Abstract
During prolonged fasting, the liver plays a central role in maintaining systemic energy homeostasis by producing glucose and ketones in processes fueled by oxidation of fatty acids liberated from adipose tissue. In mice, this is accompanied by transient hepatic accumulation of glycerolipids. We found that the hepatic expression of monoacylglycerol acyltransferase 1 (Mogat1), an enzyme with monoacylglycerol acyltransferase (MGAT) activity that produces diacylglycerol from monoacylglycerol, was significantly increased in the liver of fasted mice compared with mice given ad libitum access to food. Basal and fasting-induced expression of Mogat1 was markedly diminished in the liver of mice lacking the transcription factor PPARα. Suppressing Mogat1 expression in liver and adipose tissue with antisense oligonucleotides (ASOs) reduced hepatic MGAT activity and triglyceride content compared with fasted controls. Surprisingly, the expression of many other PPARα target genes and PPARα activity was also decreased in mice given Mogat1 ASOs. When mice treated with control or Mogat1 ASOs were gavaged with the PPARα ligand, WY-14643, and then fasted for 18 h, WY-14643 administration reversed the effects of Mogat1 ASOs on PPARα target gene expression and liver triglyceride content. In conclusion, Mogat1 is a fasting-induced PPARα target gene that may feed forward to regulate liver PPARα activity during food deprivation.
Keywords: fatty acid/oxidation, lipidomics, liver, PPARs, triglycerides, fasting, MGAT, Mogat1
During prolonged fasting, the liver receives an influx of carbon substrates from adipose tissue lipolysis and skeletal muscle lactate production and proteolysis. The liver must convert these substrates into glucose and ketones for energy use in extrahepatic tissues, most notably the brain, via processes that are fueled by high level fatty acid oxidation. In mice, the marked influx of fatty acids leads to intrahepatic triglyceride accumulation (steatosis) despite high rates of fatty acid oxidation. Although a number of studies have examined the effects of fasting on hepatic intermediary metabolism, some metabolic pathways remain understudied.
Hepatic triglyceride synthesis is generally believed to use primarily glycerol-3-phosphate as an initial substrate. Fatty acyl chains are esterified to glycerol-3-phosphate by acyltransferases to form lysophosphatidic acid and then phosphatidic acid (PA), which is converted into diacylglycerol (DAG) by the PA phosphohydrolases (1). Alternatively, monoacylglycerol (MAG), which is composed of one fatty acid and a glycerol backbone (unphosphorylated), can be directly converted into DAG through acylation by MAG acyltransferases (MGATs, gene symbol Mogats) (1, 2). The MGAT pathway is highly active in the intestine, where it is involved in dietary fat absorption (3–5). However, much less is known about the MGAT enzymes in other tissues with high rates of triglyceride synthesis, such as adipose tissue and liver. Mice have two functional MGAT isoforms, while humans have three (4–7). In mice, Mogat1 is highly expressed in liver, stomach, and adipose tissue, while Mogat2 is the intestinal isoform (4, 6, 8).
MGAT activity increases in liver during times of high fat oxidation in multiple animal models and has been suggested to retain essential fatty acids by preventing their catabolism during energy deprivation (9, 10). Recently, several groups have shown an important function of Mogat1 in obesity-related nonalcoholic fatty liver disease (9, 11–14). Mogat1 expression was induced in liver of obese mice with hepatic steatosis and the expression of Mogat1 in this context was driven by PPARγ. Strategies to suppress Mogat1 with RNAi methodology markedly improved hepatic insulin resistance (12, 13) and reduced hepatic steatosis in some models (13). While recent work with whole-body Mogat1 knockout mice has questioned the contribution of this gene to tissue MGAT activity (15), acute suppression of Mogat1 expression markedly reduced MGAT activity in steatotic liver (12), suggesting chronic compensation in knockout mice.
Herein, we demonstrate that the hepatic expression of Mogat1 and MGAT activity is increased in the liver of mice undergoing prolonged fasting. Furthermore, hepatic Mogat1 expression is dependent on PPARα, which is a master regulator of the hepatic fasting response (16, 17). We demonstrate that mice with antisense oligonucleotide (ASO)-mediated acute knockdown of Mogat1 have decreased MGAT activity and lower liver triglyceride content. Interestingly, Mogat1 suppression during fasting also led to reduced expression of PPARα target genes; an effect that was reversed by the synthetic PPARα ligand, WY-14643. These data suggest that Mogat1 is a fasting-induced PPARα target gene that may feed-forward to enhance PPARα activity and the systemic response to food deprivation.
MATERIALS AND METHODS
Animal studies
All mouse studies were approved by the Institutional Animal Care and Use Committee of Washington University. All mice were in the pure C57BL/6J background. Male whole-body PPARα-null mice were obtained from Taconic. Mice were group housed and maintained on standard laboratory chow on a 12 h light/dark cycle. On the day of the experiment, 10- to 12-week-old male and female mice were randomly assigned to receive either ad libitum (fed) access to standard laboratory chow or food deprived (fasted) for the times indicated. Fasts were timed such that all mice were euthanized at 0900. Liver tissue was harvested and immediately frozen in liquid nitrogen until further use. Blood was collected by venipuncture of the inferior vena cava in EDTA-coated tubes and plasma was frozen after collection by centrifugation.
ASO treatments were performed as previously described (12). Briefly, 8-week-old male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were given twice weekly intraperitoneal injections of ASO directed against Mogat1 [5′-GATCTTGGCCACGTGGAGAT-3′ (20-mer)] or scramble control (Ionis, Pharmaceuticals, Inc., Carlsbad, CA; 25 mg/kg body weight) for 3 weeks. Mice were then fasted for 24 h or continued to receive ad libitum access to food. Knockdown was confirmed by the quantitative (q)PCR methods described below using the following sequences against Mogat1: forward 5′- TGGTGCCAGTTTGGTTCCAG-3′ and reverse 5′-TGCTCTGAGGTCGGGTTCA-3′.
For studies involving PPARα ligand administration, mice were treated with ASO as before and given a one-time oral gavage of vehicle alone (0.5% carboxymethyl cellulose) or WY-14643 (100 mg/kg body weight; Cayman Chemical, Ann Arbor, MI) (18). Mice were then immediately fasted for 18 h and harvested as before.
mRNA isolation and gene expression analysis
Total liver RNA was isolated using RNA-BEE (Iso-Tex Diagnostics, Friendswood, TX) according to manufacturer’s instructions. RNA was reverse transcribed into cDNA using TaqMan high-capacity reverse transcriptase (Life Technologies, Woolston, WA). Quantitative RT-PCR was performed using Power SYBR green and measured on an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, CA). Results were normalized to 36B4 expression and calculated using the 2−ΔΔct method and shown as arbitrary units relative to control groups. Primer sequences are listed in Table 1.
TABLE 1.
