollment interview included age, race/ethnicity, sex, education, cigarette smoking, and alcohol consumption. Smoking and alcohol consumption were categorized as current, former, or never. BMI was calculated as weight in kilograms divided by height in meters squared. Diabetes mellitus and hypertension were defined by physicians’ clinical diagnosis based on patients’ International Classification of Diseases (ICD-9) codes and/or the use of blood pressure- and glucose-lowering agents. A history of CVD was defined as documented coronary artery disease (CAD), myocardial infarction, stroke, or heart failure based on ICD-9 codes in EMRs 1 year before the pre-ESRD program enrollment.
Statistical analyses
Continuous variables were compared using Wilcoxon rank sum test and are expressed as a median and interquartile range (IQR), whereas categorical variables were compared using the chi-square test and are expressed as a frequency (percentage). All statistical analyses were performed in SAS (version 9.4; SAS Institute Inc., Cary, NC). The two-sided statistical significance level was set at α = 0.05. Associations among baseline lipid levels, risk of ESRD, and all-cause mortality were estimated using multivariable Cox regression analysis. The baseline lipid level was modeled as a continuous as well as binary exposure variable. The dose-response relationship was characterized using a restricted cubic spline model, with three knots located at the tenth, fiftieth, and ninetieth percentiles of the overall distribution of each lipid component.
We used semiparametric group-based trajectory modeling (GBTM) to characterize the follow-up-period trajectories of all lipid profiles of the patients enrolled in the CMUH pre-ESRD Program. Briefly, the PROC TRAJ macro developed using the SAS software fits a semiparametric mixture model to longitudinal data by using the maximum likelihood method (31–33). This approach is useful when the number of subgroups and other information such as the trajectory shapes of each subgroup are unknown. We focused on two-group solutions (e.g., high vs. normal for T-CHO) for all lipid trajectories considering the sample size and the facilitation of meaningful statistical interpretation.
We evaluated the prospective associations of lipid trajectories with risk of dialysis initiation and mortality by using Cox proportional hazards models with follow-up time as the time scale and baseline age as covariate. The models were adjusted progressively (see the footnotes of Tables 2 and 3). Because the inherent renal function status itself may introduce confounding regarding the effect of lipids on dialysis risk and mortality, we adjusted multiple domains of renal function, including baseline eGFR, longitudinal eGFR trajectories, and primary etiologies of CKD, in the final model to avoid residual confounding. To further characterize the risk of dialysis associated with lipid trajectories, we performed a competing risk analysis according to the protocol of Fine and Gray (34) to minimize the potential bias introduced by a competing risk of death. Due to missing data on some explanatory variables (e.g., UPCR up to 34.9%), we further performed multiple imputations with a fully conditional method in SAS, an iterative Markov chain Monte Carlo procedure, to replace the missing values for protein-to-creatinine ratio (PCR), comorbidities, and medications with imputed values. We specified the number of imputations as 20 and iterations as 100. We also conducted a sensitivity analysis by using a dyslipidemia trajectory based on the probability of blood lipid level being defined as dyslipidemia on the basis of the NCEP ATP III criteria (supplemental Fig. S2) at each follow-up measurement.
TABLE 2.
HRs (95% CIs) of progression to dialysis by baseline level and trajectory group of each lipid component
| Baseline Serum Lipid and Longitudinal Trajectory | Number | Cases | Person-Years | Incidencea | Crude HR (95% CI) | Model 1 [Adjusted HR (95% CI)] | Model 2 [Adjusted HR (95% CI)] | Model 3 [Adjusted HR (95% CI)] | Model 4 [Adjusted HR (95% CI)] | Model 5 [Adjusted HR (95% CI)] |
| Risk of dialysisb | ||||||||||
| T-CHO | ||||||||||
| Baseline (per 20 mg/dl increase) | 4,647 | 797 | 15,685.6 | 50.8 | 1.05 (1.03, 1.08) | 1.03 (1.00, 1.06) | 1.04 (1.01, 1.06) | 1.02 (1.00, 1.05) | 1.08 (1.05, 1.12) | 1.06 (1.03, 1.10) |
| P | <0.001 | 0.027 | 0.005 | 0.071 | <0.001 | <0.001 | ||||
| Trajectory: normal (Ref) | 3,235 | 518 | 10,516.6 | 49.3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: high | 1,412 | 279 | 5,169.0 | 54.0 | 1.13 (0.97, 1.30) | 0.94 (0.81, 1.10) | 0.98 (0.84, 1.15) | 0.97 (0.83, 1.14) | 1.25 (1.07, 1.46) | 1.21 (1.03, 1.42) |
| P | 0.109 | 0.432 | 0.829 | 0.710 | 0.005 | 0.019 | ||||
| TG | ||||||||||
| Baseline (per 20 mg/dl increase) | 4,647 | 797 | 15,685.6 | 50.8 | 1.02 (1.01, 1.03) | 1.02 (1.01, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.01 (1.00, 1.02) | 1.00 (0.99, 1.02) |
| P | <0.001 | <0.001 | 0.012 | 0.035 | 0.178 | 0.373 | ||||
| Trajectory: normal (Ref) | 4,045 | 686 | 13,668.8 | 50.2 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: high | 602 | 111 | 2,016.9 | 55.0 | 1.12 (0.91, 1.36) | 1.05 (0.85, 1.29) | 0.95 (0.77, 1.17) | 0.90 (0.72, 1.13) | 0.91 (0.72, 1.14) | 0.92 (0.73, 1.17) |
| P | 0.285 | 0.649 | 0.639 | 0.364 | 0.406 | 0.496 | ||||
| LDL-C | ||||||||||
| Baseline (per 10 mg/dl increase) | 2,846 | 299 | 10,340.9 | 28.9 | 1.04 (1.02, 1.07) | 1.03 (1.00, 1.05) | 1.04 (1.01, 1.06) | 1.02 (1.00, 1.05) | 1.06 (1.03, 1.09) | 1.05 (1.02, 1.08) |
| P | <0.001 | 0.025 | 0.003 | 0.041 | <0.001 | 0.001 | ||||
| Trajectory: normal-low (Ref) | 1,928 | 199 | 6,567.6 | 30.3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: normal | 918 | 100 | 3,773.3 | 26.5 | 0.84 (0.66, 1.06) | 0.72 (0.56, 0.93) | 0.84 (0.65, 1.09) | 0.90 (0.69, 1.18) | 1.25 (0.96, 1.62) | 1.24 (0.95, 1.62) |
| P | 0.139 | 0.012 | 0.193 | 0.444 | 0.095 | 0.112 | ||||
| HDL-C | ||||||||||
| Baseline (per 10 mg/dl increase) | 2,118 | 278 | 7,611.1 | 36.5 | 0.98 (0.88, 1.09) | 0.93 (0.83, 1.04) | 0.98 (0.87, 1.09) | 0.94 (0.84, 1.05) | 1.02 (0.92, 1.13) | 1.01 (0.91, 1.14) |
| P | 0.740 | 0.690 | 0.672 | 0.276 | 0.687 | 0.802 | ||||
| Trajectory: high-normal (Ref) | 415 | 43 | 1,575.5 | 27.3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: normal | 1,703 | 235 | 6,035.6 | 38.9 | 1.44 (1.05, 1.99) | 1.67 (1.19, 2.34) | 1.42 (1.01, 2.01) | 1.37 (0.96, 1.96) | 1.19 (0.84, 1.69) | 1.17 (0.81, 1.69) |
| P | 0.025 | 0.003 | 0.045 | 0.081 | 0.336 | 0.415 |
Bold values denote significant difference between the groups at P < 0.05. Model 1: adjusted for age at entry, gender, BMI, smoking, alcohol, and education; Model 2: further adjusted for diabetes, hypertension, CVD, and primary etiologies of CKD; Model 3: further adjusted for baseline medications listed in Table 1; Model 4: further adjusted for baseline eGFR and eGFR trajectories; Model 5: further adjusted for baseline PCR.
