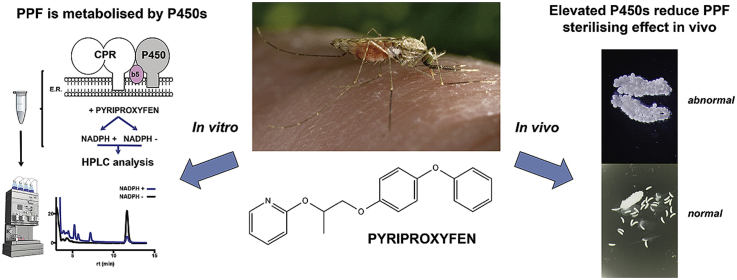
Abstract
Pyrethroid resistance is widespread in the malaria vector Anopheles gambiae leading to concerns about the future efficacy of bednets with pyrethroids as the sole active ingredient. The incorporation of pyriproxyfen (PPF), a juvenile hormone analogue, into pyrethroid treated bednets is being trialed in Africa. Pyrethroid resistance is commonly associated with elevated levels of P450 expression including CYPs 6M2, 6P2, 6P3, 6P4, 6P5, 6Z2 and 9J5. Having expressed these P450s in E. coli we find all are capable of metabolizing PPF. Inhibition of these P450s by permethrin, deltamethrin and PPF was also examined. Deltamethrin and permethrin were moderate inhibitors (IC50 1–10 μM) of diethoxyfluorescein (DEF) activity for all P450s apart from CYP6Z2 (IC50 > 10 μM), while PPF displayed weaker inhibition of all P450s (IC50 > 10 μM) except CYP's 6Z2 and 6P2 (IC50 1–10 μM). We found evidence of low levels of cross resistance between PPF and other insecticide classes by comparing the efficacy of PPF in inhibiting metamorphosis and inducing female sterility in an insecticide susceptible strain of An. gambiae and a multiple resistant strain from Cote d’Ivoire.
Keywords: Pyriproxyfen, Insecticide resistance, P450, Olyset Duo
Abbreviations: PPF, pyriproxyfen; ALA, 5-Aminolevulinic acid; DEF, diethoxyfluorescein; CPR, cytochrome P450 reductase
Graphical abstract
Highlights
-
•
Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance.
-
•
Pyrethroids may synergise pyriproxyfen activity.
-
•
Pyriproxyfen efficacy is compromised in a multiple resistant An. gambiae strain.
-
•
Resistance monitoring for pyriproxyfen chemistry is recommended.
1. Introduction
Malaria control is reliant on the use of insecticides. The dramatic reductions in malaria cases in Africa that have occurred over the last 15 years have been largely attributed to methods targeting the adult mosquito, primarily via the use of long lasting insecticidal nets (LLINs) treated with pyrethroids and, to a lesser extent indoor residual spraying (IRS) with pyrethroids and DDT and, more recently, carbamates and organophosphates (Bhatt et al., 2015, Ranson and Lissenden, 2016). Resistance to pyrethroids is now widespread in the major malaria vectors in Africa with resistance to other classes of public health insecticides also on the increase (Ranson and Lissenden, 2016). There is therefore an urgent need both for new insecticides to maintain the efficacy of these proven tools, and for new tools to reduce malaria transmission by the mosquito.
Pyriproxyfen (PPF) is a juvenile hormone analogue that inhibits metamorphosis. It has been used for several decades to protect against cotton pests (Carriere et al., 2012) and its extremely low toxicity to humans has also enabled applications in public health such as addition to water storage containers to control Aedes populations (Darriet and Corbel, 2006, Lee, 2001). As PPF is active in very low concentrations, the active ingredient can be disseminated by the insect itself; this autodissemination route was shown to be effective at controlling Aedes populations in trials in Peru (Devine et al., 2009). For malaria vectors, difficulties in identifying and treating the diverse breeding sites for malaria vectors have so far largely confined larviciding for malaria control to easy to reach urban areas but the possibility of using autodissemination strategies to distribute PPF to target Anopheles oviposition sites in rural areas is being explored. Currently, however, control of Anopheles mosquitoes is more commonly targeted at the adult stage, hence the impact of PPF on embryogenesis, shows the greatest promise for malaria control. Exposure to PPF effectively sterilizes female mosquitoes and has also been shown to reduce adult longevity (Ohashi et al., 2012, Ngufor et al., 2014). Sumitomo Chemicals Ltd has developed a LLIN incorporating both permethrin and PPF. This Olyset Duo®, net has been shown to be effective in laboratory and experimental hut trials (Aiku et al., 2006, Ngufor et al., 2014, Ohashi et al., 2012, Tsunoda et al., 2013) and is currently being evaluated in a randomised control trial in Burkina Faso to compare the efficacy of this combination net with conventional Olyset nets (Tiono et al., 2015).
However, concerns have been raised about the performance of Olyset Duo against pyrethroid resistant populations (Koffi et al., 2015). An experimental hut study of Olyset Duo carried out in an area where the Anopheles gambiae population has high levels of both target site and metabolic resistance to pyrethroids found no significant difference in the number of sterile mosquitoes in huts with Olyset Duo compared to control huts (Koffi et al., 2015).
Resistance to PPF has been reported in other insects including the greenhouse whitefly Trialeurodes vaporiarium (Karatolos et al., 2012) and the sweet potato whitefly, Bemisia tabaci (Rami Horowitz et al., 2003). Although the mechanisms of resistance have not been fully described, elevated levels of genes involved in insect P450 and GST activity appear to be involved.
Several P450 enzymes have been implicated in the development of metabolic insecticide resistance in An. gambiae but a relatively small subset of this large enzyme family are consistently found up-regulated in pyrethroid resistant populations (David et al., 2013; Ingham et al., 2014). This candidate list is predominated by three subfamilies of the CYP6 P450s: CYP6P, CYP6M and CYP6Z, but also includes CYP9J5 (Hemingway et al., 2013, Toé et al., 2015) and the two CYP4G enzymes, CYP4G16 and 17 (Jones et al., 2013; Toé et al., 2015). CYP6M2 and CYP6P3 are confirmed pyrethroid metabolisers but are also active against insecticides from other insect classes (Mitchell et al., 2012, Muller et al., 2008, Stevenson et al., 2011). In contrast the CYP4G enzymes do not have detectable activity against insecticides but instead are believed to confer resistance via reducing insecticide uptake (Balabanidou et al., 2016).
Here, we expressed the CYPs 6M2, 6P1, 6P2, 6P3, 6P4, 6P5, 6Z2 and 9J5 in Escherichia coli and assessed their ability to metabolize PPF. We also assessed the likelihood of cross resistance between PPF and other insecticide classes by comparing the efficacy of PPF in inhibiting metamorphosis and inducing female sterility in an insecticide susceptible strain of An. gambiae and a multiple resistant strain from Cote d’Ivoire.