Primer sequences for qPCR
| Mouse | Forward | Reverse |
| 36B4 | gca gac aac gtg ggc tcc aag cag at | ggt cct cct tgg tga aca cga agc cc |
| Mogat1 | tgg tgc cag ttt ggt tcc ag | tgc tct gag gtc ggg ttc a |
| Mogat2 | tgg gag cgc agg tta cag a | Cag gtg gca tac agg aca ga |
| Mgll | cgg act tcc aag ttt ttg tca ga | gca gcc act agg atg gag atg |
| Atgl | gga cac ctc aat aat gtt ggc | ctt gag cag cta gaa caa tg |
| Dgat1 | tgc agt ttg gag acc gcg agt t | cac cca ttt gct gct gcc atg t |
| Napepld | agc gcc aag cta tca gta tcc | tca gcc atc tga gca cat tcg |
| Lpin1 | ccc tcg att tca agg cac ct | gca gcc tgt ggc aat tca |
| Lpin2 | gaa gtg gcg gct ctc tat ttc | aga ggg tta cat cag gca agt |
| Angptl4 | cat cct ggg acg aga tga act | tga caa gcg tta cca cag gc |
| Apoc3 | ggt cca gga tgc gct aag ta | tgc tcc agt agc ctt tca gg |
| Acox1 | gga tgg tag tcc gga gaa ca | agt ctg gat cgt tca gaa tca ag |
| Cpt1a | gga cgc gcc cat cg | cca ctg tag cct ggt ggg t |
| Acadm | gga aat gat caa caa aaa aag tat tt | atg gcc gcc aca tca ga |
| Acadl | cct ccg ccc gat gtt gtc att c | gct gtc cac aaa agc tct ggt gac ac |
| Bdh1 | ttc ccc ttc tcc gaa gag c | ccc aga ggg tgc atc tca tag |
| Hmgcs2 | acc tgc ggg cct tgg at | ggt gaa aag gct ggt tgt ttc c |
| Pck1 | ggg tgc aga atc tcg agt tg | cac cat cac ctc ctg gaa ga |
| Ppara | act acg gag ttc acg cat gtg | ttg tcg tac acc agc ttc agc |
| Pparg1 | gga aga cca ctc gca ttc ctt | gta atc agc aac cat tgg gtc a |
| Pparg2 | tcg ctg atg cac tgc cta tg | gag agg tcc aca gag ctg att |
| Crebh | cct gtt tga tcg gca gga c | cgg ggg acc ata atg gag a |
| Ppargc1a | cgg aaa tca tat cca acc ag | tga gga ccg cta gca agt ttg |
Liver and plasma metabolites
Frozen liver tissue (100 mg/ml) was homogenized in ice-cold PBS and lipids were solubilized in 1% sodium deoxycholate via vortexing and heating at 37°C for 5 min. Hepatic triglyceride content was measured using a commercially available triglyceride colorimetric assay kit as previously described (19). Plasma samples were analyzed as previously described (12). Briefly, plasma NEFAs were measured using a colorimetric assay from WAKO (Wako Diagnostics, Richmond, VA). Plasma triglyceride was measured using the Thermo Fisher kit and plasma ketone bodies were measured using a 3-hydroxybutyrate enzymatic assay (WAKO). For blood glucose measurements, blood was procured from the tail just prior to euthanization, and glucose was measured using a OneTouch Ultra glucometer (LifeScan Inc.). Plasma insulin and glucagon were measured by Singulex immunoassay and mouse Mercodia glucagon ELISA – 10 μL, respectively, by the Washington University Core Laboratory for Clinical Studies.
MGAT enzymatic activity assay
MGAT activity was determined as described (12) with slight modification. Membrane fractions were isolated from 50 mg of liver tissue by homogenization in membrane buffer [50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 250 mM sucrose, and complete protease inhibitors tablets (Roche Diagnostics, Indianapolis, IN)]. Homogenates were precleared of whole cell debris at 500 g for 10 min at 4°C followed by ultracentrifugation at 21,000 g for 1 h at 4°C. The resultant pellet was suspended by vigorous pipetting in membrane buffer without protease inhibitors. Fifty micrograms of membrane were incubated in 5 mM MgCl2, 1.25 mg/ml BSA, 200 mM sucrose, 100 mM Tris-HCl (pH 7.4), 20 μM 14C-oleoyl-CoA (American Radiolabeled Chemicals), and 200 μM sn-2-oleoylglycerol for 5 min. The reaction was terminated with 50 μl of 1% phosphoric acid. Lipids were extracted in 2:1 v/v% CHCl3:methanol and separated by thin-layer chromatography in hexane/ethyl ether/acetic acid (80:20:1, v/v/v %). Samples were run against standards for FFA, DAG, and triglyceride and corresponding spots were scraped from the plate and 14C-radioactivity was measured via scintillation counter. Backgrounds were calculated from reaction mixtures without membrane fractions.
PPARα activity assay
Mice were treated with ASO for 3 weeks as described above and then fasted overnight. Nuclear fractions were isolated from fresh liver samples according to the manufacture’s protocol (Nuclear Extraction Kit 10009277; Cayman Chemical). Briefly, 250 mg of liver tissue were homogenized in 750 μl of ice-cold hypotonic buffer supplemented with 1 mM DTT and 0.01% NP-40 using a Dounce homogenizer. After a 15 min incubation on ice, the cytosol was removed via a 300 g spin at 4°C for 10 min. The pellets were resuspended in 500 μl of hypotonic buffer for an additional 15 min on ice. Cells were lysed with the addition of 1% NP-40 and cytosolic fractions removed after a 14,000 g spin at 4°C for 30 s. Nuclei were lysed in 100 μl of nuclear extraction buffer for 30 min. The nuclear fractions were removed following a 14,000 g spin at 4°C for 10 min and then immediately flash-frozen in liquid nitrogen.
PPARα activity was measured according to manufacturer’s protocol (PPARα Transcription Factor Assay Kit 10006915; Cayman Chemical). Briefly, 25 μg of nuclear lysates were added in duplicate to each well of the ELISA plate and incubated overnight at 4°C. The plate was read at 450 nm and the results were normalized to control ASO-treated fasted mice following the subtraction of nonspecific binding wells as the blank.
Liver and serum lipid extraction for LC/MS
Liver and serum samples were treated with 3 ml of 2:1 chloroform:methanol. Liver samples were homogenized in the presence of the following internal standards: U-13C-labeled palmitate (100 pmol/mg), C17:0 MAG (10 pmol/mg), and C17:0 DAG (10 pmol/mg). For serum samples, all internal standards were adjusted to 10 pmol/μl serum. Lipid extraction was performed by vortexing for 1 min and bath sonication for 30 min. After adding 1 ml of 0.1 M Tris buffer, samples were vortexed (1 min) and bath sonicated (30 min). The organic and aqueous layers were then separated by centrifugation at 3,000 g for 20 min. The organic layer was collected. Chloroform (1 ml) was mixed with the aqueous layer followed by vortexing, bath sonication, and centrifugation as described above. The organic layers were combined, dried by N2 gas, and reconstituted in 200 μl of 9:1 methanol:chloroform. The extract was transferred to an LC vial for MS analysis.
LC/MS analysis
LC/MS analysis of liver and serum extracts was performed by using an Agilent 6530 or 6540 quadrupole TOF mass spectrometer with an ESI source. Separations were achieved by using an Agilent 1290 capillary UPLC system. Samples were either analyzed in positive ionization mode with a Cortex T3 column (2.7 μm, 150 × 2.1 mm inner diameter; Waters) or in negative ionization mode with a Luna aminopropyl column (3 μm, 150 × 2.0 mm internal diameter; Phenomenex). The T3 column was used with the following mobile phases: A = 95% water, 5% methanol, 5 mM ammonium acetate, 0.1% formic acid (pH 3.14); B = 90% isopropanol and 10% methanol. The following linear gradient was applied: 0 min, 30% B; 10 min, 70% B; 30 min, 87.5% B; 31 min, 100% B; 40 min, 100% B. The aminopropyl column was used with following mobile phases: A = 95% water, 5% acetonitrile, 10 mM ammonium acetate, and 10 mM ammonium hydroxide; B = 95% acetonitrile and 5% water. The following linear gradient was applied: 0 min, 95% B; 2 min, 95% B; 15 min, 50% B; 20 min, 0% B; 25 min, 0% B. The injection volumes were 2 μl. The flow rate was 200 μl/min. The column temperature was maintained at 37°C. Mass detection was set from m/z 100 to 1,700 with a scan rate of 1 spectra/s. The ESI source parameters were: nebulizer pressure, 30 psi; nozzle voltage, 1,000 V; sheath gas temperature, 300°C; flow rate, 11 liters/min; drying gas, 12 liters/min; gas temperature, 300°C; capillary voltage, 3,000 V; fragmentor voltage, 100 V. Data analysis was performed with the Agilent Profinder software. The absolute quantitation of lipids in picomoles per milligram of liver or picomoles per microliter of serum was achieved by measuring the area under the extracted ion chromatogram peak. Data were normalized to an internal standard (U-13C palmitate for FFAs, C17:0 MAG for MAGs) to correct for extraction efficiency.