Incidence = number of incident dialysis cases/person-years × 1,000.
With competing risk analysis for death.
TABLE 3.
HRs (95% CIs) of all-cause mortality by baseline level and trajectory group of each lipid component
| Baseline Serum Lipid and Longitudinal Trajectory | Number | Cases | Person-Years | Incidencea | Crude HR (95% CI) | Model 1 [Adjusted HR (95% CI)] | Model 2 [Adjusted HR (95% CI)] | Model 3 [Adjusted HR (95% CI)] | Model 4 [Adjusted HR (95% CI)] | Model 5 [Adjusted HR (95% CI)] |
| All-cause mortality | ||||||||||
| T-CHO | ||||||||||
| Baseline (per 20 mg/dl increase) | 4,647 | 404 | 15,712.6 | 25.7 | 0.86 (0.82, 0.90) | 0.92 (0.87, 0.97) | 0.93 (0.88, 0.98) | 0.96 (0.90, 1.01) | 0.97 (0.92, 1.02) | 0.96 (0.91, 1.01) |
| P | <0.001 | 0.001 | 0.009 | 0.097 | 0.222 | 0.141 | ||||
| Trajectory: normal (Ref) | 3,235 | 320 | 10,537.4 | 30.4 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: high | 1,412 | 84 | 5,175.2 | 16.2 | 0.51 (0.40, 0.64) | 0.73 (0.57, 0.94) | 0.79 (0.62, 1.01) | 0.85 (0.66, 1.09) | 0.87 (0.68, 1.11) | 0.85 (0.66, 1.10) |
| P | <0.001 | 0.013 | 0.064 | 0.203 | 0.262 | 0.212 | ||||
| TG | ||||||||||
| Baseline (per 20 mg/dl increase) | 4,647 | 404 | 15,712.6 | 25.7 | 0.97 (0.95, 1.00) | 1.00 (0.98, 1.02) | 0.99 (0.97, 1.01) | 0.99 (0.96, 1.01) | 0.98 (0.96, 1.01) | 0.98 (0.96, 1.01) |
| P | 0.016 | 0.836 | 0.371 | 0.268 | 0.191 | 0.163 | ||||
| Trajectory: normal (Ref) | 4,045 | 360 | 13,689.6 | 26.3 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: high | 602 | 44 | 2,023.0 | 21.8 | 0.83 (0.61, 1.13) | 1.22 (0.89, 1.68) | 1.08 (0.78, 1.49) | 0.99 (0.71, 1.38) | 0.95 (0.68, 1.33) | 0.95 (0.68, 1.32) |
| P | 0.239 | 0.221 | 0.633 | 0.948 | 0.768 | 0.748 | ||||
| LDL-C | ||||||||||
| Baseline (per 10 mg/dl increase) | 2,846 | 176 | 10,347.9 | 17.0 | 0.92 (0.88, 0.97) | 0.95 (0.90, 0.99) | 0.96 (0.92, 1.01) | 0.96 (0.91, 1.01) | 0.97 (0.92, 1.02) | 0.97 (0.92, 1.02) |
| P | <0.001 | 0.029 | 0.134 | 0.139 | 0.206 | 0.175 | ||||
| Trajectory: normal-low (Ref) | 1,928 | 135 | 6,573.7 | 20.5 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: normal | 918 | 41 | 3,774.1 | 10.9 | 0.48 (0.34, 0.68) | 0.66 (0.46, 0.94) | 0.79 (0.55, 1.13) | 0.84 (0.58, 1.21) | 0.90 (0.62, 1.30) | 0.89 (0.62, 1.29) |
| P | <0.001 | 0.021 | 0.200 | 0.356 | 0.570 | 0.540 | ||||
| HDL-C | ||||||||||
| Baseline (per 10 mg/dl increase) | 2,118 | 173 | 7,619.6 | 22.7 | 1.00 (0.88, 1.14) | 1.02 (0.90, 1.17) | 1.10 (0.96, 1.26) | 1.14 (1.00, 1.29) | 1.20 (1.06, 1.36) | 1.20 (1.06, 1.36) |
| P | 0.980 | 0.734 | 0.153 | 0.054 | 0.004 | 0.005 | ||||
| Trajectory: normal-high (Ref) | 415 | 31 | 1,576.2 | 19.7 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Trajectory: normal | 1,703 | 142 | 6,043.4 | 23.5 | 1.24 (0.84, 1.83) | 1.11 (0.74, 1.65) | 0.99 (0.66, 1.49) | 0.90 (0.59, 1.35) | 0.86 (0.57, 1.29) | 0.85 (0.56, 1.29) |
| P | 0.277 | 0.617 | 0.964 | 0.605 | 0.464 | 0.452 |
Bold values denote significant difference between the groups at P < 0.05. Model 1: adjusted for age at entry, gender, BMI, smoking, alcohol, and education; Model 2: further adjusted for diabetes, hypertension, CVD, and primary etiologies of CKD; Model 3: further adjusted for baseline medications listed in Table 1; Model 4: further adjusted for baseline eGFR and eGFR trajectories; Model 5: further adjusted for baseline PCR.
Incidence = number of incident dialysis cases/person-years × 1,000.
Exploratory subgroup analyses were performed to evaluate potential effect modification in the adjusted models. We stratified patients on the basis of age greater or less than 65 years, sex, BMI category (<24, 24–47, >27 kg/m2), smoking status, CKD stage (3 vs. 4 and 5), UPCR greater or less than 200 mg/g creatinine, serum uric acid (SUA) greater or less than 7 mg/dl, diabetes, hypertension, and CVD. Due to the comprehensiveness of the present study, a conceptual framework is provided to help readers understand a total of four exposure matrices for each lipid profile (supplemental Fig. S3).
RESULTS
Characteristics of high versus low baseline lipid values and longitudinal trajectories
Among the 4,647 participants, the median age at enrollment was 67.0 years (IQR: 56.8–75.6), with a median follow-up time of 33.3 months. The median number of lipid profile measurements recorded was 5 (IQR: 3–9). Over 15,685.6 person-years of follow-up, 797 ESRD events, and 404 deaths occurred. Incident ESRD and all-cause mortality were 50.8 and 25.7 per 1,000 person-years, respectively. Supplemental Fig. S4 presents the Pearson’s correlation coefficients among lipid profiles. T-CHO and LDL-C had the highest correlation coefficient (γ = 0.72), whereas LDL-C exhibited near-zero correlation with TG (γ = −0.02) and HDL-C (γ = 0.14). A significant inverse relationship was noted between TG and HDL-C (γ = −0.21). At baseline, 33% and 7.6% of the study population were exposed to statin and fibric acid derivative treatment, respectively (supplemental Table S1).