2. Material and methods
2.1. Reagents
Oligonucleotides were synthesized by Eurofins genomics and enzymes for DNA manipulation were supplied by Thermo Scientific. Isopropyl-ß-D-thio-galactopyranoside (IPTG), 5-aminolevulinic acid (ALA), and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) were supplied by Melford (UK). Insecticides were supplied by ChemService: 3-phenoxybenzyl (1R,S)-cis,trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate (permethrin, mixture of isomers), (S)-α-cyano-3-phenoxybenzyl (1R,3R)-cis-2,2-dimethyl-3-(2,2-dibromovinyl)-cyclopropanecarboxylate (deltamethrin) and pyriproxyfen. HPLC solvents were supplied by Fisher Scientific. Other chemicals were obtained from Sigma-Aldrich unless indicated otherwise.
2.2. Gene cloning
Total RNA was extracted with either the Arcturus PicoPure Kit (Applied Biosystems) or TRI reagent (Sigma-Aldrich) from ten adult An. gambiae mosquitoes from the Kisumu strain. Complementary DNA was prepared using Superscript III (Invitrogen) with an oligo(dT)20 primer and used as a template for amplifying full-length genes with KOD DNA polymerase (Merk Chemicals). The gene-specific primers used in these high-fidelity PCRs were designed according to the An. gambiae genome sequence (Table S1). PCR products from P450s, summarized in Table S2, were ligated into pGEM T-easy (Promega) and sequenced. For expression, the ompA leader sequence (ompA), was engineered onto the amino-terminus to direct the P450 to the E. coli outer membrane during expression as previously described (Mclaughlin et al., 2008, Stevenson et al., 2011). The ompA-leader was fused to the P450 cDNA in frame with the P450 initiation codon by fusion PCR. The ompA-leader was fused to the P450 cDNA in frame with the P450 initiation codon by fusion PCR. The ompA P450 fusion was flanked at the 5′ and 3′ ends with NdeI and NotI respectively for ligation into NdeI/NotI digested pCWori+.
For CYP6M2 and CYP6P3 a single plasmid expression system for the P450 and CPR was constructed by ligating the expression cassette (cDNA and tactac promotor) containing the An. gambiae NADPH P450 reductase (CPR) cDNA from the pACYC:AgCPR plasmid described above into the pCW:P450 expression plasmid. In the new construct the expression of each protein is under the control of its own tactac promoter so expression of both proteins is induced by the addition of IPTG to the culture.
2.3. Preparation of membranes expressing P450 and AgCPR
For dual plasmid co-expression of P450 and AgCPR (all P450s except CYP6M2 and CYP6P3), competent E. coli DH5α cells were co-transformed with pCW:P450 plasmid and pACYC:AgCPR (Stevenson et al., 2011). Cultures, generally 0.2 l, were supplemented with 1.0 mM ALA and incubated at 23 °C for 18–24 h after 1 mM IPTG induction. P450 expression, E. coli membrane isolation and determination of P450 and AgCPR content was performed as previously described (Mclaughlin et al., 2008, Stevenson et al., 2012). Samples were stored in aliquots at −80 °C. An. gambiae cytochrome b5 (b5) was prepared as described previously to supplement enzyme reactions at a 10:1 M ratio, b5:P450 (Stevenson et al., 2011).
For CYP6M2 and CYP6P3, E. coli JM109 were transformed with the single plasmid system for the co-expression of AgCPR. Cultures were grown in 10 l stirred batch, glass fermenters and were harvested and processed to produce membrane fractions containing the CYP and CPR using the procedures described above, scaled to account for the larger culture volumes. Cytochrome b5 was added to the CYP6P3 bacterial membrane preparations at a 10:1 M ratio, b5:P450. After the addition of the cytochrome b5 the membrane preparation was gently stirred at 4 °C for 30 min to allow time for the incorporation of the cytochrome b5 into the membranes before aliquotting and freezing at −80 °C.
2.4. Pyriproxyfen metabolism
Incubations of a 200 μl reaction mix containing 20 μM insecticide, 0.1 μM of the recombinant enzyme, 1.0 μM b5, 0.2 M of TrisHCl at pH 7.4, 0.25 mM MgCl2, 1 mM glucose-6-phosphate, 0.1 mM NADP+ and 1 unit/mL glucose-6-phosphate dehydrogenase (G6PDH) were carried out in the presence or absence of 10 μM piperonyl butoxide (PBO) at 30 °C with shaking (1200 rpm) for 90 min and stopped by addition of 200 μl of methanol. Shaking was carried for an additional 10 min before centrifuging the reactions at 20000 g for 20 min. 150 μl of supernatant was used for HPLC analysis. Reactions were performed in triplicate and a paired T-test of sample reactions (+NADPH) vs negative control (-NADPH) used for statistical measurements of substrate depletion.
For reciprocal IC50 measurements of CYP6P3 metabolism of PPF and permethrin, enzyme reactions were carried out with PPF or permethrin added as inhibitors (at concentrations ranging from of 0–1.6 mM or 0–3.2 mM respectively) to the reaction mix to titer their effect on the insecticide turnover (fixed at 20 μM). Three replicates of positive and negative control reactions were run for each P450/inhibitor combination.
2.5. High-pressure liquid chromatography (HPLC) analysis
Samples were analyzed by high-pressure liquid chromatography, HPLC (Agilent 1100 series). The quantity of insecticide remaining in the samples was determined by reverse-phase HPLC with a monitoring absorbance at 232 nm using a C18 column, Acclaim 120, Thermo Scientific. 100 μl of sample was loaded with a flow-rate 1 ml/min at 23 °C into an isocratic mobile phase 90% methanol and 10% water. The retention time for PPF is 7.4 min and for PBO 7.1 min.
The same conditions were applied for HPLC analysis for the PPF/permethrin inhibition assays. Retention time for trans- and cis-permethrin is 11.8 min and 14.1 min respectively.
2.6. Mass spectrometry analysis for pyriproxyfen metabolism
PPF metabolism by CYP6P3 was examined by mass spectrometry to confirm oxidation and identify the metabolites produced. Aliquots (5 μl) of organic solvent-quenched reaction supernatant were injected onto a high resolution Thermo Q-Exactive mass spectrometer (MS) that was coupled to a 1290 series Agilent LC system. The chromatographic separation was performed on a Waters Acquity BEH C18 (2.1 × 50 mm; 1.7 μm) analytical column at 30 °C using a mixture of water (0.1% formic acid) (A) and acetonitrile (0.1% formic acid) (B) as mobile phase. In the 12 min run time the gradient program was as follows: 5% B in 0–1 min; 5% B to 100% B in 1–8 min; 100% B in 8–10 min; 100% B to 5% B in 10–10.1 min; 5% B in 10.1–12 min. During the analysis the MS was operated in positive ion full scan mode (mass range: 100–1000 m/z) at 35 K resolution using a constant heated electrospray capillary temperature (320 °C), spray voltage (3500 V), sheath gas (55 arbitrary units) and auxiliary gas flow rate (10 arbitrary units).