Statistical analysis
Data were analyzed via SPSS software. Independent samples t-tests, one-way ANOVA, or factorial ANOVAs with Bonferonni corrections were performed. Prior to analysis, normality and equal variance of samples was determined by Kolmogorov-Smirnov testing and Levene testing, respectively. If these conditions were violated logarithmic transformation intended to produce data to satisfy normality and equal variance assumptions were used. Otherwise, nonparametric methods (e.g., Kruskal-Wallis for one-way ANOVAs with Mann-Whitney U post hoc analysis and Friedman’s test for two-way ANOVAs) were used as an alternative to the more standard analyses. P < 0.05 was considered significant.
RESULTS
Mogat1 expression is induced in fasted liver in a PPARα-dependent manner
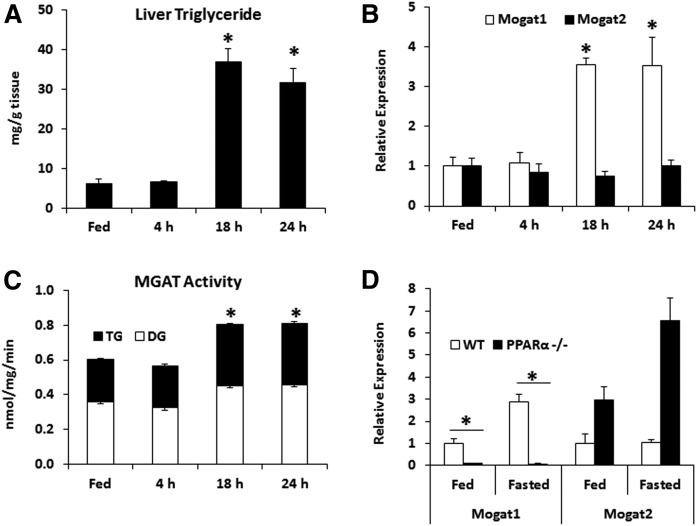
Fasting is a physiologic stimulus associated with high rates of hepatic fatty acid oxidation and increased accumulation of hepatic triglyceride. We fasted mice for 4, 18, or 24 h and found that intrahepatic triglyceride content was increased after 18 and 24 h of fasting (Fig. 1A). Given our interest in the MGAT enzymes that are involved in glycerolipid synthesis, we also quantified their gene expression and found that fasting induced the expression of Mogat1, but not Mogat2 (Fig. 1B). Total hepatic MGAT activity was also increased after 18 and 24 h fasting (Fig. 1C).
Fig. 1.
Mogat1 gene expression and MGAT activity are increased during fasting-induced steatosis and are PPARα dependent. Male C57BL/6J mice were fasted for the times indicated or given free access to food (Fed) prior to euthanization. A: Liver triglycerides are increased during prolonged fasting in mice compared with fed controls. B: Gene expression of Mogat1, but not Mogat2, is significantly upregulated during fasting. C: MGAT activity was measured in hepatic membranes from fasting or fed mice and total activity was significantly increased after 18 and 24 h of fasting. D: Mogat1 induction during fasting (24 h) is PPARα dependent. Data are shown as mean ± SE; *P < 0.05 from fed state unless otherwise indicated; n = 3–6.
Previous work has shown that Mogat1 is target gene of the PPAR family of nuclear receptors, particularly PPARγ (12, 13, 20). However, in this experiment, the expression of Mogat1 was highly dependent on PPARα. Indeed, the fed and fasted expression of Mogat1 was markedly diminished in whole-body PPARα-null mice compared with wild-type controls, whereas Mogat2 expression tended to be increased in whole-body PPARα-null mice in both fed and fasted conditions (Fig. 1D). These data demonstrate that Mogat1, but not Mogat2, is induced in liver by fasting and that its expression is highly dependent on PPARα.
Mogat1 ASO treatment lowers Mogat1 expression and activity in liver and adipose tissue
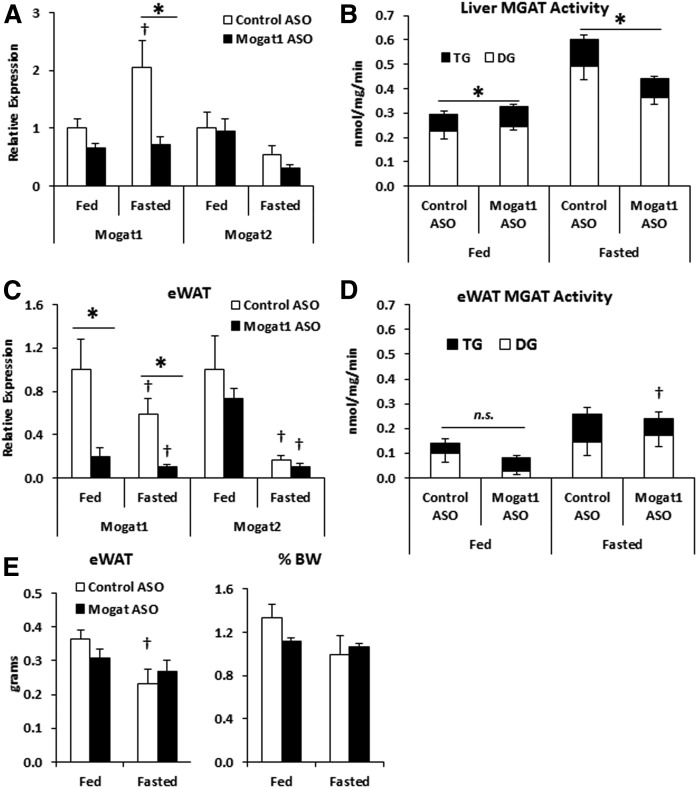
We utilized an acute model of Mogat1 knockdown using ASOs to determine Mogat1 function during fasting (12). Mice were treated for 3 weeks with ASOs directed against Mogat1 or scramble control, and then fasted for 24 h or continued receiving ad libitum access to food. As expected, Mogat1 ASO suppressed hepatic Mogat1 gene expression (Fig. 2A). Mogat1 knockdown did not lead to a compensatory increase in Mogat2 expression in liver (Fig. 2A). Furthermore, 24 h fasting increased total hepatic MGAT activity, while Mogat1 knockdown led to reduced MGAT activity during fasting, indicating the contribution of Mogat1 to total hepatic MGAT activity in fasting (Fig. 2B). Previous work has suggested that ASOs administered by intraperitoneal injection may affect the expression of the targeted gene in adipose tissue. Indeed, epididymal white adipose tissue (eWAT) Mogat1 expression was significantly reduced following ASO treatment (Fig. 2C). However, eWAT tissue MGAT activity was decreased by ASO treatment only in the fed state (Fig. 2D). Furthermore, Mogat1 ASO treatment did not affect adipose tissue weight (Fig. 2E). Fasting reduced blood glucose levels in both fasting conditions, but was not significantly different among ASO treatment groups (Table 2). Interestingly, plasma glucagon levels were increased by Mogat1 ASO treatment in both the fed and fasted states when compared with control ASO treatment (Table 2). However, plasma insulin and glucose levels were unaffected by Mogat1 ASO treatments, suggesting that glucagon levels do not effect glucose homeostasis in these mice (Table 2).
Fig. 2.
Mogat1 ASO treatment suppresses fasting-induced Mogat1 expression and MGAT activity. Male C57BL/6J mice were injected (intraperitoneally) twice weekly with ASOs targeted against Mogat1 or scramble control (25 mg/kg) for 3 weeks prior to fasting (24 h) and liver harvest. A: Fasting mice receiving Mogat1 ASO had significantly lower Mogat1, but not Mogat2, gene expression during fasting compared with controls. B: Mogat1 ASO knockdown suppresses fasting-induced MGAT activity compared with controls. C: Fasting lowered eWAT expression of Mogat1, and Mogat1 was suppressed in both states by Mogat1 ASO treatments. D: eWAT MGAT activity was lowered by Mogat1 ASO treatment in the fed state. E: eWAT weight decreased with fasting in control-treated mice, but was not significantly changed by the ASO treatment. Data are shown as mean ± SE. *P < 0.05 where indicated; †P < 0.05 versus fed state; n = 4–5.