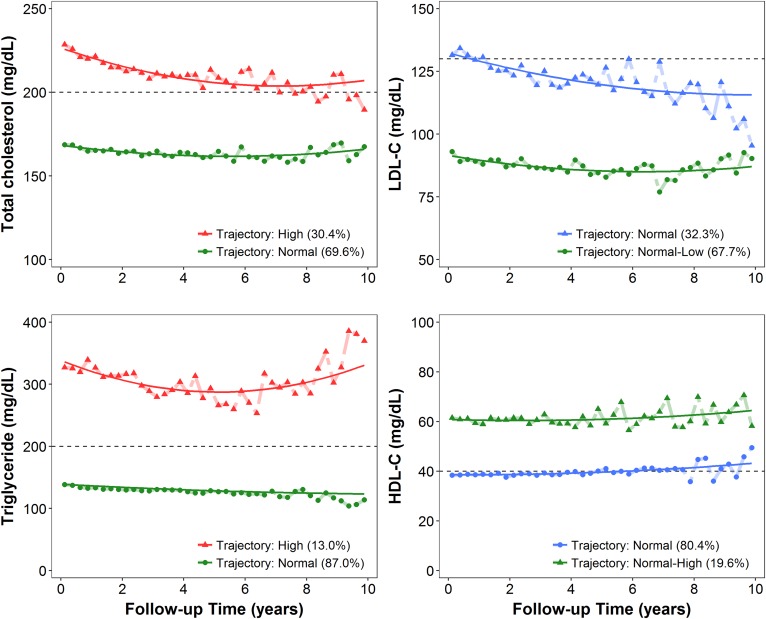
Two distinct trajectories were identified using the GBTM for each lipid component (Fig. 1). For T-CHO and TG, the two trajectories were exactly separated using the NCEP ATP III cutoffs for hypercholesterolemia (T-CHO >200 mg/dl) and hypertriglyceridemia (TG >200 mg/dl) and labeled as “high” and “normal,” respectively (Fig. 1). For LDL-C, both trajectories were below the NCEP ATP III LDL-C cutoff of 130 mg/dl (borderline-high) and were designated normal and “normal-low”. By contrast, the two distinct HDL-C trajectories were above 40 mg/dl and were labeled as “normal-high” and normal (Fig. 1). The reference trajectory group of each lipid component in the association analysis was colored green consistently in Fig. 1. The baseline demographics and clinical characteristics according to serum lipid trajectories defined by GBTM and complemental data were summarized in Table 1 and supplemental Table S1. Corresponding information by baseline lipid profile stratified by cutoff values of NCEP ATP III criteria was provided in supplemental Table S2. Patients with a high baseline or trajectory of T-CHO were younger at enrollment, more likely to be female, more likely to have diabetes and CVD, and had higher baseline hemoglobin, serum albumin, phosphate, TG, LDL-C, HDL-C, TG/HDL-C ratio, eGFR, and UPCR than those with the normal baseline or T-CHO trajectory (Table 1; supplemental Tables S1, S2). The high T-CHO trajectory was additionally associated with a lower level of baseline serum creatinine, potassium, and urine creatinine. Compared with patients with a normal baseline or trajectory of TG, those with a high TG status were younger and tended to be diabetic and hypertensive. High TG status was also associated with higher levels of SUA, calcium, and urine creatinine, which was different from what was observed for the T-CHO trajectory (Table 1; supplemental Tables S1, S2). For LDL-C, the demographic and baseline biochemical characteristics were similar to those for T-CHO; however, the high LDL-C trajectory was not associated with a lower TG/HDL ratio and the prescription of lipid-lowering agents (Table 1; supplemental Tables S1, S2). Regarding HDL-C, both baseline and longitudinal status were not associated with age or serum albumin and hemoglobin levels, but they were inversely associated with BMI, SUA level, and TG/HDL ratio. Patients with a high baseline and trajectory HDL-C status were also less likely to have diabetes, hypertension, and CVD (Table 1; supplemental Tables S1, S2).
Fig. 1.
Serum lipid trajectories as defined through GBTM by using serial quarterly average levels of each lipid component, including T-CHO, TG, LDL-C, and HDL-C, over the course of CKD. The solid lines represent the averaged estimated trajectory, whereas the points represent the averaged observed trajectories. Green represents the reference group in the association analyses, red indicates that the level of lipid in the trajectory group is stably higher than the cutoff value (black dashed lines) according to the NCEP ATP III criteria, and blue indicates that the lipid level in the trajectory group was stably lower than or close to the NCEP ATP III cutoff value.
TABLE 1.
Baseline demographic and clinical characteristics according to serum lipid trajectories defined on the basis of GBTM
| Variables | Total (n = 4,647) | T-CHO | TG | LDL-C | HDL-C | ||||
| Trajectory 1: Normal (n = 3,235) | Trajectory 2: High (n = 1,412) | Trajectory 1: Normal (n = 4,045) | Trajectory 2: High (n = 602) | Trajectory 1: Normal-Low (n = 1,928) | Trajectory 2: Normal (n = 918) | Trajectory 1: Normal (n = 1,703) | Trajectory 2: Normal-High (n = 415) | ||
| Age at entry (years) | 67.0 (56.8, 75.6) | 69.2 (59.4, 76.9) | 61.2 (51.4, 71.3) | 67.8 (57.5, 76.0) | 60.4 (51.5, 71.4) | 68.0 (59.0, 76.1) | 61.6 (51.5, 72.3) | 67.5 (58.3, 75.6) | 67.7 (56.8, 75.8) |
| Female | 1,922 (41.4) | 1,200 (37.1) | 722 (51.1) | 1,665 (41.2) | 257 (42.7) | 744 (38.59) | 401 (43.68) | 607 (35.64) | 232 (55.9) |
| BMI (kg/m2) | 24.4 (22.1, 27.2) | 24.4 (22.1, 27.2) | 24.5 (22.1, 27.2) | 24.2 (21.9, 27.0) | 25.9 (23.8, 28.4) | 24.8 (22.6, 27.5) | 24.7 (22.6, 27.2) | 25.3 (23.2, 27.9) | 23.5 (21.3, 26.4) |
| Initial CKD stage | |||||||||
| 1 | 199 (4.3) | 106 (3.3) | 93 (6.6) | 171 (4.2) | 28 (4.7) | 85 (4.42) | 82 (8.95) | 85 (5) | 35 (8.43) |
| 2 | 376 (8.1) | 230 (7.1) | 146 (10.4) | 322 (8.0) | 54 (9.0) | 184 (9.56) | 131 (14.3) | 198 (11.65) | 41 (9.88) |
| 3 | 2,139 (46.1) | 1,510 (46.7) | 629 (44.7) | 1,856 (46.0) | 283 (47.1) | 1,032 (53.61) | 488 (53.28) | 842 (49.53) | 216 (52.05) |
| 4 | 1,226 (26.4) | 869 (26.9) | 357 (25.4) | 1,064 (26.4) | 162 (27.0) | 463 (24.05) | 163 (17.79) | 422 (24.82) | 100 (24.1) |
| 5 | 698 (15.1) | 516 (16.0) | 182 (12.9) | 624 (15.5) | 74 (12.3) | 161 (8.36) | 52 (5.68) | 153 (9) | 23 (5.54) |
| Diabetes | 1,896 (40.8) | 1,389 (42.9) | 507 (35.9) | 1,582 (39.1) | 314 (52.2) | 1,069 (55.45) | 344 (37.47) | 1,093 (64.18) | 201 (48.43) |