2.7. Diethoxyfluorescein metabolism
Diethoxyfluorescein (DEF) substrate was dissolved in DMSO, with final concentration of 2% per assay. All test compounds were dissolved in DMSO, with a final solvent concentration <2% per assay. For calculation of the kinetic parameters (KM and Vmax), each P450 was used at a final concentration of 10 nM (1 pmol/reaction) and DEF concentrations in the range: 0, 0.31, 0.63, 1.25, 2.5, 5, 10 and 20 μM. DEF reactions were carried out at 25 °C in 50 mM KPi at pH 7.4 containing 1 mM glucose-6-phosphate (G6P), 0.1 mM NADP+, 0.25 mM MgCl2, and cytochrome b5 at a 10:1 M ratio, b5:P450. NADP+ and G6P were excluded from the minus NADPH controls.
Variable ligand concentrations were used for IC50 calculations with DEF used at ∼ KM for each P450 (i.e. 0.5, 1.4, 0.7, 1.0, 3.5 and 0.5 μM for CYP6M2, CYP6P2, CYP6P3, CYP6P4, CYP9J5, and CYP6Z2 respectively) and 0.1 μM P450. Three replicates of positive and negative control reactions were run for each P450/substrate combination in opaque white 96-well (flat-based) plates in triplicate. The fluorescent reactions were monitored in a fluorescence plate-reader (Ex = 485 nm, Em = 520 nm) continuously over 20 min time period after the addition of NADPH regenerating system. The rate of fluorescent molecules produced per P450 molecule per min (turnover) was determined by linear regression of the measurements between 3 min and 10 min after the reactions began. The Michaelis-Menten and IC50 fitting calculations were performed using Graphpad Prism 6. Data were fitted to the dose-response model and plots with R2 < 0.95 were rejected.
2.8. In vivo studies
Two strains of mosquitoes were used to assess the impact of exposure to PPF on life history. The Kisumu strain of An. gambiae originates from Kenya and is susceptible to all insecticide classes used in public health whereas the Tiassalé strain from Cote d'Ivoire shows resistance to four classes (pyrethroids, carbamates, organophosphates and the organochlorine DDT) (Edi et al., 2012). Resistance in the Tiassalé strain is mediated by multiple mechanisms, including the overexpression of cytochrome P450s, notably CYP6P3, 6P4 and 6M2 (Edi et al., 2014). Both mosquito strains were reared in the insectaries at the Liverpool School of Tropical Medicine under a 12:12 photoperiod at 27 °C and 70–80% humidity.
To measure the effect of PPF on metamorphosis, SumiLarv®0.5G (Sumitomo Chemicals Ltd) was ground into a fine powder and dissolved in water to prepare a stock solution of 1000 ppm SumiLarv (50 ppm active ingredient). The solution was left overnight dissolving on a magnetic stirrer, protected from light. Serial dilutions were prepared and the following PPF concentrations were tested: 0.001 ppb, 0.005 ppb, 0.07 ppb, 0.1 ppb, 1 ppb, 5 ppb and 10 ppb. Four replicates of 25 3rd instar mosquitoes were exposed to each of the SumiLarv concentrations in paper cups for up to 8 days. Larvae were fed Tetramin® baby fish food every day and cups covered with netting to prevent adults escaping. The number of live and dead larvae, pupae and adults was recorded every 24 h until all individuals were emerged as adults or dead. Adults and dead pupae were removed daily. The Dose Effect function on XLSTAT (Microsoft) was used to estimate the concentration resulting in 50% emergence inhibition (EI50).
To compare the impact of PPF on adult mosquitoes of the two strains, we measured the ability of this compound to impair ovary development. Borosilicate glass tubes (30 cm long, 11 mm wide) were impregnated with three different concentrations of PPF (ai): 0.55 mg/m2, 2.75 mg/m2 and 5.5 mg/m2. An additional tube impregnated only with the solvent (acetone) was used as a negative control. Tubes were used on the day of preparation. Two groups of fifteen 5–7 days old female mosquitoes from Tiassalé and Kisumu strains were tested for each concentration (n = 30). After 60 min acclimation in paper cups, they were transferred to the glass tubes and exposed for 3 min. Mosquitoes were then returned to the paper cups and left for 24 h with a 10% sucrose solution. 24 h after exposure the mosquitoes were bloodfed and any mosquitoes which did not feed were removed. Mosquitoes were retained in insectary conditions with access to sugar water for five days and then dissected and the morphology of the ovaries assessed as normal (loose, well developed eggs) or abnormal (non-detachable, bubble-like eggs). Dead mosquitoes or mosquitoes not presenting egg development were discarded and removed from the analysis.
To confirm that formulated products containing PPF also impaired egg development, mosquitoes were exposed to Olyset Duo nets, nets containing 1% PPF only (supplied by Sumitomo Chemicals Ltd), conventional Olyset nets or untreated nets. Batches of 10 3–5 days old Tiassalé mosquitoes were exposed to the nets for 3 min following WHO standard protocols for cone bioassays. Twenty-four hours after the exposure, mosquitoes were bloodfed and left in paper cups with 10% sucrose solution. Dead and non-bloodfed mosquitoes were removed from the experiment. After 5 days surviving mosquitoes were dissected and the morphology of the ovaries examined as described above. To assess the effects of short exposures to PPF LLINs, an additional set of cone bioassays was performed, as described above, except exposure time was reduced to 30 s.
3. Results and discussion
This study was motivated by concerns over cross resistance between pyrethroid insecticides and the insect growth regulator, PPF. Cross resistance between insecticide classes with different modes of action, mediated by cytochrome P450s, has previously been demonstrated in An. gambiae (Edi et al., 2014, Mitchell et al., 2012) and here we investigated whether these same P450 enzymes can also metabolize PPF. CYP6M2, CYP6P3 and CYP6Z2 have previously been expressed in bacterial expression systems but we extended the panel of recombinant enzymes to include a further three An. gambiae CYP6P P450s implicated in pyrethroid resistance in Cote d’Ivoire (Edi et al., 2014) and CYP9J5, which has been found over expressed in pyrethroid resistant populations from Bioko Island (Hemingway et al., 2013) and Burkina Faso (Toé et al., 2015).
3.1. Functional expression of P450s in E. coli
P450s require electrons from NADPH-cytochrome P450 oxido-reductase (CPR) for catalysis, thus new candidate An. gambiae P450s were co-expressed with AgCPR in E. coli using ompA and pelB leader sequences to direct the enzymes to the inner bacterial membrane as previously with CYP6M2 and CYP6P3 (Muller et al., 2008, Stevenson et al., 2011). CYP's 6P1, 6P2, 6P4, 6P5, 9J5 and 6M1 were co-transformed with AgCPR-pACYC for E. coli expression, producing characteristic CO-reduced spectra indicative of active P450 (Fig. S1). The yields of P450 were in the range 10–100 nmol/l, with CYP6P5 producing the lowest quantities of P450 (∼10 nmol/L; Table S3). CYPs 6P1 and 6M1 failed to express functional P450.