TABLE 2.
Plasma parameters of ASO-treated mice
| Control ASO: Fed | Mogat1 ASO: Fed | Control ASO: Fasted | Mogat1 ASO: Fasted | |
| Blood glucose (mg/dl) | 192.4 ± 6.0 | 175.0 ± 9.38 | 105.2 ± 12.3a | 97.6 ± 10.9a |
| Plasma Insulin (pg/ml) | 699.4 ± 41.6 | 709.6 ± 41.1 | 234.4 ± 38.5 | 329.2 ± 36.4 |
| Plasma Glucagon (pg/ml) | 9.6 ± 1.5 | 35.4 ± 6.4b | 10.9 ± 4.9 | 33.0 ± 6.6 (P = 0.09) |
Blood was procured from the tail vein at the time of euthanization after fasting (24 h) or ad libitum feeding (Fed) for blood glucose measurements. Plasma was collected after euthanization. Insulin and glucagon levels were measured using ELISAs. Data are shown as mean ± SE (n = 5).
P < 0.05 versus fed state.
P < 0.05 versus control littermates in the same feeding condition.
Reducing hepatic and adipose tissue Mogat1 expression results in lower intrahepatic triglyceride content
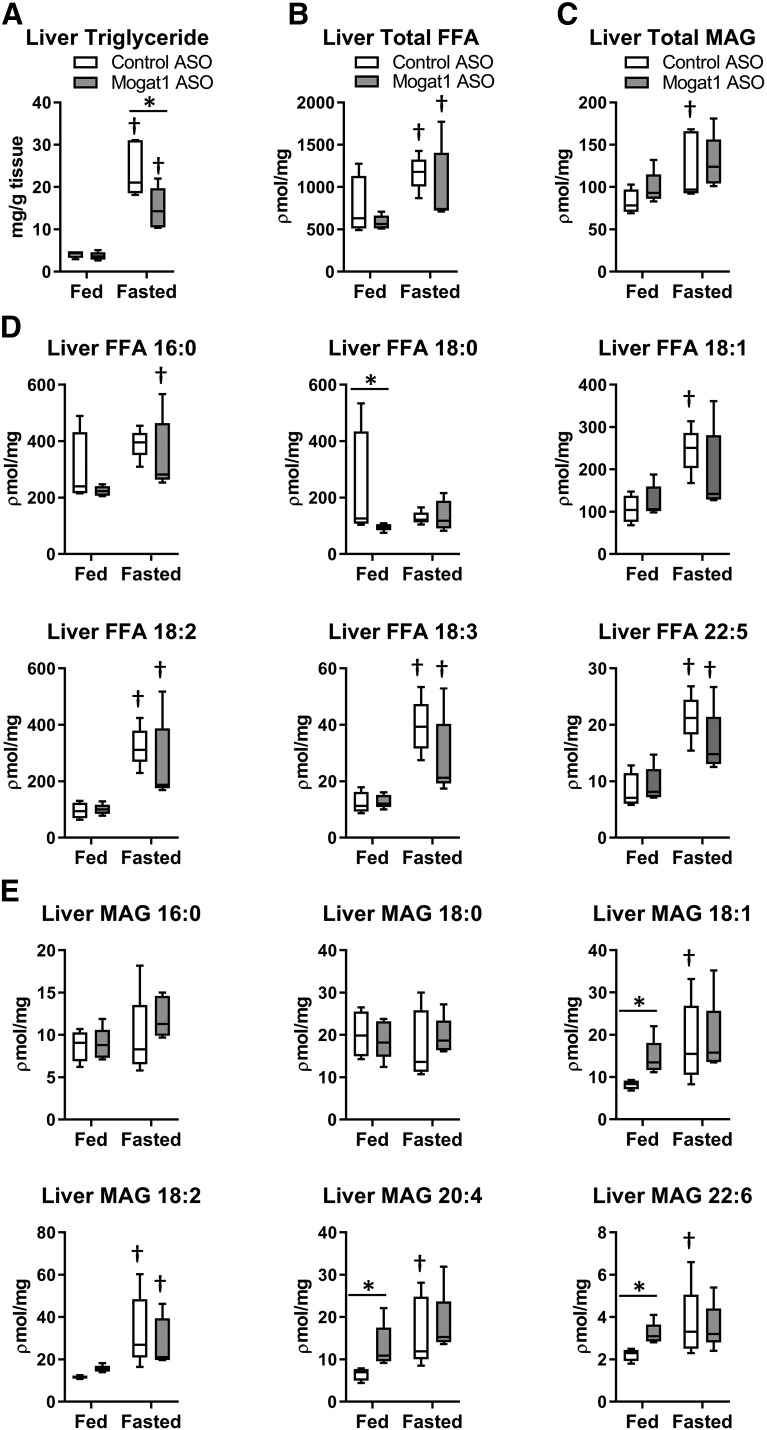
We assessed the effects of Mogat1 ASO on hepatic steatosis and found that fasting increased the intrahepatic content of triglyceride in both ASO groups (Fig. 3A), but compared with control ASO, Mogat1 ASO led to reduced intrahepatic triglyceride accumulation in fasted liver. LC/MS analyses were used to determine that fasting increased the abundance of FFAs and MAGs in liver (Fig. 3B, C), including increases in many species of these lipids (Fig. 3D, E). However, Mogat1 ASO did not affect the accumulation of total hepatic FFAs or MAGs. Interestingly, several long-chain unsaturated MAGs were increased with Mogat1 ASO treatment in the fed state, including MAG 20:4 (2-arachidonoylglycerol).
Fig. 3.
Mogat1 ASO treatment lowers hepatic triglyceride during fasting. A: Mogat1 ASO treatment lowered hepatic triglyceride content during the fasted state compared with control ASO-treated mice. B, C: Total hepatic FFA and MAG were not different between ASO treatment groups as measured by LC/MS. D: Individual hepatic FFA species measured by LC/MS. E: Long-chain unsaturated MAG species were increased by fasting, but only increased by Mogat1 ASO in the fed state. Data are shown as box and whisker plots, *P < 0.05, where indicated, †P < 0.05 vs. fed state; n = 4–5.