| Hypertension | 2,755 (59.3) | 1,939 (59.9) | 816 (57.8) | 2,349 (58.1) | 406 (67.4) | 1,247 (64.68) | 518 (56.43) | 1,176 (69.05) | 253 (60.96) |
| CVD | 1,777 (38.2) | 1,402 (43.3) | 375 (26.6) | 1,561 (38.6) | 216 (35.9) | 906 (46.99) | 267 (29.08) | 841 (49.38) | 155 (37.35) |
| Baseline biochemical profiles | |||||||||
| T-CHO (mg/dl) | 186 (161, 214) | 173 (152, 196) | 218 (196, 249) | 184 (159, 211) | 203 (176, 234) | 176 (154, 202) | 208 (187, 238) | 183 (158, 210) | 192 (168, 223) |
| TG (mg/dl) | 138 (97, 203) | 128 (91, 187) | 162 (117, 242) | 127 (92, 175) | 284 (216, 386) | 145 (101, 215) | 141 (105, 195) | 155 (114, 230) | 113 (79, 152) |
| LDL-C (mg/dl) | 105 (84, 127) | 99 (79, 119) | 121 (101, 146) | 105 (85, 128) | 98 (76, 120) | 97 (78, 114) | 126 (111, 145) | 102 (82, 124) | 103 (81, 129) |
| HDL-C (mg/dl) | 40.2 (34.2, 48.4) | 39.1 (33.2, 46.3) | 43.6 (37.2, 53.0) | 40.9 (34.5, 49.4) | 37.5 (32.3, 43.1) | 39.4 (33.8, 47.1) | 42.7 (37.1, 50.9) | 37.9 (32.9, 43.3) | 55.8 (48.9, 63.7) |
| eGFR (ml/min/1.73 m2) | 34.5 (20.2, 50.2) | 33.5 (19.5, 49.4) | 36.8 (22.2, 52.7) | 34.3 (20.1, 49.9) | 35.3 (21.5, 52.1) | 39.6 (25.2, 53.3) | 46.3 (31.2, 58.9) | 38.4 (24.7, 53.8) | 41.5 (27.0, 55.0) |
| Hemoglobin (g/dl) | 11.4 (9.8, 13.1) | 11.3 (9.7, 13.0) | 11.5 (10.1, 13.4) | 11.3 (9.8, 13.1) | 11.9 (10.1, 13.8) | 11.7 (10.1, 13.3) | 12.3 (10.7, 13.8) | 11.9 (10.3, 13.5) | 11.7 (10.1, 13.0) |
| SUA (mg/dl) | 7.30 (6.20, 8.50) | 7.30 (6.19, 8.50) | 7.35 (6.30, 8.42) | 7.30 (6.20, 8.40) | 7.60 (6.46, 8.90) | 7.20 (6.10, 8.40) | 7.30 (6.20, 8.40) | 7.34 (6.20, 8.50) | 7.00 (5.90, 8.04) |
| Serum creatinine (mg/dl) | 1.79 (1.33, 2.77) | 1.82 (1.36, 2.86) | 1.70 (1.25, 2.58) | 1.80 (1.34, 2.80) | 1.74 (1.29, 2.60) | 1.60 (1.25, 2.27) | 1.45 (1.09, 1.93) | 1.63 (1.25, 2.36) | 1.51 (1.10, 2.04) |
| Serum albumin (g/dl) | 4.00 (3.60, 4.30) | 4.00 (3.70, 4.30) | 4.00 (3.40, 4.30) | 4.00 (3.60, 4.30) | 4.15 (3.72, 4.43) | 4.10 (3.70, 4.35) | 4.05 (3.60, 4.30) | 4.03 (3.68, 4.30) | 4.00 (3.53, 4.30) |
| TG/HDL-C ratio | 3.60 (2.26, 6.05) | 3.50 (2.20, 5.68) | 4.06 (2.53, 6.99) | 3.22 (2.09, 4.90) | 7.73 (5.62, 10.79) | 3.73 (2.30, 6.14) | 3.38 (2.36, 5.46) | 4.20 (2.76, 6.73) | 1.96 (1.37, 3.13) |
| Urine PCR (mg/g) | 762 (209, 2119) | 626 (192, 1,727) | 1,149 (293, 3,331) | 731 (200, 2,004) | 1,129 (306, 2,871) | 477 (164, 1,584) | 662 (177, 2,225) | 643 (200, 2,192) | 545 (155, 2,010) |
The bold values denote significant difference between the groups at P < 0.05.
Associations of dialysis risk and all-cause mortality with baseline lipid values
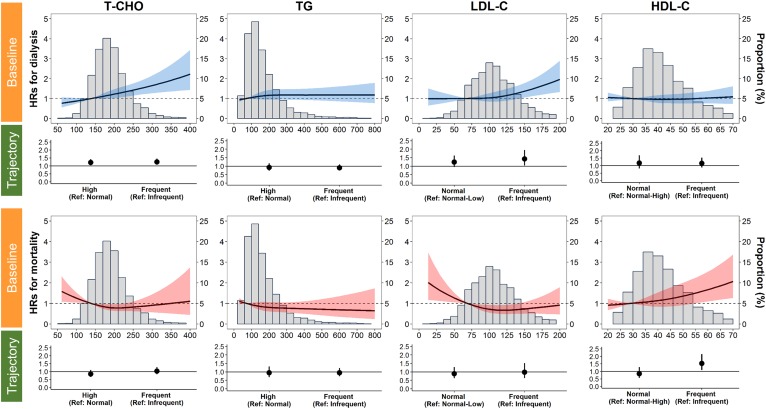
At baseline, we found that each 20 mg/dl increase in T-CHO and LDL-C was associated with a 6% (95% CI, 3–10; P < 0.001) and 5% (95% CI, 2–8; P = 0.001) increase, respectively, in the risk of ESRD (Table 2). When we stratified baseline levels of each lipid component into abnormal versus normal status according to the NCEP ATP III recommendations (Fig. 1), the patients with baseline hypercholesterolemia and hyper-LDL cholesterolemia experienced 36% (95% CI, 15–61; P < 0.001) and 33% (95% CI, 2–74; P = 0.035) higher risk of ESRD, respectively (supplemental Table S3). For all-cause mortality, the fully adjusted hazard ratios (HRs) for each 10 mg/dl increase in the level of HDL-C and the status of having an HDL-C level below 40 mg/dl were 1.20 (95% CI, 1.06–1.36; P = 0.005) and 0.58 (95% CI, 0.41–0.82; P = 0.002) (Table 3, supplemental Table S4). Regarding the dose-response relationship between baseline lipid levels and the risk of progression to dialysis, a positive linear relationship was significant above 200 and 150 mg/dl for T-CHO and LDL-C, respectively (Fig. 2). Regarding mortality, HDL-C had a linear dose-response relationship above the level of 60 mg/dl, whereas no dose-response relationship was observed for T-CHO, TG, or LDL-C (Fig. 2).
Fig. 2.
Adjusted HRs for ESRD requiring dialysis and all-cause mortality according to the baseline serum level of each lipid component. Solid lines represent adjusted HRs based on restricted cubic splines for baseline lipids. The shaded areas represent upper and lower 95% CIs. Reference was set at the tenth percentile of baseline lipid levels. Fully adjusted HRs (Tables 2 and 3, Model 5) comparing dyslipidemia-prone trajectory to reference trajectory of each lipid component are also plotted. Upper panels: Risk of ESRD requiring dialysis (blue). Lower panels: All-cause mortality (red). Variables adjusted are the same as those shown in Model 5 in Tables 2 and 3.
Association of dialysis risk and all-cause mortality with longitudinal lipid trajectories
The crude Kaplan-Meier survival curves in supplemental Fig. S5 show that participants with a high T-CHO trajectory during the follow-up had significantly lower overall survival (log-rank test, P < 0.001), but equivalent dialysis-free survival compared with those with a normal T-CHO trajectory.