CYP6M2 and CYP6P3 have previously been expressed following co-transformation with AgCPR on separate plasmids. Here, these enzymes were co-expressed in tandem with AgCPR on a single from the P450 expression plasmid, pCWori + to facilitate scaled 10 l fermentor expression. Tandem expression from the single plasmid produced higher CPR:P450 ratios. The fluorogenic substrate DEF was used to estimate the kinetic parameters of the tandemly expressed recombinant proteins against dual plasmid protein expression (Table 1) and used in dose-response experiments to determinate the inhibitory effect of permethrin, deltamethrin and pyriproxyfen in its metabolism. As expected, since CPR is rate limiting, the Vmax values were 3–6 fold higher using the single plasmid in tandemly expressed membranes compared with dual plasmid expression, consistent with the elevated levels of CPR. The KM value is the concentration of DEF required to reach max reaction velocity and independent of the enzyme concentration. The single versus double plasmid KM values for CYP6M2 (0.4 vs 0.5 μM) and CYP6P3 (0.7 vs 0.9 μM) were similar, again consistent with higher CPR levels increasing reaction rates through enhanced electron transfer rather than effects on substrate binding.
Table 1.
Kinetic parameters for DEF.
| P450 | Kinetic parameters for DEF |
|||
|---|---|---|---|---|
| Single plasmid expression |
Dual plasmid expression |
|||
| KM (μM) | Vmax (RFU/sec) | KM (μM) | Vmax (RFU/sec) | |
| CYP6M2 | 0.4 ± 0.02 | 902.2 ± 106.99 | 0.5 ± 0.01 | 292.5 ± 38.05 |
| CYP6P3 | 0.7 ± 0.10 | 54.4 ± 6.52 | 0.9 ± 0.09 | 14.8 ± 4.89 |
| CYP6P2 | nt | nt | 1.4 ± 0.02 | 300.6 ± 12.00 |
| CYP6P4 | nt | nt | 1.0 ± 0.04 | 24.2 ± 1.26 |
| CYP9J5 | nt | nt | 3.4 ± 0.21 | 265.1 ± 28.75 |
| CYP6Z2 | nt | nt | 0.5 ± 0.3 | 43.7 ± 8.9 |
nt = not tested; (mean ± SD).
3.2. PPF metabolism
The ability of the An. gambiae P450s to metabolize PPF was tested by measuring substrate turnover (substrate disappearance over time) in the presence and absence of NADPH. We also included PBO in parallel reactions as further validation of P450 induced substrate depletion (Fig. S2). PBO is an inhibitor of P450 monoxygenase activity and a common insecticide synergist (Vijayan et al., 2007). All seven P450s metabolized PPF to some degree with the percentage PPF depletion ranging from 24.78% for CYP9J5 to 100% for CYP6P3, and PPF depletion was inhibited by PBO for each P450 tested (Table 2). Since E. coli membranes expressing CYP6M2 and CYP6P3 had higher levels of CPR, the rates of activity were not comparable with the rest of the P450s. However, it is notable that, with the exception of CYP6P5, all members of the CYP6P family and CYP6Z2 produced high levels of PPF depletion (58–100%). CYP6Z2 is of interest since it is found overexpressed in pyrethroid resistant populations of An. gambiae, but metabolises the pyrethroid metabolites 3-phenoxbenzoic alcohol and aldehyde rather than the parent compound (Chandor-Proust et al., 2013). Here CYP6Z2 appears to play a direct role in the primary metabolism of PPF, thus may have an influential role in PPF clearance and potentially insecticide resistance.
Table 2.
Pyriproxyfen metabolism by mosquito P450s.
| P450 | % PPF depletion |
Inhibition ratio (%)a | |
|---|---|---|---|
| −PBO | + PBO | ||
| CYP6M2 | 30.93 ± 4.65 | 1.44 ± 0.67 | 95.3 |
| CYP6P2 | 58.03 ± 1.35 | 6.45 ± 3.22 | 91.0 |
| CYP6P3 | 100.0 ± 0.01 | 8.68 ± 1.74 | 91.3 |
| CYP6P4 | 81.63 ± 0.63 | 4.44 ± 1.99 | 94.6 |
| CYP6P5 | 39.96 ± 1.04 | 2.40 ± 2.01 | 95.4 |
| CYP9J5 | 24.78 ± 2.13 | 0.08 ± 0.15 | 99.6 |
| CYP6Z2 | 66.26 ± 3.02 | 8.41 ± 1.58 | 87.3 |
Reduction of PPF depletion in percentage caused by the inhibitory effect of PBO; (mean ± SD).
Mass spectrometry analysis of the PPF metabolites generated by CYP6P3, the strongest metabolizer was carried out to confirm oxidation and identify possible metabolites. Expected metabolites included 4′-OH-PPF, 5″-OH- PPF and 5″,4′-OH-PPF, that have been previously identified from the in vitro metabolism of PPF by microsomes from housefly larvae (Zhang et al., 1998). CYP6P3 generated three metabolite peaks (Fig. S3). The extracted ion chromatograms of [M+H]+ generated two peaks (6.9 min and 7.1 min), with molecular mass (m/z = 338.1387) corresponding to the addition of a hydroxyl group (m/z = 16), consistent with 4′-OH-pyriproxyfen and 5″-OH-pyriproxyfen production. Furthermore, the data showed signs of a metabolite with molecular mass 32 m/z larger than PPF, equivalent to a double hydroxylation, potentially 5″,4′-OH-PPF resulting from secondary metabolism of 5″-OH-PPF and/or 4′-OH-PPF metabolites. This was, however, not confirmed chromatographically as analytical reference standards were not available for the analysis. Further collision mass spectrometry or NMR is required to confirm the identity of the metabolites.