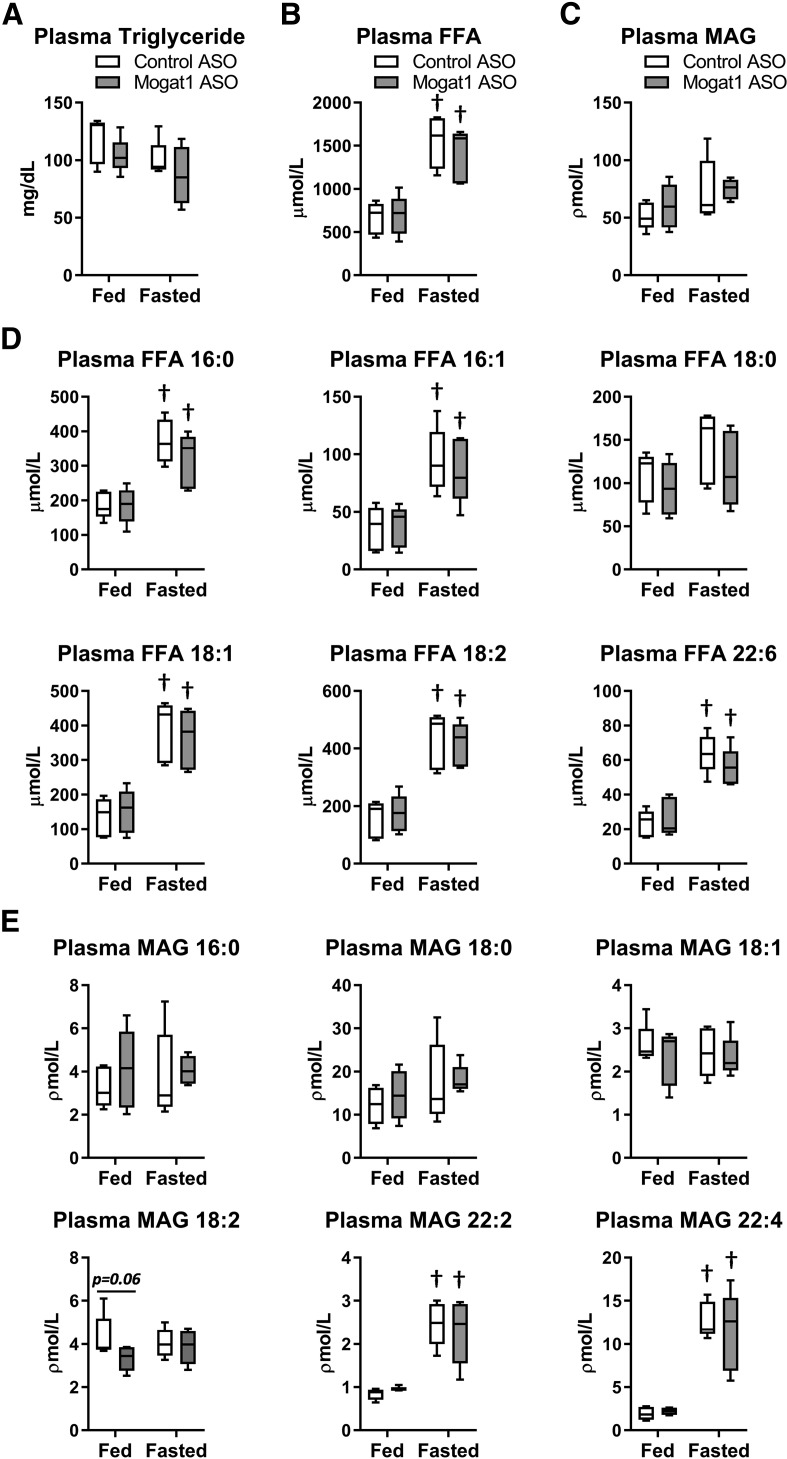
We also assessed plasma metabolite concentrations after Mogat1 knockdown. Triglyceride concentrations were not affected by Mogat1 ASO, though concentrations tended to be lower compared with control ASO-treated mice after fasting (Fig. 4A). As expected, fasting increased the abundance of FFAs in plasma (Fig. 4B), including increases in many fatty acid species (Fig. 4D). There was no effect of Mogat1 ASO on the concentrations of FFAs. It was also determined that MAG was abundant in the plasma of both fed and fasted mice and there was no significant change in total MAG with fasting (Fig. 4C). Interestingly, some MAG species (22:2 and 22:4) were robustly increased in plasma during fasting (Fig. 4E); but again, knockdown of Mogat1 in liver and adipose tissue did not affect the abundance of these species.
Fig. 4.
Mogat1 ASO treatment does not alter fasting plasma lipid levels compared with control ASO-treated mice. A: Plasma triglycerides were measured by colorimetric assay and not affected by fasting or ASO treatment. B, C: Plasma FFAs and MAGs were measured by LC/MS, and FFAs were elevated by fasting in both groups. D, E: Individual plasma FFAs and MAGs were elevated during fasting, but not changed by either ASO. Data are shown as box and whisker plots; *P < 0.05 where indicated; †P < 0.05 versus fed state; n = 5.
Mogat1 ASO treatment lowers fasting-induced PPARα target gene expression
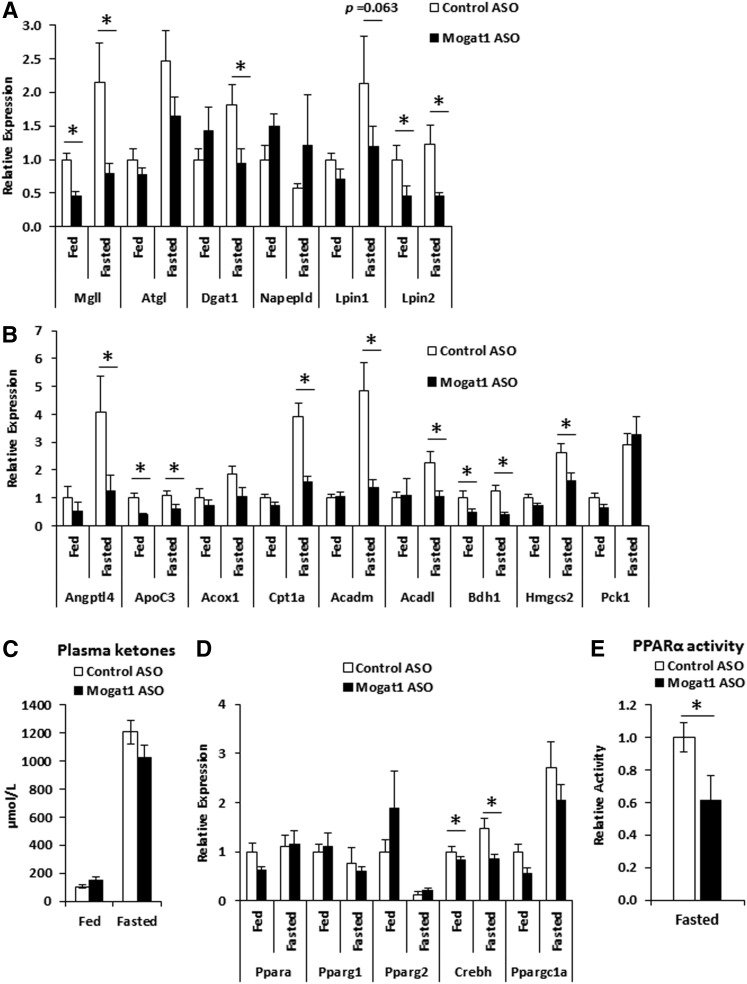
We assessed the expression of several enzymes involved in lipid metabolism in liver of mice treated with Mogat1 ASO and found that Mogat1 ASO treatment unexpectedly led to decreased fasting-induced expression of Mgll, Dgat1, and Lpin2 (Fig. 5A). Because many of these genes are known PPARα targets, we also examined the expression of other genes regulated by PPARα in the context of Mogat1 knockdown. Surprisingly, expression of several other PPARα targets involved in FFA oxidation, lipid metabolism, and ketone generation was reduced with Mogat1 knockdown (Fig. 5B). These genes included Angptl4, Apoc3, Cpt1a, Acadm, Acadl, Bdh1, and Hmgcs2. However, fasting-induced expression of the non-PPARα target gene, Pck1, encoding a gluconeogenic enzyme, was not affected by Mogat1 ASO. To confirm that the effects of Mogat1 ASO treatment were not due to off target effects of ASO treatment, we treated additional mice with an ASO targeting a separate Mogat1 sequence. Indeed, Mogat1 ASO treatment reduced Mogat1 expression during fasting and reduced several genes involved in PPARα-mediated fatty acid oxidation, including Acadm and Acadl (supplemental Fig. S1). This pattern of metabolic gene expression suggested that hepatic fatty acid oxidation and ketogenesis might be affected by Mogat1 ASO treatment. Total plasma ketone concentrations, which were increased by 24 h fasting, were not significantly affected by Mogat1 ASO, but tended to be lower compared with control ASO-treated mice (Fig. 5C).
Fig. 5.