However, in the fully adjusted multivariable Cox regression model, CKD patients with a high T-CHO trajectory did not have higher risk of all-cause mortality (adjusted HR, 0.85; 95% CI, 0.66–1.10; P = 0.212) compared with those with a normal T-CHO trajectory (Table 3, Model 5). In contrast, compared with those with a normal T-CHO trajectory, the fully adjusted HRs (95% CI) of CKD patients with a high T-CHO trajectory for the risk of progression to ESRD were 1.21 (95% CI, 1.03–1.42; P = 0.019) (Table 2, Model 5). For LDL-C trajectories, similar results were obtained in the Kaplan-Meier curve analysis for dialysis-free and overall survival (supplemental Fig. S6). No difference in dialysis-free and overall survival was observed between TG trajectories (supplemental Fig. S7); whereas, a normal-high HDL-C trajectory had better dialysis-free survival shown on Kaplan-Meier curve (supplemental Fig. S8). Regarding the trajectories of TG, LDL-C, and HDL-C, no significant difference in risk of ESRD and death was observed between patients with abnormal and reference trajectories (Tables 2, 3). However, when using GBTM to classify the probability trajectory of being hypo-HDL cholesterolemic (HDL-C <40 mg/dl at each measurement occasion), patients with relatively persistent hypo-HDL cholesterolemia had a 53% (95% CI, 9–115; P = 0.014) higher risk of mortality compared with those with less frequent hypo-HDL cholesterolemia (supplemental Table S4).
Subgroup analysis
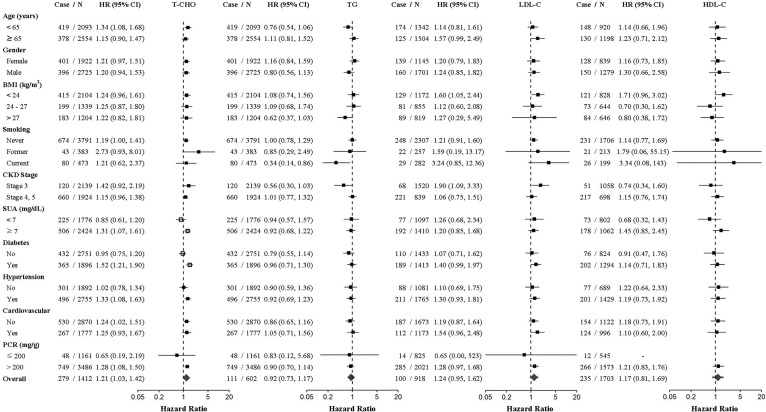
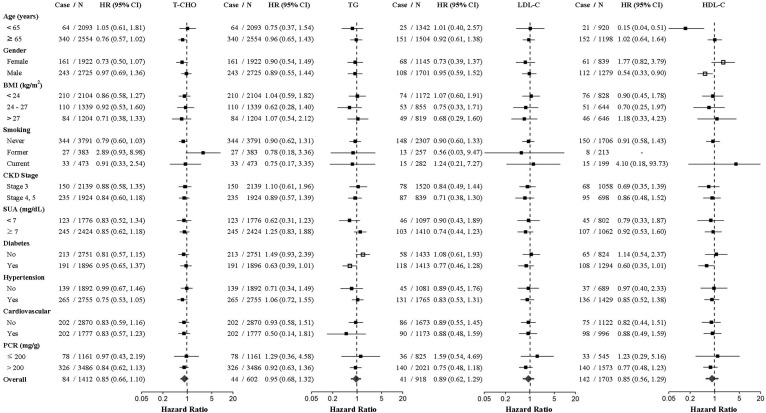
Results of the exploratory subgroup analysis revealed that the effect of a high T-CHO trajectory on the risk of progression to dialysis was modified by diabetic and hyperuricemic status, with more pronounced risk estimates in patients with diabetes (HR, 1.52; 95% CI, 1.21–1.90; P < 0.001) or hyperuricemia (HR, 1.28; 95% CI, 1.08–1.50; P = 0.003) (Fig. 3A). Regarding all-cause mortality, patients with a high TG trajectory exhibited a higher risk of death among patients without diabetes compared with those with a normal TG trajectory (HR,1.49; 95% CI, 0.93–2.39; P for interaction = 0.012) (Fig. 3B). Furthermore, the association between HDL-C trajectory and death was significantly modified by sex (P = 0.005). The risk of death was substantially lower in men with a normal longitudinal HDL-C trajectory (nearly 40 mg/dl) compared with those with a normal-high HDL-C trajectory (HR, 0.54; 95% CI, 0.33–0.90; P = 0.018).
Fig. 3.
A: HRs for the risk of CKD progression to dialysis comparing the dyslipidemia-prone versus reference lipid trajectories for each lipid component (T-CHO, TG, LDL-C, and HDL-C). HRs were adjusted for the variables in Model 5 in Table 2, except for the stratifying variables. Estimated two-sided P < 0.05 for the interaction between lipid trajectories and participants’ characteristics are indicated by unfilled squares. B: HRs for the risk of all-cause mortality comparing the dyslipidemia-prone versus reference lipid trajectories for each lipid component (T-CHO, TG, LDL-C, and HDL-C). HRs were adjusted for the variables in Model 5 in Table 3, except for stratifying variables. Estimated two-sided P < 0.05 for the interaction between lipid trajectories and participants’ characteristics are indicated by unfilled squares.
DISCUSSION
To the best of our knowledge, this is the first study to describe lipid trajectories by using a GBTM to evaluate the associations between both baseline and longitudinal change in lipid profile and the risk of progression to ESRD and death. Our results suggested that higher baseline levels of T-CHO and LDL-C and a high T-CHO trajectory were associated with a higher risk of incident ESRD in patients with CKD. The prognostic role of longitudinal T-CHO trajectory in the development of ESRD was especially prominent among CKD patients with concomitant diabetes or hyperuricemia. Hyperlipidemic status, including hypercholesterolemia and hyper-LDL cholesterolemia, both at the time of enrollment and over the course of the pre-ESRD program was a risk factor for progression to ESRD, but not for all-cause mortality. Contrary to common belief, our data showed that baseline HDL-C levels were positively associated with all-cause mortality. However, the association was in a different direction using longitudinal HDL-C trajectory data. When using GBTM to classify the probability trajectory of being hypo-HDL cholesterolemic (HDL-C <40 mg/dl at each measurement occasion), we found that the persistent status of hypo-HDL was significantly associated with all-cause mortality (supplemental Table S4). In CKD patients without diabetes, a high TG trajectory is also a significant mortality risk factor. Discrepant statistical inference derived from baseline value and longitudinal trajectory indicates that further studies are required to determine an optimal goal of HDL-C, which may decrease mortality risk in the CKD population. More importantly, our research suggests that a systematic approach to analyze both baseline and longitudinal lipid data is mandatory in future studies.