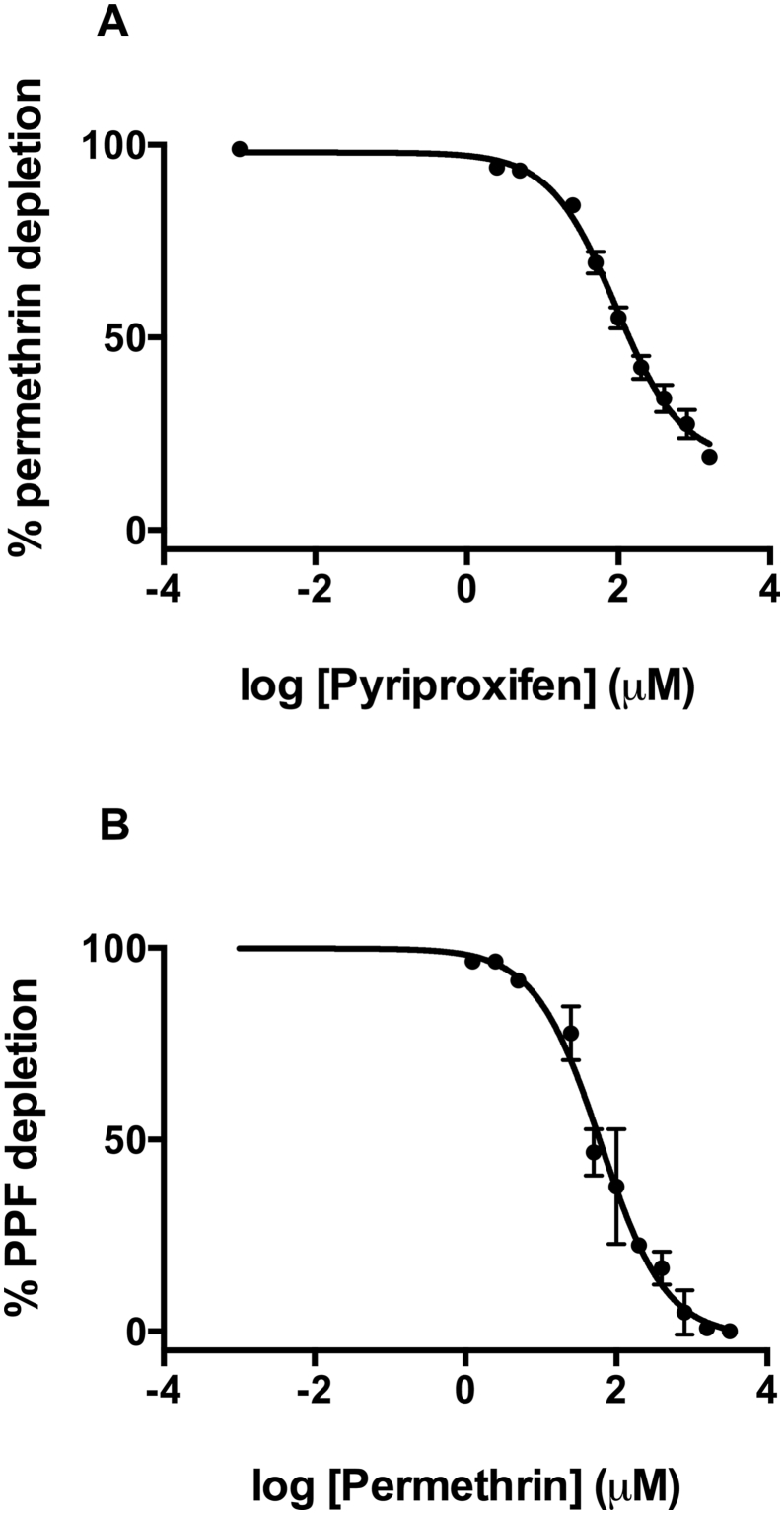
Since PPF is being used in combination with pyrethroids in bednets we were interested in potential synergistic effects. We therefore measured the IC50 values of PPF, deltamethrin and permethrin to compare relative strengths of DEF metabolism inhibition against the P450s (Table 3). The fluorescent substrate, DEF, was used for monitoring P450 activity using a 96 well microtiter plate format. In drug screens, compounds are generally categorized according to their activity as P450 inhibitors as potent inhibitors (IC50 < 1 μM), moderate (IC50 1–10 μM) and weak inhibitors (IC50 > 10 μM) (Krippendorff et al., 2007). Using these criteria, PPF displayed moderate inhibition of DEF metabolism for CYP's 6Z2 and 6P2, with the remainder being weakly inhibited. Deltamethrin and permethrin were moderate inhibitors of DEF activity for all P450s apart from CYP6Z2, which was weakly inhibited by both pyrethroids. As Olyset Duo nets contain both PPF and permethrin, we also measured PPF inhibition of permethrin metabolism by CYP6P3 and vice versa (Fig. 1). CYP6P3 was chosen as it is one of the P450s most frequently found at elevated levels of expression in pyrethroid resistant populations of An. gambiae. Permethrin produced slightly stronger inhibition of PPF metabolism (IC50 = 61.2 μM) than PPF inhibition of permethrin metabolism (IC50 = 92.7 μM). Overall, the in vitro results suggest that the pyrethroids deltamethrin and permethrin are slightly stronger inhibitors (2–3 fold) than PPF against the pyrethroid metabolizing P450s tested.
Table 3.
IC50 values for mosquito P450s.
| P450 | IC50 (μM) |
||
|---|---|---|---|
| Pyriproxyfen | Deltamethrin | Permethrin | |
| CYP6M2 | 14.14 | 4.24 | 8.07 |
| CYP6Z2 | 2.23 | 13.99 | 13.72 |
| CYP6P2 | 9.95 | 4.97 | 8.61 |
| CYP6P3 | 15.82 | 3.17 | 6.77 |
| CYP9J5 | 18.96 | 6.05 | 6.47 |
Fig. 1.
Determination of IC50values of permethrin and PPF in P450 metabolism. Dose-response analysis of the inhibitory effect of (A) pyriproxyfen on permethrin metabolism and (B) permethrin on pyriproxyfen metabolism.
These data indicate that PPF can be metabolized by a wide range of P450s associated with pyrethroid resistance. In mosquitoes that have elevated levels of expression of one or more of these enzymes, it is feasible that enhanced metabolism of PPF could reduce the efficacy of this juvenile hormone analogue. To test this hypothesis we performed PPF bioassays on insecticide susceptible and resistant strains.
3.3. Bioefficacy of PPF against insecticide resistant mosquitoes
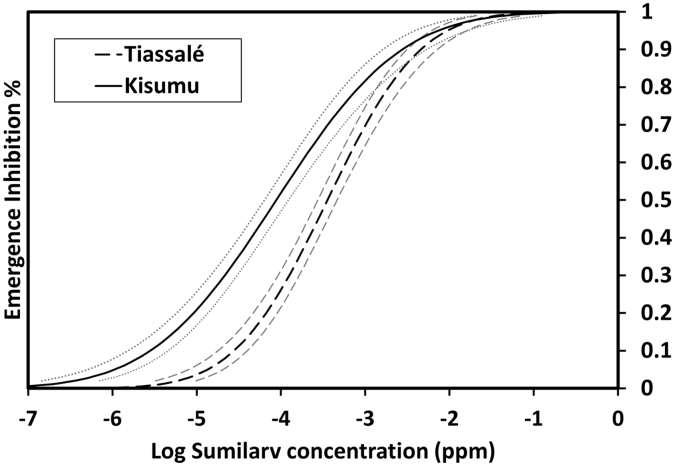
The impact of exposure to PPF on metamorphosis and embryogenesis was compared in the insecticide susceptible Kisumu strain and the multi resistant Tiassalé strain from Cote d’Ivoire. The dose-response tests showed that SumiLarv® 0.5G affected adult mosquito emergence in both strains but the minimum dose that inhibited 100% emergence was 1 ppb in the insecticide susceptible Kisumu strain and 10 ppb in the Tiassalé strain. The concentration that resulted in 50% inhibition of emergence (EI50) for the susceptible Kisumu strain was 0.088 ppb (95% confidence intervals 0.064–0.123 ppb) (Fig. 2), similar to values reported for An. gambiae s.l. in other studies (0.025 ppb Kawada (1993), 0.13 ppb (Mbare et al., 2013)). In contrast, the EI50 for the Tiassalé strain was 0.356 ppb ai (0.274–0.463) approximately 4-fold higher than the Kisumu strain. It is important to note that we cannot directly link the higher EI50 for PPF to the presence of elevated P450s in the Tiassale strain given that only one pyrethroid resistant population was evaluated and it is not known whether the same P450s found elevated in adults of this strain are also up-regulated at the larval stage. Furthermore, the field dose of SumiLarv 0.5G ranges from 10 to 50 ppb so it is likely that the product would still inhibit development of the Tiassalé strain under field conditions. However, given the trajectory of increasing pyrethroid resistance in both Anopheles (Ranson and Lissenden, 2016) and Aedes mosquitoes (Bariami et al., 2012) and the growing interest in use of PPF to target immature populations of these vectors (Kiware et al., 2015; Abad-Franch et al., 2015) it is important that further evaluation of PPF efficacy against field populations is carried out.