Changes in gene expression of triglyceride synthesis pathway enzymes and PPARα target genes and PPARα activity during prolonged fasting in mice following Mogat1 ASO treatment. Male C57BL/6J mice were injected (intraperitoneally) twice weekly with ASOs targeted against Mogat1 or scramble control (25 mg/kg) for 3 weeks prior to fasting (24 h) and liver harvest. A: Fasting mice receiving Mogat1 ASO had significantly lower hepatic gene expression of glycerolipid enzymes compared with control ASO-treated mice. B: Hepatic PPARα target gene expression. C: Gene expression of transcriptional regulators. D: Nuclear PPARα activity in fasted mice. Data shown as mean ± SE; *P < 0.05 where indicated; n = 6–10.
The data above suggest that Mogat1 may modulate the hepatic fasting response via a mechanism involving PPARα. The expression of the transcription factor, Crebh, which is known to be a PPARα target gene, was decreased by Mogat1 ASO (Fig. 5D). Mogat1 knockdown did not affect the expression of Ppara, Pparg1, Pparg2, or the PPAR coactivator, Ppargc1a, in fasted liver (Fig. 5D). To further confirm that Mogat1 ASO treatment lowers PPARα activity, nuclei from ASO-treated mice fasted for 18 h were isolated and assayed for PPARα activity. These results indicated a 40% reduction in PPARα activity in mice treated with Mogat1 ASO (Fig. 5E), similar to the magnitude of reduction seen in the expression of PPARα targets in these mice.
PPARα ligand rescues triglyceride metabolism in Mogat1-deficient mice during fasting
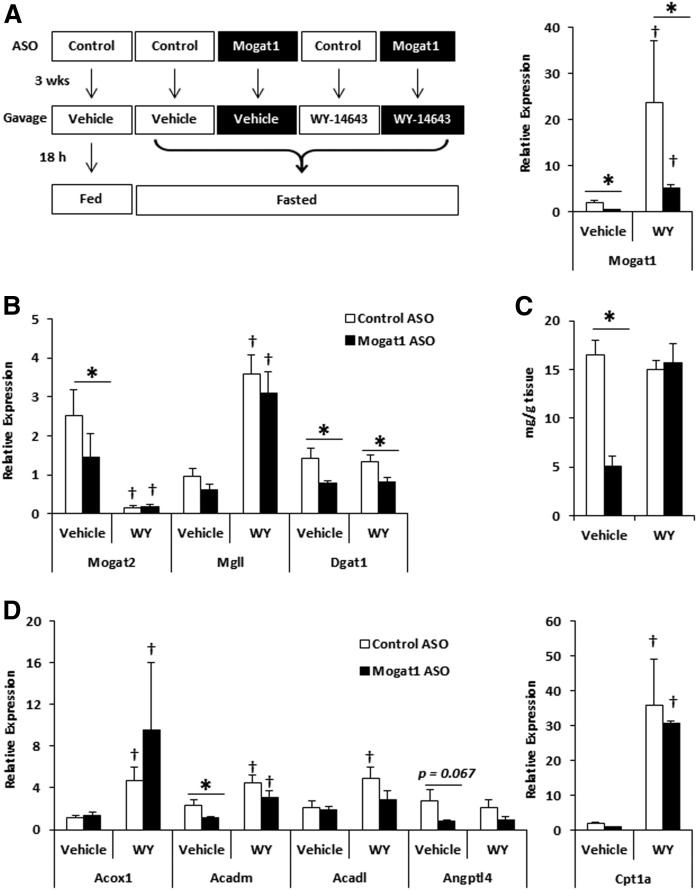
To determine whether stimulating PPARα activity would reverse the effects of Mogat1 knockdown on gene expression and rescue the metabolic phenotype of fasting, we administered a single oral gavage of vehicle or the potent PPARα agonist, WY-14643, to mice treated with control or Mogat1 ASO at the onset of an 18 h fast (Fig. 6A). The duration of fasting in this study was shorter (18 vs. 24 h) compared with most of the studies described above. Gene expression analyses revealed that PPARα ligand treatment of fasted mice strikingly induced the expression of Mogat1 (Fig. 6A), consistent with this gene being a target of PPARα. Conversely, Mogat2 expression was actually suppressed by WY-14643 administration. Ligand administration rescued the expression of Mgll, but did not change Dgat1 (Fig. 6B). Again, liver triglyceride content was reduced in fasted mice receiving Mogat1 ASO compared with ASO controls (Fig. 6C). In contrast to the longer 24 h fasting studies described above, Mogat1 ASO led to reduced plasma triglyceride and ketone concentrations after 18 h fasting (Table 3). WY-14643 treatment corrected hepatic triglyceride levels and plasma triglyceride and ketone concentration to that of ASO scramble control-treated mice receiving WY-14643 (Fig. 6C, Table 3). Lastly, the expression of several other PPARα target genes, including Acox1, Acadm, and Cpt1a, during fasting was rescued by WY-14643 (Fig. 6D). These data are suggestive that Mogat1 deficiency leads to reduced PPARα activity and that providing exogenous PPARα ligand reverses the effects of Mogat1 depletion on these parameters.
Fig. 6.
Changes in gene expression of PPARα target genes during prolonged fasting in mice following Mogat1 ASO treatment and WY-1463 gavage. Male C57BL/6J mice were injected (intraperitoneally) twice weekly with ASO targeted against Mogat1 or scramble control (25 mg/kg) for 3 weeks. Prior to fasting (18 h) and liver harvest, mice received an oral gavage of either vehicle (0.5% carboxymethyl cellulose) or PPARα ligand WY-14643 (100 mg/kg). A: Diagram of treatment groups and Mogat1 gene expression. B: Gene expression of triglyceride pathway enzymes normalized to fed control ASO vehicle gavaged mice. C: WY-14643 rescues hepatic triglyceride content in Mogat1 ASO-treated mice during fasting. D: Hepatic PPARα target gene expression. Data are shown as mean ± SE; *P < 0.05 versus control ASO group as indicated; †P < 0.05 versus vehicle gavaged mice within the same ASO treatment; n = 5.
TABLE 3.
- Plasma parameters in the WY-14643 study
| Control ASO (Fed) Vehicle | Control ASO (Fasted) Vehicle | Mogat1 ASO (Fasted) Vehicle | Control ASO (Fasted) WY-14643 | Mogat1 ASO (Fasted) WY-14643 | |
| Triglycerides (mg/dl) | 82.57 ± 6.7 | 167.4 ± 31.8 | 84.02 ± 7.1a | 119.1 ± 15.8 | 111.9 ± 9.8 |
| NEFA (mmol/l) | 0.38 ± 0.08 | 1.04 ± 0.1 | 0.73 ± 0.04 | 0.97 ± 0.11 | 1.08 ± 0.31 |
| Ketones (μmol/l) | 132.4 ± 9.3 | 1,276.4 ± 69.1 | 891.7 ± 144.4* | 1023.5 ± 132.6 | 988.6 ± 76.1 |
| Blood glucose (mg/dl) | 157 ± 4.5 | 79.4 ± 3.3 | 84.4 ± 3.0 | 89.2 ± 3.6 | 82.6 ± 1.8 |
Plasma was collected after oral gavage of vehicle or WY-14643 (100 mg/kg) and fasting (18 h) or fed. Data are shown as mean ± SE; fed controls shown for reference (n = 5).
P < 0.05 versus Control ASO (fasted) vehicle-treated mice.
DISCUSSION
MGAT activity may catalyze an important step in lipid metabolism, but the regulation and metabolic effects of this enzyme in tissues other than intestine are poorly understood. Here, we provide evidence that Mogat1 and MGAT activity is induced in liver of fasted mice and that this response occurs in a PPARα-dependent manner. MGAT activity contributes to hepatic triglyceride storage during fasting, possibly directly via its enzymatic activity and indirectly by regulating expression of other glycerolipid biosynthetic enzymes. Our data suggest that MGAT activity may indirectly regulate the expression of key enzymes in fatty acid metabolism by impacting PPARα activity. Indeed, Mogat1 ASO treatment reduced the expression of many PPARα target genes as well as PPARα activity in a DNA binding assay, and administering a synthetic PPARα ligand to mice with Mogat1 knockdown overcame the impaired activation of the expression of lipid metabolic enzymes. These findings suggest a novel role for Mogat1 in the hepatic fasting response.