We discovered that baseline T-CHO and LDL-C values and hyperlipidemia (hypercholesterolemia or hyper-LDL cholesterolemia) were linearly associated with the risk of progression to ESRD when holding eGFR trajectories constant, but not with all-cause mortality. The null associations of T-CHO, TG, and LDL-C with mortality were maintained longitudinally. In the general population, T-CHO and LDL-C are conventionally recognized as important risk factors for CVD and CVD-related mortality despite a recent debate (35). However, the positive association between T-CHO and all-cause mortality in the general population has been consistently disproved in various countries, challenging the “the lower the better” concept in lipid management (36). Nonetheless, the latest KDIGO guideline suggests wider use of statin or statin/ezetimibe for all patients older than 50 years and with CKD stages 1–2 or 3–5, respectively, due to the significant cardiovascular risk reduction that was shown in the SHARP trial (12, 37), but not for the CKD progression, despite the finding by a recent meta-analysis that statin might reduce the decline in eGFR in patients with CKD (38). Our findings support the role of baseline T-CHO and LDL-C at enrollment in the pre-ESRD program for predicting rapid progression to ESRD. However, because of the observational nature of the present study, we could not determine whether use of statins retarded the decline in kidney function with target levels of T-CHO and LDL-C similar to those in the NCEP ATP III criteria in CKD populations (29). Furthermore, the null associations among T-CHO, LDL-C, and all-cause mortality in CKD populations are consistent with the ongoing debate on the inverse relationship between statin use and all-cause mortality, particularly in general older populations (35, 39, 40). Because CKD is also part of an aging process, the observation of a potential null relationship between cholesterol and risk of mortality in patients with advanced CKD is unsurprising (41, 42). Based on our findings, it may be rational to embrace the “the earlier the better” concept by initiating statin therapy in patients with early stage CKD, because the risk of progression to ESRD conferred by baseline hyperlipidemia (e.g., T-CHO) outweighs that conferred by the longitudinal trajectories over the course of CKD (supplemental Table S5, Model 6). Baseline lipid profile may represent an individual’s overall status of endothelial dysfunction and chronic inflammation (43). For instance, the correlation coefficient (γ = 0.279) between baseline UPCR and T-CHO was high, which implied that T-CHO may serve as a surrogate marker for endothelial dysfunction or glomerular injury (44). Future research should focus on the therapy intensity, statin type, and role of alternative lipid-lowering agents other than statins, such as fish oil, during the early stages of CKD.
In CKD populations, the relationship between TG level and risk of death remains uncertain, as does the relationship between TG and risk of progression to ESRD (45, 46). In the present study, the TG level at baseline and its longitudinal change pattern were not associated with a rapid decline in eGFR and risk of mortality, which is concordant with the current KDIGO guideline that downplays the indication of fibric acid derivatives in CKD populations (12). However, in the subgroup analysis, we discovered that a longitudinal trajectory of TG that was stably higher than 200 mg/dl was associated with risk of death among CKD patients without diabetes. This finding is novel and has not been carefully evaluated in the current literature (47, 48). A large-scale trial must be conducted to determine whether TG control should be focused on CKD patients without diabetes. However, the lack of harmful effects of a high TG trajectory on all-cause mortality in diabetic patients with CKD challenges the latest American Diabetes Association practice guideline for targeting a TG level below 150 mg/dl (49). More experimental research is required to explore the underlying pathogenesis and determine whether nephrologists should target this level in diabetic patients with CKD or simply adopt the “one-size-fits-all” approach of the KDIGO guideline.
Our findings on HDL-C were partially consistent with those of some previous studies. The MDRD study demonstrated a protective effect of high baseline HDL-C on a declining eGFR among patients with CKD, but our study showed no renal protective effect of baseline HDL-C at the enrollment of the pre-ESRD program (19). Nonetheless, we did find that a trajectory of frequent hypo-HDL-C cholesterolemia over the disease course was linked to higher all-cause mortality in patients with CKD. Concordant to our findings derived from baseline HDL-C, a recent prospective study by Zewinger et al. (50) showed no association between baseline HDL-C and all-cause mortality. In patients with CAD or at high risk of CAD, two multicenter clinical trials failed to prove the beneficial effects of pharmacologically increased HDL-C levels on coronary artery event protection (51, 52). In a recent large cohort study of 116,508 individuals, extremely high HDL-C levels were found to be associated with high mortality risk (53). Other large cohort studies conducted in Canada (Cardiovascular Health in Ambulatory Care Research Team) and by the US Department of Veterans Affairs revealed similar uncertain findings on the cardioprotective effect of HDL-C (54, 55). The level and functional heterogeneity of HDL-C may be genetically and environmentally (e.g., uremic status) influenced in CKD populations (56). Therefore, serial changes of HDL-C level over the CKD course may better reflect the functionality of the kidney to catabolize HDL-C, which could be a potential biomarker for disease progression in CKD. The components of HDL-C are dynamic, and different subpopulations of HDL-C can be classified by size, density, electrophoretic mobility, and apo composition. Although the effect of the size of HDL-C shifting toward atherogenic small particles in patients with CKD remains controversial, this provides a possible explanation of the lack of protective effects of high HDL-C at baseline in this population (57, 58). For instance, increasing evidence suggests the pathogenic importance of dysfunctional HDL-C in mediating endothelial inflammation and hypertension through Toll-like receptor-2 (TLR-2) activation (59). Furthermore, the resolution of the current lipid classification might be insufficient to accurately portray the risk profiles of functional heterogeneity of HDL-C in patients with CKD. Our results provide strong impetus for future research on risk characterization and stratification of HDL-C subclassifications (e.g., HDL2-C and HDL3-C) in CKD. In addition, the sex-specific effect of HDL-C modification on mortality must be verified by more prospective studies. Inconsistent results among baseline HDL-C value, longitudinal HDL-C trajectory, and longitudinal hypo-HDL cholesterolemia trajectory would also need to be verified in future large studies, which should also focus on assessing the optimal range of HDL-C in CKD populations (60).
The strength of the present study was the well-designed patient registry that employed EMRs, which greatly improved the aggregation of biochemical data. The median number of repeated measurements of eGFR and lipid profiles was five, which enabled us to obtain detailed insight into the longitudinal lipid trajectories in patients with CKD. However, several limitations of the study must be discussed. First, we did not evaluate the longitudinal pattern of statin use, and adherence patterns with which to evaluate the effectiveness of lipid-lowering agents were unavailable. Nonetheless, serum lipid measurements are excellent biomarkers of internal doses of lipids, integrating all sources of variation affecting “lipid exposure” at the individual level, including lipid metabolism, statin therapy, dietary modification, and adherence pattern. Second, the threat of residual confounding can never be completely excluded in an observational study that employs registry-based EMRs. Information, such as genetic variations, dietary patterns, and environmental factors such as air pollution and heavy metal exposure, was unavailable for analysis. Third, this study was performed at a single tertiary medical center, which might limit the generalizability of our findings. For instance, racial difference in the regulation of lipid metabolism may be significant, and how lipid metabolism responds to changing kidney function and lipid-lowering agents may also be different between races.
In conclusion, T-CHO and LDL-C levels were related to accelerated CKD progression to ESRD; therefore, applying lipid-lowering agents at an earlier stage of CKD may retard the progression of CKD. Strict control of TG may also have some benefit for overall survival in CKD patients without diabetes. Our findings inform clinical practice to consider the “the earlier the better” approach in patients with CKD. The inconsistent relationships of baseline HDL-C levels and longitudinal HDL-C trajectories with all-cause mortality in the Taiwanese CKD population warrant further investigation to define the optimal range of HDL-C. Although our study provides new insight into the old controversy concerning the prognostic role of lipid profile in CKD populations, large experimental trials are vital for clarifying why, when, and how to optimize dyslipidemia in CKD, which is an increasing burden on health care systems worldwide.