Fig. 2.
SumiLarv emergence inhibition curves for two strains of An. gambiae. Emergence inhibition dose response curves for the insecticide susceptible Kisumu strain (continuous line) and insecticide resistant Tiassalé strain (dotted line). The grey dotted lines represent 95% upper and lower limits.
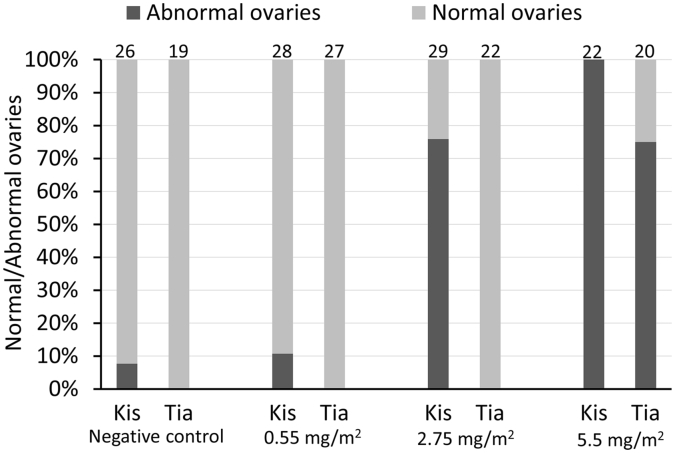
As the sterilizing effect of PPF on mosquito populations has also received considerable attention for malaria control, with clinical trials of the PPF/permethrin Olyset Duo LLIN ongoing (Tiono et al., 2015, Sagnon et al., 2015) we also compared inhibition of embryogenesis in our susceptible and resistant populations. In a narrow bore glass tube assay, designed to ensure complete contact with the PPF for the duration of the assay, mosquitoes from the insecticide susceptible Kisumu strain were completely sterilized after a 3 min exposure to 5.5 mg/m2 whereas only 75% of Tiassalé mosquitoes were sterilized by this dose. At half this dose, PPF had no impact on ovary development in Tiassalé but resulted in 76% of Kisumu mosquitoes being sterilized (Fig. 3). Thus higher concentrations of PPF are needed to sterilize the pyrethroid resistant strain, consistent with the effect on metamorphosis described above.
Fig. 3.
Effect of pyriproxyfen on egg development for two strains of An. gambiae. Proportions of normal/abnormal ovaries of mosquitoes from Tiassale and Kisumu strains exposed to three different concentrations of pyriproxyfen. The number over each bar corresponds to the sample size for each treatment.
3.4. Interactions between PPF and permethrin
The in vitro analysis demonstrated that the same P450s can bind and metabolize PPF and pyrethroids. This interaction could have a synergizing effect with one chemical essentially reducing the rate of depletion of the other such that both chemicals are more potent when used in combination. As the pyrethroid IC50s were generally lower than for PPF (with the exception of CYP6Z2), pyrethroids might be expected to have a stronger enhancing effect on PPF activity than vice versa. However, the differences in IC50 values were small (∼2 fold) and, as the in vivo concentrations in mosquitoes after exposure to products containing PPF and/or permethrin are unknown, such predictions in isolation are highly speculative. Furthermore, as shown above, the performance of PPF varies between strains and thus the impact of combining the two chemistries in vector control products may depend on the level of expression of P450s in the strain.
To investigate this further we evaluated the performance of LLINs containing a single active ingredient versus the combination Olyset Duo LLIN in cone bioassays. The Tiassalé strain was exposed to four net types, mortality recorded 24 h after exposure and surviving mosquitoes were offered a bloodmeal. Ovary development was assessed after a further 5 days. As expected very low mortality was observed in the mosquitoes exposed to untreated or PPF only nets (Table 4). Mortality was higher after exposure to Olyset Duo nets than conventional Olyset nets (2-tailed z test, p = 0.02). All bloodfed mosquitoes exposed to the Olyset or untreated nets developed normal ovaries. In contrast all of the mosquitoes exposed to the 1% PPF net were sterilized. The number of surviving mosquitoes that successfully bloodfed from the Olyset Duo arm was small but surprisingly only 60% of these mosquitoes were sterilized. The differential sterilizing effect of nets containing 1% w/w PPF alone and Olyset Duo (with 1% PPF and 2% permethrin) was confirmed in follow up cone bioassay study in which mosquitoes were only exposed for 30 s. Here >87% of Tiassalé mosquitoes exposed to PPF nets (n = 34 dissections) were sterilized versus 0% for untreated nets (n = 39) and only 16% for Olyset Duo nets (n = 38) (Table S4).
Table 4.
Impact of exposure to LLINs containing permethrin and/or pyripoxyfen on mosquito mortality and egg development. An. gambiae Tiassalé strain were exposed to the LLINs for 3 min. Mortality was measured 24 h later and surviving mosquitoes offered a blood meal. Ovary dissections were performed 5 days later. n bloodfed accounts for the number of surviving mosquitoes that fed on blood and survived for five days until ovary dissections.
| LLIN | n | Mortality % (95%CI) | n bloodfed | Abnormal | % Sterilized |
|---|---|---|---|---|---|
| Untreated | 45 | 2.22 (0.12–13.2) | 23 | 0 | 0 |
| Olyset | 46 | 30.4 (18.2–45.9) | 19 | 0 | 0 |
| Olyset Duo | 46 | 54.3 (39.2–68.8) | 10 | 6 | 60 |
| PPF | 46 | 2.17 (0.11–13.0) | 35 | 35 | 100 |
Taken together these results suggest that PPF increases the efficacy of permethrin but permethrin reduces the efficacy of PPF. This is supported by data showing higher mortality rates in huts with Olyset Duo than with Olyset in areas with resistant mosquitoes (Ngufor et al., 2014 in Benin, Koffi et al., 2015 in Cote d’Ivoire) (although no increase in the proportion of mosquitoes sterilized in huts containing PPF only nets versus Olyset Duo nets was observed, as our laboratory data would have predicted). It is important to note that these experiments are conducted on formulated products, and although the concentration of permethrin and PPF does not differ between net types, they may differ in their bleed rates affecting the bioavailability of the two active ingredients. Further laboratory and field evaluations, against vectors with differing levels of metabolic resistance, are needed to better predict the performance of combination products, such as Olyset Duo, in the field.