Several observations suggest that Mogat1 plays a role in coordinating the hepatic fasting response, potentially via PPARα-dependent and -independent mechanisms. As discussed below, deactivation of Mogat1 suppressed expression of several PPARα target genes encoding enzymes involved in fatty acid oxidation and ketogenesis. Consistent with this, plasma ketone concentration, which is an indirect readout for hepatic fatty acid oxidation and ketogenesis, was reduced by Mogat1 knockdown after 18 h fasting. Plasma triglyceride content was also significantly reduced in mice receiving Mogat1 ASO and fasted for 18 h. We postulate that this effect may be secondary to diminished expression of the secreted LPL inhibitors, Angptl4 and Apoc3 (21–23), which have been suggested to reduce adipocyte reuptake of triglycerides in fasting conditions to direct them to other tissues (23). Further studies using tracers are required to fully measure metabolic flux with Mogat1 deactivation. However, the data presented in this study suggest that hepatic Mogat1 is sufficient to affect systemic energy metabolism via its direct enzymatic and indirect transcriptional regulatory effects.
PPARα not only controls the capacity for fatty acid oxidation but also triglyceride synthesis in liver (16, 17). Whereas previous work has shown that PPARγ is an important regulator of Mogat1 expression in steatotic liver, in this study we show using whole-body PPARα-null mice that this member of the PPAR family controls Mogat1 expression in nonsteatotic fed liver and the induction of Mogat1 that occurs with fasting. In fact, many genes regulating triglyceride metabolism, including Gpat1 and Dgat1, are directly regulated by PPARα activation (24–27). Previous work has shown that chromatin in the Mogat1 promoter is occupied by PPARα and directly responds to this nuclear receptor (27). In addition, the induction in Mogat1 expression by either fenofibrates or fasting is abolished in hepatocyte-specific PPARα-knockout mice (28). Interestingly, Mogat2 is unchanged by fasting, and its expression is actually downregulated by WY-14643 treatment and increased in PPARα-null mice, suggesting that distinct regulatory circuits control the expression of these two isoforms.
The present data suggest that Mogat1 expression is regulated by PPARα and also that the induction of this enzyme serves as a feed-forward mechanism to further enhance PPARα activity. Although many aspects of the mechanisms still need to be worked out, one possible explanation is that MGAT activity is important for generating or retaining an endogenous ligand for PPARα in liver. Increased MGAT activity during energy deprivation was first noted by Xia et al. in 1993, (10) long before the cloning of the enzymes that catalyze this enzymatic activity. Subsequent publications suggest that MGAT activity spares essential fatty acids from oxidation by reesterifying these lipids in the context of lipolytic stimuli (9, 29). In the original studies, MGAT1 was shown to prefer unsaturated long-chain acyl-CoAs as a substrate (5). Whether one of these essential fatty acids or complex lipids is serving as an endogenous ligand for PPARα or another complex lipid downstream of MGAT activity remains to be determined. Recently, deficiency in MAG lipase (Mgl1), another enzyme that catabolizes MAG, has been shown to improve insulin resistance and lower hepatic triglyceride accumulation (30). One important substrate for Mgl1 is 2-arachidonoylglycerol, an endogenous cannabinoid (endocannabinoid). Some endocannabinoids are reported to be PPARα ligands (31, 32). As MGAT activity converts MAG into DAG, thereby lowering the endogenous MAG pool, it is possible that MGAT may also be involved in endocannabinoid signaling. Hepatic 2-arachidonoylglycerol (MAG 20:4) increased with Mogat1 ASO treatment in the fed state suggesting a possible link between Mogat1 and endocannabinoids. The effects of 2-arachidonoylglycerol elevation on PPARα activity and whether other endocannabinoids are affected remain to be tested. MGAT activity may also not be unique in its effects on PPAR activity among lipid synthesis enzymes. Dgat1-null mice also have impaired PPARα activation in liver, muscle, and cardiac tissue (33). Furthermore, lipin 1, a glycerol-3-phosphate pathway PA phosphohydrolase, directly binds and coactivates PPARα to amplify fatty acid oxidation (34). Therefore, PPARα may be controlled by flux through the triglyceride pathway and through direct interactions with triglyceride synthetic enzymes themselves.
A limitation of the current study is that the ASO targeting Mogat1 leads to diminished expression of this enzyme systemically (mainly liver and adipose tissue) rather than a tissue-specific manner. This may be important because we have recently shown that Mogat1 is robustly induced in adipocytes during differentiation and is involved in reesterifying fatty acids to prevent their efflux from these cells (35). There are several points that suggest that the present observations are not due to adipose effects of the ASO. Although Mogat1 expression was decreased in adipose tissue of Mogat1 ASO-treated mice compared with controls, there was no effect on MGAT activity in the fasted state. There was also no observed effect of the Mogat1 ASO on plasma FFAs, MAG concentration, or the relative abundances of several species of lipids. Lastly, our previous work has suggested that the effects of adipocyte MGAT activity on fatty acid retention is most important in the fed state and does not affect lipolytic flux in the fasted state (35). Nonetheless, we cannot exclude the possibility that depletion of Mogat1 in adipose tissue by the ASO does not factor into the phenotype of our mice.
In conclusion, we have shown Mogat1 as a PPARα-regulated acyltransferase responsible for increased hepatic MGAT activity during fasting. We have also provided evidence for feed-forward regulation of PPARα activity by Mogat1. Further work using liver-specific knockout of multiple Mogat isoforms may provide clearer evidence for MGAT function during fasting. This work provides a basis for conducting future in vivo tracer studies to determine the contributions of MGAT activity toward whole-body lipid metabolism during fasting.