Supplementary Material
Footnotes
Abbreviations:
- CAD
- coronary artery disease
- CKD
- chronic kidney disease
- CMUH
- China Medical University Hospital
- eGFR
- estimated glomerular filtration rate
- EMR
- electronic medical record
- ESRD
- end-stage renal disease
- GBTM
- group-based trajectory modeling
- HDL-C
- HDL cholesterol
- HR
- hazard ratio
- IQR
- interquartile range
- KDIGO
- Kidney Disease: Improving Global Outcomes
- LDL-C
- LDL cholesterol
- NCEP ATP III
- National Cholesterol Education Program-Adult Treatment Panel III
- PCR
- protein-to-creatinine ratio
- SUA
- serum uric acid
- T-CHO
- total cholesterol
- TG
- triglyceride
- UPCR
- urine protein-to-creatinine ratio
This work was supported by Ministry of Science and Technology of Taiwan Grant 106-2314-B-039-041-MY3. All authors have completed the International Committee of Medical Journal Editors uniform disclosure form (available on request from the corresponding author) and declare that they have no competing interests. The results presented in this paper have not been published previously in whole or in part, except in abstract format.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Mikolasevic I., Zutelija M., Mavrinac V., and Orlic L.. 2017. Dyslipidemia in patients with chronic kidney disease: etiology and management. Int. J. Nephrol. Renovasc. Dis. 10: 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaziri N. D. 2006. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 290: F262–F272. [DOI] [PubMed] [Google Scholar]
- 3.Kasiske B. L. 1998. Hyperlipidemia in patients with chronic renal disease. Am. J. Kidney Dis. 32: S142–S156. [DOI] [PubMed] [Google Scholar]
- 4.Sarnak M. J., Bloom R., Muntner P., Rahman M., Saland J. M., Wilson P. W., and Fried L.. 2015. KDOQI US commentary on the 2013 KDIGO clinical practice guideline for lipid management in CKD. Am. J. Kidney Dis. 65: 354–366. [DOI] [PubMed] [Google Scholar]
- 5.Vaziri N. D. 2014. Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clin. Exp. Nephrol. 18: 265–268. [DOI] [PubMed] [Google Scholar]
- 6.Chmielewski M., Rutkowski B., and Lindholm B.. 2009. Mechanisms and clinical implications of lipid disorders in chronic kidney disease. Clin. Lipidol. 4: 449–456. [Google Scholar]
- 7.Vaziri N. D., Liang K., and Parks J. S.. 2001. Down-regulation of hepatic lecithin:cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int. 59: 2192–2196. [DOI] [PubMed] [Google Scholar]
- 8.Moradi H., Vaziri N. D., Kashyap M. L., Said H. M., and Kalantar-Zadeh K.. 2013. Role of HDL dysfunction in end-stage renal disease: a double-edged sword. J. Ren. Nutr. 23: 203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwan B. C., Kronenberg F., Beddhu S., and Cheung A. K.. 2007. Lipoprotein metabolism and lipid management in chronic kidney disease. J. Am. Soc. Nephrol. 18: 1246–1261. [DOI] [PubMed] [Google Scholar]
- 10.Noor S., Zuberi N. A., Fatima F., Iqbal T., and Ullah K.. 2014. Status of lipid profile in different stages of chronic kidney disease. Ann. Abbasi Shaheed Hosp. Karachi Med. Dent. Coll. 19: 62–66. [Google Scholar]
- 11.Bulbul M. C., Dagel T., Afsar B., Ulusu N. N., Kuwabara M., Covic A., and Kanbay M.. 2018. Disorders of lipid metabolism in chronic kidney disease. Blood Purif. 46: 144–152. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. 2013. KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney Int. Suppl. 3: 259–305. [Google Scholar]
- 13.Fox C. S., Larson M. G., Leip E. P., Culleton B., Wilson P. W., and Levy D.. 2004. Predictors of new-onset kidney disease in a community-based population. JAMA. 291: 844–850. [DOI] [PubMed] [Google Scholar]
- 14.Muntner P., Coresh J., Smith J. C., Eckfeldt J., and Klag M. J.. 2000. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 58: 293–301. [DOI] [PubMed] [Google Scholar]
- 15.Mänttäri M., Tiula E., Alikoski T., and Manninen V.. 1995. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 26: 670–675. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffner E. S., Kurth T., Curhan G. C., Glynn R. J., Rexrode K. M., Baigent C., Buring J. E., and Gaziano J. M.. 2003. Cholesterol and the risk of renal dysfunction in apparently healthy men. J. Am. Soc. Nephrol. 14: 2084–2091. [DOI] [PubMed] [Google Scholar]
- 17.Hsu C. Y., Bates D. W., Kuperman G. J., and Curhan G. C.. 2000. Diabetes, hemoglobin A(1c), cholesterol, and the risk of moderate chronic renal insufficiency in an ambulatory population. Am. J. Kidney Dis. 36: 272–281. [DOI] [PubMed] [Google Scholar]
- 18.Rahman M., Yang W., Akkina S., Alper A., Anderson A. H., Appel L. J., He J., Raj D. S., Schelling J., Strauss L., et al. 2014. Relation of serum lipids and lipoproteins with progression of CKD: the CRIC study. Clin. J. Am. Soc. Nephrol. 9: 1190–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunsicker L. G., Adler S., Caggiula A., England B. K., Greene T., Kusek J. W., Rogers N. L., and Teschan P. E.. 1997. Predictors of the progression of renal disease in the Modification of Diet in Renal Disease Study. Kidney Int. 51: 1908–1919. [DOI] [PubMed] [Google Scholar]
- 20.Washio M., Okuda S., Ikeda M., Hirakata H., Nanishi F., Onoyama K., Yoshimura T., and Fujishima M.. 1996. Hypercholesterolemia and the progression of the renal dysfunction in chronic renal failure patients. J. Epidemiol. 6: 172–177. [DOI] [PubMed] [Google Scholar]
- 21.Ozsoy R. C., van der Steeg W. A., Kastelein J. J., Arisz L., and Koopman M. G.. 2007. Dyslipidaemia as predictor of progressive renal failure and the impact of treatment with atorvastatin. Nephrol. Dial. Transplant. 22: 1578–1586. [DOI] [PubMed] [Google Scholar]
- 22.Chen S. C., Hung C. C., Kuo M. C., Lee J. J., Chiu Y. W., Chang J. M., Hwang S. J., and Chen H. C.. 2013. Association of dyslipidemia with renal outcomes in chronic kidney disease. PLoS One. 8: e55643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massy Z. A., Nguyen Khoa T., Lacour B., Descamps-Latscha B., Man N. K., and Jungers P.. 1999. Dyslipidaemia and the progression of renal disease in chronic renal failure patients. Nephrol. Dial. Transplant. 14: 2392–2397. [DOI] [PubMed] [Google Scholar]
- 24.Lin C. M., Yang M. C., Hwang S. J., and Sung J. M.. 2013. Progression of stages 3b-5 chronic kidney disease–preliminary results of Taiwan national pre-ESRD disease management program in Southern Taiwan. J. Formos. Med. Assoc. 112: 773–782. [DOI] [PubMed] [Google Scholar]
- 25.Tsai C. W., Chiu H. T., Huang H. C., Ting I. W., Yeh H. C., and Kuo C. C.. 2018. Uric acid predicts adverse outcomes in chronic kidney disease: a novel insight from trajectory analyses. Nephrol. Dial. Transplant. 33: 231–241. [DOI] [PubMed] [Google Scholar]
- 26.Tsai C.W., I. W. Ting, H. C. Yeh, and C. C. Kuo. 2017. Longitudinal change in estimated GFR among CKD patients: a 10-year follow-up study of an integrated kidney disease care program in Taiwan. PLoS One. 12: e0173843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Kidney Foundation. 2002. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am. J. Kidney Dis. 39 (2 Suppl. 1): S1–S266. [PubMed] [Google Scholar]
- 28.Anandaraja S., Narang R., Godeswar R., Laksmy R., and Talwar K. K.. 2005. Low-density lipoprotein cholesterol estimation by a new formula in Indian population. Int. J. Cardiol. 102: 117–120. [DOI] [PubMed] [Google Scholar]