4. Conclusions
Given that pyrethroid resistant populations of malaria vectors are now ubiquitous in Africa, it is important to evaluate the possible impact of this resistance on the performance of any new vector control tools. In this study we demonstrated that a subset of mosquito P450 enzymes responsible for elevated pyrethroid metabolism in insecticide resistant mosquitoes can also metabolize PPF. As metabolic resistance is an increasingly problematic resistance mechanism in African malaria vectors, there is a very real concern that PPF resistance may already be present in field population of Anopheles mosquitoes. Although the levels of PPF resistance we observed in the current study are low, continual monitoring for resistance to this chemistry should be undertaken in any area employing PPF as a larvicide or considering PPF use for adult mosquito control.
Funding
The research leading to these results has received funding from the European Union Seventh Framework Programme FP7 (2007–2013) under grant agreement no 265660 AvecNet. N.G was supported by the ‘Francisco Jose de Caldas’ PhD fellowship from the Colombian Administrative Department of Science, Technology and Innovation (COLCIENCIAS), Call No 529.
Acknowledgements
We thank Sumitomo Chemicals Ltd for providing the samples of bednets and SumiLarv for this study and Dr Dave Weetman for helpful comments on the manuscript.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ibmb.2016.09.001.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Abad-Franch F., Zamora-Perea E., Ferraz G., Padilla-Torres S.D., Luz S.L.B. Mosquito-disseminated pyriproxyfen yields high breeding-site coverage and boosts juvenile mosquito mortality at the neighborhood scale. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiku A.O., Yates A., Rowland M. Laboratory evaluation of pyriproxifen treated bednets on mosquito fertility and fecundity. A preliminary study. West Afr. J. Med. 2006;25:22–26. doi: 10.4314/wajm.v25i1.28240. [DOI] [PubMed] [Google Scholar]
- Bhatt S., Weiss D.J., Cameron E., Bisanzio D., Mappin B., Dalrymple U. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526 doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanidou V., Kampouraki A., MacLean M., Blomquist G.J., Tittiger C., Juarez M.P., Mijailovsky S.J., Chalepakis G., Anthousi A., Lynd A., Antoine S., Hemingway J., Ranson H., Lycett G.J., Vontas J. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1608295113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariami V., Jones C.M., Poupardin R., Vontas J., Ranson H. Gene amplification, ABC transporters and cytochrome P450s: unraveling the molecular basis of pyrethroid resistance in the dengue vector. Aedes aegypti. PLoS Negl. Trop. Dis. 2012;6:e1692. doi: 10.1371/journal.pntd.0001692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere Y., Ellers-Kirk C., Hartfield K., Larocque G., Degain B., Dutilleul P., Dennehy T.J., Marsh S.E., Crowder D.W., Li X., Ellsworth P.C., Naranjo S.E., Palumbo J.C., Fournier A., Antilla L., Tabashnik B.E. Large-scale, spatially-explicit test of the refuge strategy for delaying insecticide resistance. PNAS. 2012;109:775–780. doi: 10.1073/pnas.1117851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandor-Proust A., Bibby J., Régent-Kloeckner M., Roux J., Guittard-Crilat E., Poupardin R., Riaz M.A., Paine M., Dauphin-Villemant C., Reynaud S., David J.-P.P., Regent-Kloeckner M., Roux J., Guittard-Crilat E., Poupardin R., Riaz M.A., Paine M., Dauphin-Villemant C., Reynaud S., David J.-P.P., Régent-Kloeckner M., Roux J., Guittard-Crilat E., Poupardin R., Riaz M.A., Paine M., Dauphin-Villemant C., Reynaud S., David J.-P.P. The central role of mosquito cytochrome P450 CYP6Zs in insecticide detoxification revealed by functional expression and structural modelling. Biochem. J. 2013;455:75–85. doi: 10.1042/BJ20130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darriet F., Corbel V. Laboratory evaluation of pyriproxyfen and spinosad, alone and in combination, against Aedes aegypti larvae. J. Med. Entomol. 2006;43:1190–1194. doi: 10.1603/0022-2585(2006)43[1190:leopas]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- David J., Ismail H.M., Chandor-proust A., Ingraham M.J., John M., Paine I. Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecticides on Earth Role of cytochrome P450s in insecticide resistance: impact on the control of mosquito-borne diseases and use of insecti. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2013;368 doi: 10.1098/rstb.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine G.J., Perea E.Z., Killeen G.F., Stancil J.D., Clark S.J., Morrison A.C. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc. Natl. Acad. Sci. 2009;106:11530–11534. doi: 10.1073/pnas.0901369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edi C.V., Djogbénou L., Jenkins A.M., Regna K., Muskavitch M.A.T., Poupardin R., Jones C.M., Essandoh J., Kétoh G.K., Paine M.J.I., Koudou B.G., Donnelly M.J., Ranson H., Weetman D. CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10:e1004236. doi: 10.1371/journal.pgen.1004236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edi C.V., Koudou B.G., Jones C.M., Weetman D., Ranson H. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Cote d'Ivoire. Emerg Infect Dis. 2012;18:1508–1511. doi: 10.3201/eid1809.120262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J., Vontas J., Poupardin R., Raman J., Lines J., Schwabe C., Matias A., Kleinschmidt I. Country-level operational implementation of the global plan for insecticide resistance management. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9397–9402. doi: 10.1073/pnas.1307656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham V.A., Jones C.M., Pignatelli P., Balabanidou V., Vontas J., Wagstaff S.C., Moore J.D., Ranson H. Dissecting the organ specificity of insecticide resistance candidate genes in Anopheles gambiae: known and novel candidate genes. BMC Genom. 2014;15:1018. doi: 10.1186/1471-2164-15-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C.M., Haji K.A., Khatib B.O., Bagi J., Mcha J., Devine G.J., Daley M., Kabula B., Ali A.S., Majambere S., Ranson H. The dynamics of pyrethroid resistance in Anopheles arabiensis from Zanzibar and an assessment of the underlying genetic basis. Parasit. Vectors. 2013;6:343. doi: 10.1186/1756-3305-6-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawada H., Shono Y., Ito T., Abe Y. Laboratory evaluation of insect growth regulators against several species of anopheline mosquitoes. Jpn J. Sanit. Zool. 1993;44:349–353. [Google Scholar]