Supplementary Material
Footnotes
Abbreviations:
- ASO
- antisense oligonucleotide
- DAG
- diacylglycerol
- eWAT
- epididymal white adipose tissue
- MAG
- monoacylglycerol
- Mogat
- monoacylglycerol acyltransferase gene
- MGAT
- monoacylglycerol acyltransferase
- PA
- phosphatidic acid
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants T32 DK07120 (A.J.L., training grant), P30 DK052574 (K.S.M.), R56 DK111735 (A.M.H.), and R01 DK078187 (B.N.F); National Heart, Lung, and Blood Institute Grant K99 HL136658 (K.S.M.); and American Diabetes Association Grant 1-17-IBS-109 (A.M.H.). The Core services of the Diabetes Research Center (P30 DK020579) and the Nutrition Obesity Research Center (P30 DK56341) at the Washington University School of Medicine also supported this work. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Coleman R. A., and Lee D. P.. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43: 134–176. [DOI] [PubMed] [Google Scholar]
- 2.Shi Y., and Cheng D.. 2009. Beyond triglyceride synthesis: the dynamic functional roles of MGAT and DGAT enzymes in energy metabolism. Am. J. Physiol. Endocrinol. Metab. 297: E10–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chon S-H., Zhou Y. X., Dixon J. L., and Storch J.. 2007. Intestinal monoacylglycerol metabolism: developmental and nutritional regulation or monoacylglycerol lipase and monoacylglycerol acyltransferase. J. Biol. Chem. 282: 33346–33357. [DOI] [PubMed] [Google Scholar]
- 4.Hall A. M., Kou K., Chen Z., Pietka T. A., Kumar M., Korenblat K. M., Lee K., Ahn K., Fabbrini E., Klein S., et al. . 2012. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J. Lipid Res. 53: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yen C-L. E., Stone S. J., Cases S., Zhou P., and Farese R. V.. 2002. Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase. Proc. Natl. Acad. Sci. USA. 99: 8512–8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao J., Lockwood J., Burn P., and Shi Y.. 2003. Cloning and functional characterization of a mouse intestinal acyl-CoA:monoacylglycerol acyltransferase, MGAT2. J. Biol. Chem. 278: 13860–13866. [DOI] [PubMed] [Google Scholar]
- 7.Yue Y. G., Chen Y. Q., Zhang Y., Wang H., Qian Y-W., Arnold J. S., Calley J. N., Li S. D., Perry W. L., Zhang H. Y., et al. . 2011. The acyl coenzymeA:monoacylglycerol acyltransferase 3 (MGAT3) gene is a pseudogene in mice but encodes a functional enzyme in rats. Lipids. 46: 513–520. [DOI] [PubMed] [Google Scholar]
- 8.Yen C-L. E., and Farese R. V.. 2003. MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J. Biol. Chem. 278: 18532–18537. [DOI] [PubMed] [Google Scholar]
- 9.Mostafa N., Bhat B. G., and Coleman R. A.. 1993. Increased hepatic monoacylglycerol acyltransferase activity in streptozotocin-induced diabetes: characterization and comparison with activities from adult and neonatal rat liver. Biochim. Biophys. Acta. 1169: 189–195. [DOI] [PubMed] [Google Scholar]
- 10.Xia T., Mostafa N., Bhat B. G., Florant G. L., and Coleman R. A.. 1993. Selective retention of essential fatty acids: the role of hepatic monoacylglycerol acyltransferase. Am. J. Physiol. 265: R414–R419. [DOI] [PubMed] [Google Scholar]
- 11.Cortés V. A., Curtis D. E., Sukumaran S., Shao X., Parameswara V., Rashid S., Smith A. R., Ren J., Esser V., Hammer R. E., et al. . 2009. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 9: 165–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall A. M., Soufi N., Chambers K. T., Chen Z., Schweitzer G. G., McCommis K. S., Erion D. M., Graham M. J., Su X., and Finck B. N.. 2014. Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice. Diabetes. 63: 2284–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y. J., Ko E. H., Kim J. E., Kim E., Lee H., Choi H., Yu J. H., Kim H. J., Seong J-K., Kim K-S., et al. . 2012. Nuclear receptor PPARγ-regulated monoacylglycerol O-acyltransferase 1 (MGAT1) expression is responsible for the lipid accumulation in diet-induced hepatic steatosis. Proc. Natl. Acad. Sci. USA. 109: 13656–13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soufi N., Hall A. M., Chen Z., Yoshino J., Collier S. L., Mathews J. C., Brunt E. M., Albert C. J., Graham M. J., Ford D. A., et al. . 2014. Inhibiting monoacylglycerol acyltransferase 1 ameliorates hepatic metabolic abnormalities but not inflammation and injury in mice. J. Biol. Chem. 289: 30177–30188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agarwal A. K., Tunison K., Dalal J. S., Yen C-L. E., Farese R. V., Horton J. D., and Garg A.. 2016. Mogat1 deletion does not ameliorate hepatic steatosis in lipodystrophic (Agpat2-/-) or obese (ob/ob) mice. J. Lipid Res. 57: 616–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kersten S., Seydoux J., Peters J. M., Gonzalez F. J., Desvergne B., and Wahli W.. 1999. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 103: 1489–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leone T. C., Weinheimer C. J., and Kelly D. P.. 1999. A critical role for the peroxisome proliferator- activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc. Natl. Acad. Sci. USA. 96: 7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rusli F., Deelen J., Andriyani E., Boekschoten M. V., Lute C., van den Akker E. B., Müller M., Beekman M., and Steegenga W. T.. 2016. Fibroblast growth factor 21 reflects liver fat accumulation and dysregulation of signalling pathways in the liver of C57BL/6J mice. Sci. Rep. 6: 30484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Z., Gropler M. C., Norris J., Lawrence J. C., Harris T. E., and Finck B. N.. 2008. Alterations in hepatic metabolism in fld mice reveal a role for lipin 1 in regulating VLDL-triacylglyceride secretion. Arterioscler. Thromb. Vasc. Biol. 28: 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J. H., Song S. J., Kim A., Choi Y., Seok J. W., Kim H. J., Lee Y. J., Lee K. S., and Kim J.. 2016. Suppression of PPARγ-mediated monoacylglycerol O-acyltransferase 1 expression ameliorates alcoholic hepatic steatosis. Sci. Rep. 6: 29352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y., Azrolan N., Connell A. O., Walsh A., and Breslow J. L.. 1990. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 249: 790–793. [DOI] [PubMed] [Google Scholar]
- 22.Yoshida K., Shimizugawa T., Ono M., and Furukawa H.. 2002. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43: 1770–1772. [DOI] [PubMed] [Google Scholar]
- 23.Cushing E. M., Chi X., Sylvers K. L., Shetty S. K., Potthoff M. J., and Davies B. S. J.. 2017. Angiopoietin-like 4 directs uptake of dietary fat away from adipose during fasting. Mol. Metab. 6: 809–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das A. K., Aquilina J. W., and Hajra A. K.. 1983. The rapid induction of liver glycerophosphate acyltransferase in mice by clofibrate, a hypolipidemic agent. J. Biol. Chem. 258: 3090–3093. [PubMed] [Google Scholar]
- 25.Rakhshandehroo M., Sanderson L. M., Matilainen M., Stienstra R., Carlberg C., de Groot P. J., Müller M., and Kersten S.. 2007. Comprehensive analysis of PPARalpha-dependent regulation of hepatic lipid metabolism by expression profiling. PPAR Res. 2007: 26839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakhshandehroo M., Hooiveld G., Müller M., and Kersten S.. 2009. Comparative analysis of gene regulation by the transcription factor PPARalpha between mouse and human. PLoS One. 4: e6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sankella S., Garg A., and Agarwal A. K.. 2016. Characterization of the mouse and human monoacylglycerol O-acyltransferase 1 (Mogat1) promoter in human kidney proximal tubule and rat liver cells. PLoS One. 11: e0162504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montagner A., Polizzi A., Fouché E., Ducheix S., Lippi Y., Lasserre F., Barquissau V., Régnier M., Lukowicz C., Benhamed F., et al. . 2016. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut. 65: 1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostafa N., Bhat B. G., and Coleman R. A.. 1994. Adipose monoacylglycerol: acyl-coenzyme A acyltransferase activity in the white-throated sparrow (Zonotrichia albicollis): characterization and function in a migratory bird. Lipids. 29: 785–791. [DOI] [PubMed] [Google Scholar]
- 30.Taschler U., Radner F. P., Heier C., Schreiber R., Schweiger M., Schoiswohl G., Preiss-Landl K., Jaeger D., Reiter B., Koefeler H. C., et al. . 2011. Monoglyceride lipase deficiency in mice impairs lipolysis and attenuates diet-induced insulin resistance. J. Biol. Chem. 286: 17467–17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fu J., Gaetani S., Oveisi F., Verme J. L., Serrano A., de Fonseca F. R., Rosengarth A., Luecke H., Giacomo B. D., Tarzia G., et al. . 2003. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 425: 90–93. [DOI] [PubMed] [Google Scholar]
- 32.Guzmán M., Lo Verme J., Fu J., Oveisi F., Blázquez C., and Piomelli D.. 2004. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor alpha (PPAR-alpha). J. Biol. Chem. 279: 27849–27854. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Yu S., Khan R. S., Ables G. P., Bharadwaj K. G., Hu Y., Huggins L. A., Eriksson J. W., Buckett L. K., Turnbull A. V., et al. . 2011. DGAT1 deficiency decreases PPAR expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J. Lipid Res. 52: 732–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finck B. N., Gropler M. C., Chen Z., Leone T. C., Croce M. A., Harris T. E., Lawrence J. C. Jr., and Kelly D. P.. 2006. Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 4: 199–210. [DOI] [PubMed] [Google Scholar]
- 35.Liss K. H. H., Lutkewitte A. J., Pietka T., Finck B. N., Franczyk M., Yoshino J., Klein S., and Hall A. M.. 2018. Metabolic importance of adipose tissue monoacylglycerol acyltransferase 1 in mice and humans. J. Lipid Res. 59: 1630–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.