- 29.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002. Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106: 3143–3421.. [PubMed]
- 30.Levey A. S., Bosch J. P., Lewis J. B., Greene T., Rogers N., and Roth D.. 1999. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann. Intern. Med. 130: 461–470. [DOI] [PubMed] [Google Scholar]
- 31.Nagin D. S., Lynam D., Raudenbush S., and Roeder K.. 1999. Analyzing developmental trajectories: a semiparametric, group-based approach. Psychol. Methods. 4: 139–157. [Google Scholar]
- 32.Nagin D. S., and Odgers C. L.. 2010. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 6: 109–138. [DOI] [PubMed] [Google Scholar]
- 33.Jones B. L., Nagin D. S., and Roeder K.. 2001. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol. Methods Res. 29: 374–393. [Google Scholar]
- 34.Fine J. P., and Gray R. J.. 1999. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 94: 496–509. [Google Scholar]
- 35.Ravnskov U., Diamond D. M., Hama R., Hamazaki T., Hammarskjold B., Hynes N., Kendrick M., Langsjoen P. H., Malhotra A., Mascitelli L., et al. 2016. Lack of an association or an inverse association between low-density-lipoprotein cholesterol and mortality in the elderly: a systematic review. BMJ Open. 6: e010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamazaki T., Okuyama H., Ogushi Y., and Hama R.. 2015. Towards a paradigm shift in cholesterol treatment. A re-examination of the cholesterol issue in Japan. Ann. Nutr. Metab. 66 (Suppl. 4): 1–116. [DOI] [PubMed] [Google Scholar]
- 37.Baigent C., Landray M. J., Reith C., Emberson J., Wheeler D. C., Tomson C., Wanner C., Krane V., Cass A., Craig J., et al. 2011. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 377: 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanguankeo A., Upala S., Cheungpasitporn W., Ungprasert P., and Knight E. L.. 2015. Effects of statins on renal outcome in chronic kidney disease patients: a systematic review and meta-analysis. PLoS One. 10: e0132970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vos E., Rose C. P., and Biron P.. 2013. Point: why statins have failed to reduce mortality in just about anybody. J. Clin. Lipidol. 7: 222–224. [DOI] [PubMed] [Google Scholar]
- 40.Weverling-Rijnsburger A. W., Blauw G. J., Lagaay A. M., Knook D. L., Meinders A. E., and Westendorp R. G.. 1997. Total cholesterol and risk of mortality in the oldest old. Lancet. 350: 1119–1123. [DOI] [PubMed] [Google Scholar]
- 41.Kooman J. P., Kotanko P., Schols A. M., Shiels P. G., and Stenvinkel P.. 2014. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 10: 732–742. [DOI] [PubMed] [Google Scholar]
- 42.Cholesterol Treatment Trialists’ (CTT) Collaboration, Herrington W. G., Emberson J., Mihaylova B., Blackwell L., Reith C., Solbu M. D., Mark P. B., Fellstrom B., Jardine A. G., et al. 2016. Impact of renal function on the effects of LDL cholesterol lowering with statin-based regimens: a meta-analysis of individual participant data from 28 randomised trials. Lancet Diabetes Endocrinol. 4: 829–839. [DOI] [PubMed] [Google Scholar]
- 43.Stenvinkel P., Carrero J. J., Axelsson J., Lindholm B., Heimburger O., and Massy Z.. 2008. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle? Clin. J. Am. Soc. Nephrol. 3: 505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zandi-Nejad K., Eddy A. A., Glassock R. J., and Brenner B. M.. 2004. Why is proteinuria an ominous biomarker of progressive kidney disease? Kidney Int. Suppl. 66 (Suppl. 92): S76–S89. [DOI] [PubMed] [Google Scholar]
- 45.Cases A., and Coll E.. 2005. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int. Suppl. 68 (Suppl. 99): S87–S93. [DOI] [PubMed] [Google Scholar]
- 46.Visconti L., Benvenga S., Lacquaniti A., Cernaro V., Bruzzese A., Conti G., Buemi M., and Santoro D.. 2016. Lipid disorders in patients with renal failure: role in cardiovascular events and progression of chronic kidney disease. J. Clin. Transl. Endocrinol. 6: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chawla V., Greene T., Beck G. J., Kusek J. W., Collins A. J., Sarnak M. J., and Menon V.. 2010. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin. J. Am. Soc. Nephrol. 5: 1582–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Navaneethan S. D., Schold J. D., Arrigain S., Thomas G., Jolly S. E., Poggio E. D., Schreiber M. J. Jr., Sarnak M. J., and Nally J. V. Jr.. 2012. Serum triglycerides and risk for death in stage 3 and stage 4 chronic kidney disease. Nephrol. Dial. Transplant. 27: 3228–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.American Diabetes Association. 2017. Standards of medical care in diabetes. Diabetes Care. 40 (Suppl. 1): S1–S134. [DOI] [PubMed] [Google Scholar]
- 50.Zewinger S., Speer T., Kleber M. E., Scharnagl H., Woitas R., Lepper P. M., Pfahler K., Seiler S., Heine G. H., Marz W., et al. 2014. HDL cholesterol is not associated with lower mortality in patients with kidney dysfunction. J. Am. Soc. Nephrol. 25: 1073–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz G. G., Olsson A. G., Abt M., Ballantyne C. M., Barter P. J., Brumm J., Chaitman B. R., Holme I. M., Kallend D., Leiter L. A., et al. ; dal-OUTCOMES Investigators . 2012. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 52.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J., Komajda M., Lopez-Sendon J., Mosca L., Tardif J. C., Waters D. D., et al. 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122. [DOI] [PubMed] [Google Scholar]
- 53.Madsen C. M., Varbo A., and Nordestgaard B. G.. 2017. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur. Heart J. 38: 2478–2486. [DOI] [PubMed] [Google Scholar]
- 54.Ko D. T., Alter D. A., Guo H., Koh M., Lau G., Austin P. C., Booth G. L., Hogg W., Jackevicius C. A., Lee D. S., et al. 2016. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J. Am. Coll. Cardiol. 68: 2073–2083. [DOI] [PubMed] [Google Scholar]
- 55.Bowe B., Xie Y., Xian H., Balasubramanian S., Zayed M. A., and Al-Aly Z.. 2016. High density lipoprotein cholesterol and the risk of all-cause mortality among US veterans. Clin. J. Am. Soc. Nephrol. 11: 1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rysz-Górzyńska M., and Banach M.. 2016. Subfractions of high-density lipoprotein (HDL) and dysfunctional HDL in chronic kidney disease patients. Arch. Med. Sci. 12: 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Boer I. H., and Brunzell J. D.. 2014. HDL in CKD: how good is the “good cholesterol?”. J. Am. Soc. Nephrol. 25: 871–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Untersteller K., Meissl S., Trieb M., Emrich I. E., Zawada A. M., Holzer M., Knuplez E., Fliser D., Heine G. H., and Marsche G.. 2018. HDL functionality and cardiovascular outcome among nondialysis chronic kidney disease patients. J. Lipid Res. 59: 1256–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Speer T., Rohrer L., Blyszczuk P., Shroff R., Kuschnerus K., Krankel N., Kania G., Zewinger S., Akhmedov A., Shi Y., et al. 2013. Abnormal high-density lipoprotein induces endothelial dysfunction via activation of Toll-like receptor-2. Immunity. 38: 754–768. [DOI] [PubMed] [Google Scholar]
- 60.Martin S. S., Khokhar A. A., May H. T., Kulkarni K. R., Blaha M. J., Joshi P. H., Toth P. P., Muhlestein J. B., Anderson J. L., Knight S., et al. ; Lipoprotein Investigators Collaborative (LIC) . 2015. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur. Heart J. 36: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.