- Karatolos N., Williamson M.S., Denholm I., Gorman K., ffrench-Constant R.H., Bass C. Over-expression of a cytochrome P450 is associated with resistance to pyriproxyfen in the greenhouse whitefly trialeurodes vaporariorum. PLoS One. 2012;7 doi: 10.1371/journal.pone.0031077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiware S.S., Corliss G., Merrill S., Lwetoijera D.W., Devine G., Majambere S., Killeen G.F. Predicting scenarios for successful autodissemination of pyriproxyfen by malaria vectors from their resting sites to aquatic habitats; description and simulation analysis of a field-parameterizable model. PLoS One. 2015;10:e0131835. doi: 10.1371/journal.pone.0131835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffi A.A., Ahoua Alou L.P., Djenontin A., Kabran J.-P.K., Dosso Y., Kone A., Moiroux N., Pennetier C. Efficacy of Olyset(®) Duo, a permethrin and pyriproxyfen mixture net against wild pyrethroid-resistant Anopheles gambiae s.s. from Côte d’Ivoire: an experimental hut trial. Parasite. 2015;22:28. doi: 10.1051/parasite/2015028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippendorff B.-F., Lienau P., Reichel A., Huisinga W. Optimizing classification of drug-drug interaction potential for CYP450 isoenzyme inhibition assays in early drug discovery. J. Biomol. Screen. Off. J. Soc. Biomol. Screen. 2007;12:92–99. doi: 10.1177/1087057106295897. [DOI] [PubMed] [Google Scholar]
- Lee D.-K. Field evaluation of an insect growth regulator, pyriproxyfen, against Aedes togoi larvae in brackish water in South Korea. J. Vector Ecol. 2001;26:39–42. [PubMed] [Google Scholar]
- Mbare O., Lindsay S.W., Fillinger U. Dose-response tests and semi-field evaluation of lethal and sub-lethal effects of slow release pyriproxyfen granules (Sumilarv(R)0.5G) for the control of the malaria vectors Anopheles gambiae sensu lato. Malar. J. 2013;12:94. doi: 10.1186/1475-2875-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mclaughlin L.A., Niazi U., Bibby J., David J.P., Vontas J., Hemingway J., Ranson H., Sutcliffe M.J., Paine M.J.I. Characterization of inhibitors and substrates of Anopheles gambiae CYP6Z2. Insect Mol. Biol. 2008;17:125–135. doi: 10.1111/j.1365-2583.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- Mitchell S.N., Stevenson B.J., Muller P., Wilding C.S., Egyir-Yawson A., Field S.G., Hemingway J., Paine M.J.I., Ranson H., Donnelly M.J. Identification and validation of a gene causing cross-resistance between insecticide classes in Anopheles gambiae from Ghana. Proc. Natl. Acad. Sci. U. S. A. 2012;109:6147–6152. doi: 10.1073/pnas.1203452109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P., Warr E., Stevenson B.J., Pignatelli P.M., Morgan J.C., Steven A., Yawson A.E., Mitchell S.N., Ranson H., Hemingway J., Paine M.J.I., Donnelly M.J. Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngufor C., N'Guessan R., Fagbohoun J., Odjo A., Malone D., Akogbeto M., Rowland M. Olyset Duo?? (a pyriproxyfen and permethrin mixture net): an experimental hut trial against pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus in southern Benin. PLoS One. 2014;9 doi: 10.1371/journal.pone.0093603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi K., Nakada K., Ishiwatari T., Miyaguchi J., Shono Y., Lucas J.R., Mito N. Efficacy of pyriproxyfen-treated nets in sterilizing and shortening the longevity of Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 2012;49:1052–1058. doi: 10.1603/me12006. [DOI] [PubMed] [Google Scholar]
- Rami Horowitz A., Gorman K., Ross G., Denholm I. Inheritance of pyriproxyfen resistance in the whitefly, Bemisia tabaci (Q biotype) Arch. Insect Biochem. Physiol. 2003:177–186. doi: 10.1002/arch.10115. [DOI] [PubMed] [Google Scholar]
- Ranson H., Lissenden Insecticide resistance in African Anopheles mosquitos: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016:187–196. doi: 10.1016/j.pt.2015.11.010. (in press) [DOI] [PubMed] [Google Scholar]
- Sagnon N., Pinder M., Tchicaya E.F., Tiono A.B., Faragher B., Ranson H., Lindsay S.W. To assess whether addition of pyriproxyfen to long-lasting insecticidal mosquito nets increases their durability compared to standard long-lasting insecticidal mosquito nets: study protocol for a randomised controlled trial. Trials. 2015;16:195. doi: 10.1186/s13063-015-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B.J., Bibby J., Pignatelli P., Muangnoicharoen S., O'Neill P.M., Lian L.Y., Muller P., Nikou D., Steven A., Hemingway J., Sutcliffe M.J., Paine M.J.I. Cytochrome P450 6M2 from the malaria vector Anopheles gambiae metabolizes pyrethroids: sequential metabolism of deltamethrin revealed. Insect Biochem. Mol. Biol. 2011;41:492–502. doi: 10.1016/j.ibmb.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Stevenson B.J., Pignatelli P., Nikou D., Paine M.J.I. Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: developing new tools to combat insecticide resistance. PLoS Negl. Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiono A.B., Pinder M., N'Fale S., Faragher B., Smith T., Silkey M., Ranson H., Lindsay S.W. The AvecNet Trial to assess whether addition of pyriproxyfen, an insect juvenile hormone mimic, to long-lasting insecticidal mosquito nets provides additional protection against clinical malaria over current best practice in an area with pyrethroid-resist. Trials. 2015;16:1–12. doi: 10.1186/s13063-015-0606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toé K.H., N'Falé S., Dabiré R.K., Ranson H., Jones C.M. The recent escalation in strength of pyrethroid resistance in Anopheles coluzzi in West Africa is linked to increased expression of multiple gene families. BMC Genomics. 2015;16:1–11. doi: 10.1186/s12864-015-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda T., Kawada H., Huynh T.T.T., Luu L., Le S.H., Tran H.N., Vu H.T.Q., Le H.M., Hasebe F., Tsuzuki A., Takagi M. Field trial on a novel control method for the dengue vector, Aedes aegypti by the systematic use of Olyset® Net and pyriproxyfen in Southern Vietnam. Parasit. Vectors. 2013;6:6. doi: 10.1186/1756-3305-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan V.A., Sathish Kumar B.Y., Ganesh K.N., Urmila J., Fakoorziba M.R., Makkapati A.K. Efficacy of piperonyl butoxide (PBO) as a synergist with deltamethrin on five species of mosquitoes. J. Commun. Dis. 2007;39:159–163. [PubMed] [Google Scholar]
- Zhang L., Kasai S., Shono T. In vitro metabolism of pyriproxyfen by microsomes from susceptible and resistant housefly larvae. Arch. Insect Biochem. Physiol. 1998;37:215–224. doi: 10.1002/(SICI)1520-6327(1998)37:3<215::AID-ARCH4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.